Abstract
Regulated nuclear-cytoplasmic trafficking is a well-established mechanism utilized by cells to regulate adaptive and maladaptive responses to acute oxidant stress. Commonly associated with endoplasmic reticulum stress, the bZIP transcription factor CCAAT/enhancer-binding protein homologous protein (CHOP/DDIT3) mediates the cellular response to redox stress with effects on cellular growth, differentiation, and survival. We show through functional analyses that CHOP contains a conserved, compound pat4/bipartite nuclear localization signal within the basic DNA-binding domain. Using phylogenetic analyses and mass spectrometry, we now show that Ser107 located within the linker region of the bipartite NLS domain is a substrate for phosphorylation under standard culture conditions. Studies using the S107E phospho-mimic of CHOP indicate that changes in the charge properties at this residue regulate CHOP’s nuclear-to-cytoplasmic ratio. And while co-stimulation with the SERCA inhibitor thapsigargin induced injury in cells expressing wild-type CHOP, the S107A point-mutant blocked this response. These findings indicate that phosphorylation within the bipartite NLS exerts regulatory effects on both the subcellular localization and toxic potential of DDIT3/CHOP. Future studies geared towards defining the relevant kinase/phosphatase networks that converge on the phosphorylation-regulated NLS (prNLS) phosphoepitope may provide an opportunity to constrain cellular damage in the context of acute ER stress.
Keywords: DDIT3/CHOP, nuclear localization signal, post-translational modification, growth arrest, apoptosis, nuclear-cytoplasmic transport
1. Introduction
Endoplasmic reticulum stress responses (ERSRs) perform critical cellular housekeeping functions that help cells cope with environmental stress by regulating translation rates and the turnover of unfolded client proteins that accumulate under conditions of nutrient starvation and oxidant stress. ERSRs also regulate survival and cell death decisions after ischemia and other harmful stimuli through effects on transcription [1, 2]. Cloned in 1990, gene 153 induced upon growth arrest and after DNA damage (gadd153), also known as the C/EBP homologous protein (CHOP-10) [3], is a 29-kDa protein recognized as a central player in the ER stress response. CHOP belongs to the basic leucine zipper (bZIP) family of transcription factors and functions as an obligate heterodimer, binding with the CAAT/enhancer-binding protein beta (C/EBP-β) [4], ATF3 [5], and cJun [6]. CHOP activation is observed following a variety of physiological stressors, including amino acid starvation [7], viral infection [8], and with the accumulation of unfolded proteins [2]. Consequently, homozygous deletion of CHOP reduces rates of ER-stress induced apoptosis in multiple neurological conditions, including the acute response to acute injury [9].
Regulated nuclear-cytoplasmic trafficking is a well-established mechanism utilized by cells to regulate the cellular transcriptional response to acute oxidant stress. While the core region regulating CHOP’s nuclear localization has been mapped to the basic DNA-binding domain [10], the precise cis-acting elements and putative modifiers of NLS activity remain unknown. In one report, Jauhiainen et al. found that of the 175 genes regulated by CHOP, over two-thirds were transcriptionally repressed, supporting CHOP’s function as a dominant-negative factor [11]. Using a model system by which CHOP subcellular localization could be controlled, the authors also found that while nuclear CHOP regulated proliferation and apoptosis/survival genes, the cytoplasmic form affected genes associated with cell migration. Thus, defining how CHOP nucleocytoplasmic shuttling occurs could help resolve fundamental questions regarding the signaling mechanisms regulating the quantitative and qualitative outputs of CHOP-dependent transcription.
To date, several post-translational modifications (PTMs) that influence CHOP’s function have been identified. For example, phosphorylation at Ser30 by AMPKα1 facilitates CHOP ubiquitination proteasomal degradation in macrophages [12], while ubiquitination of lysine residues within AA10–26 similarly promotes CHOP turnover [13]. With respect to CHOP’s transcriptional activity, p38MAPK-dependent phosphorylation at Ser78/81 induces its activity [14], while CKII-dependent effects at Ser14/15/30/31 induce transcriptional silencing [6]. That said, there remain no reports linking specific PTMs with changes in CHOP’s subcellular localization. CHOP’s subcellular localization is regulated by physiological factors, including ER stress and BDNF-induced signaling, whose effects oppose each other [15]. And, in addition to acting as a dominant-negative inhibitor of C/EBP-β dependent transcription [3], maintaining stoichiometric parity between C/EBP-β with CHOP levels is an important factor in supporting CHOP accumulation within the nucleus [10].
In this study, we performed a series of structure-function analyses to identify whether conserved cis-elements bordering CHOP’s nuclear localization domain influences its subcellular localization and toxic potential. Our findings indicate that CHOP contains both monopartite and bipartite elements that synergize to drive nuclear localization. We also show that the phosphorylation regulated-NLS (prNLS) motif located within the linker region of the bipartite NLS alters the subcellular distribution and toxicity of CHOP. And we further explore the role that C/EBP-β plays in supporting both processes. These observations broaden our understanding of the cis-and trans-acting factors governing CHOP’s activity and provide a path forward to identify physiologically relevant signaling pathways that influence CHOP’s latent apoptotic potential under conditions of redox stress.
2. Materials and methods
2.1. Plasmid constructs and site-directed mutagenesis
The plasmid pEGFP-C2 was purchased from Clontech (Carlsbad, CA). CHOP 6:mCHOP-WT-9E10-pcDNA1 (Addgene # 21913) was provided as a gift from David Ron. The vector pCIG3-IRES-GFP (Addgene # 78264) was provided as a gift from Felicia Goodrum. pEGFP-CHOP-V1 was generated by transferring the CHOP cDNA as an XhoI-XbaI PCR fragment into pEGFP-C2. To generate pEGFP-CHOP V2–9 block deletion mutants, pEGFP-CHOP-V1 was subjected to PCR using 5’ and 3’ primers harboring XhoI and XbaI sites, respectively. PCR products were digested, gel-purified, and cloned into pEGFP-C2. The alanine (K103A, S107) and glutamic acid (S107E) mutants were generated using the two-stage, PCR-based method published by Barettino et al. [16]. For N:C and survival analyses, the CHOP cDNA was transferred by PCR into pCIG3-IRES-GFP as an XhoI-BamHI PCR-generated fragment. Restriction endonucleases were obtained from New England Biolabs (Ipswitch, MA), PCR-based cloning was confirmed by Sanger sequencing (GeneWiz, South Plainfield, NJ), and transfection-ready DNA was prepared using the endotoxin-free plasmid midi system (Qiagen, Germantown, MD). See the supplementary methods for details regarding the mutagenesis procedures and primer sequences.
2.2. Cell culture, drug treatments, and transfections
Vero and Neuro2A cell lines were obtained from the ATCC (Gaithersburg, MD) and maintained in DMEM/HG containing 10% fetal bovine serum (FBS) without antibiotics. Cell culture grade DMSO and thapsigargin were obtained from Sigma-Aldrich (St. Louis, MO). Transfections were performed using the in-tube method [17]. Briefly, for every 250,000 cells, 7.5μL Lipofectamine 3000 (Invitrogen, Carlsbad, CA) was diluted in OPTIMEM and added to 500ng DNA diluted in OPTIMEM with p3000 reagent. The appropriate number of cells was added to the preformed DNA:lipid complex and allowed to stand at room temperature for 40 minutes before plating in 6-well culture plates. For C/EBP-β:CHOP co-transfection experiments, C/EBP-β was co-transfected with CHOP at a 4:1 ratio unless otherwise noted. The dose of the CHOP-EGFP expression plasmids was titrated to achieve transfection efficiency at 10% to minimize the non-physiological effects of enforced expression.
2.3. Halo-Tag cloning, protein purification, and mass spectrometry analyses.
Full details are provided in the supplemental methods. Briefly, The CHOP cDNA was transferred from CHOP 6:mCHOP-WT-9E10-pcDNA1 into the Halo-expression plasmid (pFN21A) and transfected into Neuro2A cultures using Lipofectamine (Thermo Fischer, Waltham, MA). Forty-eight hours post-transfection, Halo-CHOP protein was purified from whole-cell lysates using Halo-resin, washed, and released from the column by TEV-dependent cleavage (Promega, Woods Hollow, WI). Purified protein was then separated on a Tris-glycine 4–12% gradient gel (Novex, Thermo Fischer, Waltham, MA), identified by silver staining and subjected to in-gel digestion with 10 ng/mL trypsin (Pierce) overnight at 37°C. Extracted peptides were analyzed by spectrometry analyses using a 30 cm C18 column with 1.8 μm beads (Sepax), with an Easy nLC-1200 HPLC (Thermo Fisher), connected to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher). Peptides were eluted off the column using a multi-step gradient at a flow rate of 300 nL/min, scanning over a range of 375–1500 m/z. MS2 scans were collected in the Orbitrap, and the raw data were analyzed using the SEQUEST search engine within the Proteome Discoverer software platform (V2.2, Thermo Fisher), using the SwissProt mouse database (download date 06/11/19). Oxidation of methionine, acetylation of lysine, and phosphorylation of serine, threonine, and tyrosine were set as variable modifications. The Target Decoy PSM Validator node was used as the FDR calculator, while the ptmRS node was employed to help localize modification sites.
