Abstract
Background:
There are limited data describing the presenting characteristics and outcomes among US persons with HIV (PWH) requiring hospitalization for coronavirus disease 2019 (COVID-19).
Methods:
We performed a case series of all PWH sequentially admitted with COVID-19 from 8 March 2020 to 23 April 2020 at three hospitals in Atlanta, Georgia. Sociodemographic, clinical and HIV-associated characteristics were collected.
Results:
Of 530 confirmed COVID-19 cases hospitalized during this period, 20 occurred among PWH (3.8%). The median age was 57 (Q1–Q3, 48–62) years, 65% were men, and 85% were non-Hispanic Black. Presenting median symptom duration was 5 (Q1–Q3, 3–7) days; cough (90%), fever (65%), malaise (60%) and dyspnea (60%) were most common. On admission, 40% of patients required oxygenation support and 65% had an abnormal chest radiograph. Median length of hospitalization was 5 (Q1–Q3, 4–12) days, 30% required intensive care, 15% required intubation, and 15% died. Median CD4+ cell count prior to admission was 425 (Q1–Q3, 262–815) cells/μl and 90% of patients had HIV-1 RNA less than 200 copies/ml. Half of the patients had at least five comorbidities; hypertension (70%), dyslipidemia (60%) and diabetes (45%) were most prevalent. All three patients who died had CD4+ cell count more than 200, HIV suppression and each had a total of five comorbidities.
Conclusion:
The multisite series in the Southern United States provides characteristics and early outcomes of hospitalized PWH with COVID-19. Nearly all patients had controlled HIV and a high comorbidity burden. Additional study of COVID-19 among PWH is needed to determine the role of age, comorbidities and HIV control in mediating COVID-19 presentation and its sequelae.
Keywords: comorbidity burden, coronavirus disease 2019, HIV, persons living with HIV, severe acute respiratory syndrome coronavirus 2
Introduction
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pandemic continues to spread unabated globally, with more than 1 million confirmed cases reported in the United States. Coronavirus disease 2019 (COVID-19), a novel pneumonia caused by SARS-CoV-2, particularly devastates those of older age and with multimorbidity. The Centers for Disease Control and Prevention categorizes people with ‘poorly controlled HIV or AIDS’ at higher risk than the general population for severe illness from COVID-19 [1]; however, supportive data are lacking. The Southeast persists as the epicenter of the United States HIV epidemic with the majority of new diagnoses, lower rates of virologic suppression and higher rates of deaths from late-stage AIDS than other parts of the country [2]. To understand how COVID-19 may affect persons with HIV (PWH) in the Southern United States, a prematurely aging population with a high comorbidity burden [3,4], we analyzed cases among hospitalized PWH in Atlanta, Georgia.
Methods
PWH admitted with COVID-19 at one of three hospitals in Atlanta, Georgia between 8 March 2020 and 23 April 2020 were recorded. The included hospitals were: first, Grady Memorial Hospital, a 961-bed public safety-net hospital; second, Emory University Hospital, a 587-bed tertiary academic medical center; and third, the Atlanta Veterans Affairs Medical Center, a 466-bed hospital serving veterans in the metropolitan and surrounding areas. COVID-19 was confirmed by detection of SARS-CoV-2 by reverse transcription-PCR (RT-PCR) from a respiratory specimen.
Sociodemographic, clinical and HIV-associated characteristics were manually extracted from the electronic medical record. Non-AIDS comorbidities were included if documented by a healthcare provider in the outpatient setting or during hospitalization. Viral suppression was defined as HIV-1 RNA less than 200 copies/ml. The research was approved by the Emory University Institutional Review Board.
Results
Of 530 confirmed COVID-19 cases admitted during this period, 20 occurred among PWH (3.8%). The median age was 57 (Q1–Q3, 48–62) years, 65% were men, and 85% were non-Hispanic Black; 60% were admitted to the public safety-net hospital. Although 50% denied recent travel or a known exposure, seven (35%) reported known sick contacts. Three of these were nursing home residents, two had ill household contacts, one was recently incarcerated, and one interacted with clients from Italy at the workplace in Atlanta (Table 1 ). Median symptom duration on admission was 5 (Q1–Q3, 3–7) days; cough (90%), fever (65%), malaise (60%) and dyspnea (60%) were most common. Two patients experienced both anosmia and ageusia.
Table 1.
Clinical characteristics, comorbidities and outcomes among persons living with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia.
