Abstract
Background:
Chronic diseases, such as opioid use disorder (OUD) require a multifaceted scientific approach to address their evolving complexity. The Council for the Advancement of Nursing Science’s (Council) four nursing science priority areas (precision health; global health, determinants of health, and big data/data analytics) were established to provide a framework to address current complex health problems.
Purpose:
To examine OUD research through the nursing science priority areas and evaluate the appropriateness of the priority areas as a framework for research on complex health conditions.
Method:
OUD was used as an exemplar to explore the relevance of the nursing science priorities for future research.
Findings:
Research in the four priority areas is advancing knowledge in OUD identification, prevention, and treatment. Intersection of OUD research population focus and methodological approach was identified among the priority areas.
Discussion:
The Council priorities provide a relevant framework for nurse scientists to address complex health problems like OUD.
Keywords: Precision health, Big data and Data analytics, Determinants of health, Global health, Opioid use disorder research
Opioid Use Disorder Research and Nursing Science Priority Areas
Opioid use disorder (OUD) is a complex chronic disease and increasing cause of death, with approximately 130 people in the United States dying daily after an opioid overdose (Iwanicki et al., 2018). OUD mortalities include deaths from illicit drug abuse and prescription drug misuse. Multiple factors contribute to OUD and its negative outcomes including prescriber practices, stigma of the disease, misconceptions regarding opioid use in pain management, economic and political forces, and the vulnerable populations often affected (Davis, Green, & Beletsky, 2017; Faul, Bohm, & Alexander, 2017).
In contrast to the growing recognition that population health problems and chronic diseases, such as OUD, are complex and continually changing, the research methods used to tackle these issues can be limited. Individuals are inherently complex and the environment in which we live, provides an evolving, open system that changes in an ongoing and reciprocal cycle (Greenhalgh & Papoutsi, 2018). Within these systems, complex health problems require research that spans discipline-specific silos and is nontraditional and iterative (Marshall, 2017). Research in OUD prevention, treatment, and management must reflect a diverse array of methods and approaches to be successful (Kuehner-Hebert, 2017; Zirui, 2017). Nurses are well-positioned to conduct OUD research with a broad portfolio of distinct, yet interconnected, methods and approaches, allowing them to study heterogeneous populations across the lifespan (Grady, 2017; K. A. Russell, 2017).
Complexity of OUD
Most opioid misuse originates from a prescription for pain, and the majority of patients who overdose on prescription opioids are taking their medications in a manner other than as prescribed or are using opioids prescribed to someone else (Nelson, Juurlink, & Perrone, 2015). However, the illicit use of opioids (e.g., heroin and nonprescribed fentanyl) also contributes to the growing incidence of OUD and is responsible for the majority of opioid-related deaths (Knopf, 2016). There is considerable heterogeneity amid general patterns of who is at risk for OUD and mortality related to OUD (King, Fraser, Boikos, Richardson, & Harper, 2014). For example, although men were more likely to overdose, relative increases in opioid-related deaths were greater among women with more than a 400% increase in women as compared to a 265% increase in men since 1999 (Centers for Disease Control and Prevention 2019, October 31). This heterogeneity related to risk factors and profiles of those individuals for OUD and opioid related deaths contributes to the challenges of OUD research.
The communities, policies, clinical practices, financial, and political environments that contribute to the incidence and prevalence of OUD constitute part of a complex national and global system, some of which respond to and interact with each other in intricate and often unpredictable ways (Crowley, Kirschner, Dunn, & Bornstein, 2017; Davis et al., 2017). Grassroots initiatives are frequently spurred by local decisive events that lead a community to react in ways that may conflict with state or national efforts to combat OUD. Further, state and national adoption of Prescription Drug Monitoring Program tracking to control OUD may have the unintentional consequence of adversely influencing national health initiatives to decrease all substance use disorders. Tracking prescribing practices may decrease clinicians’ prescribing of opioids for pain, resulting in the abuse of alternate, and often more readily available substances, such as alcohol, marijuana, heroin, or other prescribed medications intended to treat anxiety, depression, or insomnia to control their pain (Sajid et al., 2016). The consideration of these interacting agencies and their influence on individual and community-level outcomes are integral to answering OUD research questions.
Council Nursing Science Research Priority Areas
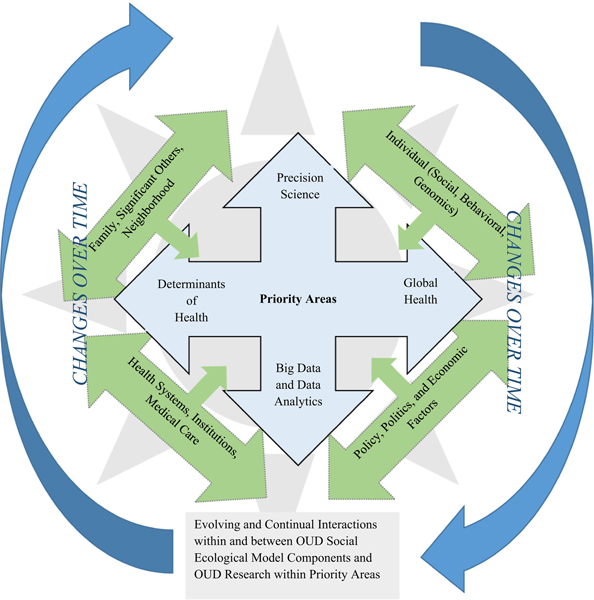
In 2016, the American Academy of Nursing asked the Science Committee of Council for the Advancement of Nursing Science to identify national nursing science priorities. The Science Committee identified four thematic areas: (a) Precision science; (b) Big data and data analytics; (c) Determinants of health; and (d) Global health (Eckardt et al., 2017). The priority areas reflect the diversity of nursing science while demonstrating its intersection with other scientific disciplines investigating complex epidemics and diseases and provide the national community of nurse scientists with an outline of national nursing science priorities to inform future collaborations, lines of scientific inquiry, and resource allocation in alignment with the American Academy of Nursing’s mission and goals. The priority areas often overlap in theoretical and conceptual foundations, populations of interest, and complex health and research questions to be answered. These common elements of the priority areas provide an organic framework to support research of complex health epidemics (Figure 1) such as OUD (Eckardt et al., 2017).
Figure 1 –
Relationship of social ecological complexity of opioid use disorder and nursing science priority areas.