2.4. Western blotting
Protein concentration in samples derived from whole cell and post-fractionation lysates was determined by the DC protein assay (Biorad, Hercules, CA). For N:C studies, an equal amount of protein was loaded for nuclear and cytoplasmic fractions. Samples were boiled for ten minutes in Laemmeli buffer under reducing conditions and resolved by electrophoresis on polyacrylamide gels. Proteins were transferred to PVDF membranes and blocked in TBS-T (50 nM Tris-HCl [pH 8.0], 0.9% NaCl, and 0.1% Tween-20) containing 5% nonfat dry milk for 1 h at room temperature. Primary antibodies used in this study include CHOP 1:1000 (L63F7, Cell Signaling Technology, Danvers, MA), C/EBP-β 1:5000 (C-19, Santa Cruz Biotechnology), GFP 1:2000 (B-2, Santa Cruz Biotechnology), Lamin-B1 1:500 (L-5, Thermo Fischer Scientific), HSP90 1:2000 (C45G5, Cell Signaling Technology). HRP-conjugated goat anti-mouse and goat anti-rabbit antisera were used at a 1:2000 dilution (Azure Biosystems, Dublin, CA). Membranes were developed using Clarity Western ECL substrate (Bio-Rad) and imaged on the Azure biosystems platform. Densitometry was performed using ImageJ (version 1.51)[18]. The area under the sample curve was determined using the „analyze gel’ function, setting the local background with the line tool. Western blotting experiments were replicated twice with technical replicates denoted in the respective figures and figure legends.
2.5. Bioinformatic sequence analyses
The mouse CHOP FASTA sequence (AAH13718) was analyzed using predict-NLS for nuclear localization (http://cubic.bioc.columbia.edu/predictNLS/) and NetPhos 3.1 to define putative kinase domains (http://www.cbs.dtu.dk/services/NetPhos/). Cross-species amino-acid homology comparisons were performed using the alignment program CLC Bio Protein Workbench (Cambridge, MA) and the following species: Homo sapiens (Human, P35638), Mus musculus (Mouse, P35639), Bos taurus (Bovine, Q0IIB6), Rattus norvegicus (Rat, Q62857) and Ovis aries (Sheep, R9ZWD5). Sequence annotations used in this study were based on regions listed by UniProtKB (http://www.uniprot.org/uniprot/P35638).
2.6. Nuclear localization analyses
Qualitative analyses of nuclear-cytoplasmic distribution were conducted using confocal microscopy. Transient transfections were performed using the in-tube method described in section 2.2 above, scaling ratios for plating in 24-well plates. Cells were plated on plasma-cleaned 12mm round coverslips (Thermo Fisher Scientific) coated with poly-D-lysine (5 μg/cm2) and cultured for 24 hours. Samples were rinsed with PBS, fixed with cold 4% PFA for 15 minutes, counterstained with Hoechst 33342 nuclear stain (F.C. 10 μM), rinsed again in PBS, and mounted in mowiol-based mounting media. Fluorescence confocal microscopy images were acquired on an Olympus FV1000 at 60x using a 60x oil objective with a numerical aperture of 1.42. For quantitative N:C analyses, Vero cultures were transfected with GFP or CHOP constructs and allowed to express for 24 hours before collecting and isolating the cytoplasmic and nuclear fractions using the NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific, Waltham, MA). Protein lysates were analyzed as described above, and quantitative densitometry was performed to calculate the nuclear to cytoplasmic ratio of each construct by dividing the area under the curve of the nuclear signal by the cytoplasmic signal.
2.7. Cell growth and viability assays
Vero cultures were plated at 20,000 cells per well in 96-well plates (Greiner, Cat # 655090) in phenol-red-free media. Cultures were treated with 10, 50, 100 nM Tg, or DMSO for 4, 8, or 24h in 100 μL of treatment media per well. A 2.33x solution of NucBlue (Hoechst 33343, 3.5 drops/mL, Thermo Fisher) and ethidium homodimer (EthD-1, 2.33 μL/mL, Invitrogen) was added to each well at 75 μL per well. Assay components were added directly to the well without washing to avoid cell loss. The plate was incubated at 37°C for 20 minutes before reading the plate on the Lionheart imaging platform (Biotek, Winooski, VT) and analyzing total (Hoechst+) and dead (EthD-1+) cells using Gen5 software. Each data point (n=3) reflects the average count of between 1281 to 2294 total cells depending on the treatment condition. Data are presented as either the proliferative index representing the percent of drug-treated samples relative to DMSO treated controls. For the live-dead assays described in Fig. 7, Vero cultures were transfected with pCIG3-CHOP-IRES-GFP and pSG5-C/EBP-β plasmids at a 4:1 ratio of CHOP to C/EBP-β. Cultures were imaged at 24 or 48h, or at 24h following 50 nM Tg or DMSO treatment for 4h. NucBlue and EthD-1 were added as above, and the plate was imaged and analyzed using a Celigo Image Cytometer (Nexelcom, San Diego, CA). The data were presented as the fraction of EthD-1+ dead cells relative to the population of transfected cells expressing GFP. The proliferation and survival assays were repeated twice.
Fig. 7. Modification at prNLS residue Ser107 alters CHOP-dependent toxicity.
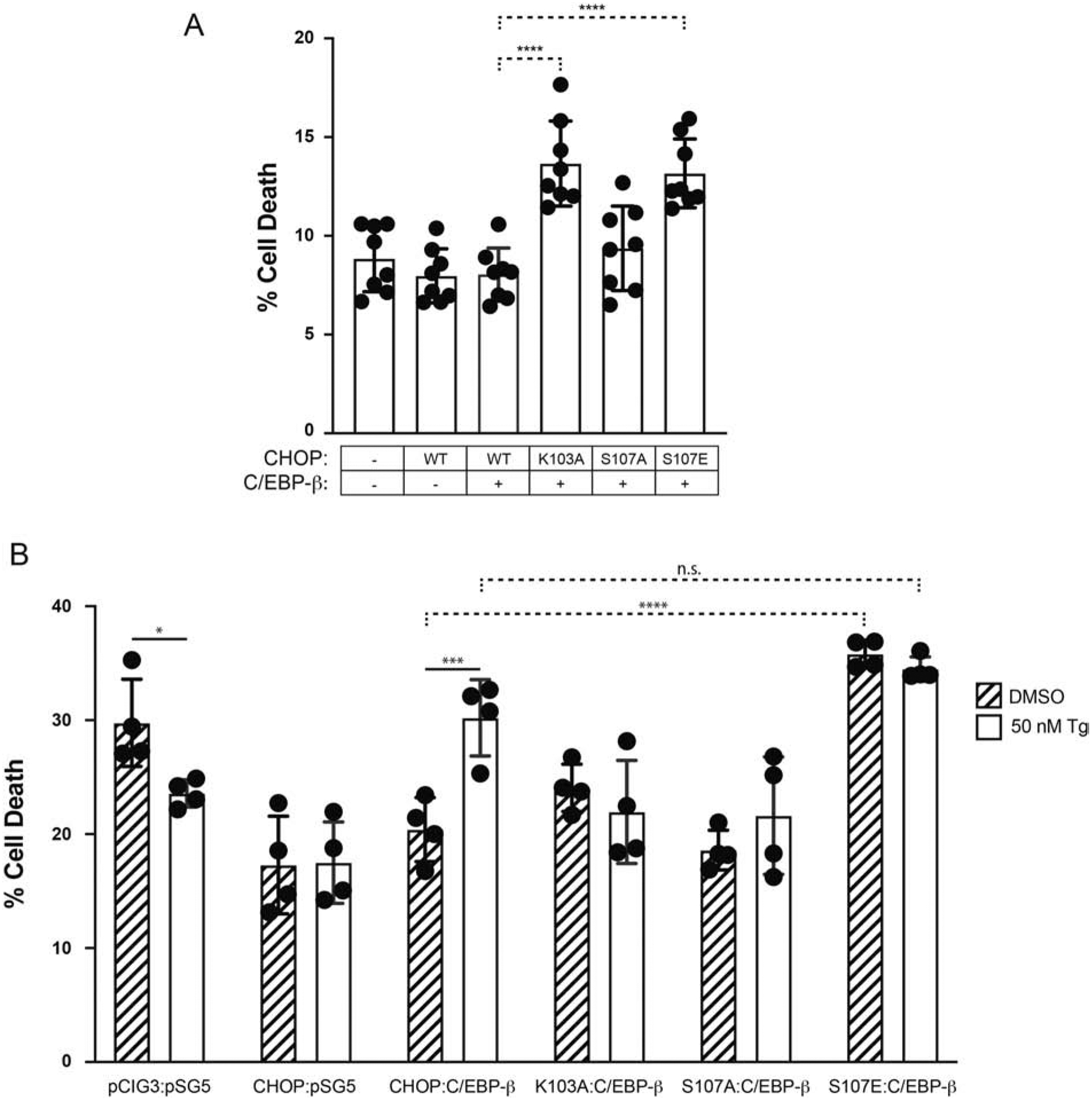
(A) Basal toxicity of pCIG3-CHOP-IRES-GFP relative to prNLS mutants (K103A, S107A, S107E) and the vector control (pCIG3-IRES-GFP) in Vero cells 24h post-transfection. Data are expressed as the fraction of EthD-1 positive (dead) per GFP+ cells; n=9. (B) Exposure of transfected Vero cultures to DMSO vs. Tg (50 nM) reveals conditional toxic properties of CHOP and the various prNLS mutants. Rates of cell death reflect the proportion of EthD-1(+)/GFP(+) cells 24 hours post-transfection; n=4. In both cases, CHOP and C/EBP-β were delivered at a 4:1 ratio. * p ≤ 0.05, *** p ≤ 0.001, **** p ≤ 0.0001.
2.8. Statistical analyses
Data analyses were performed with Graphpad Prism (Version 8), and all data are expressed as the mean ± SD. Significance testing was performed using either Student’s t testing or ANOVA with Tukey’s Test of post-hoc analyses in all cases except for Fig. 6 for which SIDAK multiple comparison testing was performed. p values ≤ 0.05 were considered statistically significant.