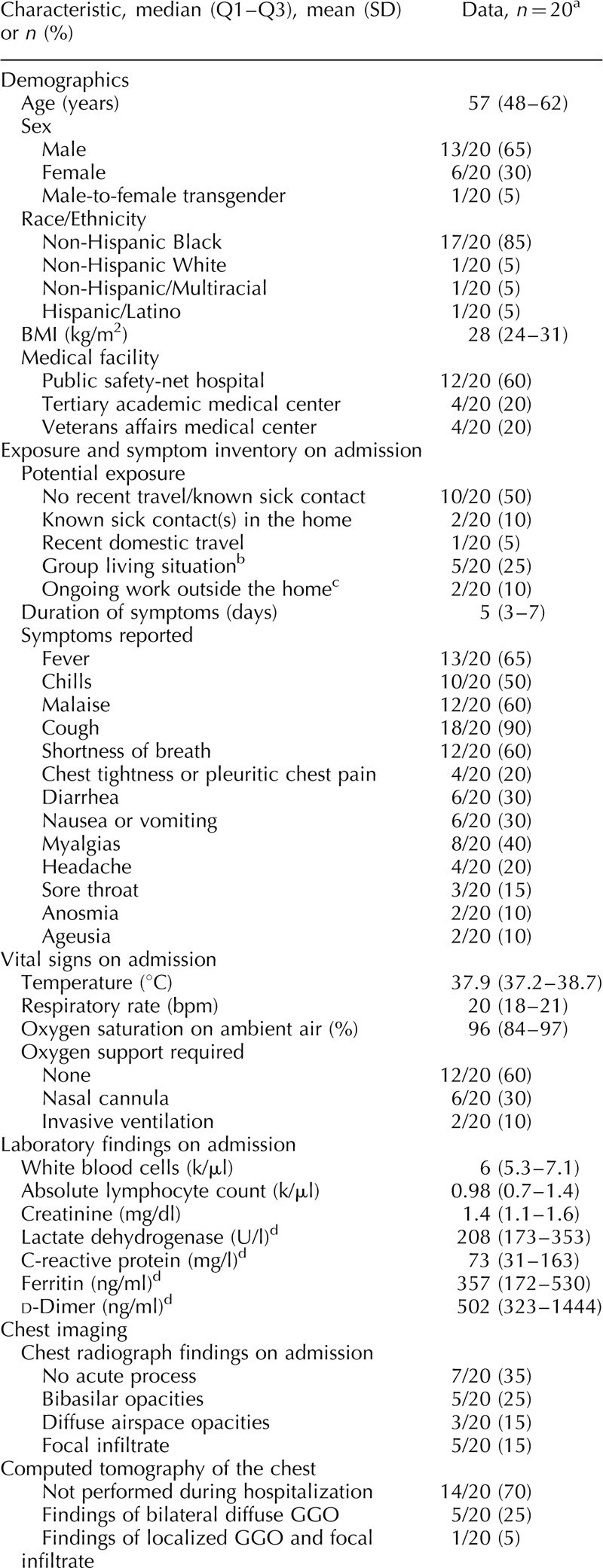
Table 1 (Continued).
Clinical characteristics, comorbidities and outcomes among persons living with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia.
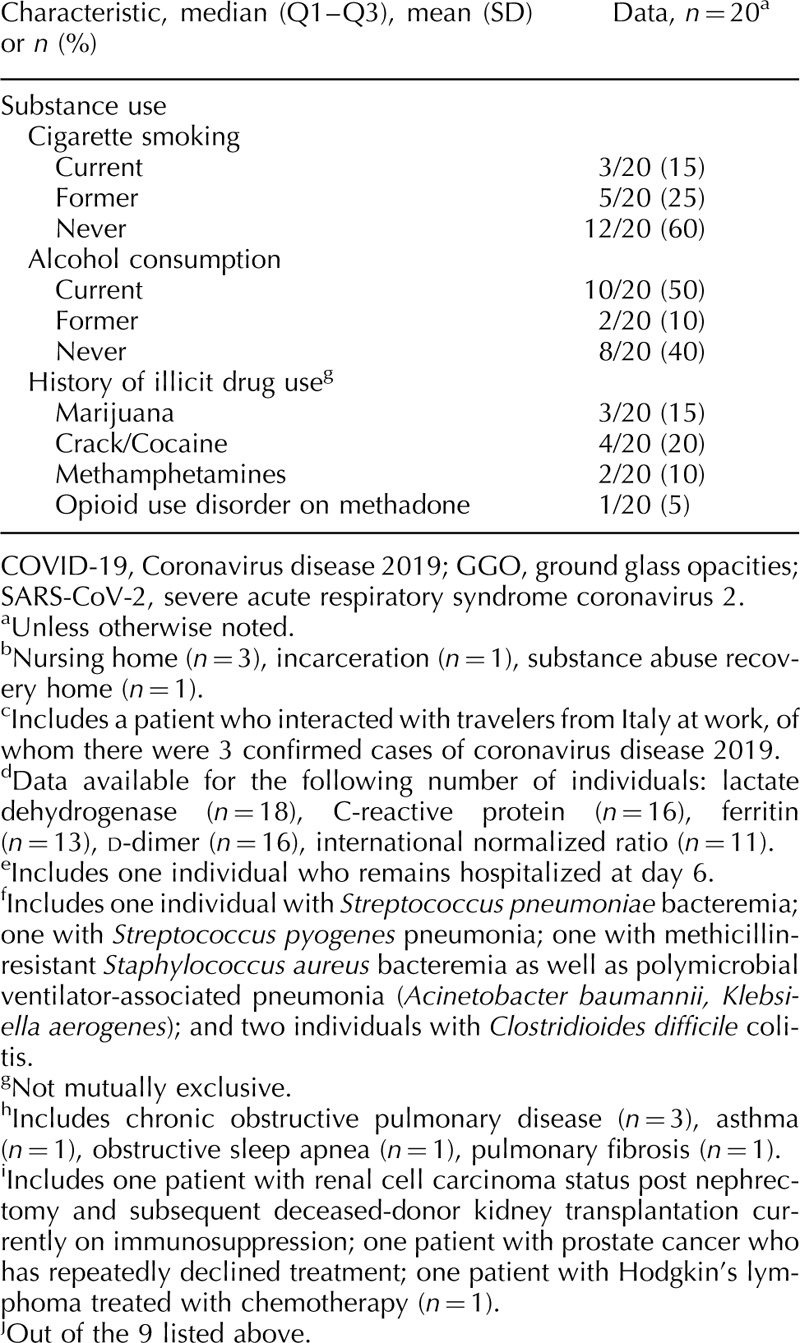
Overall, presenting vital sign abnormalities were subtle (Table 1 ). Forty percent of patients required oxygenation support in the emergency department including two patients who were intubated on arrival. Absolute lymphocyte count was depressed, and serum creatinine, lactate dehydrogenase, C-reactive protein and d-dimer were all elevated (Table 1 ). Chest radiograph was abnormal in 65% of patients, with 40% showing diffuse multifocal or bibasilar opacities and 15% demonstrating a focal infiltrate. Over half (55%) of patients received a therapeutic for SARS-CoV-2 including open-label hydroxychloroquine +/− azithromycin or clinical trial enrollment. Median length of hospitalization was 5 (Q1–Q3, 4–12) days, 30% required intensive care, 15% required intubation, and 15% died. One patient remained admitted to date requiring high-flow oxygen support via nasal cannula on hospital day 6.
