Abstract
Background and purpose
In patients with acute ischemic stroke and atrial fibrillation (AF), treatment with low molecular weight heparin (LMWH) increases early hemorrhagic risk without reducing early recurrence and there is limited data comparing warfarin to direct oral anticoagulant (DOAC) therapy. We aim to compare the effects of the treatments above on the risk of 90-day recurrent ischemic events and delayed symptomatic intracranial hemorrhage (d-sICH).
Methods
We included consecutive patients with acute ischemic stroke and AF from the Initiation of Anticoagulation after Cardioembolic stroke (IAC) study pooling data from stroke registries of 8 comprehensive stroke centers across the United States. We compared recurrent ischemic events and d-sICH between each of the following groups in separate cox-regression analyses: 1) DOAC versus warfarin and 2) Bridging with heparin/LMWH versus no bridging, adjusting for pertinent confounders to test these associations.
Results
We identified 1,289 patients who met the “bridging versus no bridging” analysis inclusion criteria and 1,251 patients who met the “DOAC versus warfarin” analysis inclusion criteria. In adjusted cox-regression models, bridging (versus no bridging) treatment was associated with a high risk of d-sICH (HR 2.74 95% CI 1.01 – 7.42) but a similar rate of recurrent ischemic events (HR 1.23 95% CI 0.63 – 2.40). Furthermore, DOAC (versus warfarin) treatment was associated with a lower risk of recurrent ischemic events (HR 0.51 95% CI 0.29 – 0.87) but not d-sICH (HR 0.57 95% CI 0.22 – 1.48).
Conclusion
Our study suggests that patients with ischemic stroke and AF would benefit from the initiation of a DOAC without bridging therapy. Due to our study limitations, these findings should be interpreted with caution pending confirmation from large prospective studies.
Keywords: stroke, atrial fibrillation, anticoagulation, treatment
Introduction
Cardioembolic stroke is associated with increased risk of mortality and morbidity.1 Moreover, it carries a relatively high risk of early recurrent ischemic events2, 3 but also the highest risk for hemorrhagic transformation amongst ischemic stroke subtypes.4, 5 Therefore, treatment decisions aimed at reducing early recurrence without increasing hemorrhagic complications are challenging, but critical for good patient outcomes.
Previous studies of stroke survivors with atrial fibrillation (AF) have shown that treatment with low molecular weight heparin (LMWH) increases the risk of hemorrhagic complications without reducing early recurrence.2, 6 However, these studies were limited in that they did not account for high risk conditions (e.g., presence of cardiac thrombus or valvular heart disease) that may have biased physicians’ treatment decisions. In addition, there is a distinct paucity of data comparing direct oral anticoagulant (DOAC) to warfarin treatment to determine the risk of early recurrence and major bleeding.
In this study, we sought to determine the risk of recurrent ischemic events and delayed symptomatic intracranial hemorrhage (d-sICH) across different anticoagulation strategies. Specifically, we examined the association of treatment with LMWH or heparin (LMWH/heparin) bridging therapy with early recurrent ischemic events and d-sICH, and then compared the rates of these events between patients who were taking warfarin versus DOACs.
Methods
Study cohort
This is a retrospective analysis of the Initiation of Anticoagulation after Cardioembolic stroke (IAC) data, which is a multicenter retrospective collaboration of 8 comprehensive stroke centers across the United States within the years 2015 and 2018. The IAC study team pooled data from ischemic stroke registries of the collaborating sites of consecutive patients with acute ischemic stroke and AF. Patients with mechanical heart valves were excluded from the analysis. Institutional Review Board approval was obtained from each of the participating centers. De-identified data may be shared upon reasonable request to the corresponding author.
Primary Predictors:
The primary predictors were: 1) bridging therapy with treatment dose heparin or LMWH therapy versus no bridging therapy and 2) DOAC versus warfarin treatment.
Outcomes
The primary outcomes in all analyses were: 1) recurrent ischemic events and 2) delayed d-sICH, both within 90-days of stroke onset. We defined recurrent ischemic events as recurrent stroke, TIA, and systemic arterial embolism and d-sICH as neurological deterioration in the setting of any new or worsening hemorrhage detected by brain CT occurring after initiation of anticoagulation therapy, with the hemorrhage being the likely cause of the neurological deterioration.7
In all participating centers, patients discharged with a diagnosis of stroke were scheduled to have an in-person clinic visit at 90 days. In addition, in 3 out of 8 centers, pre-specified phone calls were performed at the 30-day (in one center) and 90-day (in two centers) time points that assessed for recurrent ischemic and hemorrhagic outcomes. Outcomes were preferentially abstracted from the 90-day patient follow up visit and pre-specified 90-day phone calls. In the sites assessing 90-day outcomes by phone and for patients not showing up to their 90 day visits, three attempts were made on different occasions to contact the patient or health care provider by phone. If unsuccessful, then outcomes were assessed by chart review of hospitalization and other outpatient visit and outside hospital records. All outcomes were abstracted by the study local research assistant and confirmed by the site principal investigator. Multiple queries were sent to the participating sites regarding study outcomes and other variables in our dataset and several data cross-checks were performed to confirm the integrity of the data sent by individual sites.
