Abstract
Nature teaches us that form precedes function, yet structure and function are intertwined. Such is the case with synapse structure, function, and plasticity underlying learning, especially in the hippocampus, a crucial brain region for memory formation. As the hippocampus matures, enduring changes in synapse structure produced by long-term potentiation (LTP) shift from synaptogenesis to synapse enlargement that is homeostatically balanced by stalled spine outgrowth and local spine clustering. Production of LTP leads to silent spine outgrowth at P15, and silent synapse enlargement in adult hippocampus at 2 hours, but not at 5 or 30 minutes following induction. Here we consider structural LTP in the context of developmental stage and variation in the availability of local resources of endosomes, smooth endoplasmic reticulum and polyribosomes. The emerging evidence supports a need for more nuanced analysis of synaptic plasticity in the context of subcellular resource availability and developmental stage.
Introduction
Analysis of LTP provides a powerful window into cellular mechanisms of learning. Hence, LTP is mostly studied in the hippocampus, a brain region required to form memories. The importance of prior activation history and specific induction paradigms are increasingly emphasized to understand mechanisms of LTP.[1–3] Dendritic spines are tiny protrusions that stud the surface of dendrites and host most of the excitatory synapses throughout the brain. The importance of context arises even when single spine synapses are potentiated by glutamate uncaging.[3] Most experiments image changes in spine structure as a proxy for synapse growth, and usually end within an hour after onset of potentiation. Such experiments have revealed exquisite detail about molecular and cellular mechanisms controlling spine structural plasticity during the early phase of LTP. Here we consider more enduring structural LTP in the context of developmental stage and availability of local resources.
LTP Enhances Synaptogenesis at P15 but Stalls Spine Outgrowth in Adults
To investigate enduring LTP, hippocampal slices are prepared, allowed to rest for 3–4 hours, and then test pulses are delivered at a frequency of one per 2 minutes for 30–40 minutes to establish baseline response. Then LTP is induced with a pattern of theta-burst stimulation (TBS) that fully saturates LTP.[4, 5] The number and frequency of test pulses is matched in control and LTP conditions for varying times post-TBS. Three-dimensional reconstruction from serial section electron microscopy (3DEM) obtained at different times post-TBS provides time-series snapshots of the underlying structural plasticity.
In stratum radiatum of rat hippocampal area CA1, 3DEM shows that spine density reaches about a third of adult levels by postnatal day (P)15 (Fig. 1). Prior work shows this density reaches ~80% of adult levels one week later at P21.[6] Thus, P15 is an age when the rate of natural synaptogenesis is high. In P15 rat hippocampal slices, control test pulses markedly reduce spine outgrowth over time (Fig. 1B). The TBS counteracts inhibited spine outgrowth and the resulting LTP enhances spinogenesis by 2 hours (Fig. 1B), but not at 5 or 30 minutes after TBS. The LTP-related synaptogenesis adds small dendritic spines, while the density of large spines remains essentially stable across time for both the LTP and control conditions. At P15, synapse dimensions on the LTP-related new spines are comparable to those on control small spines. Synapse dimensions on large spines are also comparable across perfusion-fixed, control, and LTP conditions.[5, 7]
Figure 1:
LTP enhances synaptogenesis at P15 but stalls spine outgrowth in adults. A) 3DEMs of dendrites from oblique dendrites in s. radiatum of hippocampal area CA1 at P15 representing the 50th percentile rank. Spine density in the 2-hour (2h) control is less than (red <) the 2h LTP condition. B) Quantification of spine density (spines/ μm) length of dendrite in s. radiatum of perfusion-fixed hippocampus (PF), and after 5 minutes (5’), 30’, and 2 hr in the control or LTP conditions. C) 3DEMs of dendrites from adult hippocampal s. radiatum representing the 50th percentile rank. D) Plots show spine density in the 2h control is greater than (>) in the 2h LTP condition. Comparing PF between ages in B and D reveals natural age-dependent synaptogenesis. In all graphs, data were controlled for spine head diameter (HD). The LTP effects were not evident at 5 or 30 minutes; hence, these data are not plotted here, for simplicity, but they are available in the original publications. (Yellow – dendrite, green – smooth endoplasmic reticulum, red – excitatory postsynaptic density surface area. Gray asterisks indicate significant (p<0.05) differences between the indicated condition and PF; red asterisks show significant LTP effects. The medium blue asterisk in D, shows significant differences between time points for small spines. Adapted from Bourne and Harris, 2011; Bell et al., 2014; Watson et al., 2016; Kulik et al., 2019).