Purpose
The purpose of this manuscript was to examine OUD research from the perspective of the four nursing science priority areas and evaluate the appropriateness of the priority areas as a framework for research on complex health conditions, such as OUD. Strengths, challenges, and limitations of this approach are discussed.
Findings
Priority 1: Precision Science
OUD is the consequence of a complex interplay between life experiences and social environments as well as individual genetic, behavioral, psychological, and neurobiological vulnerabilities (Belzeaux, Lalanne, Kieffer, & Lutz, 2018). Applying biology, behavior, and environmental considerations in understanding OUD is essential to build knowledge that can precisely target the right interventions to the right individuals at the right time (Corwin, Redeker, Richmond, Docherty, & Pickler, 2019).
Individuals who are physically and psychologically dependent on illicit drugs can be genetically predisposed and/or have a family history of drug addiction. Genetic predisposition can also influence responses to addiction treatment (Bühler et al., 2015; Ghanbari & Sumner, 2018; Xuei et al., 2006). Genome-wide association studies have demonstrated connections between OUD and genetic variants (Agrawal, Edenberg, & Gelernter, 2016; Ducci & Goldman, 2012) involving any of the three opioid receptor genes (OPRM1, OPRD1, and OPRK1). Mutations alter clinical opioid effects and affect susceptibility to drug addiction (Haerian & Haerian, 2013). Among the OPRM1 gene variants, the A118G (rs1799971) locus is the most frequent coding region for polymorphisms in most populations. There are also overlaps among genes associated with opioids, nicotine, and alcohol addiction (Reyes-Gibby, Yuan, Wang, Yeung, & Shete, 2015) that may predispose to abuse of all three substances.
Other pathways affecting the brain’s reward system and implicated in OUD include dopaminergic, noradrenergic, glutamatergic, and GABAergic systems (Reed, Butelman, Yuferov, Randesi, & Kreek, 2014). Epigenetic studies suggest that exposure to drugs of abuse can induce changes in gene expression, and change response to opioid use and OUD treatment (Andersen, Dogan, Beach, & Philibert, 2015). Metabolomic studies identified biomarkers related to opioid dependence and lifestyle choices in brain hippocampus and prefrontal cortex in opioid addicted animal models (Deng et al., 2012).
Precision health approaches to OUD must also include behavioral and psychosocial characteristics, as well as social determinants and biological factors to provide personalized targeted care (Eckardt et al., 2017). Researchers using this approach to precision heath research have successfully advanced knowledge of OUD occurrence and treatment. For example, links between affective disorders and OUD may emerge from several different pathways, including prolonged OUD that can lead to depression or use of opioids to manage anxiety (Gros, Milanak, Brady, & Back, 2013). Predictors of OUD are not limited to mental health comorbidities or exposure. However, research that incorporates behavioral and psychological factors such as mental health may assist in the identification of a common biomarker to advance OUD prevention and treatment research.
Geospatial data such as census data, crime data, Emergency Medical Services (EMS) data, health system data, prescription drug monitoring data, or crowdsourcing locations of discarded needles to precise geographical locations, show that OUD is not equally distributed geographically within or across communities (Bearnot, Pearson, & Rodriguez, 2018; Wright et al., 2014). This unequal distribution is exacerbated by regional imbalances in treatment capacity, having effects on policy formulation, programmatic prevention activities, rescue resources, and transition to medication-assisted treatment (Langabeer et al., 2019). Medical prescription of opioids shows significant variations across states and regions which can inform high-risk regions that would benefit from testing policies or evidence-based interventions to achieve optimal opioid treatment (Mazumdar, McRae, & Islam, 2015; Piper, Shah, Simoyan, McCall, & Nichols, 2018). Employing data-driven identification of high-risk locations for overdose can inform key stakeholders where to deploy and locate limited treatment resources to obtain maximum benefit (Dworkis, Taylor, Peak, & Bearnot, 2017). Recent research examined spatial access to OUD treatment and emergency medical services using a composite of street network distances, driving times and distance decay relationships, and service availability in relation to fentanyl overdose, noting a lack of uniformity across the state of New Hampshire (Cao, Stewart, Wish, Artigiani, & Sorg, 2019).
Geospatial data can also provide information for policy decisions regarding use of resources to combat OUD (Bird, McAuley, Perry, & Hunter, 2016; Dworkis et al., 2017; Dworkis, Weiner, Liao, Rabickow, & Goldberg, 2018). Historical and current data also provide a registry of policy and intervention history that can be examined across time, and levels (such as national, state, and local policy adoption) that include cross-level interactions and competing models to provide causal estimates of OUD policy intervention (Bird, McAuley, Munro, Hutchinson, & Taylor, 2017; McAuley et al., 2017). For example, Eckardt and Erlanger (2018) used propensity score matching across a hierarchical model within a longitudinal design to examine 17 years of forensic, emergency health services, and legislative data to provide causal estimates of policy implementation across neighborhoods to prevent and decrease opioid-related deaths in Suffolk County, NY. Though the results of the study support existing policy implementation in decreasing deaths, it also estimated that the neighborhood effect on opioid-related death prevention was significant when controlling for state and national policies. These findings substantiate the need for interdisciplinary research at local levels to inform the unique models required for accurate neighbourhood-centered prevention and treatment (Eckardt, et al., 2020).
Summary
There are limitations associated with a precision health approach to OUD research. Most notably, protection of participants’ privacy and the confidentiality of their data and specimens cannot be assured as new advances in omics and technology may lead to unforeseen future breaches of privacy and confidentiality. Additionally, data used in secondary analyses were often collected for non-research purposes jeopardizing the validity of these measures to answer the intended research questions that a randomized control trial with prospective assignment to treatment would afford (Derose, Contreras, Coleman, Koebnick, & Jacobsen, 2013; Hunt, Lee, Harrison, & Smith, 2018). However, recognizing biobehavioral risk factors for OUD, screening for risk, and creating plans to address these in the treatment of pain, may lead to evidence-based treatment algorithms to prevent the development of OUD in susceptible individuals (Cheatle, 2016). Nurse researchers studying OUD, whether to prevent its onset, avoid overdose, or facilitate hand-offs to medication-assisted treatment, can incorporate geospatial techniques to inform their work. The effects of the opioid epidemic that are of central interest to nursing science, such as the effects of overdose rampant in a community or the exposure of children to discarded needles in the neighborhoods where they live, play, and learn, could be mitigated by focusing on high risk geographic locations.
