Abstract
We developed a high-performance liquid chromatography mass spectrometry method for quantitating iohexol in 50 μL human plasma. After acetonitrile protein precipitation, chromatographic separation was achieved with a Shodex Asahipak NH2P-50 2D (5 μm, 2 × 150 mm) column and a gradient of 0.1% formic acid in acetonitrile and 0.1% formic acid in water over a 10 min run time. Mass spectrometric detection was performed on a Micromass Quatromicro triple-stage bench-top mass spectrometer with electrospray, positive-mode ionization. The assay was linear from 1–500 μg/mL for iohexol, proved to be accurate (101.3–102.1%) and precise (<3.4%CV), and fulfilled Food and Drug Administration (FDA) criteria for bioanalytical method validation. Recovery from plasma was 53.1–64.2% and matrix effect was trivial (−3.4 to −1.3%). Plasma freeze thaw stability (97.4–99.4%), stability for 5 months at −80 °C (95.5–103.3%), and stability for 4 h at room temperature (100.6–103.3%) were all acceptable. This validated assay using a deuterated internal standard will be an important tool in measuring iohexol clearance and determining glomerular filtration rate (GFR) in patients.
Keywords: iohexol, tandem mass spectrometry, assay, validation
1. Introduction
Iohexol is a widely used marker for measuring glomerular filtration rate (GFR) which is a major indicator of kidney function. Previously reported assays for iohexol have been developed, yet most do not use an isotopic internal standard (IS), which increases robustness, nor are validated according to U.S. Food and Drug Administration (FDA) guidance, Suppl.Table 1
Because cancer is a disease of the elderly, patients with cancer commonly also have impaired kidney function, as part of the normal age-related decline in kidney function [1]. Kidney function has always been an important metric used to determine therapy and guide dosing in oncology. However, the accuracy of the historic formulas used to estimate kidney function, such as creatinine clearance, have been called in to question as they often fail to capture true GFR, and the potential for developing new approaches to determine kidney function, including through iohexol clearance, has recently gained increasing attention in the field of oncology [1, 2]. To support preclinical studies and clinical trials in oncology that will utilize iohexol as a marker for GFR in the context of the optimal dosing of carboplatin (ClinicalTrials.gov Identifier: NCT03997370), we developed and validated a simple liquid chromatography tandem mass spectrometric (LC-MS/MS) assay to accurately and precisely quantitate iohexol in human plasma with a clinically relevant concentration range.
Previously reported iohexol bioanalytical assays (Suppl.Table 1) suffer from not covering clinically relevant concentration ranges, large sample volumes HPLC flow rates or run times, do not use internal standards or are not validated according to recent or any bioanalytical guidance.
2. Experimental
2.1. Chemicals and reagents
Iohexol (99.97% D0/(D5+D0)) was manufactured by GE Healthcare, (Shanghai, China) and obtained from UPMC Pharmacy and [D5]-iohexol (IS, (99.96% D5/(D5+D0))) was purchased from ALSACHIM (Graffenstaden, France), see Suppl.Fig. 1 for structures. Acetonitrile, methanol, and water (all HPLC grade) were purchased from Fisher Scientific (Fairlawn, NJ, USA). Formic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA). Control heparinized and EDTA human plasma was purchased from Lampire Biological Laboratories (Pipersville, PA, USA). Nitrogen for mass spectrometric applications was purified with a nitrogen generator (Parker Balston, Haverhill, MA, USA).
2.2. Chromatography
The LC system consisted of an Agilent (Palo Alto, CA, USA) 1100 SL autosampler kept at ambient temperature, an Agilent 1100 Binary Pump, and a HILIC Shodex (New York, NY USA) NH2P-50 2D (5 μm, 2×150 mm) column kept at ambient temperature. Mobile phase solvent A consisted of 0.1% formic acid in acetonitrile and mobile phase solvent B consisted of 0.1% formic acid in water. A flow rate of 0.4 mL/min was maintained throughout analysis. The initial mobile phase composition was 10% solvent B and increased linearly to 50% over 2.0 min where it was held until 4.0 min. Between 4.0 and 4.1 min, the system returned to initial conditions with re-equilibration until 10 min, followed by injection of the next sample. The injection volume was 1 μL, and the total run time was 10 min.
