Abstract
An association between early CD4+ T cell immune reconstitution (IR) and survival after T-replete allogenic hematopoietic cell transplantation (HCT) was previously reported. Here we report validation of this relationship in a separate cohort including recipients of ex vivo T cell depleted (TCD)-HCT. We studied the relation between CD4+IR and clinical outcomes.
A retrospective analysis on children/young-adults receiving their first allogenic-HCT for any indication between January-2008 and December-2017 was performed. We related early CD4+IR (defined as achieving >50 CD4+ T cells/μL on two consecutive measures within 100 days of HCT) to overall survival (OS), relapse, non-relapse mortality (NRM), event-free survival (EFS) and acute graft-versus-host-disease (aGvHD). Fine and Gray competing risk models and cox-proportional hazard models were used.
In this analysis, 315 patients with a median age of 10.4 years (IQR 5.0–16.5 years) were included. The cumulative incidence (CI) of CD4+IR at 100 days was 66.7%; in the entire cohort; 54.7% in TCD (N=208) (HR 0.47, P<0.001); 90.0% in uCB (N=40) and 89.6% in T-replete(N=47) HCT recipients. In multi-variate analyses not achieving early CD4+IR was a predictor for inferior OS (HR 2.35, 95%CI 1.46–3.79, P<0.001) and EFS (HR 1.80, 95%CI 1.20–2.69, P=0.004) and for increased NRM (HR: 6.58, 95% CI 2.82–15.38, P<0.001). No impact of CD4+IR on relapse or aGvHD was found. Within the TCD group, similar associations were observed.
In this HCT-cohort, including recipients of TCD-HCT, we confirmed that early CD4+IR was an excellent predictor for outcomes. Finding strategies to predict or improve CD4+IR may influence outcomes.
Keywords: Immune reconstitution, Hematopoietic transplant, T cell reconstitution
Introduction
For selected patients with hematological malignancies or life-threatening non-malignant hematological disorders, allogenic Hematopoietic Cell Transplantation (HCT) is a potentially curative option. Though safety and efficacy have improved, there remains considerable nonrelapse mortality (NRM), Graft-versus-Host-Disease (GvHD) and relapse. In conventional (T-replete) HCT (cHCT), early lymphocyte reconstitution has been associated with increased chances for survival, mostly driven by lower infection related NRM.[1] CD4+ T cell reconstitution (CD4+IR) appears to protect against infection-related death and disease recurrence.[2–6] In these studies, numeric and functional reconstitution of CD4+ T cell immunity has been established to correlate with outcomes across different hematopoietic transplant platforms in both pediatric and adult patients. The time to CD4+IR and the numeric threshold evaluated in these studies varied with most using evaluating the threshold of 200 CD4+ T cells/uL (extrapolated from the HIV literature) at 6 months or later. Earlier detection of (poor) CD4+IR is potentially modifiable and could impact clinical decision making.
The association between early CD4+IR and outcomes was described in pediatric recipients of T-replete allogenic HCT.[3, 7] In these analyses CD3, CD8 and NK reconstitution were not predictive of outcomes. In a retrospective landmark analysis of TCD-HCT, CD4+IR at 6 months was associated with superior outcomes in univariate analysis, but not in multivariate analysis for OS and EFS.[8] No assessment has been made of the impact of early CD4+IR on outcomes in pediatric and young adult patients undergoing ex vivo TCD HCT.
To validate the finding that „early‟ (within 100 days) CD4+IR is a predictor for outcomes in an independent cohort of patients, including recipients of TCD HCT, we performed a retrospective cohort analysis relating early CD4+ IR (as previously defined) to clinical outcomes of OS, NRM, EFS, relapse and acute GvHD (aGvHD).
Methods
Study design and population
We performed a retrospective analysis of children and young adults receiving their first allogenic HCT between January 2008 and December 2017 at Memorial Sloan Kettering Cancer Center, New York City, United States. All consecutive patients transplanted by the pediatric Bone Marrow Transplantation Service were included. No restrictions applied in terms of indication, age, remission status or comorbidities. Data was prospectively captured in the institutional BMT database. Clinical data was linked to numeric reconstitution of CD3, CD4, CD8 and NK cells measured after transplant by flow cytometry. Patients were included and data collected after Institutional Review Board (IRB) approval of this retrospective research protocol (IRB 19–379).
Outcomes of interest
The primary outcomes of interest were CD4+IR and OS. Other outcomes of interest include: NRM, EFS and grade II-IV aGvHD. We also evaluated CD8+IR, CD3+IR and Natural Killer Cell (NK) IR in relation to these outcomes. CD4+ IR was defined, as previously described as having more than or equal to 50×106 CD4+ T cells per liter blood at two consecutive measurements within 100 days of HCT.[3, 7, 9] If there was one measurement ≥ 50×106 within 100 days, and a second measurement above 50×106 within 2 months of the first measurement we considered the first time point as the time of achieving CD4+IR. If there was only one measurement, or no second measurement above 50×106 we considered that early CD4+IR was not achieved. Patients without CD4+ measurements, no second measurement within 2 months of the first, or who died before the median time of CD4+ reconstitution were excluded from analyses. The thresholds defined for CD8, CD3 and NK immune reconstitution were 50×106/L, 100×106/L and 200×106/L respectively.
OS was defined as time between HCT and date of death or date of last follow-up. NRM was defined as death not caused by relapse. EFS was defined as time between HCT and date of event or date of last-follow up in patients without an event. Relapse, NRM and subsequent infusions (boost or second transplant) were considered events. GvHD was diagnosed in accordance with the National Institutes of Health (NIH) criteria. [10]
Statistical analyses
For categorical variables, proportions were used to describe baseline patient characteristics. For all numeric variables, median and interquartile range (IQR) were calculated. Baseline characteristics of excluded patients were compared with included patients using the two-proportions z-test with continuity correction for proportions. The Fisher’s Exact probability test was used for observed values under 5.
