Abstract
The discovery of a highly selective putative sigma-1 (σ1) receptor agonist, PRE-084, has revealed the numerous potential uses of this receptor subtype as a therapeutic target. While much work has been devoted to determining the role of σ1 receptors in normal and pathophysiological states in the nervous system, recent work suggests that σ1 receptors may be important for modulating functions of other tissues. These discoveries have provided novel insights into σ1 receptor structure, function, and importance in multiple intracellular signaling mechanisms. These discoveries were made possible by σ1 receptor-selective agonists such as PRE-084. The chemical properties and pharmacological actions of PRE-084 will be reviewed here, along with the expanding list of potential therapeutic applications for selective activation of σ1 receptors.
Keywords: Sigma-1 receptor, neuroprotection, therapeutics, PRE-084, cardioprotection, endothelial protection
Biology of σ1 receptors
The σ receptors were first discovered through pharmacological methods, and were originally classified as an opioid receptor based on behavioral observations by Martin et al., who named it the sigma/opioid receptor1. However, Martin also reported that activation of σ receptors by benzomorphan derivatives did not cause analgesia in a manner similar to the parent molecule and classic opioid receptor agonist, morphine. Rather, benzomorphans were found to trigger delusions and psychosis similar to other opioids1. Su et al. used the putative σ opioid receptor ligand SKF-10047 to identify a protein that had a nanomolar affinity for SKF- 10047 and no affinity for the opioid antagonist naloxone2. The SKF-10047-binding site, as shown by Su, has higher affinity for dextrorotatory benzomorphans like (+) SKF-10047 and (+) pentazocine than for levorotatory isomers, in contrast to opioids that have higher sensitivity to (−) benzomorphans2. This finding suggested that the protein identified by Su might not be a true opioid receptor2. There was also early confusion about σ receptors and the NMDA receptor’s PCP binding site, which is also targeted by (+) SKF-100473. This was later resolved with binding assays using ligands known to be more selective for respective binding sites, such as (+)pentazocine to label σ receptors and TCP to identify PCP-sites. Such studies resolved that σ receptors were indeed not the PCP binding site of the NMDA receptor4–7.
Two subtypes of σ receptors have been described based upon pharmacological properties, σ1 and σ2. Quiroin et al distinguished between the σ1 and σ2 receptors, reporting that each has distinct functions8. They showed that ligands such as pentazocine, carpetapentane and dextromethorphan have high affinity to σ1 receptors and low affinity to σ2 receptors, whereas ligands such as haloperidol and DTG have equal affinity for both subtype8. The σ1 receptor, but not σ2 receptor, was also characterized by having phenytoin and GTP sensitivity8.
The gene that encodes σ1 receptors was first successfully cloned by Hanner et al. in 19969. Since then, the σ1 receptors have gained considerable attention pertaining to their function in cell biology, molecular biology, cancer, immunology, and behavioral neuroscience10. On the other hand, σ2 receptors are thought to be involved in cellular processes related to neuropathy10, as well as cancer biology and have been proposed as a potential drug target in cancer therapy11. Moreover, σ2 receptor ligands have been shown to have neuroprotective effects in Alzheimer’s disease experimental models12,13. In 2017, Alon et al. reported that the gene TMEM97 encodes the σ2 receptor protein14.
The σ1 receptor is now known to be a chaperone protein that is ubiquitously expressed and typically located in the endoplasmic reticulum, primarily in the mitochondria-associated ER membranes (MAM)15. Encoded by the SIGMAR1 gene, the σ1 receptor is a non-G-protein- coupled transmembrane protein16. Schmidt et al. provided a comprehensive overview of the crystal structure of the human σ1 receptor17. Based on their crystallographic description, the structure of the σ1 receptor contains a trimeric organization with one transmembrane domain for each protomer mediated by the carboxy-terminal membrane-adjacent domains17. The activity of sigma-1 receptor seems to be dependent on its oligomeric state, which affects and is affected by ligand binding18. Moreover, the mono/dimeric state allows its activity as chaperone and high oligomeric states or monomeric are dormant forms of the receptor19. Various agonists seem to affect σ1 receptor oligomeric state differently. For example, haloperidol tends to stabilize them in a higher oligomeric state, whereas (+)-pentazocine has the opposite effect of stabilizing lower order oligomers (i.e. the dimers)18.
The σ1 receptor is ubiquitously expressed in mammals. Hanner et al. were the first to discover the high liver expression of σ1 receptor9. In addition, the σ1 receptor has been shown to be expressed abundantly in the intestine, kidney, white pulp of the spleen, adrenal gland, brain, placenta and the lung20. Also, nearly all cancer cell lines express the σ1 receptor; with one of the few exceptions being MCF-7 cells21. In brain it was reported that σ1 receptors are expressed primarily in cranial nerve nuclei, mesencephalon, red nucleus, periaqueductal gray matter and substantia nigra, as well as in some diencephalic structures including paraventricular and ventromedial hypothalamic nuclei20. While much of the work on the physiological and pathophysiological function of σ1 receptors has focused on the brain, numerous studies have demonstrated that these receptors play an important role in autonomic neuron function22–24. More recently, studies have shown very high mRNA levels in vascular tissue and some other tissues that are cataloged in the GTEx portal database (Fig. 1). Bhuiyan et al. have confirmed the expression of σ1 receptors in rat left ventricle25, while Trujillo et al. have successfully showed its expression in rat lymphatic vessels26. More recently, σ1 receptors have been detected in cultured endothelial cells27.
Fig. 1: Gene expression level of σ1 in different human tissues:
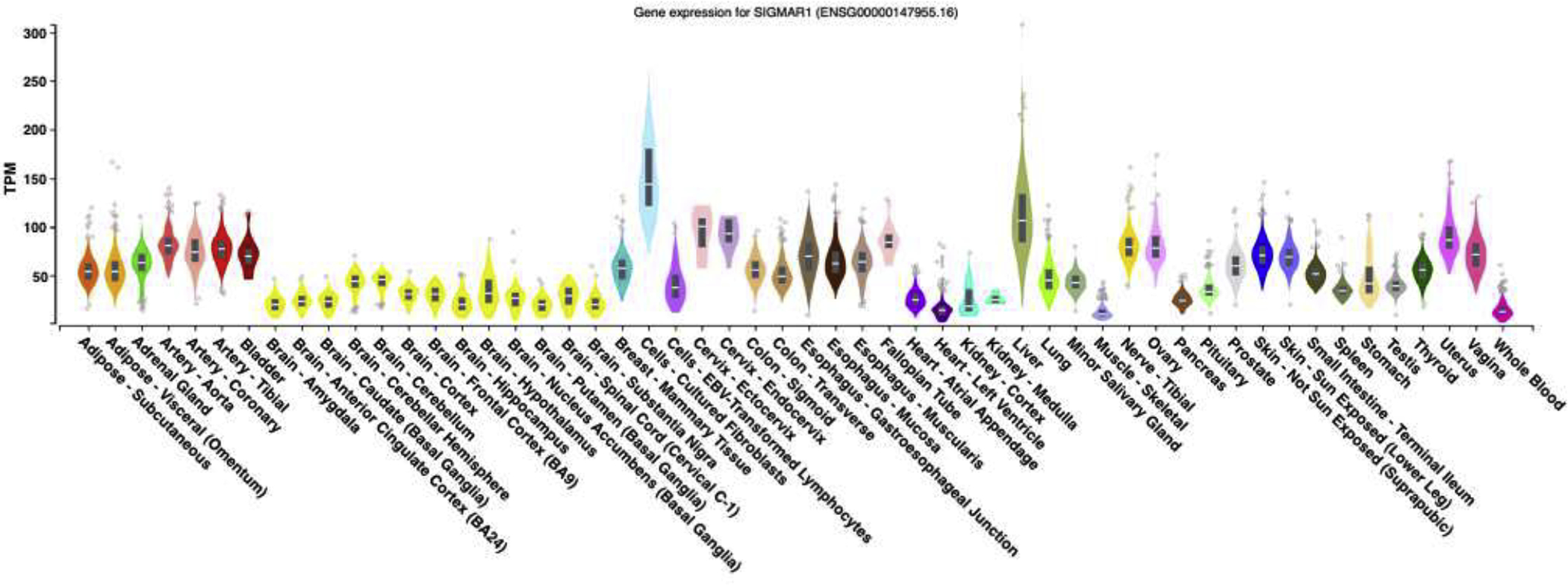
Data obtained from GTex portal database, GTEx Analysis Release V8 (dbGaP Accession phs000424.v8.p2). Gene code ID (ENSG00000147955.16), location chr9: 34634722–34637809. Date accessed November 18th, 2019. Gene description: sigma non-opioid intracellular receptor 1 [Source: HGNC Symbol; Acc: HGNC: 8157]. The x-axis shows different tissues tested. The y-axis represents transcripts per million (TPM). Number of examined samples for each tissue ranges from n=4 (kidney: medulla) to n=803 (skeletal muscle).
