Abstract
Glucose and lactate provide energy for cellular function in the brain, and serve as an important carbon source in the synthesis of a variety of biomolecules. Thus, there is a critical need to quantitatively monitor these molecules in situ on a time scale commensurate with neuronal function. In this work, carbon-fiber microbiosensors were coupled with fast-scan cyclic voltammetry to monitor glucose and lactate fluctuations at a discrete site within rat striatum upon electrical stimulation of the midbrain projection to the region. Systematic variation of stimulation parameters revealed the distinct dynamics by which glucose and lactate respond to the metabolic demand of synaptic function. Immediately upon stimulation, extracellular glucose and lactate availability rapidly increased. If stimulation was sufficiently intense, concentrations then immediately fell below baseline in response to incurred metabolic demand. The dynamics were dependent on stimulation frequency, such that more robust fluctuations were observed when the same number of pulses was delivered at a higher frequency. The rates at which glucose was supplied to, and depleted from, the local recording region were dependent on stimulation intensity, and glucose dynamics led those of lactate in response to the most substantial stimulations. Glucose fluctuated over a larger concentration range than lactate as stimulation duration increased, and glucose fell further from baseline concentrations. These real-time measurements provide an unprecedented direct comparison of glucose and lactate dynamics in response to metabolic demand elicited by neuronal activation.
Keywords: Fast-scan cyclic voltammetry, Biosensor, In vivo, Carbon-fiber microelectrode, Astrocyte-to-neuron lactate shuttle, Neuroenergetics
Graphical Abstract

INTRODUCTION
The brain consumes about 20% of the body’s total energy in order to sustain normal brain function, despite making up only ~2% of the overall mass [1,2]. The majority of this energetic demand is attributed to neuronal activation and the exocytotic release of chemical neurotransmitters [1,3,4]. Dysregulation in brain metabolism has been linked to many neurodegenerative and dopamine-associated diseases, such as Parkinson’s and Alzheimer’s diseases, and even drug addiction and obesity [5–10]. In order to get a better sense of adaptations associated with these disease states and to inform potential therapeutic strategies, it is imperative to understand the rates at which specific energetic substrates are supplied, and subsequently used, to meet the metabolic demands associated with neurotransmission. Such investigations have traditionally focused on glucose, due to its central role in the generation of adenosine triphosphate (ATP) through the mitochondrial electron transport chain. In cells, glucose is broken down via glycolysis to generate two molar equivalents of ATP, and pyruvate. Pyruvate can then undergo oxidative phosphorylation in the mitochondria through the tricarboxylic acid (TCA) cycle, which generates ~38 molecules of ATP per one molecule of pyruvate [11,12].
Compelling evidence suggests that lactate also plays an important role in meeting neuroenergetic demands [11,13–19]. Lactate has been implicated in important neural functions, including long-term memory formation, cortical spreading depression, and neuroprotection [20–23]. A disruption in the brain’s ability to supply or break down lactate has been linked to several neurological disorders, such as panic and attention deficit/hyperactivity disorders [24–28]. One of the most prevalent models to describe glucose and lactate use in the brain is the astrocyte-to-neuron lactate shuttle (ANLS) hypothesis, first described by Pellerin and Magistretti [11,14,16,20,21,24,25]. According to this model, energy metabolism in the brain is thought to be compartmentalized such that glycolysis is largely accomplished by astrocytes. Some of the pyruvate produced by glycolysis is converted to lactate via lactate dehydrogenase, and transported into the extracellular space. Lactate can be taken up by cells and converted back to pyruvate, and thus used to produce ATP as described above. However, there is also evidence to support the more straightforward mechanism whereby neurons take up glucose directly to generate ATP via both glycolysis and oxidative metabolism without the use of lactate as an energetic substrate [26,27]. Additionally, astrocytes are not the only glial cells that play a role in the delivery of energetic fuel in the brain; pericytes can modulate the supply of these neuroenergetic substrates, as they can constrict and dilate capillaries and arterioles [(28)]. Overall, many details remain unclear regarding the specific role of each of these molecules in meeting neuroenergetic demand.
Illuminating the dynamics of glucose and lactate with respect to the time course of neuronal activation has been difficult to accomplish due to a lack of analytical tools and techniques capable of selective measurements in real time. Two of the most prevalent strategies for monitoring these substrates in the living brain are microdialysis sampling followed by ex vivo analysis, and amperometric biosensing. Microdialysis studies have shown that, over several minutes of prolonged stimulation, glucose levels decrease as lactate levels increase [(17,29)]. However, microdialysis is an equilibrium-based sampling technique that requires sufficient sampling time (generally over several minutes). As such, these studies do not show how glucose and lactate fluctuate on a second-by-second timescale. By contrast, electrochemical techniques are popular for detecting neurotransmitters because of the ability to monitor these analytes in situ, on a millisecond timescale [(30–32)]. Glucose and lactate, however, are difficult to study using electrochemistry because neither is inherently redox active. Therefore, it is common to employ enzymes that selectively target non-electrochemically active substrates and generate a redox-active reporter molecule, typically hydrogen peroxide, to indirectly detect glucose and lactate. Consistent with microdialysis studies, in vivo measurements using enzyme-modified amperometric sensors have demonstrated that glucose levels decrease in response to prolonged neuronal activation, as extracellular lactate concentrations increase [(33,34)]. However, amperometry is inherently a non-selective measurement approach, and interfering species can convolute the signal. Thus, amperometric biosensors typically incorporate chemically-selective polymeric membranes into the sensing scheme, which are designed to exclude interferents. These membranes substantially limit temporal resolution because they also hinder the diffusion of the target analyte to the sensor surface. In addition, they necessitate the use of separate probes for separate analytes, typically implanted in opposing hemispheres [(33,34)]. As such, it remains unclear how glucose and lactate fluctuate relative to one another on a subsecond timescale at discrete recording sites in brain tissue.
The purpose of this study was to investigate the dynamics with which each of these molecules is supplied and utilized to meet metabolic demand in the rat dorsal striatum. Enzyme-modified carbon-fiber microelectrodes were coupled with background-subtracted fast-scan cyclic voltammetry (FSCV) to monitor glucose and lactate with subsecond (msec) and high spatial (μm) resolution [(35–38)]. The principal advantage of FSCV over amperometric detection strategies is that it can provide both qualitative and quantitative information. The characteristic oxidation and reduction peaks that are revealed in voltammograms of different neurotransmitters enable identification of the released substance, in addition to concentration information based on the measured current. As such, chemically selective coatings that serve to eliminate interferents are not necessary, allowing for faster measurements. Using this approach, we have previously demonstrated that glucose availability rapidly increases to meet metabolic demand in the striatum, upon midbrain electrical stimulation to induce DA release [(38)]. Herein, the midbrain stimulation parameters were systematically varied to reveal how the extracellular availability of striatal glucose and lactate scales with stimulation frequency and duration. The results provide in vivo evidence of the time course with which both of these neuroenergetic substrates are immediately supplied, and subsequently utilized, in the physiological response to metabolic demands associated with neuronal activation.
MATERIALS AND METHODS
Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. All in vitro electrochemical experiments were conducted in 0.01 M phosphate buffered saline (PBS), pH 7.4. All aqueous solutions were prepared in doubly distilled water, ≥18 MΩ·cm (Millipore Milli-Q, Billerica, MA). Glucose oxidase (GOx; specific activity: 100 U/mg at 37°C) originated from Aspergillus niger. Lactate oxidase (LaOx; specific activity: 20 U/mg at 37°C) originated from Aerococcus viridans. Chitosan originated from shrimp shells with a deacetylation percentage of ≥75% and an approximate molecular weight of 190–375 KDa, based on viscosity (medium molecular weight, practical grade). β-ᴅ-Glucose was obtained from VWR International (West Chester, PA). Glucose stock solution was prepared in PBS (pH 7.4) and underwent mutarotation for 24 hr at room temperature before use. Lactate stock solution was prepared from sodium ʟ-lactate and mixed with PBS (pH 7.4) and stored at 4 °C for 24 hr before use.
Microelectrode Fabrication
Glass-insulated carbon-fiber microelectrodes were fabricated as described previously [(32)]. Briefly, a single T-650 carbon fiber (7-μm diameter, Cytec Industries, West Patterson, NJ) was aspirated into a glass capillary tube (1.0-mm external diameter and 0.50-mm internal diameter, A-M Systems, Carlsburg, WA) and heat pulled using a vertical micropipette puller (Narishige, Tokyo, Japan) to form two sealed microelectrodes. The carbon fiber extending beyond the tapered glass seal was manually cut under an optical microscope to 100 μm in length. A stainless-steel lead wire with conductive silver paint (GC Electronics, Rockford, IL) was inserted into the capillary for electrical contact with the fiber.
