Abstract
6-Formylindolo (3, 2-b) Carbazole (FICZ) is a ligand of aryl hydrocarbon receptor (AHR) which regulates Th17 release of IL-17 and IL-22 production. Earlier, we showed that ethanol combined with burn injury suppresses Th17 responses and disrupts intestinal barrier leading to increased gut bacterial growth and translocation. Since IL-22 is known for its role in intestinal barrier maintenance, we determined whether treatment of mice with FICZ restores T cell IL-22 release and protects intestine barrier following ethanol and burn injury. Wildtype and Rag1−/− mice were gavaged with ~2.9g/kg ethanol or water, and given a ~12.5% total body surface area burn. Mice were given FICZ (5μg) in resuscitation fluid. FICZ treatment of wildtype mice normalized IL-22 and IL-17 in lamina propria and spleen T cells, as well as increased CYP1A1 expression in spleen T cells. This was accompanied by improved gut motility, decreased copy number of small intestine total bacteria and Enterobacteriaceae, attenuation of intestinal tissue levels of IL-6, KC, IL-18, decreased apoptosis, and prevention of gut leakiness following ethanol and burn injury. However, FICZ treatment of Rag1−/− mice did not improve any of the parameters listed after ethanol and burn injury. Additional data generated using mice treated with recombinant IL-22 alone or in combination with anti-IL-18 antibody suggest that full protection of gut barrier integrity requires both IL-18 inhibition and IL-22 restoration following ethanol and burn injury. Together our findings suggest that AHR ligand FICZ may have better therapeutic potential for maintenance of gut barrier function after ethanol and burn injury.
Keywords: Alcohol, burn Injury, T cells, gut barrier integrity, FICZ
1. Introduction
The human intestine contains approximately one trillion microorganisms. Under normal conditions, these microorganisms aid in the digestion of food and protect the host by limiting pathogenic bacterial colonization in the intestinal lumen and stimulating immune responses against pathogens (1,2). Under abnormal conditions, such as trauma/burn injury and inflammatory bowel disease, the balance between the bacterial flora and intestinal barrier is disturbed. In these situations, intestinal bacteria can translocate from the intestinal lumen to other organs, such as the mesenteric lymph nodes (MLN), spleen, liver, and blood, which results in systemic inflammation, sepsis, and multiple organ dysfunction (MOD) and failure (MOF).(3–5).
Excessive consumption of alcohol (ethanol) remains a major risk factor for all types of traumatic injuries including burn injury. Previous studies using hospitalized patients have found those who have consumed ethanol prior to burn injury have higher incidence of infection, multiple organ failure, and mortality (6–8). A major cause of enhanced morbidity and mortality in these patients is the increased incidence of bacterial infections and suppressed immune defense to invading pathogens (9–13). Furthermore, clinical and experimental evidence suggests that burn injury results in suppressed immune effector responses and disruption of the intestinal barrier (9–12,14). Previous studies from our laboratory have shown that ethanol intoxication combined with burn injury suppresses T cell proliferation, IL-2, and IFN-γ release, which was accompanied by an increase in bacterial growth, intestinal permeability, and bacterial translocation (15–18). We further observed that ethanol intoxication combined with burn injury also decreases Th17 effector functions in intestinal lymphoid organs (mesenteric lymph nodes and Peyer's patches), as well as in systemic immune organs (spleen) (19,20).
Additionally, studies have indicated that Th17 effector cytokines IL-22 and IL-17 play an important role in maintenance of intestinal immune homeostasis and intestinal barrier function. IL-17 induces formation of tight junctions by regulating the expression of claudin-1, claudin-2, and occludin in epithelial cells and reduces gut permeability following DSS-induced colitis (21,22). IL-22 induces protective mucus production in a mouse model of ulcerative colitis (23). In addition, IL-22 induces antimicrobial peptides Reg3β and Reg3γ against C. rodentium infection in mice (24). We observed that the suppression of Th17 effector cytokines IL-17 and IL-22 following ethanol and burn injury is accompanied by a decrease in the expression of Aryl hydrocarbon receptor (AHR) and CYP1A1 in T cells (19,20). AHR is a ligand-dependent transcription factor, which upon binding to its ligand, translocates from the cytoplasm to the nucleus where it dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT). The AHR-ARNT dimer binds upstream of target genes, which contain xenobiotic-responsive element (XRE) consensus sequences, such as the cytochrome P450 family 1 gene, CYP1A1, to induce transcription (25,26). AHR activation is also involved in Th17 cell differentiation and regulates IL-17 and IL-22 release (25,26). Our laboratory has shown promising protective effects of treatment with recombinant IL-22 in mitigating bacterial overgrowth and restoring intestinal barrier function following ethanol and burn injury, though it did not result in complete recovery (17,18).
Parallel studies have identified Interleukin-18 (IL-18) as a pro-inflammatory mediator in ethanol intoxication and burn injury. IL-18 is produced by both immune and non-immune cells, and is a key driver of the inflammation and neutrophil recruitment (27,28). Previous studies from our laboratory as well as reports by others have shown that the presence of IL-18 can lead to tissue cell death in experimental models of infection, burn and ischemia reperfusion injury (29–31). Additional findings from our laboratory have demonstrated that administration of neutralizing anti-IL-18 antibodies significantly restores occludin and claudin-1 expression in intestinal epithelial cells of rats following ethanol and burn injury (29).
In this study, we treated mice with an AHR agonist 6-Formylindolo (3, 2-b) Carbazole (FICZ) to determine whether AHR activation modulates the intestinal laminal propria (LP) Th17 cell response and protects the gut barrier after ethanol and burn injury. We observed that mice treated with the AHR ligand FICZ prevented decreases in IL-22 and IL-17, which was accompanied by reduced IL-18 production, and gut leakiness after ethanol and burn injury. Since AHR is expressed in several types of immune cells, we used Rag1−/− mice lacking mature T and B cells to further determine whether treatment with AHR agonist FICZ protected the intestinal barrier in a T cell-dependent manner. Our results suggest that mice treated with FICZ have decreased intestinal bacteria growth and gut leakiness in a T cell-dependent manner following ethanol and burn injury.
2. Materials and methods
2.1. Animals and reagents
Male C57/BL6 mice (22-25 g) were obtained from Charles River Laboratories (Wilmington, MA). Rag1−/− mice and wildtype mice were obtained from Jackson laboratories (Bar Harbor, ME). IL-6 enzyme-linked immunosorbent assay (ELISA) kit was obtained from BD Biosciences (San Diego, CA). IL-17 and KC ELISA kits were obtained from R&D Systems (Minneapolis, MN). IL-22 and IL-18 ELISA kits, PE IL-22 (Clone 1H8PWSR, Catalog # 12-7221-82, eBioscience™), eFluor450 IL-17 (Clone eBio17B7 Catalog # 48-7177-82 eBioscience™) and APC-eFluor780 CD3 antibodies (Clone 17A2Catalog # 47-0032-82, eBioscience™), and Fixable Viability Dye eFluor506 were obtained from eBioscience (San Diego, CA). Cell Death Detection ELISA kit was obtained from Roche (Catalog # 11920685001, Indianapolis, IN). FITC-dextran (4D) was obtained from Sigma-Aldrich (St. Louis, MO). Primers to CYP1A1 and β-actin, MirVana miRNA Isolation Kit, High Capacity cDNA Reverse Transcription Kit, and TaqMan Gene Expression Master Mix were obtained from Life Technologies (Grand Island, NY). AHR agonist, 6-formylindolo (3, 2-b) carbazole (FICZ) was obtained from Abeam (Cambridge, MA).
