Abstract
Mobilized peripheral blood (mPB) hematopoietic stem (HSCs) and progenitor (HPCs) cells are primary sources for hematopoietic cell transplantation (HCT). Successful HCT requires threshold numbers of high-quality HSCs to reconstitute hematopoiesis long-term. Nevertheless, considerable percentages of patients and healthy donors fail to achieve required thresholds of HSCs with current mobilization regimens. In this present study we demonstrate that similar to mouse bone marrow (BM) and human cord blood, collection and processing of mouse Granulocyte Colony Stimulating Factor (G-CSF)-, AMD3100/Plerixafor- or G-CSF plus AMD3100/Plerixafor-mobilized HSCs in 3% O2 results in enhanced numbers of rigorously-defined phenotypic and for G-CSF – and G-CSF plus AMD3100/Plerixafor – mPB enhanced functionally-engrafting HSCs. These results may be of potential clinical utility.
Keywords: Mobilized Peripheral Blood, G-CSF, AMD3100/Plerixafor, Lowered O2 Tension, Hematopoietic Stem Cell Transplantation
Graphical Abstract
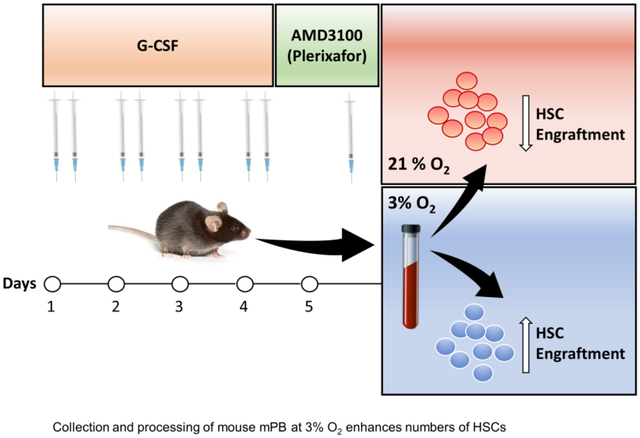
Introduction
Growth factor mobilized peripheral blood (mPB) is a primary source of hematopoietic stem (HSCs) and progenitor (HPCs) cells for autologous and allogeneic hematopoietic cell transplantation (HCT) (1). Granulocyte Colony Stimulating Factor (G-CSF) is still a main mobilizing factor for collection of mPB HSCs and HPCs (1,2). However, this mobilization procedure does not always yield optimal therapeutic numbers of transplantable HSCs, and up to 40% of mPB donors fail to mobilize sufficient numbers of CD34+ cells especially for harder to mobilize patients (1,3–5). CD34 is a marker used to identify populations of human cells that are greatly enriched for HPCs, and that also contain HSCs (1,3–5). AMD3100/Plerixafor is a rapid mPB agent for mice (1,6), and man (1,6,7), and adding AMD3100/plerixafor to conventional G-CSF mobilization procedures increased output of HSCs and HPCs from mice (1,6), healthy human donors (1,8), and from patients with non-Hodgkin’s lymphoma, multiple myeloma, lymphoma, and Fanconi anemia (9–13). Conventional mobilization regimens can entail multiple apheresis sessions, extended hospitalization stays, and financial burdens (1,14), and still may not yield enough HSCs from some donors who may be harder to mobilize. Hence, we evaluated the effects of collection and processing of mPB cells at lowered (3%) O2 tension to see if we could mimic the enhanced numbers of detectable HSCs seen for mouse bone marrow (BM) and human cord blood when these cells were collected and processed at 3% O2, compared to that in ambient air (~21% O2) (15). We found that collection and processing of G-CSF-, AMD3100/Plerixafor-, and the combination of G-CSF plus AMD3100/Plerixafor-mPB from mice at 3% O2 yield significantly increased numbers of rigorously-defined phenotypic and for G-CSF-and G-CSF plus AMD3100/Plerixafor enhanced numbers of functionally engrafting HSCs.
Methods
Mice.
Mice used for Peripheral Blood (PB) and BM collections and transplantation were C57Bl/6J (CD45.2+), Boy/J (CD45.1+), and B6xBoy/J F1 (F1; dual CD45.2+/CD45.1+) mice (8- to 10-week-old). All mice were obtained from the In Vivo Therapeutics Core at the Indiana University School of Medicine. All injections were approved by the Indiana University Use and Care of Animals Committee. Mice husbandry and care were performed under 21–24°C temperature- and 12-hour light,12-hour dark cycle); group-housed according to age, sex, and genotype; and fed ad libitum. Mice used were males and females; all mice were matched for age and sex for each experiment.
G-CSF and AMD3100/Plerixafor injections.
To mobilize mouse HSCs and HPCs, we injected C57BL/6 mice with 2.5μg/100μl G-CSF (Amgen, Thousand Oaks, CA) subcutaneously (s.c) twice daily for four days and collected PB on day 5. AMD3100/Plerixafor (Selleckchem, Houston, TX) was given as a single 20μg injection s.c. on day 5, and PBS s.c. injections to non-AMD3100/Plerixafor control groups. Mice were euthanized for PB collection 12 hours after the last G-CSF injection and one-hour post-AMD3100/Plerixafor–injection (6).
BM harvest and PB collection.
All BM and PB collections were performed in a hypoxia chamber, maintained at 3% O2, 5% CO2, and N2 balance. All reagents and supplies were equilibrated in the 3%O2 hypoxia chamber for at least 16 hours prior to cell collection time (15). To isolate PB mononuclear cells (PBMCs), PB was collected by intracardiac aspiration with heparinized syringes immediately after euthanizing mice. PBMC were isolated using lymphocyte mammal Ficoll (CEDARLANE, Burlington, ON, Canada) with gradient centrifugation isolation. All tubes were sealed with parafilm to maintain the 3% O2 during centrifugation outside the hypoxia chamber. BM was harvested by flushing femurs with PBS. All subsequent techniques, flow cytometry staining, and fixation, HPC colony assays, and injections for transplantation were performed in the hypoxia chamber. For ambient air control groups, we removed half of the cells from the hypoxia chamber to ambient air for at least 60 minutes before applying equivalent techniques in ambient air. We had previously shown that cells collected immediately in air showed similar effects to cells collected in hypoxia and then equilibrated to air for 1 hour (15). Cells assessed by colony-forming cell assays were incubated at 5% O2, 5% CO2 regardless of how cells were collected and processed, as this allows detection of optimal growth (16).
