Abstract
Background:
Prenatal alcohol exposure (PAE) is associated with a variety of structural abnormalities in the brain, including several within the para-limbic system. Children with PAE have higher rates of internalizing disorders, including depression and anxiety, which may be related to underlying limbic system anomalies.
Method:
Children ages 8–16 with PAE (n=41) or without PAE (n= 36) underwent an MRI of the brain and parents completed behavioral questionnaires about their children. Semi-automated procedures (FreeSurfer) were used to derive para-limbic volumes from T1 weighted anatomical images.
Results:
There were significant group differences (PAE vs. non-exposed controls) in the caudate, hippocampus, and the putamen; children with PAE had smaller volumes in these regions even after controlling for total intracranial volume. A trend-level association was seen between caudate volume and internalizing symptoms in children with PAE; smaller caudate volumes (presumably reflecting less optimal neurodevelopment) were associated with higher levels of anxiety and depression symptoms in these children.
Conclusions:
Caudate structure may be disproportionately affected by PAE and may be associated with the later development of internalizing symptoms in those affected by PAE.
Keywords: Fetal Alcohol Spectrum Disorder, Brain, Anxiety, Depression, Children
INTRODUCTION
Children with prenatal alcohol exposure (PAE) are well-documented as having a variety of neurodevelopmental abnormalities that contribute to emotional, behavioral and neurocognitive challenges. Those with PAE have high rates of externalizing disorders such as attention-deficit/hyperactivity disorder (ADHD) and disruptive behavior disorders as well as internalizing conditions such as anxiety disorders and depression (Famy et al., 1998, Hellemans et al., 2010, O'Connor et al., 2002, Weyrauch et al., 2017). Attempts to link underlying neuropathology with these outcomes have revealed a wide array of structural brain abnormalities in PAE, including smaller volumes in multiple areas such as in the para-limbic system. This study specifically focused on examining associations between brain abnormalities in the para-limbic system and internalizing symptoms in children with PAE.
Weyrauch and colleagues (2017) conducted a systematic review of 26 studies investigating psychiatric comorbidity in 5894 children with confirmed PAE. The authors computed weighted prevalence rates of 14.1% for depression and 7.8% for anxiety disorder across studies. For context, the expected population prevalence rates in this age range were 3.5% for depression and 0.7% for anxiety disorder. The high rate of depression is notable considering that adolescent depression is associated with increased risk for both suicide (the second leading cause of death in adolescents) and adult depression (Weissman et al., 1999, Centers for Disease Control and Prevention, 2019). Further evidence for the lifelong implications of PAE comes from a longitudinal study of 433 young adults with PAE that found that 23% had at least one suicide attempt by the age of 25 (Streissguth et al., 1996). Comparatively, Nock and colleagues (2013) found that the lifetime prevalence of suicide attempts in adolescents ages 13–18 was 4.1% and another study conducted by Piscopo et al. (2016) utilizing the National Survey on Drug Use and Health found that 1.6% of young adults ages 18–25 had attempted suicide in the past 12 months. The high rates of internalizing disorders in children and adolescents with PAE highlight the need to fully characterize and understand the specific neural mechanisms involved. Thus far, to the authors’ knowledge, there are no prior studies examining the underlying neurobiological factors that may contribute to higher rates of internalizing behavior in individuals with PAE; existing studies describe the increased incidence as a “secondary disability.”
The focus of the current study was guided by prior structural and functional magnetic resonance imaging (MRI) research on major depressive disorder (MDD). These studies have extensively investigated the para-limbic system (Figure 1) — specifically the hippocampus, amygdala, putamen, and caudate — as these regions are part of the mesolimbic dopamine system and hypothalamic-pituitary adrenal axis, which play key roles in depressive symptomology (Nestler and Carlezon Jr, 2006, Pariante and Lightman, 2008). Internalizing disorders are associated with smaller volumes in these regions as shown in a meta-analysis of 4118 adults with depression and 3545 controls (Arnone et al., 2012). Of these regions, the hippocampus has the most consistent and robust abnormalities (Schmaal et al., 2016, McKinnon et al., 2009, Videbech and Ravnkilde, 2004, Campbell et al., 2004). Unfortunately, there are not yet any large-scale meta-analyses of MRI studies focusing specifically on children and adolescents with internalizing disorders. However, individual studies have revealed similar findings in young patients with depression. For example, a longitudinal study examining growth in the hippocampus, amygdala, and putamen in 86 adolescents ages 12–16 found specific volumetric abnormalities in the hippocampus, amygdala, and putamen that were predictive of depressive disorders (Whittle et al., 2014). Abnormal functional connectivity between the amygdala, hippocampus, parahippocampus, and brainstem was also observed in adolescents and young adults with depression and the degree of abnormality in connectivity correlated with depressive symptoms (Cullen et al., 2014).
Figure 1. Para-limbic regions.
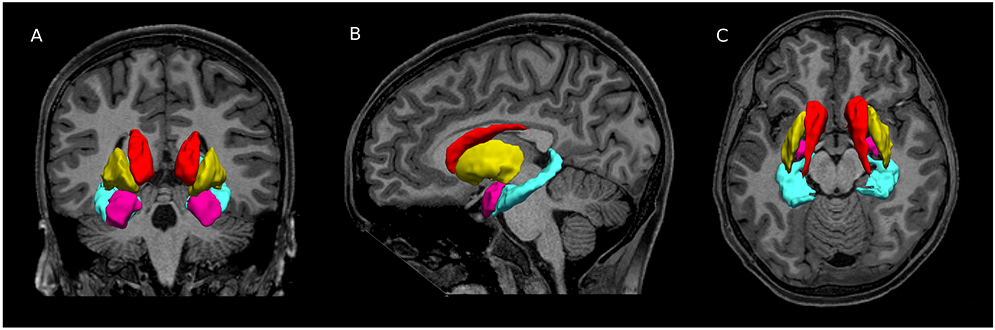
MRI structural images of a 13 year old male with prenatal alcohol exposure. Blue = hippocampus, pink= amygdala, red= putamen, and yellow = caudate.
Children and adolescents with PAE show a range of brain abnormalities due to the teratogenic effects of alcohol prenatally, including small overall brain volume and volumetric differences in specific regions. Some of these affected regions overlap with para-limbic regions identified in studies of internalizing disorders. In a meta-analysis of 32 MRI studies of PAE, Donald et al. (2015) identified consistent abnormalities in the hippocampus, caudate, and the putamen. Amygdala volume differences are less consistently observed, with some studies finding smaller volumes (Riikonen et al., 2005a, Nardelli et al., 2011) and other studies finding no differences from controls (Riikonen et al., 1999, Archibald et al., 2001, Roussotte et al., 2012). While there have been numerous studies attempting to identify volumetric brain abnormalities in children with PAE, there has not yet been research specifically attempting to identify relationships between volumetric brain regions and internalizing behaviors.