Langa et al. successfully generated σ1 receptor knockout mice to enable study of the definitive functions of this receptor in vivo28. Using this model, work by Sabino et al. suggests that σ1 receptors inversely modulate depressive-like behavior in mice29. Mice deficient in σ1 receptors displayed increased depressive-like behavior in the forced swim test, with significantly greater immobility behavior than matched wild-type mice29. The involvement of σ1 receptors in plasticity and synaptic transmission was evaluated by Snyder et al., who showed that σ1 receptor knockout mice do not have altered neuronal excitability or post synaptic function, but do have reduced long-term potentiation compared to control mice30. Moreover, using σ1 receptor knockout mice revealed the importance of this receptor in dentate gyrus neurogenesis, as σ1 receptor knockout mice displayed enhanced proliferation of progenitor cells, a reduction in both survival and neurite outgrowth in newborn neuron, and downregulation of NMDA receptors in these cells31.
The σ1 receptor has been shown to bind to different molecular partners that play an important role in its effects in different pathologies. For instance, co-immnoprecipitation revealed the binding between σ1 receptor and plasmalemmal potassium and calcium channels which reduced outward potassium current and inhibited L-type calcium channels as well as acid sensing ion channels22,32,33. In addition, bimolecular fluorescence complementation assay revealed the binding between σ1 receptor and cannabinoid receptor-1 protein which is involved in NMDAR activity34. Moreover, the partnership between NMDAR and σ1 receptor is thought to enhance synaptic plasticity and be neuroprotective in schizophrenia35.
Chemistry and pharmacology of PRE-084
The non-specific σ receptor ligand, phencyclidine, was used as the parent molecule to develop the highly selective σ1 receptor agonist, PRE-084, by Su et al.36. The structure of PRE-084 is reported in Figure 2. This molecule contains the three pharmacophoric moieties for a σ receptor ligand: 1) a hydrophobic cluster, 2) an amine group and 3) an intermediate chain36. Conformational analysis showed that PRE-084 matched the pharmacophore models for σ receptor binding with minimal cross reactivity with many other receptors36. PRE-084 had an IC50 of 44 nM in the sigma receptor assay, an IC50 of more than 100,000 nM for PCP receptors and an IC50 higher than 10,000 nM in a variety of other receptor systems36. The binding between σ1 receptors and PRE-084 was also confirmed by the use of reverse phase liquid chromatography in mouse blood, brain, and spinal cord, which was the first in vivo analysis of PRE-084 binding37. The same study showed that PRE-084 was stable in biological matrices for at least 24 hours37. Of note, PRE-084 was shown to promote the dissociation of σ1 immunoglobulin protein (BiP) complex allowing its activity as chaperone15.
Fig. 2: Chemical Structure of PRE-084:
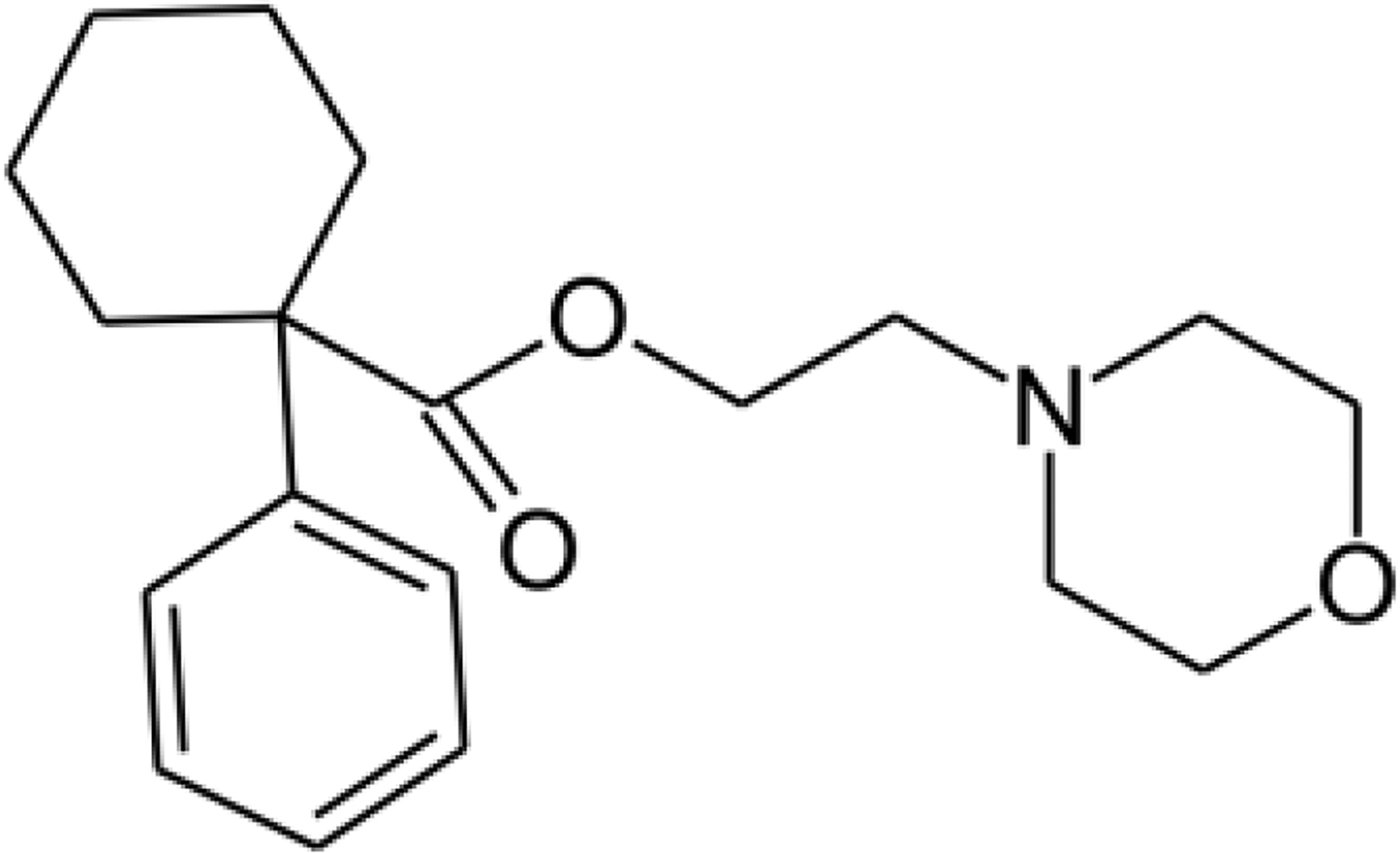
2-(4-Morpholino) ethyl-1-phenylcyclohexane-1-carboxylate. The active site is the arylcycloalkalyl group, the amine group and the intermediate chain while the phenyl group remains inactive.