2.3. Mass spectrometry
A Waters (Milford, MA, USA) Quattro Micro triple-stage, benchtop quadrupole mass spectrometer with electrospray ionization in positive-mode, multiple reaction monitoring (MRM) mode was used for mass spectrometric detection. The mass spectrometer settings were as follows: capillary voltage 4.0 kV; cone voltage 40 V; source temperature 120 °C; desolvation temperature 450 °C; cone gas flow 50 L/h; desolvation gas flow 550 L/h; collision voltage 20 V; and both quadrupoles 1 and 3 with low mass and high mass resolution set at 12.0. A dwell time of 0.20 s and an interscan delay of 0.02 s were used. Monitored MRM m/z transitions were 821.5>803.5 for iohexol and 826.5>808.5 for [D5]-iohexol.
2.4. Preparation of calibration standards and quality control samples
Stock solutions of iohexol were diluted from clinical dosing solution at 10 mg/mL in water and stored on the benchtop at room temperature. Stock solutions of [D5]-iohexol were prepared independently at 1 mg/mL in 1x PBS (pH 7.4) and stored on the benchtop at room temperature. On the day of the assay, iohexol stock was diluted 10-fold in water to obtain a mixture working stock of 1 mg/mL and 0.1 mg/mL. These calibration solutions were diluted in human plasma to produce the following iohexol concentrations: 1, 3, 10, 30, 100, 300, and 500 μg/mL. For each calibration series, zero and blank samples were also prepared from 50 μl of control plasma. Quality control (QC) stock solutions were prepared independently and stored at −80 °C. These solutions were diluted in human plasma to produce the following QC samples of either: Lower Limit of Quantitation (LLOQ) 1 μg/mL, QC Low (QCL) 2 μg/mL; QC Mid (QCM) 20 μg/mL, and QC High (QCH) 400 μg/mL. The QCM value was chosen to represent the approximate midpoint of the calibration range in log space: QCM≈ LLOQ*(SQRT(ULOQ/LLOQ)). On the day of the assay, [D5]-iohexol was diluted to 0.2 mg/mL in water as an IS working stock.
2.5. Sample preparation
A volume of 50 μL of the standard, QC, or sample plasma was pipetted into a microfuge tube and 10 μL 0.2 mg/mL [D5]-iohexol was added. A total of 500 μL of acetonitrile was then added followed by vortexing for 1 min on a Vortex Genie-2 set at 10 (Model G-560 Scientific Industries, Bohemia, NY, USA). Samples were centrifuged at 14,000 × g at room temperature for 5 min. Supernatants were transferred to autosampler vials followed by injection of 1 μL into the LC-MS/MS system.
2.6. Validation procedures
2.6.1. Calibration curve and lower limit of quantitation (LLOQ)
Calibration standards and blanks were prepared (see paragraph 2.4 and 2.5) and analyzed in triplicate to establish the calibration range with acceptable accuracy and precision, as previously described [3].
2.6.2. Accuracy and precision
The accuracy and precision of the assay were determined by analyzing samples at the LLOQ, QCL, QCM, and QCH concentrations in 6 replicates each in 3 analytical runs, together with independently prepared, triplicate calibration curves, as previously described [3].
2.6.3. Selectivity and specificity
To investigate whether endogenous matrix constituents interfered with the assay, six individual batches of control, drug-free human plasma were processed and analyzed according to the described procedure. Responses of analytes at the LLOQ concentrations were compared with the response of the blank samples. Relevant cross-talk of iohexol and IS was characterized by detection in other multiple reaction monitoring (MRM) channels at the upper limit of quantitation (ULOQ).
Carry-over was assessed by injecting plasma samples with 500 μg/mL iohexol and 200 μg/mL IS, followed by serial plasma blank injections.