Co-variates
Demographic factors:
Age at the time of admission and gender.
Clinical variables:
Vascular risk factors (history of hypertension, history of diabetes, history of prior stroke or TIA, active smoking), CHA2DS2-Vasc score, and NIHSS score.
Medications prior to admission:
Anticoagulant use.
Neuroimaging and vascular imaging variables:
Presence of intracranial or extracranial stenosis atherosclerosis with ≥ 50% luminal narrowing in the territory of the stroke, largest ischemic stroke lesion volume, hemorrhagic transformation on brain imaging (CT or MRI) prior to initiation of anticoagulation.5 The choice of brain imaging at baseline (CT vs. MRI) was at the discretion of the treating physician.
Echocardiographic variables:
Severe left atrial enlargement (determined by left atrial diameter or volume), moderate to severe valvular heart disease involving the aortic or mitral valves, intracardiac thrombus or spontaneous echocardiographic contrast (SEC), and ejection fraction.
In-hospital treatments:
Time to start anticoagulation.
Analytical plan
Data from sites were pooled and queries were sent to assure accuracy of data, as indicated. We excluded patients who were lost to follow up, had non-outcome related death within 90 days, as well as those who were not started on anticoagulation or in whom the time to starting anticoagulation could not be confirmed. Included patients were stratified to the two primary predictor subgroups: 1) Bridging versus no bridging and 2) DOAC versus warfarin. In the DOAC versus warfarin analysis, patients who were started on one class of medication (eg. DOAC) and switched to another (eg. warfarin) prior to an outcome event were excluded.
We then performed cox regression analyses to determine the association between the primary predictors and ischemic events (stroke/TIA/systemic embolism) and d-sICH, adjusting for pertinent pre-specified confounders based on the outcome of interest. In addition, Kaplan Meier survival analyses to determine the above-mentioned associations. We also performed cox-regression analyses including patients with non-outcome related death and when the exact time of death was not recorded, the time of death was imputed as day 90. Analysis was done using SPSS version 25.0 (Chicago, IL) and a two-tailed p-value < 0.05 was considered statistically significant.
Results
We included 2,084 patients from 8 comprehensive centers in the United States. Details about patients included from each site are shown in supplementary table I. Of these, 1,289 patients met the “bridging versus no bridging” analysis inclusion criteria and 1,251 patients met the “DOAC versus warfarin” analysis inclusion criteria (Tables 1 and 2). Figure 1 shows the study flow chart and reasons for exclusion. We found that 203 (15.8%) patients were started on LMWH/heparin versus 1,086 (84.2%) who did not receive LMWH/heparin bridging (Figure 1). Among patients bridged with heparin or LMWH, the median time in days from starting bridging therapy to initiating oral anticoagulation was shorter in the warfarin group as opposed to the DOAC group [(1 (3) vs. 4 (8), p = 0.002]. The median time from index event to therapeutic INR in patients started on warfarin was 8 days with an interquartile range of 4 to 13 days. For the comparison between DOAC versus warfarin (n = 1251 patients), 862 (69.0%) patients were started on a DOAC (95.6% received factor Xa inhibitors). Of those started on anticoagulation, treatment types did not vary between patients included and excluded from the analysis; LMWH/heparin bridging (15.7% versus 16.0%, p = 0.924) and DOAC treatment (68.9% versus 63.9%, p = 0.142). The median (interquartile range) time in days to recurrent ischemic event was 11 (5–36) and to delayed sICH was 16 (9.5 – 27.25).
Table 1.
Baseline characteristics and outcomes of patients treated with versus without low molecular weight heparin or heparin (LMWH/heparin) bridging
| Bridging (n = 203) |
No bridging (n = 1086) |
p-value | |
|---|---|---|---|
| Age (median, IQR) | 74 (18) | 78 (16) | 0.001 |
| Sex (% female) | 45.3% (92) | 50.5% (548) | 0.194 |
| Hypertension (%) | 88.7% (180) | 82.8% (899) | 0.038 |
| Diabetes (%) | 39.4% (80) | 32.4% (352/1085) | 0.062 |
| Prior stroke or TIA (%) | 27.6% (56) | 30.4% (330) | 0.453 |
| CHA2DS2-Vasc score (median, IQR) | 5 (2) | 4 (3) | 0.637 |
| On anticoagulation prior to index event (%) | 35.1% (71/202) | 40.8% (443) | 0.138 |
| NIHSS score (median, IQR) | 8 (13) | 8 (12) | 0.667 |
| Largest ischemic lesion size | <0.001 | ||
| < 10 mL (%) | 24.6% (47) | 45.7% (460) | |
| 10–20 mL (%) | 24.6% (47) | 19.6% (197) | |
| 20–40 mL (%) | 21.9% (42) | 14.6% (147) | |
| mL (%) | 11.0% (21) | 7.1% (71) | |
| >60 mL (%) | 17.8% (34) | 13.0% (131) | |
| Ipsilateral atherosclerosis with 50% - 99% luminal narrowing (%) | 16.1% (32/199) | 16.8% (180/1071) | 0.918 |
| Early hemorrhagic transformation (%) | 22.7% (46) | 16.0% (174) | 0.025 |
| Cardiac thrombus/SEC (%) | 8.5% (17/200) | 2.5% (25/1020) | <0.001 |
| Valvular heart disease (%) | 21.0% (42/200) | 22.5% (229/1020) | 0.710 |
| Severe left atrial enlargement (%) | 35.5% (60/169) | 38.7% (347/896) | 0.439 |
| Ejection fraction (median %, IQR) | 55 (15) | 60 (15) | 0.004 |
| Time to initiating anticoagulation (median days, IQR) | 2 (3) | 5 (8) | <0.001 |
| Recurrent ischemic events (%) | 5.9% (12) | 6.9% (75) | 0.760 |
| Delayed symptomatic intracranial hemorrhage (%) | 4.4% (9) | 1.0% (11) | 0.002 |
Table 2.