Flow cytometry immunophenotyping.
This analysis was performed by incubating PB or BM cells with fluorochrome conjugated anti-mouse antibodies (1 mg/106 cell) in PBS + 2% BSA in ice for 30 minutes. The 3% O2 group was stained inside the hypoxia chamber, and the ambient air group was stained in ambient air. All groups were fixed with fresh 2% paraformaldehyde in PBS (Thermo Fisher Scientific, Waltham, MA) for 30 minutes. Antibodies were purchased from (BD Biosciences, San Diego, CA) or (BioLegend, San Diego, CA). Antibodies used were; APC mouse lineage antibody cocktail, BD Bioscience, Cat. 51–9003632, APC-H7 anti-mouse CD117, BD Bioscience, Cat. 560185, PE/Dazzle 594 anti-mouse Sca1, Biolegend, Cat. 108138, PE anti-mouse CD34, BD Bioscience, Cat. 551387, BV421 anti-mouse CD135, Biolegend, Cat. 135314, PerCP-Cy5.5 anti-mouse CD16/32, BD Bioscience, Cat. 560540, BV786 anti-mouse CD127, BD Bioscience, Cat. 563748.
Data acquisition was conducted in LSRII flow cytometer (BD Biosciences, San Diego, CA). Data analysis was performed using FlowJo 10.6.2 software (TreeStar, WA). Gates were plotted using fluorescence-minus-one controls. Percentages of each cell population and that of live cells were used to calculate absolute numbers of phenotypically- defined HSC and HPC per ml blood and per femur. The analysis of phenotyped- HSCs and HPCs was as follows, with absolute numbers of each cell type calculated per ml PB or per femur. Long term (LT)-HSC were defined as Lin−c-Kit+ Sca1+ CD34− Flt3−, short-term (ST)-HSC as Lin− c-Kit+ Sca1+ CD34+ Flt3−, multipotent progenitors MPP as Lin − c-Kit+ Sca1+ 1 CD34+ Flt3+, common myeloid progenitors (CMP) as Lin− c-Kit+ Sca1− CD34int FcgRlo, granulocyte-macrophage progenitors (GMP) as Lin− c-Kit+ Sca1− CD34hi FcgRhi, megakaryocyte-erythrocyte progenitors (MEP) as Lin− c-Kit+ Sca1− CD34+/− FcgR− and common lymphoid progenitors (CLP) Lin− c-Kitlo Sca1+ Flt3+ IL7R+ (17).
HPC assays.
Cells were plated at 5×104 or 1×105 cells/mL in 1% methylcellulose culture medium with 30% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA), 1 U/mL recombinant human erythropoietin, 50 ng/mL recombinant mouse stem cell factor (R&D Systems, Minneapolis, MN), 0.1 mM 2-mercaptoethanol, 2 mM L-glutamine, 0.1 mM hemin Sigma Aldrich (St. Louis, MO), and 5% vol/vol pokeweed mitogen spleen conditioned medium. Cells were incubated at 5% CO2 and 5% O2 in a humidified chamber, and granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitor cells were scored at day 6 of incubation (15,16) and are shown as cells/femur or as cells/ml of mPB.
Transplantation.
Recipient F1 (CD45.1+ CD45.2+) mice were fed uniprim for one week and irradiated with one dose of 950 cGy, 24 hours before transplantation. Irradiated F1 mice were transplanted with limiting dilutions of (C57BL/6 CD45.2+) donor cells and 100,000 Boy/J (CD45.1+) BM competitor cells. Injections were done via intravenous (i.v.) injections in the hypoxia chamber for the 3% O2 group and in ambient air for the ambient air control group. Competitor Boy/J mice cells were collected in air and injected after the donor cells for both hypoxia and air collected cells. The method of injection inside the hypoxia chamber has been detailed (15).
Chimerism of F1 mouse PB was assessed at various time points by submandibular vein bleeding. Mice were sacrificed four months after transplantation, and then BM cells were analyzed by flow cytometry for HSCs, HPCs, and lymphoid and myeloid lineages. Competitive repopulating units (CRUs) were calculated and plotted using ELDA software (bioinf.wehi.edu.au/software/elda/), and 1.5 ×106 of primary recipient’s bone marrow cells were transplanted into secondary recipients to assess the self-renewal capacity of donor HSCs. (15,16,18–20).
Statistical analysis.
GraphPad Prism 8 was used for Statistical analysis. Two-tailed Student’s t-test was used for analysis between two groups. P values are designated as *p < 0.05, **p < 0.05, ***p < 0.005. Error bars represent SEM unless stated otherwise.
Results and Discussion
Effects of harvesting and processing G-CSF-, AMD3100/Plerixafor- or G-CSF/AMD3100/Plerixafor- mobilized PB cells in hypoxia (3% O2) vs. in ambient air (~21% O2) were assessed by phenotypic analysis of HSCs and HPCs, for functional HPCs as assessed by colony assay, by engraftment of HSCs in lethally irradiated congenic mice (as determined by chimerism over time), and by calculation of competitive repopulating cells (CRUs; a measure of the numbers of functional HSCs), and by secondary engraftment to assess the self-renewal capacity of donor HSCs.
G-CSF Mobilization.
Flow cytometry immunophenotyping analysis of G-CSF mPB HSCs and HPCs demonstrated a significant two-fold increase in long-term HSC (LT-HSC) when cells were collected and processed in 3% O2 compared to ambient air (Fig. 1Ai). Numbers of MPPs were significantly decreased in the hypoxia group (Fig. 1Aiii), as were numbers of GMPs (Fig. 1Av). ST-HSCs, CMPs, MEPs, and CLPs (Fig. 1Aii, iv, vi, vii) were not statistically different between the 2 groups. A sample flow plot is shown in Fig. 1Aviii.
Figure 1. Numbers of G-CSF mobilized HSCs and HPCs per ml blood.