2.6.4. Extraction recovery and matrix effect
We determined the extraction recovery of iohexol from plasma by comparing the absolute response of an extract of control plasma to which the analyte had been added after protein precipitation, with the absolute response of an extract of plasma to which the same amount had been added before protein precipitation. The matrix effect by plasma matrix components was defined as the change of the absolute response of an extract of control plasma to which analyte had been added after the protein precipitation relative to the absolute response of solvent to which the same amount of the analyte had been added. Experiments were performed in replicates of four at the QCL, QCM and QCH concentrations.
2.6.5. Stability
The stability of iohexol in plasma at −80 °C was determined by assaying samples before and after storage for 5 months. The effect of 3 freeze/thaw cycles analyte concentrations on plasma was evaluated by assaying samples after they had been frozen (−80 °C) and thawed on 3 separate days and comparing the results with those of freshly prepared samples. The stability of iohexol in plasma during sample preparation was evaluated by assaying samples before and after 4 h of storage at room temperature and ambient light. All stability testing in plasma was performed in replicates of four at the QCL, QCM and QCH concentrations. To evaluate the stability of iohexol in reconstituted samples in the autosampler, we re-injected QC samples and calibration curves approximately 72 h after the first injection and compared the concentrations derived from the second injection with those derived from the first injection using the initial duplicate calibration curve, and relative to a fresh duplicate calibration curve. The results of the second runs were expressed as a percentage of their respective values in the first runs.
2.6.6. Additional validation items
The impact of hemolysis was assessed by adding 10% (v/v) pre-hemolyzed whole blood to blanks, LLOQ, QCL, QCM, and QCH samples (n=4) and incubating at room temperature for 4 h.
Heparinized plasma was evaluated as an alternative anticoagulated matrix to the standard EDTA plasma by analyzing QCL, QCM, and QCH samples (n=4) against both heparin and EDTA plasma. Mouse plasma was evaluated similarly to determine assay validity for mouse plasma.
The ability to analyze urine was evaluated by adding 10% (v/v) human urine to QCL, QCM, and QCH samples (n=4).
Dilutional integrity was shown by preparing plasma samples (n=3) at 1,000 μg.mL and analysis after 10-fold dilution (to 100 μg/mL) with control plasma.
2.7. Application of the assay
To document the potential applicability of the assay, we determined the pharmacokinetics of iohexol after IV administration of a 300 mg/kg dose of iohexol in saline (10 mL/kg) to female CFW (Swiss-Webster) mice. EDTA blood was collected by cardiac puncture after euthanization by CO2 inhalation at terminal time points (n=3 mice/time) of 2.5, 5, 10, 15, 30, 60 and 120 min after administration followed by centrifugation (12,000 × g for 4 min) to obtain plasma. Plasma was immediately stored at −80 °C, until LC-MS/MS analysis. After iohexol quantitation, compartmental pharmacokinetic analysis was performed using ADAPT5 and naively pooled data [4].
2.8. Incurred sample reanalysis and cross-validation
Upon completion of the validation, incurred sample re-analysis was performed on the mouse samples described above.
For external validation, we participated in an external quality assessment scheme (Equalis AB, Uppsala, Sweden). Values of unknown samples were reported and later compared to Equalis declared weighed-in concentrations of iohexol.