Fig. 6. Phosphomimetic substitution at Ser107 inhibits C/EBP-β-induced CHOP nuclear localization.
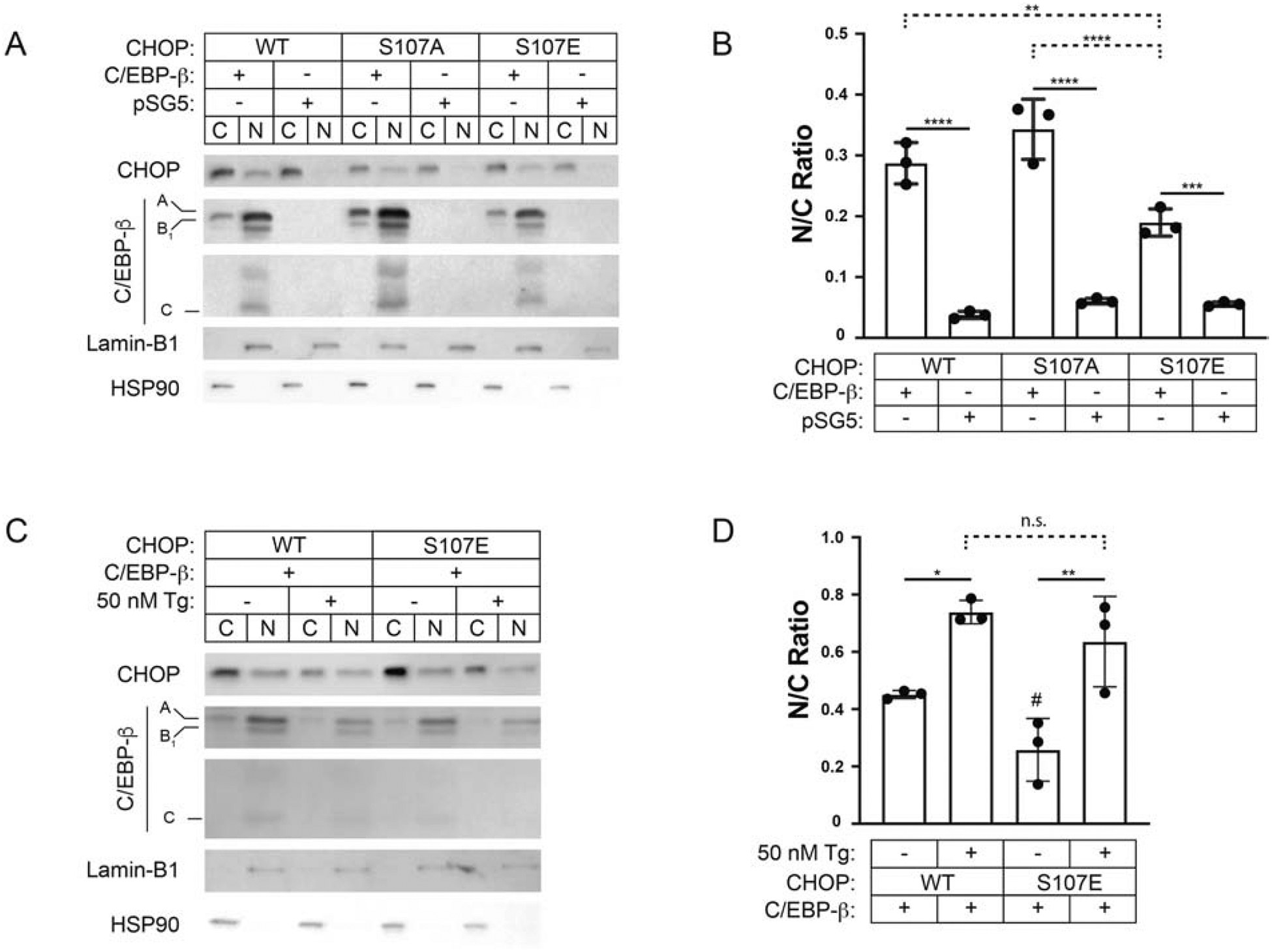
(A) Western blotting for CHOP, C/EBP-β, Lamin-B1, and HSP90 of nuclear and cytoplasmic cell fractions from Vero cultures collected at 24h after acute transfection with WT CHOP, CHOP Ser107Ala, or Ser107Glu with WT C/EBP-β or the control vector (pSG5) at a ratio of 1:4. (B) Densitometry is represented as the ratio of nuclear to cytoplasmic CHOP signal; n=3. (C-D) Western blotting and densitometry of nuclear and cytoplasmic cell fractions from Vero cultures transfected with either WT CHOP or CHOP Ser107Glu with either C/EBP-β or pSG5 at a ratio of 1:4 and 4h after treating cultures with 50nM thapsigargin or DMSO control; n=3. S107E DMSO vs. WT DMSO, # p=0.04. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
3. Results
3.1. DDIT3/CHOP localizes to cytoplasmic and nuclear compartments
To study the dynamics of CHOP nucleocytoplasmic trafficking in the setting of ER stress, we first established the treatment conditions that would induce endogenous CHOP protein expression and nuclear translocation without causing overt cell death. Results show that thapsigargin (Tg) induced growth arrest in Vero cultures within four hours that was both dose- (10, 50, and 100 nM) and time-dependent (Fig. 1A). These changes were not associated with an increase in death at 24 hours (Fig. 1B) based on levels of EthD-1 staining in the 100 nM Tg vs. DMSO treated groups (7.7% ± 1.5 vs. 8.3 % ± 5.7, one-way ANOVA, p = 0.81). Although 10 nM Tg was sufficient to induce growth arrest, exposure to 50 nM Tg was required to induce CHOP protein in the whole cell lysate (Fig. 1C). We then measured the CHOP nuclear to cytoplasmic ratio (N:C) at early (4h) and delayed (24h) time points, observing a time- and dose-dependent increase in CHOP N:C ratios (N/C at 4h, 50 vs. 100 nM Tg: 3.43 ± 0.19 vs. 4.12 ± 0.14, p < 0.005; at 24h: 4.65 ± 0.19 vs. 6.23 ± 0.28, p < 0.0001; 50 nM and 100 nM at 4 vs 24h, p < 0.0001), despite total protein amounts remaining consistent at each time point (Fig. 1D).
Fig. 1. Thapsigargin induces CHOP in both the nuclear and cytoplasmic compartments.
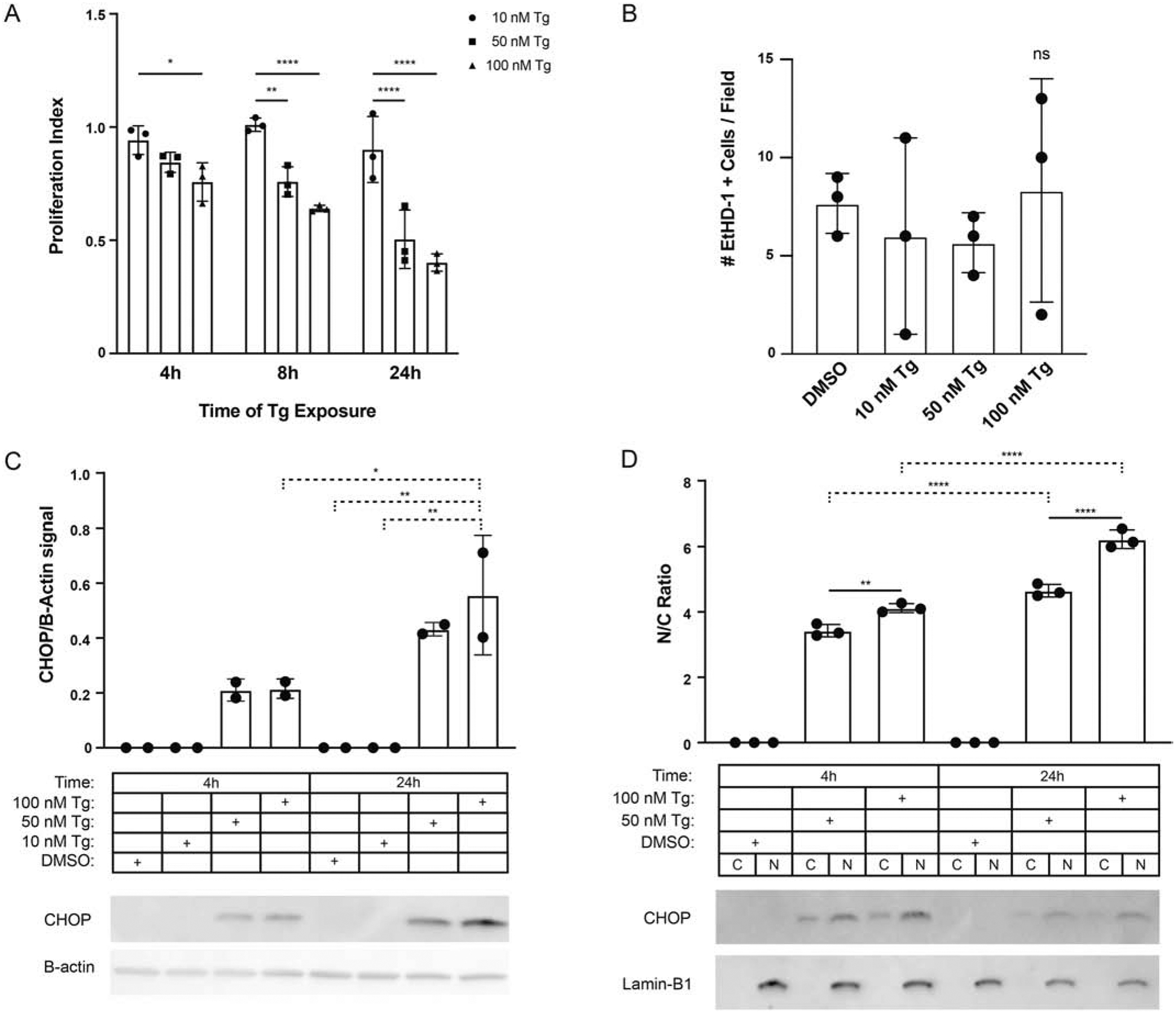
(A) Proliferation analyses of Vero cultures at 4, 8, and 24h exposed to thapsigargin (Tg) 10, 50, and 100nM. The proliferative index represents the number of nuclei in Tg treated wells normalized to DMSO; n=3. (B) Effects of Tg treatment on cell injury measured as the total number of ethidium homodimer (EthD-1) positive cells per field; n=3. (C) Western analyses for CHOP and β-actin expression in whole-cell lysates following exposure to 10, 50, or 100 nM Tg vs. DMSO control at 4 and 24 hours. Values reflect CHOP signal by densitometry normalized to β-actin; n=2. (D) Western analyses for CHOP and Lamin-B1 expression nuclear and cytoplasmic cell fractions from Vero cultures treated with 50 and 100nM thapsigargin or DMSO control at 4 and 24 hours. Values reflect the ratio of nuclear to cytoplasmic CHOP signal by densitometry; n=3. * p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001.