The current study compares para-limbic structural volumes in children and adolescents with and without PAE and evaluates the associations between brain structure and internalizing symptoms. Understanding these associations may contribute to insights with important implications for intervention in young people with PAE who have internalizing disorders. We hypothesized that participants with PAE would exhibit smaller volumes in the hippocampus, amygdala, putamen, and caudate. Additionally, we predicted that children with PAE would exhibit higher levels of internalizing symptoms and these symptoms would be associated with smaller volumes in the aforementioned regions. Because the sample of controls did not have elevated risk of exhibiting internalizing symptoms, our analyses did not assess relationships between the volume and internalizing symptoms for these individuals.
MATERIALS AND METHODS
Participants
All participants enrolled in the study were part of the fourth iteration of the Collaborative Initiative on FASD (CIFASD). Information about the CIFASD project is available in previous CIFASD publications (Mattson et al., 2010) and at www.cifasd.org. For the current study, participants with PAE were recruited from the University of Minnesota Fetal Alcohol Spectrum Disorder (FASD) Clinic between 2017 and 2019. PAE histories were obtained via clinical interviews and review of records. Acceptable sources of PAE histories included retrospective maternal report, social service, legal, adoption, and/or medical records. Participants were included in the PAE group if there was a history of heavy PAE (>13 drinks per week or >4 drinks per occasion at least once per week during pregnancy) or when such exposure was suspected in a child with a Fetal Alcohol Syndrome (FAS) diagnosis based on dysmorphology. In some cases, detailed history about exposure amounts was unattainable and decisions about inclusion or exclusion were made on the available evidence. For example, PAE was inferred if the mother was known to have had an alcohol use disorder and had contact with the police or social services during the pregnancy. Although in some cases other drugs were used, in all cases, alcohol was the primary and most commonly used substance of abuse. Participants were included in the non-exposed control group if there was a reliable history of only very minimal (<1 drink/week, never >2 drinks on any one occasion) or no reported exposure during pregnancy.
Recruitment methods for control participants included community advertising, mailings to control participants from other studies who agreed to be contacted, online advertising, and referrals from other study participants. Advertisements and flyers were placed in neighborhoods and online locations chosen to maximize the ethnic, racial, and socioeconomic diversity of the control participants.
Control participants were excluded for parent-reported history of prenatal substance exposure (other than tobacco and caffeine) and for diagnosed psychiatric conditions. Psychiatric co-morbidity was not an exclusion criterion for participants with PAE because it is well-recognized that co-morbidity is a common feature of PAE (Streissguth and O’Malley, 2000). Additional exclusion criteria for all participants included another developmental disorder (e.g., Autism), very low birthweight (<1500 grams), neurological conditions ( e.g., epilepsy), severe psychiatric disability that would prevent participation ( e.g., psychosis or mania), substance use by the participant, or contraindications to MRI scanning (e.g. implanted medical device, dental braces, and/or claustrophobia).
Procedures
Participants completed an MRI scan, cognitive testing and a brief physical exam after they assented to the study procedures and the informed consent process was completed with a parent. Parents also completed standardized assessments regarding the child’s behavior. All study procedures were reviewed and approved by the University of Minnesota’s Institutional Review Board (IRB). Participants were compensated per IRB guidelines for their time and provided travel reimbursement.
Classification
The standardized physical examination was completed by a trained dysmorphologist (KLJ) who had not previously met the child and who was blinded to the child’s PAE status. Key physical features assessed included ratings of the vermillion border of the upper lip and philtrum, measurement of palpebral fissure length, and measurement of occipital-frontal circumference. Height and weight were also recorded. As specified in Hoyme’s clinical guidelines (2016), Center for Disease Control growth charts (Kuczmarski et al., 2000) were used for height and weight percentiles and Nellhaus data (1968) were used for head circumference percentiles. Stromland’s norms (1999) were used to determine palpebral fissure length percentiles because of the close fit to actual growth trajectories (Astley, 2011). The University of Washington’s 4-Digit Diagnostic System “lipometer” (a photo-based grading system) was used to classify vermillion and philtrum scores. FASD classifications were made using the modified Institute of Medicine (IOM) criteria (Hoyme et al., 2016) (Table 1).
Table 1.
Characteristics of participants included in final analyses
| N(%) or mean (SD) | PAE (n=41) |
Control (n=36) |
Statistical Test |
|---|---|---|---|
| Age | 11.66 (2.42) | 12.08 (2.68) | t(75)= −0.732, p= 0.466 |
| Gender | |||
| Male | 20 (48.78%) | 19 (52.78%) | x2(1)= 0.123 p= 0.726 |
| Female | 21 (51.22%) | 17 (47.22%) | |
| Racial Categories | |||
| White | 16 (39.02%) | 34 (94.44%) | x2(5)=26.910 p< 0.001 |
| Black or African American | 7 (17.07%) | 0 (0.00%) | |
| American Indian/Alaska Native | 3 (7.32%) | 0 (0.00%) | |
| Asian | 2 (4.88%) | 1 (2.78%) | |
| Asian | 1 (2.44%) | 1 (2.78%) | |
| Native Hawaiian or Other Pacific Islander | 12 (31.71%) | 1 (2.78%) | |
| More than One Race | 12 (31.71%) | 1 (2.78%) | |
| Highest Level of Parental Education | x2(4)=18.359 p<.001 | ||
| High School | 1 (2.40%) | 3 (8.30%) | |
| Some College | 12 (29.30%) | 0 (%) | |
| Technical School | 8 (19.50%) | 3 (8.30%) | |
| 4-Year College | 6 (14.60%) | 14 (38.9%) | |
| Graduate School | 14 (34.10%) | 16 (44.40%) | |
| Nicotine/Tobacco Exposure | 7 (17.07% %) | 0 (%) | x2(1)=6.761 p=.009 |
| Alcohol Exposure | |||
| Alcohol Confirmed | 34 (82.93%) | ||
| Alcohol Suspected | 7 (17.07% %) | ||
| Other Drug Exposure | |||
| None | 9 (21.95%) | ||
| Drug Exposure Suspected | 10 (24.39%) | ||
| Drug Exposure Confirmed | 22 (53.66%) | ||
| Dysmorphic Facial Features | |||
| Lip (score 4 or 5) | 13 (31.71%) | 3 (8.33%) | x2(1)= 3.993 p= 0.046 |
| Philtrum (score 4 or 5) | 17 (41.46%) | 3 (8.33%) | x2(1)= 7.523 p= 0.006 |
| Palpebral Fissure (≤10th percentile) | 3 (7.32%) | 3 (8.33%) | x2(1)= 0.275 p= 0.600 |
| ≥ 2 Facial Features Present | 13 (31.71%) | 1 (2.78%) | x2(1)= 9.356 p= 0.025 |
|
Growth Deficiency (≤10th percentile) Height Weight |
|||
| Height | 4 (9.76%) | 0 (0.00%) | x2(1)= 2.842 p= 0.092 |
| Weight | 1 (2.44%) | 2 (5.56%) | x2(1)= 0.932 p= 0.334 |
| Deficient Brain Growth (≤10th percentile) a | |||
| Occipital-Frontal Circumference (OFC) | 5 (12.20%) | 0 (0.00%) | x2(1)= 4.013 p= 0.045 |
| IOM Diagnostic Category | |||
| FAS | 1 (2.44%) | ||
| Partial FAS | 12 (29.26%) | ||
| ARND | 26 (63.41%) |
Two participants in the PAE group and 10 participants in the control group did not have available physical exam information for analysis. Of the seven participants in the PAE group with suspected alcohol exposure, four were included because they met the study criteria for pFAS ; One participant had adoption records indicating maternal alcohol use; One participant had a corroborating report from the biological father indicating maternal alcohol use and also had two siblings with pFAS diagnoses; the final participant had a biological sibling with an FASD diagnosis and record of the removal of multiple other siblings from the biological mother’s home due to alcohol abuse. Additionally, the latter participant had a thin vermillion border at a previous point in development according to medical records.