3. Results and Discussion
3.1. Validation of the assay
3.1.1. Chromatography
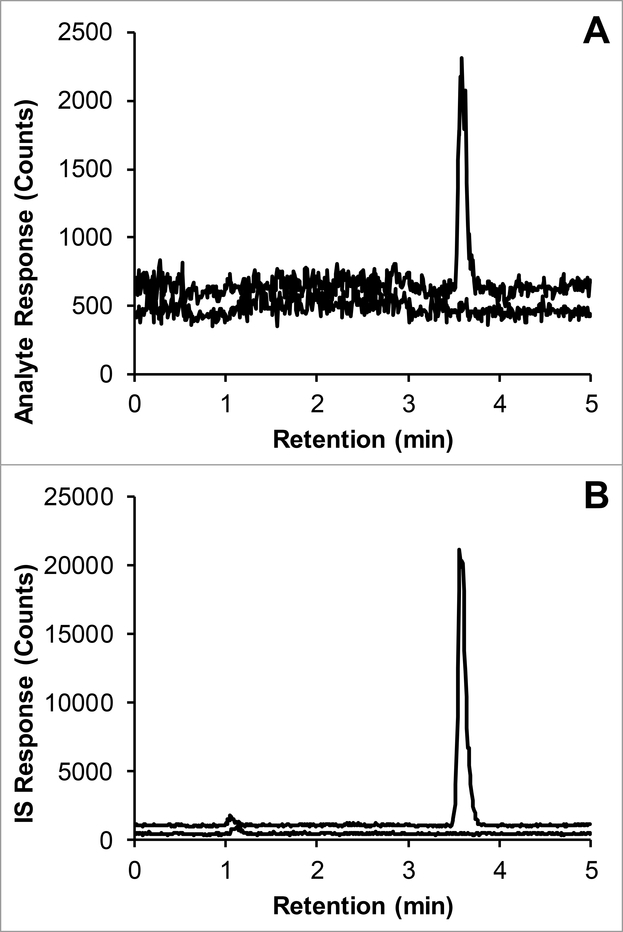
The approximate retention time of iohexol was 3.6 min, with a corresponding capacity factory of 3.3, with a void time of 0.8 min. Representative chromatograms of the LLOQ and IS in plasma are displayed in Fig. 1.
Fig. 1.
Representative chromatograms of: A) iohexol (m/z 821.5>803.5; 3.5 min) added to control plasma at the LLOQ concentration of 1 μg/mL (top trace with an offset of 150 counts) and control human plasma (bottom trace); B) [D5]-iohexol IS (m/z 826.5>808.5; 3.5 min) added to control plasma at a concentration of 0.2 mg/mL (top trace with an offset of 600 counts) and control human plasma (bottom trace).
3.1.2. Calibration curve and LLOQ
The selected assay range of 1–500 μg/mL fulfilled the FDA criteria for the LLOQ concentration and the calibration curve (calibrators within ± 15% of nominal concentrations, with LLOQ within ± 20%, and 75% and a minimum of six non-zero calibrators meeting these criteria in each run) [5]. Accuracies and precisions for each concentration from triplicate calibration curves prepared on three separate days are reported in Suppl.Table 2. A representative calibration curve and the corresponding correlation and regression coefficient are shown in Suppl.Fig. 2.
3.1.3. Accuracy and precision
The range of QC based accuracies was 101.3 to 102.1%. The intra- and inter-assay precisions for the tested concentrations (LLOQ, QCL, QCM, QCH) were all within the defined acceptance criteria (accuracy: ± 15% of nominal concentrations; except ± 20% at LLOQ, precision: within-run and between runs: ± 15% CV, except ± 20% CV at LLOQ) (Table 1) [5].
Table 1.
Assay performance data for the quantitation of LLOQ, QCL, QCM, and QCH of iohexol concentrations in human plasma.
| Concentration (μg/mL) | Accuracy (%) | Intra-assay precision (%) | Inter-assay precision (%) |
|---|---|---|---|
| 1 (LLOQ) | 103.1 | 4.4 | * |
| 2 (QCL) | 101.3 | 3.4 | 3.3 |
| 20 (QCM) | 102.1 | 3.0 | 6.9 |
| 400 (QCH) | 101.8 | 2.6 | * |
n=18; 6-fold results, each in 3 separate runs, for each concentration.
The mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variation due to the performance of the assay in different runs [6]. LLOQ, lower limit of quantitation; QCL, QC Low; QCM, QC Mid; QCH, QC High.
3.1.4. Selectivity and specificity
The chromatograms of six individual control plasma samples contained no co-eluting peaks >20% of the analyte areas at the LLOQ concentration (interference ≤1.2%), and no co-eluting peaks >5% of the IS area (interference ≤0.09%).