PRE-084 was used in animal models of several CNS pathologies and it was administered in mice in a wide range of doses (from 0.1 to 64.0 mg/kg)37. Marra et al studied the pharmacokinetic profile of PRE-084 in comparison of the other σ1 receptor agonist RC-33 in CD1 mice38. They injected the mice Intraperitoneally with 10mg/ml PRE-084 and drew blood serially from retro mandibular plexus at 7 time points ending at 8 hours38. Using liquid chromatography and mass spectrometry, PRE-084 concentration was measured in different samples. PRE-084 showed a maximal concentration (Cmax) of 659.0 ± 117.1 ng/ml (Tmax of 5 min) with an area under the curve of 45516.4 ± 8386.4 ng/ml*min38. The half life of PRE-084 was shown to be 195.517.5 PRE- 084 was shown to be rapidly distributed to CNS showing a concentration in brain, spinal cord and plasma at the Tmax (5 min) of 773.6 ± 26.8 ng/g, 871.5 ± 77.3 ng/g and 651.5 ± 72.6 ng/ml respectively38.
Despite its characterization as being highly selective for σ1 receptors, there is the potential for off-target effects of PRE-084, which is expected to increase with the dose or concentration applied to the biological system used. In our recent study in which we investigated the role of σ1 receptors in human umbilical vein endothelial cell (HUVEC) monolayer permeability, we found that PRE-084 could elicit enhanced barrier function of the monolayers in a concentration-dependent manner27. However, we obtained a particularly interesting finding when we tested the role of σ1 receptors in this model by siRNA-mediated depletion. Under these conditions, we expected that the effect of PRE-084 would be abolished, which is what we observed, but with an additional surprising finding. We found that when PRE- 084 was applied to HUVEC that had significant depletion of σ1 receptors not only was the barrier-enhancing property of PRE-084 abolished as expected, but in addition, PRE-084 actually caused a decrease in monolayer barrier function27. This finding suggests that off- target effects of PRE-084 are certainly possible, particularly in this case in which σ1 receptor expression was diminished, allowing for binding to other targets for which PRE-084 may have affinity.
Impacts of PRE-084 on neural tissue:
Activation of σ1 receptors by PRE-084 has been shown to be neuroprotective, and PRE-084 is an enhancer of neurodevelopment in various models. For example, Saulite at al reported that σ1 receptor activation with PRE-084 could facilitate differentiation of skin mesenchymal stem cells to Schwann cells by upregulation of myelin basic protein expression compared to controls39. Another study showed that PRE-084 improved neurite elongation of cerebellar granule neurons, probably by TrKB signaling40. Neurite elongation by PRE-084 was also linked to protein kinase C (PKC) signaling on motoneurons41. These findings support the potential use of σ1 receptor agonists in neurological diseases such as motor and cognitive dysfunction, stroke and other types of brain injury.
PRE-084 and motor function:
There are many lines of evidence supporting that PRE-084 is a neuroprotective agent in motor neuron disease models. Spinal muscular atrophy (SMA) is a motor disease that involves an increase in microglial cell activation. In a mouse mode of SMA, Cervero et al. found that following the application of PRE-084 to SMA mice, the ratio of M1/M2 microglial phenotype was restored, indicating a reduction in microglial activation and concomitant neuroinflammation. There were however some limitations, as PRE-084 did not improve motor neuron atrophy in these mice, and its beneficial effects were not sufficient to decrease motor neuron degeneration42.
Parkinsonism is a complex neurological disorder involving dysautonomia, sensory deficits and motor impairment43–45. In a mouse model of Parkinsonism, PRE-084 significantly improved spontaneous forelimb use. These effects were accompanied by increased density of dopaminergic fibers in the most denervated striatal regions and a modest recovery of dopamine levels. In addition, PRE-084 caused upregulation of neurotrophic factors (BDNF and GDNF) and their downstream effector pathways (extracellular signal regulated kinases 1/2 and Akt)46.
PRE-084 was also found to be beneficial in an amyotrophic lateral sclerosis model, SOD1-G93A mice (ALS mice)47. When PRE-084 was applied in these animals at pre- symptomatic or early symptomatic stages, there was a significant improvement in locomotor function and motoneuron survival48. Mancuso et al. further investigated the protective effects of PRE-084 in ALS mice49. Their study confirmed the previous finding that PRE-084 improved motor function and motor neuron survival in ALS mice. Additionally, PRE-084 extended survival in both female and male mice by more than 15 %49. The mechanisms responsible for these improved outcomes were attributed to induction of protein kinase C-specific phosphorylation of the NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor and a reduction of the microglial reactivity compared with untreated mice49. Collectively, these studies suggest the potential therapeutic use of σ1 receptor agonists such as such as PRE- 084 in ALS.
Hyrskyluoto et al. have targeted PRE-084 as a therapeutic agent in Huntington disease. Their study showed that PRE-084 could increase cell survival and counteract the deleterious effects of N-terminal mutant huntingtin proteins in neuronal PC6.3 cells. Specifically, PRE-084 increased cellular antioxidants by activating the NF-κB pathway that is compromised by the mutant huntingtin proteins, supporting the future use of σ receptors agonists as therapeutics for Huntington disease50.
PRE-084 was also used in other motoneuron-injury models. Penas et al. reported that PRE-084 increased GDNF and BiP expression and promoted neuroprotection after root avulsion injury51. Furthermore, PRE-084 attenuated head twitch response in mice caused by 2,5-dimethoxy-4-iodoamphetamine52,53. However, PRE-084 has not been beneficial in all neurological diseases tested. For example, PRE-084 did not ameliorate seizures in different animal models of this disorder54,55
PRE-084 and cognitive disorders:
PRE-084 is the σ1 receptor ligand that has been mostly studied in cognitive functions, particularly learning and memory. PRE-084 attenuated amnesia in pharmacological models such as MK-801 induced amnesia56, amnesia induced by amyloid beta peptide57, or hypoxia/ischemia induced amnesia58,59,60. Moreover, PRE-084 attenuated amnesia in aged animals61,62.