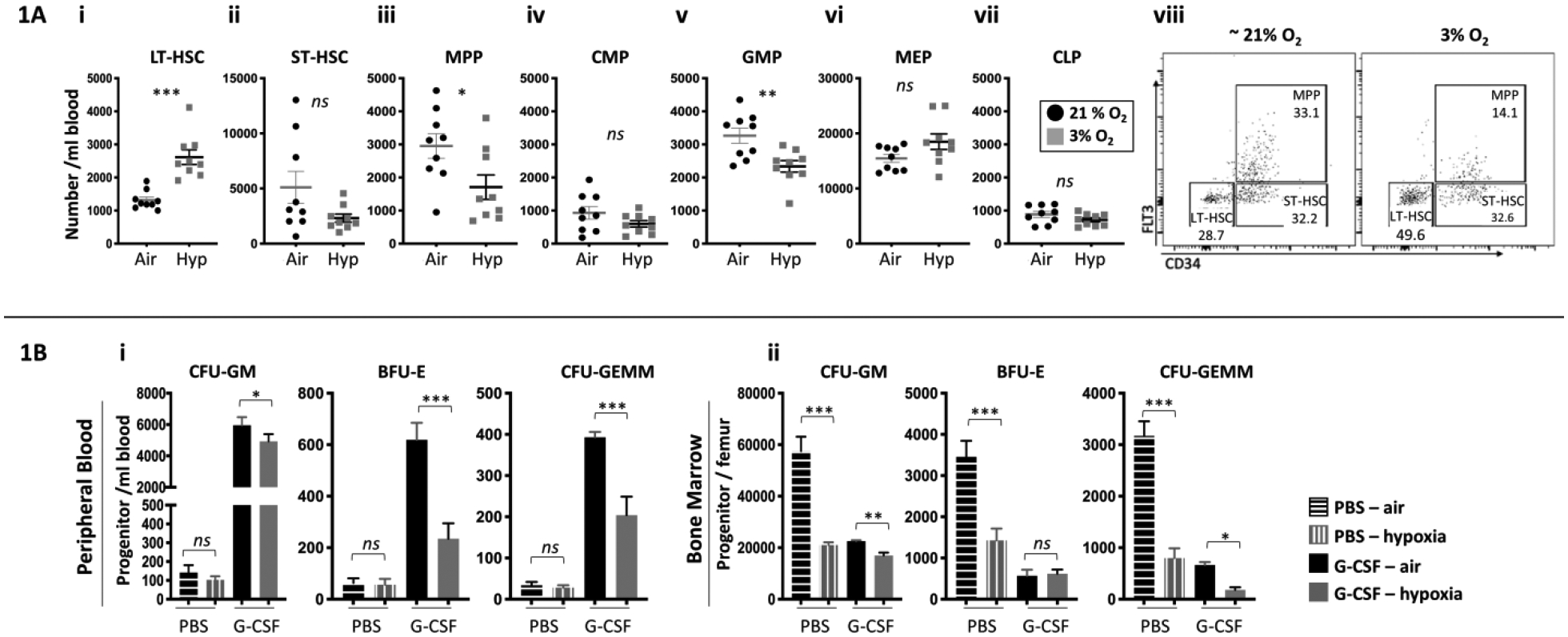
A combination of three independent experiments is shown; each experiment assessed three mice. PB and BM was harvested and processed in a hypoxic chamber (3% O2, 5% CO2, N 2) or ambient air (~21% O2) from G-CSF mobilized C57BL/6 mice, and analyzed for HSC and HPC numbers. Numbers of LT-HSCs (Ai), ST-HSCs (Aii), MPPs (Aiii), CMPs (Aiv), GMPs (Av), MEPs (Avi) and CLPs (Avii) per ml blood were assessed by flow cytometry. A flow cytometry dot plot representative of LT-HSCs, ST-HSC and MPP (Aviii). Progenitor cell numbers and were analyzed using a functional HPC colony assay examining CFU-GM, BFU-E, and CFU-GEMM per ml PB (Bi) and per femoral BM (Bii). Data are presented as mean± SEM. *p < 0.05, **p < 0.05, ***p < 0.005 when analyzed by Student’s t test.
Multi-cytokine stimulated HPC colony assay was performed for mPB and BM cells from mice treated with G-CSF. In PB, numbers of G-CSF mPB CFU-GM, BFU-E, and CFU-GEMM collected and processed in 3%O2 were significantly reduced compared to the ambient air condition (1Bi). There was also a significant reduction in BM CFU-GM, BFU-E and CFU-GEMM collected/processed in 3%O2 (1Bii). These results are similar to that we reported for BM HPCs (15).
Results of phenotypically defined populations of HSCs do not always recapitulate HSC function (21,22). We conducted competitive transplantation assays to assess engrafting and repopulating capacity of donor HSC (Fig. 2Ai, schematic of protocol). Donor PB (Fig. 2Aii) and BM (Fig. 2Aiii) chimerism at month 4 showed that hypoxia collected/processed cells manifested significantly increased engraftment. Limiting dilution analysis (LDA) to calculate numbers of functional HSCs, demonstrated increased competitive repopulating units (CRUs) in the 3% O2 group vs. ambient air control for PB (Fig. 2Bi, Bii), and for BM (2Biii, iv), in the engrafted mice. There was no myeloid/lymphoid lineage bias in the donor cell engraftment in PB of primary mice (Fig. 2Bv) The hypoxia collected/processed donor cells also showed significantly increased repopulation in secondary mice at month 4 (Fig. 2Bvi), demonstrating that hypoxia-collected and processed cells had increased numbers of self-renewing HSCs.
Figure 2. Engrafting efficiency of G-CSF mPB collected and processed in 3% O2 vs. ambient air (~21% O2), assessed by competitive BM transplantation and limiting dilution analysis.