Comparisons were made between three graft types: 1) conventional T replete HCT (cHCT) including recipients of both bone marrow (BM) and peripheral blood stem cells (PBSC); 2) unrelated cord blood (uCB) and 3) TCD BM or PBSC. For the purposes of this analysis, haploidentical TCD in combination with uCB was included with uCB and matched sibling conventional BM in combination with related uCB (private bank stored) was included with conventional BM. For TCD HCT, depletion of T cells was performed ex vivo. We assessed potential differences between three different TCD techniques used (soybean agglutination and sheep erythrocyte depletion; CD34+ selection using ISOLEX 300i Magnetic Cell Separator with sheep erythrocyte depletion; and CD34+ selection using CliniMacs, Miltenyi. In the patient cohort in this study, ATG was infrequently used in conventional transplant regimens, was not used in regimens for cord blood HCT, and was almost uniformly used in regimens for TCD HCT for prevention of rejection. Cumulative incidence (CI) of CD4+IR was plotted according to different graft types using Fine and Gray competing risk models. For NRM, relapse-related death was considered a competing event. For aGvHD, death was considered a competing event. CD4+ T cell count was assessed as a time-dependent variable. EFS and OS were plotted as Kaplan-Meier survival curves. The log-rank test was used to calculate P values.
Predictor analysis was done using cox-proportional hazard models. CD4+ IR was assessed as a categorical variable. Other variables considered are shown in Tables 1,2 and 5S. P values < 0.05 were considered statistically significant and included in multivariate analysis. If HLA mismatch and donor type were both associated with an outcome, only donor type was included in multivariate analysis. Univariate analysis and multivariate analysis were also performed to identify predictors for CD4 IR (Table 1S and 2S)
Table 1.
Characteristics of Included Patients.
| All patients (N=315) | |
|---|---|
| Sex, female (%, n) | 40.3% (127) |
| Median age at HCT (years, IQR) | 10.4 (5.0 – 16.5) |
| Year of transplantation | |
| – 2008 – 2011 | 41.9% (132) |
| – 2012 – 2014 | 30.2% (95) |
| – 2015 – 2017 | 27.9% (88) |
| Diagnosis | |
| – Malignancy | 69.8% (220) |
| – AML | 25.1% (79) |
| – ALL | 31.4% (99) |
| – Other (CML, JMML, Leukemia other, FAζ, MDS, NHL/HD, Sarcoma) | 13.3% (42) |
| – Non-malignant | 30.2% (95) |
| – Group 1: ALD, SSD, Thal, SCN, CGD, | 5.4% (17) |
| – Group 2: DBA, CMT, DC,FA, SAA, PNH, SAA, SDS | 12.1% (38) |
| – Group 3: CID, SCID, HLH, LAD, WAS, AHA | 12.7% (40) |
| Disease type malignancy ALL vs AML | |
| – ALL in CR1/CR2 | 27.0% (85) |
| – ALL in CR3+ or other | 4.4% (14) |
| – AML in CR1 | 14.0% (44) |
| – AML in CR2/CR3+/other | 11.1% (35) |
| Stem cell source (%, n) | |
| – Unmodified (T-replete) graft | 18.7% (59) |
| -Bone Marrow + Cord Blood | 2.5% (8) |
| – T cell depleted graft* | 66.0% (208) |
| – Cord blood | 12.7% (40) |
| Donor relation / match | |
| – MMRD | 8.9% (28) |
| – MMUD | 24.4% (77) |
| – MRD | 28.6% (90) |
| – MUD | 25.4% (80) |
| – Cord blood | 12.7% (40) |
| CMV serostatus recipient/donor | |
| – R-/D- | 37.1% (117) |
| – R-/D+ | 12.4% (39) |
| – R+/D- | 19.7% (62) |
| – R+/D+ | 30.8% (97) |
| Conditioning | |
| – Ablative | 95.2% (300) |
| – Non-ablative | 3.5% (11) |
| – None | 1.3% (4) |
| Conditioning regimen | |
| – Chemotherapy based± | 63.2% (199) |
| – TBI basedº | 35.6% (112) |
| – None | 1.3% (4) |
| Follow-up time (years, IQR) | 3.2 (1.3 – 6.0) |
Chemotherapy include different combination of following agents
Busulfan/Cyclophosphamide/Clofarabine/Fludarabine/Melphalan/Mesna/Thio-TEPA
In combination with chemotherapy
Nine FA (2.9%) patients with malignancy were included in the malignancy group.
the following techniques for ex vivo TCD were used: Soybean Agglutination (SBA-E-; n=30), Isolex/E- (n=33) and CliniMACs (n=145; see methods).
Table 2.
Multivariate analysis for NRM, OS, EFS and aGvHD.