3.2. CHOP’s functional NLS contains both mono- and bipartite motifs
While the CHOP NLS has been mapped to the basic DNA binding domain (bDBD) [10, 13], the structural topology and existence of functional regulatory elements remain unexplored. Using in silico analyses, we identified two candidate NLS motifs within CHOP’s basic region. These included a monopartite (pat4) NLS (aa102–105) and an overlapping bipartite NLS (aa103–120) separated by a linker region ten residues in length. To discern the effects of the monopartite NLS from the full bipartite NLS on subcellular distribution, we constructed a series of EGFP-CHOP block deletion mutants that bisected the NLS region into N- and C-terminal segments (Fig. 2A). Using laser confocal microscopy, we found that full-length CHOP fused to EGFP (aa6–168) exhibited predominantly nuclear localization compared to EGFP alone in representative images (Fig. 2B). Similar results were obtained with constructs that retained both the putative monopartite and bipartite NLS motifs (i.e., aa25–168, aa6–135, aa90–135, and aa90–168). As expected, the aa6–90 fusion lacking both the basic DNA binding and leucine zipper domains exhibited predominantly cytoplasmic localization. The aa90–135 fusion, which includes both putative NLS motifs, exhibits the strongest nuclear localization. The aa90–118 construct exhibits a subcellular pattern most similar to full-length CHOP despite including an uninterrupted monopartite NLS. Although the C-terminal portion of the bipartite NLS (aa118–135) is insufficient to drive enhanced nuclear signal, it enhanced nuclear localization of the aa90–118 segment, indicating that the bipartite functionally contributes to CHOP’s NLS motif. Though some of these constructs fall below the size limit for passive diffusion through the nuclear pore complex [19], there are clear differences between the EGFP control and the NLS-containing constructs. To quantify the observed differences, we performed Western analyses on nucleocytoplasmic fractions from the acute Vero transfections (Fig. 2C–D) and found that the highest N:C ratio was attained with the aa90–135 construct. We also observed marked variability in the relative abundance between EGFP-CHOP and other block-deletion fusion proteins (Fig. 2C). Specifically, constructs lacking the known N-terminal ubiquitin instability domain (aa 10–26) exhibited improved basal stability [13]. These results indicate that while the monopartite NLS is sufficient to drive CHOP nuclear localization, the inclusion of the intact bipartite NLS element further enhances this effect.
Fig. 2. Mapping the CHOP bipartite NLS.
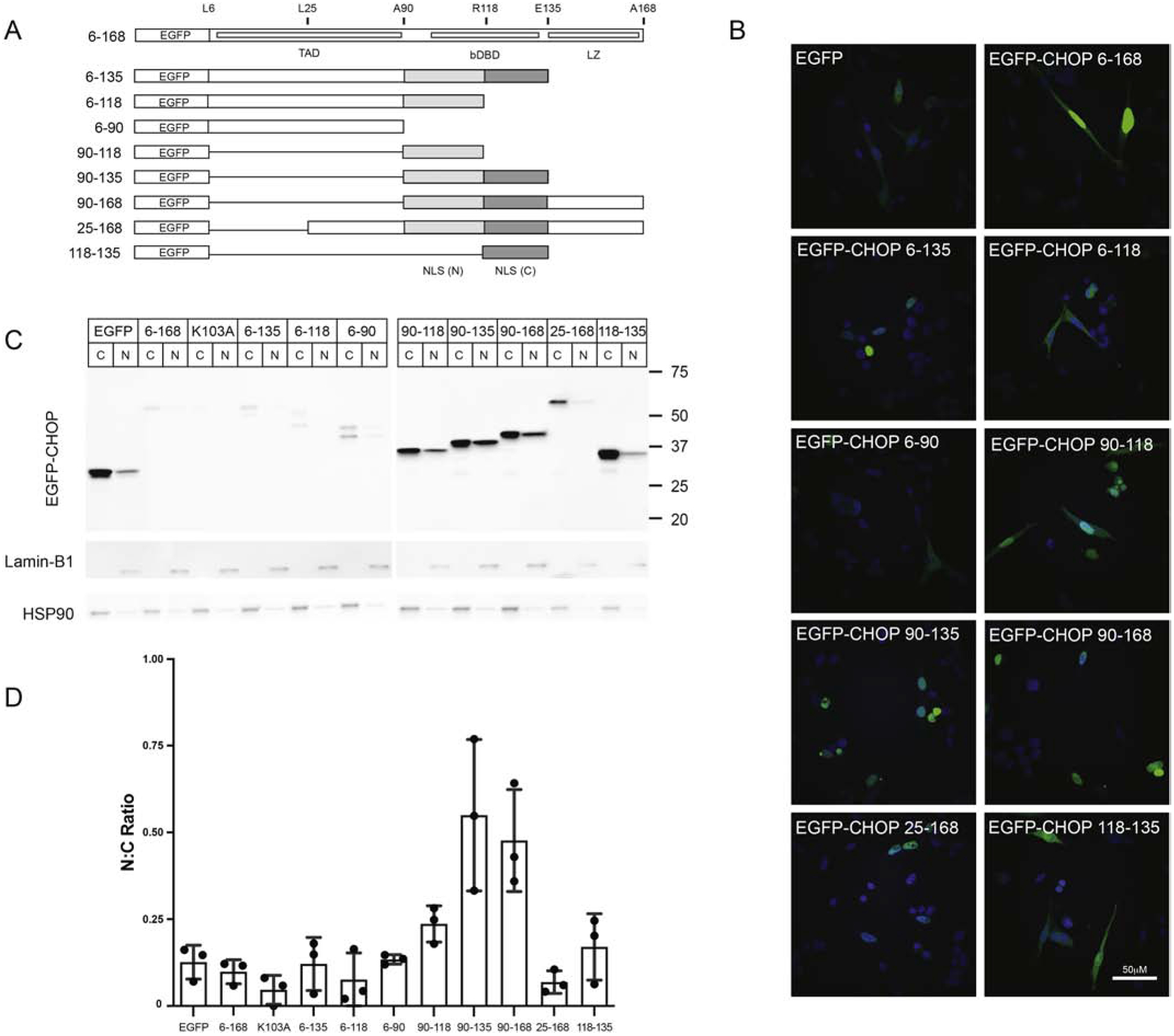
(A) Motif structure of CHOP/DDIT3 and the EGFP-CHOP deletion constructs used in Western and confocal analyses. Structures include the amino-terminal transcriptional activation (TAD, aa1–90), central basic DNA binding domain (bDBD; aa101–130) and carboxy-terminal leucine zipper domain (LZ; aa135–168). The N- and C-terminal portions of the nuclear localization sequence region, including the bipartite NLS spanning the dDBD are shown. (B) Confocal imaging of acutely transfected EGFP-CHOP block deletions constructs in Vero at 24h following acute transfection. (C) Western blotting for CHOP, Lamin-B1, and HSP90 of nuclear and cytoplasmic cell fractions from Vero cultures collected at 24h after acute transfection with the EGFP-CHOP block deletion constructs. (D) Densitometry results are represented as the ratio of nuclear to cytoplasmic CHOP signal; n=3.
3.3. C/EBP-β enhances CHOP nuclear localization
In addition to the cis-acting elements present within the bipartite NLS, CHOP’s subcellular distribution is also regulated by trans-acting factors. Consistent with published reports [10], we found that the heterodimeric factor C/EBP-β facilitated CHOP nuclear import when delivered at equal ratios (Fig. 3A, WT vs. -). After transfection, Western analyses demonstrate that Vero cells produce both full-length and internally translated forms of C/EBP-β commonly known as the liver-enriched activator protein (LAP) and liver-enriched inhibitory protein (LIP) [20]. To investigate the relative contribution of LIP vs. LAP to CHOP’s nuclear import, we conducted N:C Western analyses using a series of cDNAs harboring mutations at select C/EBP-β internal translational start sites required to generate LAP (ΔA, ΔB1) and LIP (ΔC) isoforms, and the upstream open reading frame (ΔD) located in the 5’ end of the transcript. Results indicate that no single species was required for nuclear import (Fig. 3A, N:C range: 0.23 to 0.51). To optimize conditions for CHOP nuclear import, we varied the ratio of CHOP to C/EBP-β plasmid from 1:4 to 4:1 delivered by transfection to Vero cultures. The delivery of CHOP alone produces low levels of nuclear CHOP (Fig. 3B, 0.02 ± 0.01). When WT C/EBP-β is delivered in a high ratio to CHOP, CHOP N:C ratios attain their highest level (0.65 ± 0.40), and the N:C ratio approaches one. When CHOP is delivered in excess (1:4), the amount of total nuclear CHOP decreases, and the N:C ratio falls steadily, approaching levels achieved with CHOP alone (0.10 ± 0.02).