Baseline characteristics and outcomes of patients treated with direct oral anticoagulant (DOAC) versus warfarin (n=1,251).
| Warfarin treatment (n = 389) |
DOAC treatment (n = 862) |
p-value | |
|---|---|---|---|
| Age (median, IQR) | 76 (17) | 78 (17) | 0.266 |
| Sex (% female) | 49.4% (192) | 50.3% (434) | 0.760 |
| Hypertension (%) | 87.7% (341) | 82.0% (707) | 0.013 |
| Diabetes (%) | 31.4% (122) | 34.6% (298/861) | 0.272 |
| Prior Stroke or TIA (%) | 31.1% (121) | 29.5% (254) | 0.594 |
| CHA2DS2-Vasc score (median, IQR) | 5 (2) | 4 (2) | <0.001 |
| On anticoagulation prior to index event (%) | 46.3% (180) | 37.8% (326) | 0.006 |
| NIHSS score (median, IQR) | 6 (11) | 8 (12) | 0.117 |
| Ischemic stroke size | 0.006 | ||
| < 10 mL (%) | 50.6% (173) | 39.7% (325) | |
| 10–20 mL (%) | 14.6% (50) | 23.1% (189) | |
| 20–40 mL (%) | 16.1% (55) | 15.0% (123) | |
| 40–60 mL (%) | 7.3% (25) | 7.4% (61) | |
| >60 mL (%) | 11.4% (39) | 14.8% (121) | |
| Ipsilateral atherosclerosis with 50% - 99% luminal narrowing (%) | 17.2% (65/378) | 16.6% (142/855) | 0.805 |
| Early hemorrhagic transformation (%) | 14.6% (57) | 18.0% (155) | 0.166 |
| Cardiac thrombus/SEC (%) | 4.8% (17/358) | 2.9% (24/825) | 0.121 |
| Valvular heart disease (%) | 26.2% (94/358) | 20.0% (165/825) | 0.018 |
| Severe left atrial enlargement (%) | 38.7% (105/271) | 38.3% (293/766) | 0.885 |
| Ejection fraction (median, IQR) | 55 (15) | 60 (15) | <0.001 |
| Time to initiating anticoagulation (median, IQR) | 2 (6) | 5 (8) | <0.001 |
| Recurrent ischemic events (%) | 10.0% (39) | 5.3% (46) | 0.003 |
| Delayed symptomatic intracranial hemorrhage (%) | 2.1% (8) | 1.2% (10) | 0.303 |