Information from available clinical and research cognitive testing and standardized parent reports was used to determine the presence or absence of neurobehavioral impairment (one of the diagnostic criteria). Impairment was defined by scores 1.5 standard deviations or more from the mean in the direction of deficit. Hoyme outlined four categories of neurobehavioral functioning: global intellectual ability (IQ or IQ index score), behavioral and self-regulation, other cognitive function (memory, executive functioning, specific learning impairment, or visual spatial processing), and adaptive functioning. All participants in the PAE group exhibited dysfunction in at least two of the four described domains, except for two participants who only demonstrated impairments in one domain.
MRI Acquisition and Processing
Structural MRI data were acquired at the University of Minnesota’s Center for Magnetic Resonance Research on two 3T Siemens Prisma-fit scanners (Siemens, Erlangen, Germany) equipped with standard 32-channel head coils. For each participant, a T1-weighted (T1w) and T2-weighted (T2w) scan was acquired using pulse sequences and acquisition parameters developed for the human connectome project (https://www.humanconnectome.org/) aging and development studies (Harms et al., 2018). The HCP-A/D structural protocol includes two advantages for this study. First, the multi-echo MPRAGE T1w acquisition generates images with lower geometric distortion caused by susceptibility effects. Second, both the T1w and T2w pulse sequences include volumetric navigators that are used evaluate each k-space line for motion and reject and reacquire k-space lines that exceed a motion threshold, reducing motion effects in the data. Acquisition parameters are described in Table 2.
Table 2.
MRI Scan Parameters
| Sequence | Imaging Parameters |
|---|---|
| T1-weighted | TR = 2500 ms, TE = 1.8/3.6/5.4/7.2 ms, TI = 1000 ms, 208 slices, voxel size = 0.8 mm isotropic, FOV = 256 mm, flip angle = 8 degrees |
| T2-weighted | TR = 3200 ms, TE = 564 ms, 208 slices, voxel size = 0.8 mm isotropic, FOV = 256 mm, flip angle = 120 degrees |
NOTE: TR= repetition time, TE=echo time; FOV=Field of view, ms=milliseconds.
Structural MRI data were initially processed using the PreFreeSurfer stage of the Human Connectome Project’s Minimal Preprocessing Pipeline (v4.0.1) (Glasser et al., 2013) in order to align the T1w and T2w images, perform bias field and gradient distortion corrections, and register the data to MNI space. FreeSurfer version 6.0.0 was used for cortical parcellation and subcortical segmentation of the T1w volume (surfer.nmr.mgh.harvard.edu) (Dale, Fischl, & Sereno, 1999; Fischl et al., 2002). The T2w volume was included in the FreeSurfer processing stream to improve definition of the pial surface. Processing included removal of non-brain tissue, automated Talairach transformation, intensity normalization, tessellation of the grey matter / white matter boundary, topology correction, surface deformation, automated cortical labelling and volumetric segmentation. Para-limbic structural volumes used for these analyses were derived from the FreeSurfer “aseg” labels generated during this automated segmentation. Additionally, estimates of total intracranial volume (eTIV) were derived from standard FreeSurfer processing. To maximize reproducibility, our MRI preprocessing pipeline has been “containerized” using Singularity (Kurtzer, Sochat, & Bauer, 2017). This container will be made available upon request.
To ensure the accuracy of the FreeSurfer segmentation and parcellation, data were processed using the ENIGMA2 and ENIGMA3 quality assurance protocols available from the ENIGMA consortium (http://enigma.ini.usc.edu) (Hibar et al., 2015). An experienced evaluator (DJR) visually inspected images of the volumetric segmentation and cortical parcellation for each subject. Histograms of volume measurements for each of the para-limbic regions were evaluated and outlier subjects were flagged for further review. In cases of significantly aberrant FreeSurfer processing (such as failed boundary identification), the subject’s data were excluded from further analyses.
Parent-Child Reports
Internalizing measures were obtained from the Child Behavior Checklist (CBCL) (Achenbach, 1991) and the Behavior Assessment System for Children – Third Edition (BASC-3) (Reynolds, 2015). Both measures are parent-report questionnaires of typical and atypical child behavior. The assessments were completed by the child’s parent. Both measures yield t-scores (mean=50, standard deviation=10) with higher t-scores indicating greater symptom severity. The Cronbach’s alpha for the six selected scores was 0.948 reflecting a high level of consistency.
The CBCL (Achenbach, 1991) consists of 113 questions based on a three-point Likert scale (0=Not true (as far as you know), 1=Somewhat or sometimes true, 2=Very true or often true). Parents are asked to reflect on their child’s behavior in the last six months. Although the questionnaire covers a variety of domains, only relevant internalizing domains were included in the main analyses: Anxious/Depressed, Withdrawn/Depressed, and Internalizing Problems summary scores. Additionally, the Externalizing Problems summary score was used as a measure of divergent validity for the analyzed ROIs. While the CBCL does generate a Total Problems Score that is an averaged score of the Internalizing and Externalizing Problems summary scores, this measure was not used in any analyses.
The BASC-3 (Reynolds, 2015) is composed of 173 questions reflecting the caregiver’s observations over the last several months. Parents use a 4-point scale: Never, Sometimes, Often, and Almost Always. The following scores were utilized in the analyses: Anxiety and Depression scale scores, and the Internalizing Problems summary score.
Intelligence (IQ) Scores
Although IQ has not previously been identified to be a significant moderator for internalizing in children with PAE (Khoury et al., 2018), IQ scores were analyzed in relation to these measures to ensure that there was not a relevant relationship to the analyses. These scores were based on the full scale IQ scores from the Weschler Intelligence Scale for Children Fifth Edition (WISC-V). The full scale IQ score is derived from scores on domains of verbal comprehension, visual spatial, fluid reasoning, working memory, and processing speed. The mean standard score is 100 with a standard deviation of 15.
RESULTS
Demographics
Participants were 8–16 years old at the time of their study participation. A total of 80 participants (44 with PAE & 36 Controls) met inclusion criteria and were included in the study. Table 1 contains the demographics for the 77 participants (41 with PAE & 36 Controls) who were included in the analyses after eliminating the three participants with excessive movement during the MRI scan and aberrant image processing (detail of the quality assurance procedures are found in the MRI Acquisition and Processing section).
Chi-Square analyses were used to test for differences in gender, race, and income (based on parent report) between the PAE and control groups. Gender and ethnicity (Hispanic or Latinx) did not significantly differ between the groups; however, there were race differences with significantly more self-identified white individuals in the control group than in the PAE group (x2(1)=25.857 p< 0.001; Table 1). Additionally, the control group fell in significantly higher family income categories than the group with PAE on average (x2(6)=19.424 p=0.004). An independent sample t-test determined that there was not a group difference in age between the control and PAE groups (t(75)= −0.732, p= 0.466).