The effects of control stimulation and TBS in adult rats (P60–75) are opposite from those found at P15 (Fig. 1C, D). Relative to perfusion-fixed brain, spine density is initially reduced in slices from adult hippocampus. Over time, delivery of control test pulses results in recovery of small spines. TBS stalls the small spine recovery while the density of larger, presumably more stable, spines is unchanged (Fig. 1D). These findings show profound developmental differences in the response of hippocampal neurons to saturating induction of LTP.
Resource dependent synapse enlargement and synaptogenesis
Multiple subcellular resources contribute locally to structural LTP. Smooth endoplasmic reticulum (SER) is a continuous internal membrane system that extends from the cell body into dendrites and into some spines. The SER regulates calcium and the synthesis and trafficking of lipids and proteins.[8] In locations where the SER elaborates, ER exit sites abound and can deliver resources of membrane and proteins to synapses.[9] The spine apparatus is a structure elaborated from SER into membrane sheets separated by dense plates containing the actin binding protein synaptopodin. In addition to its SER-related functions, the spine apparatus also may acquire Golgi-like properties that could provide post-translational modification of transmembrane proteins, although this function appears to be lacking in young dendrites.[10]
Local protein synthesis is another critical resource for structural LTP and is evidenced in electron microscopy by the presence of monosomes, polyribosomes (PR), or rough endoplasmic reticulum, all of which can be found in dendrites and spines. https://synapseweb.clm.utexas.edu/141-dendritic-spines-25 Local protein synthesis is required for normal synaptogenesis during development and for enduring LTP and learning.[11, 12] The PR are more readily identified in 3DEM than monosomes or RER; hence, their quantification provides a conservative estimate of local protein synthesis in various locations at the time when the tissue was fixed.