All patients had SARS-CoV-2 detected by RT-PCR on initial specimen testing (19/20 specimens were collected by nasopharyngeal swab and 1/20 by mini-bronchoalveolar lavage). Three patients had repeat testing performed for discharge planning. Each individual had two nasopharyngeal swabs obtained several days after their initial test, including one individual who had a negative test result on both repeat tests (obtained on hospital days 18 and 20) and two individuals who had positive test results on both repeat tests (obtained on hospital days 7 and 10, and on hospital days 12 and 14, respectively).
Fifty-five percent of patients had HIV diagnosed at least 10 years ago. The most frequently reported HIV acquisition risk factor was heterosexual behavior (55%), followed by MSM activity (25%) and injection drug use (10%). Median absolute CD4+ cell count and percentage prior to admission were 425 (Q1–Q3, 262–815) cells/μl and 29% (Q1–Q3, 21–36), respectively, and 90% of patients had viral suppression (Table 1 ). In the 2 years prior to hospitalization, 82% of patients were continuously virologically suppressed over a median of 4.5 (Q1–Q3, 3.3–5.0) HIV care visits per patient. All 20 patients were prescribed antiretroviral therapy prior to hospitalization, and 95% reported therapy adherence on admission. Eighty percent were prescribed an integrase strand transfer inhibitor-containing regimen, most commonly bictegravir/emtricitabine/tenofovir alafenamide (35%) or dolutegravir/abacavir/lamivudine (25%).