Controlling for Total Intracranial Volume
To determine if it was necessary to control for eTIV in the following analyses, a t-test compared eTIV between the control and PAE groups. As expected, participants with PAE had significantly smaller eTIV than controls (t(75)=−2.200, p=0.031). Therefore, in all of the following analyses, eTIV was controlled for. Because there were not significant differences in age or sex between the groups, we did not control for age or sex in any of the following analyses.
Race and SES
Because there were race and family income differences between the groups (PAE vs. controls), we ran additional analyses to determine if there were differences in any of the regions of interest (ROI) volumes (caudate, hippocampus, putamen, or amygdala) or internalizing measures that could have contributed to bias in the results. Two general linear models (GLM) controlling for eTIV were completed with both race and group (PAE vs. control) as independent variables, and the ROIs and internalizing measures as dependent variables, respectively. There were no significant race or group by race interaction effects for either model. Thus, we did not control for race in any of the following analyses. We completed a parallel set of analyses, but included a categorical measure of family income (ranges) as an independent variable in addition to group. For the model examining difference in the ROIs, there was not a significant effect of income category nor was there a significant group by income interaction. The model testing for differences in internalizing measures was significant for income category (Wilks’ Lambda = 0.375, F(36, 226.718)= 1.576, p= 0.026), but there was not a significant group by income interaction effect (Wilks’ Lambda= 0.438, F(36, 226.718)= 1.303, p= 0.128. Therefore, income was not considered in the following analyses.
Group differences in para-limbic regional volumes
To test for group (PAE vs. control) differences in brain volume in four ROIs, a GLM was used with four dependent measures (hippocampus, amygdala, putamen, and caudate) and eTIV entered as a covariate. Because our hypotheses did not predict a lateralized effect for volumes, volumes from the left and right hemisphere were combined to create bilateral volumes for all ROIs. We report adjusted p-values from Holm-Bonferroni corrections with an alpha of 0.05 in order to account for multiple comparisons. The overall model was significant for group, Wilks’ Lambda = 0.737, F(4, 71), p< 0.001. Follow-up univariate tests revealed smaller volumes in the PAE group compared to control group in the hippocampus (F(2,74)=9.917, corrected p-value (adjp)= 0.006; Table 3), caudate (F(2,74)=17.810, adjp < 0.001), and putamen (F(2, 74)=5.128, adjp= 0.050). There was not a significant difference between the PAE and control group for amygdala volume (F(2,74)= 3.807, adjp= 0.055).
Table 3.
Group differences in para-limbic volumes
| Para-limbic Region | Estimated Marginal Mean |
SE | F | p-value | Corrected p-value |
Cohen’s d |
|---|---|---|---|---|---|---|
| Hippocampus | PAE= 7918.32 | 98.02 | 9.917 | 0.002 | 0.006** | 0.78 |
| Control= 8377.23 | 104.82 | |||||
| Caudate | PAE= 7403.13 | 107.14 | 17.810 | < 0.001 | < 0.001*** | 0.98 |
| Control= 8075.31 | 114.57 | |||||
| Putamen | PAE= 10596.47 | 135.31 | 4.040 | 0.026 | 0.050* | 0.53 |
| Control= 11052.03 | 144.70 | |||||
| Amygdala | PAE= 3200.55 | 40.842 | 3.807 | 0.055 | 0.055 | 0.45 |
| Control= 3319.02 | 43.68 |
NOTE: Corrected p-values are derived from a Holm-Bonferroni correction. SE= standard error; PAE= prenatal alcohol exposure. Degrees of freedom = 74 for all analyzed regions.
corrected p ≤ .05,
corrected .05 < p ≤ .01,
corrected p ≤ .001
Group differences in internalizing measures
Internalizing data were available for all but four participants (39 with PAE & 34 Controls). Descriptive data outlining the number of at-risk and clinical scores for each group can be found in Table 4. Independent sample t-tests were used to evaluate group differences (PAE vs. controls) for the internalizing measures from the BASC-3 and CBCL. As expected, there were significant group differences for all measures. In all cases, those with PAE had more internalizing symptomology than controls. Full results are in Table 5.
Table 4.
Number of at risk and clinical scores on internalizing measures by group (PAE vs. Control)
| Behavioral Measure | PAE | Control | |
|---|---|---|---|
| CBCL | |||
| Internalizing Problems | At Risk | 10 | 5 |
| Clinical | 16 | 1 | |
| Anxious/Depressed | At Risk | 12 | 4 |
| Clinical | 13 | 1 | |
| Withdrawn/Depressed | At Risk | 14 | 6 |
| Clinical | 6 | 2 | |
| BASC-3 | |||
| Internalizing | At Risk | 16 | 3 |
| Clinical | 6 | 3 | |
| Anxiety | At Risk | 3 | 4 |
| Clinical | 11 | 3 | |
| Depression | At Risk | 17 | 1 |
| Clinical | 11 | 2 |
NOTE: CBCL = Child Behavior Checklist; PAE= prenatal alcohol exposure; BASC-3 = Behavior Assessment System for Children 3rd Edition.
Table 5.
Group differences in internalizing measure
| Behavioral Measure | Mean | SD | t | df | p-value | Corrected p-value |
Cohen’s d |
|---|---|---|---|---|---|---|---|
| CBCL | |||||||
| Internalizing | PAE=63.077 | 11.777 | 4.752 | 71.000 | < 0.001 | < 0.001*** | 1.117 |
| Control= 50.294 | 11.096 | ||||||
| Anxious/Depressed | PAE= 64.564 | 11.189 | 4.123 | 66.545 | < 0.001 | < 0.001*** | 0.954 |
| Control= 55.500 | 7.427 | ||||||
| Withdrawn/Depressed | PAE= 60.923 | 9.114 | 4.040 | 62.993 | < 0.001 | < 0.001*** | 0.932 |
| Control= 53.941 | 5.400 | ||||||
| BASC-3 | |||||||
| Internalizing Problems | PAE= 61.947 | 12.440 | 4.252 | 71.000 | < 0.001 | < 0.001*** | 1.000 |
| Control= 50.429 | 10.525 | ||||||
| Anxiety | PAE= 59.211 | 13.521 | 2.386 | 71.000 | 0.020 | 0.020* | 0.559 |
| Control= 51.771 | 13.075 | ||||||
| Depression | PAE= 64.053 | 10.235 | 5.484 | 71.000 | < 0.001 | < 0.001*** | 1.284 |
| Control= 50.743 | 10.492 |
NOTE: Corrected p-values are derived from a Holm-Bonferroni correction. CBCL = Child Behavior Checklist; BASC-3 = Behavior Assessment System for Children 3rd Edition; PAE= prenatal alcohol exposure.
corrected p ≤ .05,
corrected .05 < p ≤ .01,
corrected p ≤ .001
Internalizing measures and IQ
In order to rule out a relationship between IQ and internalizing symptoms, several analyses were conducted using the WISC-V full IQ score. First, an independent sample t-test was completed between the PAE and Control groups to test for IQ score differences. As expected, the PAE group had significantly lower IQ scores (t(74)= −7.166, p <.001). Next, bivariate correlations were run to determine if there were significant relationships between IQ and any of the internalizing measures in the PAE or Control groups, respectively. Neither group had significant relationships between IQ and internalizing. P-values for the PAE group ranged from .351-.634, while p-values for the Control group ranged from .534-.795.