Endocytic, secretory, and recycling components also contribute to LTP under age and time-dependent constraints.[7, 9] These subcellular structures are highly dynamic with rapid rates of turnover. Thus, their presence or absence relative to time post-TBS is also a conservative reflection of their roles in spine formation and regulated synaptic growth.
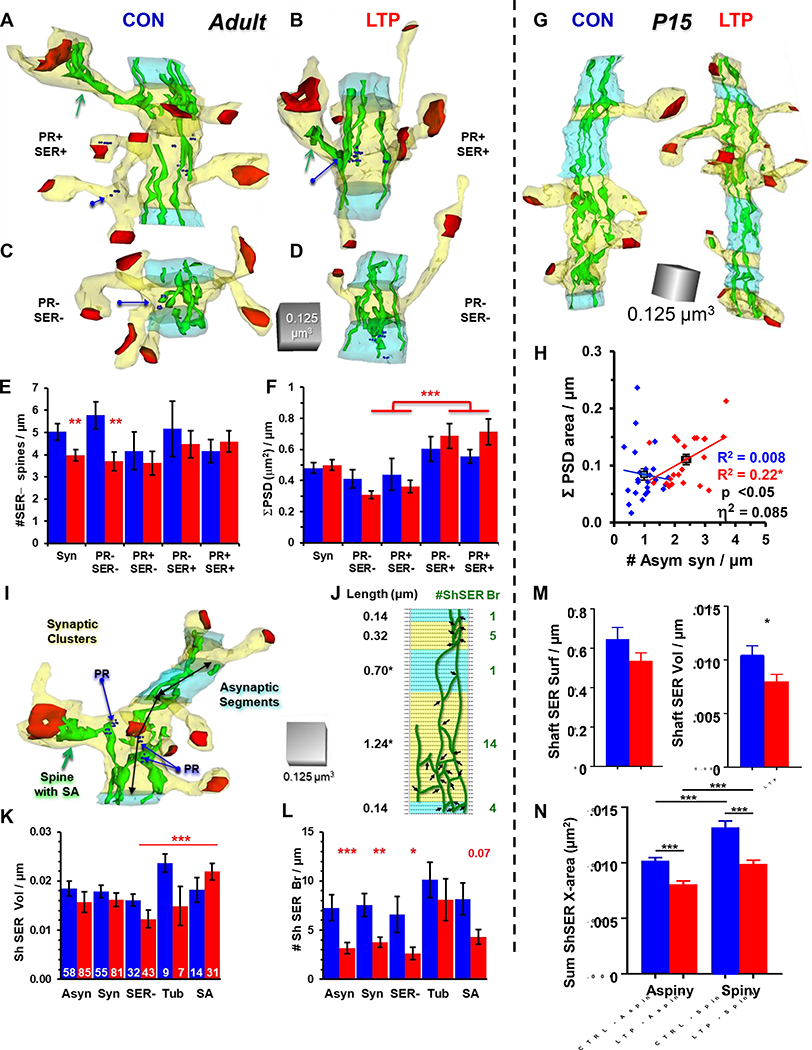
In adults, only 10–15% of dendritic spines in s. radiatum of hippocampal area CA1 contain a tubule of SER or a fully elaborated spine apparatus (Fig. 2A).[13] There is no change in this overall low frequency of spines that contain SER. By 2 hours after induction of LTP more of the SER-containing spines have acquired a spine apparatus (Fig. 2B). The relative decrease in spines containing a single tubule of SER suggests that the spine apparatus could be elaborated locally in a spine from a single tubule of SER following LTP.[14]
Figure 2:
Role of SER and polyribosomes in supporting synaptic growth after LTP. A) Electron micrographs and A’) 3DEMs of dendritic spines without SER, with a simple tubule of SER, or a fully elaborated spine apparatus (SA). B) Following LTP in adults, the frequency of SER containing spines does not change; however, there is a significant shift from a single tubule (T) to the SA form of SER (*, p<0.5, n= number of spines in each condition). C) Electron micrograph and C’) 3DEM of spine containing a polyribosome, but no SER. D) The LTP-related synapse enlargement is minimal in spines lacking PR or SER, is greater on spines that retain PR, and is greatest on spines containing SER. Each graph illustrates the actual PSD areas, controlled for head diameter, and plotted on a log-normal scale, with correlation values (R2), and results of ANCOVA (p values and effect sizes, η2). E) At P15, most of the new spines produced 2 hours after LTP induction have small synapses and contain no SER. F) 3DEM of dendritic segment from P15 illustrating secretory compartments increase in spines after LTP. G) At P15, the increased secretory elements are primarily small vesicles (sv) or recycling compartments (RC) while some are also coated pits (cp) coated vesicles (cv) or large clear vesicles (LV). Amorphous vesicles and degradative structures are not elevated significantly.