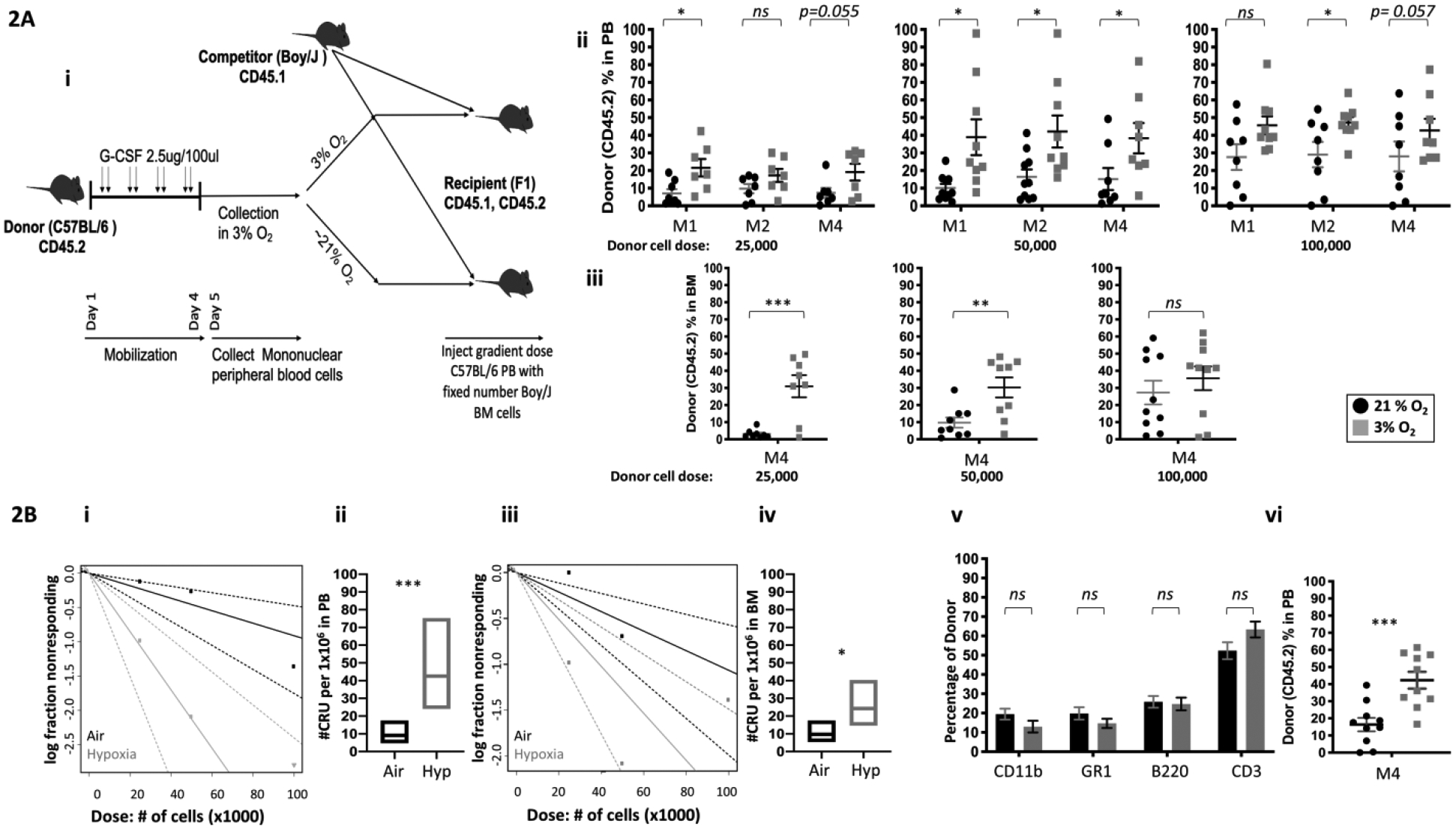
A combination of 2 independent experiments, n=8–10 recipient mice per group is shown. Donor G-CSF mobilized PB cells (CD45.2+) were injected i.v.at doses of 25,000, 50,000, and 100,000, into lethally irradiated F1 host mice (CD45.1+CD45.2+) in air or hypoxia. Competitor Boy/J BM cells (CD45.1+) were injected i.v. at 100,000 cell dose in air (Ai; diagrammatic representation). The percentage of donor-derived cells in the PB was assessed by flow cytometry at months (M) 1, 2 and 4 (Aii). The percentage of donor-derived cells in the BM was assessed at month 4 (Aiii). Poisson statistical analysis from the limiting dilution transplantation PB data (Bi) and BM (Biii) are shown. Solid line indicates best-fit linear model. Dotted lines represent 95% confidence intervals. Squares represent the percentage of negative mice for each dose. Triangles indicate that all tested mice were positive in this group. Numbers of CRUs in 106 cells in PB (Bii) and BM (Biv). The myeloid (CD11b+ and GR1+)/ lymphoid (CD3+ and B220+) ratio in the PB at month 4 was assessed by flow cytometry (Bv). Engraftment in secondary recipients at month 4 (BVi). Data are presented as mean± SEM. *p < 0.05, **p < 0.05, ***p < 0.005, when analyzed by Student’s t test.
G-CSF plus AMD3100/Plerixafor Mobilization.
AMD3100/Plerixafor mobilizes an improved graft (6) with a unique transcriptional profile (23–26). We assessed effects of collection and processing of G-CSF alone-, AMD3100/Plerixafor alone-, and the combination of AMD3100/Plerixafor plus G-CSF- mPB cells in 3% vs. ambient air (~21%) O2 levels. Numbers of phenotypically defined LT-HSCs collected/processed in ambient air followed the same trends as published (6) for G-CSF-, AMD3100/Plerixafor- and AMD3100/ Plerixafor plus G-CSF-BM, with the combination of AMD3100/ Plerixafor plus G-CSF showing clear synergy over that of combination of the G-CSF and AMD3100/Plerixafor each alone (Fig. 3i). Importantly, the low oxygen collected and processed mPB LT- HSCs and ST-HSCs from donor mice given AMD3100/Plerixafor plus G-CSF were superior to these cells collected in air (Fig. 3i and 3ii). For MPPs, there was synergy for the MPP groups collected/processed at 3% O2 vs. ambient O2 but the 3% O2 groups were significantly decreased compared to ambient air (Fig. 3iii). All the groups (G-CSF, AMD3100/Plerixafor, and G-CSF plus AMD3100/Plerixafor) of functional HPCs, as defined by colony assay, showed enhanced mPB numbers in order of G-CSF plus AMD3100/Plerixafor > G-CSF>AMD3100/Plerixafor, for the ambient air groups, with each greater than that of the hypoxia groups (fig. 3iv–vi).