DOAC = Direct Oral Anticoagulant; SEC = Spontaneous Echocardiographic Contrast
Figure 1.
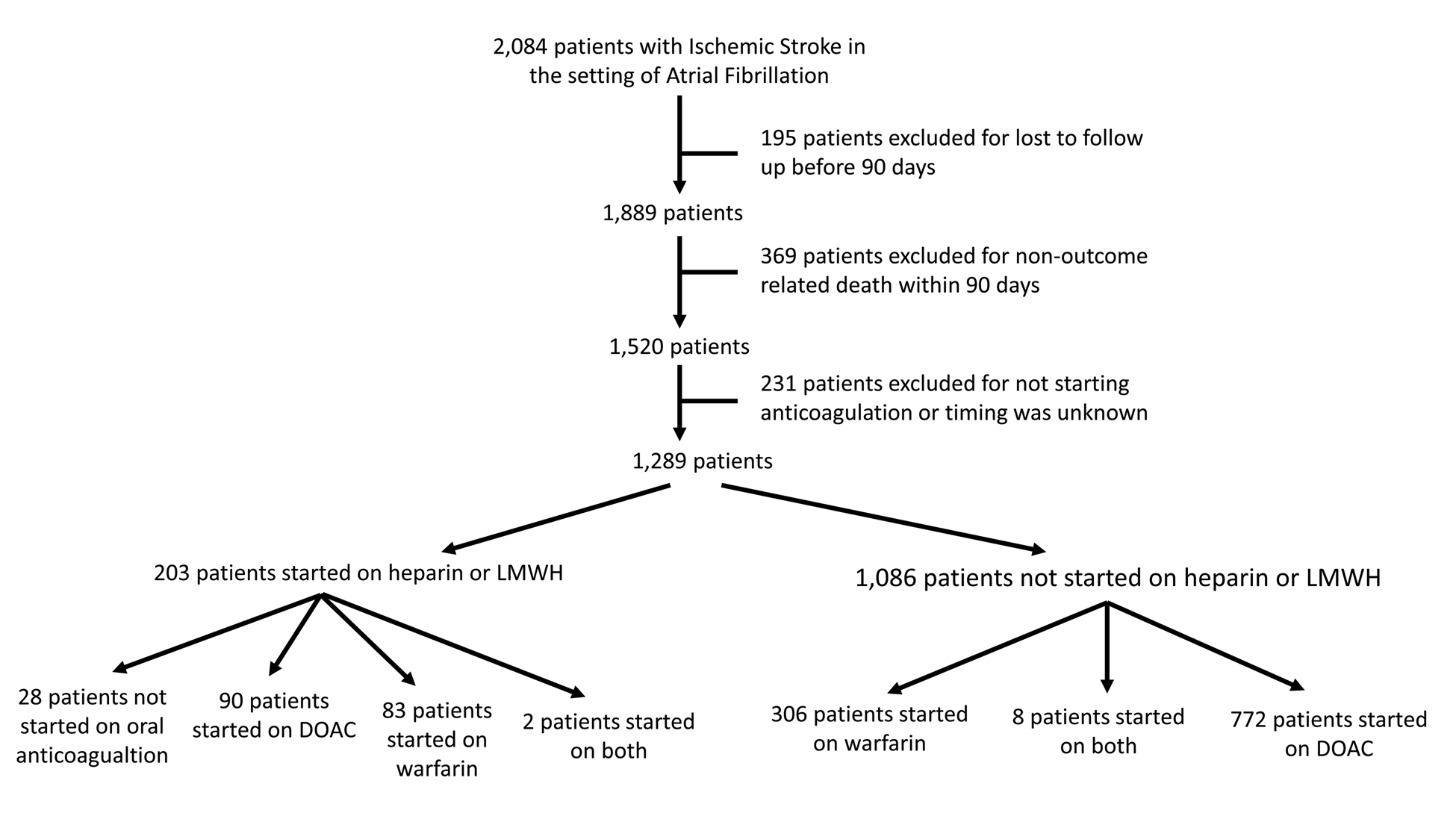
Figure 1 shows the study flow chart showing patients included vs. those included.
Comparison of LMWH/heparin bridging versus no bridging therapy
The baseline characteristics between patients started on LMWH/heparin bridging versus no bridging are shown in Table 1. In univariate analyses, bridging with LMWH/heparin (versus no bridging) was associated with a significantly higher rate of d-sICH (4.4% versus 1.0%, p = 0.002). However, there was no association with recurrent ischemic events (5.9% versus 6.9%, p = 0.760) (Table 1). Figure 2 shows the Kaplan Meier curve of recurrent ischemic events and d-sICH within 90 days, indicating a higher risk of d-sICH with LMWH/heparin bridging.
Figure 2.
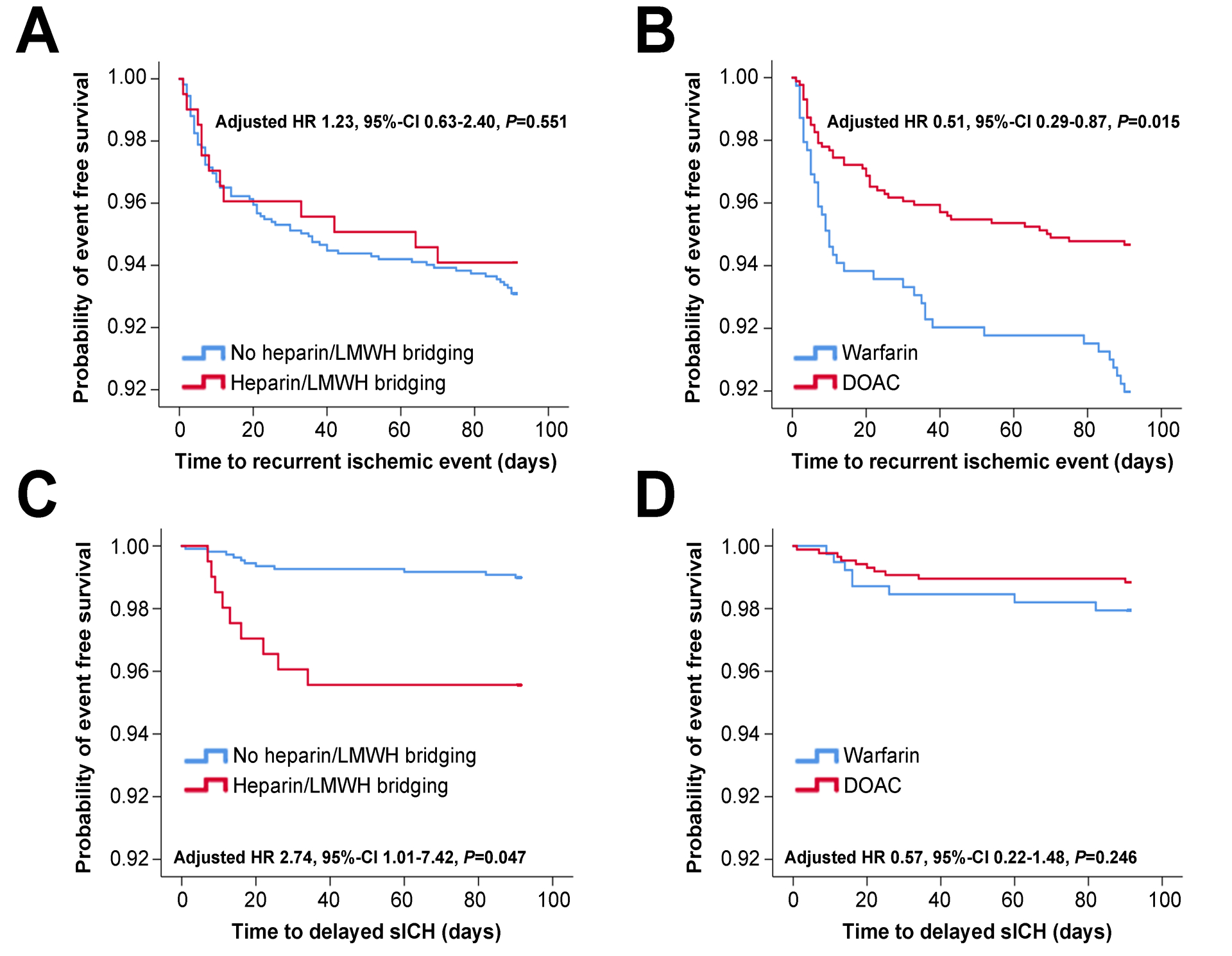
Figure 2 shows KM survival analyses of the risk of delayed symptomatic intracranial hemorrhage and recurrent ischemic events based on treatment type: left side shows bridging vs. no bridging and right side shows DOAC vs. warfarin treatment.
In an unadjusted cox-regression model, bridging treatment was not associated with recurrent ischemic events (HR 0.85, 95% CI 0.46 – 1.57, p = 0.611) (Table 3). The lack of an association persisted after adjusting for potential confounders (HR 1.23, 95% CI 0.63 – 2.40, p = 0.551) (Table 3, model 3).
Table 3.
Cox regression models showing effect of low molecular weight heparin or heparin (LMWH/heparin) bridging versus no bridging and direct oral anticoagulant (DOAC) versus warfarin on recurrent ischemic events adjusting for potential confounders.