Cross-talk calculations were performed and concentrations of either iohexol (500 μg/mL) or IS (500 μg/mL) were analyzed and their relative signals were compared. Results showed 0.02% cross talk of IS to analyte (at assay IS concentrations of 200 μg/mL, corresponding to 0.04 μg/mL iohexol, which is 4% of the LLOQ), and 0.07% cross talk of analyte to IS (at assay ULOQ concentrations of 500 μg/mL, corresponding to 0.35 μg/mL, which is 0.2% of the applied IS concentration).
Carry-over in the final assay was 0.018%, and 0.023% for iohexol and IS, respectively, see Suppl.Fig. 3.
3.1.5. Extraction recovery and matrix effect
The recoveries of iohexol ranged from 53.1% to 64.2% (CV 4.2% to 9.7%). Matrix effect ranged from −1.3 to 3.4% (CV 1.2% to 7.4%) (Table 2).
Table 2.
Recoveries of iohexol from human plasma and respective matrix eifects in human plasma extract, with coefficients of variation (CV).
| Concentration (μg/mL) | Recovery (%) | CV (%) | Matrix effect (%) | CV (%) |
|---|---|---|---|---|
| 2 (QCL) | 62.6 | 9.7 | 0.8 | 7.4 |
| 20 (QCM | 64.2 | 7.5 | 3.4 | 1.2 |
| 400 (QCH) | 53.1 | 4.2 | −1.3 | 2.3 |
n=4, for each concentration. QCL, QC Low; QCM, QC Mid; QCH, QC High
3.1.6. Stability
The stability of the analyte after 3 freeze thaw cycles (−80 °C to RT) was between 97.4 to 99.4%. Long-term stability of iohexol in plasma at −80 °C was adequate with recovery between 95.5 to 103.3%. The ratio of analyte to IS of plasma extracts of iohexol at the quality control concentrations, when reconstituted and kept in the autosampler for 72 h, was between 100.0 to 107.0% of the initial response (CV 4.7–9.1%), while with fresh calibration curves, these values were 86.1–90.8% of the initial response (CV 2.1–3.3%) (Suppl.Table 3) and the run passed the requirements set by the FDA (accuracy ± 15% of nominal) [7].
3.1.7. Additional validation items
Presence of hemolysis did not result in extra peaks in blank plasma and did not impact quantitation with an accuracy of 99.9–101.0% (CV 3.2–8.3%).
Heparinized plasma was free of interference and calibrators prepared in heparinized plasma used to quantitate QC samples resulted in adequate performance with an accuracy of 99.9–104.6% (CV 1.7–3.3%).
Mouse plasma calibrators performed adequately as well with an accuracy of 93.2–105.9% (CV 1.1–3.9%) and no extra peaks in blank plasma.
Urine, diluted in 9 parts control plasma, did not impact quantitation with an accuracy of 96.2–105.5% (CV 2.1–3.1%), suggesting urine samples can be analyzed after 10-fold dilution in control plasma.
Dilutional integrity was confirmed with 101.4% accuracy (CV of 1.6%) meeting the FDA requirements (accuracy ± 15% of nominal, and precision: ± 15% CV) [7].
3.2. Development
Aiming for maximum accuracy and precision, we kept the assay as simple as possible with minimum sample handling steps. The target concentration range of 1–500 μg/mL, allowed a single step “dilute and shoot” approach.
We initially separated the two well-described iohexol isomers [6, 7]. We confirmed that after freshly dissolving iohexol from powder, the ratio of the isomers (area peak 1/2 = 0.072) changes in time and after approximately 3 days will have reached a stable equilibrium at room temperature (area peak 1/2 = 0.17). For simplicity and because iohexol solutions prepared from powder are not encountered clinically, we ultimately chose not to separate iohexol isomers in our quantitative assay and instead, we decided to pursue a gradient that would ensure co-elution. Given the identified stability of iohexol at room temperature in the clinical dosing solution and exact concentration information from the manufacturer, we used the clinical formulation as stock solution to generate calibrators and QCs.