Figure 3. Numbers of G-CSF, AMD3100/Plerixafor, and G-CSF plus AMD3100/Plerixafor mobilized HSCs and HPCs per ml blood.
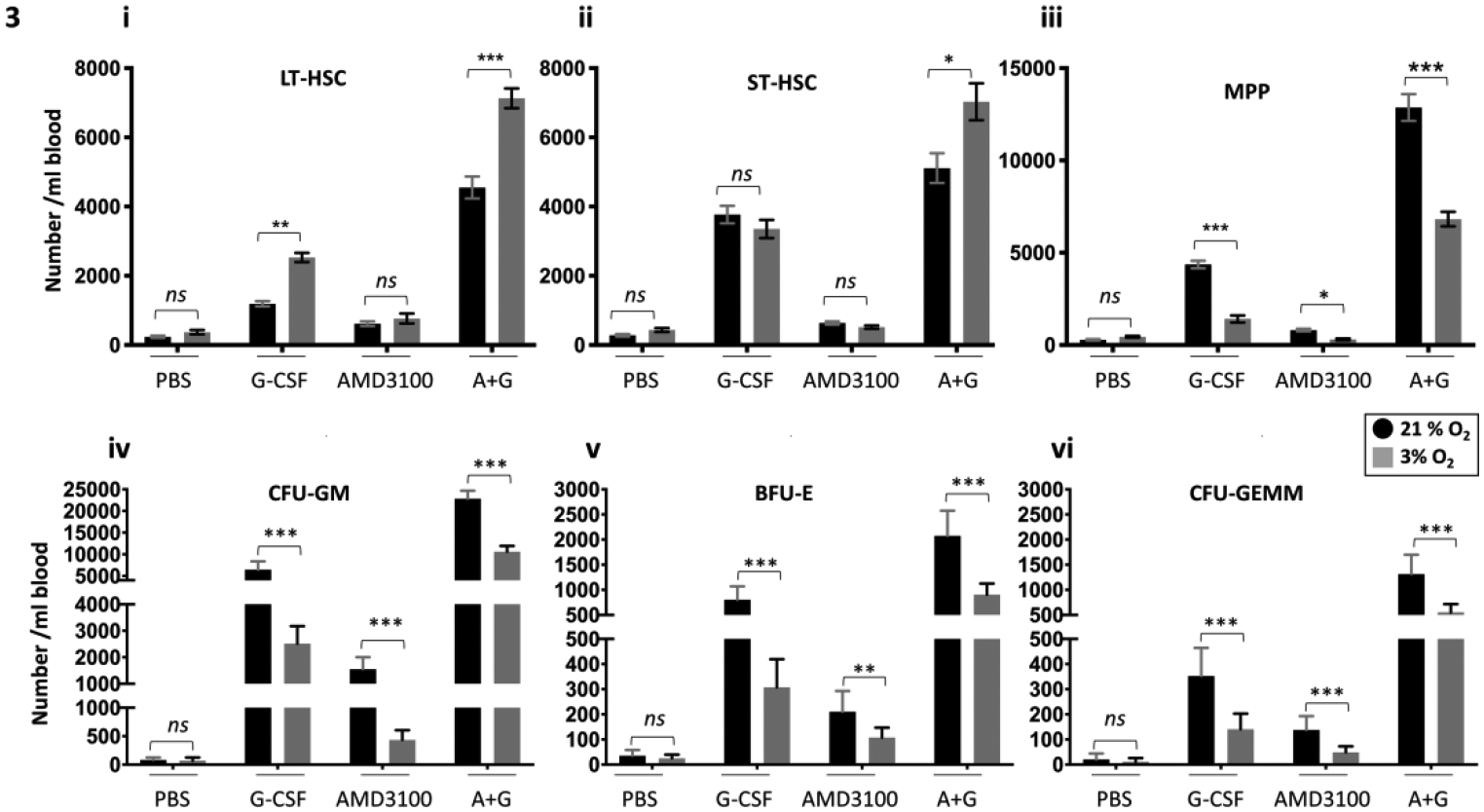
A combination of three independent experiments is shown; each experiment included three mice. PB and BM were harvested and processed in a hypoxic chamber (3% O2, 5% CO2, N2) or ambient air (~21% O2) from G-CSF and/or AMD3100/Plerixafor mobilized C57BL/6 mice and analyzed for HSC and HPC numbers. Numbers of LT-HSCs (Ai), ST-HSCs (Aii), and MPPs (Aiii) per ml blood were assessed by flow cytometry. Progenitor cell numbers were analyzed using a functional HPC colony assay examining CFU-GM (iv), BFU-E (v), and CFU-GEMM (vi) in blood. Data are presented as mean± SEM. *p < 0.05, **p < 0.05, ***p < 0.005, when analyzed by Student’s t test.
Competitive transplantation assays were performed to assess engrafting and repopulating capacity of G-CSF- and AMD3100/Plerixafor- mPB HSCs. In similar manner to G-CSF mobilized HSCs, G-CSF plus AMD3100/Plerixafor mPB donor cells collected and processed in 3% O2 showed significantly higher engraftment at month 4 in PB (Fig. 4i) and BM (Fig. 4ii). LDA demonstrated significantly increased CRUs in the 3%O2 collection/processing group compared to that of ambient air in PB (Fig. 4iii) and BM (Fig. 4iv). The hypoxia collected/processed donor cells also demonstrated increased numbers of self-renewing HSCs as shown by significantly increased chimerism in secondary mice at month 2 (Fig. 4v).
Figure 4. Engrafting efficiency of G-CSF plus AMD3100/Plerixafor mPB collected and processed in 3% O2 vs. ambient air (~21% O2), assessed by competitive BM transplantation and limiting dilution analysis.