Microbiosensor Fabrication
Glucose and lactate microbiosensors were fabricated using an aqueous solution of 2% chitosan prepared in 87-mM acetic acid (pH~5.3), as described previously [(35–38)]. GOx deposition solution (20 mg/mL) was prepared by slowly mixing 10,000 U of GOx with 5 mL of 2% chitosan until homogenous at room temperature. LaOx solution was prepared by dissolving 100 U of LaOx in 200 μL of deionized water, and mixing slowly with 800 μL of 2% chitosan until homogenous at room temperature. Enzyme/chitosan solutions were stored for at least 24 hr at 4 °C before use. Microelectrodes were pre-conditioned with a triangular voltammetric waveform (−0.4 V to 1.4 V) applied at a scan rate of 400 V/sec for 30 min at 60 Hz, followed by 10 Hz for another 30 min. Immediately following conditioning, the microelectrode was submerged in the enzyme/chitosan solution, and an electrodeposition potential of −3 V (vs Ag/AgCl reference) was applied for 30 s before the electrode was slowly removed from solution. Each microbiosensor was visually inspected under a microscope to ensure no distortion in the hydrogel membrane, and then stored in PBS (pH 7.4) at 4 °C.
Microbiosensor Characterization
Microbiosensors were characterized in vitro prior to in vivo use, as described previously (35–37). All in vitro data were collected at room temperature in a custom-built, grounded Faraday cage containing a flow-injection apparatus. A micromanipulator (World Precision Instruments, Inc., Sarasota, FL) was used to position an electrode into a custom electrochemical cell. A continuous flow of 0.01-M PBS (pH 7.4) was delivered from a syringe pump (New Era Pump Systems, Inc., Wantagh, NY) at a flow rate of 1.00 mL/min. Electrochemical data acquisition, including waveform output, signal processing, and data analysis, was achieved using a custom instrument (Universal Electrochemistry Instrument, University of North Carolina at Chapel Hill, Department of Chemistry, Electronics Facility) in conjunction with data acquisition cards (National Instruments, Austin TX) used for measuring current and synchronizing the electrochemical cell with the flow-injection system. This was carried out using High Definition Cyclic Voltammetry (HDCV) Acquisition Software (University of North Carolina at Chapel Hill, Department of Chemistry, Electronics Facility). Microbiosensors were conditioned using a triangular voltammetric waveform (0.1 V to 1.4 V, vs Ag/AgCl reference) applied at a scan rate of 400 V/s at 60 Hz for 20 min, followed by another 15 min at 10 Hz All electrochemical data were collected at 10 Hz with low-pass Bessel filtering (2 KHz).
Animal Experiments
All animal care and use procedures followed Institutional Animal Care and Use Committee (IACUC) and the NIH’s Guide for the Care and Use of Laboratory Animal guidelines. Male, Sprague-Dawley rats (n=7, 280–500 g, Charles River, Wilmington, MA) were allowed at least two days to acclimate to the facility. Rats were pair housed in a temperature- and humidity-controlled room on a 12:12 light-dark cycle with idle access to food and water.
Animals were anesthetized with 4% isoflurane (5 min, 2 L/min O2) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Isoflurane levels were maintained at ~2.0% for the duration of the experiment. Body temperature was maintained at 37 °C with a heating pad, and peripheral blood glucose was monitored using OneTouch® Ultra 2 and OneTouch® Ultra Blue test strips (LifeScan IP Holdings, Inc.) to ensure that glucose concentrations remained relatively stable over the course of the experiment. Holes were drilled for electrode placement according to coordinates from the Paxinos and Watson rat brain atlas [(39)]. Glucose- and lactate-sensitive biosensors were placed side-by-side, ~1 mm apart at the tip, and lowered into the dorsal striatum (+1.2 mm anteroposterior (AP), +1.0–2.5 mm mediolateral (ML) relative to bregma; −5.0–6.0 mm dorsoventral (DV) relative to skull). A Ag/AgCl reference electrode was placed in the contralateral forebrain and was secured using a gold screw (J. I. Morris Co., Southbridge, MA) and dental cement (Lang Dental Mfg. Co., Inc, Wheeling, IL). A bipolar stimulating electrode (Plastics One, Roanake, VA) was implanted in the dopaminergic midbrain (−5.8 mm AP, +1.1 ± 0.3 mm ML relative to bregma; −8.4–8.6 mm DV relative to skull), ipsilateral to the sensors. A triangular waveform (0.1 V–1.4 V, 400 V/s, 10 Hz) was applied to both electrodes simultaneously versus the same reference electrode using a multichannel Universal Electrochemical Headstage (University of North Carolina at Chapel Hill, Department of Chemistry, Electronics Facility), until performance stabilized. Electrical stimulations consisted of 60, 120, or 240 biphasic pulses (300–600 μA; 2 ms pulse width) applied at either 30 or 60 Hz. All electrical stimulations were repeated in triplicate in each animal subject. After each experiment, the biosensors were carefully removed and calibrated in vitro to determine sensitivity to glucose, lactate, DA, and H2O2, within 24 hr of in vivo use.
Histology
Electrode placement was histologically verified in two animals. Rats were deeply anesthetized using isoflurane and a potential of +10 V (vs Ag/AgCl reference) was applied to the working electrode to lesion brain tissue at the recording site. Immediately thereafter, 60 mL of PBS was perfused through the heart, followed by 60 mL of formalin, after which the brain was quickly removed and stored in 10% formalin/10% sucrose solution. These were later flash frozen with dry ice and 40-μm slices were prepared using a microtome (Leica SM2010 R Sliding Microtome, Leica Biosystems, Buffalo Grove, IL). The tissue was mounted onto a glass slide and allowed to dry for 24 hr. Then, the tissue was rehydrated with 100%, 95%, and 70% ethanol and deionized water, followed by a staining solution of a 1% Cresyl violet. The stained tissue was then rinsed with deionized water, 70%, 95%, and 100% ethanol. Drops of mounting solution were applied to the slide and a cover slip was carefully placed on top. The tissue was observed under a microscope to confirm electrode placement using the rat brain atlas [(39)].
Data and Statistical Analysis
The limit of detection (LOD) was defined as three standard deviations from the noise. All data are described as mean values with standard error of the mean (SEM). Per subject, replicates of each stimulation were averaged. As appropriate, two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons posthoc analyses were used for statistical analysis of the data, unless otherwise noted. All statistical analyses were accomplished using GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA), with significance designated as p < 0.05.
RESULTS
Our lab has previously described GOx- and LaOx-modified carbon-fiber microelectrodes for the in situ detection of extracellular glucose and lactate fluctuations in the brain, respectively [(35–38)]. The real-time measurements are accomplished by coupling the microbiosensor(s) with background-subtracted FSCV. With this strategy, the applied potential is linearly swept from a starting (or holding) potential to a switching potential, then back, at ~400 V/sec. These measurements are typically completed in < 10 msec and repeated at a frequency of 10 Hz. Fig. 1a (left) depicts the voltammetric waveform used in this work, which sweeps from +0.1 V to +1.4 V and back. When using a 400 V/sec scan rate, a large background current is generated (black), predominantly arising from the rapid charging and discharging of the electrical double-layer at the electrode surface. This background charging current can mask faradaic currents produced by the redox activity of species that are present at low concentrations at the sensor surface (Fig. 1a, middle panels). Fortunately, the background current is stable over several seconds, and can thus be subtracted to clearly reveal faradaic currents measured in response to specific analytes (Fig. 1a, right) [(32)]. This is advantageous, because a voltammogram is generated that enables analyte identification. For instance, Fig. 1b presents voltammetric data collected at a bare carbon-fiber microelectrode, shown in the form of a color plot for the simultaneous detection of 50-μM hydrogen peroxide (H2O2) and 1-μM dopamine (DA). With this waveform, sensitivity to DA is reduced as compared to the waveform that is typically used to target DA (which uses a holding potential of −0.4 V) [(32)]. However, both DA and H2O2 can be identified by the position of the voltammetric peaks evident in the voltammogram (inset) and quantified using calibration factors specific to each analyte at a given electrode. DA oxidation to DA-o-quinone is evident at ~0.6V. The oxidation of H2O2 to hydrogen and oxygen gas generates a single peak at ~1.2V (evident on the reverse scan herein) [(40)]. Glucose and lactate, however, are not inherently electroactive. As such, they do not generate a quantifiable signal at a bare carbon electrode. When the electrode is modified by immobilizing the appropriate oxidase enzyme in a chitosan matrix at the sensing surface, H2O2 is enzymatically produced in response to the presence of the specific enzyme substrate (glucose or lactate) and oxygen co-substrate, and readily detected (Fig. 1c, d) [(35–38)].