Fig. 3. C/EBP-β facilitates CHOP’s nuclear localization.
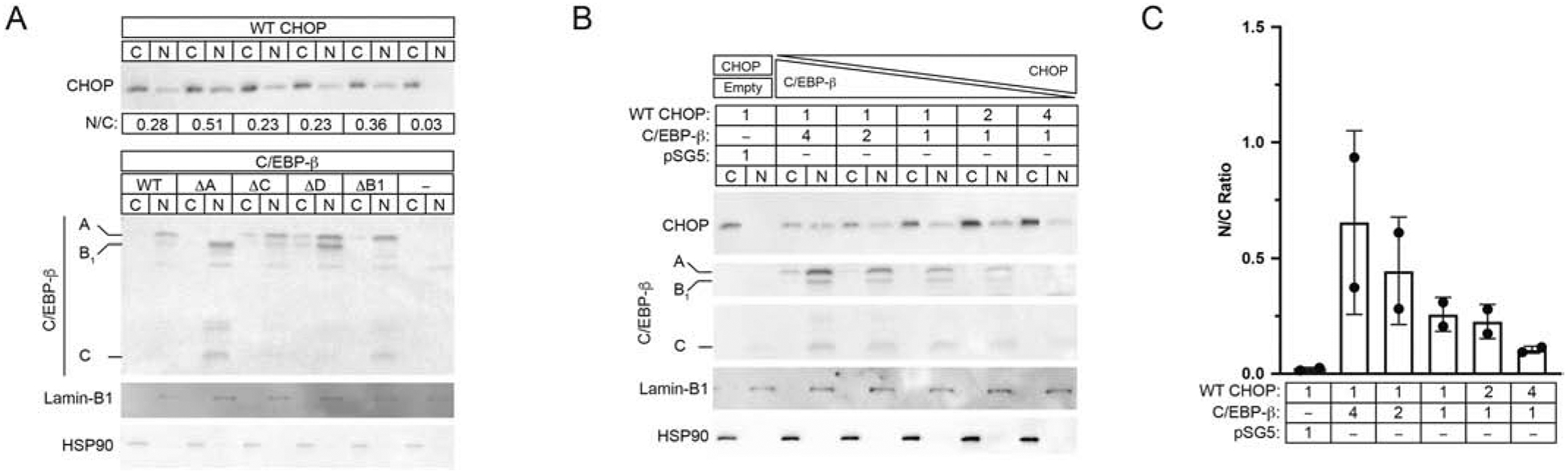
(A) Effects of internal translation products of C/EBP-β on CHOP N:C ratios. Western analyses for CHOP and C/EBP-β in Veros receiving equal amounts (1:4 ratio) of CHOP and either pSG5-C/EBP-β-WT or one of four C/EBP-β translation mutants (ΔA-ΔD). Ratios based on CHOP N:C densitometry and the major translational products (A, B1, C) of C/EBP-β are denoted. (B-C) Effects of varying of CHOP:C/EBP-β on CHOP N:C distribution in transfected Vero cultures. Relative ratios of CHOP, C/EBP-β, or empty vector (pSG5) are shown. Densitometry values shown in the histogram represent the N:C ration for CHOP; n=2. Nuclear (Lamin-B1) and cytoplasmic (HSP90) markers are shown as controls for the fractionation procedure.
3.4. Targeted disruption of the pat4 NLS reduces CHOP’s nuclear localization
The CHOP NLS and surrounding basic DNA binding domain exhibit a high degree of conservation between species (Fig. 4A). Interestingly, non-conserved residues (asterisk) fall either outside the NLS or are within the linking region of the bipartite NLS. While “rules” regarding the composition of the linker region are specific to length, it is unknown whether AA substitutions in this region should influence NLS behavior. Alignments indicate that the predicted mono- and bipartite NLS sequences overlap at residues 103 and 104 (Fig. 4A). Results from the block deletion studies in Figure 2 indicate that the pat4 NLS is sufficient to drive CHOP’s nuclear localization. To determine whether this motif is also necessary and whether CHOP contains a functional NLS [21], we introduced Lys103Ala mutation within the monopartite NLS, which leaves the predicted bipartite NLS motif intact. In transfected Vero cultures, CHOP Lys103Ala exhibited a four-fold reduction in the N:C ratio relative to WT CHOP (0.04 ± 0.01 vs. 0.01 ± 0.01, p = 0.004). However, when C/EBP-β is co-transfected at a 4:1 ratio to CHOP, this effect is abrogated, and the N:C ratios of WT and Lys103Ala are statistically equivalent (0.28 ± 0.08 vs. 0.20 ± 0.05, p = 0.24). These data indicate that when CHOP is present in stoichiometric excess, the pat4 motif is a driver of nuclear localization: loss of the pat4 NLS results in lower N:C ratios, revealing that, while the predicted bipartite sequence is still intact, the bipartite NLS alone is not sufficient to drive baseline CHOP nuclear localization. The Lys103 residue, and thus the intact pat4 NLS motif is necessary for driving CHOP’s baseline localization to the nucleus. However, mutation of this motif can be compensated by C/EBP-β in trans.
Fig. 4. Molecular interrogation of the CHOP pat4 NLS.
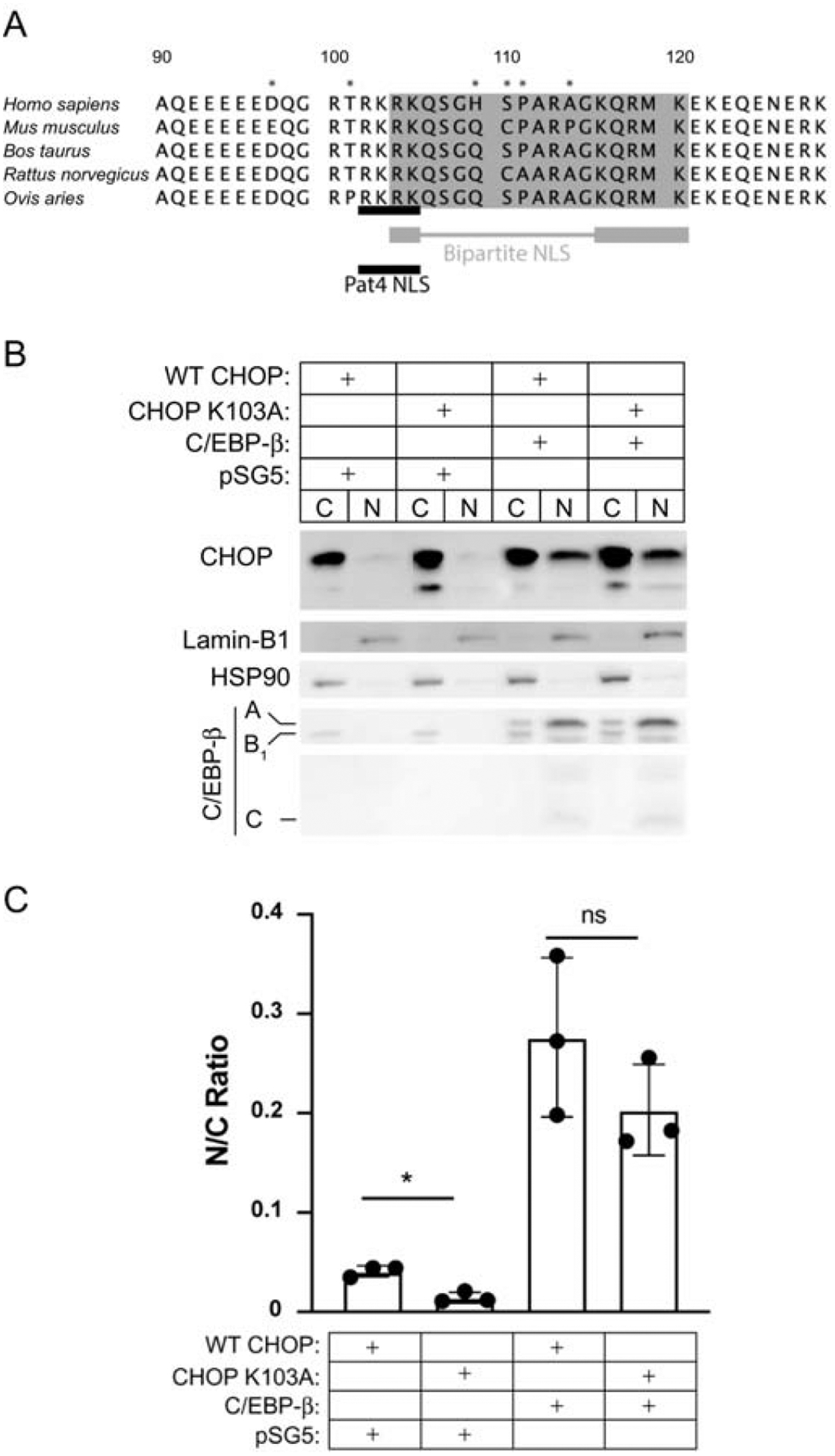
(A) CHOP amino acid sequence alignments from the genera Homo, Mus, Bos, Rattus, and Ovis. The predicted pat4 (monopartite) NLS is shown with a black bar. The bipartite NLS is shown in grey, with the functional residues indicated by the thick bars, and linker region indicated by the thin bar. Non-conserved residues are noted with an asterisk. (B) Western blotting for CHOP, C/EBP-β, Lamin-B1, and HSP90 of nuclear and cytoplasmic cell fractions from Vero cultures collected at 24h after acute transfection with WT CHOP or K103A and WT C/EBP-β or empty backbone at a ratio of 1:4. (C) Densitometry is represented as the ratio of nuclear to cytoplasmic CHOP signal; n=3. * p ≤ 0.05
3.5. Identification of a novel linker region phosphoepitope
The phosphorylation of CHOP’s N-terminal domain modulates its transcriptional activity with phosphorylation at Ser78 and Ser81 enhancing [14], and phosphorylation at Ser14–15 and Ser30–31 inhibiting its activity [6]. There is also precedent establishing that the localization of bZIP proteins, including C/EBP-β, can be regulated by post-translational modification [22, 23]. Since CHOP subcellular localization appears to be conditionally regulated, we asked whether phosphorylation of residues proximate to the NLS may play a similar role. Bioinformatics analyses conducted using the NetPhos 3.1 [24] prediction tool correctly identified several known kinase sites (Fig. S1). These included Ser14, 15, 30, 31 identified as consensus sites for CK II (0.518–0.650) as well as residues Ser78 and 81 known to serve as sites for the p38-MAPK (0.997, 0.668). Also, the search identified a pair of putative consensus sites with high similarity scores including Thr101 (0.889) recognized by the PKC family of kinases [14, 25] and the Ser107 site recognized by PKA (0.764), PKB (0.708), RSK (0.619), and PKG (0.528). To investigate whether any of the predicted phosphorylation sites were utilized under standard passage conditions, we performed tandem mass spectrometry analyses on purified recombinant CHOP protein using the Halo-Tag system (Fig. 5A–B). Peptide coverage of the CHOP protein was achieved from Thr75-Arg99, Lys105-Lys116, and Met119-Arg146 (Fig. 5C). Based on the coverage obtained, we identified three novel phosphoepitopes. Two residues were located within the n-terminal transactivating domain (Thr75 or Ser76, and Ser84 or Ser85), and a third residue (Ser107) was identified within the bipartite NLS linking region. Phosphorylation at Ser81, a previously reported site [14], was also identified.