Priority 2: Big Data and Data Analytics
Big data and data analytics (i.e. informatics and technology) through data synthesis and integration support research approaches investigating the complexities of OUD epidemiology and treatment. Big data science is the application of mathematical techniques to large data sets to infer probabilities for prediction and find novel patterns to enable data driven decisions. The different types of data that big data affords, coupled with the non-linear assumptions allowed with these analytics, provide a medium for complex, iterative, and openmodel approaches to OUD research. For example, promising results demonstrating the efficacy of opioid-sparing medication for pain control that were not apparent with a classical approach were achieved through the use of divergent, yet iterative, models (Juul et al., 2017). Additionally, big data and data modeling simulations and extensions often include comparisons among competing models (Clancy, Effken, & Pesut, 2008) reflecting the heterogeneity in risk profiles and treatment models in OUD. Profiles for patients at risk of adverse events for varying classes and combinations of opioids were developed through data mining of the U.S. Food and Drug Administration Adverse Events Reporting System (Min, Osborne, Kowalski, & Prosperi, 2018).
To advance the validity and reliability of big data analytics methodological approaches, the National Academies of Sciences, Engineering, and Medicine Board on Mathematical Sciences and Analytics convened symposia and webinars on data, modeling, and policy outlining the use and integration of national data registries for OUD research (Springer, Korthuis, & Del Rio, 2018). Multiple funding mechanisms for a big data approach to OUD research have also spurred advances in knowledge generated using this approach (Institute of Human Virology at the University of Maryland School of Medicine, 2018; Knopf, 2018; Medica-Safe, 2019). The advances in resources and education available to assist OUD researchers using big data and data analytics approaches provide a rich and promising source of data to construct knowledge across all populations experiencing OUD disease. The big data approach to OUD research is cost-effective, reflective of true population characteristics, feasible (involving existing large data registries that can be combined), and does not require enrolling large numbers of new participants (J. Russell, 2017; Sharp & Melnik, 2015). However, education and training specific to the unique ethical considerations in big data research regarding human subjects’ autonomy, beneficence, and justice are essential as the human subject can easily be viewed as a line of data, a waveform, or a cell when the human interaction with the subject is limited to their data (Butler, Becker, & Humphreys, 2018).
Summary
Big data in OUD is advancing knowledge in OUD identification, prevention, and treatment (J. Russell, 2017). The strengths of using big data in OUD research include its representativeness, feasibility, robustness, and cost-effectiveness. Nursing research that applies big data methods needs to be more visible for reproducibility, peer review, and education (Westra et al., 2017). Additionally, discussions with content experts and relevant stakeholders for convergence on the right questions to ask during data collection improve the probability of returning appropriate and useful data (Lau & Staccini, 2019; Rowe, 2019) The weaknesses of a big data approach to OUD research include the absence of opportunities to verify data analysis conclusions with participants, a lack of ability to determine reason for often missing data, the constraints associated with the use of data originally collected for nonresearch purposes; the lack of availability of standardized data, variation in feature selection method, and methodological control being limited to analytic approaches.
Priority 3: Determinants of Health
Models of health determinants (HD) are increasingly used to explain complex relationships among social aspects of a human life. HDs are nonmedical factors that can affect overall health and health outcomes and include personal, social, economic, and environmental factors that are often interactive. Considering OUD from a HDs perspective may help to unravel the complexity of factors thought to influence opioid use, initiation, continuation, effectiveness of treatment options, and ultimately opioid-related health outcomes. Below we present three populations (neonates, veterans, and oncological patients) impacted by OUD through a HDs perspective.
A major population affected in the OUD epidemic is the birth of addicted infants who then experience neonatal abstinence syndrome (NAS), resulting in short-term neonatal withdrawal and more long-term neurodevelopmental, cognitive, and behavioral effects (Lester & Lagasse, 2010; Maguire et al., 2016; Sandtorv et al., 2018). There are no standardized protocols for the treatment of NAS, and some of the most often used treatments such as swaddling, demand feeding, dim lighting, and noise reduction lack evidence of effectiveness (Wachman, Schiff, & Silverstein, 2018; Walsh et al., 2018). Initial treatments for NAS are primarily nonpharmacologic, but pharmacological interventions such as reintroducing opioids with eventual weaning are also used (Hudak & Tan, 2012). However, barriers to seeking treatment for OUD are increased for pregnant women due to social stigma, and are uniquely complicated for adolescents with unplanned pregnancy (Spada, Kmiec, Glance, & Gopalan, 2019; Sutter, Gopman, & Leeman, 2017).
The most desirable approach to NAS is prevention of OUD during pregnancy. Unfortunately, there is little understanding of the causes of perinatal OUD, thus, prevention interventions are limited. To solve this problem, application of a social determinants framework to describe the potential causes of OUD during pregnancy is necessary with a specific focus on the interaction among personal, social, economic, and environmental determinants. For example, while there is a higher incidence of perinatal OUD in rural residents, particularly those with mental illness (Kozhimannil, Chantarat, Ecklund, Henning – Smith, & Jones, 2019), little is known about the contributions of social (e.g., family support, education) or economic factors particularly pertinent in rural communities.
Chronic pain, a condition that affects 40% to 70% of veterans is the leading cause of disability with significantly adverse effects on veterans’ lives (Department of Veterans Affairs, 2015). The interaction of chronic pain with depression, PTSD, anxiety and other mental health conditions in veterans and others (Legarreta, Bueler, DiMuzio, McGlade, & Yurgelun-Todd, 2018) adds to the complexity of the study of pain and its consequences. The Veterans Administration identified four approaches for treating chronic pain including self-management, nondrug treatments, nonopioid drug treatments, and opioids (Department of Veteran Affairs; Department of Defense 2017). Yet, opioids are the most often used because of their effectiveness in treating pain.
Health determinants of veterans include personal circumstances (e.g., educational level; health behaviors including smoking and alcohol use); social (e.g., gender discrimination); economic (e.g., income, employment status, and type of work); and environmental factors (e.g., location of residence, access to healthcare, and available health insurance). These determinants of health often place the veteran at risk due to their increased propensity for smoking and alcohol abuse, homelessness, unemployment, and limited access to health care in an over-burdened and often disjointed health care delivery network (Doran et al., 2016; Oliva et al., 2016). A focus on these factors and their interactions in relation to OUD is critical to conducting research and providing intervention for these individuals.