| Variable | N = | Multivariate HR | 95% CI | P value* | ++ |
|---|---|---|---|---|---|
| OS | |||||
| Age (continuous) | 315 | 1.01 | 0.98 – 1.05 | 0.400 | |
| Malignant disease (Y/N) | 220/95 | 3.06 | 1.46 – 6.39 | 0.003 | * |
| Donor relation/match: | |||||
| – MRD (reference) | 90 | 1.00 | |||
| – MMRD | 28 | 1.79 | 0.70 – 4.54 | 0.221 | |
| – MMUD | 77 | 1.39 | 0.71 – 2.72 | 0.333 | |
| – MUD | 80 | 1.10 | 0.54 – 2.11 | 0.798 | |
| – Cord Blood | 40 | 1.97 | 0.92 – 4.22 | 0.082 | |
| TBI based conditioning (Y/N) CMV matching | 112/199 | 1.29 | 0.80 – 2.07 | 0.292 | |
| – (Mis)match CMV: R-/D- | 116 | 1.00 | |||
| – (Mis)match CMV: R-/D+ | 40 | 1.31 | 0.58 – 2.96 | 0.525 | |
| – (Mis)match CMV: R+/D- | 59 | 2.32 | 1.29 – 4.18 | 0.004 | * |
| – (Mis)match CMV: R+/D+ | 100 | 1.95 | 1.08 – 3.55 | 0.028 | * |
| CD4+ IR (N/Y) | 107/208 | 2.32 | 1.44 – 3.75 | < 0.001 | * |
| NRM | |||||
| Age (continuous) | 315 | 1.04 | 0.997 – 1.09 | 0.066 | |
| T cell depletion (Y/N) | 208/107 | 1.85 | 0.37 – 9.24 | 0.455 | |
| Donor relation/match | |||||
| – MRD | 90 | 1.00 | |||
| – MMRD | 28 | 2.07 | 0.60 – 7.11 | 0.246 | |
| – MMUD | 77 | 1.43 | 0.51 – 4.03 | 0.497 | |
| – MUD | 80 | 0.73 | 0.22 – 2.36 | 0.596 | |
| – Cord blood (unrelated) | 40 | 2.40 | 0.39 – 14.67 | 0.343 | |
| Recipient CMV serostatus (+/−) | 159/156 | 2.12 | 1.06 – 4.22 | 0.034 | * |
| CD4+ IR (N/Y) | 107/208 | 6.58 | 2.82 – 15.38 | <0.001 | * |
| EFS | |||||
| Malignant disease (Y/N) | 220/95 | 1.80 | 1.08 – 3.03 | 0.025 | * |
| T cell depletion (Y/N) | 208/107 | 1.03 | 0.51 – 2.07 | 0.943 | |
| Donor relation/match: | |||||
| – MRD (reference) | 90 | 1.00 | |||
| – MMRD | 28 | 2.66 | 1.19 – 5.97 | 0.018 | * |
| – MMUD | 77 | 1.92 | 0.98 – 3.77 | 0.056 | |
| – MUD | 80 | 1.67 | 0.87–1.99 | 0.122 | |
| – Cord blood (unrelated) | 40 | 1.72 | 0.78 – 3.78 | 0.177 | |
| TBI based conditioning (Y/N) CMV (mis)match | 112/199 | 0.76 | 0.88 – 1.99 | 0.185 | |
| – R-/D- (reference) | 116 | 1.00 | |||
| – R-/D+ | 40 | 0.97 | 0.49 – 1.95 | 0.938 | |
| – R+/D- | 59 | 1.86 | 1.12 – 3.08 | 0.016 | * |
| – R+/D+ | 100 | 1.50 | 0.92 – 2.44 | 0.013 | |
| No CD4IR (no/yes) | 107/208 | 1.79 | 1.20 – 2.68 | 0.005 | * |
| aGvHD | |||||
| Age categorical: < 10.15 yrs | 155 | 1.00 | |||
| Age categorical: > 10.15 yrs | 160 | 1.46 | 0.88 – 2.42 | 0.140 | |
| malignant/non-malignant) | 220/95 | 1.90 | 0.95 – 3.80 | 0.069 | |
| T cell depletion (Y/N) | 208/107 | 0.56 | 0.25 – 1.25 | 0.157 | |
| Donor relation/match | |||||
| – MRD (reference) | 90 | 1.00 | |||
| – MMRD | 28 | 1.91 | 0.63 – 5.79 | 0.256 | |
| – MMUD | 77 | 1.80 | 0.75 – 4.36 | 0.191 | |
| – MUD | 80 | 1.70 | 0.75 – 3.83 | 0.203 | |
| – Cord Blood | 40 | 3.57 | 1.64 – 7.77 | 0.001 | |
| * | |||||
Significance level, p< 0.0
Cumulative incidence curves of NRM and GvHD, and Kaplan-Meier curves of OS and EFS were plotted according to CD4+IR at day 100. The curves were adjusted for significant predictors from multivariate analysis in order to achieve equality of risk in all groups, using competing risk regression. Analyses were performed with R version 1.2.1335 (R core team, Austria, 2018), using the following packages; ggplot2, lme4, dplyr, plyr, survival, cmprsk, survminer, cr17.
Results
Study population
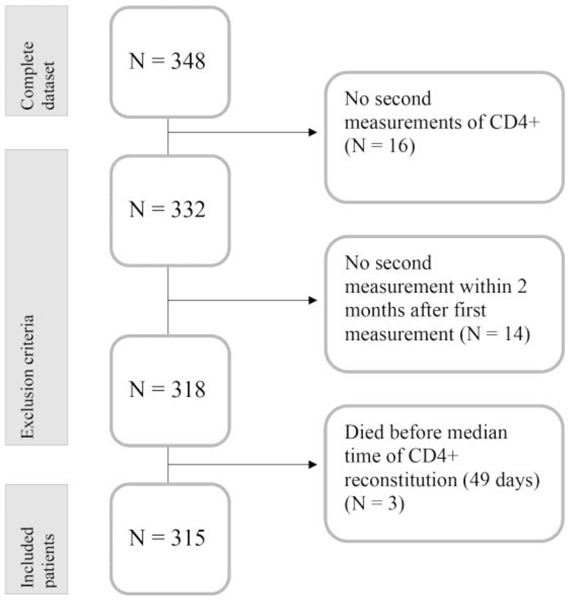
A total of 348 patients were considered for analysis of whom 33 (9.5%) were excluded (Figure 1) Thirty excluded patients had no second measurement of CD4+ T cells (N=16; 4.6%) or no second measurement within 2 months of the first (N=14; 4.0%) and 3 (0.9%) patients had measurements performed but had not achieved CD4+IR when they died before the median time of CD4+IR. The majority of excluded patients died (Table 1S, N=27, 81.8%).
Figure 1.