Fig. 1:
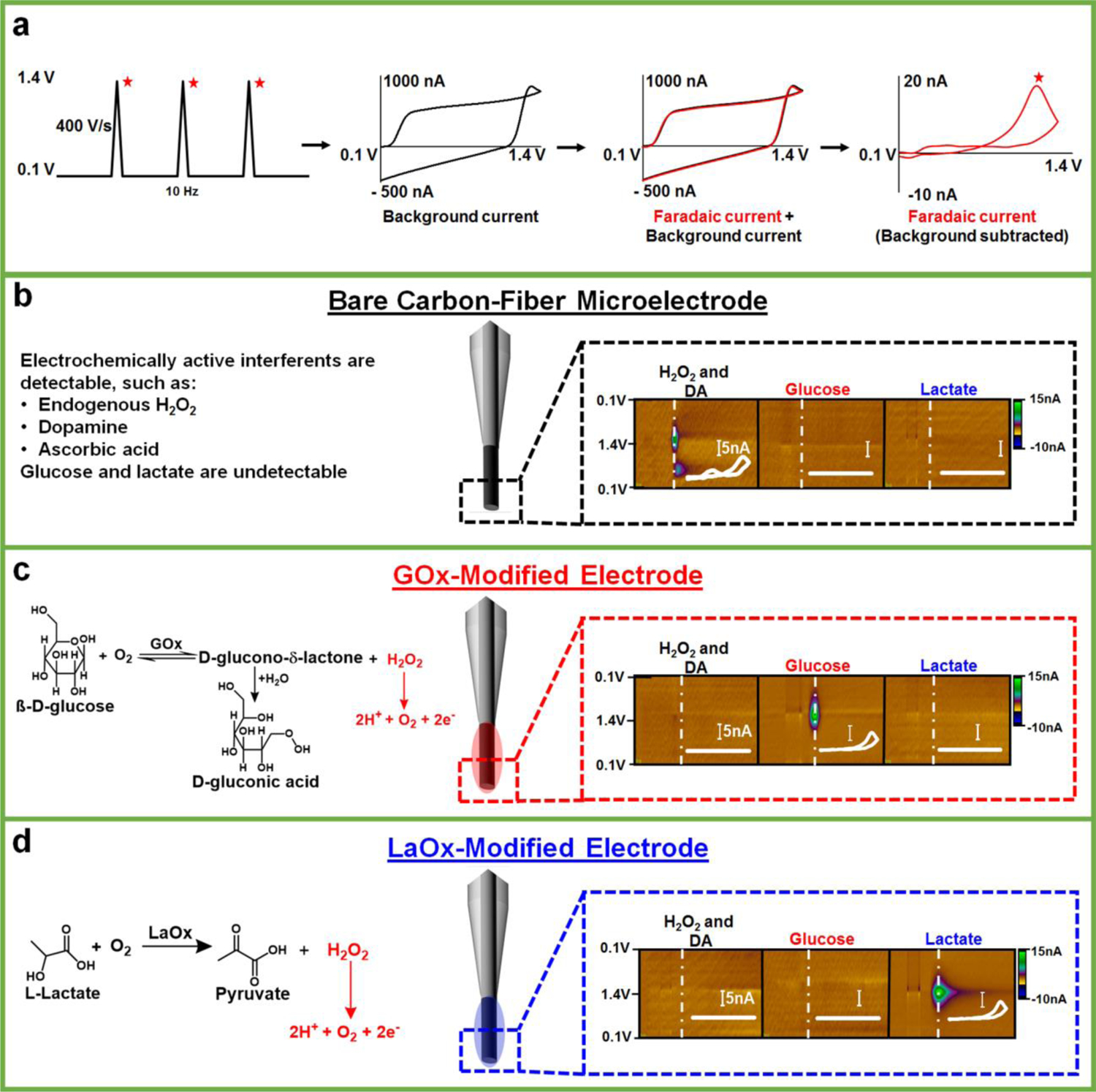
Carbon-fiber microbiosensors coupled with FSCV. (a) The triangular waveform, ranging from +0.1 V to 1.4 V, is applied at 10 Hz. This generates a large background current (black), which is subtracted to reveal faradaic current (right, red) specific to a given analyte, for example H2O2. (b-d) Voltammetric detection of H2O2, DA, glucose, and lactate at bare and enzyme-modified carbon-fiber microelectrodes, with appropriate enzymatic reactions (if applicable). Representative color plots are shown with inset background subtracted cyclic voltammograms (scale bar is 5 nA). H2O2 (50 μM) and DA (1 μM) can be simultaneously detected on bare microelectrodes with this waveform, but glucose (2.6 mM) and lactate (1 mM) are not inherently electroactive and, therefore, are undetectable. By contrast, glucose and lactate can be detected at microelectrodes modified with the (appropriate) oxidase enzyme, but standards of H2O2 (50 μM) and DA (1 μM) produce little signal [(35–38)].
The voltammetric approach enables identification and quantification of H2O2 even in the presence of other electroactive molecules, eliminating the need for additional polymeric membranes that are typically required to impart selectivity in amperometric measurements [(38)]. The addition of any membrane can substantially slow diffusion of the target analyte to the sensor surface, compromising temporal resolution. However, the simple chitosan matrix only minimally slows detection of glucose and lactate, as their interaction with the enzyme at the biosensor/solution interface triggers the production of H2O2 within the membrane itself, without requiring diffusion through the matrix. A comparison of the rise time (10–90% of the maximum current) of the signal for a H2O2 standard (50 μM, t10–90% = 1.13 ± 0.05 sec) on a bare electrode to that for 2.6-mM glucose (t10–90%,GOx=0.96 ± 0.08 sec) or 1-mM lactate (t10–90%,LaOx = 1.11 ± 0.08 sec) recorded using the respective microbiosensors revealed no significant difference (F2,6 = 0.55, p ≥ 0.05, one-way ANOVA, n=3 each sensor, data not shown). Thus, the coupling of enzyme-modified carbon-fiber microelectrodes with FSCV has enabled the selective measurement of glucose and lactate on a subsecond timescale [(35–38)].
DA is undoubtedly the most well-characterized neurotransmitter studied using FSCV, and voltammetric measurements of DA in the striatum have helped to link DA signaling to reward-related learning, goal-directed behavior, and action initiation [(8,32,41–49)]. However, relatively little is known about the dynamics with which chemical substrates are supplied to striatal tissue to fuel DA release, reuptake, and the resulting postsynaptic activation (or inhibition) of local cells. To investigate this, a bipolar stimulating electrode was implanted in the ventral tegmental area (VTA)/substantia nigra (SN) complex of anesthetized rats to directly depolarize the midbrain projection to striatum. GOx- and LaOx- microbiosensors were positioned in the dorsal striatum, to record fluctuations of glucose and lactate elicited in response to metabolic demand evoked by the resultant neuronal activity. This experimental set-up is depicted in Fig. 2a (left), which directly compares striatal glucose dynamics with those of lactate evoked in response to a 120-pulse stimulation applied at 60 Hz. Representative color plots (Fig. 2a, right) and concentration versus time traces (Fig. 2b) are presented for both species detected simultaneously using microbiosensors implanted in a side-by-side configuration.
Fig. 2:
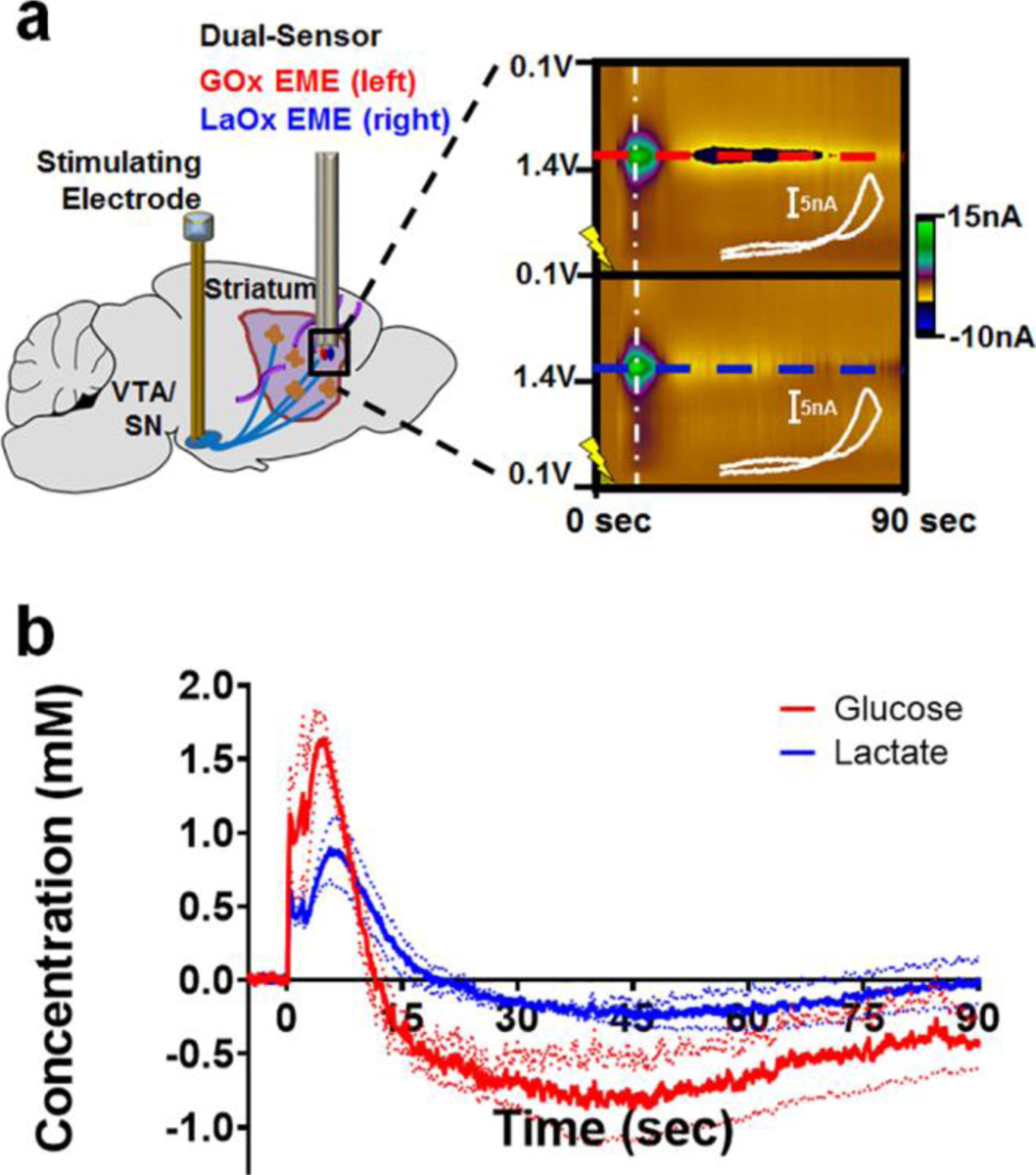
Voltammetric monitoring of glucose and lactate in the rat striatum in response to electrical stimulation of the midbrain. (a) Left, A bipolar stimulating electrode was implanted into the VTA/SN complex of anesthetized rats to evoke striatal DA release while GOx- and LaOx- microbiosensors monitored resultant fluctuations of glucose and lactate in the dorsal striatum. Right, Raw data shown in the form of a color plot for glucose (top) and lactate (bottom). The time of stimulation is indicated by the lightning bolt. (b) The concentration versus time traces for both species detected simultaneously (n=3 animals). Note that a stimulation glitch confounds the data during the application of stimulation pulses (0–2 sec). Glucose (red) concentrations rose before those of lactate (blue). Glucose concentrations were also first to fall below baseline, and remained below baseline for a longer period of time than those of lactate.