| Analysis 1) LMWH/heparin bridging versus no bridging | Analysis 2) DOAC versus warfarin | |
|---|---|---|
| Unadjusted | 0.85 (0.46 – 1.57), p = 0.611 | 0.52 (0.34 – 0.80), p = 0.003 |
| Model 1 | 0.84 (0.45 – 1.54), p = 0.567 | 0.52 (0.34 – 0.80), p = 0.003 |
| Model 2 | 0.86 (0.45 – 1.64), p = 0.648 | 0.54 (0.34 – 0.86), p = 0.009 |
| Model 3 | 1.23 (0.63 – 2.40), p = 0.551 | 0.51 (0.29 – 0.87), p = 0.015 |
| Model 4 | 1.26 (0.62 – 2.53), p = 0.525 | 0.50 (0.29 – 0.87), p = 0.014 |
Model 1: adjusted for age and sex, Model 2: adjusted for CHA2DS2-Vasc, ipsilateral atherosclerosis, prior stroke/TIA, and ischemic lesion > 10 mL. Model 3: Model 2 + valvular heart disease, severe LAE, and SEC/thrombus. Model 4: model 3 + time to start anticoagulation
Conversely, in unadjusted cox-regression LMWH/heparin bridging was associated with a significantly increased risk for d-sICH (HR 4.47, 95% CI 1.85 – 10.77, p = 0.001) (Table 4). After adjusting for pertinent confounders, bridging therapy remained associated with an almost 3-fold risk for d-sICH (adjusted HR 2.74 95% CI 1.01 – 7.42, p = 0.047) (Table 4 model 3). Adding time to initiation of anticoagulation into the models did not meaningfully change the results (Tables 3 and 4, model 4). Furthermore, to determine the effect of unmeasured confounders on this association, we calculated the E-value.8 We calculated an E-value of 4.92, indicating that our observed HR of 2.74 could be explained away by an unmeasured confounder that was associated with both the treatment and the outcome by an HR of 4.92-fold each, above and beyond the measured confounders.
Table 4.