2.2. Mouse model of acute ethanol intoxication and burn injury and FICZ treatment
As described previously (17–19,32), 22-25g wildtype and Rag1−/− male mice were randomly divided into four groups: sham vehicle, sham vehicle treated with FICZ, burn ethanol, and burn ethanol treated with FICZ. In the ethanol treated group, mice were gavaged with 0.4 ml of 25% ethanol (~2.9g/Kg) and vehicle group mice were gavaged with 0.4 ml of water. Four hours after the gavage, mice were anesthetized with a mixture of ketamine and xylazine by intraperitoneal injection (IP) and transferred into a template fabricated to expose ~12.5% of the total body surface area (TBSA). TBSA was calculated by using Meeh’s formula as described by Walker and Mason (33). For burn injury, mice were immersed in a ~90°C water bath and sham mice were immersed in a ~37°C water bath for ~7 seconds. Mice were resuscitated with 1.0 ml physiological saline by IP injection. For FICZ treated group, mice were given FICZ (5μg) in 1.0ml physiological saline by IP injection. For anti-IL-18 antibody and recombinant IL-22 (rIL-22) treatment groups, mice were treated with anti-IL-18 antibody (1mg/kg, MBL International, Woburn, MA) alone or in combination with rIL-22 (1mg/kg GenScript, Piscataway Township, NJ) in 1.0ml physiological saline by IP injection at time injury. After recovery from anesthesia, mice were returned to their cages and allowed food and water ad libitum. In this series of experiments, we observed an ~20% overall mortality within 24 hours after ethanol and burn injury. Wildtype mice treated with FICZ did not show any mortality after injury. However nearly 50% Rag−/− mice did not survive after ethanol and burn injury regardless of FICZ treatment. All the animal procedures were carried out in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Loyola University Chicago Health Sciences Division Institutional Animal Care and Use Committee (IACUC # 2016010).
2.3. Isolation of small intestinal lamina propria (LP) cells
LP cells were isolated as previously described (34). One day after injury, mice were euthanized and the abdominal cavity was exposed via midline incision. ~8 cm of the distal small intestine was collected, and fat tissue and Peyer’s patches (PPs) were removed from each animal. The harvested small intestine segments were opened longitudinally, cut into 4-5 cm pieces, and washed in cold PBS. Small intestines were incubated in HBSS supplemented with 10mMol/L HEPES, 50μg/ml gentamicin, 100U/ml penicillin, 100μg/ml streptomycin, 5mM EDTA and 1mM DTT (predigestion solution) for 20 min at 37°C with agitation to remove epithelial cells. Tissues were then cut into small pieces and incubated in RPMI-1640 supplemented with 2mMol/L L-glutamine, 10mMol/L HEPES, 50μg/ml gentamicin, 100U/ml penicillin, 100μg/ml streptomycin, 10% FCS, 0.5mg/ml Collagenae D (Roche), 200 units/ml DNAse I (Sigma-Aldrich), and 0.5mg/ml Dispase II (Roche) for 20 min. at 37°C with shaking, repeated 2-3 times. The digested tissues were passed through 70 μM cell strainers and centrifuged at 1200 rpm for 10 min at 20°C. Cell pellets were suspended in a 40% fraction of Percoll and overlaid with 80% fraction of Percoll. LP cells were collected at the 40/80 interface after centrifuging at 2400 rpm for 20 min at 20°C. The cells were washed and resuspended in cold PBS with 2% FCS.
2.4. Flow cytometry
Isolated LP cells (1x106) were stimulated with PMA (10ng/ml) and ionomycin (50ng/ml) in cell culture medium for 2 h. Following stimulation, GolgiPlug Protein Transport Inhibitor (1 μl/ml, BD) was added to the culture medium for another 3 h. The cells were harvested, washed with staining buffer (PBS with 5% FCS), and incubated with Fixable Viability Dye (1μl/ml, eBioscience) for 30 min for dead cell staining. Anti-CD16/32 antibodies were added to cells to block Fc Receptor. The cells were stained with APC-eFluor780 anti-mouse CD3 antibody for 30 min and washed with staining buffer two times. Intracellular staining (IL-17 and IL-22) was performed using BD Cytofix/Cytoperm Fixation/Permeabilization Kit according to the manufacture’s instruction. Flow cytometry data were collected using the FACS Canto II (BD Biosciences), and analyzed by FlowJo software (Tree Star Inc.).
2.5. Isolation of spleen T cells
For isolation of T cells, spleens were gently crushed in HBSS supplemented with 10mMol/L HEPES, 50μg/ml gentamicin, 100U/ml penicillin, and 100μg/ml streptomycin. Cell suspensions were centrifuged at 1200 rpm for 10 min at 10°C. The red blood cells were lysed by adding 9ml of sterile-distilled H2O followed by 1ml of 10x phosphate-buffered saline (PBS) and centrifuged at 1200 rpm for 10 min at 10°C. The supernatant was discarded and the cells were washed. 106-107 total spleen cells were resuspended in 90μl of separation buffer (PBS containing 0.5% BSA and 2 mMol/L EDTA) and incubated with 10μl of CD90 (Thy1.2) MicroBeads (MiltenyiBiotec, Auburn, CA) for 15 min at 4°C. The cells were washed with separation buffer and run through separation columns (Miltenyi Biotec) in a magnetic field. Purified T cells were obtained by flushing out magnetically labeled cells from the separation columns (19).
2.6. Measurement of cytokines
As previously described (19), isolated spleen T cells (5x105 cells/well) were cultured in RPMI-1640 supplemented with 2mMol/L L-glutamine, 10mMol/L HEPES, 50μg/ml gentamicin, 100U/ml penicillin, 100μg/ml streptomycin, and 10% FCS (complete RPMI-1640) in 96-well plates pre-coated with anti-CD3 (5μg/ml) in the presence of soluble anti-CD28 (1μg/ml) at 37°C and 5% CO2 for 48 h. The supernatants were harvested to determine IL-17 and IL-22 levels using ELISA kits according to the manufacturer’s instructions.
For the measurement of small intestine tissue inflammatory cytokines, ~8 cm of distal small intestines were homogenized using Cell Lysis buffer with Protease Inhibitor Cocktail (Cell Signaling Technology, Danvers, MA) and supernatants were collected to determine IL-6, KC, IL-18, and apoptosis by their respective ELISA kits according to the manufacture’s instruction and normalized with protein(32).
2.7. Measurement of spleen T cell CYP1A1 mRNA expression
Spleen T cells (5x105 cells/well) were cultured in complete RPMI-1640 in 96-well plates pre-coated with anti-CD3 (5μg/ml) in the presence of anti-CD28 (1μg/ml) at 37°C and 5% CO2 for 48h. Cells were collected for extraction of total RNA by using mirVana miRNA Isolation Kit according to the manufacturer’s instructions. The total RNA concentration was determined by Nanodrop spectrophotometer (Thermo Scientific). 1μg of the total RNA was used for cDNA reverse transcription using a High Capacity cDNA Reverse Transcription Kit according to the manufacturer’s instructions. CYP1A1 expression was analyzed by RT-PCR and normalized with β-actin.
2.8. Intestinal permeability
As previously described (17), one-day after injury, mice were anesthetized. During the entire procedure, mice remained anesthetized with isoflurane by using Vaporstick Small Animal Anesthesia Machine (Smiths medical PM Inc. Waukesha, WI). The abdominal cavity was opened, and renal artery and renal vein in both kidneys were ligated. A 10-cm-long segment of distal small intestine was ligated at both ends without damaging intestinal and mesenteric structures. An 18-gauge BD Insyte Autoguard Shielded IV catheter (Monsey, NY) was inserted into the ligated intestinal segment from the distal end, and 0.10 mL of PBS containing 25 mg/mL of FITC-dextran was infused into the intestinal lumen via catheter. Following FITC-dextran infusion, the abdominal skin was sutured. Blood was drawn via cardiac puncture at 90 min after FITC-dextran injection and plasma was separated. Plasma samples were analyzed for FITC-dextran concentration by using a fluorescence spectrophotometer at an excitation wavelength of 480 nm and emission wavelength of 520 nm using Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments, Inc, Winooski, VT).
2.9. Intestinal transit
In order to assess both gut transit and permeability in the same mouse, we gavaged mice with 0.4ml of 22mg/ml FITC-dextran in PBS and euthanized 3h after the gavage. To determine permeability, blood was collected via cardiac puncture and used for the measurement of FITC-dextran levels (3). For measurement of gut transit, contents from the stomach, small intestine (proximal, middle and distal), and large intestine were collected and weighed. The contents were suspended in PBS (1:5 ratio based on weight:volume) and centrifuged at 10,000rpm for 10 min at room temperature. The supernatants were collected for the measurement of FITC-dextran levels.