The microbiosensors were lowered into the dorsal striatum until glucose and lactate signals were optimized. Systematic variation of the stimulation conditions affords the ability to characterize glucose and lactate dynamics. The LOD, defined as three times the standard deviation of the noise, was found to be 7.6 ± 0.9 μM for glucose and 5.1 ± 0.6 μM for lactate. Biphasic electrical stimulations were delivered in a counterbalanced fashion to the VTA/SN (60, 120, or 240 pulses, applied at 60 Hz) corresponding to 1-, 2-, and 4-sec stimulation durations, respectively. The averaged traces, presented in Fig. 3a and b, demonstrate current recorded for both analytes with respect to time, normalized to the 120-pulse stimulation. Note that, throughout this work, a stimulation glitch convolutes the signal collected while electrical pulses were delivered (grey boxes in Fig. 3–4, 6). Nonetheless, it is clear that the extracellular availability of both glucose (red) and lactate (blue) is dynamic on the seconds timescale, and that the evoked concentrations scale with increasing stimulation duration (Fig. 3c; analyte, F1,18 = 13.6, p < 0.01; pulses, F2,18 = 18.62, p < 0.0001; analyte x pulse interaction, F2,18 = 6.09, p < 0.01). Significantly more glucose was measured in response to the 240-pulse stimulation, as compared to the shorter 60- and 120-pulse stimulations (p < 0.01 – p < 0.0001). As such, extracellular glucose increased in concentration significantly more than did lactate in response to the longest (240-pulse) stimulation (p < 0.001), but not the 60- or the 120-pulse stimulation (p > 0.05). Interestingly, the time of maximal glucose and lactate availability varied based on stimulation duration at 60 Hz (Fig. 3d; analyte, F1,18 = 14.07, p < 0.01; pulses, F2,18 = 9.92, p < 0.01; analyte x pulse interaction, F2,18 = 5.09, p < 0.05), such that the time at which peak extracellular lactate availability was recorded in response to the 240-pulse, stimulation was greater than that for the corresponding 60-pulse stimulation (p < 0.001, Sidak post-hoc test). By contrast, extracellular glucose availability consistently peaked ~5 sec after stimulation onset (5.2 ± 0.1 sec; n=4 animals), regardless of stimulation duration. Thus, glucose availability peaked significantly before that of lactate (7.6 ± 0.4 sec) in response to the longest stimulation (240 pulses, n=4 animals, p < 0.01, Sidak post-hoc test). These values are summarized in Table 1.
Fig. 3:
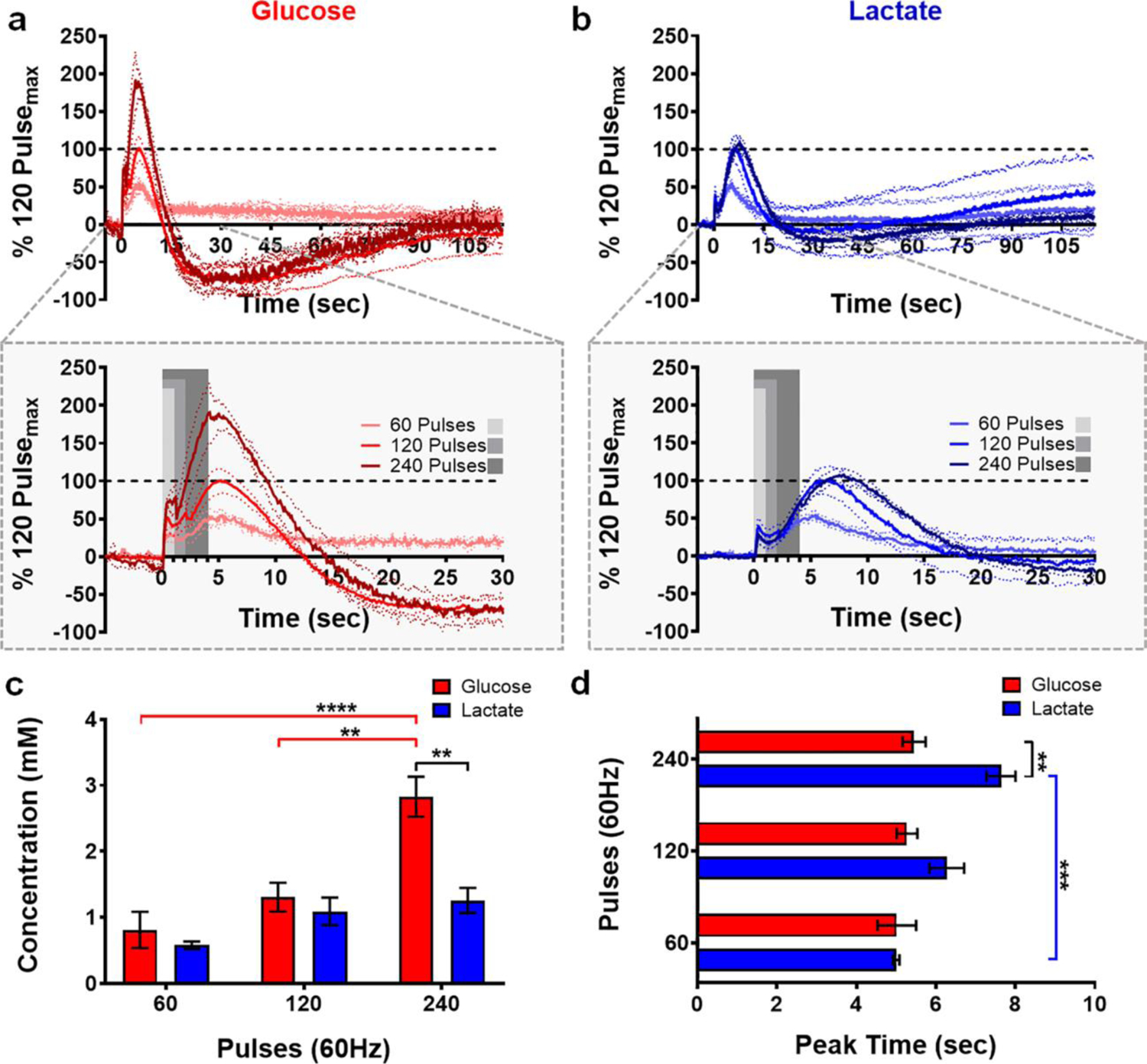
Extracellular glucose and lactate dynamics as a function of stimulation duration. Glucose (a, red) and lactate (b, blue) concentration profiles elicited in response to stimulation duration (60 Hz, n = 4 animals). Current is normalized with respect to the 120-pulse stimulation. A stimulation glitch confounds the data during the application of stimulation pulses (gray boxes). (c) Glucose availability scales with increasing stimulation duration (** p < 0.01 - **** p < 0.0001). Extracellular glucose availability increased significantly more than that of lactate in response to the 240-pulse stimulation (**p < 0.01). (d) Extracellular glucose concentrations consistently peaked ~5 sec after stimulation onset, regardless of stimulation duration. Contrastingly, it took more time to reach maximum lactate availability in response to the 240-pulse stimulation (*** p < 0.001). Consequently, glucose peaked before lactate in response to this stimulation (** p < 0.01).