Outline of patients included and excluded from analysis
The most frequent causes of death in these patients included relapse (N=5, 15.2%), infection (N=6, 18.2%), GvHD (N=5, 15.2%), and multi-organ failure (N=5, 15.2%) for NRM in these 33 patients of 61% (Table 1S). Excluded patients less frequently received TCD HCT (45.5% versus 66% p=0.031) and there were no differences in diagnosis between excluded and included patients.
Median age at HCT was 10.4 years (IQR 5.0 – 16.5 years); malignancy (220/315, 69.8%) was the most frequent indication for HCT. Median follow-up time was 3.2 years (IQR 1.3 – 6.0) (Table 1). For the purposes of this analysis the conditioning regimens were divided into total body irradiation (TBI) and non TBI-based. GvHD-prophylaxis consisted of in vitro TCD (208/315, 66.0%) with CD3 < 10e4/kg (with (N=188) or without ATG. Recipients of conventional transplant the majority of whom were matched sibling HCT, received calcineurin inhibitor based prophylaxis (cyclosporine or tacrolimus) with methotrexate (15mg/m2 on day +1, 10mg/m2 on Day+3,+6 and +11). Recipients of cord blood transplant received CsA/mycophenylate (MMF) prophylaxis (N=40).
Outcomes
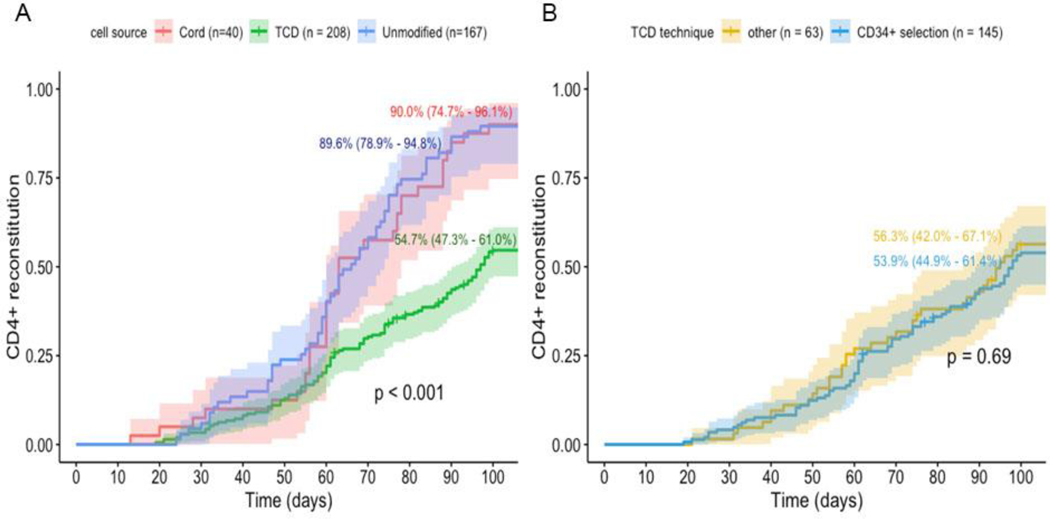
Median number of CD4+ T cell measurements during follow-up was 8 (range 1– 52), with a median of 4 (range 1–15) measurements performed within 100 days. For the 315 patients included in the analyses, the CI of CD4+ IR at 100 days was 66.7% (95% CI 61.0% - 71.6%) and median time to CD4+IR was 49 days (IQR 32–62 days). Both CI of CD4+IR and median time to CD4+IR differed by graft type and were inferior in recipients of TCD HCT. In uCB transplants median time to CD4+IR was 30 days (IQR 31 – 59), in cHCT median time to CD4+IR was 40 days (IQR 31–59days), and in TCD HCT median time to CD4+IR was 57days (IQR 39–74days). For TCD the CI of CD4+ IR was 54.7% (95% CI 47.3–61.0%), while for uCB this was 90.0% (95% CI 74.7–96.1%) and for T-replete BM 89.6% (95% CI 78.9–94.8%; Figure 2A). No difference in CD4+IR was found associated with TCD technique (CliniMaCs vs Isolex or SBA-E-Figure 2B).
Figure 2.
A. Cumulative incidence (CI) CD4+ T cell reconstitution according to transplant type / manipulation (cell source). CD4+ IR was defined as having greater than or equal to 50×106 CD4+ T cells/L in 2 consecutive measurements within 100 days after SCT. B. Cumulative incidence of CD4+ T cell reconstitution according to TCD technique: “Isolex/E- and SBA-E-- combined‟ versus CliniMACS, Miltenyi.
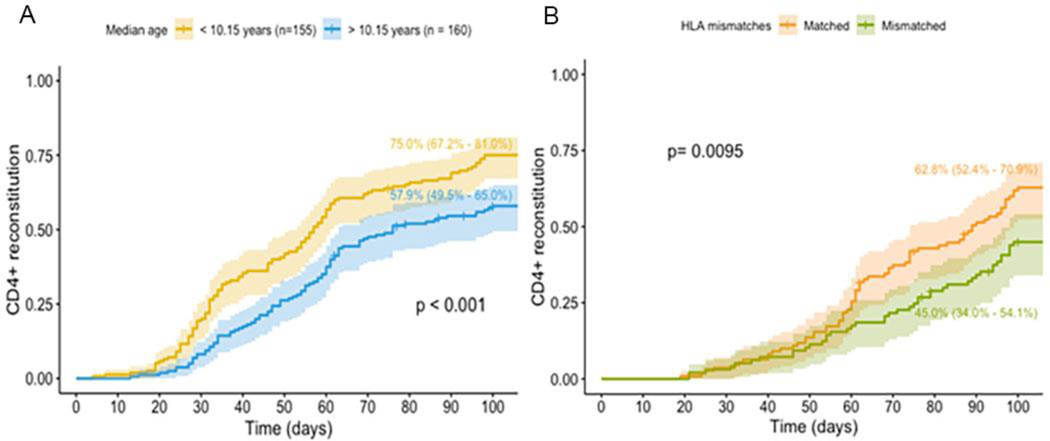
Older age at HCT (Hazard ratio (HR): 0.97, 95% CI 0.95 – 0.99; p=0.002; Figure 3A) and transplant from an HLA mismatched donor (45 vs 62.8% p=.0095. Figure 3B). were independent multivariate (MV) predictors of not achieving CD4+IR (Table 3).