We explored various “dilute and shoot” protein precipitation approaches. Protein precipitation with acid was done with either a 10% perchloric acid solution or 10% trifluoroacetic acid solution added to plasma at a ratio of 1 volume acid to 9 volumes plasma. The perchloric acid protein precipitation proved to be superior as the supernatant was visually clear compared to precipitation with TFA that produced opaque supernatant. The protein precipitation with perchloric acid was followed by a dilution step with water (20 μL supernatant + 80 μL water) in the HPLC vial. Protein precipitation with acetonitrile consisted of adding 500 μL acetonitrile to 50 μL of plasma sample followed by direct injection of the resulting supernatant. Overall, acetonitrile was then chosen as the preferred protein precipitation reagent because it did not involve a dilution step and avoided the hazards of using acids.
For chromatographic separation we evaluated the following five columns: Phenomenex Synergi hydro RP 80A (50×2.0 mm, 4 μm), Phenomenex Synergi hydro RP 80A (100×2.0 mm, 4 μm), Phenomenex Kinetex 100 Å (50×2.0 mm, 2.6 μm), Luna PFP(2) (150×2 mm, 3 μm), and Shodex Asahipak NH2P-50 2D HILIC (150×2 mm, 5 μm). All columns demonstrated retention <5 min and adequate peak shape. The Asahipak NH2P-50 2D HILIC column was selected because the chosen sample preparation with acetonitrile resulted in a >90% organic solvent sample composition, which is not just compatible with, but even ideal for a HILIC starting gradient [8].
3.2.1. Extraction efficiency and the use of methanol for protein precipitation
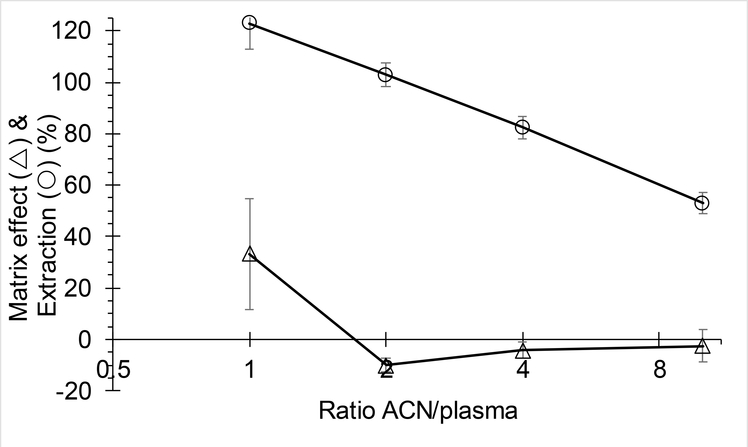
A 100% extraction recovery for iohexol was expected because it is a polar compound with physiological distribution limited to extracellular body water and no plasma protein binding [9]. To our surprise, extraction with acetonitrile was only approximately 50%, and repeat experiments confirmed this finding. We next designed an experiment where control plasma was treated with acetonitrile (followed by centrifuging) to precipitate proteins, and only then iohexol was added. The samples were subsequently vortexed and centrifuged, and the supernatant injected. This experiment, the technical intermediate between spiked plasma and spiked supernatant, resulted in an intermediate extraction recovery of 63%. This extraction recovery suggests that the majority of loss during extraction is due to association of iohexol with the protein precipitate and that this even occurs after proteins have been precipitated, suggesting a phenomenon on the surface of the protein precipitate. We next confirmed that this phenomenon was dependent on the ratio of acetonitrile to plasma, with a diminishing extraction efficiency going from 1 to 10 parts acetonitrile to plasma, see Fig. 2.
Fig. 2.
Matrix effect and extraction efficiency of iohexol from plasma at various ratios of acetonitrile to plasma, plotted on log-scale (mean±SD, n=3).