There is significant evidence supporting the use of σ1 receptor agonists as therapeutics for the treatment of Alzheimer’s disease and Alzheimer’s-related dementias. PRE-084 attenuated β-amyloid induced cell death63. In addition, PRE-084 ameliorated the learning deficits and lipid peroxidation in Alzheimer’s disease mouse model64. It also decreased tau hyperphosphorylation and amyloid β deposition in a different mouse model65. In a non transgenic Alzheimer’s disease mouse model generated by amyloid-β 25–35 peptide-injection, PRE-084 protected mitochondrial respiration66. This finding suggests potential therapeutic benefits of σ1 receptor activation in Alzheimer’s disease especially because mitochondrial dysfunction was shown to be a trigger of Alzheimer’s disease pathobiology67. In a second study, PRE-084 abolished the inhibitory effect of amyloid-β on long-term potentiation in the pyramidal layer of the CA1 of rat hippocampus68. Similarly, PRE-084 attenuated the learning and memory loss in brain ischemia/reperfusion induced vascular dementia model, possibly through NR2A-CaMKIV-TORC1 pathway60. An additional σ1 receptor agonist, blarcamesine, has advanced through phase II clinical trials in Alzheimer’s disease patients, in which cognitive decline was reported to be reduced69. At the time of this writing, blarcamesine is entering phase III clinical trials for Alzheimer’s disease.
In addition, σ1 receptor agonism has been shown to alleviate blood-brain barrier dysfunction in a vascular dementia mouse model70, an effect that suggests σ1 receptors as a therapeutic target for vascular dementia especially because BBB disruption is a key factor in vascular dementia disease pathology71. Other cellular effects of PRE-084 that may contribute to improved outcomes in dementia are the promotion of neurite growth in a primary neuronal culture72 and protection against glutamate induced excitotoxicity in primary hippocampal neurons73
Dopamine has been shown to enhance cognition and attention74,75. Hong et al. have shown that PRE-084 and other σ1 receptor agonists can facilitate the interaction between σ1 receptor receptors and dopamine receptors, which in turn stabilizes a conformational change in dopamine receptors that facilitates dopamine binding76 along with enhancement of dopamine levels77. In addition to dopamine, NMDA was studied as a target for cognitive enhancement78. Some studies provide evidence that PRE-084 also increases the expression of NMDA receptors in rat hippocampus79,80. A finding that was consistent with a previous finding of Martina et al. is that σ1 receptor activation potentiates NMDA receptor responses and long-term potentiation by preventing SK currents81. PRE-084 rescued the impairment of learning and memory in brain ischemia/reperfusion model by a mechanism involving upregulation of NMDA receptor 2A60. These findings further support the potential use of σ1 agonists in cognitive disorders.
Impact of PRE-084 on stroke and brain injury:
PRE-084 has been suggested to have neuroprotective effects against excitotoxic brain injury in newborn mice. PRE-084 treatment resulted in a decrease in cell death, as indicated by reduced TUNEL positivity and caspase-3 activation. In addition, there were fewer isolectin B4-positive cells, suggesting a decrease in activated microglial cells73. The σ1 receptor agonist PRE-084 reduced infarct volume, neurological deficits, and pro-inflammatory cytokines while enhancing anti-inflammatory cytokines after embolic stroke in rats82. This finding was consistent with the use of other σ receptor agonists in stroke using middle cerebral artery occlusion models in rats83–85. PRE-084 was also reported to protect against endoplasmic reticulum stress-mediated apoptosis in mice following cerebral ischemia/reperfusion injury86. This was further supported by another study that indicated the possible involvement of NR2A- induced pathway to regulate brain-derived neurotrophic factor87. Another stroke study showed that PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats82.
Table 1 summarizes some of the applications of PRE-084 in the last few years with the appropriate doses/concentrations that were used. Interestingly, the dose-range of usage of PRE-084 in vivo in mice was very similar across different models.
Table 1:
Different application an doses/concentrations of PRE-084 in different disease models.
| 2 | Didn’t affect HIV Infectivity105 | Macrophages | 1 μM or 10 μM | Added to culture media | 1 h | Omar Vélez Ló pez, 2018 |
| 4 | Ameliorates Myocardial Ischemia-Reperfusion Induced Apoptosis88 | Rats | 1 mg/kg | Intraperitoneal injection | 1 h | Qi-Jun Gao, 2018 |
| 6 | Inhibit catecholamine secretion due to block of nicotinic acetyl choline receptors107 | Mice adrenal chromaffin cells | 5 μM, 10 μM | Added to culture media | 20 s | Rebecca L. Brindley, 2017 |
| 8 | Enhanced differentiation into Schwann cells39 | Human skin mesenchymal cells | 0.3–3 μM, up to 200 μM | Added to culture media | 24 hours | L. Saulite, 2017 |
| 10 | Protects against excitotoxic neonatal brain injury73 | Mice | 0.1 μg/g | Intraperitoneal | 1 hour | E Griesmaier, 2012 |
Cardio-protective actions of PRE-084
Gao et al. have highlighted the important advantage of PRE-084 in cardio-protection. In a rat model of myocardial ischemia/reperfusion, pre-surgical administration of PRE-084 maintained cardiac function and reduced myocardial apoptosis. The proposed mechanism was a reduction of Bax and cleaved‑caspase 3 expression associated with increased expression of Bcl‑2, and phosphorylation of protein kinase B and endothelial nitric oxide synthase on their activation sites88. This observation is consistent with recent findings that σ1 receptor activation with afobazole enhances nitric oxide production in lymphatic endothelial cells, which results in vasorelaxation of rat mesenteric collecting lymphatic vessels26.
Endothelial barrier-protective actions of PRE-084
In addition to neuroprotection and cardio-protection, our recent study has shown the significance of σ1 receptor activation with PRE-084 in maintenance of endothelial barrier function in cultured HUVEC monolayers. We found that PRE-084 can enhance endothelial barrier function in a concentration- and σ1 receptor-dependent manner27. We also observed that PRE-084 enhances endothelial bioenergetics in the form of glycolysis parameters. In addition, PRE-084 could partially counteract endothelial barrier dysfunction caused by the mitochondrial disrupting agent Carbonyl Cyanide Chlorophenylhydrazone (CCCP)27. These findings support another recent observation that the σ1 receptor may have protective properties for the blood-brain barrier70. Activation of the σ1 receptor is also known to regulate neuroinflammation, specifically astrocytosis and microglial activation, which in turn limits their production of inflammatory cytokines such as IL-1β, IL-10, TNF-α and metalloproteinases. This effect can indirectly impair the blood-brain-barrier89–92. Also, given that endothelial barrier disruption and endothelial dysfunction is a main characteristic of many disease models such as stroke93, myocardial infarction94, hemorrhagic shock and resuscitation95 or inflammation96, our recent findings suggest potential uses of PRE-084 to promote endothelial health in such pathologies.
σ1 receptor agonists in clinical trials
Some σ1 receptor ligands have advanced to clinical trials for various neurological conditions. For example, the σ1 receptor agonist, SA-4503 (cutamesine)97, has been studied in clinical trials for stroke patients where it was administered starting 48 hours to 72 hours following stroke at the doses of either 1 mg/day or 3 mg/day for 28 days97. Cutamesine was proven safe and well tolerated by patients, and the while the drug failed to reach significant effect on functional endpoints, the dose of 3 mg/day improved NIH stroke scale outcomes in patients with greater pretreatment deficits97. More research is being conducted on the therapeutic effects of cutamesine in other models such as retinal photoreceptor restoration, cardiac arrest and depression98–100. Another σ1 receptor agonist, igmesine, significantly improved depression in a phase II clinical trial101. An additional σ1 receptor agonist with mild muscarinic-modulating activity, ANAVEX2–73 (blarcamesine), has been tested in Phase I and II clinical trials in patients with Alzheimer’s disease and Parkinson’s disease with dementia69.