Fig. 4:

Extracellular glucose and lactate dynamics as a function of stimulation frequency. Glucose (a) and lactate (b) concentration profiles elicited in response to 120-pulse stimulations delivered at 30 Hz and 60 Hz (normalized to 60 Hz, n = 4). A stimulation glitch confounds the data during the application of stimulation pulses (gray boxes). (c) Glucose availability scaled with stimulation frequency (*p < 0.05). (d) Extracellular glucose and lactate concentrations peaked with similar latencies after the onset of the 120-pulse stimulation, regardless of stimulation frequency.
Fig. 6:

The total concentration range of striatal glucose and lactate fluctuations evoked in response to each stimulation condition. (a) A representative trace depicting the initial increase and subsequent decrease in extracellular glucose and lactate concentrations evoked by a 60-Hz stimulation delivering 240-pulses. (b) The total range in extracellular glucose availability reflects the increased metabolic demand elicited by more intense stimulations. Extracellular glucose concentrations fluctuated over a larger range than those of lactate in response to the strongest stimulation (*p < 0.05 - ****p < 0.0001).
Table 1:
Quantification of glucose and lactate dynamics in rat striatum.
| Glucose | |||||||
|---|---|---|---|---|---|---|---|
| Stimulation Parameters | Maximum Concentration (mM) | Peak Time (sec) | Rate of Increase (mM/sec) | Fall Under Baseline (mM) | Time Below Baseline (sec) | Total Range (mM) | Rate of Decrease (mM/sec) |
| 30 Hz 120 Pulses | 0.7 ± 0.2 | 6.2 ± 0.6 | 0.11 ± 0.04 | --- | --- | 1.1 ± 0.2 | 0.05 ± 0.01 |
| 60 Hz 60 Pulses | 0.8 ± 0.3 | 5.0 ± 0.5 | 0.17± 0.07 | --- | --- | 1.1 ± 0.4 | 0.05 ± 0.01 |
| 60 Hz 120 Pulses | 1.3 ± 0.2 | 5.3 ± 0.2 | 0.26 ± 0.06 | −1.2 ± 0.2 | 90 ± 10 | 2.5 ± 0.4 | 0.09 ± 0.01 |
| 60 Hz 240 Pulses | 2.8 ± 0.3 | 5.5 ± 0.3 | 0.53 ± 0.07 | −1.1 ± 0.2 | 80 ± 10 | 4.0 ± 0.4 | 0.17 ± 0.03 |
| Lactate | |||||||
| Stimulation Parameters | Maximum Concentration (mM) | Peak Time (sec) | Rise Rate (mM/sec) | Fall Under Baseline (mM) | Time Below Baseline (sec) | Total Range (mM) | Rate of Decrease (mM/sec) |
| 30 Hz 120 Pulses | 0.62 ± 0.09 | 6.3 ± 0.9 | 0.11 v 0.02 | --- | --- | 0.6 ± 0.1 | 0.04 ± 0.03 |
| 60 Hz 60 Pulses | 0.58 ± 0.06 | 5.00 ± 0.08 | 0.13 ± 0.02 | --- | --- | 0.7 ± 0.1 | 0.03 ± 0.01 |
| 60 Hz 120 Pulses | 1.1 ± 0.2 | 6.3 ± 0.4 | 0.17 ± 0.02 | −0.2 ± 0.3 | 50 ± 20 | 1.3 ± 0.2 | 0.06 ± 0.01 |
| 60 Hz 240 Pulses | 1.3 ± 0.2 | 7.6 ± 0.4 | 0.16 ± 0.01 | −0.36 ± 0.02 | 50 ±10 | 1.6 ± 0.1 | 0.056 ± 0.003 |
Next, the effects of stimulation frequency were investigated, because burst firing of midbrain DA neurons is functionally important for reward-related learning, goal-directed behavior, and action initiation [8,31,38–46]. It has been clearly shown that burst firing of DA cells augments the amount of DA released from striatal terminals [(50)]. This process requires energy, and also elicits neural activity (or inhibition) in subsets of nearby medium spiny neurons (MSNs) [(51–53)], further augmenting the local energetic demand. Stimulation pulses (120) were delivered to the midbrain at frequencies of 30 and 60 Hz (stimulation durations of 4 and 2 sec, respectively). The averaged traces for glucose and lactate concentrations recorded in the extracellular space are plotted in Fig. 4a and b, respectively, normalized to the 60-Hz trace. As expected, the magnitude of extracellular energy availability was dependent on stimulation frequency overall (Fig. 4c; analyte, F1,12 = 0.51, p > 0.05; frequency, F1,12 =9.89, p < 0.01; analyte x frequency interaction F1, 12 = 0.25, p > 0.05) with a specific effect between 30 and 60 Hz (4- vs. 2-sec durations for delivery of 120 pulses) evident for glucose (p < 0.01, Sidak post-hoc test). The latency required for these energetic substrates to peak in the extracellular space did not shift as stimulation frequency was modified (Fig. 4d). All values are reported in Table 1.
All stimulations elicited an immediate increase in extracellular glucose and lactate concentrations in the striatum. However, only delivery of 120–240 pulses at a frequency of 60 Hz created a metabolic demand sufficient to then consistently drop glucose and lactate concentrations below baseline, as observed in Fig. 3a and b. This is quantified in Fig. 5a (analyte, F1,12=18.26, p < 0.01; pulse, F1, 12 = 0.07, p > 0.05; analyte x pulse interaction, F1, 12 = 0.30, p > 0.05). Glucose concentrations fell further below baseline than did those of lactate in response to both the 120-pulse and the 240-pulse stimulations at 60 Hz (p < 0.05, Sidak post-hoc test; Fig. 5a). In fact, glucose concentrations fell to a minimum value that was nearly the same amplitude as the prior increase in concentration, but in the opposite direction. Minimum glucose concentrations were −91.6 ± 0.3 % and −40.1 ± 0.2 % of the amplitude of the prior increase in extracellular glucose evoked by the 120-pulse (2-sec) and 240-pulse (4-sec) stimulations, respectively. By contrast, lactate concentrations fell less drastically, to values that were −17 ± 2 % and −28.3 ± 0.2 % of the increase in concentration that immediately preceded the drop. Although there was a trend for a difference between these analytes, the amount of time that glucose and lactate concentrations remained below baseline (Fig. 5b) was not dependent on stimulation duration (analyte, F1,12= 4.31, p =0.06; pulse, F1, 12 = 0.14, p > 0.05; analyte x pulse interaction, F1, 12 = 0.08, p > 0.05).
Fig. 5:

Delivery of 120 and 240 pulses at a frequency of 60 Hz created a metabolic demand sufficient to drop glucose and lactate concentrations well below baseline. (a) Glucose concentrations were depleted significantly more than those of lactate in response to both the 120-and 240-pulse stimulations (*p < 0.05). (b) Overall, differences in the amount of time that glucose and lactate concentrations remained below baseline were not dependent on stimulation duration.
The total concentration range (i.e., the overall magnitude of the initial increase and the subsequent fall, labeled in Fig. 6a) was quantitatively compared in Fig. 6b (analyte, F1,24 = 29.26, p < 0.0001; stimulation parameter, F3,24 = 19.70, p < 0.0001; analyte x stimulation interaction, F3,24 = 5.60, p < 0.01). Glucose concentrations fluctuated over a larger range than did lactate across stimulation conditions. The total range in extracellular glucose was significantly greater when a train of 120 pulses was delivered at 60 Hz as compared to 30 Hz (p < 0.05), and it also scaled with the number of pulses delivered at 60 Hz (treatment, p < 0.05 - p < 0.0001). These data reflect the increased metabolic demand elicited by a more intense stimulation profile. Importantly, extracellular glucose concentrations fluctuated over a significantly larger range than those of lactate in response to the most intense stimulation (60 Hz, 240-pulses, p < 0.0001). These values are reported in Table 1.
Next, the rate at which glucose and lactate concentrations rose in the extracellular space was calculated by dividing the maximum concentration of a given species by the time at which the peak occurred (Fig. 7a). Overall, the rate at which the neuroenergetic substrates were supplied to the local recording site was dependent on stimulation intensity (analyte, F1,24 = 16.44, p < 0.001; treatment, F3,24 = 10.49, p < 0.0001; analyte x treatment, F3,24=6.78, p < 0.01). Glucose and lactate were supplied at the same rate in response to the more modest stimulations. However, the concentrations of glucose rose at a significantly faster rate when 240 pulses were delivered at 60 Hz, as compared to all other conditions (p < 0.01 – p < 0.0001). As such, when the most substantial 60 Hz stimulation was delivered, glucose concentrations rose significantly faster than those of lactate (p < 0.001). By contrast, the rate at which lactate was supplied generally remained constant across these stimulation conditions. All calculated values are reported in Table 1.
Fig. 7.