Figure 3.
A. Cumulative incidence CD4+ T cell reconstitution according to median age in whole cohort. B. Cumulative incidence CD4+ T cell reconstitution according to HLA-matching in patients undergoing HCT with a TCD graft.
Table 3.
Multivariate analysis of CD4+ T cell IR (immune reconstitution).
| End point | N | = HR | 95% CI | P value | Significance level |
|---|---|---|---|---|---|
| Age (continue) | 315 | 0.97 | 0.95 – 0.99 | 0.002 | * |
| Transplant type (cell source) | |||||
| – T replete (BM/Cord, Cord, Unmodified graft) (reference) | 107 | 1.00 | |||
| – TCD | |||||
| 208 | 0.47 | 0.31 – 0.70 | < 0.001 | * | |
| Donor relation / match | |||||
| – MRD (reference) | 90 | 1.00 | |||
| – MMRD | 28 | 0.58 | 0.30 – 1.10 | 0.094 | |
| – MMUD | 77 | 0.50 | 0.31 – 0.82 | 0.005 | * |
| – MUD | 80 | 0.86 | 0.58 – 1.28 | 0.452 | |
| – Cord blood (unrelated) | 40 | 1.13 | 0.74 – 1.72 | 0.570 | |
Patients receiving uCB or TCD HCTs had lower OS (p =0.017) and EFS (p=0.024) compared to children receiving a cHCT. CI of NRM was higher (p=0.011) in patients receiving TCD transplants (16.7%, 95% CI 11.3–22.1%) compared to cHCT (1.5%, 95% CI 0.0–4.4%) or uCB HCT (5.0%, 95% CI 0.0–11.8%). (Table 2 and Table 2S ) The most frequent causes of NRM were GvHD (13/37, 35.1%) and infection (10/37, 27.0%) (Table 3S). EFS was lower in TCD and uCB HCT (p=0.024) compared to cHCT. In patients with a malignancy as the indication for HCT, graft type was not associated with relapse; however the uCB group had a higher incidence of relapse since the indication for transplant was more commonly malignancy in the uCB group (37/40) than in the unmodified (32/67) or TCD group (151/208) the lower EFS and OS related to relapse. In addition, achieving or not achieving CD4+IR was not associated with relapse (Supplemental Figure 1S). Incidence of grade II-IV aGvHD was higher for patients receiving an uCB (52.5%, 95% CI 36.7–68.3%) compared to TCD (17.8%, 95% CI 12.6–23.0%) and cHCT (17.9%, 95% CI 8.7–27.2%; p< 0.001).
Outcomes according to CD4 IR
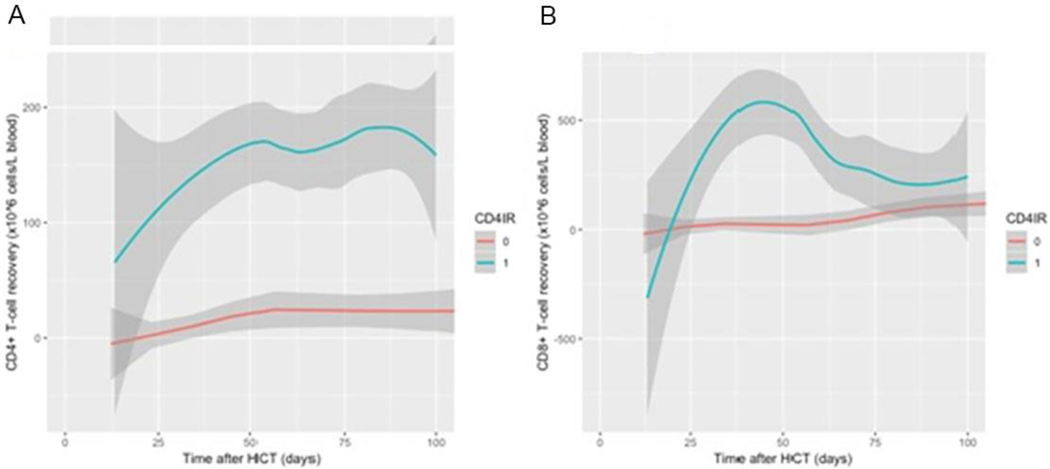
In patients achieving versus not achieving CD4+IR, median CD4+ T cell count at day 100 after HCT was 117.5×106/L and 0.0×106/L with non-overlapping interquartile ranges (IQR 74.0 – 179.3) and IQR 0.0 – 11.0). Continues measurement of CD4+ and CD8+ by CD4+IR also demonstrates that early CD4+IR is a marker of subsequent robust CD4 and CD8 reconstitution (Figure 4).
Figure 4.