Determinants of health can also contribute to poor outcomes among persons with cancer who are at risk for OUD (Hacker, Reynolds, & Uppal, 2018). Opioids are a mainstay in the treatment of advanced and metastatic cancer-related pain and end-of-life symptoms. However, oncologists face difficulty in prescribing opioids to treat pain due to initiatives to combat OUD such as required prior authorization and dispensed pill limits (Page & Blanchard, 2019). Pain management is a delicate balance of pain control and avoidance of OUD when patients develop addiction to opioids in order to control severe pain or misuse prescribed opioids to self-medicate for diagnosis-related depression or anxiety (Guitart et al., 2018). Pain is prevalent among patients with cancer; 66% of patients with advanced or metastatic cancer report pain and 38% of all patients report moderate or severe pain (van den Beukenvan Everdingen, Hochstenbach, Joosten, Tjan-Heijnen, & Janssen, 2016). Socioeconomic factors such as income, health literacy and health insurance affect quality of life and pain management options for oncology patients. Additional research on the interaction between social determinants of health and cancer treatment, symptoms and quality of life is critically important to inform patient education and policy decisions.
Summary
The primary strength of applying models of health determinants to the study of causes of OUD or the development of interventions to prevent or treat OUD is the comprehensive and holistic nature of viewing health with a broader lens. The use of a health determinants framework allows consideration of the multiple and cross-sectional nature of health, emphasizes the role of communities as well as individuals in the promotion of wellness, and supports our understanding the origins of health, and illness, are multifactorial, and thus, to achieve health and reduce illness, multiple interventions are needed. The possible weaknesses associated with using health determinants models in OUD research include nonrepresentativeness of registry level data, bias in self-reported data; missing determinants in the models, and missing data.
Priority 4: Global Health
The prevalence for opioid use in adults worldwide was estimated at 0.37% in 2015. The high-income North America region had the highest rates of cannabis, opioid, and cocaine dependence and mortality rates of 6.9 deaths/100,000 people (Peacock et al., 2018). Mortality rates were highest in low-and middle-income countries that were more populated and where data and data quality are limited (Charlson et al., 2016). The common misconception that OUD is a moral weakness or a willful choice persists globally. OUD must be considered a health problem in order to increase treatment levels, reduce sequela, and save lives. The economic and human costs are substantial with estimated disability-adjusted life-years (DALYs) for illicit drug use at 27.8 million worldwide (Peacock et al., 2018). Mental, neurological, and substance use disorders frequently co-occur and accounted for 10.4% of global DALYs in 2010 (Whiteford, Ferrari, Degenhardt, Feigin, & Vos, 2015). DALYs peaked in early adulthood for mental and substance use disorders. Overall DALYs were highest in Eastern Europe/Central Asia and lowest in East Asia/the Pacific (Whiteford et al., 2015). Opioids are also the most common cause of poisoning, after alcohol intoxication in patients presenting to North American emergency departments (Nelson, Juurlink, & Perrone, 2015).
Adolescence is the peak time for initiation of substance use, with tobacco and alcohol usually preceding the use of illicit drugs (Degenhardt, Stockings, Patton, Hall, & Lynskey, 2016). Substantial variation is noted between countries in levels, types, and sequences of substance use in young people. Results from the Global Burden of Disease 2013 study suggested the burden attributable to substance use increased substantially in adolescence and young adulthood (Charlson et al., 2016) from 1990 to 2013. In young men aged 20 to 24 years, alcohol and illicit substance use are responsible for 14% of total health burden. Illicit drug burden is higher in the high-income countries of the United States, Canada, Australia, New Zealand, and Western Europe (Charlson et al., 2016).
Public health policy should target OUD interventions using a social-ecological model (Sallis et al., 2006). At the population-level, best practices include legislative measures to reduce prescriptions and to compel insurers to pay for treatment (Patel et al., 2016). At the community-level, best practices include life-skills training in schools to build social and emotional competencies. At the healthcare delivery level, integrating treatment OUD into primary care may lead to greater clinician attention to specific needs of these patients (Olsen & Sharfstein, 2014). The costs of providing a significantly scaled up package of specified cost-effective interventions for prioritized mental health, neurological, and substance abuse disorders in low-income and lower-middle-income countries is estimated in the U.S. at $3 to 4 per person per year (Patel et al., 2016). Since OUDs are chronic in nature and can be disabling, costs should largely be assumed by the government (Florence, Zhou, Luo, & Xu, 2016). Currently, less than 1% of assistance for health and government spending on health in low-income and middle-income countries is allocated to the care of people with OUD. Achieving the health gains associated with prioritized interventions will require financial resources as well as committed and sustained efforts to address a range of other barriers to treatment such as paucity of human resources, lack of support at all levels, and stigma (Patel et al., 2016).
Summary
Opioid use disorder is a global health problem that does not vary significantly between countries in prevalence, disability, and mortality. Of note, the high-income North American region has the highest rates of cannabis, opioid and cocaine dependence as well as mortality rates. Stigma that varies globally across countries and within cultures has hindered adequate care and research. Addressing OUD as a health problem will facilitate prevention, treatment, and recovery worldwide.
Discussion and Recommendations
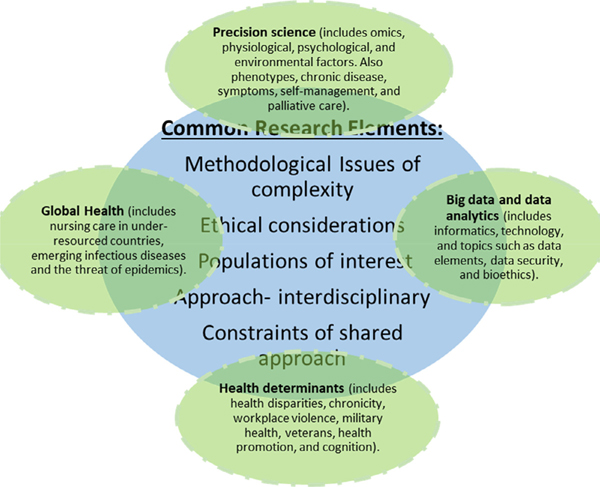
The chronic, relapsing nature of opioid addiction means most patients are never “cured,” and the best outcome is long-term recovery. Although multifaceted approaches are needed to successfully address the opioid epidemic, an important first step is prevention (Nelson et al., 2015). Nurse scientists are producing knowledge in OUD research through studies that examine various at-risk populations using integrated and novel approaches (LaBelle, Han, Bergeron, & Samet, 2016; Vyas, LeBaron, & Gilson, 2018) that align with the identified four priority areas. Though each have distinct properties, the priority areas often intersect regarding populations of interest, methodological considerations, interdisciplinary element, constraints of shared approaches, and ethical considerations in research (Figure 2).