The assay using the described protein precipitation with acetonitrile was completely validated and used in the below reported application, incurred sample reanalysis, and cross-validation. Next, based on suggestions by Dr Siegmiller (see acknowledgements) who had experience using methanol to isolate iohexol from plasma, we evaluated the extraction efficiency when using methanol instead of acetonitrile, changing nothing else in the assay, which indeed resulted in complete extraction recovery, see Suppl.Table 4. We fully validated this methanol extraction variant method, which also fulfilled all criteria stipulated by the FDA guidance for method validation (Suppl.Table 5–Suppl.Table 6, and Suppl.Fig. 4–Suppl.Fig. 5). The ratio of analyte to IS of plasma extracts of iohexol at the quality control concentrations, when reconstituted and kept in the autosampler for 72 h, was between 98.7 to 100.5% of the initial response (CV 0.7–1.7%), while with fresh calibration curves, these values were 94.2–97.7% of the initial response (CV 0.7–1.8%) (Suppl.Table 7) and the run passed the requirements set by the FDA [7]. Six individual control plasma samples contained no co-eluting peaks >20% of the analyte areas at the LLOQ concentration (interference ≤3.9%), nor co-eluting peaks >5% at the IS (interference ≤0.06%). Presence of hemolysis did not result in extra peaks in blank plasma and did not impact quantitation with an accuracy of 98.1–99.8% (CV 2.6–4.3%). Deviations of 4th quarter 2019 Equalis external validation samples (57.5 and 77.4 μg/mL weighed-in values) were −0.7% and −5.0%.
3.3. Application of the assay
The assay was capable of quantitating iohexol in mice and pharmacokinetic compartmental modelling revealed a two-compartment model best fit the data (Fig. 3) with pharmacokinetic parameters and model performance as detailed in Suppl.Table 8. Visual inspection of standardized residuals of model of predictions demonstrated adequate model-fit as both a function of time and concentration (data not shown). Because iohexol is a marker for GFR, the clearance that was determined in this analysis equates to GFR in these mice. As such, the GFR of 0.0197 mL/min/g measured in our strain of mice is in line with the previously published value of 0.0124 mL/min/g [10].
Fig. 3.
Plasma concentrations of iohexol (○) in mice after a 300 mg/kg intravenous bolus dose. Concentration-time profile displaying the observed data (○) and the two-compartment model (—) of best fit.
3.4. Incurred sample reanalysis and cross-validation
Re-analysis of 21 samples yielded the following results: no samples with a difference larger than 20%; a −1.5% average difference, a 4.0% average absolute difference.
Deviations of 3rd quarter 2019 Equalis external validation samples (38.2 and 96.1 μg/mL weighed-in values) were −0.8% and 1.2%.
4. Conclusion
The objective of this study was to develop and validate an analytical method for the quantitation of iohexol in human plasma. Our use of a HILIC column ensured adequate retention of this highly polar analyte. Our objective is to apply this assay to samples obtained from patients who received a 5 mL bolus of iohexol (ClinicalTrials.gov Identifier: NCT03997370), with sampling between 2 and 6 hours post dose which will result in concentrations well within our assay range [11, 12]. Previously reported assays for the quantitation of iohexol in human plasma or serum are listed in Suppl.Table 1. The current assay has several advantages over those previously described, which include the use of relevant concentration ranges without sample dilutions, a small sample volume, a simple and easily implementable sample preparation, a short run time, and utilization of a deuterated IS that ensures robust performance. The suitability of iohexol as a renal filtration marker is, in large part, based on its highly hydrophilic nature with an apparent volume of distribution of 0.27 L/kg, indicating limited distribution in the extracellular water that does not easily penetrate membranes [9]. Yet, with the exception of Killbride (Chirobiotic T [13]) and Annesley (Oasis HLB [14]), all previously published bioanalytical assays are based on C8 or C18 reversed phase columns, which may not retain polar analytes such as iohexol very well, an issue that has been previously pointed out, as opposed to our use of a HILIC column that provided significant retention with a capacity factor of 3.0 [14].