Associations between brain volumes and internalizing measures
Partial correlations, controlling for eTIV, were conducted between three bilateral regional volumes (caudate, hippocampus and putamen) and the internalizing measures across the entire sample (Table 6). The amygdala was not included in these analyses because its volume did not significantly differ between the groups. Before Holm correction, the caudate significantly correlated with all internalizing measures from the BASC-3 and CBCL. After correction, the caudate remained significantly correlated with the internalizing problems summary score (r= −.374, p= 0.002; adjp= 0.012) and the anxious/depressed score (r= −.362, p= 0.002; adjp= 0.012). Neither the hippocampal nor putamen volumes significantly correlated with any of the internalizing measures after correcting for multiple comparisons.
Table 6.
Partial correlations of bilateral regions and internalizing measures controlling for eTIV
| Internalizing Measure | Caudate | Hippocampus | Putamen |
|---|---|---|---|
| CBCL | |||
| Internalizing | −0.374a* | 0.160 | −0.043 |
| Anxious/Depressed | −0.362 a* | 0.118 | −0.136 |
| Withdrawn/Depressed | −0.301b | −0.058 | −0.106 |
| BASC-3 | |||
| Internalizing Problems | −0.276b | −0.189 | 0.001 |
| Anxiety | −0.241 b | −0.071 | −0.021 |
| Depression | −0.277 b | −0.241* | −0.009 |
NOTE: CBCL = Child Behavior Checklist; BASC-3 = Behavior Assessment System for Children 3rd Edition; eTIV= total intracranial volume.
denotes an uncorrected p-value .01 ≤ p ≤ .05.
denotes an uncorrected p-value ≤ .01,
corrected p ≤ .05
Because there were group differences (PAE vs. control) in both caudate volume and internalizing symptoms that could inflate the correlations between the two, a second partial correlation analysis was conducted in the PAE group; these analyses also included eTIV. In the PAE group, these correlations showed a trend-level relationship between caudate volume and anxiety measures on both the CBCL (r= −.329, p= 0.053; Figure 2) and the BASC-3 (r= −0.296, p= 0.085; Figure 3); smaller caudate volume was associated at a trend level with higher levels of anxiety symptoms (Table 7). In the control group, there were no trends or significant correlations between the internalizing measures and caudate volume.
Figure 2. A scatterplot of bilateral caudate volumes and CBCL anxiety T-scores in the PAE group.
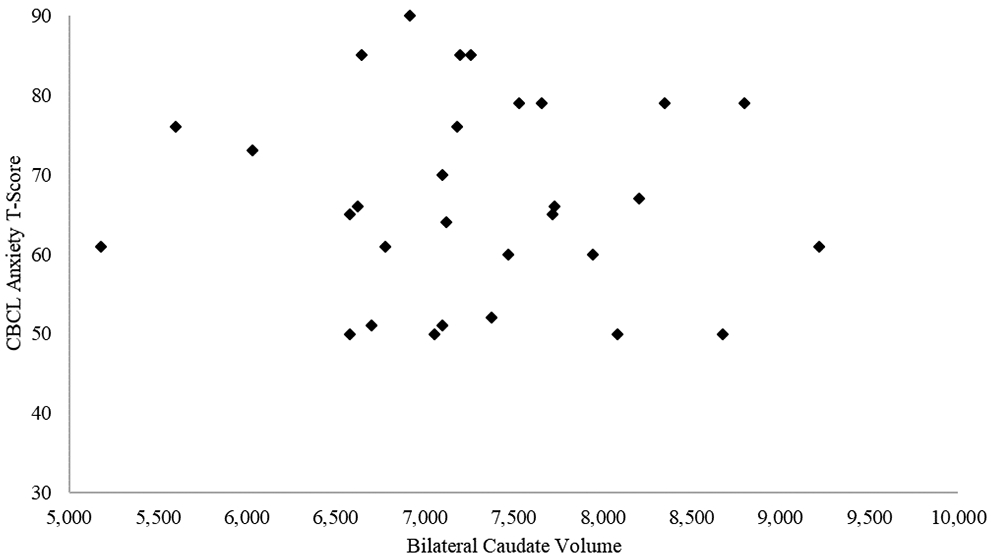
NOTE: CBCL = Child Behavior Checklist; PAE= prenatal alcohol exposure.
Figure 3 – A scatterplot of bilateral caudate volumes and BASC-3 anxiety T-scores.
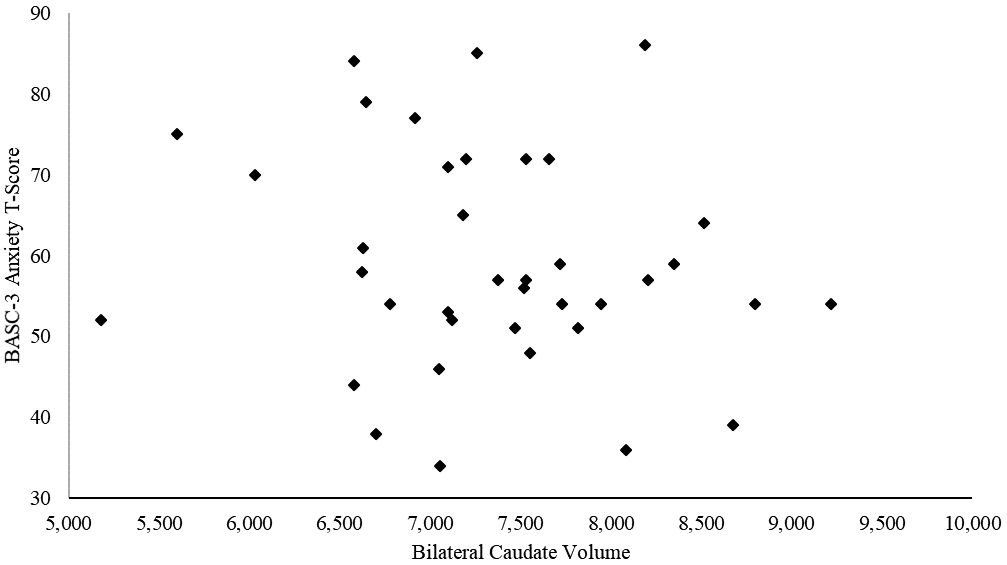
NOTE: BASC-3 = Behavior Assessment System for Children 3rd Edition; PAE= prenatal alcohol exposure.
Table 7.
Partial correlations of bilateral caudate volumes and internalizing measures controlling for eTIV separated by group
| Internalizing Measure | Caudate (PAE) |
|---|---|
| CBCL | |
| Internalizing | −0.279 |
| Anxious/Depressed | −0.329c |
| Withdrawn/Depressed | −0.232 |
| BASC-3 | |
| Internalizing Problems | −0.207 |
| Anxiety | −0.296 c |
| Depression | −0.174 |
NOTE. eTIV= total intracranial volume, PAE= prenatal alcohol exposure, BASC-3 = Behavior Assessment System for Children 3rd Edition; CBCL = Child Behavior Checklist.
uncorrected p-values .05≤ p ≤ .10.