The burden of non-AIDS comorbidities was high in this cohort, with 50% of patients having at least five comorbidities, and only 15% with no comorbidities (Table 1 ). The most prevalent comorbidities were hypertension (70%), dyslipidemia (60%), diabetes (45%) and psychiatric illness (40%). Nine patients were prescribed an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker on their medication list prior to admission. Forty percent of patients reported current/former cigarette smoking, 50% currently used alcohol and 20% reported a history of crack/cocaine use. One patient had chronic active hepatitis B virus and five patients had a history of hepatitis C virus, including two who spontaneously cleared and three who achieved sustained virologic response after treatment with direct-acting antivirals.
A summary of demographic, comorbidity, HIV-specific and treatment characteristics for the three patients whom died is provided in Table 2.
Table 1 (Continued).
Clinical characteristics, comorbidities and outcomes among persons living with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia.
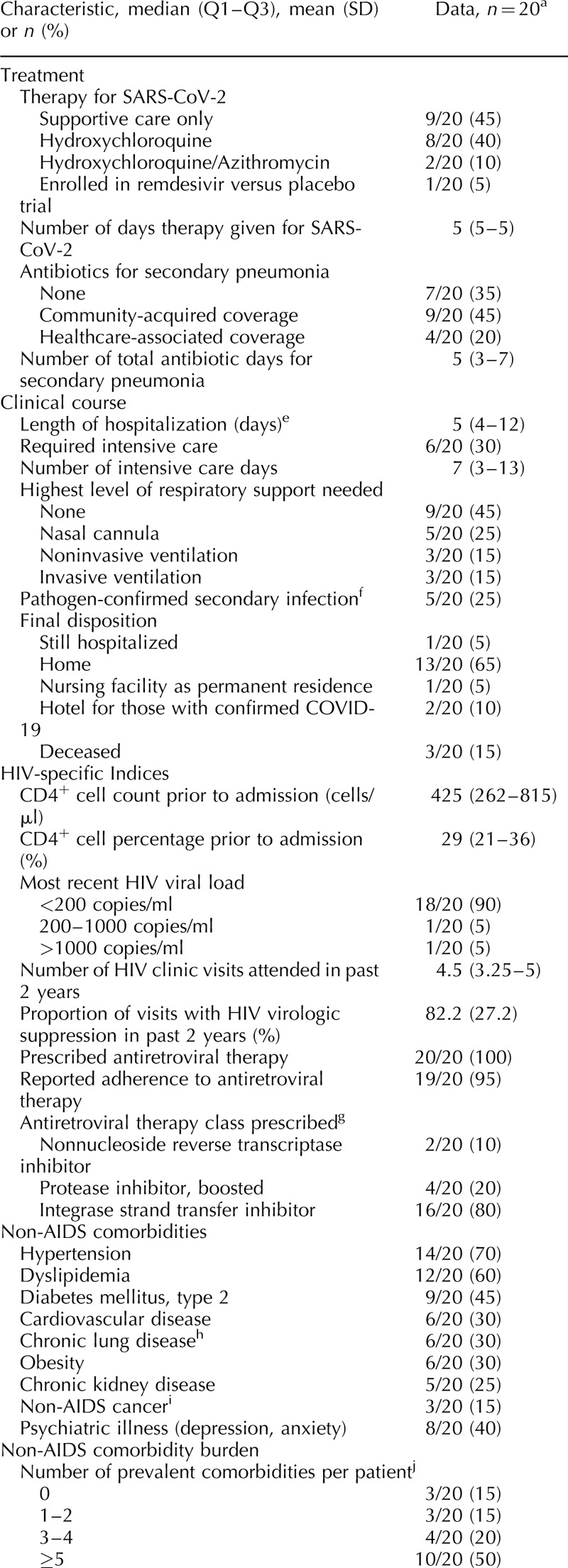
Table 2.
Characteristics of persons with HIV who died of coronavirus disease 2019 in Atlanta, Georgia.

Discussion
The multisite series of PWH hospitalized with COVID-19 in Atlanta is notable for several reasons. The median age of 57 years is 4–6 years younger than reports of non-HIV hospitalized COVID-19 US cohorts [5,6]. Half of PWH in this study had at least five comorbidities, an alarming burden that exceeds previous reports of hospitalized COVID-19 cases including adults in New York and California [5,6] and specifically among PWH in Spain [7]. Among hospitalized PWH in this series, nearly all had CD4+ cell counts at least 200 and viral suppression (inclusive of all three patients who died, Table 2), despite the three hospitals’ catchment areas treating significant proportions of demographically similar PWH with uncontrolled HIV and AIDS [8]. It is possible that the premature onset of multimorbidity among PWH considerably impacts SARS-CoV-2 infection and illness severity, distinct from HIV infection or its associated immunosuppression.