The frequency of PR-containing spines (Fig. 2C) also changes over the time course of LTP in adult hippocampus. The PR+ spine frequency is elevated at 5 and 30 minutes after TBS; however, by 2 hours post-TBS PR+ spines are reduced relative to controls at the same time point.[13] This effect is dependent on the induction protocol, because the PR remain elevated in spines for at least 2 hours following tetanus-induced LTP in adult hippocampus.[15] Growth in the postsynaptic density (PSD) surface area was greatest on spines that contained SER, regardless of whether PR were present in the spine (Fig. 2D). This growth was not limited by spine head size. Although PR and SER were rarely captured in the same spine, their spine synapses were as large as the spines containing SER alone in both control and LTP conditions (Fig. 2D). More work is needed to determine whether monosomes or RER are differentially expressed across time following induction of LTP, which could reflect synthesis of different populations of proteins.[16]
The SER rarely occurs in electron micrographs of dendritic spines from developing neurons. This rare occurrence might reflect the highly dynamic state of SER making quick visits without stopping to stabilize a tubule or form a spine apparatus.[17] At P15, the LTP-enhanced synaptogenesis involves formation of spines that lack SER (Fig. 2E).[7] Instead the new small spines contain more secretory compartments, especially large and small vesicles (Fig. 2F, G). These vesicles are likely derived from ER exit sites or recycling endosomes.[7, 9]
At P15, the PR are elevated for at least 2 hours after TBS saturated LTP, especially at the base of dendritic spines.[11] PR frequency is also high in spines that form during control stimulation in adult hippocampus. These results suggest that highly dynamic, age-dependent, and state-dependent utilization of local resources supports synaptogenesis during LTP in developing neurons or recovering adult slices, and the enlargement of synapses following LTP in adults.
Maturation of Homeostasis and Spine clustering
Recent experiments using optogenetics, live imaging, and computational models suggest that clusters of spines cooperate to enhance the efficacy of particular inputs during plasticity and learning.[18–24] The redistribution of subcellular resources could be critical in determining where such spine clustering hotspots arise. During LTP, do the enlarging synapses on SA-containing spines sequester resources and prevent neighboring spine outgrowth, or do they share with neighbors and deprive distant spine outgrowth?
To answer this question, clusters are defined by the overlapping origins of spines and shaft synapses (Fig. 3). The spine/synapse clusters are surrounded by asynaptic dendritic regions (>120 nm) without intervening spine origins or shaft synapses. In adults, some clusters contain resource rich spines (Fig. 3A, B), while other clusters have no SER or PR in the spines, only in the dendritic shaft (Fig. 3C, D). In adults, spine outgrowth is stalled in synaptic clusters that have no resource-rich spines (PR- SER-spines, Fig. 3E). Clusters having at least one SER+ spine recover the same spine density as controls (Fig. 3E). Total synaptic weight is measured as the summed PSD surface area across all synapses per unit length of dendrite in the cluster. In adults, the total synaptic weight is balanced across all synaptic clusters (Fig. 3F). However, total synaptic weight is elevated following LTP, in synaptic clusters that had SER+ spines (Fig. 3F). Thus, in adults, LTP engages a homeostatic process that enlarges some synapses, creates spine clusters around them, and stalls distant spine outgrowth to balance total excitatory synaptic input along the dendrite.[13] At P15, the LTP-enhanced synaptogenesis results in a greater total synaptic weight per length of dendrite (Fig. 3G, H). Together these findings suggest that LTP preserves the normal process of synaptogenesis in the developing system but encounters a profound regulation of total synaptic weight as the potentiated synapses enlarge in adults. The regulation occurs at a distance from the enlarging spine, which shares its resources locally to create a cluster of stronger dendritic spines.
Figure 3:
Resource regulation of spine clusters. A-D) Representative dendritic segments from adult hippocampal slices under control and LTP conditions. Resource rich clusters have spines that contain PR or SER and resource poor clusters have no PR- or SER-containing spines. Each reconstruction is at about the 50th percentile rank within condition by spine density within the synaptic cluster (yellow) which is surrounded by an asynaptic region (light blue that is least 120 nm long, and averages 250 nm in both control and LTP conditions). E) The density of spines without SER is reduced overall in the synaptic clusters (Syn) following LTP, and this effect only occurred in clusters that lacked resource-rich spines (**p<0.01). F) Summed PSD area is balanced across all synaptic clusters and is greater following LTP in clusters that have resource-rich spines (***p<0.001). G) Representative dendritic segments from P15 hippocampal slices under control and LTP conditions. H) Synaptogenesis following LTP increases the mean (black squares) summed PSD area in proportion to the increase in spine density, with an overall effect size of 8% (η2). I-J) Calculating the number of SER branches per synaptic cluster or asynaptic segment length in adults. K) The volume of SER in the dendritic shaft increases following LTP in those clusters that had a spine with a spine apparatus (SA). L) Overall, the number of SER branches in the dendritic shaft decreases following LTP in adults but is retained in the clusters that contain spines with SER tubules (Tub) or SA. M) At P15, Shaft SER volume decreased as does the N) complexity of shaft SER in both aspiny and spiny segments. (Adapted from: Chirillo et al., 2019; Watson et al., 2015; and Kulik et al., 2019).