Externalizing problems group differences and volumetric associations
In order to put the internalizing findings in a broader context, the externalizing problems sum score from the CBCL was also analyzed for group differences and associations with para-limbic volumes. As expected, externalizing behavior was more common in the PAE group compared to controls, t(71) = 11.237, p< 0.001. Nonetheless, two partial correlational analyses of caudate volume and externalizing symptoms, controlling for eTIV, revealed no significant effect for either the PAE group (PAE: r= 0.021, p= 0.903) or the control group (Control: r= −0.094, p= 0.603). Therefore, the relationship between caudate volumes and internalizing symptoms appears to be specific as opposed to being a function of a broad association between severity of brain effects and severity of behavioral effects.
DISCUSSION
This study aimed to compare para-limbic structural volumes between children with and without PAE and examine potential associations between brain structures and internalizing symptoms. Based on prior studies, it was hypothesized that the putamen, caudate, hippocampus, and amygdala volumes would be significantly smaller in those with PAE compared to controls due to the teratogenic effects of PAE. The data revealed volumetric differences in the caudate, hippocampus, and putamen (smaller in the PAE group) but no difference in the amygdala. High levels of parent-reported internalizing symptoms were expected in the PAE group and these were observed in the data. We hypothesized that the degree of para-limbic abnormality (as indicated by smaller regional volumes) would be associated with measures of internalizing symptoms. Of the regions that were significantly smaller in the PAE group (hippocampus, caudate, and putamen), the caudate was associated at a trend-level with the internalizing measures.
PAE-related volumetric associations in the basal ganglia, a region encompassing both the putamen and caudate, have been found in humans (Mattson et al., 1992, 1996, Archibald et al., 2001, Fryer et al., 2012, Inkelis et al., 2020) and in animal models (Mattson et al., 1994). In fact, the basal ganglia tends to be one of the most disproportionately affected areas in those with PAE (Nardelli et al., 2011, Archibald et al., 2001). Basal ganglia structures have been suggested to have multiple functions: the putamen is important for motor function as evidenced by its significant motor cortex connectivity, and the caudate receives input from the frontal and temporal lobes (Leh et al., 2007), which implies greater involvement with higher level behavior. Our data show volumetric differences in the PAE group in both ROIs, but trend-level associations only between internalizing behaviors and the caudate. Previous studies have associated smaller caudate volume with neurobehavioral deficits commonly exhibited by children with FASD, including difficulties in cognitive control (Fryer et al., 2012) and deficits in inhibitory performance (Fryer et al., 2007). The current study expands these findings to include suggestions of linkage to internalizing behaviors.
Although the current analyses revealing a trend toward smaller caudate volume in association with higher reported internalizing symptoms in children with PAE is novel, previous work has demonstrated this general association in a non-alcohol exposed samples. For example, smaller ganglio-thalamic-ovoid volumes, which include the basal ganglia and thalamus, in six-week-old infants has been linked to greater internalizing symptoms at 18 and 36 months per maternal report on the CBCL even when controlling for maternal psychopathology and head circumference (Herba et al., 2010). The association between smaller basal ganglia volumes and internalizing symptoms is seen throughout the life span – including during adolescence and adulthood (Arnone et al., 2012).
Furthermore, in cases with a specific injury to the caudate, such as calcification (Gluck-Vanlaer et al., 1996) or gliosis (Bhatia et al., 1993), greater depressive symptomology is seen. Internalizing symptoms are also seen in Huntington’s disease and Parkinson’s disease, both of which are categorized by basal ganglia abnormalities, specifically bilateral caudate atrophy (Bonelli et al., 2006, Muslimović et al., 2005). In these two diseases, depressive symptoms often present prior to other symptoms such as motor disturbance (Mendez et al., 1989, Muslimović et al., 2005).
The current study also revealed smaller hippocampal volumes in those with PAE compared to controls, which is consistent with previous literature in PAE (Nardelli et al., 2011, Willoughby et al., 2008). Although smaller hippocampal volumes are commonly found in adolescents with depression (Hulvershorn et al., 2011), we did not observe significant correlations between hippocampal volume and any of the internalizing measures. This lack of correlation may be due to differences in the etiology of internalizing symptoms for those with neurodevelopmental disorders like FASD compared to those with later-emerging conditions, including those with adolescent/adult onset of internalizing symptoms.
Lastly, previous literature on amygdala volume differences in children with PAE has been mixed - with some studies showing differences (Riikonen et al., 2005a, Nardelli et al., 2011) and others not finding differences (Archibald et al., 2001, Riikonen et al., 1999, Roussotte et al., 2012). It is important to note that some of the studies only found significant differences in amygdala volume when total intracranial volume was not controlled for in the analyses (Nardelli et al., 2011, Riikonen et al., 2005b). This study found that amygdala volumes trended towards being smaller in the PAE group. However, the analyses of this region did have a limited effect size for this region, Cohen’s d =.45, which is generally considered to be small to medium effect size.
Limitations
In evaluating the findings presented here, it is important to acknowledge one limitation of the study is the relative lack of participant diversity in terms of race and socioeconomic status (SES) as represented by family income. In this study, we did not find group by income interaction effects or group by race interaction effects in caudate volume or internalizing symptomology, but there were differences in race and SES between the PAE and control groups. Previous literature examining behavioral disturbances in children with PAE versus controls with matched age, sex, SES, ethnicity, and verbal IQ did find elevated levels of internalizing behavior on the CBCL in children with PAE (Mattson and Riley, 2000), suggesting that the effects seen in the current study are not likely due to a confound with race/SES. Future studies that are able to better match and/or account for these important potential confounds will help to further confirm these results.
The sample was also limited in terms of size and age range. The small sample size limited the power of the analyses, which could have prevented relationships between the internalizing symptoms and brain volumes from being detected. Future research would benefit from a larger, more robust sample to better decipher relevant group differences. Additionally, the sample in this paper only examined relationships in internalizing for children 8–16. While this perhaps does give some indication of relationships between para-limbic volumes and internalizing in late childhood and early adolescence, it does not necessarily describe this relationship across the lifespan for individuals with PAE. Furthermore, in this age group specifically it would be advantageous to obtain adolescent self-report of symptoms for internalizing behaviors. Gomez et al.(2014) found that the CBCL and the corresponding Youth Self-Report were particularly convergent in internalizing domains, thus illustrating the importance of adolescent self-report of symptoms in conjunction with parent reports.
A final limitation to note is the lack of biological maternal mental health diagnosis information. Because all participants in the PAE group were adopted, this information was not accessible. While there is literature suggesting that environmental factors (i.e. adoptive parental depression) play a more compelling role in the development of internalizing symptoms than genetic influences (Silberg et al., 2010, Singh et al., 2011), including this information in future analyses will contribute to the understanding of the underlying mechanisms of internalizing behaviors.