2.10. Determination of small intestine fecal bacteria
One day after injury, mice were euthanized and the contents from the distal small intestine were collected. Genomic bacterial DNA was purified from mouse fecal samples using the Qiagen DNA Stool Mini Kit according to the manufacturer’s instruction. Real-time PCR was used to quantify bacterial ribosomal small subunit (SSU) 16S rRNA gene abundance, as described previously (3,18). Primers targeting SSU rRNA genes of microorganisms at the domain level (Total Bacteria) and at the family level (Enterobacteriaceae) were used. Primers included 340F: (ACTCCTACGGGAGGCAGCAGT) and 514R: (ATTACCGCGGCTGCTGGC) for total bacteria analyses and 515F: (GTGCCAGCMGCCGCGGTAA) and 826R: (GCCTCAAGGGCACAACCTCCAAG) for Enterobacteriaceae analyses (Thermo Fisher Scientific). 10-fold dilution standards were made from purified genomic DNA from reference bacteria (ATCC, Manassas, VA). All reactions were run using SYBR green (Bio-Rad, Flercules, CA) at 95°C for 3’, followed by 40 cycles of 95°C for 15” and a 63°C (Total Bacteria) or 67°C (Enterobacteriaceae) for 60” using a Step One Plus Real-Time PCR instrument (Applied Biosystems, Foster City, CA).
2.11. Statistical Analysis
The data are presented as means ± standard error of the mean (SEM) and were analyzed with one-way analysis of variance (ANOVA) with Tukey-Kramer Multiple Comparisons Test, or Student’s two-tailed t test (In-Stat; GraphPad Software Inc., La Jolla, CA, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of FICZ on release of Th17 effector cytokines IL-17 and IL-22 after ethanol and burn injury
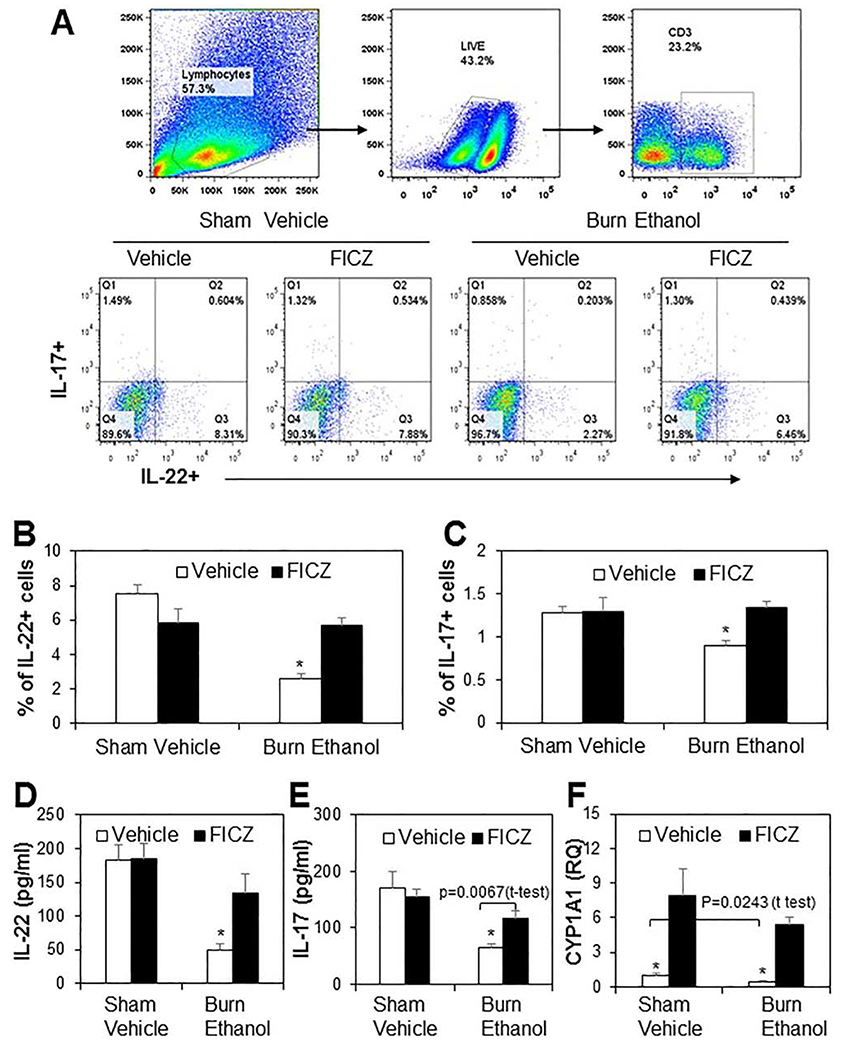
Previous studies from our laboratory have shown that ethanol intoxication combined with burn injury significantly suppresses the release of Th17 cytokines, IL-17 and IL-22, in cells isolated from MLN, PP, and spleen. This was accompanied with decreased expression of transcription factor AHR and its downstream molecular target CYP1A1 (19). We have also demonstrated that direct stimulation of T cells with FICZ prevented decreases in IL-22, but not IL-17 following ethanol and burn injury. In this study, we treated mice with FICZ at time of injury to determine whether FICZ normalized Th17 cell responses after ethanol and burn injury. The cells were isolated from the LP and stained with Fixable Viability Dye to exclude dead cells. We then examined IL-22 and IL-17 expression in CD3+ T cells. As shown as in Fig. 1, there was a significant decreased in expression of IL-22 (Fig. 1B) and IL-17(Fig. 1C) in burn ethanol mice compared to sham vehicle mice. However, treatment of mice with FICZ at the time of injury prevented the decreased expression of IL-22 and IL-17 in LP cells from burn ethanol mice. Since the population of IL-22 and IL-17 was very low in LP cells, we confirmed this finding by isolating T cells from the spleen and stimulating with anti-CD3 and anti-CD28 for 48 h to determine IL-22 and IL-17 levels in culture medium. There were significant decreases in IL-22 (Fig. 1D) and IL-17 (Fig. 1E) levels in splenic T cells isolated from burn ethanol mice compared to sham vehicle mice. Similar to LP cells, treatment of mice with FICZ normalized IL-22 and IL-17 release following ethanol and burn injury. We also determined the expression of CYP1A1, a downstream target of AHR. We observed that there was a significant decrease in CYP1A1 expression in spleen T cells isolated from burn ethanol mice compared to sham vehicle mice (p=0.0243, t-test). Treatment of mice with FICZ increased CYP1A1 expression 8-fold in sham vehicle mice and 12-fold in burn ethanol mice (Fig. 1F).
Figure 1. Effect of FICZ on Th17 effector cytokines IL-17 and IL-22 release in T cells after ethanol and burn injury.
One day after injury, mice were euthanized and distal small intestine was collected for LP cell isolation. Isolated LP cells (1x106) were stimulated with PMA (10ng/ml) and Ionomycin (50ng/ml) in cell culture medium for 2 h. GolgiPlug Protein Transport inhibitor (1 μl/ml) was add in culture medium for another 3 h. The cells were harvested and stained with Fixable Viability Dye, APC-eFluor780 Anti-mouse CD3, eFluor450 anti-mouse-IL-17 and PE anti-mouse IL-22 antibodies. LP cells were gated for live/CD3+ cells and then gated for IL-17 and IL-22 (Fig. 1A). The percentage of IL-22+ cells is shown in Fig. 1B and IL-17+ cells is shown in Fig. 1C. Spleen T (5 x 105 cells/well) were cultured with plate-bound anti-CD3 (5μg/ml) and anti-CD28 (1μg/ml) for 48 h and supernatants were collected to determine IL-22 (Fig. 1D), IL-17 (Fig. 1E), and CYP1A1 mRNA expression (Fig. 1F). Values are means ± SEM from four to eight animals per group (In Fig. 1A, B and C, sham vehicle n = 5, sham vehicle + FICZ n = 5, burn ethanol n = 8 and burn ethanol + FICZ n = 7. In Fig. 1D, E, and F, sham vehicle n = 5, sham vehicle + FICZ n = 4, burn ethanol n = 7 and burn ethanol + FICZ n = 6). P*<0.05 compared to other groups.