The synaptic crosstalk between the LTP-enlarged spines that preserves and strengthens its neighbors in a cluster could be mediated by the SER in the dendritic shaft via a local spread of calcium release form the stores.[25, 26] To test this hypothesis using 3DEM in adult hippocampal slices, the volume of SER is measured and normalized across the dendritic shaft length of the synaptic clusters and asynaptic segments (Fig. 3I). Shaft SER complexity was determined by counting the total number of branches in each segment (Fig 3J). Although shaft SER volume was similar over all asynaptic and synaptic clusters, it was greatest in synaptic clusters that had at least one spine with a spine apparatus, especially following LTP (Fig. 3K). At P15, the SER surface area and volume in the dendritic shaft is also reduced 2 hours post-TBS (Fig. 3M). When the SER complexity is measured as the summed cross-sectional area (X-sect) in each cluster, both aspiny and spiny segments show a decreased complexity following LTP (Fig. 3N). At first glance the similar outcomes for shaft SER appear to conflict with the opposite outcomes for spinogenesis at P15 and synapse enlargement in adults. At both ages, a drop in SER complexity and associated ER exit sites could reflect production of vesicles that would support spine outgrowth at P15 and synapse enlargement in adults, with developmental shifts in the specific cargoes being targeted following LTP.
Silent formation and enlargement of synapses
Curiously, synaptogenesis and synapse enlargement appear to be silent at P15 and adult hippocampus. Enhanced synaptogenesis with LTP (P15) or recovery of spines during control stimulation in adults are both silent. This conclusion is obvious from looking at the time course of spine formation during control stimulation or LTP relative to the physiological response across time during LTP experiments (Fig. 4A). In adults, if the spines that recovered in response to control stimulation were active, then the physiological response should climb as the spine number increases over hours. Instead, the physiological response to test pulses is stable for hours. Following TBS, the level of potentiation is fully saturated by 5 minutes; however, both synaptogenesis at P15 and synapse enlargement in adults does not occur at 5 or 30 minutes but is observed instead at 2 hours, yet the potentiated response remained stable. The quiet spinogenesis is not surprising, because newly formed spines typically do not contain AMPA receptors and the unsilencing of synapses by their addition has long been an integral mechanism of LTP in young hippocampus (Fig. 4B).[27] However, it is perhaps more surprising that enlargement of the PSD surface area in adults is also not observed at 5 or 30 minutes when LTP is saturated, but takes time, during which the physiological response is stable. This silent PSD enlargement is not due to the absence of postsynaptic receptors but instead to the absence of presynaptic vesicles that creates a silent zone across from the PSD (Fig. 4C).[28]
Figure 4:
Model for silent synaptogenesis and synapse enlargement. A) Saturation of LTP and stable control responses. B) During STP at P15, GluAR are added to existing PSDs. By 120 min during LTP, new GluAR-lacking spines emerge (orange spines with light blue PSDs). C) In adults, some spines have GluAR-containing portions of the PSD, that are never-the-less silent because there are no presynaptic vesicles opposed to those zones (light blue zones in PSD, at 4 spines with presynaptic axonal boutons also illustrated; for simplicity, the other presynaptic axons are not illustrated at P15 or in adults). Zones of the PSD with presynaptic vesicles are red, being both pre- and postsynaptically active. In adults, the new spines that emerge during control stimulation lack GluARs. Induction of LTP blocks spine outgrowth (X’s) and fills presynaptic zones with vesicles (red arrow). By 120 minutes, new PSD areas are added that lack presynaptic active zones (blue arrow)