Fig. 5. CHOP is phosphorylated at Ser107 under basal conditions.
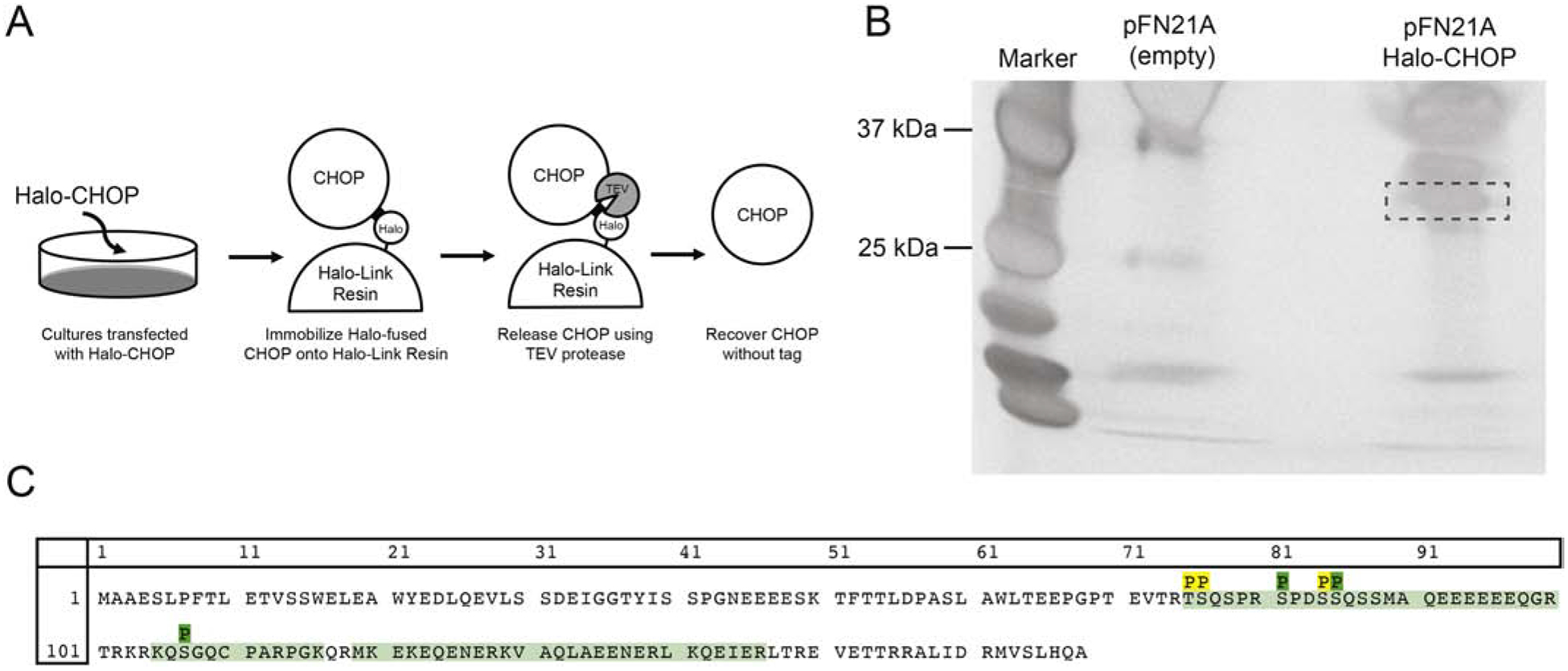
(A) Schematic of Halo-CHOP purification procedure. Forty-eight hours following transfection of either pFN21A-Halo-CHOP or the empty vector into N2a cultures, the Halo-CHOP fusion protein in whole-cell lysates binds to Halo-Link resin. After sequential washes, TEV protease is used to release CHOP from the Halo-tag linker. (B) Results from the silver staining procedure demonstrate the isolation of the purified CHOP species (dashed box) not present in pFN21A transfected lysates. (C) The amino acid sequence for mouse CHOP with the peptide coverage from the tandem mass spectrometry analysis in light green. The phosphorylation events (P) include both novel (Thr75/Ser76, Ser84/85, and Ser107) and published (Ser81) epitopes. Sites in yellow represent uncertainty in the analysis as to which neighboring residue is truly phosphorylated based on that fragment’s mass to charge ratio.
3.6. Ser107 regulates CHOP’s subcellular distribution
To study the potential role of Ser107 phosphorylation in regulating CHOP localization, we generated both kinase-resistant (Ser107Ala) and phosphomimic (Ser107Glu) point mutants and compared their behavior against the wild-type protein. When delivered without C/EBP-β, there were no differences between the N:C ratios for WT CHOP, Ser107Ala, or the Ser107Glu phosphomimic (0.04 ± 0.01 vs. 0.06 ± 0.01, and 0.06 ± 0.01, p > 0.89) (Fig. 6 A–B). However, delivery of C/EBP-β and CHOP at a ratio of 4:1 revealed that in unstimulated cells, Ser107Glu is resistant to nuclear localization relative to WT CHOP (0.19 ± 0.02 vs. 0.28 ± 0.03, p = 0.007). Conversely, Ser107Ala exhibited N:C ratios at WT levels, well above Ser107Glu (0.34 ± 0.05 vs. 0.19 ± 0.02, p = 0.0001). We next asked whether the phosphorylation status would influence thapsigargin-induced nuclear localization of exogenously expressed CHOP as observed for endogenous CHOP (Fig. 1D). Results show that treatment with Tg (50 nM, 4h) increased exogenous WT CHOP N:C ratios (0.45 ± 0.01 vs. 0.74 ± 0.04, p = 0.03) (Fig. 6C–D). Given the observed effects on basal localization, we hypothesized that the Ser107Glu modification would also block Tg-dependent changes on the CHOP N:C ratio. Again, Ser107Glu exhibited a reduced N:C ratio relative to WT CHOP in DMSO controls (0.45 ± 0.01 vs. 0.26 ± 0.11, p = 0.04). However, Ser107Glu remained sensitive to Tg-induced N:C redistribution (0.64 ± 0.16, p = 0.006). These data indicate that while phosphorylation within the NLS linker region moderates CHOP’s localization under basal conditions, the Ser107 phosphoepitope appears dispensable for Tg-induced redistribution.
3.7. Modification at the prNLS motif Ser107 alters CHOP-dependent toxicity
Since substitutions at the Ser107 site can alter CHOP’s N:C ratio, we reasoned that mutants that influence nuclear levels of CHOP should also affect cellular growth and survival. To test this, we transfected cultures with the vectors pCIG3-CHOP-IRES-GFP and pSG5-C/EBP-β plasmids at a 4:1 ratio, measuring the proportion of CHOP-expressing cells (GFP-positive) that co-label with the cell death marker ethidium homodimer. After 24 hours, the levels of injury induced either by CHOP alone or the combination of CHOP with C/EBP-β (7.98 ± 1.37% vs. 8.05 ± 1.33% dead) were comparable to those seen in controls receiving the empty vectors (pSG5/pCIG3, 8.84 ± 1.67% dead) (Fig. 7A). However, death in both the Lys103Ala:C/EBP-β (13.66 ± 2.15% dead, p<0.0001) and Ser107Glu:C/EBP-β (13.16 ± 1.74% dead, p<0.0001) groups were both higher compared to the WT CHOP:C/EBP-β group.