3.2. Effect of FICZ on gut transit and permeability after ethanol and burn injury
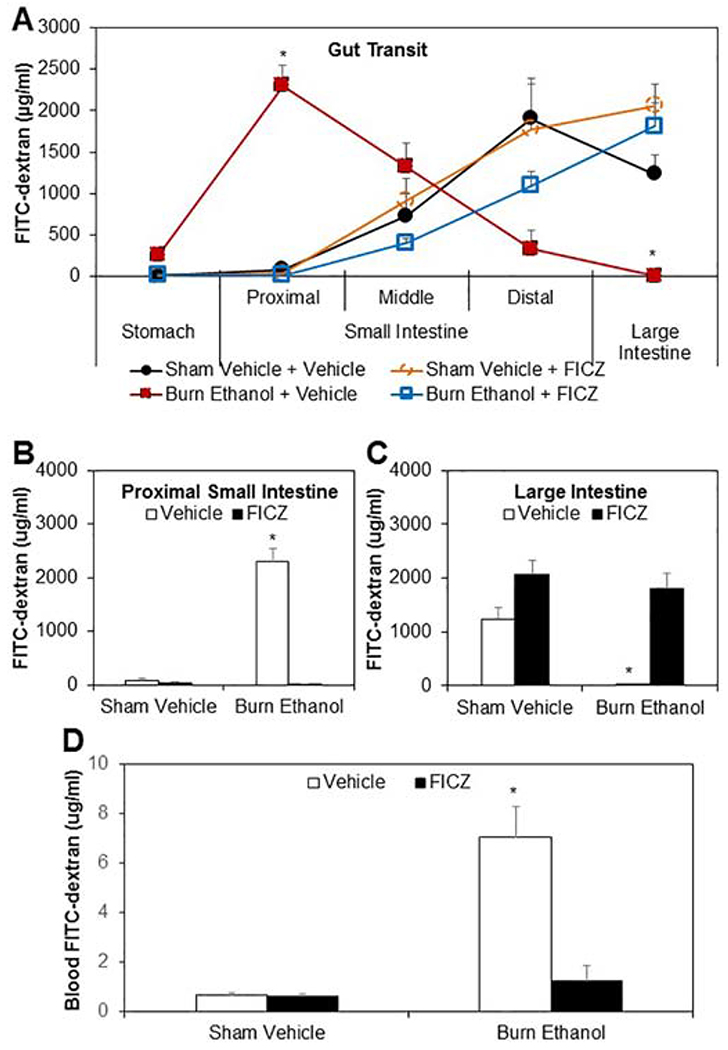
Previous studies have suggested that Th17 effector cytokines IL-22 and IL-17 play a critical role in maintaining the intestinal barrier through mechanisms such as inducing antimicrobial peptides Reg3β and Reg3γ, increasing expression of tight junctions claudin and occludin, and preventing increase in epithelial permeability (22,24,35). In this study, we determined whether ethanol and burn injury influenced gut transit and increase intestinal permeability, and whether treatment of mice with FICZ can influence intestine motility and permeability after ethanol and burn injury. As shown in Fig. 2, there was significant delay in intestinal transit in mice receiving a combined insult of ethanol and burn injury compared to sham vehicle mice (Fig. 2A). The accumulation of FITC-dextran in the proximal small intestine feces was 2302.05±250.95 μg/ml in burn ethanol mice and 80.59±46.99 μg/ml in sham vehicle mice (Fig. 2B), however, in the large intestine the accumulation of FITC-dextran was 2.95±0.66 μg/ml in burn ethanol mice and 1234.11±226.91 μg/ml in sham vehicle mice (Fig. 2C). Treatment of mice with FICZ improved gut transit following ethanol intoxication and burn injury.
Figure 2. Effect of FICZ on gut transit and permeability after ethanol and burn injury.
One day after injury, mice were gavaged with 0.4ml of 22mg/ml FITC-dextran in PBS. 3h after gavage, mice were euthanized. The contents from stomach, small intestine (proximal, middle and distal) and large intestine were collected to measure FITC-dextran levels (Fig. 2A). The FITC-dextran level in the contents collected from proximal small intestine was shown in Fig. 2B and from large intestine was shown in Fig. 2C. Blood also was collected to measure FITC-dextran levels (Fig. 2D). Values are means ± SEM from five to eleven animals per group (sham vehicle n = 5, sham vehicle + FICZ n = 6, burn ethanol n = 11 and burn ethanol + FICZ n = 10). P*<0.05 compared to other groups.
To further confirm the protective role of FICZ in intestinal tissue damage, we determined intestinal permeability. Similar to previous findings, results shown in Fig. 2D suggest a significant increase (10-fold) in FITC-dextran accumulation in the blood of burn ethanol mice compared to sham vehicle mice after gavage with FITC-dextran. Treatment of mice with FICZ prevented the increased accumulation of FITC-dextran in the blood of mice following ethanol intoxication and burn injury.
3.3. Effect of FICZ on gut bacterial growth after ethanol and burn injury
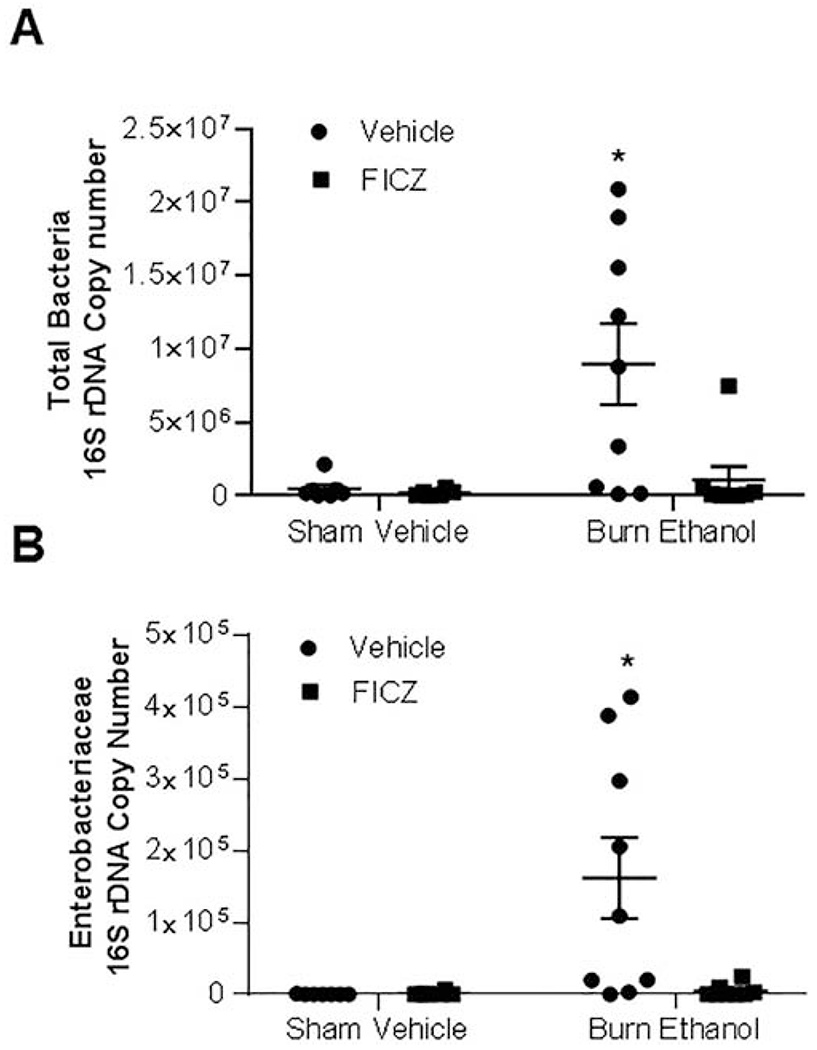
Previous studies from our laboratory have shown that ethanol and burn injury results in a large overgrowth of total bacteria and Enterobacteriaceae in the small intestine of mice (17,18,36). We performed 16S ribosomal RNA amplification by real-time PCR to quantify the number of total bacteria and Enterobacteriaceae present in the luminal content of small intestines following ethanol and burn injury and different treatments (Fig. 3A and 3B). Our findings demonstrate a 19-fold increase in total bacteria (Fig. 3A) and 883-fold increase in Enterobacteriaceae (Fig. 3B) compared to sham vehicle mice. Treatment of mice with FICZ prevented total bacteria and Enterobacteriaceae overgrowth following ethanol and burn injury.