Presynaptic axons track postsynaptic changes
Presynaptic plasticity is also developmentally regulated by LTP.[28–30] At P15, more presynaptic boutons form to accommodate the LTP-induced synaptogenesis. In adults, fewer presynaptic boutons accompany stalled spine outgrowth after LTP. At both ages, a drop in presynaptic vesicles remains for at least 2 hours after TBS-induction of LTP, especially in boutons with mitochondria.[29] This drop could reflect the elevated recycling of presynaptic vesicles detected 30 minutes post induction of LTP.[31] However, recent findings suggest that the vesicle surface area associated with this drop provides enough membrane to account for an LTP-associated growth in presynaptic bouton surface area.[32] These findings suggest that a pool of presynaptic vesicles are available to maintain the well-known coordination between presynaptic and postsynaptic dimensions throughout life. It will be interesting to learn whether the presynaptic effects, specific to nascent zone formation and axon expansion, might in turn influence spine cluster formation after LTP.
Other considerations
Several other factors may contribute to the maturation of homeostasis and dendritic spine clustering. We focused here on the extent to which dendritic shaft SER and the associated ER exit sites may serve to define regions of dendritic spine clustering. The post-LTP spread of numerous other molecules may be restricted to individual spines or short regions of the dendritic shaft.[2, 33, 34] Differential expression of calcium-permeable AMPA receptors could influence the range over which a calcium influx may enhance spinogenesis following LTP during development.[25] Improved methods are needed to identify the specificity of LTP expression among the spines in and outside the clusters.[35] It will be interesting to know how local resource availability influences outgrowth and stabilization of dendritic spines in vivo during learning.[18, 19, 36] Perisynaptic astroglia, microglia, and local inhibition may also serve to control the maturation and location of dendritic spine clustering.[37–39] Ultimately, all of these resources speak to mechanisms that may regulate information content at synapses throughout the brain.[40]
Conclusion
Despite dramatic structural plasticity, and daily turnover of synaptic proteins, memories stored in synapses show remarkable tenacity. Synapse stabilization appears to require reactivation, especially during sleep.[41, 42] Failure of synapses to form, grow, or remodel is likely responsible for many developmental and age-related disorders.[43] It remains unclear whether dendritic spine loss is a cause or consequence. Observing that dendrites retain immature varicosities and filopodia in developmental disorders is not sufficient to explain the cause. Dendrites are almost spine-free in seizure disorders, which may reflect homeostatic down regulation of excitatory input. However, the remaining spines host multiple synapses, suggesting they try to compensate for input loss. A disruption in spine structure could undo critical biochemical compartmentation needed to isolate calcium-intense reactions from the dendritic shaft to avoid disruption in microtubules and trafficking of organelles and proteins. Synapses on spines that contain a spine apparatus undergo the most enlargement following LTP and these spines are preferentially reduced in Alzheimer’s disease. Knowing whether dendritic spine responses are a cause or consequence is fundamental to deciding whether to target presynaptic, postsynaptic and/or perisynaptic glial components. Here we show evidence supporting the need for more nuanced analysis of synaptic plasticity in the context of subcellular resource availability and developmental stage.
Highlights.
Structural LTP produces opposite effects at synapses on immature versus adult dendrites.
Local subcellular compartments mediate synaptogenesis at P15 and synapse enlargement in adults.