A Continuous CD4+ T cell recovery (x106 T cells) within 100 days after transplantation, according to CD 4 reconstitution (no CD4+IR in red line “0” and CD4+IR in blue line “1”) . B. Continuous values of CD8+ T cell recovery (x106 T cells) within 100 days after transplantation, according to CD 4 reconstitution (no CD4+IR in red line “0” and CD4+IR in blue line “1”)
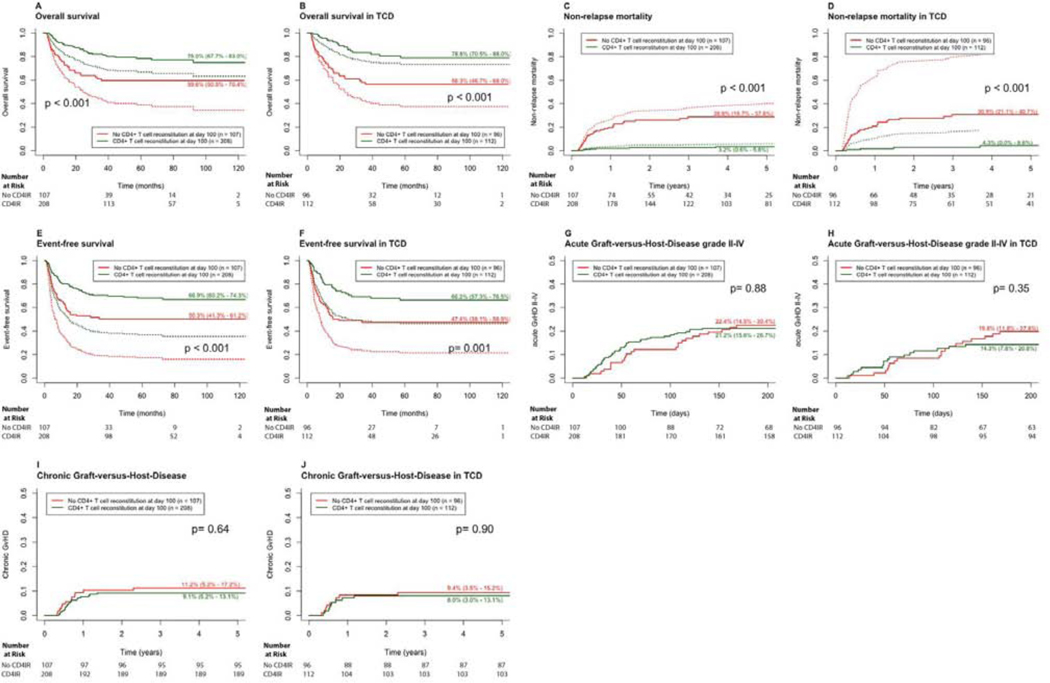
As demonstrated in Figure 5 (A,C, and E) patients without CD4+IR had lower OS (59.6%) and EFS (50.3%) and higher NRM (28.8%), compared to patients with CD4+IR (75.0%, 66.9% and 3.2% respectively; p < 0.001). In univariate and multivariate analysis (Table 2, 2S and 5S), CD4+IR was an independent predictor for OS (HR: 2.35, 95% CI 1.46 – 3.79, p < 0.001), EFS (HR: 1.80, 95% CI 1.20 – 2.69, p=0.004) and NRM (HR: 6.57, 95% CI 2.82 – 15.38, p < 0.001). In a subset analysis of TCD HCT recipients only (Figure 5B,D and F), achieving CD4+IR was similarly associated with superior OS (p < 0.001), NRM (p < 0.001), and EFS (p=0.001). No associations were found between CD4+IR and aGvHD (Figure 5G, 5H).
Figure 5.
Outcomes according to CD4+ T cell IR. Plots on the left (A, C, E) show results in all (whole cohort) patients. Plots on the right (B, D, F) show results in T cell depleted patients. Red lines show patients without CD4 IR, green lines patients with CD4 IR. Dotted lines show adjusted curves; curves for NRM, OS and EFS are adjusted for significant predictors from multivariate analysis (Table 2). (A) NRM according to CD4 IR. (B) NRM according to CD4 IR in TCD. (C) OS according to CD4 IR. (D) OS according to CD4 IR in TCD. (E) EFS according to CD4 IR. (F) EFS according to CD4 IR in TCD. (G) aGvHD according to CD4 IR. (H) aGvHD according to CD4 IR in TCD. (I) cGvHD (all) according to CD4 IR. (J) cGvHD (all) according to CD4 IR in TCD.
Other predictors for OS, EFS and NRM (Tables 2 and Table 2S, Table 4S, and Table 5S) included: recipient CMV seropositivity (OS and EFS); mismatch between donor and recipient in CMV serostatus (OS and NRM); transplant for malignancy (OS and EFS); and transplant from mismatched related donors (EFS). In multivariate analysis CD3+IR was associated with these outcomes of interest, but given that CD8+IR was not, and that 95% of patients who reconstituted CD4 also reconstituted CD3, this appears to be driven by the CD4+IR. In univariate analysis, NK+IR was not associated with these outcomes of interest. In addition, higher aGvHD was only associated with uCB HCT ((HR 3.57, 95% CI 1.64 – 7.77, p = 0.001).
Discussion
In a large, independent cohort that included recipients of TCD HCT we validated that early CD4+IR, of 50/μL by day 100 after HCT, is an excellent early and simple biomarker for clinical outcomes in pediatric and young adult patients undergoing HCT. Importantly, in contrast to prior studies evaluating ALC and NK reconstitution,[1, 11] the predictive value of early CD4+IR was demonstrated across transplant indications and approaches. The incidence of early CD4+IR was found to be graft dependent, with fewer patients achieving early CD4+IR after TCD HCT, compared to uCB or cHCT. These findings stress the importance of thorough monitoring of immune reconstitution early after HCT.
The retrospective nature of this analysis is a limitation. Also, the exclusion of approximately 9% of patients who did not have adequate CD4 monitoring is not ideal. This led disproportionately to exclusion of recipients of unmodified grafts, which may have influenced the analysis. However given that 8 of 13 recipients of unmodified transplants in the excluded group were excluded because there was no second measurement of CD4+ T cells, it may have led to an underestimation of recipients of conventional HCT achieving CD4 IR. Alternatively, if one assumed that none of the 33 excluded patients achieved CD4+IR it would accentuate the finding of high NRM in patients who do not achieve CD4+IR as 61% of the excluded patients died of NRM. Thus, it is likely that the effects seen would have been more pronounced if these patients were included. Therapy prior to transplant for malignant disease could influence post-transplant immune reconstitution. Few of the patients in this cohort received CAR T cells or Blinatumomab prior to transplant, but will be interesting to evaluate prospectively. While damage to the thymus from prior therapies may impair thymic derived T cell reconstitution; at the time point of interest (100 days) we would not expect to see a significant contribution to T cell reconstitution from the thymus. In addition, as a group, the most heavily pretreated patients were the recipients of CB transplant and they all reconstituted.