CONCLUSION
Children and adolescents with PAE are disproportionately affected by internalizing disorders – at substantially higher rates than the general population. This cross-sectional study represents an initial step in understanding the neurodevelopmental correlates of internalizing symptoms in PAE. Future plans for this study include following this sample longitudinally (15 month interval between MRI scans) to determine group trends in internalizing behaviors and changes in para-limbic structural volumes over critical stages of subsequent development.
Acknowledgments
Sources of Support: All of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Support for this research was provided by the NIAAA (5U01AA026102, 5U01AA014834, 5U24AA014815, 5U24AA014811, 5U24AA014815-16, 3U24AA014815-16S1), the National Institute of Biomedical Imaging and Bioengineering (NIBIB P41 EB027061), the Biotechnology Research Center (P41 EB015 894), the NINDS Institutional Center Core Grants to Support Neuroscience Research (P30 NS076408), and the High Performance Connectome Upgrade for Human 3T MR Scanner (1S10OD017974-01).
Footnotes
Conflicts of Interest: None to report.
REFERENCES
- ACHENBACH TM 1991. Manual for the Child Behavior Checklist/4-18 and the 1991 Profile. Burlington: Department of Psychiatry, University of Vermont. [Google Scholar]
- ARCHIBALD SL, FENNEMA-NOTESTINE C, GAMST A, RILEY EP, MATTSON SN & JERNIGAN TL 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental medicine and child neurology, 43, 148–154. [PubMed] [Google Scholar]
- ARNONE D, MCINTOSH A, EBMEIER K, MUNAFÒ M & ANDERSON I 2012. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European Neuropsychopharmacology, 22, 1–16. [DOI] [PubMed] [Google Scholar]
- ASTLEY SJ 2011. Canadian palpebral fissure length growth charts reflect a good fit for two school and FASD clinic-based U.S. populations. J Popul Ther Clin Pharmacol, 18, e231–41. [PubMed] [Google Scholar]
- BHATIA K, DANIEL S & MARSDEN C 1993. Familial parkinsonism with depression: a clinicopathological study. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 34, 842–847. [DOI] [PubMed] [Google Scholar]
- BONELLI R, KAPFHAMMER H-P, PILLAY S & YURGELUN-TODD D 2006. Basal ganglia volumetric studies in affective disorder: what did we learn in the last 15 years? Journal of neural transmission, 113, 255–268. [DOI] [PubMed] [Google Scholar]
- CAMPBELL S, MARRIOTT M, NAHMIAS C & MACQUEEN GM 2004. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry, 161, 598–607. [DOI] [PubMed] [Google Scholar]
- CENTERS FOR DISEASE CONTROL AND PREVENTION, N. C. F. I. P. A. C. 2019. 10 Leading Causes of Death by Age Group, United States: - 2017. [Google Scholar]
- CULLEN KR, WESTLUND MK, KLIMES-DOUGAN B, MUELLER BA, HOURI A, EBERLY LE & LIM KO 2014. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71, 1138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALD KA, EASTMAN E, HOWELLS FM, ADNAMS C, RILEY EP, WOODS RP, NARR KL & STEIN DJ 2015. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatrica, 27, 251–269. [DOI] [PubMed] [Google Scholar]
- FAMY C, STREISSGUTH AP & UNIS AS 1998. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry, 155, 552–4. [DOI] [PubMed] [Google Scholar]
- FRYER SL, MATTSON SN, JERNIGAN TL, ARCHIBALD SL, JONES KL & RILEY EP 2012. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 36, 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER SL, TAPERT SF, MATTSON SN, PAULUS MP, SPADONI AD & RILEY EP 2007. Prenatal alcohol exposure affects frontal–striatal BOLD response during inhibitory control. Alcoholism: clinical and experimental research, 31, 1415–1424. [DOI] [PubMed] [Google Scholar]
- GLUCK-VANLAER N, FALLET A, PLAS J & CHEVALIER JF 1996. [Depression and calcinosis of the basal ganglia: apropos of a case]. Encephale, 22, 127–31. [PubMed] [Google Scholar]
- GOMEZ R, VANCE A & GOMEZ RM 2014. Analysis of the convergent and discriminant validity of the CBCL, TRF, and YSR in a clinic-referred sample. Journal of Abnormal Child Psychology, 42, 1413–1425. [DOI] [PubMed] [Google Scholar]
- HARMS MP, SOMERVILLE LH, ANCES BM, ANDERSSON J, BARCH DM, BASTIANI M, BOOKHEIMER SY, BROWN TB, BUCKNER RL & BURGESS GC 2018. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage, 183, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLEMANS KG, SLIWOWSKA JH, VERMA P & WEINBERG J 2010. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev, 34, 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBA CM, ROZA SJ, GOVAERT P, VAN ROSSUM J, HOFMAN A, JADDOE V, VERHULST FC & TIEMEIER H 2010. Infant brain development and vulnerability to later internalizing difficulties: the Generation R study. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 1053–1063. [DOI] [PubMed] [Google Scholar]
- HOYME HE, KALBERG WO, ELLIOTT AJ, BLANKENSHIP J, BUCKLEY D, MARAIS AS, MANNING MA, ROBINSON LK, ADAM MP, ABDUL-RAHMAN O, JEWETT T, COLES CD, CHAMBERS C, JONES KL, ADNAMS CM, SHAH PE, RILEY EP, CHARNESS ME, WARREN KR & MAY PA 2016. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULVERSHORN LA, CULLEN K & ANAND A 2011. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain imaging and behavior, 5, 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INKELIS SM, MOORE EM, BISCHOFF-GRETHE A & RILEY EP 2020. Neurodevelopment in Adolescents and Adults with Fetal Alcohol Spectrum Disorders (FASD): a Magnetic Resonance Region of Interest Analysis. Brain Research, 146654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHOURY JE, JAMIESON B & MILLIGAN K 2018. Risk for Childhood Internalizing and Externalizing Behavior Problems in the Context of Prenatal Alcohol Exposure: A Meta-Analysis and Comprehensive Examination of Moderators. Alcoholism: Clinical and Experimental Research, 42, 1358–1377. [DOI] [PubMed] [Google Scholar]
- KUCZMARSKI RJ, OGDEN CL, GRUMMER-STRAWN LM, FLEGAL KM, GUO SS, WEI R, MEI Z, CURTIN LR, ROCHE AF & JOHNSON CL 2000. CDC growth charts: United States. Adv Data, 1–27. [PubMed] [Google Scholar]
- LEH SE, PTITO A, CHAKRAVARTY MM & STRAFELLA AP 2007. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neuroscience letters, 419, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTSON SN & RILEY EP 2000. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism: Clinical and Experimental Research, 24, 226–231. [PubMed] [Google Scholar]