In summary, this is the first assay that was validated according to 2018 FDA bioanalytical guidance, and that uses an isotopic IS, which takes into account the polar nature of iohexol. This assay will be a valuable tool in measuring iohexol clearance and determining GFR for evaluation of kidney function.
Supplementary Material
Highlights.
Iohexol is a widely used marker for measuring glomerular filtration rate (GFR)
Accurate GFR measurement is important in treatment decisions in oncology
We validated an iohexol LC-MS/MS assay from 1–500 μg/mL in 0.05 mL plasma
Acknowledgements
We are grateful for the helpful suggestions and critical feedback of Dr. Jesse Seegmiller from the Department of Laboratory Medicine and Pathology at the University of Minnesota.
Funding
Support: Grant UM1 CA186690, U24 CA247643 (NCI-CTEP) and R50 CA211241. This project used the UPMC Hillman Cancer Center Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by NCI P30 CA47904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Casal MA, Nolin TD, Beumer JH, Estimation of Kidney Function in Oncology, Clinical Journal of the American Society of Nephrology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beumer JH, Inker LA, Levey AS, Improving Carboplatin Dosing Based on Estimated GFR, American journal of kidney diseases : the official journal of the National Kidney Foundation 71(2) (2018) 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim KP, Parise RA, Holleran JL, Lewis LD, Appleman L, van Erp N, Morris MJ, Beumer JH, Simultaneous quantitation of abiraterone, enzalutamide, N-desmethyl enzalutamide, and bicalutamide in human plasma by LC-MS/MS, Journal of pharmaceutical and biomedical analysis 138 (2017) 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].D’Argenio DZS, A.; Wang X, ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software, Biomedical Simulations Resource, Los Angeles, 2009. [Google Scholar]
- [5].U.S. Department of Health and Human Services Food and Drug Administration, Guidance for Industry-Bioanalytical Method Validation, U.S.Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation; and Research Center for Veterinary Medicine, 2001. [Google Scholar]
- [6].Nyssen L, Delanaye P, Le Goff C, Peeters S, Cavalier E, A simple LC-MS method for the determination of iohexol and iothalamate in serum, using ioversol as an internal standard, Clinica chimica acta; international journal of clinical chemistry 463 (2016) 96–102. [DOI] [PubMed] [Google Scholar]
- [7].Foster SJ, Sovak M, Isomerism in iohexol and ioxilan. Analysis and implications, Investigative radiology 23 Suppl 1 (1988) S106–9. [DOI] [PubMed] [Google Scholar]
- [8].Hemstrom P, Irgum K, Hydrophilic interaction chromatography, Journal of Separation Science 29(12) (2006) 1784–1821. [DOI] [PubMed] [Google Scholar]
- [9].Olsson B, Aulie A, Sveen K, Andrew E, Human pharmacokinetics of iohexol. A new nonionic contrast medium, Investigative radiology 18(2) (1983) 177–82. [DOI] [PubMed] [Google Scholar]
- [10].Qi Z, Breyer MD, Measurement of glomerular filtration rate in conscious mice, Methods in molecular biology 466 (2009) 61–72. [DOI] [PubMed] [Google Scholar]
- [11].Krutzen E, Back SE, Nilsson-Ehle I, Nilsson-Ehle P, Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate, The Journal of laboratory and clinical medicine 104(6) (1984) 955–61. [PubMed] [Google Scholar]
- [12].Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G, Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate, Journal of the American Society of Nephrology : JASN 6(2) (1995) 257–63. [DOI] [PubMed] [Google Scholar]
- [13].Kilbride HS, Stevens PE, Eaglestone G, Knight S, Carter JL, Delaney MP, Farmer CK, Irving J, O’Riordan SE, Dalton RN, Lamb EJ, Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly, American journal of kidney diseases : the official journal of the National Kidney Foundation 61(1) (2013) 57–66. [DOI] [PubMed] [Google Scholar]
- [14].Annesley TM, Clayton LT, Ultraperformance liquid chromatography-tandem mass spectrometry assay for iohexol in human serum, Clinical chemistry 55(6) (2009) 1196–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.