The σ agonist, afobazole, went through multiple clinical trials in Russia, and is approved for use in Russia as an anxiolytic agent102. Afobazole showed higher tolerability and patient acceptability for the treatment of anxiety in comparison to benzodiazepines102,103. Afobazole was tested in patients with generalized anxiety disorders and adjustment disorders102. Afobazole caused a significant reduction of Hamilton Anxiety Rating Scale total score that exceeded diazepam103. After treatment completion, no withdrawal symptoms were noted in the afobazole group while diazepam withdrawal syndrome was observed in (68%) patients in that study103. More potential therapeutic applications and use of σ1 agonists will be highlighted in this review.
Concluding Remarks
Several lines of investigation suggest diverse potential therapeutic uses of σ1 receptor agonists. In this review, we included some of the recent experimental uses of PRE-084 in different disease models. Despite its existence for almost 30 years, PRE-084 is still an important prototypical drug to uncover potential therapeutic benefits of σ1 receptors in central and peripheral diseases. The beneficial effects of σ1 receptor activation include but not limited to enhancement of motor and cognitive functions in neurodegenerative diseases and cardio- protection as summarized in Fig. 3. Most research projects studying σ1 receptor functions to date have been focused on brain and neurological functions. However, σ1 receptor expression levels are high in other tissues. Notably, the σ1 receptor mRNA levels in the liver, blood vessels, bladder, and in cultured fibroblasts have been reported to be higher than in the brain, suggesting new avenues for investigating functional significance of σ1 receptor function in these tissues. The recent finding of expression of σ1 receptors in lymphatic vessels and the connection to endothelial nitric oxide synthase26,88 as well as the findings that PRE-084 could promote endothelial barrier function27,70 also suggest the potential use of σ1 receptor agonists for a wider therapeutic utility.
Fig 3.
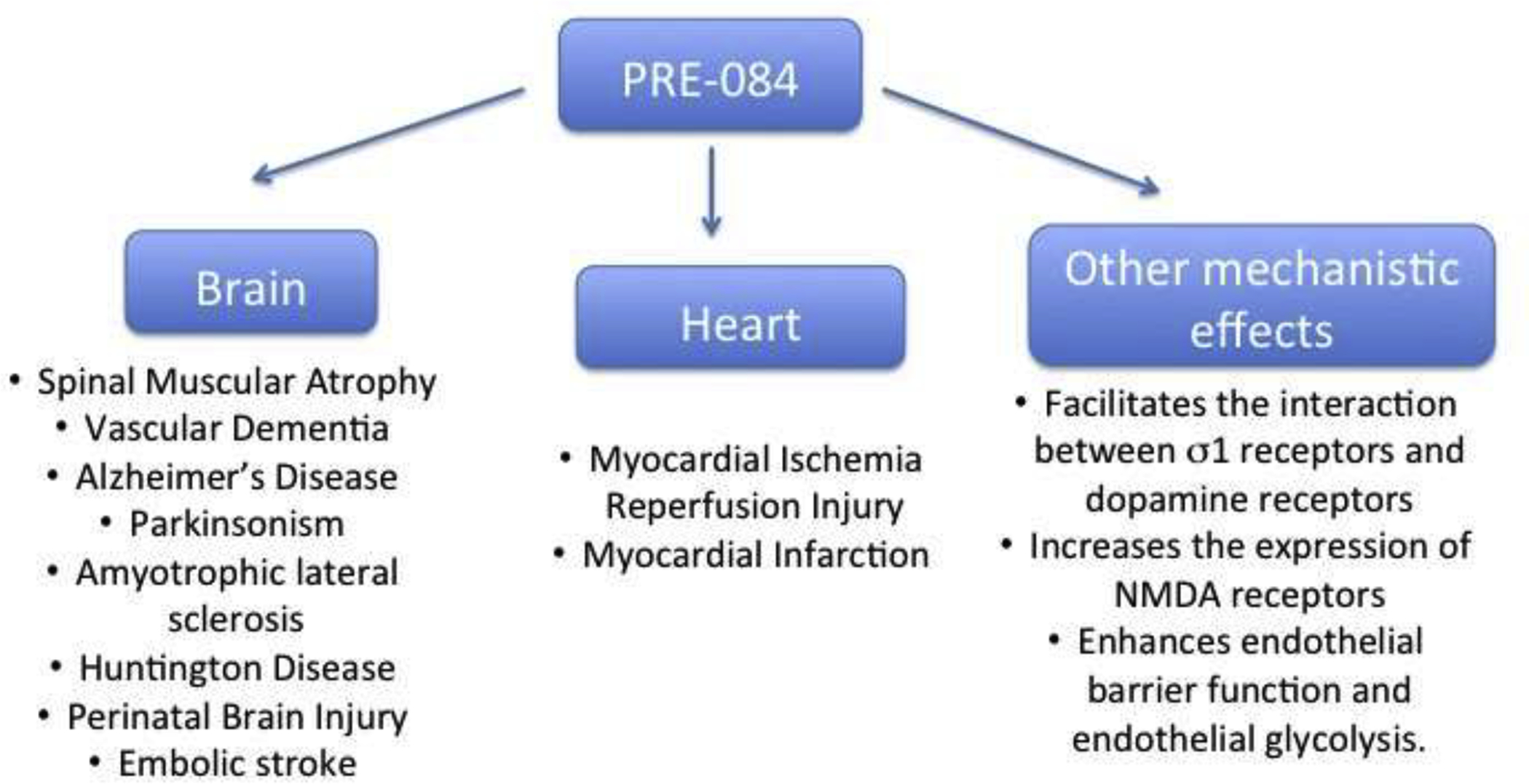
Summary of uses and applications of PRE-084 in disease models.