Cox regression models showing effect of low molecular weight heparin or heparin (LMWH/heparin) bridging versus no bridging and direct oral anticoagulant (DOAC) versus warfarin on delayed symptomatic intracranial hemorrhage adjusting for potential confounders.
| Analysis 1) LMWH/heparin bridging versus no bridging | Analysis 2) DOAC versus warfarin | |
|---|---|---|
| Unadjusted | 4.47 (1.85 – 10.77), p = 0.001 | 0.56 (0.22 – 1.42), p = 0.224 |
| Model 1 | 4.20 (1.73 – 10.18), p = 0.002 | 0.57 (0.23 – 1.45), p = 0.240 |
| Model 2 | 3.48 (1.42 – 8.55), p = 0.007 | 0.48 (0.19 – 1.21), p = 0.119 |
| Model 3 | 2.74 (1.01 – 7.42), p = 0.047 | 0.57 (0.22 – 1.48), p = 0.246 |
| Model 4 | 2.74 (0.90 – 8.34), p = 0.075 | 0.57 (0.22 – 1.48), p = 0.246 |
Model 1: adjusted for age and sex, Model 2: adjusted for age, sex, Ischemic lesion > 10, early hemorrhagic transformation, Model 3: age, sex, Ischemic lesion > 10, early hemorrhagic transformation and both predictors (bridging and DOAC treatment), model 4: model 3 + interval to start anticoagulation
Comparison of patients treated with DOAC versus warfarin
Baseline characteristics of patients started on DOAC versus warfarin are shown in table 2. DOAC treatment was associated with a lower rate of recurrent ischemic events within 90 days (5.3% versus 10.0%, p = 0.003) without a significant difference in rate of d-sICH (1.2% versus 2.0%, p = 0.303) (Table 2). Figure 2 shows the Kaplan Meier curve of recurrent ischemic evens within 90 days, demonstrating the lower risk for DOAC, compared to warfarin, without a significant difference in d-sICH risk.
In an unadjusted cox-regression, DOAC treatment was associated with a reduced risk of recurrent ischemic events (HR 0.52, 95% CI 0.34 – 0.80, p = 0.003) (Table 3). This association persisted after adjusting for potential confounders (HR 0.51, 95% CI 0.29 – 0.87, p = 0.015) (Table 3 model 3). Furthermore, to further investigate the effect of confounders on this association, we calculated the E-value8 for this association and it was 3.33 indicating that our observed HR of 0.51 could be explained away by an unmeasured confounder that was associated with both the treatment and the outcome by an HR of 3.33-fold each, above and beyond the measured confounders.
Conversely, in an unadjusted cox-regression there was no statistically significant difference in the risk of d-sICH between DOAC versus warfarin treatment (HR 0.56, 95% CI 0.22 – 1.42, p = 0.224) (Table 4). The lack of association persisted after adjusting for potential confounders (HR 0.57, 95% CI 0.22 – 1.48, p = 0.246) (Table 4 model 3). Including time to start anticoagulation in both models, the results remained unchanged (Tables 3 and 4, model 4).
Additional analyses
We performed additional analyses including patients with a non-outcome related death. There were 369 patients with non-outcome related death, among which 23.3% were started on anticoagulation. Patients with non-outcome related death were older (81.2 ± 11.2 vs. 76.2 ± 11.7, p < 0.001), had higher median (IQR) admission NIHSS [8 (12) vs. 21 (11), p <0.001) and were more likely to have > 60 mL infarct size (47.6% vs. 16.3%, p < 0.001). The day of death was recorded on 52.6% (194/369) of patients. In cox regression analyses, in fully adjusted models (model 4): bridging vs. no bridging therapy was associated with increased risk of d-sICH (HR 2.72 95% CI 0.89 – 8.29, p = 0.079) without any reduction in the risk of ischemic events (HR 1.19 95% CI 0.59 – 2.41, p = 0.624). Furthermore, in fully adjusted models (model 4): DOAC vs. warfarin treatment was associated with a lower risk of recurrent ischemic events (HR 0.50 95% CI 0.29 – 0.87, p = 0.014) but no reduction in the risk of d-sICH (HR 0.56 95% CI 0.22 – 1.45, p = 0.233).
Moreover, we ran the DOAC vs. warfarin treatment analysis excluding patients who were bridged with heparin or LMWH. In fully adjusted models, DOAC treatment was associated with reduced risk of recurrent ischemic events (HR 0.53 95% CI 0.29 – 0.98, p = 0.041) without any significant difference in the risk of d-sICH (HR 0.60 95% CI 0.17 – 2.09, p = 0.424). Event rates by treatment type are shown in supplementary Figure I.
Discussion
The ideal approach to anticoagulation in patients with AF and acute ischemic stroke remains uncertain. Leveraging data from a large retrospective, multicenter study of a well-defined cohort of patients with stroke and AF, we explored the association of the most commonly utilized anticoagulant strategies and their association with the outcomes of recurrent ischemic events and intracranial hemorrhage.
First, we examined the association of LMWH/heparin bridging treatment with the outcomes of interest. Overall, our observations are in line with prior studies7, 9 showing that bridging therapy is associated with significantly increased risk of d-sICH without reducing the risk for recurrent ischemic events. Second, we sought to determine the association of DOAC versus warfarin therapy with the outcomes. In this analysis, we found that DOAC treatment was associated with a significantly reduced risk of recurrent ischemic events without increasing the risk for d-sICH. This finding differs from a recent analysis pooling data from European and Japanese prospective cohort studies.10 However, in contrast to the abovementioned study we focused on the event rate within the first 90 days as opposed to long-term outcomes precluding direct comparison. Nevertheless, it is noteworthy that the direction of association (i.e., numerically lower event rate with DOAC for hemorrhagic and ischemic events) is similar in both studies. Importantly, these associations persisted after adjusting for pertinent confounders and factors that have been associated with early stroke recurrence or bleeding risk.11
Mechanism of associations
There are severe potential mechanisms by which patients treated with DOACs (versus warfarin) had a lower risk of recurrent ischemic events. First, after initiation of warfarin therapy, it typically takes several days until the therapeutic INR is reached.12 Second, on average patients have a therapeutic INR less than 50% of the time with significant fluctuations throughout the course of treatment.13, 14 Third, warfarin treatment has been hypothesized to cause an initial transient hypercoagulable state by inhibiting protein C and S, which are endogenous anticoagulants.15 In contrast, DOAC treatment is associated with a rapid onset, more stable therapeutic window, and no early hypercoagulability. That said, based on our study physicians tend to use warfarin over DOAC in relatively sicker patients such as those moderate to severe aortic/mitral valve disease, bioprosthetic valves, and cardiac thrombus which may have contributed to this finding.