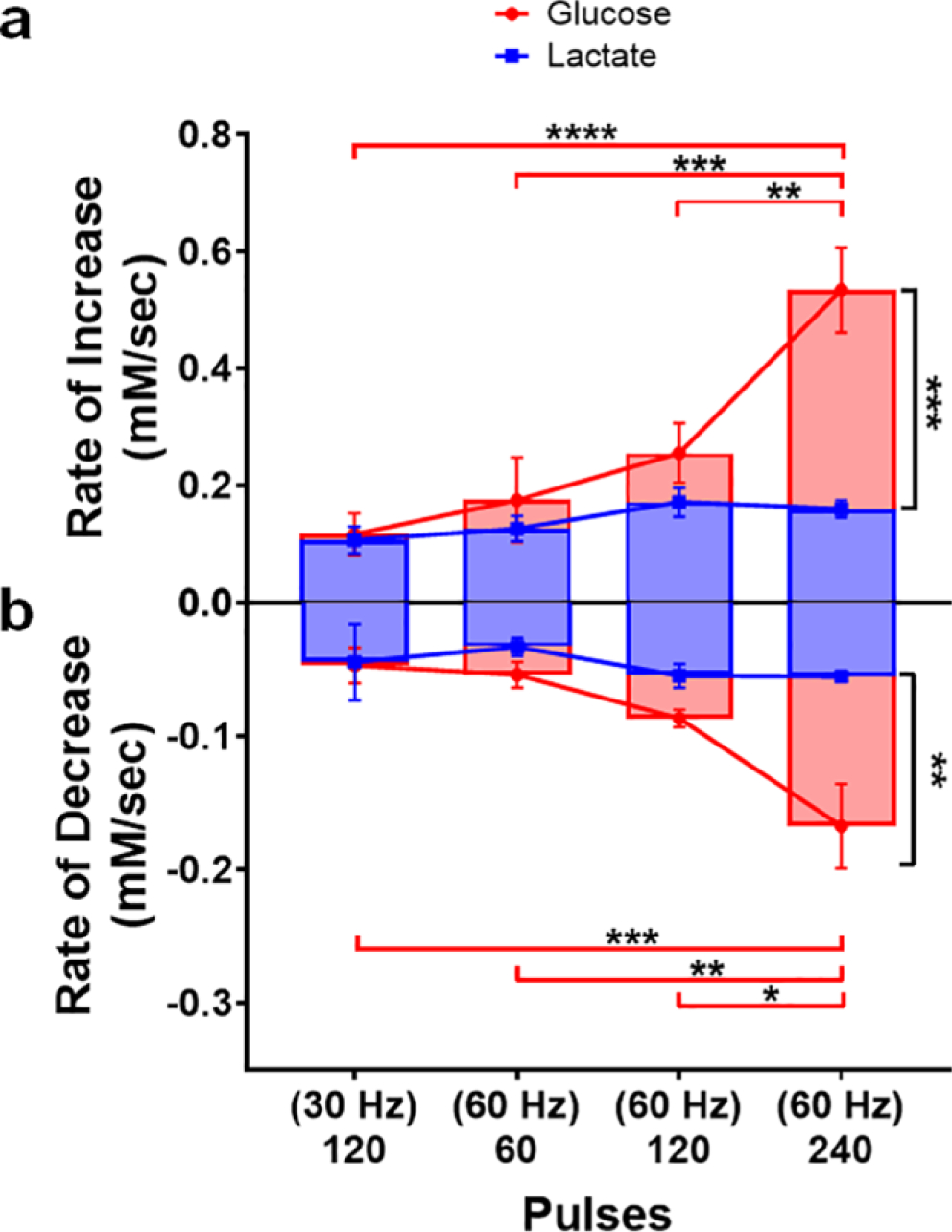
The relative rates of striatal glucose and lactate dynamics evoked in response to midbrain stimulation. (a) Rates at which extracellular glucose and lactate concentrations increased were calculated by dividing the amplitude of the increase by peak latency. Glucose and lactate were supplied at the same rate in response to the more modest stimulations, but glucose concentrations rose significantly faster as the number of stimulation pulses (60 Hz) increased (** p < 0.01 - **** p < 0.0001). (b) The relative rates at which glucose and lactate concentrations fell were similarly calculated, but the total concentration range covered by the initial rise and subsequent fall was divided by the time between the peak and the valley in the concentration trace. Glucose and lactate were consumed at the same rate in response to modest stimulations, but glucose was consumed at increasingly faster rates as the stimulation intensified (*p < 0.05 - ***p < 0.001). Conversely, the rate of lactate consumption remained consistent across all stimulation conditions investigated.
The relative rates at which glucose and lactate concentrations fell in the extracellular recording environment were similarly calculated by dividing the total range of the concentration fluctuation by the time between the peak and the subsequent valley in the concentration trace (Fig. 7b). The rate of decrease was broadly dependent on the intensity of the stimulation (analyte, F1,24 = 12.42, p < 0.01; treatment, F3,24 = 7.06, p < 0.01; analyte x treatment, F3,24 = 4.15, p < 0.05). Glucose and lactate concentrations decreased at the same rate in response to the more modest stimulations. However, extracellular glucose concentrations decreased at increasingly faster rates as the stimulation intensified (p < 0.05 – p < 0.001). Interestingly, the rate at which lactate was consumed was relatively constant across all stimulation conditions. Overall, these data support the hypothesis that glucose is the primary energy substrate, playing the role of ‘first responder’, with lactate playing an important (but secondary) role.
DISCUSSION
While the astrocyte-to-neuron ratio in most brain regions is approximately 10:1, neurons are believed to be the main consumers of brain energy, consuming approximately 75–80% of the total energy used by the brain [(4,12,15,19,24,25,54)]. Vesicular exocytosis and recycling are undoubtedly energetically demanding processes, as is the activation (or inhibition) of local neurons and non-neuronal support cells [(1,3)]. It is imperative for fuel to be supplied rapidly and efficiently, possibly from multiple sources, in order to meet the energetic demand incurred by synaptic transmission. Enlisting astrocytes to shuttle additional energetic substrates to neurons would be strategically favorable in meeting a significant metabolic demand.
One of the most predominant models to describe glucose and lactate use in the brain is the ANLS hypothesis, graphically depicted in Fig. 8 [(11,14–16,20,24,25,55)]. According to this influential but controversial theory, glucose is transported from blood capillaries across the blood-brain barrier via glucose transporter 1 (GLUT1). It is transported directly into neurons via GLUT3, or preferentially shuttled into astrocytes via GLUT1. Glucose taken up by astrocytes can be converted to pyruvate to meet a metabolic need, shuttled to the neurons, or stored as glycogen for future use. Some of the pyruvate generated in the astrocytes is converted to lactate using lactate dehydrogenase 5 (LDH5), which is present at high concentrations in astrocytes [(15–20,24,55,56)]. Lactate can then be shuttled from the astrocytes via monocarboxylate transporters (MCT) 1 or 4. Neurons can take lactate up via MCT2, where it is converted back to pyruvate via LDH1, which is present at high concentrations in neurons [15–20,24,48,49]. The converted pyruvate is then repurposed to the TCA cycle to generate a substantial amount of ATP [(15–20,24,55,56)]. Thus, a neuron could potentially avoid glycolysis entirely by opting to meet metabolic demand through oxidative metabolism. However, the ANLS hypothesis may not tell the whole story. There is certainly evidence in support of the parsimonious hypothesis that neurons take up glucose directly and generate ATP via both glycolysis and oxidative metabolism, and that the use of lactate for fuel may not be stoichiometrically sound [(57–61)]. Controversy remains over the preference for neurons to undergo oxidative metabolism versus glycolysis, leaving the specific role for lactate as a neuroenergetic substrate in the brain widely debated [(57–63)].
Fig. 8:
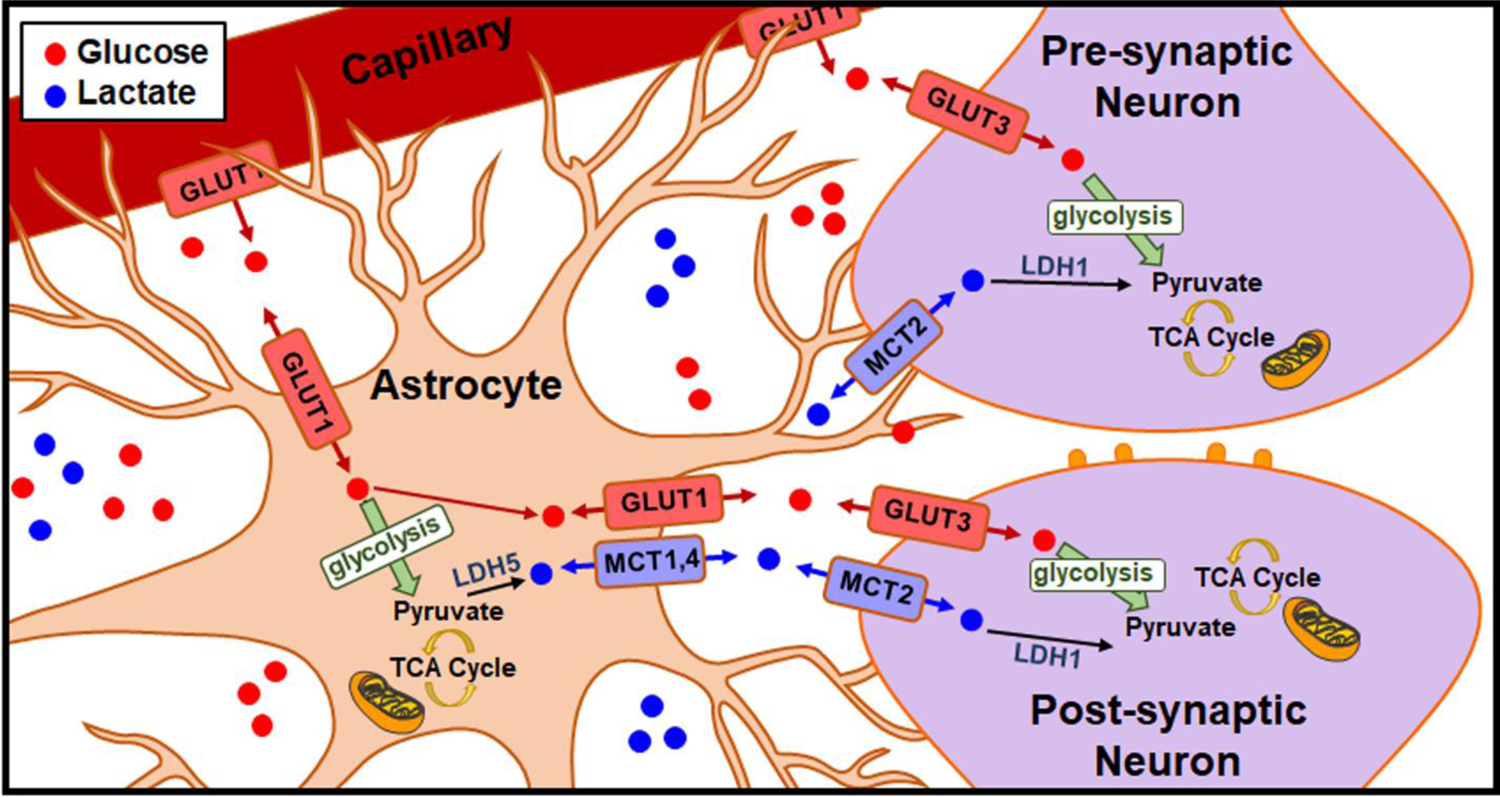
The ANLS hypothesis, first described by Magestretti and Pellerin in 1994 [(11,14–16,20,24,25,55)].