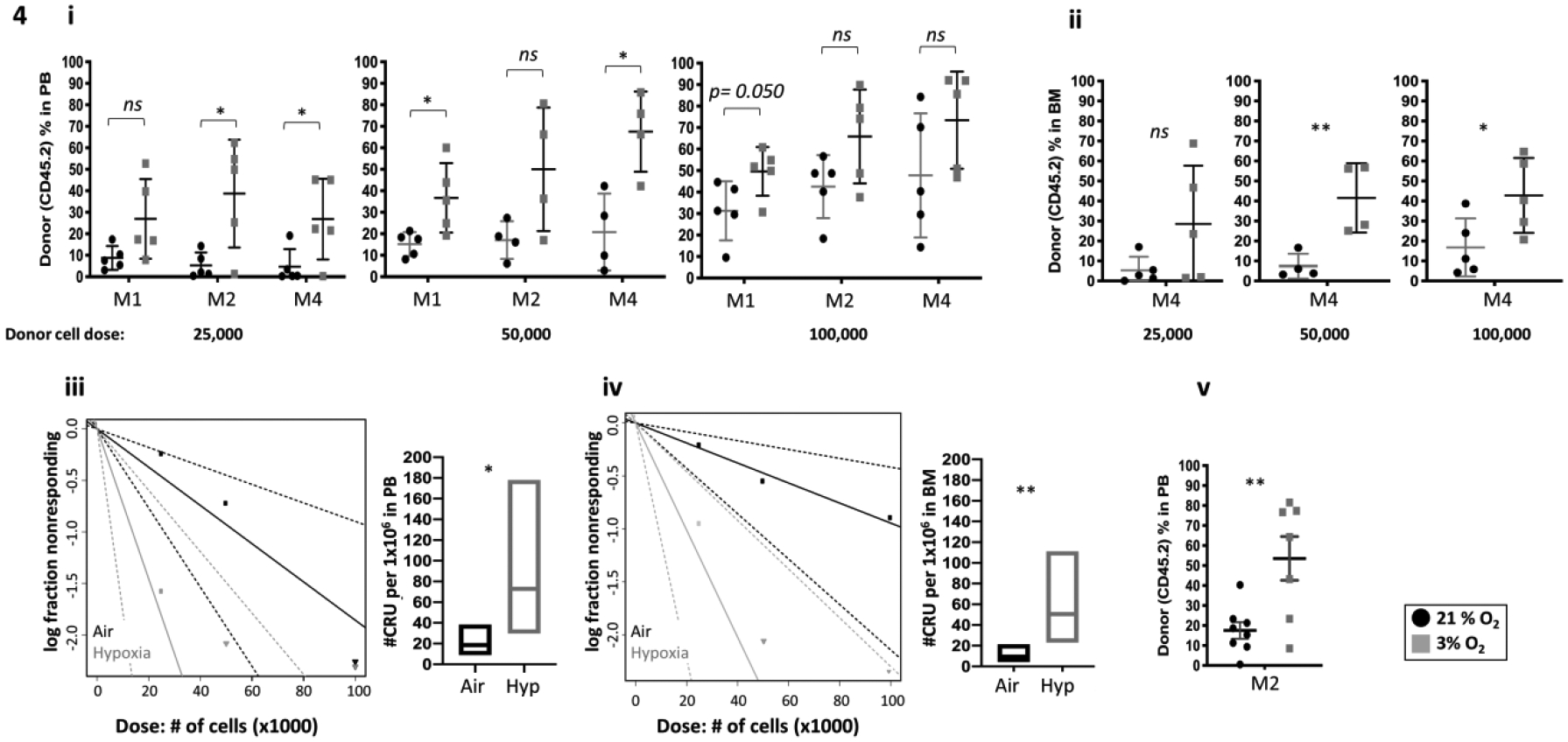
A single transplant is shown, n=5 recipient per group. Donor G-CSF and AMD3100/Plerixafor mobilized PB cells (CD45. 2+) were injected i.v. at doses of 25,000, 50,000, and 100,000 cells, into lethally irradiated F1 host mice (CD45.1+CD45.2+) in air or hypoxia. Competitor Boy/J BM cells (CD45.1+) were injected i.v. at 100,000 cell in air. The percentage of donor-derived cells in the PB was assessed by flow cytometry at months 1, 2 and 4 (i). The percentage of donor-derived cells in the BM was assessed at month 4 (ii). Poisson statistical analysis of CRUs in 106 cells (iii and iv). Engraftment in secondary recipients at month 2 (v). Data are presented as mean± SD. *p < 0.05, **p < 0.05, ***p < 0.005, when analyzed by Student’s t test.
Hypoxia signaling plays a crucial role in HSC biology (27). Traditional studies of HSC function until recently have been conducted after their collection and processing in an ambient air atmosphere that greatly underestimates numbers of HSCs (15). As reported in mouse BM and human cord blood HSC, mPB HSC and HPC in 3% O2 results in an increase in HSC number and a decrease in multipotent and committed progenitors. Conversely, collection and processing in ambient air revealed an increased number of multipotent and committed progenitors and reduced HSC numbers (15). The sudden reoxygenation of HSCs serves as a stress-induced mechanism when cells are collected in ambient air, resulting in decreased LT-HSC numbers via rapid differentiation to downstream progenitors by a phenomenon that we termed Extra Physiologic Oxygen Shock/Stress EPHOSS). Cell surface protein phenotyping does not provide an accurate representation of the LT-HSC or progenitors multi-engraftment potential. However, the phenotyped- LT-HSC population includes a higher number of long-term engrafting and self-repopulating capability cells than downstream progenitors population (28).