Figure 3. Effect of FICZ on small intestine bacteria growth after ethanol and burn injury.
One day after injury, mice were euthanized and the feces from the distal part of small intestine were collected. Genomic bacterial DNA was purified from mouse fecal samples. Total bacteria (Fig. 3A) and enterobacteriaceae (Fig. 3B) were quantified by real time PCR using specific primers. Values are means ± SEM from six to nine animals per group (sham vehicle n = 7, sham vehicle + FICZ n = 6, burn ethanol n = 9 and burn ethanol + FICZ n = 8). P*<0.05 compared to other groups.
3.4. Effect of FICZ on small intestine inflammation and apoptosis after ethanol and burn
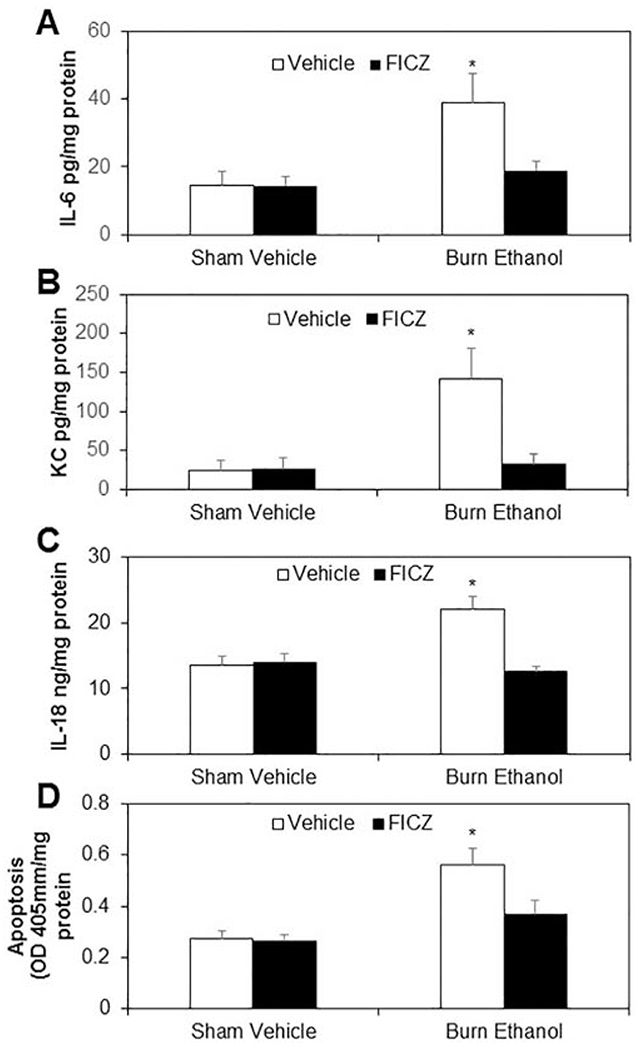
We further examined the effect of FICZ on small intestine inflammation and apoptosis. As shown as in Fig. 4, there were significant increases in IL-6 (Fig, 4A), KC (Fig. 4B), IL-18 (Fig. 4C), and apoptosis (Fig. 4D) in small intestine tissue from mice receiving ethanol and burn injury compared to sham vehicle mice. These results were similar to our previous findings (32). Treatment of mice with FICZ at the time of injury prevented increases in IL-6, KC, IL-18, and apoptosis in small intestine following ethanol and burn injury.
Figure 4. Effect of FICZ on small intestine inflammation and apoptosis after ethanol and burn injury.
One day after injury, mice were euthanized, and the distal end of the small intestine were collected and homogenized. IL-6 (Fig. 4A), KC (Fig. 4B), IL-18 (Fig. 4C) and apoptosis (Fig. 4D) were determined by using respective ELISA kits. Values are means ± SEM from seven to nine animals per group (sham vehicle n = 8, sham vehicle + FICZ n = 7, burn ethanol n = 9 and burn ethanol + FICZ n = 9). P*<0.05 compared to other groups.
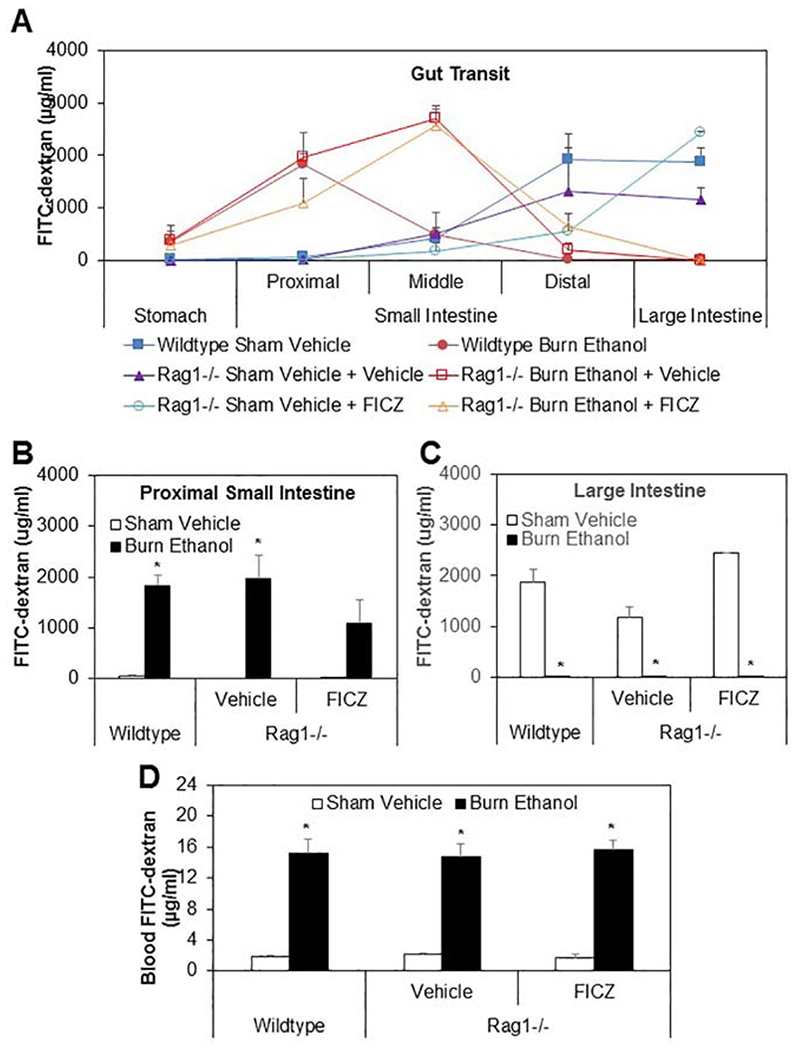
3.5. Effect of FICZ on gut transit and intestinal permeability in Rag1−/− mice after ethanol and burn injury
AHR is expressed in several types of immune cells, including B cells, DCs, macrophages, and T cells. In this experiment, we wanted to determine whether the protective effects on the intestinal barrier seen with treatment of mice with AHR agonist FICZ were dependent on T cells. To test this, we used Rag1−/− mice lacking mature T or B cells to determine gut transit after ethanol and burn injury. Mice were divided into six groups: wildtype sham vehicle, wildtype burn ethanol, Rag1−/− sham vehicle, Rag1−/− burn ethanol, Rag1−/− sham vehicle treated with FICZ and Rag1−/− burn ethanol treated with FICZ. As shown in Fig. 5, there was a significant delay in intestinal transit in wildtype burn ethanol mice and Rag1−/− burn ethanol mice regardless of FICZ treatment compared to both wildtype and Rag1−/− sham vehicle mice (Fig. 5A). In the proximal small intestinal content , the accumulation of FITC-dextran was 1828.46±200.6, 1958.98±469.92, and 1084.53± 469.26 μg/ml in wildtype burn ethanol, Rag1−/− burn ethanol, and Rag1−/− burn ethanol mice treated with FICZ respectively compared to 55.30 ±25.19, 23.29±11.68, and 11.29 ±5.15 μg/ml in wildtype sham vehicle, Rag1−/− sham vehicle, and Rag1−/− sham vehicle treated with FICZ respectively (Fig. 5B), However, in the large intestine feces, the accumulation of FITC-dextran was 15.04±12.08, 3.56±1.05, and 3.89±0.52 μg/ml in wildtype burn ethanol, Rag1−/− burn ethanol, and Rag1−/− burn ethanol mice treated with FICZ respectively compared to 1876.79±259.55, 1168.72±219.11, and 2438.94±18.56 μg/ml in wildtype sham vehicle, Rag1−/− sham vehicle, and Rag1−/− sham vehicle treated with FICZ respectively (Fig. 5C). These data suggest that treatment of Rag1−/− mice with FICZ did not improve gut transit after ethanol and burn injury. Finally, we determined whether treatment of Rag1−/− mice with FICZ modulated intestinal permeability after ethanol and burn injury. As shown as in Fig. 5D, there was no significant difference in blood FITC-dextran accumulation in all sham vehicle groups. There were significant increases in accumulation of blood FITC-Dextran levels of 15.1744±1.77, 14.77±1.71 and 15.73±1.08 μg/ml in wildtype burn ethanol, Rag1−/− burn ethanol and Rag1−/− burn ethanol treated with FICZ respectively compared to 1.85±0.19, 2.09±0.22 and 1.71±0.43μg/ml in wildtype sham vehicle, Rag1−/− sham vehicle, and Rag1−/− sham vehicle treated with FICZ respectively.