To date, the dynamics with which glucose and lactate are immediately supplied to support neuronal activation have remained unclear, as most biosensors require multiple seconds for a given measurement, and most monitor only one of these substrates at a time in a given brain region. In this work, glucose and lactate availability was monitored in real time at discrete locations in rat striatum. Upon stimulation of the midbrain projection to the region, glucose and lactate were immediately supplied to meet metabolic demand. Glucose availability scaled with stimulation duration (Fig. 3c) and frequency (Fig. 4c), whereas evoked lactate concentrations appeared more consistent, regardless of stimulation intensity. This was evident when considering the full range of concentration changes for each analyte across all stimulation parameters (Fig. 6b). Maximal glucose concentrations were consistently evident ~ 5–6 sec after stimulation began (Fig. 3d, 4d), and the latency required for either of these neuroenergetic substrates to reach maximum concentrations in response to a 120-pulse stimulation was not dependent on stimulation frequency (Fig. 4d). However, extracellular glucose availability increased more than that of lactate (Fig. 3c), and glucose concentrations changed at a faster rate (Fig. 7) in response to the stimulation with the longest duration, at the highest frequency tested (240 pulses, 60 Hz; Fig. 3c). Indeed, maximal glucose availability was evident before lactate concentrations peaked in response to this stimulation condition (Fig. 2b, 3d, 6a), suggesting that glucose serves as the neuroenergetic ‘first responder’ when stimulations are sufficiently intense. However, the latency required for lactate to reach peak concentrations was also the longest in response to this most intense stimulation (Fig. 3d) which may indicate that lactate demand (utilization) exceeded the rate of supply to the extracellular space. Intriguingly, the maximal concentrations evoked by stimulation and the duration to peak extracellular availability (Fig. 4), the range of the evoked concentration changes (Fig. 6b), and the rates of the concentration changes did not differ between glucose and lactate in response to the less intense stimulations of identical duration (4 sec; 120 pulses at 30 Hz). These findings are functionally important because manipulating the firing frequency of midbrain DA neurons can drive specific aspects of goal-directed behavior [(64,65)] and facilitate action initiation [(66)] or movement vigor [(67)],], and the role of striatal neuroenergetics in these processes remains unclear.
When the higher frequency (60 Hz) stimulations were administered for more than 1 sec, extracellular concentrations of glucose and lactate rapidly increased, but then subsequently fell below baseline for several tens of seconds before returning to pre-stimulation levels (Fig. 3a, b; Fig. 5b). Glucose concentrations were first to dip below baseline (Fig. 2b, 6a), the magnitude of the drop was greater regardless of stimulation duration (Fig. 5a), and concentrations decreased at a more rapid rate than those of lactate when the stimulation was 4 sec long (240 pulses at 60 Hz; Fig. 7b). As FSCV is a differential analytical technique, the drop below baseline suggests that striatal activation evoked by high-intensity stimulation of the dopaminergic midbrain elicited a metabolic demand sufficient to reduce the extracellular availability of both substrates below a pre-stimulation baseline. This is likely due to increased energy consumption (utilization), but activity-induced reductions in supply, storage, and synthesis may also play a part.
This work represents the first simultaneous monitoring and direct comparison of real-time glucose and lactate fluctuations at a discrete region in intact dorsal striatum. The data demonstrate that both species are rapidly supplied to meet metabolic demand with distinct dynamics. The availability of both scaled with stimulation intensity. Interestingly, extracellular lactate availability lagged that of glucose when the most intense stimulations were administered, suggesting a rate-limiting step in synthesis or transport, or simply that more lactate was being actively transported from the extracellular space. It will be interesting to extend these studies to quantification of neuroenergetic fluctuations that occur in response to more natural stimuli that activate the dorsal striatum, such as administration of psychostimulants [(46)], goal-directed behaviors [(43,44)], or vigorous locomotion [(45)]. This foundation of experiments can be expanded upon in countless ways to help clarify specific mechanisms by which glucose and lactate are supplied and utilized to meet the metabolic demands incurred by synaptic transmission.
ACKNOWLEDGEMENTS
We would like to thank Samantha K. Smith, Gregory S. McCarty, Erica M. Cullison, Brian M. Horman, Heather B. Patisaul, and Nathan W. Burnham for equipment, technical assistance and helpful discussion. Lastly, we would like to thank Pinnacle Technology Inc. for helpful discussion. This work was funded by the National Institute of Health (R43MH119870).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
DATA AVAILABILTY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: L.A. Sombers is working with Pinnacle Technologies, Inc. to commercialize these probes through a grant funded by the National Institutes of Health (R43MH119870). The other authors declare no conflict of interest.
All animal procedures followed Institutional Animal Care and Use Committee (IACUC) and North Carolina State University protocols.