The enhanced engraftment of HSC collected and processed in hypoxia can be attributed to multiple hypoxia-induced events. In mouse BM, we reported a mechanism that links ROS production to cyclophilin D recruitment to the inner mitochondrial membrane, mitochondrial permeability transition pore, and p53. Using mouse gene deletion models, we found the deletion of hif1-α and miR210 cancel hypoxia’s protective effect (15). Although the CXC chemokine receptor 4 (CXCR4) expression was decreased in mouse bone marrow LT-HSC collected and processed in hypoxia (15), CXCR4 and CXCl12 expression were increased in hypoxia in other cell types such as mononuclear phagocytes, endothelial (29), acute myeloid leukemia (AML) cell line and PB-AML cells (30) as a result of Hypoxia-inducible factor (hif1-α) stabilization. Furthermore, hif1-α promotes the activation of Nlrp3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome in conditions associated with low oxygen tension; acute lung injury (31), venous thrombosis (32) and ischemic stroke (33). In mouse HSC and HPC, Nlrp3 activation induces a release of extracellular adenosine triphosphate (eATP), which incorporates CXCR4 into membrane lipid rafts of the transplanted HSC and HPC (34). Additionally, hif1-α stabilization using Prolyl Hydroxylase Domain (PHD) inhibitor (FG-4497) was reported to enhance HSC mobilization in response to vascular endothelial growth factor receptor-2 (VEGFR2) signaling in BM endothelial cells (35).
We tested our method of collection and processing cells in 3% O2 to see if collection and processing of mPB in hypoxia could also result in increased numbers of HSCs. We found that collection and processing of G-CSF- AMD3100/Plerixafor- and G-CSF plus Plerixafor- mPB in 3% O2 resulted in significantly increased numbers of HSC and enhanced multi-lineage engraftment of HSC for the G-CSF and the G-CSF plus AMD3100/Plerixafor groups.
A primary determinant of autologous HCT success, is the ability to collect a minimum of 2×106 CD34+ cells/kg of the patient’s body weight (36). With advancements of using CRISPR-cas9 applications and reduced toxicity conditioning regimens, gene therapy and autologous HCT procedures are anticipated to expand the use of mPB to additional patient populations. Here, we propose a means to further enhance collection of mPB HSCs, when the mPB cells are collected/processed at 3% O2 vs. ambient air O2 levels. While collection and processing of mPB cells in hypoxia at present would present a clinical logistical problem, further research, such as using collection and processing of cells at cold (~4°C) temperatures, which mimics at least in part effects of hypoxia collection/processing (37), as well as other yet to be defined means could have practical implications in obtaining greater numbers of mPB HSCs for transplantation.
Acknowledgements
These studies were supported by Public Health Service Grants from the National Institutes of Health to H.E.B.: R35 HL139599 and U54 DK106846. A.A. is supported by T32 DK007519 training grant to H.E.B
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement
None of the authors have any COI to report.
References
- 1.Pelus LM, & Broxmeyer HE (2018, December). Peripheral blood stem cell mobilization; a look ahead. Curr Stem Cell Rep, 4(4), 273–281. 10.1007/s40778-018-0141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan WP, Begley CG, Juttner CA, Szer J, To LB, Maher D, McGrath KM, Morstyn G, & Fox RM (1992, March 14). Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet, 339(8794), 640–644. 10.1016/0140-6736(92)90795-5 [DOI] [PubMed] [Google Scholar]
- 3.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Neben K, Goldschmidt H, & Ho AD (2010, April). Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant, 16(4), 490–499. 10.1016/j.bbmt.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 4.Olivieri J, Attolico I, Nuccorini R, Pascale SP, Chiarucci M, Poiani M, Corradini P, Farina L, Gaidano G, Nassi L, Sica S, Piccirillo N, Pioltelli PE, Martino M, Moscato T, Pini M, Zallio F, Ciceri F, Marktel S, Mengarelli A, Musto P, Capria S, Merli F, Codeluppi K, Mele G, Lanza F, Specchia G, Pastore D, Milone G, Saraceni F, Di Nardo E, Perseghin P, & Olivieri A (2018, April). Predicting failure of hematopoietic stem cell mobilization before it starts: the predicted poor mobilizer (pPM) score. Bone Marrow Transplant, 53(4), 461–473. 10.1038/s41409-017-0051-y [DOI] [PubMed] [Google Scholar]
- 5.Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E, Hosing C, Negrin R, Petersen FB, Rondelli D, Soiffer R, Leather H, Pazzalia A, & Devine S (2014, March). Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant, 20(3), 295–308. 10.1016/j.bbmt.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, & Srour EF (2005, April 18). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med, 201(8), 1307–1318. 10.1084/jem.20041385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, & Dale DC (2003, October 15). Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood, 102(8), 2728–2730. 10.1182/blood-2003-02-0663 [DOI] [PubMed] [Google Scholar]
- 8.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, Christensen J, Wood B, Price TH, & Dale DC (2005, March). Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion, 45(3), 295–300. 10.1111/j.1537-2995.2005.04222.x [DOI] [PubMed] [Google Scholar]
- 9.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G, & Investigators. (2009, October 1). Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol, 27(28), 4767–4773. 10.1200/JCO.2008.20.7209 [DOI] [PubMed] [Google Scholar]
- 10.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G, & Investigators. (2009, June 4). Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood, 113(23), 5720–5726. 10.1182/blood-2008-08-174946 [DOI] [PubMed] [Google Scholar]
- 11.Russell N, Douglas K, Ho AD, Mohty M, Carlson K, Ossenkoppele GJ, Milone G, Pareja MO, Shaheen D, Willemsen A, Whitaker N, & Chabannon C (2013, February). Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: results of the prospective PREDICT trial. Haematologica, 98(2), 172–178. 10.3324/haematol.2012.071456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micallef IN, Stiff PJ, Nademanee AP, Maziarz RT, Horwitz ME, Stadtmauer EA, Kaufman JL, McCarty JM, Vargo R, Cheverton PD, Struijs M, Bolwell B, & DiPersio JF (2018, June). Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report. Biol Blood Marrow Transplant, 24(6), 1187–1195. 10.1016/j.bbmt.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean-Sebastien Diana SM, Thierry Leblanc, Chloé Couzin, Alessandra Magnani, Elisa Magrin, Matthieux Bendavid, Laure Joseph, Marianne Delville, Stephane Blanche, Jean Soulier, Marina Cavazzana, Francois Lefrere. (2019). A New Step in Understanding of Fanconi Patients Peripheral Stem Cell Harvesting, a Bridge to Gene Therapy. Blood, 134 [Google Scholar]