Figure 2 –
Intersection of nursing science priority areas and common research elements.
There are strengths in using each of the priority areas as a framework for future OUD. This framework contributes to the sum being greater than the parts, and could leverage nurse scientists’ knowledge and lines of inquiry to address some of the most daunting health issues of today, such as OUD. The nursing priorities framework also provides opportunities for interdisciplinary research for improved outcomes across multiple populations.
Future OUD Research Using the Priority Areas
To date in precision science, no common biomarkers have been found to assist in identifying individuals predisposed to develop OUD, response to OUD treatment, or relapse. However, promising genetic information from the body’s opioid receptors identifies potential future targets for screening and treatment that can be further explored. Big data for OUD research continues to be archived and organized for data sharing across platforms. Ongoing integration of these data may produce predictive algorithms of OUD risk or interventions that will inform prevention and management. As health determinants of OUD risk and profiles for effective targeted treatment emerge, future research and refinement of treatment models will need to be tested to provide prevention and treatment therapies for different populations. Additionally, a focus of future studies with inclusion of global participants in OUD research would provide a more representative sample of the human population and strengthen inference from findings.
Conclusion
The four nursing science priority areas provide a framework to address the research needs of today’s complex chronic health problems such as OUD. The strength in this research priority framework is the breadth of OUD research approaches possible and the interdisciplinary science that can occur across the translational science continuum using these four priority areas. However, as with all research frameworks, there are challenges with using the four identified priority areas. These challenges include funding constraints, access to participants, and rigor and responsibility in the conduct of research.
Many OUD studies that have advanced knowledge in identification and treatment of this deadly chronic disease have been carried out by nurse scientists within the priority areas. For example, a precision science approach has provided information using geospatial data for nurses to use real-time data to treat OUD-related overdoses in New York (Hallas et al., 2019). Effective interventions such as methadone maintenance, mental health interventions, and behavioral therapies, can help prevent and treat OUD. A social determinants approach advanced the management of chronic pain in primary care settings for those identified as at risk for substance use disorder (Wiedemer, Harden, Arndt, & Gallagher, 2007). Public health policy should target OUD interventions using a social-ecological model at the population, community, and delivery levels with a big data approach examining years of policy effect at a local level (Eckardt & Erlanger, 2018) or a global health approach to explain perceived social support among women undergoing methadone maintenance treatment in Iran (Raheimi, Jalali, & Jalali, 2018). Large gaps in data about drug use worldwide remain and much of what is known comes from studies in high-income countries completed decades ago. Gains against OUD will require committed and sustained efforts to address a range of barriers such as a paucity of human resources, lack of support at all levels, and stigma. The critical linkages of common populations of interest, ethical considerations, and interdisciplinary approaches between the four priority areas strengthen the required scientific framework to tackle the research challenges associated with this chronic disease.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- Agrawal A, Edenberg HJ, & Gelernter J (2016). Meta-Analyses of Genome-Wide Association Data Hold New Promise for Addiction Genetics. Journal Of Studies On Alcohol And Drugs, 77(5), 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AM, Dogan MV, Beach SRH, & Philibert RA (2015). Current and future prospects for epigenetic biomarkers of substance use disorders. Genes, 6(4), 991–1022, doi: 10.3390/genes6040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearnot B, Pearson JF, & Rodriguez JA (2018). Using publicly available data to understand the opioid overdose epidemic: Geospatial distribution of discarded needles in Boston, Massachusetts. American Journal Of Public Health, 108(10), 1355–1357, doi: 10.2105/AJPH.2018.304583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzeaux R, Lalanne L, Kieffer BL, & Lutz P-E (2018). Focusing on the opioid system for addiction biomarker discovery. Trends In Molecular Medicine, 24(2), 206–220, doi: 10.1016/j.molmed.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Bird SM, McAuley A, Munro A, Hutchinson SJ, & Taylor A (2017). Prison-based prescriptions aid Scotland’s National Naloxone Programme. Lancet, 389 (10073), 1005–1006, doi: 10.1016/S0140-6736(17)30656-6. [DOI] [PubMed] [Google Scholar]
- Bird SM, McAuley A, Perry S, & Hunter C (2016). Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006–10) versus after (2011–13) comparison. Addiction, 111(5), 883–891, doi: 10.1111/add.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler K-M, Giné E, Echeverry-Alzate V, Calleja-Conde J, de Fonseca FR, & López-Moreno JA (2015). Common single nucleotide variants underlying drug addiction: more than a decade of research. Addiction Biology, 20(5), 845–871, doi: 10.1111/adb.12204. [DOI] [PubMed] [Google Scholar]
- Butler JM, Becker WC, & Humphreys K (2018). Big Data and the Opioid Crisis: Balancing Patient Privacy with Public Health. Journal of Law, Medicine & Ethics, 46 (2), 440–453, doi: 10.1177/1073110518782952. [DOI] [PubMed] [Google Scholar]
- Cao Y, Stewart K, Wish E, Artigiani E, & Sorg MH (2019). Determining spatial access to opioid use disorder treatment and emergency medical services in New Hampshire. Journal of Substance Abuse Treatment, 101, 55–66, doi: 10.1016/j.jsat.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Vital signs: Prescription painkiller overdoses: A growing epidemic, especially among women. Retrieved from https://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html