Figure 5. Effect of FICZ on gut transit and intestinal permeability in Rag1−/− mice after ethanol and burn injury.
One day after injury, wildtype and Rag1−/− mice were gavaged with 0.4ml of 22mg/ml FITC-dextran in PBS. Three hours following gavage, mice were euthanized. The contents from stomach, small intestine (proximal, middle and distal) and large intestine were collected to measure FITC-dextran levels (Fig. 5A). The FITC-dextran level in the contents collected from proximal small intestine was shown in Fig. 5B and from large intestine was shown in Fig. 5C. The blood also was collected to measure FITC-dextran levels (Fig. 5D). Values are means ± SEM from three to five animals per group (wildtype sham vehicle n = 4, Rag−/− sham vehicle n = 3, Rag−/− sham vehicle + FICZ n = 3, wildtype burn ethanol n = 5, Rag−/− burn ethanol n = 4, and Rag−/− burn ethanol + FICZ n = 4). P*<0.05 compared to respective sham vehicle.
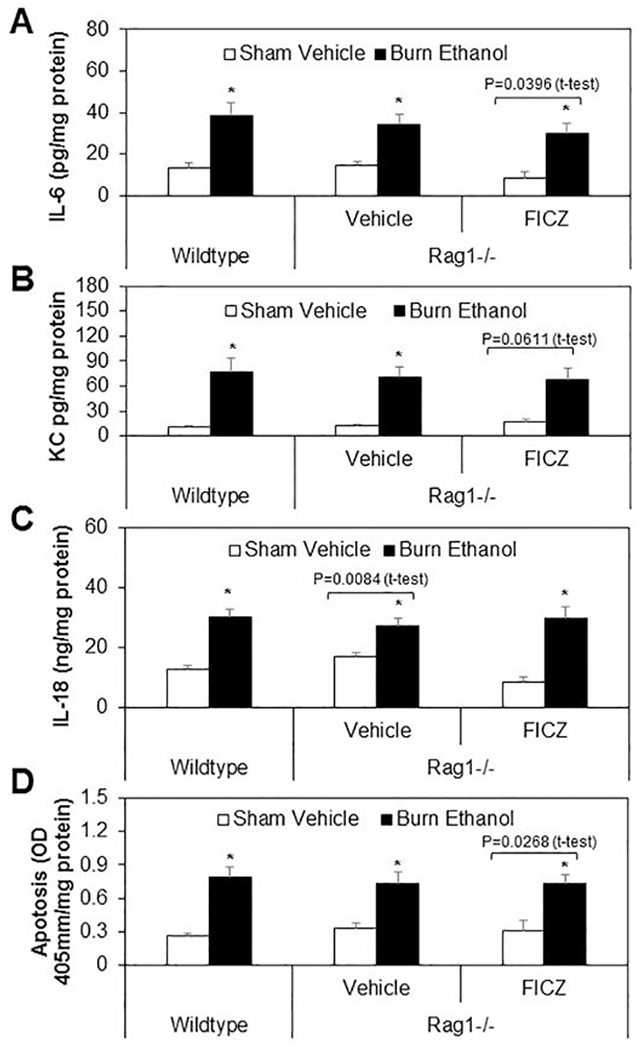
3.6. Effect of FICZ on small intestine inflammation and apoptosis in Rag1−/− mice after ethanol and burn
We further tested whether treatment of Rag1−/− mice with FICZ influenced small intestine inflammation and apoptosis. As shown as in Fig. 6, there was no significant difference in IL-6 (Fig. 6A), KC (Fig. 6B), IL-18(Fig. 6C), and apoptosis (Fig. 6D) in small intestine homogenates from sham vehicle wildtype mice and Rag1−/− mice regardless of FICZ treatment. Furthermore, increases in IL-6, KC, IL-18, and apoptosis were observed not only in wildtype burn ethanol mice, but also in Rag1−/− mice receiving ethanol and burn injury compared to all sham vehicle mice. In addition, treatment of Rag1−/− mice with FICZ did not prevent intestinal inflammation and apoptosis following ethanol and burn injury.
Figure 6. Effect of FICZ on small intestine inflammation and apoptosis in Rag1−/− mice after ethanol and burn.
One day after injury, wildtype and Rag1−/− mice were euthanized and the distal ends of the small intestines were collected and homogenized. IL-6 (Fig. 6A), KC (Fig. 6B), IL-18 (Fig. 6C) and apoptosis (Fig. 6D) were determined by using respective ELISA kits. Values are means ± SEM from three to nine animals per group (wildtype sham vehicle n = 7, Rag−/− sham vehicle n = 6, Rag−/− sham vehicle + FICZ n = 3, wildtype burn ethanol n = 8, Rag−/− burn ethanol n = 9, and Rag−/− burn ethanol + FICZ n = 9). P*<0.05 compared to respective sham vehicle.
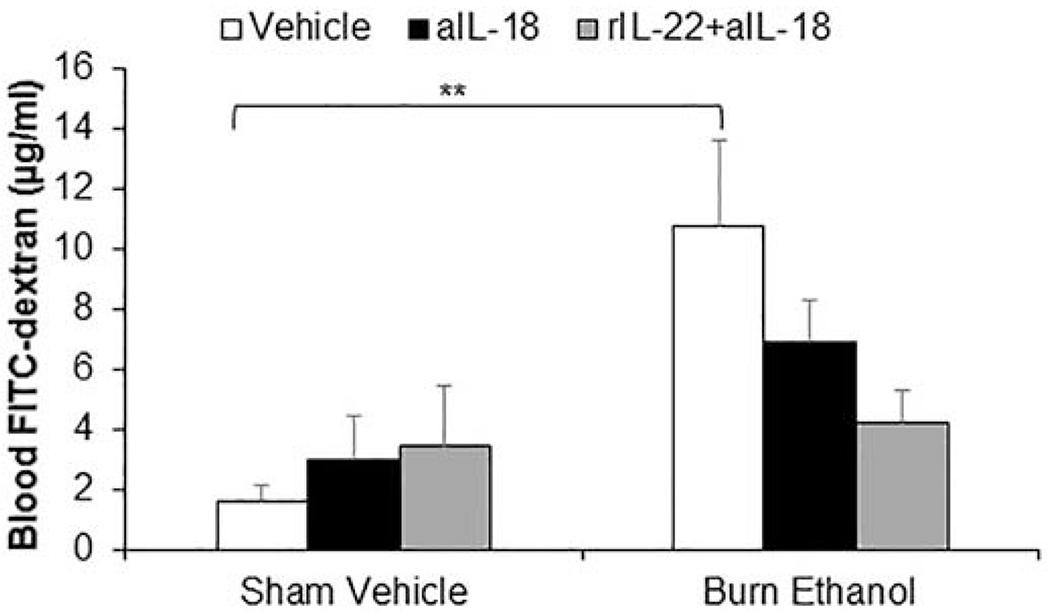
3.7. Treatment of mice with IL-22 combined with αIL-18 antibody is needed to restore gut barrier leakiness following alcohol and burn injury.