A unified theory of resource-dependent silent spinogenesis and synapse enlargement is presented.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Segal M, Dendritic spines: Morphological building blocks of memory. Neurobiol Learn Mem, 2017. 138: p. 3–9. [DOI] [PubMed] [Google Scholar]
- 2.Nakahata Y and Yasuda R, Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization. Front Synaptic Neurosci, 2018. 10: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruijssen DLH and Wierenga CJ, Single Synapse LTP: A Matter of Context? Front Cell Neurosci, 2019. 13: p. 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne JN and Harris KM, Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus, 2011. 21(4): p. 354–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson DJ, et al. , LTP enhances synaptogenesis in the developing hippocampus. Hippocampus, 2016. 26(5): p. 560–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirov SA, Goddard CA, and Harris KM, Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology, 2004. 47(5): p. 640–648. [DOI] [PubMed] [Google Scholar]
- 7.Kulik YD, et al. , Structural plasticity of dendritic secretory compartments during LTP-induced synaptogenesis. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez G, et al. , ER Proteostasis Control of Neuronal Physiology and Synaptic Function. Trends Neurosci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen AB, et al. , Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy MJ and Hanus C, Architecture and Dynamics of the Neuronal Secretory Network. Annu Rev Cell Dev Biol, 2019. 35: p. 543–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostroff LE, et al. , Shifting patterns of polyribosome accumulation at synapses over the course of hippocampal long-term potentiation. Hippocampus, 2018. 28(6): p. 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry FE, et al. , Mechanistic target of rapamycin is necessary for changes in dendritic spine morphology associated with long-term potentiation. Mol Brain, 2017. 10(1): p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirillo MA, et al. , Local resources of polyribosomes and SER promote synapse enlargement and spine clustering after long-term potentiation in adult rat hippocampus. Sci Rep, 2019. 9(1): p. 3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedlicka P and Deller T, Understanding the role of synaptopodin and the spine apparatus in Hebbian synaptic plasticity - New perspectives and the need for computational modeling. Neurobiol Learn Mem, 2017. 138: p. 21–30. [DOI] [PubMed] [Google Scholar]
- 15.Bourne JN, et al. , Polyribosomes are increased in spines of CA1 dendrites 2 h after the induction of LTP in mature rat hippocampal slices. Hippocampus, 2007. 17(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 16.Biever A, et al. , Monosomes actively translate synaptic mRNAs in neuronal processes. Science, 2020. 367(6477). [DOI] [PubMed] [Google Scholar]
- 17.Konietzny A, et al. , Myosin V regulates synaptopodin clustering and localization in the dendrites of hippocampal neurons. J Cell Sci, 2019. 132(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank AC, et al. , Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat Commun, 2018. 9(1): p. 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Boustani S, et al. , Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 2018. 360(6395): p. 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hruska M, et al. , Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat Neurosci, 2018. 21(5): p. 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloss EB, et al. , Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nat Neurosci, 2018. 21(3): p. 353–363. [DOI] [PubMed] [Google Scholar]
- 22.Bhaduri A, et al. , Spiking Neural Classifier with Lumped Dendritic Nonlinearity and Binary Synapses: A Current Mode VLSI Implementation and Analysis. Neural Comput, 2018. 30(3): p. 723–760. [DOI] [PubMed] [Google Scholar]
- 23.Stein IS and Zito K, Dendritic Spine Elimination: Molecular Mechanisms and Implications. Neuroscientist, 2018: p. 1073858418769644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastellakis G and Poirazi P, Synaptic Clustering and Memory Formation. Front Mol Neurosci, 2019. 12: p. 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dittmer PJ, Dell’Acqua ML, and Sather WA, Synaptic crosstalk conferred by a zone of differentially regulated Ca(2+) signaling in the dendritic shaft adjoining a potentiated spine. Proc Natl Acad Sci U S A, 2019. 116(27): p. 13611–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell M, et al. , Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium. J Gen Physiol, 2019. 151(8): p. 1017–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent-Lamarre P, Lynn M, and Beique JC, The Eloquent Silent Synapse. Trends Neurosci, 2018. 41(9): p. 557–559. [DOI] [PubMed] [Google Scholar]