Funding
This work was supported by the National Institute of Health grant number (R01GM120774), American Heart Association Grant-In-Aid (17GRNT33661273) and an Edith Wright Hartley PhD Graduate Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Martin WR, Eades CG, Thompson JA, Huppler RE & Gilbert PE The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. The Journal of pharmacology and experimental therapeutics 197, 517–532 (1976). [PubMed] [Google Scholar]
- 2.Su TP Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. The Journal of pharmacology and experimental therapeutics 223, 284–290 (1982). [PubMed] [Google Scholar]
- 3.Vaupel DB Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. European journal of pharmacology 92, 269–274, doi: 10.1016/0014-2999(83)90297-2 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Gundlach AL, Largent BL & Snyder SH Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. The Journal of neuroscience : the official journal of the Society for Neuroscience 6, 1757–1770 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Largent BL, Gundlach AL & Snyder SH Pharmacological and autoradiographic discrimination of sigma and phencyclidine receptor binding sites in brain with (+)-[3H]SKF 10,047, (+)-[3H]-3-[3-hydroxyphenyl]-N-(1-propyl)piperidine and [3H]-1-[1-(2-thienyl)cyclohexyl]piperidine. The Journal of pharmacology and experimental therapeutics 238, 739–748 (1986). [PubMed] [Google Scholar]
- 6.Largent BL, Gundlach AL & Snyder SH Sigma receptors on NCB-20 hybrid neurotumor cells labeled with (+)[3H]SKF 10,047 and (+)[3H]3-PPP. European journal of pharmacology 124, 183–187, doi: 10.1016/0014-2999(86)90142-1 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Tam SW (+)-[3H]SKF 10,047, (+)-[3H]ethylketocyclazocine, mu, kappa, delta and phencyclidine binding sites in guinea pig brain membranes. European journal of pharmacology 109, 33–41, doi: 10.1016/0014-2999(85)90536-9 (1985). [DOI] [PubMed] [Google Scholar]
- 8.Quirion R et al. A proposal for the classification of sigma binding sites. Trends in pharmacological sciences 13, 85–86, doi: 10.1016/0165-6147(92)90030-a (1992). [DOI] [PubMed] [Google Scholar]
- 9.Hanner M et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proceedings of the National Academy of Sciences of the United States of America 93, 8072–8077, doi: 10.1073/pnas.93.15.8072 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada K, Migita K, Matsushima Y & Kamei C Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders. Neural regeneration research 14, 1893–1894, doi: 10.4103/1673-5374.259609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford KW, Coop A & Bowen WD sigma(2) Receptors regulate changes in sphingolipid levels in breast tumor cells. European journal of pharmacology 443, 207–209, doi: 10.1016/s0014-2999(02)01581-9 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Yi B et al. Small molecule modulator of sigma 2 receptor is neuroprotective and reduces cognitive deficits and neuroinflammation in experimental models of Alzheimer’s disease. Journal of neurochemistry 140, 561–575, doi: 10.1111/jnc.13917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzo NJ et al. Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PloS one 9, e111899, doi: 10.1371/journal.pone.0111899 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alon A et al. Identification of the gene that codes for the sigma2 receptor. Proceedings of the National Academy of Sciences of the United States of America 114, 7160–7165, doi: 10.1073/pnas.1705154114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi T & Su TP Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610, doi: 10.1016/j.cell.2007.08.036 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Hong W & Werling LL Evidence that the sigma(1) receptor is not directly coupled to G proteins. European journal of pharmacology 408, 117–125, doi: 10.1016/s0014-2999(00)00774-3 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Schmidt HR et al. Crystal structure of the human sigma1 receptor. Nature 532, 527–530, doi: 10.1038/nature17391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra AK et al. The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. The Biochemical journal 466, 263–271, doi: 10.1042/BJ20141321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gromek KA et al. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. The Journal of biological chemistry 289, 20333–20344, doi: 10.1074/jbc.M113.537993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi T & Su T The sigma receptor: evolution of the concept in neuropsychopharmacology. Current neuropharmacology 3, 267–280, doi: 10.2174/157015905774322516 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilner BJ, de Costa BR & Bowen WD Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. The Journal of neuroscience : the official journal of the Society for Neuroscience 15, 117–134 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H & Cuevas J Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. Journal of neurophysiology 87, 2867–2879, doi: 10.1152/jn.2002.87.6.2867 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Zhang H & Cuevas J sigma Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. The Journal of pharmacology and experimental therapeutics 313, 1387–1396 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Christopher K & Cuevas J Sigma Receptor Activation Inhibits Voltage-Gated Sodium Channels in Rat Intracardiac Ganglion Neurons. Int J Physiol Pathophysiol Pharmacol 2, 1–11 (2009). [PMC free article] [PubMed] [Google Scholar]
- 25.Bhuiyan MS, Tagashira H, Shioda N & Fukunaga K Targeting sigma-1 receptor with fluvoxamine ameliorates pressure-overload-induced hypertrophy and dysfunctions. Expert opinion on therapeutic targets 14, 1009–1022, doi: 10.1517/14728222.2010.509348 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Trujillo AN et al. Modulation of mesenteric collecting lymphatic contractions by sigma1-receptor activation and nitric oxide production. American journal of physiology. Heart and circulatory physiology 313, H839–H853, doi: 10.1152/ajpheart.00702.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motawe ZY, Farsaei F, Abdelmaboud SS, Cuevas J & Breslin JW Sigma-1 Receptor Activation-Induced Glycolytic ATP Production and Endothelial Barrier Enhancement. Microcirculation In Press, DOI: 10.1111/micc.12620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langa F et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. The European journal of neuroscience 18, 2188–2196, doi: 10.1046/j.1460-9568.2003.02950.x (2003). [DOI] [PubMed] [Google Scholar]
- 29.Sabino V, Cottone P, Parylak SL, Steardo L & Zorrilla EP Sigma-1 receptor knockout mice display a depressive-like phenotype. Behavioural brain research 198, 472–476, doi: 10.1016/j.bbr.2008.11.036 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder MA, McCann K, Lalande MJ, Thivierge JP & Bergeron R Sigma receptor type 1 knockout mice show a mild deficit in plasticity but no significant change in synaptic transmission in the CA1 region of the hippocampus. Journal of neurochemistry 138, 700–709, doi: 10.1111/jnc.13695 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Sha S et al. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS neuroscience & therapeutics 19, 705–713, doi: 10.1111/cns.12129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aydar E, Palmer CP, Klyachko VA & Jackson MB The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34, 399–410, doi: 10.1016/s0896-6273(02)00677-3 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Church J & Fletcher EJ Blockade by sigma site ligands of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones. British journal of pharmacology 116, 2801–2810, doi: 10.1111/j.1476-5381.1995.tb15929.x (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Blazquez P et al. The calcium-sensitive Sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. The international journal of neuropsychopharmacology 17, 1943–1955, doi: 10.1017/S1461145714000029 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Pabba M & Sibille E Sigma-1 and N-Methyl-d-Aspartate Receptors: A Partnership with Beneficial Outcomes. Molecular neuropsychiatry 1, 47–51, doi: 10.1159/000376549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su TP et al. Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. The Journal of pharmacology and experimental therapeutics 259, 543–550 (1991). [PubMed] [Google Scholar]