Previous studies from our laboratory have shown IL-22 administration significantly improves, but does not fully restore gut barrier leakiness one day following the combined injury (17). However, with FICZ treatment we observe a restoration of IL-22 along with a reduction in intestine inflammation and gut leakiness. Since FICZ also normalizes the levels of IL-18 which in earlier study was shown to cause a decrease in tight junction proteins expression following alcohol and burn injury (29), we sought to determine if the combined treatment of IL-22 and αIL-18 antibodies could act cooperatively to completely prevent gut barrier leakiness following alcohol and burn. Results showed that mice receiving alcohol and burn injury had significantly higher levels of FITC-dextran in circulation, which was partially reduced by αIL-18 antibody administration alone. However, as shown in Fig. 7 the combined treatment showed near complete reduction of leakiness in mice receiving alcohol and burn injury, suggesting these therapies can work in cooperatively to better prevent intestinal leakiness than either therapy individually.
Fig. 7. Both IL-22 restoration and inhibition of IL-18 are required to prevent gut barrier leakiness following ethanol and burn injury.
Mice were treated with aIL-18 antibody (1mg/Kg BW) alone or combination with rIL-22 (1mg/kg BW) at time injury. One day after injury, mice were anesthetized. The abdominal cavity was opened, and renal artery and renal vein in both kidneys were ligated. A 10-cm-long segment of distal small intestine was ligated at both ends without damaging intestinal and mesenteric structures. 0.10 mL of PBS containing 25 mg/mL of FITC-dextran was injected into the intestinal lumen. At 90 min. after injection, blood was collected to measure FITC-dextran levels (Fig. 7). Values are means ± SEM from two to seven animals per group (sham vehicle + vehicle n = 7, sham vehicle + aIL-18 antibody n = 2, sham vehicle + aIL-18 antibody + rIL-22 n = 2, burn ethanol + vehicle n = 4, burn ethanol + aIL-18 antibody n = 4 and burn ethanol + aIL-18 antibody + rIL-22 n = 4). **p<0.01 compared to sham vehicle + vehicle.
4. Discussion
The results presented in this manuscript clearly demonstrate that ethanol intoxication combined with burn injury suppresses Th17 effector cytokines IL-22 and IL-17 in LP and spleen T cells. We further found significant delays in gut transit time and increases in total bacteria (19-fold) and Enterobacteriaceae (883-fold) in small intestine feces. This was accompanied by increases in inflammatory cytokines IL-6, KC and IL-18 in intestinal tissue, as well as increased apoptosis and intestinal permeability following ethanol intoxication combined with burn injury. Treatment of mice with FICZ normalized IL-22 and IL-17 in LP and spleen T cells, as well as increased CYP1A1 expression in spleen T cells following ethanol and burn injury. Furthermore, we observed that mice treated with FICZ had normalized gut motility, decreased small intestine total bacteria and Entrobacteriaceae overgrowth, attenuated intestinal tissue IL-6, KC, IL-18, apoptosis, and prevented gut leakiness following ethanol and burn injury.
T helper cells, including Th17 cells, play a critical role in maintenance of intestinal immune homeostasis, protection of intestinal barrier function, and control of gut pathogenic bacteria (22–24,35). IL-22 knockout mice infected with C. rodentium show increased intestinal epithelial damage, bacterial translocation, sepsis, and mortality compared to wild type mice. Furthermore, when wild type mice infected with C. rodentium were administrated anti-IL-22 antibody to neutralize IL-22, they have 100% mortality after infection compared to isotype control antibody-treated mice, which all survived (24). Consistent with these findings, a previous study from our laboratory has shown that treatment of mice with recombinant IL-22 (rIL-22) either prevented the decrease in or further upregulated the release of antimicrobial peptides Reg3β and Reg3γ and intestinal permeability after ethanol and burn injury (17,18).
AHR, a ligand-dependent transcription factor, is highly expressed in Th17 cells and acts as a sensor of environmental signals that are derived from intestinal microbiota, diet, and drugs. FICZ acts as a natural high-affinity ligand for AHR (37,38). Quintana et al have indicated that AHR regulates both Treg and Th17 cell differentiation in a ligand-specific fashion. AHR activation by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) induces Treg cell differentiation, whereas AHR activation by FICZ promotes Th17 cell differentiation (38). In a previous study, we observed that ethanol intoxication combined with burn injury suppressed expression of AHR and decreased IL-17 and IL-22 release in spleen T cells. The treatment of T cells with FICZ prevented decreases in IL-22 and increased AHR-dependent CYP1A1 expression after ethanol and burn injury (19). We also observed when PP cells were treated with AHR antagonist CH-223191, IL-23-mediated restoration of IL-22 was inhibited (20). Therefore, we treated mice with FICZ at the time of injury to determine whether AHR activation modulates the Th17 cell response, reduces pro-inflammatory cytokines, and protects the gut barrier after ethanol and burn injury. We observed that treatment of mice with FICZ prevented decreases in IL-22 and IL-17, as well as modulated gut motility and prevented bacteria overgrowth, accompanied by decreased intestinal tissue inflammatory cytokines release, apoptosis and intestinal permeability. Consistent with our findings, a significant decrease in the expression of AHR in intestinal tissue from IBD patients was found compared to control; furthermore, LP cells isolated from IBD patients cultured with FICZ demonstrated increased IL-22 production. Conversely, the treatment of mice with an AHR antagonist produced more inflammatory cytokines, less IL-22, and developed severe colitis (39). AHR−/− mice have reduced IL-22 production. AHR−/− mice infected with C. rodentium rapidly lost body weight and died by day 10 post-infection, whereas control mice had no weight loss and death by day 10 post-infection. There were higher numbers of C. rodentium colonies in feces by day 5 post-infection compared to control mice. AHR−/− mice infected with C. rodentium administered with IL-22 had much less weight loss and survived after day 17 post-infection with reduced numbers of bacteria in the feces (26). Together these findings suggest that AHR-mediated Th17 effector cytokines play a role in defense against gut pathogens.
Since AHR is expressed in many tissues and cells, we used Rag1−/− mice (no mature T cells and B cells) to further characterize the role of Th17 cells in FICZ mediated protection of intestinal barrier function after ethanol intoxication combined with burn injury. Treatment of Rag1−/− mice with FICZ did not improve gut transit, reduce release of inflammatory cytokines IL-6, KC, and IL-18, reduce apoptosis in intestinal tissue, or prevent gut leakiness after ethanol and burn injury. Furthermore, we observed that ethanol combined with burn injury decreased expression of AHR and CYP1A1 in small intestine epithelial cells in wildtype mice (data not shown). We use the YAMC epithelial cell line to determine whether FICZ-mediated AHR reduced LPS-induced epithelial cell inflammation. We observed that YAMC cells cultured with LPS (10ng/ml) significantly increased IL-6 levels in cell culture medium. Treatment of YAMC cells with LPS combined with FICZ (200nM) did not influence LPS-induced IL-6 release. In addition, we used J774 cell line differentiated to DCs by culturing with GM-CSF (10ng/m) and IL-4 (10ng/ml) for 5 days to determine whether FICZ modulated LPS-induced IL-6. We observed that treatment of DCs with FICZ did not prevent increases in LPS-induced IL-6 (data not shown). These data suggest that T cells play a critical role in FICZ-mediated intestinal inflammation and barrier function after ethanol and burn injury. When Rag2 knockout mice, which lack mature T or B lymphocytes, were infected with C.rodentium, they gradually lost body weight and eventually died (24). In addition, previous studies from our laboratory found that depletion of CD3+ T cells from healthy animals led to bacterial accumulation in MLN. Furthermore, animals depleted of T cells receiving ethanol combined with burn injury resulted in bacteria translocating to multiple organs (15).
While FICZ restores gut barrier integrity in a T cell dependent fashion, our findings further suggest that IL-22 alone may not be sufficient for barrier restoration in mice receiving ethanol and burn injury. We observed that while IL-22 may be protective against microbial dysbiosis following ethanol and burn injury (17,18). It requires an inhibition of IL-18 to fully restore the barrier integrity. Interestingly IL-18 was not found to have any direct impact on bacterial dysbiosis (data not shown).