- Charlson FJ, Baxter AJ, Dua T, Degenhardt L, Whiteford HA, & Vos T (2016). Excess mortality from mental, neurological, and substance use disorders in the global burden of disease study 2010. (Third Edition). Mental, neurological, and substance use disorders: Disease control priorities: 4, doi: 10.1596/978-1-4648-0426-7. [DOI] [Google Scholar]
- Cheatle MD (2016). Biopsychosocial approach to assessing and managing patients with chronic pain. The Medical Clinics Of North America, 100(1), 43–53, doi: 10.1016/j.mcna.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Clancy TR, Effken JA, & Pesut D (2008). Applications of complex systems theory in nursing education, research, and practice. Nursing Outlook, 56(5), 248–256. [DOI] [PubMed] [Google Scholar]
- Corwin E, Redeker NS, Richmond TS, Docherty SL, & Pickler RH (2019). Ways of knowing in precision health. Nursing Outlook, 67(4), 293–301, doi: 10.1016/j.outlook.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley R, Kirschner N, Dunn AS, & Bornstein SS (2017). Health and public policy to facilitate effective prevention and treatment of substance use disorders involving illicit and prescription drugs: An American College of Physicians position paper. Annals Of Internal Medicine, 166(10), 733–736, doi: 10.7326/M16-2953. [DOI] [PubMed] [Google Scholar]
- Davis C, Green T, & Beletsky L (2017). Action, not rhetoric, needed to reverse the opioid overdose epidemic. Journal of Law, Medicine & Ethics, 45, 20–23, doi: 10.1177/1073110517703310. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Stockings E, Patton G, Hall WD, & Lynskey M (2016). The increasing global health priority of substance use in young people. The Lancet. Psychiatry, 3(3), 251–264, doi: 10.1016/S2215-0366(15)00508-8. [DOI] [PubMed] [Google Scholar]
- Deng Y, Bu Q, Hu Z, Deng P, Yan G, Duan J, et al. (2012). (1) H-nuclear magnetic resonance-based metabonomic analysis of brain in rhesus monkeys with morphine treatment and withdrawal intervention. Journal Of Neuroscience Research, 90(11), 2154–2162, doi: 10.1002/jnr.23109. [DOI] [PubMed] [Google Scholar]
- Department of Veteran Affairs; Department of Defense (2017). VA/DoD Clinical practice guideline for management of opioid therapy for chronic pain. Retrieved from http://www.healthquality.va.gov/guidelines/Pain/cot/
- Department of Veterans Affairs. (2015). Care Management for the Effective Use of Opioids (CAMEO). Retrieved from https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141700805
- Derose SF, Contreras R, Coleman KJ, Koebnick C, & Jacobsen SJ (2013). Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: the Kaiser Permanente Southern California experience. Medical Care Research And Review: MCRR, 70(3), 330–345, doi: 10.1177/1077558712466293. [DOI] [PubMed] [Google Scholar]
- Doran N, De Peralta S, Depp C, Dishman B, Gold L, Marshall R, et al. (2016). The validity of a brief risk assessment tool for predicting suicidal behavior in veterans utilizing VHA Mental Health Care. Suicide & Life-Threatening Behavior, 46(4), 471–485, doi: 10.1111/sltb.12229. [DOI] [PubMed] [Google Scholar]
- Ducci F, & Goldman D (2012). The genetic basis of addictive disorders. Psychiatric Clinics of North America, 35(2), 495–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkis DA, Taylor LA, Peak DA, & Bearnot B (2017). Geospatial analysis of emergency department visits for targeting community-based responses to the opioid epidemic. PLoS ONE, 12(3), 1–9, doi: 10.1371/jour-nal.pone.0175115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkis DA, Weiner SG, Liao VT, Rabickow D, & Goldberg SA (2018). Geospatial Clustering of Opioid-Related Emergency Medical Services Runs for Public Deployment of Naloxone. The Western Journal Of Emergency Medicine, 19(4), 641–648, doi: 10.5811/westjem.2018.4.37054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt P, Culley JM, Corwin E, Richmond T, Dougherty C, Pickler RH, et al. (2017). National nursing science priorities: Creating a shared vision. Nursing Outlook, 65(6), 726–736, doi: 10.1016/j.outlook.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Eckardt P, & Erlanger AE (2018). Lessons learned in methods and analyses for pragmatic studies. Nursing Outlook, 66(5), 446–454, doi: 10.1016/j.outlook.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Eckardt P, Erlanger AE, Caplan M, Dettling R, Delagi R, & Tomarken JL (2020). Policy Effects on Opioid Use Disorder Related Deaths: A Seventeen Year Retrospective Study. Manuscript in Preparation. [Google Scholar]
- Faul M, Bohm M, & Alexander C (2017). Methadone Prescribing and Overdose and the Association with Medicaid Preferred Drug List Policies - United States, 2007–2014. MMWR: Morbidity & Mortality Weekly Report, 66(12), 320–323, doi: 10.15585/mmwr.mm6612a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, & Xu L (2016). The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Medical Care, 54(10), 901–906, doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari R, & Sumner S (2018). Using metabolomics to investigate biomarkers of drug addiction. Trends In Molecular Medicine, 24(2), 197–205, doi: 10.1016/j.molmed.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Grady PA (2017). Advancing science, improving lives: NINR’s new strategic plan and the future of nursing science, editorial. Journal of Nursing Scholarship 247–248. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=122859240&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, & Papoutsi C (2018). Studying complexity in health services research: Desperately seeking an overdue paradigm shift. BMC Medicine, 16(1), doi: 10.1186/s12916-018-1089-4 N.PAG-N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros DF, Milanak ME, Brady KT, & Back SE (2013). Frequency and severity of comorbid mood and anxiety disorders in prescription opioid dependence. The American Journal On Addictions, 22(3), 261–265, doi: 10.1111/j.1521-0391.2012.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart J, Vargas MI, De Sanctis V, Folch J, Salazar R, Fuentes J, et al. (2018). Efficacy and Safety of Sublingual Fentanyl Tablets in Breakthrough Cancer Pain Management According to Cancer Stage and Background Opioid Medication. Drugs in R&D, 18(2), 119–128, doi: 10.1007/s40268-018-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker KE, Reynolds RK, & Uppal S (2018). Ongoing strategies and updates on pain management in gynecologic oncology patients. Gynecologic Oncology, 149(2), 410–419, doi: 10.1016/j.ygyno.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Haerian BS, & Haerian MS (2013). OPRM1 rs1799971 polymorphism and opioid dependence: Evidence from a meta-analysis. Pharmacogenomics, 14(7), 813–824, doi: 10.2217/pgs.13.57. [DOI] [PubMed] [Google Scholar]