- 14.Shaughnessy P, Chao N, Shapiro J, Walters K, McCarty J, Abhyankar S, Shayani S, Helmons P, Leather H, Pazzalia A, & Pickard S (2013, September). Pharmacoeconomics of hematopoietic stem cell mobilization: an overview of current evidence and gaps in the literature. Biol Blood Marrow Transplant, 19(9), 1301–1309. 10.1016/j.bbmt.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, Haas DM, Falah N, Kapur R, Pelus LM, Bardeesy N, Fitamant J, Ivan M, Kim KS, & Broxmeyer HE (2015, June 18). Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell, 161(7), 1553–1565. 10.1016/j.cell.2015.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Hoggatt J, O’Leary HA, Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag S, Rohrabaugh SL, Ou X, Speth J, Pelus LM, Srour EF, & Campbell TB (2012, December). Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med, 18(12), 1786–1796. 10.1038/nm.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doulatov S, Notta F, Laurenti E, & Dick JE (2012, February 3). Hematopoiesis: a human perspective. Cell Stem Cell, 10(2), 120–136. 10.1016/j.stem.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Cooper SH, et al. (2020). Experimental mouse models of mouse and human hematopoietic stem cell transplantation. In Pelus LM, and Hoggatt J (Ed.), Methods in Molecular Biology; Hematopoietic Stem Cells. Springer. Nature. [Google Scholar]
- 19.Brecher G, Bookstein N, Redfearn W, Necas E, Pallavicini MG, & Cronkite EP (1993, July 1). Self-renewal of the long-term repopulating stem cell. Proc Natl Acad Sci U S A, 90(13), 6028–6031. 10.1073/pnas.90.13.6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purton LE, & Scadden DT (2007, September 13). Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell, 1(3), 263–270. 10.1016/j.stem.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Yao C, Teng Y, Jiang R, Huang X, Liu S, Wan J, Broxmeyer HE, & Guo B (2019, December). Phorbol ester induced ex vivo expansion of rigorously-defined phenotypic but not functional human cord blood hematopoietic stem cells: a cautionary tale demonstrating that phenotype does not always recapitulate stem cell function. Leukemia, 33(12), 2962–2966. 10.1038/s41375-019-0528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capitano ML, et al. (2020). The IL-33 Receptor/ST2 Acts as a Positive Regulator of Functional Mouse Bone Marrow Hematopoietic Stem and Progenitor Cells. Blood Cells Mol Dis(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, Kollet O, Cohen NN, Margalit R, Buss EC, Baleux F, Oishi S, Fujii N, Larochelle A, Dunbar CE, Broxmeyer HE, Frenette PS, & Lapidot T (2011, August). Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia, 25(8), 1286–1296. 10.1038/leu.2011.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, & Stroncek DF (2009, September 17). Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood, 114(12), 2530–2541. 10.1182/blood-2009-04-214403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaugler B, Arbez J, Legouill S, Tiberghien P, Moreau P, Derenne S, Saas P, & Mohty M (2013, July). Characterization of peripheral blood stem cell grafts mobilized by granulocyte colony-stimulating factor and plerixafor compared with granulocyte colony-stimulating factor alone. Cytotherapy, 15(7), 861–868. 10.1016/j.jcyt.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 26.Lidonnici MR, Aprile A, Frittoli MC, Mandelli G, Paleari Y, Spinelli A, Gentner B, Zambelli M, Parisi C, Bellio L, Cassinerio E, Zanaboni L, Cappellini MD, Ciceri F, Marktel S, & Ferrari G (2017, April). Plerixafor and G-CSF combination mobilizes hematopoietic stem and progenitors cells with a distinct transcriptional profile and a reduced in vivo homing capacity compared to plerixafor alone. Haematologica, 102(4), e120–e124. 10.3324/haematol.2016.154740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Trinh T, Aljoufi A, & Broxmeyer HE (2018, June). Hypoxia Signaling Pathway in Stem Cell Regulation: Good and Evil. Curr Stem Cell Rep, 4(2), 149–157. 10.1007/s40778-018-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen JL, & Weissman IL (2001, December 4). Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A, 98(25), 14541–14546. 10.1073/pnas.261562798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, & Sica A (2003, November 3). Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med, 198(9), 1391–1402. 10.1084/jem.20030267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, & Andreeff M (2009, February 12). CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood, 113(7), 1504–1512. 10.1182/blood-2008-06-161539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang JJ, Xia J, Huang LL, & Li YC (2019, October). HIF1alpha promotes NLRP3 inflammasome activation in bleomycininduced acute lung injury. Mol Med Rep, 20(4), 3424–3432. 10.3892/mmr.2019.10575 [DOI] [PubMed] [Google Scholar]
- 32.Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, & Ashraf MZ (2017, May 2). Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A, 114(18), 4763–4768. 10.1073/pnas.1620458114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Q, Stone CR, Geng X, & Ding Y (2018, October-Dec). Hypoxia-inducible factor-1 alpha and RIP3 triggers NLRP3 inflammasome in ischemic stroke. Brain Circ, 4(4), 191–192. 10.4103/bc.bc_35_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamiak M, Abdel-Latif A, Bujko K, Thapa A, Anusz K, Tracz M, Brzezniakiewicz-Janus K, Ratajczak J, Kucia M, & Ratajczak MZ (2020, July 13). Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts. Stem Cell Rev Rep. 10.1007/s12015-020-10005-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisht K, Brunck ME, Matsumoto T, McGirr C, Nowlan B, Fleming W, Keech T, Magor G, Perkins AC, Davies J, Walkinshaw G, Flippin L, Winkler IG, & Levesque JP (2019, February 12). HIF prolyl hydroxylase inhibitor FG-4497 enhances mouse hematopoietic stem cell mobilization via VEGFR2/KDR. Blood Adv, 3(3), 406–418. 10.1182/bloodadvances.2018017566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, Sutherland DR, & Chin-Yee IH (2002, June). Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant, 29(12), 967–972. 10.1038/sj.bmt.1703575 [DOI] [PubMed] [Google Scholar]
- 37.Broxmeyer HE, Cooper S, & Capitano ML (2020, June 14). Enhanced collection of phenotypic and engrafting human cord blood hematopoietic stem cells at 4 degrees C. Stem Cells. 10.1002/stem.3243 [DOI] [PMC free article] [PubMed] [Google Scholar]