The immunologic intersection between SARS-CoV-2 and chronic HIV infection remains to be examined. Clearly, in a subset of people with COVID-19, a hyperimmune response to SARS-CoV-2 leads to severe lung damage and poor outcomes exemplified by elevated systemic levels of proinflammatory cytokines, coagulation dysfunction and dysregulated cellular responses [9]. Curiously, we and others [5] have not yet observed a high prevalence of severe COVID-19 disease among PWH in general, and particularly not among PWH with advanced immunosuppression, that is, CD4+ cell count less than 200. Further research will be necessary to understand whether the immune response to SARS-CoV-2 differs among PWH and how multimorbidity, which is common in HIV [3,4], influences clinical outcomes of COVID-19 among PWH specifically.
PWH in the Southern United States represent a group particularly vulnerable to COVID-19 severity and sequelae, given the confluence of income inequality, housing instability and food insecurity – disproportionate among minorities because of structural racism and discrimination – limiting the ability to adopt optimal infection prevention measures and augmenting comorbidity risk, which in turn confers worse outcomes [2]. The predominance of non-Hispanic Black men in this cohort reflects the demographic of HIV/AIDS in Atlanta; however, more data are needed to determine the role of racial/ethnic disparities in COVID-19 presentation and outcomes, especially as this can be exacerbated in high HIV-burden areas [10].
The indirect effects of the SARS-CoV-2 pandemic on PWH and those at risk for HIV are not yet fully understood. Closure of clinics, reduced access to medications and fewer support services may lead to decreased rates of retention in care and lower rates of virologic suppression, particularly among the most marginalized [11]. In addition, many clinics have scaled back testing for HIV and other sexually transmitted infections, and others have suspended visits for preexposure prophylaxis. The overall impact on the HIV care continuum and the Ending the HIV Epidemic initiative must be closely monitored.
It is anticipated that additional resources are needed to support the unique needs of PWH, including to increase access to SARS-CoV-2 testing and clinical trials, and more broadly to build socioeconomic and healthcare infrastructure that will enhance HIV-related, comorbidity, mental and sexual health and well being [12]. This report supports dedicated larger study of COVID-19 among PWH to determine the role of age, comorbidities, immunocompetency, HIV control and antiretroviral agents in mediating COVID-19 presentation and its sequelae.
Acknowledgements
We sincerely thank the patients, clinicians and staff at Grady Memorial Hospital, Emory University Hospital and the Atlanta Veteran's Affairs Medical Center. We also thank the Emory-Grady HIV Clinical Cohort Registry for contribution of data.
The current work was supported by the Emory Center for AIDS Research (award number P30-AI-050409) as well as the Emory Specialized Center of Research Excellence (SCORE) on Sex Differences (award number U54AG062334; to I.O.). L.F.C. is also supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) (award numbers UL1TR002378 and TL1TR002382) and C.A.M. by the NCATS of the NIH (award number KL2TR002381). C.D.L. is also supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number K23-AI124913).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): people who are at higher risk; 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. [Accessed 26 April, 2020]. [Google Scholar]
- 2.Colasanti JA, Armstrong WS. Challenges of reaching 90–90–90 in the Southern United States. Curr Opin HIV AIDS 2019; 14:471–480.. [DOI] [PubMed] [Google Scholar]
- 3.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. JAMA 2019; doi: 10.1001/jama.2019.19775. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Collins LF, Sheth AN, Mehta CC, Naggie S, Golub ET, Anastos K, et al. The prevalence and burden of non-AIDS comorbidities among women living with or at-risk for HIV infection in the United States. Clin Inf Dis 2020; ciaa204. doi: 10.1093/cid/ciaa204. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated healthcare system in California. JAMA 2020; 323:2195–2198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, Miro JM. COVID-19 in HIV Investigators COVID-19 in patients with HIV: clinical case series. Lancet HIV 2020; 7:e314–e316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV surveillance supplemental report 2019; 24 (no. 1). 2019; http://www.cdc.gov/hiv/library/reports/hiv-surveillance.htmlhttp://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Accessed 21 March 2020]. [Google Scholar]
- 9.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–2629.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 – Georgia, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:545–550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colasanti J, Kelly J, Pennisi E, Hu YJ, Root C, Hughes D, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016; 62:648–654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoptaw S, Goodman-Meza D, Landovitz RJ. Collective call to action for HIV/AIDS community-based collaborative science in the era of COVID-19. AIDS Behav 2020; 24:2013–2016.. [DOI] [PMC free article] [PubMed] [Google Scholar]