- Hallas D, Klar RT, Baldyga JA, Rattner I, Waingortin R, & Fletcher J (2019). Traditional and Nontraditional Collaborations to Improve Population Health Using Geospatial Information System Maps: Analysis of the Opioid Crisis. Journal of Pediatric Health Care, 33(3), 309–322, doi: 10.1016/j.pedhc.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Hudak ML, & Tan RC (2012). Neonatal drug withdrawal. Pediatrics, 129(2), e540–e560, doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- Hunt LJ, Lee SJ, Harrison KL, & Smith AK (2018). Secondary Analysis of Existing Datasets for Dementia and Palliative Care Research: High-Value Applications and Key Considerations. Journal of Palliative Medicine, 21 (2), 130–142, doi: 10.1089/jpm.2017.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Human Virology at the University of Maryland School of Medicine, M. (2018). Institute of Human Virology (IHV) Awarded $12M to Combat Opioid Epidemic Through Clinical Research Trials. In. [Google Scholar]
- Iwanicki JL, Severtson SG, Margolin Z, Dasgupta N, Green JL, & Dart RC (2018). Consistency between opioid-related mortality trends derived from poison center and National Vital Statistics System, United States, 2006 2016. American Journal Of Public Health, 108(12), 1639–1645, doi: 10.2105/AJPH.2018.304728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul RV, Nyberg J, Kreilgaard M, Christrup LL, Simonsson USH, & Lund TM (2017). Analysis of opioid consumption in clinical trials: a simulation based analysis of power of four approaches. Journal Of Pharmacokinetics And Pharmacodynamics, 44(4), 325–333, doi: 10.1007/s10928-017-9522-4. [DOI] [PubMed] [Google Scholar]
- King NB, Fraser V, Boikos C, Richardson R, & Harper S (2014). Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: A systematic review. American Journal Of Public Health, 104(8), e32–e42, doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf A (2018). NIDA funds research on clinical trials to measure benefits of treatment for OUDs. Alcoholism & Drug Abuse Weekly, 30(31), 1–4, doi: 10.1002/adaw.32063. [DOI] [Google Scholar]
- Knopf A (2016). CDC report shows heroin and illicit fentanyl overdoses increasing. Alcoholism & Drug Abuse Weekly, 28(2), 1–3, doi: 10.1002/adaw.30433. [DOI] [Google Scholar]
- Kozhimannil KB, Chantarat T, Ecklund AM, Henning Smith C, & Jones C (2019). Maternal opioid use disorder and neonatal abstinence syndrome among rural US residents, 2007 2014. Journal of Rural Health, 35(1), 122–132, doi: 10.1111/jrh.12329. [DOI] [PubMed] [Google Scholar]
- Kuehner-Hebert K (2017). State opioid policies could be decreasing long-term dispensing. BenefitsPRO, 1. [Google Scholar]
- LaBelle CT, Han SC, Bergeron A, & Samet JH (2016). Office-Based Opioid Treatment with Buprenorphine (OBOT-B): Statewide Implementation of the massachusetts collaborative care model in community health centers. Journal of Substance Abuse Treatment, 60, 6–13, doi: 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langabeer JR, Gourishankar A, Chambers KA, Giri S, Madu R, & Champagne-Langabeer T (2019). Disparities between US opioid overdose deaths and treatment capacity: A geospatial and descriptive analysis. Journal Of Addiction Medicine, doi: 10.1097/ADM.0000000000000523. [DOI] [PubMed] [Google Scholar]
- Lau AYS, & Staccini P (2019). Artificial Intelligence in Health: New Opportunities, Challenges, and Practical Implications. Yearbook Of Medical Informatics, 28(1), 174–178, doi: 10.1055/s-0039-1677935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarreta M, Bueler E, DiMuzio J, McGlade E, & Yurgelun-Todd D (2018). Suicide behavior and chronic pain: An exploration of pain-related catastrophic thinking, disability, and descriptions of the pain experience. Journal of Nervous & Mental Disease, 206(3), 217–222, doi: 10.1097/NMD.0000000000000799. [DOI] [PubMed] [Google Scholar]
- Lester BM, & Lagasse LL (2010). Children of addicted women. Journal of Addictive Diseases, 29(2), 259–276, doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DJ, Taylor S, Armstrong K, Shaffer-Hudkins E, Germain AM, Brooks SS, et al. (2016). Long-Term Outcomes of Infants with Neonatal Abstinence Syndrome. Neonatal Network, 35(5), 277–286, doi: 10.1891/0730-0832.35.5.277. [DOI] [PubMed] [Google Scholar]
- Marshall BDL (2017). Contextualizing complexity: When are systems science methods constructive. American Journal Of Public Health, 107(9), 1385–1386, doi: 10.2105/AJPH.2017.303873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar S, McRae IS, & Islam MM (2015). How can geographical information systems and spatial analysis inform a response to prescription opioid misuse? A discussion in the context of existing literature. Current Drug Abuse Reviews, 8(2), 104–110. [DOI] [PubMed] [Google Scholar]
- McAuley A, Bouttell J, Barnsdale L, Mackay D, Lewsey J, Hunter C, & Robinson M (2017). Evaluating the impact of a national naloxone programme on ambulance attendance at overdose incidents: a controlled time-series analysis. Addiction, 112(2), 301–308, doi: 10.1111/add.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedicaSafe, I. (2019). MedicaSafe Receives $1.0 million Grant from National Institutes of Health. In. [Google Scholar]
- Min J, Osborne V, Kowalski A, & Prosperi M (2018). Reported Adverse Events with Painkillers: Data Mining of the US Food and Drug Administration Adverse Events Reporting System. Drug Safety, 41(3), 313–320, doi: 10.1007/s40264-017-0611-5. [DOI] [PubMed] [Google Scholar]
- Nelson LS, Juurlink DN, & Perrone J (2015). Addressing the opioid epidemic: 314Chicago, Illinois: American Medical Association; In. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Nevedal A, Lewis ET, McCaa MD, Cochran MF, Konicki PE, et al. (2016). Patient perspectives on an opioid overdose education and naloxone distribution program in the U.S. Department of Veterans Affairs. Substance Abuse, 37(1), 118–126, doi: 10.1080/08897077.2015.1129528. [DOI] [PubMed] [Google Scholar]
- Olsen Y, & Sharfstein JM (2014). Confronting the stigma of opioid use disorder and its treatment. JAMA, 311(14), 1393–1394, doi: 10.1001/jama.2014.2147. [DOI] [PubMed] [Google Scholar]