The majority of patients who did not achieve early CD4+IR had extremely poor reconstitution and were recipients of TCD HCT. Early T cell reconstitution in this cohort depends on expansion of the few T cells infused with the graft or host T cells surviving conditioning. The finding that recipients of TCD HCT were less likely to achieve CD4+IR is in line with previous findings of delayed immune recovery in the setting of TCD HCT.[12, 13] The association we found between age >10 years and delayed CD4+IR is in line with a prospective study on IR, in which median CD4+ T cell counts at day 60–360 after allogeneic HCT were higher in children aged < 5 years compared to children >10 years of age. Faster immune recovery of CD4+ T cells was explained by a more dominant role of thymic CD4+ lymphopoiesis in younger children.[14] In the current analyses, the delayed CD4+IR may also be related to ATG exposure. Patients undergoing TCD HCT received weight-based dosing of ATG within 3 days of infusion of the graft. In recent studies of ATG pharmaco-kinetics (PK) /pharmaco-dynamics, patients weighing greater than 40kg (approximately weight of 10-year-old children) had high exposure of ATG. In addition, population PK analyses showed that clearance of ATG in patients above 40kg was only predicted by the absolute lymphocyte count prior to ATG dosing. Further evidence for a role of ATG in controlling the pace of immune reconstitution comes from comparing CD4+ immune reconstitution after uCB HCT performed with versus without ATG.[15–17] In the current analysis, recipients of conventional and uCB HCT did not receive ATG. Unfortunately, no samples were available to measure and prove the suggested correlation between ATG exposure and inferior CD4+IR.
In line with previous cohorts of T replete transplant recipients.[3, 7, 17] CD4+IR at day 100 was independently associated with OS, EFS and NRM. This was also in line with a prospective, randomized trial by Soiffer et al, on Anti-T-Lymphocyte Globulin (ATLG) in patients undergoing HCT.[18] Interestingly no relationship between CD4+IR at day 100 and incidence of relapse was observed. Contrarily, others found CD4+IR at day 100 to be a predictor for AML relapse-related mortality.[7, 17] The relatively low numbers of patients and a heterogenous AML population as well as selection of KIR favorable donors may have contributed to this. No association was found for CD4+IR and the incidence of aGvHD, in line with previous studies.[3, 7, 17] While a previous association has been described between CD4+ reconstitution and the subsequent development of chronic GvHD,[19] it appears that reflects a CD4+ count of greater than 200 cells/uL at a time closer to 200 days post HCT. In addition, the low incidence of chronic GvHD in both the uCB and TCD transplant groups would make such a signal difficult to detect in this cohort.
This study confirmed the finding that early CD4+IR by day 100 is an excellent predictor for OS, NRM and EFS. Importantly, this finding extends to recipients of TCD HCT. In addition to ex vivo TCD techniques, agents used in the conditioning regimen, such as ATG and Fludarabine, can influence CD4+IR in both conventional and TCD HCTs.[17, 18, 20] To aim for better IR, conditioning regimens could be optimized by individualizing dosage to target optimal exposure to achieve early CD4+IR after HCT. Including CD4+IR as a standard endpoint in patients undergoing HCT may help to guide clinical decision making. While this study did not assess the morbidity associated with infection and GvHD in patients achieving versus those not achieving CD4+IR that will be an important next step. Future studies should focus on strategies to predict and improve early CD4+ T cell reconstitution after HCT.[9, 21]
Supplementary Material
Highlights:
Early CD4+ immune reconstitution:
Defined as achieving >50 CD4+ T cells/μL on two measures within 100 days of HCT
Is an excellent predictor of outcomes after HCT, irrespective of graft type used
Has an impact on non-relapse mortality, not on relapse.
Acknowledgements:
we would like to thank Dr Rick Admiraal and Coco de Koning from the UMC Utrecht, The Netherlands for statistical training of Ichelle van Roessel.
Role of the funding source: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations:
- CD4+IR
Immune Reconstitution of 50 CD4+T cells/μL
- HCT:
hematopoietic cell Transplantation
- AHA:
autoimmune hemolytic anemia.
- ALD:
adrenoleukodystrophy.
- ALL:
acute lymphoid leukemia.
- AML:
acute myeloid leukemia.
- BM:
bone marrow.
- CGD:
chronic granulomatous disease.
- CID:
combined immunodeficiency disease.
- CML:
chronic myeloid leukemia
- CMT
congenital amegakaryocytosis
- DC
dyskeratosis congenita
- CMV:
cytomegalovirus.
- CR:
complete remission.
- D:
donor.
- DBA
Diamond Blackfan anemia
- FA:
Fanconi anemia.
- (F)HLH:
(familial) hemophagocytic lymphohistiocytosis.
- IQR:
interquartile range.
- JMML:
juvenile myelomonocytic leukemia.
- LAD:
leukocyte adhesion deficiency
- SCN:
Kostmann neutropenia
- MDS:
myelodysplastic syndrome.
- MMRD:
mismatched related donor.
- MMUD:
mismatched unrelated donor.
- MRD:
matched related donor.
- MUD:
matched unrelated donor.
- NHL/HD:
non-Hodgkin lymphoma/Hodgkin disease.
- PID:
primary immunodeficiency.
- PNH:
paroxysmal nocturnal hemoglobinuria.
- R:
recipient.
- SDS:
Schwachman-Diamond syndrome
- SD:
sickle cell disease
- SAA:
severe aplastic anemia.
- SCID:
severe combined immunodeficiency
- Thal:
Thalassemia
- TBI:
total body irradiation.