REFERENCES
- 1.Attwell D, Laughlin SB. An Energy Budget for Signaling in the Grey Matter of the Brain. J Cereb Blood Flow Metab. 2001. October;21(10):1133–45. [DOI] [PubMed] [Google Scholar]
- 2.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997. July 1;77(3):731–58. [DOI] [PubMed] [Google Scholar]
- 3.Lennie P The Cost of Cortical Computation. Curr Biol. 2003. March;13(6):493–7. [DOI] [PubMed] [Google Scholar]
- 4.Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005. November;6(11):841–9. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl DE, Metter EJ, Riege WH. Patterns of local cerebral glucose utilization determined in Parkinson’s disease by the [18F]fluorodeoxyglucose method. Ann Neurol. 1984. May;15(5):419–24. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer S Abnormalities of Glucose Metabolism in Alzheimer’s Disease. Ann N Y Acad Sci. 1991. December;640(1):53–8. [DOI] [PubMed] [Google Scholar]
- 7.Kalaria RN, Harik SI. Reduced Glucose Transporter at the Blood-Brain Barrier and in Cerebral Cortex in Alzheimer Disease. J Neurochem. 1989. October;53(4):1083–8. [DOI] [PubMed] [Google Scholar]
- 8.Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004. January;42(11):1447–58. [DOI] [PubMed] [Google Scholar]
- 10.Peppard RF, Martin WRW, Carr GD, Grochowski E, Schulzer M, Guttman M, et al. Cerebral Glucose Metabolism in Parkinson’s Disease With and Without Dementia. Arch Neurol. 1992. December 1;49(12):1262–8. [DOI] [PubMed] [Google Scholar]
- 11.Magistretti PJ, Allaman I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron. 2015. May;86(4):883–901. [DOI] [PubMed] [Google Scholar]
- 12.Watts ME, Pocock R, Claudianos C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front Mol Neurosci. 2018. June 22;11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hall G, Stømstad M, Rasmussen P, Jans Ø, Zaar M, Gam C, et al. Blood Lactate is an Important Energy Source for the Human Brain. J Cereb Blood Flow Metab. 2009. June;29(6):1121–9. [DOI] [PubMed] [Google Scholar]
- 14.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Howseman A, Zeki S, editors. Philos Trans R Soc Lond B Biol Sci. 1999. July 29;354(1387):1155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsacopoulos M, Magistretti P. Metabolic coupling between glia and neurons. J Neurosci. 1996. February 1;16(3):877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci. 1994. October 25;91(22):10625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin MC, Hopwood SE, Boutelle MG, Strong AJ. Resolving dynamic changes in brain metabolism using biosensors and on-line microdialysis. TrAC Trends Anal Chem. 2003. September;22(8):487–97. [Google Scholar]
- 18.Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, et al. The redox switch/redox coupling hypothesis. Neurochem Int. 2006. May;48(6–7):523–30. [DOI] [PubMed] [Google Scholar]
- 19.Killeen PR, Russell VA, Tannock R. Neuroenergetics. Curr Dir Psychol Sci. 2016. April;25(2):124–9. [Google Scholar]
- 20.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018. April;19(4):235–49. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell. 2011. March;144(5):810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009. May;109:10–6. [DOI] [PubMed] [Google Scholar]
- 23.Newman LA, Korol DL, Gold PE. Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. Brann D, editor. PLoS ONE. 2011. December 13;6(12):e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. Physiology. 1999. October;14(5):177–82. [DOI] [PubMed] [Google Scholar]
- 25.Pellerin L, Magistretti PJ. Neuroenergetics: Calling Upon Astrocytes to Satisfy Hungry Neurons. The Neuroscientist. 2004. February;10(1):53–62. [DOI] [PubMed] [Google Scholar]
- 26.Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JDR, et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun. 2015. November;6(1):6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehlig A, Wittendorp-Rechenmann E, Dao Lam C. Selective Uptake of [14 C]2-Deoxyglucose by Neurons and Astrocytes: High-Resolution Microautoradiographic Imaging by Cellular 14 C-Trajectography Combined with Immunohistochemistry. J Cereb Blood Flow Metab. 2004. September;24(9):1004–14. [DOI] [PubMed] [Google Scholar]
- 28.Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science. 2019. July 19;365(6450):eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uehara T, Sumiyoshi T, Itoh H, Kurachi M. Dopamine D1 and D2 receptors regulate extracellular lactate and glucose concentrations in the nucleus accumbens. Brain Res. 2007. February;1133:193–9. [DOI] [PubMed] [Google Scholar]
- 30.Hersey M, Berger SN, Holmes J, West A, Hashemi P. Recent Developments in Carbon Sensors for At-Source Electroanalysis. Anal Chem. 2019. January 2;91(1):27–43. [DOI] [PubMed] [Google Scholar]
- 31.Xiao T, Wu F, Hao J, Zhang M, Yu P, Mao L. In Vivo Analysis with Electrochemical Sensors and Biosensors. Anal Chem. 2017. January 3;89(1):300–13. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JG, Lugo-Morales LZ, Loziuk PL, Sombers LA. Real-Time Chemical Measurements of Dopamine Release in the Brain In: Kabbani N, editor. Dopamine [Internet]. Totowa, NJ: Humana Press; 2013. [cited 2019 Nov 19]. p. 275–94. Available from: http://link.springer.com/10.1007/978-1-62703-251-3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y, Wilson GS. A Temporary Local Energy Pool Coupled to Neuronal Activity: Fluctuations of Extracellular Lactate Levels in Rat Brain Monitored with Rapid-Response Enzyme-Based Sensor. J Neurochem. 1997. October;69(4):1484–90. [DOI] [PubMed] [Google Scholar]
- 34.Rocchitta G, Secchi O, Alvau MD, Farina D, Bazzu G, Calia G, et al. Simultaneous Telemetric Monitoring of Brain Glucose and Lactate and Motion in Freely Moving Rats. Anal Chem. 2013. November 5;85(21):10282–8. [DOI] [PubMed] [Google Scholar]
- 35.Smith SK, Lugo-Morales LZ, Tang C, Gosrani SP, Lee CA, Roberts JG, et al. Quantitative Comparison of Enzyme Immobilization Strategies for Glucose Biosensing in Real-Time Using Fast-Scan Cyclic Voltammetry Coupled with Carbon-Fiber Microelectrodes. ChemPhysChem. 2018. May 22;19(10):1197–204. [DOI] [PubMed] [Google Scholar]
- 36.Lugo-Morales LZ, Loziuk PL, Corder AK, Toups JV, Roberts JG, McCaffrey KA, et al. Enzyme-Modified Carbon-Fiber Microelectrode for the Quantification of Dynamic Fluctuations of Nonelectroactive Analytes Using Fast-Scan Cyclic Voltammetry. Anal Chem. 2013. September 17;85(18):8780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SK, Gosrani SP, Lee CA, McCarty GS, Sombers LA. Carbon-Fiber Microbiosensor for Monitoring Rapid Lactate Fluctuations in Brain Tissue Using Fast-Scan Cyclic Voltammetry. Anal Chem. 2018. November 6;90(21):12994–9. [DOI] [PubMed] [Google Scholar]
- 38.Smith SK, Lee CA, Dausch ME, Horman BM, Patisaul HB, McCarty GS, et al. Simultaneous Voltammetric Measurements of Glucose and Dopamine Demonstrate the Coupling of Glucose Availability with Increased Metabolic Demand in the Rat Striatum. ACS Chem Neurosci. 2017. February 15;8(2):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. Paxino’s and Watson’s The rat brain in stereotaxic coordinates. Seventh edition. Amsterdam; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier; 2014. 1 p. [Google Scholar]
- 40.Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, et al. Voltammetric Detection of Hydrogen Peroxide at Carbon Fiber Microelectrodes. Anal Chem. 2010. June 15;82(12):5205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syed ECJ, Grima LL, Magill PJ, Bogacz R, Brown P, Walton ME. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat Neurosci. 2016. January;19(1):34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamid AA, Frank MJ, Moore CI. Dopamine waves as a mechanism for spatiotemporal credit assignment [Internet]. Neuroscience; 2019. August [cited 2020 Jan 3]. Available from: http://biorxiv.org/lookup/doi/10.1101/729640 [Google Scholar]
- 43.Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013. August;500(7464):575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus–reward learning. Nature. 2011. January;469(7328):53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe MW, Dombeck DA. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016. July;535(7613):505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, et al. Phasic Dopamine Release Evoked by Abused Substances Requires Cannabinoid Receptor Activation. J Neurosci. 2007. January 24;27(4):791–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roitman MF. Dopamine Operates as a Subsecond Modulator of Food Seeking. J Neurosci. 2004. February 11;24(6):1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahapatra A. Overeating, obesity, and dopamine receptors. ACS Chem Neurosci. 2010. May 19;1(5):346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wightman RM, Amatorh C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, et al. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988. May;25(2):513–23. [DOI] [PubMed] [Google Scholar]
- 50.Sombers LA, Beyene M, Carelli RM, Mark Wightman R. Synaptic Overflow of Dopamine in the Nucleus Accumbens Arises from Neuronal Activity in the Ventral Tegmental Area. J Neurosci. 2009. February 11;29(6):1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheer JF, Aragona BJ, Heien MLAV, Seipel AT, Carelli RM, Wightman RM. Coordinated Accumbal Dopamine Release and Neural Activity Drive Goal-Directed Behavior. Neuron. 2007. April;54(2):237–44. [DOI] [PubMed] [Google Scholar]
- 52.Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Mark Wightman R, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009. September;30(6):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci. 2008. August 19;105(33):11957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyder F, Rothman DL, Bennett MR. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci. 2013. February 26;110(9):3549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bélanger M, Allaman I, Magistretti PJ. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011. December;14(6):724–38. [DOI] [PubMed] [Google Scholar]
- 56.Killeen PR, Russell VA, Sergeant JA. A behavioral neuroenergetics theory of ADHD. Neurosci Biobehav Rev. 2013. May;37(4):625–57. [DOI] [PubMed] [Google Scholar]
- 57.Dienel GA. Brain Lactate Metabolism: The Discoveries and the Controversies. J Cereb Blood Flow Metab. 2012;32:1107–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dienel GA. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain: Lactate Shuttling Does Not Satisfy Stoichiometry. J Neurosci Res. 2017. November;95(11):2103–25. [DOI] [PubMed] [Google Scholar]
- 59.Dienel GA. Lactate Shuttling and Lactate use as Fuel after Traumatic Brain Injury: Metabolic Considerations. J Cereb Blood Flow Metab. 2014. November;34(11):1736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dienel GA. Does shuttling of glycogen-derived lactate from astrocytes to neurons take place during neurotransmission and memory consolidation? J Neurosci Res. 2019. August;97(8):863–82. [DOI] [PubMed] [Google Scholar]
- 61.Fillenz M The role of lactate in brain metabolism. Neurochem Int. 2005. November;47(6):413–7. [DOI] [PubMed] [Google Scholar]
- 62.Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 2017. August;26(2):361–374.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Díaz-García CM, Yellen G. Neurons rely on glucose rather than astrocytic lactate during stimulation. J Neurosci Res. 2019. August;97(8):883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, et al. Optogenetic Interrogation of Dopaminergic Modulation of the Multiple Phases of Reward-Seeking Behavior. J Neurosci. 2011. July 27;31(30):10829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 2009. May 22;324(5930):1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.da Silva JA, Tecuapetla F, Paixão V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018. February;554(7691):244–8. [DOI] [PubMed] [Google Scholar]
- 67.Saunders BT, Richard JM, Margolis EB, Janak PH. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci. 2018. August;21(8):1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