- Page R, & Blanchard E (2019). Opioids and Cancer Pain: Patients’ Needs and Access Challenges. Journal of Oncology Practice, 15(5), 229–231, doi: 10.1200/JOP.19.00081. [DOI] [PubMed] [Google Scholar]
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. (2016). Global priorities for addressing the burden of mental, neurological, and substance use disorders. (Third Edition). Mental, neurological, and substance use disorders: Disease control priorities: 4, doi: 10.1596/978-1-4648-0426-7. [DOI] [PubMed] [Google Scholar]
- Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. (2018). Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction, 113(10), 1905–1926, doi: 10.1111/add.14234. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Shah DT, Simoyan OM, McCall KL, & Nichols SD (2018). Trends in Medical Use of Opioids in the U.S., 2006–2016. American Journal Of Preventive Medicine, 54(5), 652–660, doi: 10.1016/j.amepre.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Raheimi S, Jalali A, & Jalali R (2018). Social support among women undergoing methadone maintenance treatment in Iran. Journal Of Addictions Nursing, 29(3), 179–187, doi: 10.1097/JAN.0000000000000234. [DOI] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Yuferov V, Randesi M, & Kreek MJ (2014). Genetics of opiate addiction. Current Psychiatry Reports, 16(11), 504, doi: 10.1007/s11920-014-0504-6-504. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Yuan C, Wang J, Yeung S-CJ, & Shete S (2015). Gene network analysis shows immune-signaling and ERK1/2 as novel genetic markers for multiple addiction phenotypes: alcohol, smoking and opioid addiction. BMC Systems Biology, 9, 25, doi: 10.1186/s12918-015-0167-x-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M (2019). An Introduction to Machine Learning for Clinicians. Academic Medicine: Journal Of The Association Of American Medical Colleges, 94(10), 1433–1436, doi: 10.1097/ACM.0000000000002792. [DOI] [PubMed] [Google Scholar]
- Russell J (2017). Using big data to attack epidemic. Indianapolis Business Journal, 38(14), 6. [Google Scholar]
- Russell KA (2017). The opioid epidemic: A national response and nursing’s contribution to ending the crisis. Dean’s Notes, 38(3), 1–2. [Google Scholar]
- Sajid A, Whiteman A, Bell RL, Greene MS, Engleman EA, & Chambers RA (2016). Prescription drug monitoring program data tracking of opioid addiction treatment outcomes in integrated dual diagnosis care involving injectable naltrexone. The American Journal On Addictions, 25(7), 557–564, doi: 10.1111/ajad.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, & Kerr J (2006). An ecological approach to creating active living communities. Annual Review Of Public Health, 27, 297–322. [DOI] [PubMed] [Google Scholar]
- Sandtorv LB, Fevang SKE, Nilsen SA, Bøe T, Gjestad R, Haugland S, & Elgen IB (2018). Symptoms associated with attention deficit/hyperactivity disorder and autism spectrum disorders in school-aged children prenatally exposed to substances. Substance Abuse: Research And Treatment, 12, doi: 10.1177/1178221818765773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp MJ, & Melnik TA (2015). Poisoning deaths involving opioid analgesics - New York State, 2003–2012. MMWR: Morbidity & Mortality Weekly Report, 64(14), 377–380. [PMC free article] [PubMed] [Google Scholar]
- Spada M, Kmiec J, Glance JB, & Gopalan P (2019). Consideration of opioid agonist treatment in a pregnant adolescent: A case report and literature review. Substance Abuse, 1–5, doi: 10.1080/08897077.2019.1635970. [DOI] [PubMed] [Google Scholar]
- Springer SA, Korthuis PT, & Del Rio C (2018). Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: A Call for action after a national academies of sciences, engineering, and medicine workshop. Annals Of Internal Medicine, 169(5), 335–336, doi: 10.7326/M18-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter MB, Gopman S, & Leeman L (2017). Patient-centered Care to Address Barriers for Pregnant Women with Opioid Dependence. Obstetrics And Gynecology Clinics Of North America, 44(1), 95–107, doi: 10.1016/j.ogc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, & Janssen DJA (2016). Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. Journal Of Pain And Symptom Management, 51(6), 1070–1090, doi: 10.1016/j.jpainsymman.2015.12.340 e1079. [DOI] [PubMed] [Google Scholar]
- Vyas MB, LeBaron VT, & Gilson AM (2018). The use of cannabis in response to the opioid crisis: A review of the literature. Nursing Outlook, 66(1), 56–65, doi: 10.1016/j.outlook.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Wachman EM, Schiff DM, & Silverstein M (2018). Neonatal abstinence syndrome: Advances in diagnosis and treatment. JAMA, 319(13), 1362–1374, doi: 10.1001/jama.2018.2640. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Crowley M, Wexelblatt S, Ford S, Kuhnell P, Kaplan HC, et al. (2018). Ohio perinatal quality collaborative improves care of neonatal narcotic abstinence syndrome. Pediatrics, 141(4), 1–10, doi: 10.1542/peds.2017-0900. [DOI] [PubMed] [Google Scholar]
- Westra BL, Sylvia M, Weinfurter EF, Pruinelli L, Park JI, Dodd D, et al. (2017). Big data science: A literature review of nursing research exemplars. Nursing Outlook, 65(5), 549–561, doi: 10.1016/j.outlook.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, & Vos T (2015). The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS ONE, 10(2), 1–14, doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemer NL, Harden PS, Arndt IO, & Gallagher RM (2007). The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Medicine (Malden, Mass.), 8(7), 573–584. [DOI] [PubMed] [Google Scholar]
- Wright ER, Kooreman HE, Greene MS, Chambers RA, Banerjee A, & Wilson J (2014). The iatrogenic epidemic of prescription drug abuse: County-level determinants of opioid availability and abuse. Drug And Alcohol Dependence, 138, 209–215, doi: 10.1016/j.drugalcdep.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, et al. (2006). Association of the kappa-opioid system with alcohol dependence. Molecular Psychiatry, 11(11), 1016–1024. [DOI] [PubMed] [Google Scholar]
- Zirui S (2017). Mortality quadrupled among opioid-driven hospitalizations, notably within lower-income and disabled white populations. Health Affairs, 36(12), 2054–2061, doi: 10.1377/hlthaff.2017.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]