Bridging with LMWH/heparin has been hypothesized to expedite full anticoagulation before starting oral anticoagulation with the added potential benefit of better control in high-risk patients. Studies of patients with venous thromboembolic events have used parenteral anticoagulation prior to starting warfarin or a DOAC.16 In the acute stroke setting, bridging treatment with heparin which is easily reversible may have been used as an initial treatment in patients considered at “high risk” for early recurrence and bleeding, and once stable, they were started on a DOAC or bridged to warfarin. In addition, some patients were started on LMWH instead, possibly to achieve a more rapid therapeutic effect and to avoid the need for frequent dose adjustment. Unfortunately, our study lacks specific data on which bridging agent was used (LMWH versus heparin). Nevertheless, the mechanism as to why bridging with LMWH/heparin is associated with increased risk of d-sICH is not well understood but this finding has been shown in multiple studies.6, 17–19 Cardioembolic stroke is associated with higher risk of hemorrhagic transformation20, 21 likely due to reperfusion injury and fragile vasculature post ischemia.5 It is possible that bridging treatment resulted in a high intensity of anticoagulation possibly due to over-shooting therapeutic targets with heparin or with some overlap between the parenteral and oral agent which may have led to increased bleeding risk with bridging treatment. In our study, patients who were started on bridging treatment had larger strokes and were more likely to have early hemorrhagic transformation which may have been a contributing factor.
Clinical Implications
For patients with nonvalvular AF and ischemic stroke, DOAC therapy is the standard of care for secondary stroke prevention. However, warfarin continues to be used frequently because of lower cost, presence of absolute contraindications for DOACs, and greater physician comfort with warfarin.22 Data from randomized controlled trials have shown that DOACs are equally effective for secondary stroke prevention compared to warfarin with a lower risk of intracranial hemorrhage, other hemorrhagic complications, and death.23–25 However, those trials largely excluded patients with recent stroke26, in whom physicians face a common clinical dilemma: bridging versus no-bridging and DOAC versus warfarin? While prior studies in this patient population using clinical trial or registry data have suggested that DOAC therapy was superior to warfarin for prevention of cardiovascular events and that bridging therapy may be harmful,6, 27, 28 real-world clinical data is critical for generalizability.
Strengths and limitations
Our study has several limitations. First, the treatments were not randomly assigned, leading to potential bias in the treatment assignment across the subgroups. This may not have significantly impacted our findings given that we compared baseline characteristics between the treatments and adjusted for major confounders and had relatively high E-values. Second, approximately 9% of our patients were lost to follow up. Although this is a reasonably low rate, it does add bias. Third, TTE was used to determine variables such as thrombus and spontaneous echocardiographic contrast, which are better detected on trans-esophageal echocardiogram (TEE). However, TEE is not routinely used in clinical practice, particularly in those with cardioembolic stroke, and thus our study reflects real-world practice patterns. The small number of patients with high risk cardiac abnormalities such as cardiac thrombus, however, left us underpowered to determine whether bridging therapy is useful in this subpopulation. Fourth, our study was performed at comprehensive stroke centers, and therefore it is biased towards more complex and severe stroke patients, somewhat limiting the generalizability our findings. Fifth, although both CT and MRI use are within guideline recommended patient care and reflect clinical practice, the choice of baseline and follow-up brain imaging (CT vs. MRI) was at the discretion of the treating physician and this could have possibly introduced bias. Finally, our event rates were relatively low. This may have left our study underpowered to detect treatment. Nevertheless, our study has several notable strengths including the large sample size and breadth of predictor variables, and being a multicenter center study, which allowed us to encompass a wide range of practice patterns.
Conclusion
We show that, in patients with recent ischemic stroke and AF, bridging therapy with LMWH/heparin was associated with increased risk of d-sICH without a reduction in recurrent ischemic events. In addition, DOAC treatment, compared to warfarin, was associated with a lower risk of recurrent ischemic events, but similar rates of d-sICH. These findings suggest that patients with ischemic stroke and AF would benefit from the initiation of a DOAC without bridging therapy. Our study has several major limitations and therefore our findings should be interpreted with caution pending confirmation from large prospective studies.
Supplementary Material
Funding
This study is partially funded by the NIH grant K08NS091499.