- 28.Bell ME, et al. , Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. J Comp Neurol, 2014. 522(17): p. 3861–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HL, et al. , Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourne JN, Chirillo MA, and Harris KM, Presynaptic ultrastructural plasticity along CA3-->CA1 axons during long-term potentiation in mature hippocampus. J Comp Neurol, 2013. 521(17): p. 3898–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rey S, et al. , Nanoscale Remodeling of Functional Synaptic Vesicle Pools in Hebbian Plasticity. Cell Rep, 2020. 30(6): p. 2006–2017 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirk LM, et al. Presynaptic ultrastructure changes in response to LTP stimulation in stratum radiatum of hippocampal CA1 neuropil. in Society for Neuroscinece. 2018. San Diego. [Google Scholar]
- 33.Cugno A, et al. , Geometric principles of second messenger dynamics in dendritic spines. Sci Rep, 2019. 9(1): p. 11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colgan LA, et al. , PKCalpha integrates spatiotemporally distinct Ca(2+) and autocrine BDNF signaling to facilitate synaptic plasticity. Nat Neurosci, 2018. 21(8): p. 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwajima M, et al. , Ultrastructure of light-activated axons following optogenetic stimulation to produce late-phase long-term potentiation. bioRxiv, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J and Zuo Y, Clustered structural and functional plasticity of dendritic spines. Brain Res Bull, 2017. 129: p. 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen NJ and Eroglu C, Cell Biology of Astrocyte-Synapse Interactions. Neuron, 2017. 96(3): p. 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammond TR, Robinton D, and Stevens B, Microglia and the Brain: Complementary Partners in Development and Disease. Annu Rev Cell Dev Biol, 2018. 34: p. 523–544. [DOI] [PubMed] [Google Scholar]
- 39.Hu HY, et al. , Endocannabinoid Signaling Mediates Local Dendritic Coordination between Excitatory and Inhibitory Synapses. Cell Rep, 2019. 27(3): p. 666–675 e5. [DOI] [PubMed] [Google Scholar]
- 40.Bromer C, et al. , Long-term potentiation expands information content of hippocampal dentate gyrus synapses. Proc Natl Acad Sci U S A, 2018. 115(10): p. E2410–E2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fauth MJ and van Rossum MC, Self-organized reactivation maintains and reinforces memories despite synaptic turnover. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seibt J and Frank MG, Primed to Sleep: The Dynamics of Synaptic Plasticity Across Brain States. Front Syst Neurosci, 2019. 13: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forrest MP, Parnell E, and Penzes P, Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci, 2018. 19(4): p. 215–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biever A, et al. , Monosomes actively translate synaptic mRNAs in neuronal processes. Science, 2020. 367(6477).**This paper demonstrates for the first time that monosomes and polysomes are translating different proteins locally in dendrites and hence may regulate both the timing and type of proteins produced during synaptic plasticity.
- Hu HY, et al. , Endocannabinoid Signaling Mediates Local Dendritic Coordination between Excitatory and Inhibitory Synapses. Cell Rep, 2019. 27(3): p. 666–675 e5.*This paper demonstrates that strong activation of dendritic spine synapses that induces LTP also encourages growth of neighboring presynaptic boutons at inhibitory synapses.
- Frank AC, et al. , Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat Commun, 2018. 9(1): p. 422.*Established preferential outgrowth of dendritic spines nearer to neighboring spines in retrosplenial cortex during fear conditioning, but not in motor cortex.
- El-Boustani S, et al. , Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 2018. 360(6395): p. 1349–1354.**Used both in vivo imaging and 3DEM to establish preferential spine clustering during plasticity in visual cortex.
- Bloss EB, et al. , Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nat Neurosci, 2018. 21(3): p. 353–363.*Shows that distal dendrites have a much higher frequency of compound synapses than proximal dendritic regions suggesting that axonal input may also control spine clustering.
- Bowen AB, et al. , Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife, 2017. 6.**Provide clear evidence that secretory trafficing can occur in the absence of the Golgi apparatus such that resources can be both generated and delivered locally throughout dendrites and dendritic spines.