- MATTSON SN, RILEY EP, JERNIGAN TL, EHLERS CL, DELIS DC, JONES KL, STERN C, JOHNSON KA, HESSELINK JR & BELLUGI U 1992. Fetal alcohol syndrome: A case report of neuropsychological, MRI, and EEG assessment of two children. Alcoholism: Clinical and Experimental Research, 16, 1001–1003. [DOI] [PubMed] [Google Scholar]
- MATTSON SN, RILEY EP, JERNIGAN TL, GARCIA A, KANEKO WM, EHLERS CL & JONES KL 1994. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicology and teratology, 16, 283–289. [DOI] [PubMed] [Google Scholar]
- MATTSON SN, RILEY EP, SOWELL ER, JERNIGAN TL, SOBEL DF & JONES KL 1996. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research, 20, 1088–1093. [DOI] [PubMed] [Google Scholar]
- MCKINNON MC, YUCEL K, NAZAROV A & MACQUEEN GM 2009. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of psychiatry & neuroscience: JPN, 34, 41. [PMC free article] [PubMed] [Google Scholar]
- MENDEZ MF, ADAMS NL & LEWANDOWSKI KS 1989. Neurobehavioral changes associated with caudate lesions. Neurology, 39, 349–349. [DOI] [PubMed] [Google Scholar]
- MUSLIMOVIĆ D, POST B, SPEELMAN JD & SCHMAND B 2005. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology, 65, 1239–1245. [DOI] [PubMed] [Google Scholar]
- NARDELLI A, LEBEL C, RASMUSSEN C, ANDREW G & BEAULIEU C 2011. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 35, 1404–1417. [DOI] [PubMed] [Google Scholar]
- NELLHAUS G 1968. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics, 41, 106–14. [PubMed] [Google Scholar]
- NESTLER EJ & CARLEZON JR WA 2006. The mesolimbic dopamine reward circuit in depression. Biological psychiatry, 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- NOCK MK, GREEN JG, HWANG I, MCLAUGHLIN KA, SAMPSON NA, ZASLAVSKY AM & KESSLER RC 2013. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry, 70, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNOR MJ, SHAH B, WHALEY S, CRONIN P, GUNDERSON B & GRAHAM J 2002. Psychiatric Illness in a Clinical Sample of Children with Prenatal Alcohol Exposure. The American Journal of Drug and Alcohol Abuse, 28, 743–754. [DOI] [PubMed] [Google Scholar]
- PARIANTE CM & LIGHTMAN SL 2008. The HPA axis in major depression: classical theories and new developments. Trends in neurosciences, 31, 464–468. [DOI] [PubMed] [Google Scholar]
- PISCOPO K, LIPARI RN, COONEY J & GLASHEEN C 2016. Suicidal thoughts and behavior among adults: Results from the 2015 National Survey on Drug Use and Health. NSDUH Data Review. [Google Scholar]
- REYNOLDS CR, & KAMPHAUS RW 2015. Behavior assessment system for children (3rd ed.). Bloomington: NCS Pearson, Inc. [Google Scholar]
- RIIKONEN R, SALONEN I, PARTANEN K & VERHO S 1999. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Developmental Medicine & Child Neurology, 41, 652–659. [DOI] [PubMed] [Google Scholar]
- RIIKONEN RS, NOKELAINEN P, VALKONEN K, KOLEHMAINEN AI, KUMPULAINEN KI, KÖNÖNEN M, VANNINEN R-LS & KUIKKA JT 2005a. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-β-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biological psychiatry, 57, 1565–1572. [DOI] [PubMed] [Google Scholar]
- RIIKONEN RS, NOKELAINEN P, VALKONEN K, KOLEHMAINEN AI, KUMPULAINEN KI, KONONEN M, VANNINEN RL & KUIKKA JT 2005b. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatry, 57, 1565–72. [DOI] [PubMed] [Google Scholar]
- ROUSSOTTE FF, SULIK KK, MATTSON SN, RILEY EP, JONES KL, ADNAMS CM, MAY PA, O'CONNOR MJ, NARR KL & SOWELL ER 2012. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human brain mapping, 33, 920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMAAL L, VELTMAN DJ, VAN ERP TG, SAMANN PG, FRODL T, JAHANSHAD N, LOEHRER E, TIEMEIER H, HOFMAN A, NIESSEN WJ, VERNOOIJ MW, IKRAM MA, WITTFELD K, GRABE HJ, BLOCK A, HEGENSCHEID K, VOLZKE H, HOEHN D, CZISCH M, LAGOPOULOS J, HATTON SN, HICKIE IB, GOYA-MALDONADO R, KRAMER B, GRUBER O, COUVY-DUCHESNE B, RENTERIA ME, STRIKE LT, MILLS NT, DE ZUBICARAY GI, MCMAHON KL, MEDLAND SE, MARTIN NG, GILLESPIE NA, WRIGHT MJ, HALL GB, MACQUEEN GM, FREY EM, CARBALLEDO A, VAN VELZEN LS, VAN TOL MJ, VAN DER WEE NJ, VEER IM, WALTER H, SCHNELL K, SCHRAMM E, NORMANN C, SCHOEPF D, KONRAD C, ZUROWSKI B, NICKSON T, MCINTOSH AM, PAPMEYER M, WHALLEY HC, SUSSMANN JE, GODLEWSKA BR, COWEN PJ, FISCHER FH, ROSE M, PENNINX BW, THOMPSON PM & HIBAR DP 2016. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry, 21, 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERG JL, MAES H & EAVES LJ 2010. Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. Journal of Child Psychology and Psychiatry, 51, 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH A, D'ONOFRIO B, SLUTSKE W, TURKHEIMER E, EMERY R, HARDEN K, HEATH A, MADDEN P, STATHAM D & MARTIN N 2011. Parental depression and offspring psychopathology: a children of twins study. Psychological medicine, 41, 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISSGUTH AP, BARR HM, KOGAN J & BOOKSTEIN FL 1996. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Final report to the Centers for Disease Control and Prevention (CDC), 96–06. [Google Scholar]
- STROMLAND K, CHEN Y, NORBERG T, WENNERSTROM K & MICHAEL G 1999. Reference values of facial features in Scandinavian children measured with a range-camera technique. Scand J Plast Reconstr Surg Hand Surg, 33, 59–65. [DOI] [PubMed] [Google Scholar]
- VIDEBECH P & RAVNKILDE B 2004. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry, 161, 1957–1966. [DOI] [PubMed] [Google Scholar]
- WEISSMAN MM, WOLK S, GOLDSTEIN RB, MOREAU D, ADAMS P, GREENWALD S, KLIER CM, RYAN ND, DAHL RE & WICKRAMARATNE P 1999. Depressed adolescents grown up. Jama, 281, 1707–1713. [DOI] [PubMed] [Google Scholar]
- WEYRAUCH D, SCHWARTZ M, HART B, KLUG MG & BURD L 2017. Comorbid Mental Disorders in Fetal Alcohol Spectrum Disorders: A Systematic Review. J Dev Behav Pediatr, 38, 283–291. [DOI] [PubMed] [Google Scholar]
- WHITTLE S, LICHTER R, DENNISON M, VIJAYAKUMAR N, SCHWARTZ O, BYRNE ML, SIMMONS JG, YÜCEL M, PANTELIS C & MCGORRY P 2014. Structural brain development and depression onset during adolescence: a prospective longitudinal study. American Journal of Psychiatry, 171, 564–571. [DOI] [PubMed] [Google Scholar]
- WILLOUGHBY KA, SHEARD ED, NASH K & ROVET J 2008. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc, 14, 1022–33. [DOI] [PubMed] [Google Scholar]