- 37.Marra A et al. Development of easy-to-use reverse-phase liquid chromatographic methods for determining PRE-084, RC-33 and RC-34 in biological matrices. The first step for in vivo analysis of sigma1 receptor agonists. Biomedical chromatography : BMC 30, 645–651, doi: 10.1002/bmc.3609 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Marra A et al. Toward the identification of neuroprotective agents: g-scale synthesis, pharmacokinetic evaluation and CNS distribution of (R)-RC-33, a promising SIGMA1 receptor agonist. Future medicinal chemistry 8, 287–295, doi: 10.4155/fmc.15.191 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Saulite L, Vavers E, Zvejniece L, Dambrova M & Riekstina U The Differentiation of Skin Mesenchymal Stem Cells Towards a Schwann Cell Phenotype: Impact of Sigma-1 Receptor Activation. Molecular neurobiology 55, 2840–2850, doi: 10.1007/s12035-017-0511-9 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Kimura Y, Fujita Y, Shibata K, Mori M & Yamashita T Sigma-1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PloS one 8, e75760, doi: 10.1371/journal.pone.0075760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzman-Lenis MS, Navarro X & Casas C Selective sigma receptor agonist 2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate (PRE084) promotes neuroprotection and neurite elongation through protein kinase C (PKC) signaling on motoneurons. Neuroscience 162, 31–38, doi: 10.1016/j.neuroscience.2009.03.067 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Cervero C et al. Glial Activation and Central Synapse Loss, but Not Motoneuron Degeneration, Are Prevented by the Sigma-1 Receptor Agonist PRE-084 in the Smn2B/-Mouse Model of Spinal Muscular Atrophy. Journal of neuropathology and experimental neurology 77, 577–597, doi: 10.1093/jnen/nly033 (2018). [DOI] [PubMed] [Google Scholar]
- 43.De Pablo-Fernandez E et al. Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA neurology 74, 970–976, doi: 10.1001/jamaneurol.2017.1125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gokcal E, Gur VE, Selvitop R, Babacan Yildiz G & Asil T Motor and Non-Motor Symptoms in Parkinson’s Disease: Effects on Quality of Life. Noro psikiyatri arsivi 54, 143–148, doi: 10.5152/npa.2016.12758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolano M et al. Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain : a journal of neurology 131, 1903–1911, doi: 10.1093/brain/awn102 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Francardo V et al. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain : a journal of neurology 137, 1998–2014, doi: 10.1093/brain/awu107 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Fulton D et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601, doi: 10.1038/21218 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peviani M et al. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiology of disease 62, 218–232, doi: 10.1016/j.nbd.2013.10.010 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Mancuso R et al. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 9, 814–826, doi: 10.1007/s13311-012-0140-y (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyrskyluoto A et al. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kappaB pathway. Cell death & disease 4, e646, doi: 10.1038/cddis.2013.170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penas C et al. Sigma receptor agonist 2-(4-morpholinethyl)1 phenylcyclohexanecarboxylate (Pre084) increases GDNF and BiP expression and promotes neuroprotection after root avulsion injury. Journal of neurotrauma 28, 831–840, doi: 10.1089/neu.2010.1674 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Egawa G et al. Intravital analysis of vascular permeability in mice using two-photon microscopy. Scientific reports 3, 1932, doi: 10.1038/srep01932 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik M, Rangel-Barajas C, Mach RH & Luedtke RR The effect of the sigma-1 receptor selective compound LS-1–137 on the DOI-induced head twitch response in mice. Pharmacology, biochemistry, and behavior 148, 136–144, doi: 10.1016/j.pbb.2016.07.001 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Vavers E et al. The activity of selective sigma-1 receptor ligands in seizure models in vivo. Behavioural brain research 328, 13–18, doi: 10.1016/j.bbr.2017.04.008 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Sourbron J, Smolders I, de Witte P & Lagae L Pharmacological Analysis of the Anti-epileptic Mechanisms of Fenfluramine in scn1a Mutant Zebrafish. Frontiers in pharmacology 8, 191, doi: 10.3389/fphar.2017.00191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maurice T, Su TP, Parish DW, Nabeshima T & Privat A PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacology, biochemistry, and behavior 49, 859–869, doi: 10.1016/0091-3057(94)90235-6 (1994). [DOI] [PubMed] [Google Scholar]
- 57.Maurice T, Su TP & Privat A Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 83, 413–428, doi: 10.1016/s0306-4522(97)00405-3 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Maurice T et al. The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (sigma) receptor ligands involves both sigma1 and sigma2 sites. British journal of pharmacology 127, 335–342, doi: 10.1038/sj.bjp.0702553 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito K, Hirooka Y & Sunagawa K Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. Journal of cardiovascular pharmacology 62, 222–228, doi: 10.1097/FJC.0b013e3182970b15 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Xu Q et al. Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion. Psychopharmacology 232, 1779–1791, doi: 10.1007/s00213-014-3809-6 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Maurice T, Roman FJ, Su TP & Privat A Beneficial effects of sigma agonists on the age-related learning impairment in the senescence-accelerated mouse (SAM). Brain research 733, 219–230, doi: 10.1016/0006-8993(96)00565-3 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Maurice T Beneficial effect of the sigma(1) receptor agonist PRE-084 against the spatial learning deficits in aged rats. European journal of pharmacology 431, 223–227, doi: 10.1016/s0014-2999(01)01436-4 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Marrazzo A et al. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport 16, 1223–1226, doi: 10.1097/00001756-200508010-00018 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Meunier J, Ieni J & Maurice T The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25–35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. British journal of pharmacology 149, 998–1012, doi: 10.1038/sj.bjp.0706927 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lahmy V et al. Blockade of Tau hyperphosphorylation and Abeta(1)(−)(4)(2) generation by the aminotetrahydrofuran derivative ANAVEX2–73, a mixed muscarinic and sigma(1) receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 1706–1723, doi: 10.1038/npp.2013.70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lahmy V, Long R, Morin D, Villard V & Maurice T Mitochondrial protection by the mixed muscarinic/sigma1 ligand ANAVEX2–73, a tetrahydrofuran derivative, in Abeta25–35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Frontiers in cellular neuroscience 8, 463, doi: 10.3389/fncel.2014.00463 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreira PI, Carvalho C, Zhu X, Smith MA & Perry G Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochimica et biophysica acta 1802, 2–10, doi: 10.1016/j.bbadis.2009.10.006 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Solntseva EI, Kapai NA, Popova OV, Rogozin PD & Skrebitsky VG The involvement of sigma1 receptors in donepezil-induced rescue of hippocampal LTP impaired by beta-amyloid peptide. Brain research bulletin 106, 56–61, doi: 10.1016/j.brainresbull.2014.06.002 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Hampel H et al. Longitudinal 148-Week Extension Study for ANAVEX® 2–73 Phase 2a Alzheimer’s Disease Demonstrates Maintained Activities of Daily Living Score (ADCS-ADL) and Reduced Cognitive Decline (MMSE) for Patient Cohort on Higher Drug Concentration and Confirms Role of Patient Selection Biomarkers. J Prev Alzheimers Dis 5, p.S43 (2018). [Google Scholar]