Disclosures
Dr. Henninger is supported by K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and R44NS076272 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. He also received personal fees from Astrocyte Pharmaceuticals, Inc and grants from NICHD outside the submitted work. Dr. Liberman is supported by K23NS107643 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Dr. Mistry is funded by NIH/NINDS grant K23NS113858. Dr. Nouh received funding from Genentech. Dr Yaghi reports other from Medtronic outside the submitted work. Dr de Havenon reports funding from Regeneron pharmaceuticals and AMAG pharmaceuticals. All other authors report no disclosures or acknowledgments.
References
- 1.Henninger N, Goddeau RP Jr, Karmarkar A, Helenius J, McManus DD Atrial fibrillation is associated with a worse 90-day outcome than other cardioembolic stroke subtypes. Stroke; a journal of cerebral circulation. 2016;47:1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arboix A, Garcia-Eroles L, Oliveres M, Massons JB, Targa C. Clinical predictors of early embolic recurrence in presumed cardioembolic stroke. Cerebrovascular diseases (Basel, Switzerland). 1998;8:345–353 [DOI] [PubMed] [Google Scholar]
- 3.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: A double-blind randomised study. Haest study group. Heparin in acute embolic stroke trial. Lancet (London, England). 2000;355:1205–1210 [DOI] [PubMed] [Google Scholar]
- 4.England TJ, Bath PM, Sare GM, Geeganage C, Moulin T, O’Neill D, et al. Asymptomatic hemorrhagic transformation of infarction and its relationship with functional outcome and stroke subtype: Assessment from the tinzaparin in acute ischaemic stroke trial. Stroke. 2010;41:2834–2839 [DOI] [PubMed] [Google Scholar]
- 5.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2017;48:e343–e361 [DOI] [PubMed] [Google Scholar]
- 6.Altavilla R, Caso V, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, et al. Anticoagulation after stroke in patients with atrial fibrillation. Stroke. 2019;50:2093–2100 [DOI] [PubMed] [Google Scholar]
- 7.Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: Effect of anticoagulation and its timing: The raf study. Stroke. 2015;46:2175–2182 [DOI] [PubMed] [Google Scholar]
- 8.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the e-value. Annals of internal medicine. 2017;167:268–274 [DOI] [PubMed] [Google Scholar]
- 9.Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-k oral anticoagulants (raf-noacs) study Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, et al. Direct oral anticoagulants versus vitamin k antagonists after recent ischemic stroke in patients with atrial fibrillation. Annals of neurology. 2019;85:823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Sabin J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013;12:689–705 [DOI] [PubMed] [Google Scholar]
- 12.Harrison L, Johnston M, Massicotte MP, Crowther M, Moffat K, Hirsh J. Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy. Annals of internal medicine. 1997;126:133–136 [DOI] [PubMed] [Google Scholar]
- 13.Passman R Time in therapeutic range in warfarin-treated patients: Is very good good enough? Jama. 2016;316:872–873 [DOI] [PubMed] [Google Scholar]
- 14.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk-adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: Results of the veterans affairs study to improve anticoagulation (varia). Circulation. Cardiovascular quality and outcomes 2011;4:22–29 [DOI] [PubMed] [Google Scholar]
- 15.Freedman MD. Oral anticoagulants: Pharmacodynamics, clinical indications and adverse effects. Journal of clinical pharmacology. 1992;32:196–209 [DOI] [PubMed] [Google Scholar]
- 16.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for vte disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e496S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The international stroke trial (ist): A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet (London, England). 1997;349:1569–1581 [PubMed] [Google Scholar]
- 18.Steinberg BA, Peterson ED, Kim S, Thomas L, Gersh BJ, Fonarow GC, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: Findings from the outcomes registry for better informed treatment of atrial fibrillation (orbit-af). Circulation. 2015;131:488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallevi H, Albright KC, Martin-Schild S, Barreto AD, Savitz SI, Escobar MA, et al. Anticoagulation after cardioembolic stroke: To bridge or not to bridge? Archives of neurology. 2008;65:1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terruso V, D’Amelio M, Di Benedetto N, Lupo I, Saia V, Famoso G, et al. Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology. 2009;33:261–265 [DOI] [PubMed] [Google Scholar]
- 21.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084 [DOI] [PubMed] [Google Scholar]
- 22.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:2160–2236 [DOI] [PubMed] [Google Scholar]
- 23.Oral anticoagulants for prevention of stroke in atrial fibrillation: Systematic review, network meta-analysis, and cost effectiveness analysis. BMJ (Clinical research ed.) 2017;359:j5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: Cohort study in primary care. BMJ (Clinical research ed.) 2018;362:k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet (London, England). 2014;383:955–962 [DOI] [PubMed] [Google Scholar]
- 26.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. The Lancet. Neurology 2019;18:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xian Y, Xu H, O’Brien EC, Shah S, Thomas L, Pencina MJ, et al. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: Findings from the patient-centered research into outcomes stroke patients prefer and effectiveness research (prosper) study. JAMA neurology. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteley WN, Adams HP Jr, Bath PM, Berge E, Sandset PM, Dennis M, et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: An individual patient data meta-analysis of randomised controlled trials. The Lancet. Neurology 2013;12:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