Through this, we were able to identify that that IL-18 inhibition appears to be important in reducing the neutrophil release of MPO and intestinal tissue edema inflammatory cytokines IL-6 and KC, and is sufficient to restore epithelial cell tight junction complexes and drastically reduce apoptosis(27,29). On the other hand, IL-22 prevents microbial dysbiosis 24 hours following the combined injury. When αIL-18 antibodies and recombinant IL-22 are administered in tandem, gut barrier leakiness is restored nearly to sham vehicle levels. Collectively, our findings indicate that while both these treatments benefit the intestine independently following acute ethanol and burn injury, the combination of both IL-22 and αIL-18 antibodies act cooperatively through different mechanisms to provide broader protection to the intestinal barrier.
4.1. Conclusions
Our findings show that FICZ-mediated AHR activity plays a critical role in restoration of Th17 effector cytokines IL-22 and IL-17, as well as reducing pro-inflammatory cytokines, such as IL-18. Furthermore the findings that treatment of Rag1−/− mice with FICZ did not improve any of the parameters listed, suggesting that FICZ-mediated protection is T cell-dependent and requires both the restoration of IL-22 and neutralization of IL-18. Interestingly, our findings reported earlier (17) as well as the data presented here support the suggestion that IL-22 promotes the intestine barrier by upregulating epithelial cells proliferation and AMPS and preventing the bacteria dysbiosis. In contrast inhibition of IL-18 reduces inflammation and epithelial cell apoptosis. Together our findings suggest that AHR ligand FICZ may have beneficial effects for the treatment of patients under the influence of ethanol who sustain a burn injury.
Highlights:
Ethanol and burn injury suppresses T cell release of IL-17 and IL-22 and disrupts intestine barrier integrity.
FICZ protects intestine barrier by enhancing T cell release of IL-17 and IL-22 and decreasing IL-18 in small intestine.
FICZ-mediated protection is diminished in Rag1 knockout mice.
Acknowledgments
Grants
This study was supported by the National Institutes of Health (R01 AA015731, R01 GM128242, and T32 AA013527).
Abbreviations:
- FICZ
6-Formylindolo (3, 2-b) Carbazole
- AHR
aryl hydrocarbon receptor
- LP
lamina propria
- CYP1A1
Cytochrome P450 Family 1 Subfamily A Member 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
The authors have no conflicts of interest to declare
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference List
- 1.Macpherson AJ, and Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol 4: 478–485. [DOI] [PubMed] [Google Scholar]
- 2.Honda K, and Littman DR. 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535: 75–84. [DOI] [PubMed] [Google Scholar]
- 3.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, Gamelli RL, Kennedy RH, and Choudhry MA. 2015. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS. One. 10: e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, and Logsetty S. 2020. Burn injury. Nat. Rev. DIs. Primers. 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheatley EG, Curtis BJ, Hulsebus HJ, Boe DM, Najarro K, Ir D, Robertson CE, Choudhry MA, Frank DN, and Kovacs EJ. 2020. Advanced Age Impairs Intestinal Antimicrobial Peptide Response and Worsens Fecal Microbiome Dysbiosis Following Burn Injury in Mice. Shock 53: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thombs BD, Singh VA, Halonen J, Diallo A, and Milner SM. 2007. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: evidence from a national sample of 31,338 adult patients. Ann. Surg 245: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, and Gamelli RL. 2008. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J. Burn Care Res. 29: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis CS, Esposito TJ, Palladino-Davis AG, Rychlik K, Schermer CR, Gamelli RL, and Kovacs EJ. 2013. Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J. Burn Care Res. 34: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CS, Janus SE, Mosier MJ, Carter SR, Gibbs JT, Ramirez L, Gamelli RL, and Kovacs EJ. 2013. Inhalation injury severity and systemic immune perturbations in burned adults. Ann. Surg 257: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messingham KA, Faunce DE, and Kovacs EJ. 2002. Alcohol, injury, and cellular immunity. Alcohol 28: 137–149. [DOI] [PubMed] [Google Scholar]
- 11.Albright JM, Kovacs EJ, Gamelli RL, and Schermer CR. 2009. Implications of formal alcohol screening in burn patients. J. Burn Care Res. 30: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Hammer AM, Rendon JL, and Choudhry MA. 2015. Intestine immune homeostasis after alcohol and burn injury. Shock 43: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inatsu A, Kogiso M, Jeschke MG, Asai A, Kobayashi M, Herndon DN, and Suzuki F. 2011. Lack of Th17 cell generation in patients with severe burn injuries. J. Immunol 187: 2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwacha MG, and Chaudry IH. 2002. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int. J. Mol. Med 10: 239–243. [PubMed] [Google Scholar]
- 15.Choudhry MA, Fazal N, Goto M, Gamelli RL, and Sayeed MM. 2002. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am. J. Physiol Gastrointest. Liver Physiol 282: G937–G947. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Chaudry IH, and Choudhry MA. 2009. ERK and not p38 pathway is required for IL-12 restoration of T cell IL-2 and IFN-gamma in a rodent model of alcohol intoxication and burn injury. J. Immunol 183: 3955–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rendon JL, Li X, Akhtar S, and Choudhry MA. 2013. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock 39: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer AM, Morris NL, Cannon AR, Khan OM, Gagnon RC, Movtchan NV, van L, Li I,X, Gao B, and Choudhry MA. 2017. Interleukin-22 Prevents Microbial Dysbiosis and Promotes Intestinal Barrier Regeneration Following Acute Injury. Shock 48: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Cannon AR, Hammer AM, Morris NL, and Choudhry MA. 2017. IL-23 restoration of Th17 effector function is independent of IL-6 and TGF-beta in a mouse model of alcohol and burn injury. J. Leukoc. Biol 102: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rendon JL, Li X, Brubaker AL, Kovacs EJ, Gamelli RL, and Choudhry MA. 2014. The role of aryl hydrocarbon receptor in interleukin-23-dependent restoration of interleukin-22 following ethanol exposure and burn injury. Ann. Surg 259: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinugasa T, Sakaguchi T, Gu X, and Reinecker HC. 2000. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, and Cua DJ. 2015. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 43: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, and Mizoguchi A. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest 118: 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, and Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 25.Esser C, and Rannug A. 2015. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev 67: 259–279. [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, and Zhou L. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 36: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar S, Li X, Chaudry IH, and Choudhry MA. 2009. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2- production and intestinal edema following alcohol intoxication and burn injury. Am. J. Physiol Gastrolntest. Liver Physiol 297: G340–G347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanishi K, Yoshimoto T, Tsutsui H, and Okamura H. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol 19: 423–474. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Akhtar S, and Choudhry MA. 2012. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim. Biophys. Acta 1822: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimesaat MM, Grundmann U, Alutis ME, Fischer A, Gobel UB, and Bereswill S. 2016. The IL-23/IL-22/IL-18 axis in murine Campylobacter jejuni infection. Gut Pathog. 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouzounidis N, Giakoustidis A, Poutahidis T, Angelopoulou K, Iliadis S, Chatzigiagkos A, Zacharioudaki A, Angelopoulos S, Papalois A, Papanikolaou V, and Giakoustidis D. 2016. Interleukin 18 binding protein ameliorates ischemia/reperfusion-induced hepatic injury in mice. Liver Transpl. 22: 237–246. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Akhtar S, Kovacs EJ, Gamelli RL, and Choudhry MA. 2011. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J. Burn Care Res. 32: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker HL, and Mason AD Jr. 1968. A standard animal burn. J. Trauma 8: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 34.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, and Neurath MF. 2007. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc 2: 2307–2311. [DOI] [PubMed] [Google Scholar]
- 35.Kolls JK, McCray PB Jr., and Chan YR. 2008. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol 8: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammer AM, Khan OM, Morris NL, Li X, Movtchan NV, Cannon AR, and Choudhry MA. 2016. The Effects of Alcohol Intoxication and Burn Injury on the Expression of Claudins and Mucins in the Small and Large Intestines. Shock 45: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, and Rannug U. 2009. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J. Biol. Chem 284: 2690–2696. [DOI] [PubMed] [Google Scholar]
- 38.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, and Weiner HL. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71. [DOI] [PubMed] [Google Scholar]
- 39.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, Macdonald TT, Pallone F, and Monteleone G. 2011. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141: 237–48, 248. [DOI] [PubMed] [Google Scholar]