Given its ability to influence CHOP N:C ratios, we hypothesized Tg treatment would increase toxicity in CHOP-transfected cultures. While the CHOP:C/EBP-β transfected group exposed to DMSO exhibited levels of death comparable to CHOP alone (Fig. 7B), exposure to low-dose Tg induced cell death (20.40 ± 2.80% vs. 30.21 ± 3.35%, p=0.0002). Conversely, cultures receiving either CHOP alone (CHOP:pSG5) or those co-transfected with either Lys103Ala:C/EBP-β or Ser107Ala:C/EBP-β were resistant to Tg-induced toxicity. Again, co-expression of Ser107Glu:C/EBP-β without Tg-stimulation was toxic relative to CHOP:C/EBP-β (35.18 ± 1.21% vs. 20.40 ± 2.80%, p<0.0001). And although Tg increased CHOP N:C ratios in Ser107Glu transfected cultures (Fig. 6D), it did not induce cell death above levels observed in the DMSO-treatment group (35.81 ± 1.21% dead vs. 34.49 ± 1.06%, p = 0.80) (Fig. 7B).
4. Discussion
In the current study, we demonstrate that the bZIP transcription factor CHOP contains a conserved, compound pat4/bipartite nuclear localization signal within its basic DNA-binding domain. Though we do not interrogate which nuclear pore complex or import pathway that regulates CHOP’s NLS, our functional analysis establishes the necessary (Fig. 4) and sufficient (Fig. 2) sequences that constitute and validate CHOP’s predicted NLS (Fig. 4A) [21, 26]. We also define the regulatory properties of associated cis elements, including a novel phosphoepitope Ser107 located within the linker region of the bipartite NLS. Importantly, we found that manipulation of the charge properties at Ser107 influences both the subcellular distribution and toxic potential of DDIT3/CHOP. Notably, we also found that the stoichiometric relationship between CHOP and its heterodimeric binding partner C/EBP-β is an important determinant of CHOP’s nuclear localization, independent of discrete prNLS modifications. Collectively, these data expand our appreciation for the repertoire of mechanisms involved in constraining CHOP-dependent toxicity under both physiological and pathological conditions stemming from pharmacologically-induced ER stress.
The compartmentalization of transcription from translation is a hallmark of eukaryotic cells. While small molecules can passively diffuse across the nuclear envelope through nuclear pore complexes (NPCs), proteins larger than 40 kDa rely on active transport mediated by the β-karyopherin family of soluble transport receptors that recognize cargo nuclear localization signals (NLS) [19]. The classical NLS is that of the SV40 large T antigen [27]. One of the two subclasses of the monopartite NLS is a four-residue pattern known as ‘pat4’. It is composed of four basic amino acids, or three basic amino acids and either a Histidine or Proline residue. In contrast, the bipartite NLS, first identified in Xenopus nucleoplasmin by Robbins et al. [28], consists of two basic residues, a ten-residue linker, and another basic region consisting of at least three basic residues out of five. It is thought that these complex NLS motifs target proteins to particular NPCs and influence their ultimate sub-nuclear localization. Posttranslational modification of epitopes within or proximal to a classical NLS (prNLS) provides an opportunity to link subcellular localization of proteins to physiologically salient stimuli. For example, Akt alters the cellular distribution of FOXO1, mTOR, and the Akt inhibitor Tribbles 3 (TRIB3/NIPK/SKIP3) through the modification of such prNLS sites [29, 30]. In this case, regulated phosphorylation at CHOP Ser107 may provide the cell the means to respond quickly, converting physiological changes into actionable molecular and cellular responses.
The basic DNA binding and leucine zipper regions of CHOP are conserved within the bZIP family and across species [31]. Variability within the leucine zipper region encodes specificity with regard to the hetero- and homo-dimerization potential of individual factors [32]. Additional post-translational modifications within these domains are believed to refine these interactions that, in turn, convey effects on cell survival, apoptosis, proliferation, and differentiation. PTM-dependent regulation of bZIP spatial regulation and activity is also described. For example, the core structure of the C/EBP-β NLS, described by Williams and colleagues, possesses bipartite-like characteristics [33]. Disruption of the PKA Ser239 phosphoepitope within a region spanning the first motif of the NLS and bZIP domain inhibits C/EBP-β nuclear translocation [22]. Moreover, later studies revealed that TNF-α induced phosphorylation at Ser239 mediates inducible nuclear exclusion through reciprocal regulation of both NLS and NES segments [23]. While there is evidence in the literature supporting dynamic shifts in CHOP’s subcellular localization in human SY5Y neuroblastoma cells exposed to either BDNF or the ER-stress agent tunicamycin [15], the molecular mechanisms regulating these responses remain unknown.
The observed effects of Ser107 modification on N:C ratios did not match our predictions regarding CHOP’s toxic potential. We initially hypothesized that constructs with higher N:C ratios would exhibit greater toxicity. And while logic dictates that the Ser107Ala should exhibit increased toxicity relative to the phosphomimic Ser107Glu, we observed the opposite result. In fact, while the Ser107Ala transfected group was resistant to thapsigargin-induced toxicity, expression of the Ser107Glu induced toxicity in Vero cells, mimicking levels observed following Tg-exposure. Based on these observations, our working model posits that phosphorylation at Ser107 alters the local charge properties of the NLS that interfere with its ability to interact with one or more components of the nuclear pore complex and reduce the efficiency of import (Fig. 8), particularly under conditions where levels of C/EBP-β are low. While the Ser107 epitope falls outside the leucine zipper domain, we cannot exclude potential steric effects that altering the charge properties within the NLS linker region may have on CHOP heterodimerization. And while the Ser107Glu phospho-mimic exhibited a lower basal N:C ratio, thapsigargin exposure corrected the ratio to levels approximating that of wild-type CHOP (Fig. 6C–D). While unexpected, this result underscores the importance of alternate residues and trans-acting influences governing stress-induced CHOP redistribution.
Fig. 8. The phosphoepitope Ser107 regulates CHOP toxicity.

The residue Ser107 is located within the conserved linker region of the bipartite NLS in proliferating Veros. Under conditions where CHOP is not phosphorylated at Ser107, C/EBP-β facilitates CHOP accumulation in the nucleus and promotes survival. However, compared to the kinase-resistant form (S107A), the phosphomimic form of CHOP S107E induces cytotoxicity.
In this respect, we found the stoichiometry between CHOP and C/EBP-β to be a critical factor in establishing CHOP N:C ratios (Fig. 3B–C). In MEF cultures lacking C/EBP-β, exogenous C/EBP-β and a construct expressing only the LIP form both enhance CHOP nuclear localization. While LIP is the minimal sequence that enhances CHOP nuclear localization [10], it was unclear whether LIP was absolutely required. Our studies utilizing internal methionine start site mutants of C/EBP-β indicate that the LIP form is dispensable in this regard (Fig. 3A). Notably, while disruption of the monopartite NLS with the Lys103Ala substitution disrupted CHOP nuclear import (Fig. 4), co-transfection of C/EBP-β corrected this defect. This observation is consistent with prior reports indicating that C/EBP-α facilitates the nuclear localization of mutated forms of C/EBP-β lacking a functional NLS [33]. These dynamics may play a particularly important role in how CHOP functions under conditions of oxidative stress. In prior studies, we observed marked changes in the prevailing pattern of bZIP expression in cortical neurons exposed to chronic hypoxia. Specifically, hypoxic injury was associated with the loss of total C/EBP-β and reciprocal induction of both CHOP and its alternate binding partner ATF4 [34]. In light of the current findings, it is interesting to speculate that ATF4 may compensate for the loss of C/EBP-β in this and other contexts to support CHOP import with attendant changes in bZIP-directed transcription.
CHOP has generally been considered a pro-death gene due to its repressive effects on cell survival genes, including BCL2 and stimulatory effects on pro-death targets (i.e., DR5, TRB3, ERO1L) [3, 35–37]. CHOP also transactivates genes involved in the unfolded protein, nutrient starvation, and mitochondrial unfolded protein responses and conveys cytoprotection by reducing redox-related stress through the inducible expression of genes including carbonic anhydrase (CAVI-b) [38]. How then might conditional regulation of Ser107 tie in with physiological regulation of these CHOP-dependent ER stress responses? In addition to influencing NLS function, modification of the Ser107 epitope within the bipartite NLS linking region may influence CHOP’s DNA-binding activity. Consistent with this idea, phosphorylation within the DNA binding domain of the bZIP proteins C/EBP-β and BATF has been shown to inhibit their ability to bind DNA [23, 31, 39]. The Ser107 site within the basic region exhibits is conserved in over 95% (37/39) of species (data not shown). And the fact that Ser107 lies immediately adjacent to the core basic residues required for bZIP-DNA interactions [31] increases the likelihood that modification at Ser107 influence CHOP-dependent transcription. Beyond quantitative effects on CHOP DNA binding, the issue regarding how CHOP discriminates between adaptive and maladaptive responses remains an important yet outstanding question. There is evidence indicating that the ability of bZIP dimer pairs to discriminate between genomic targets relies primarily on amino acids in the basic and liner region, just n-terminal to the start of the leucine zipper [40, 41]. While further analysis is required to establish whether Ser107 exerts this effect, it is clear that the complement of cellular CHOP exists as a quasispecies with respect to post-translational modifications that in aggregate dictate its nuclear accumulation, transcriptional potency, and target specificity. With this in mind, further exploration regarding the upstream kinase and phosphatase signals regulating prNLS phosphorylation may provide an opportunity to fine-tune CHOP’s spatial distribution and transcriptional output, thereby constraining its latent pro-apoptotic potential under conditions of oxidative stress.