- 70.Liu DY et al. Sigma-1 receptor activation alleviates blood-brain barrier dysfunction in vascular dementia mice. Experimental neurology 308, 90–99, doi: 10.1016/j.expneurol.2018.07.002 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Ueno M et al. Blood-brain barrier damage in vascular dementia. Neuropathology : official journal of the Japanese Society of Neuropathology 36, 115–124, doi: 10.1111/neup.12262 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Page M, Pacico N, Ourtioualous S, Deprez T & Koshibu K Procognitive Compounds Promote Neurite Outgrowth. Pharmacology 96, 131–136, doi: 10.1159/000436974 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Griesmaier E et al. Neuroprotective effects of the sigma-1 receptor ligand PRE-084 against excitotoxic perinatal brain injury in newborn mice. Experimental neurology 237, 388–395, doi: 10.1016/j.expneurol.2012.06.030 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Nieoullon A Dopamine and the regulation of cognition and attention. Progress in neurobiology 67, 53–83, doi: 10.1016/s0301-0082(02)00011-4 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Costa A et al. Dopamine treatment and cognitive functioning in individuals with Parkinson’s disease: the “cognitive flexibility” hypothesis seems to work. Behavioural neurology 2014, 260896, doi: 10.1155/2014/260896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong WC et al. The sigma-1 receptor modulates dopamine transporter conformation and cocaine binding and may thereby potentiate cocaine self-administration in rats. The Journal of biological chemistry 292, 11250–11261, doi: 10.1074/jbc.M116.774075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garces-Ramirez L et al. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biological psychiatry 69, 208–217, doi: 10.1016/j.biopsych.2010.07.026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collingridge GL et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64, 13–26, doi: 10.1016/j.neuropharm.2012.06.051 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pabba M et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 11325–11338, doi: 10.1523/JNEUROSCI.0458-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang XJ, Liu LL, Jiang SX, Zhong YM & Yang XL Activation of the zeta receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience 177, 12–22, doi: 10.1016/j.neuroscience.2010.12.064 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Martina M, Turcotte ME, Halman S & Bergeron R The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. The Journal of physiology 578, 143–157, doi: 10.1113/jphysiol.2006.116178 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allahtavakoli M & Jarrott B Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain research bulletin 85, 219–224, doi: 10.1016/j.brainresbull.2011.03.019 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Behensky AA, Katnik C, Yin H & Cuevas J Activation of Sigma Receptors With Afobazole Modulates Microglial, but Not Neuronal, Apoptotic Gene Expression in Response to Long-Term Ischemia Exposure. Frontiers in neuroscience 13, 414, doi: 10.3389/fnins.2019.00414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katnik C et al. Treatment with afobazole at delayed time points following ischemic stroke improves long-term functional and histological outcomes. Neurobiology of disease 62, 354–364, doi: 10.1016/j.nbd.2013.10.011 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Katnik C et al. Activation of sigma1 and sigma2 receptors by afobazole increases glial cell survival and prevents glial cell activation and nitrosative stress after ischemic stroke. Journal of neurochemistry 139, 497–509, doi: 10.1111/jnc.13756 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Zhao X et al. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis : an international journal on programmed cell death 24, 157–167, doi: 10.1007/s10495-018-1495-2 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Xu Q et al. Sigma-1 receptor in brain ischemia/reperfusion: Possible role in the NR2A-induced pathway to regulate brain-derived neurotrophic factor. Journal of the neurological sciences 376, 166–175, doi: 10.1016/j.jns.2017.03.027 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Gao QJ et al. Sigma-1 Receptor Stimulation with PRE-084 Ameliorates Myocardial Ischemia-Reperfusion Injury in Rats. Chin Med J (Engl) 131, 539–543, doi: 10.4103/0366-6999.226076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, Saul A, Roon P & Smith SB Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America 113, E3764–3772, doi: 10.1073/pnas.1521749113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.da Fonseca AC et al. The impact of microglial activation on blood-brain barrier in brain diseases. Frontiers in cellular neuroscience 8, 362, doi: 10.3389/fncel.2014.00362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ajmo CT Jr., Vernon DO, Collier L, Pennypacker KR & Cuevas J Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Current neurovascular research 3, 89–98, doi: 10.2174/156720206776875849 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Hall AA, Herrera Y, Ajmo CT Jr., Cuevas J & Pennypacker KR Sigma receptors suppress multiple aspects of microglial activation. Glia 57, 744–754, doi: 10.1002/glia.20802 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffmann A et al. Early Blood-Brain Barrier Disruption in Ischemic Stroke Initiates Multifocally Around Capillaries/Venules. Stroke 49, 1479–1487, doi: 10.1161/STROKEAHA.118.020927 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Bauersachs J et al. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 100, 292–298, doi: 10.1161/01.cir.100.3.292 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Alves NG, Trujillo AN, Breslin JW & Yuan SY Sphingosine-1-Phosphate Reduces Hemorrhagic Shock and Resuscitation-Induced Microvascular Leakage by Protecting Endothelial Mitochondrial Integrity. Shock 52, 423–433, doi: 10.1097/SHK.0000000000001280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beard RS Jr. et al. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nature communications 7, 12823, doi: 10.1038/ncomms12823 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urfer R et al. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke 45, 3304–3310, doi: 10.1161/STROKEAHA.114.005835 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Shimazawa M, Sugitani S, Inoue Y, Tsuruma K & Hara H Effect of a sigma-1 receptor agonist, cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Experimental eye research 132, 64–72, doi: 10.1016/j.exer.2015.01.017 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Qin J et al. Activation of Sigma-1 Receptor by Cutamesine Attenuates Neuronal Apoptosis by Inhibiting Endoplasmic Reticulum Stress and Mitochondrial Dysfunction in a Rat Model of Asphyxia Cardiac Arrest. Shock 51, 105–113, doi: 10.1097/SHK.0000000000001119 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Liu X et al. Chronic stimulation of the sigma-1 receptor ameliorates autonomic nerve dysfunction and atrial fibrillation susceptibility in a rat model of depression. American journal of physiology. Heart and circulatory physiology 315, H1521–H1531, doi: 10.1152/ajpheart.00607.2017 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Pande A et al. A placebo-controlled trial of igmesine in the treatment of major depression. European Neuropsychopharmacology 9: S138 (1999). [Google Scholar]
- 102.Reutova MA, Siuniakov SA, Siuniakov TS & Neznamov GG [Anxiolytic afobazole action self-evaluated by patients with anxiety-asthenic disorders]. Eksperimental’naia i klinicheskaia farmakologiia 73, 6–12 (2010). [PubMed] [Google Scholar]
- 103.Syunyakov TS & Neznamov GG [Evaluation of the therapeutic efficacy and safety of the selective anxiolytic afobazole in generalized anxiety disorder and adjustment disorders: Results of a multicenter randomized comparative study of diazepam]. Terapevticheskii arkhiv 88, 73–86, doi: 10.17116/terarkh201688873-86 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Matsushima Y et al. Effects of fluvoxamine on nerve growth factor-induced neurite outgrowth inhibition by dexamethasone in PC12 cells. Bioscience, biotechnology, and biochemistry 83, 659–665, doi: 10.1080/09168451.2018.1553607 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Lopez OV et al. Sigma-1 Receptor Antagonist (BD1047) Decreases Cathepsin B Secretion in HIV-Infected Macrophages Exposed to Cocaine. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 14, 226–240, doi: 10.1007/s11481-018-9807-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bol’shakova AV et al. Neuroprotective Effect of sigma1-Receptors on the Cell Model of Huntington’s Disease. Bulletin of experimental biology and medicine 164, 252–258, doi: 10.1007/s10517-017-3968-7 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Brindley RL, Bauer MB, Hartley ND, Horning KJ & Currie KPM Sigma-1 receptor ligands inhibit catecholamine secretion from adrenal chromaffin cells due to block of nicotinic acetylcholine receptors. Journal of neurochemistry 143, 171–182, doi: 10.1111/jnc.14149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang K et al. Sigma-1 Receptor Plays a Negative Modulation on N-type Calcium Channel. Frontiers in pharmacology 8, 302, doi: 10.3389/fphar.2017.00302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Voronin MV & Kadnikov IA Contribution of Sigma-1 receptor to cytoprotective effect of afobazole. Pharmacology research & perspectives 4, e00273, doi: 10.1002/prp2.273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Katnik C, Guerrero WR, Pennypacker KR, Herrera Y & Cuevas J Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. The Journal of pharmacology and experimental therapeutics 319, 1355–1365, doi: 10.1124/jpet.106.107557 (2006). [DOI] [PubMed] [Google Scholar]