- WAS:
Wiskott Aldrich Syndrome
Footnotes
Conflict of Interest: None of authors has relevant financial interest related to this topic. Some of authors have non-related financial interest: JJB consulting Magenta, Advanced clinical, Bluebird bio, Bluerock, Omeros, Takeda, Avrobio, CK consulting Mesoblast, Novartis, SP consulting
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L, Superata J, Kurlander R, Singh A, Childs R, Barrett AJ, Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation, Biol Blood Marrow Transplant 13(10) (2007) 1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, Weissinger E, Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation, Front Immunol 7 (2016) 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Admiraal R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, Wolfs TFW, Nierkens S, Boelens JJ, Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation, J Allergy Clin Immunol 140(6) (2017) 1643–1650 e9. [DOI] [PubMed] [Google Scholar]
- [4].Berger M, Figari O, Bruno B, Raiola A, Dominietto A, Fiorone M, Podesta M, Tedone E, Pozzi S, Fagioli F, Madon E, Bacigalupo A, Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality, Bone Marrow Transplant 41(1) (2008) 55–62. [DOI] [PubMed] [Google Scholar]
- [5].Fedele R, Martino M, Garreffa C, Messina G, Console G, Princi D, Dattola A, Moscato T, Massara E, Spiniello E, Irrera G, Iacopino P, The impact of early CD4+ lymphocyte recovery on the outcome of patients who undergo allogeneic bone marrow or peripheral blood stem cell transplantation, Blood Transfus 10(2) (2012) 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB, Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation, Bone Marrow Transplant 37(12) (2006) 1119–28. [DOI] [PubMed] [Google Scholar]
- [7].Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, van Tol MJ, Knibbe CA, Bredius RG, Boelens JJ, Association between antithymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis, Lancet Haematol 2(5) (2015) e194–203. [DOI] [PubMed] [Google Scholar]
- [8].Goldberg JD, Zheng J, Ratan R, Small TN, Lai KC, Boulad F, Castro-Malaspina H, Giralt SA, Jakubowski AA, Kernan NA, O’Reilly RJ, Papadopoulos EB, Young JW, van den Brink MR, Heller G, Perales MA, Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant, Leuk Lymphoma 58(8) (2017) 1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Koning C, Langenhorst J, van Kesteren C, Lindemans CA, Huitema ADR, Nierkens S, Boelens JJ, Innate Immune Recovery Predicts CD4(+) T Cell Reconstitution after Hematopoietic Cell Transplantation, Biol Blood Marrow Transplant 25(4) (2019) 819–826. [DOI] [PubMed] [Google Scholar]
- [10].Lee SJ, Classification systems for chronic graft-versus-host disease, Blood 129(1) (2017) 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim DH, Kim JG, Sohn SK, Sung WJ, Suh JS, Lee KS, Lee KB, Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation, Br J Haematol 125(2) (2004) 217–24. [DOI] [PubMed] [Google Scholar]
- [12].Oshrine BR, Li Y, Teachey DT, Heimall J, Barrett DM, Bunin N, Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood, Biol Blood Marrow Transplant 19(11) (2013) 1581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ho VT, Soiffer RJ, The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation, Blood 98(12) (2001) 3192–204. [DOI] [PubMed] [Google Scholar]
- [14].Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, LatosGrazynska E, Krol M, Boguslawska-Jaworska J, Chybicka A, Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery, Br J Haematol 118(1) (2002) 74–89. [DOI] [PubMed] [Google Scholar]
- [15].Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, Herrera MI, Reynolds CG, Alyea EP, Ho VT, Koreth J, Armand P, Chen YB, Ballen K, Soiffer RJ, Antin JH, Cutler CS, Ritz J, Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation, Biol Blood Marrow Transplant 18(4) (2012) 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Koning C, Gabelich JA, Langenhorst J, Admiraal R, Kuball J, Boelens JJ, Nierkens S, Filgrastim enhances T-cell clearance by antithymocyte globulin exposure after unrelated cord blood transplantation, Blood Adv 2(5) (2018) 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, Boelens JJ, Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation, Blood 128(23) (2016) 2734–2741. [DOI] [PubMed] [Google Scholar]
- [18].Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, Rybka W, Artz A, Porter DL, Shea TC, Boyer MW, Maziarz RT, Shaughnessy PJ, Gergis U, Safah H, Reshef R, DiPersio JF, Stiff PJ, Vusirikala M, Szer J, Holter J, Levine JD, Martin PJ, Pidala JA, Lewis ID, Ho VT, Alyea EP, Ritz J, Glavin F, Westervelt P, Jagasia MH, Chen YB, Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation, J Clin Oncol 35(36) (2017) 4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wiegering V, Keupp A, Frietsch M, Fiessler C, Haas K, Haubitz I, Beyersdorf N, Wolfl M, Schlegel PG, Eyrich M, Role of B cells in chronic graft-versus-host disease after allogeneic stem cell transplantation in children and adolescents, Br J Haematol 186(5) (2019) e133–e137. [DOI] [PubMed] [Google Scholar]
- [20].Langenhorst JB, Dorlo TPC, van Maarseveen EM, Nierkens S, Kuball J, Boelens JJ, van Kesteren C, Huitema ADR, Population Pharmacokinetics of Fludarabine in Children and Adults during Conditioning Prior to Allogeneic Hematopoietic Cell Transplantation, Clin Pharmacokinet 58(5) (2019) 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Langenhorst J, van Kesteren C, van Maarseveen E, Kuball J, de Witte M, Nierkens S, Dorlo T, Huitema A, Boelens J-J, 440 - Individualized Fludarabine Dosing for Predictable Immune Reconstitution and Increased Survival Chances after Allogeneic Hematopoietic Cell Transplantation, Biology of Blood and Marrow Transplantation 24(3, Supplement) (2018) S306–S307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.