Supplementary Material
Fig. S1. CHOP-10 predicted phosphorylation epitopes. The amino acid number and kinase consensus sequence are reported, along with the predicted kinase and the output score from the NetPhos 3.1 server. Phosphorylation has been reported at Ser14, 15, 30, and 31 by Casein kinase 2 [6], at Ser30 by AMP-activated protein kinase alpha-1 [12], and Ser 78, 81 by p38 mitogen-activated protein kinase [14]. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
Highlights.
CHOP/DDIT3 contains a conserved, compound Pat4/bipartite nuclear localization signal within the basic DNA-binding domain.
The NLS linker region phosphoepitope Ser107 is modified under basal conditions.
S107 phosphorylation reduces the nuclear-to-cytoplasmic ratio of CHOP.
The S107E CHOP point mutant mimics thapsigargin-induced toxicity in vitro while alanine-substitution at this position renders CHOP non-toxic.
Funding
This work was supported by grants from the American Heart Association to JCB (18PRE34020109) and National Institutes of Health MWH (NS060764). The authors have no conflicts of interest to declare related to this work.
Abbreviations
- BATF
B-cell activating transcription factor
- bDBD
Basic DNA binding domain
- BDNF
Brain-derived neurotrophic factor
- bZIP
Basic leucine zipper
- C/EBP-β
CAAT/enhancer-binding protein beta
- CHOP
C/EBP homologous protein
- DDIT3
DNA-damage inducible transcript 3
- DMSO
Di-methyl sulfoxide
- DR5
Death receptor 5
- ERO1-L
Endoplasmic oxidoreductin-1-like
- ERSR
Endoplasmic reticulum stress response
- LAP
Liver-enriched inhibitory protein
- LIP
Liver-enriched activator protein
- N:C
Nuclear:cytoplasmic
- NES
Nuclear export sequence
- NLS
Nuclear localization sequence
- prNLS
phosphorylation regulated-NLS
- PTM
Post-translational modification
- Tg
Thapsigargin
- TRB3
Tribbles 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufman RJ, et al. , The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol, 2002. 3(6): p. 411–21. [DOI] [PubMed] [Google Scholar]
- 2.Rao RV, Ellerby HM, and Bredesen DE, Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ, 2004. 11(4): p. 372–80. [DOI] [PubMed] [Google Scholar]
- 3.Ron D and Habener JF, CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev, 1992. 6(3): p. 439–53. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett TW, et al. , Physical and functional association between GADD153 and CCAAT/enhancer-binding protein beta during cellular stress. J Biol Chem, 1996. 271(24): p. 14285–9. [DOI] [PubMed] [Google Scholar]
- 5.Chen BP, Wolfgang CD, and Hai T, Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol, 1996. 16(3): p. 1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ubeda M and Habener JF, CHOP Transcription Factor Phosphorylation by Casein Kinase 2 Inhibits Transcriptional Activation. Journal of Biological Chemistry, 2003. 278(42): p. 40514–40520. [DOI] [PubMed] [Google Scholar]
- 7.Jousse C, et al. , Amino acid limitation regulates CHOP expression through a specific pathway independent of the unfolded protein response. FEBS Lett, 1999. 448(2–3): p. 211–6. [DOI] [PubMed] [Google Scholar]
- 8.Medigeshi GR, et al. , West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol, 2007. 81(20): p. 10849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang K and Kaufman RJ, From endoplasmic-reticulum stress to the inflammatory response. Nature, 2008. 454(7203): p. 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiribau CB, et al. , Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol, 2010. 30(14): p. 3722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jauhiainen A, et al. , Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PloS one, 2012. 7(4): p. e33208–e33208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai X, et al. , Phosphorylation of CHOP (C/EBP Homologous Protein) by the AMP-Activated Protein Kinase Alpha 1 in Macrophages Promotes CHOP Degradation and Reduces Injury-Induced Neointimal Disruption In Vivo. Circulation research, 2016. 119(10): p. 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohoka N, et al. , Critical and functional regulation of CHOP (C/EBP homologous protein) through the N-terminal portion. J Biol Chem, 2007. 282(49): p. 35687–94. [DOI] [PubMed] [Google Scholar]
- 14.Wang X and Ron D, Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Vol. 272 1996. 1347–9. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, et al. , Brain-derived neurotrophic factor suppresses tunicamycin-induced upregulation of CHOP in neurons. J Neurosci Res, 2007. 85(8): p. 1674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barettino D, et al. , Improved method for PCR-mediated site-directed mutagenesis. Nucleic Acids Res, 1994. 22(3): p. 541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halterman MW, et al. , In-tube transfection improves the efficiency of gene transfer in primary neuronal cultures. J Neurosci Methods, 2009. 177(2): p. 348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider CA, Rasband WS, and Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 2012. 9(7): p. 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart M, Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol, 2007. 8(3): p. 195–208. [DOI] [PubMed] [Google Scholar]
- 20.Calkhoven CF, Müller C, and Leutz A, Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes & development, 2000. 14(15): p. 1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 21.Frangioni JV and Neel BG, Use of a general purpose mammalian expression vector for studying intracellular protein targeting: identification of critical residues in the nuclear lamin A/C nuclear localization signal. J Cell Sci, 1993. 105 (Pt 2): p. 481–8. [DOI] [PubMed] [Google Scholar]
- 22.Chinery R, et al. , Antioxidant-induced nuclear translocation of CCAAT/enhancer-binding protein beta. A critical role for protein kinase A-mediated phosphorylation of Ser299. J Biol Chem, 1997. 272(48): p. 30356–61. [DOI] [PubMed] [Google Scholar]
- 23.Buck M, et al. , C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell, 2001. 8(4): p. 807–16. [DOI] [PubMed] [Google Scholar]
- 24.Blom N, Gammeltoft S, and Brunak S, Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol, 1999. 294(5): p. 1351–62. [DOI] [PubMed] [Google Scholar]
- 25.Maytin EV, et al. , Stress-Inducible Transcription Factor CHOP/gadd153 Induces Apoptosis in Mammalian Cells via p38 Kinase-Dependent and -Independent Mechanisms. Experimental Cell Research, 2001. 267(2): p. 193–204. [DOI] [PubMed] [Google Scholar]
- 26.Damelin M, Silver PA, and Corbett AH, Nuclear protein transport. Methods Enzymol, 2002. 351: p. 587–607. [DOI] [PubMed] [Google Scholar]
- 27.Kalderon D, et al. , A short amino acid sequence able to specify nuclear location. Cell, 1984. 39(3 Pt 2): p. 499–509. [DOI] [PubMed] [Google Scholar]
- 28.Robbins J, et al. , Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell, 1991. 64(3): p. 615–23. [DOI] [PubMed] [Google Scholar]
- 29.Harreman MT, et al. , Regulation of nuclear import by phosphorylation adjacent to nuclear localization signals. J Biol Chem, 2004. 279(20): p. 20613–21. [DOI] [PubMed] [Google Scholar]
- 30.Meur G, et al. , Nucleo-cytosolic shuttling of FoxO1 directly regulates mouse Ins2 but not Ins1 gene expression in pancreatic beta cells (MIN6). J Biol Chem, 2011. 286(15): p. 13647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinson C, Acharya A, and Taparowsky EJ, Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta, 2006. 1759(1–2): p. 4–12. [DOI] [PubMed] [Google Scholar]
- 32.Newman JR and Keating AE, Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science, 2003. 300(5628): p. 2097–101. [DOI] [PubMed] [Google Scholar]
- 33.Williams SC, Angerer ND, and Johnson PF, C/EBP proteins contain nuclear localization signals imbedded in their basic regions. Gene expression, 1997. 6(6): p. 371–385. [PMC free article] [PubMed] [Google Scholar]
- 34.Halterman MW, et al. , Loss of c/EBP-beta activity promotes the adaptive to apoptotic switch in hypoxic cortical neurons. Mol Cell Neurosci, 2008. 38(2): p. 125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luethy JD, et al. , Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem, 1990. 265(27): p. 16521–6. [PubMed] [Google Scholar]
- 36.Batchvarova N, Wang XZ, and Ron D, Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). The EMBO journal, 1995. 14(19): p. 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyadomari S and Mori M, Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death & Differentiation, 2004. 11(4): p. 381–389. [DOI] [PubMed] [Google Scholar]
- 38.Matthews TA, et al. , Expression of the CHOP-inducible carbonic anhydrase CAVI-b is required for BDNF-mediated protection from hypoxia. Brain Research, 2014. 1543: p. 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deppmann CD, et al. , Phosphorylation of BATF regulates DNA binding: a novel mechanism for AP-1 (activator protein-1) regulation. Biochem J, 2003. 374(Pt 2): p. 423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agre P, Johnson PF, and McKnight SL, Cognate DNA binding specificity retained after leucine zipper exchange between GCN4 and C/EBP. Science, 1989. 246(4932): p. 922–6. [DOI] [PubMed] [Google Scholar]
- 41.Metallo SJ and Schepartz A, Distribution of labor among bZIP segments in the control of DNA affinity and specificity. Chem Biol, 1994. 1(3): p. 143–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CHOP-10 predicted phosphorylation epitopes. The amino acid number and kinase consensus sequence are reported, along with the predicted kinase and the output score from the NetPhos 3.1 server. Phosphorylation has been reported at Ser14, 15, 30, and 31 by Casein kinase 2 [6], at Ser30 by AMP-activated protein kinase alpha-1 [12], and Ser 78, 81 by p38 mitogen-activated protein kinase [14]. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.


