Abstract
With technological advances, multi-gene panel testing has become increasingly used to identify patients at risk for hereditary breast cancer (HBC). There are currently evidence-based interventions and breast cancer screening strategies that exist for cancer prevention and early detection among patients with HBC. Moreover, in addition to the personal impact of identifying HBC, this information may be shared with at-risk family members to amplify the benefits of testing and subsequent care among those at high risk. Opportunities and challenges with the utilization of updated multi-gene panel testing for HBC, including: (a) tumor sequencing with germline consequences; (b) genetic counseling implications; and (c) strategies to improve the communication of genetic test results to family members will be reviewed. With the advances and expansion of genetic testing, all health care providers need to be updated on both the importance and complexities of HBC counseling and testing, in order to optimize patient care.
Keywords: BRCA, hereditary breast cancer, multi-gene panel, next-generation sequencing, tumor-based testing
1|. INTRODUCTION
Breast cancer is the most common cancer diagnosed in women worldwide, with approximately 5%–10% of cases attributed to high-penetrance hereditary breast cancer (HBC) genes,1,2 with an additional 20% attributed to moderate and low penetrance genes.3,4 Cancer risks associated with high-penetrance genes may be defined as >fourfold risk (and generally 10- to 20-fold risk), compared to moderate-penetrance genes with a twofold-fourfold risk of cancer.5,6 Prior studies suggest that the majority of HBC are attributed to the BRCA1 and BRCA2 (BRCA) genes7; however, there are “non-BRCA” inherited breast cancer genes which include both high (eg, PALB2, TP53, PTEN, and CDH1)8 and moderate (eg, ATM, CHEK2) penetrance genes.6 Additionally, there are single nucleotide polymorphisms (SNPs) identified within or outside of genes, which impart <twofold risks, thus may be considered to impart “low penetrance” cancer risks.9
Identification of HBC enables early detection and prevention of cancers and may impact therapeutic options.10 Specifically, BRCA mutation carriers have a 60%–70% lifetime risk of developing breast cancer,11 a 40% or greater risk of developing a second primary breast cancer,12 and up to a 44% lifetime risk of developing ovarian cancer.11,12 These risks may be reduced by 90% or more through preventive options such as risk-reducing mastectomy (RRM) and risk-reducing oophorectomy (RRSO).13 Analogous to the BRCA genes, other HBC genes have various health risks with corresponding management recommendations to mitigate elevated risks.6
With tremendous technological advances due to next-generation sequencing (NGS) technologies, there has been a shift to simultaneously test multiple genes through multi-gene panel testing, including genes which may not be clinically indicated based on personal or family history (FH) of cancer.14 These multi-gene cancer panels may include high and moderate-penetrance genes, in addition to “preliminary evidence” genes with uncertain or unproven cancer associations, and SNPs to generate a polygenic risk score. In addition to broader approaches to germline testing for hereditary cancers, tumor testing (including tumor sequencing) presents a tremendous opportunity to identify patients who may have HBC and require germline genetic testing for confirmation. The purpose of this article is to review newer approaches to germline genetic testing and screening for HBC, including tumor sequencing with germline consequences, updates in multi-gene panel testing, genetic counseling implications, and communication of genetic test results to family members.
2|. TESTING FOR GERMLINE MUTATIONS
Despite the longstanding availability of testing for hereditary cancer, including the BRCA genes, only a small proportion of the at-risk population has been tested. In fact, recent data suggest that <20% of patients with breast or ovarian cancer who meet national criteria for HBC testing are tested.15 Moreover, testing focused primarily on the BRCA genes resulted in an underdiagnosis of HBC, missing up to 10% of women with “non-BRCA” genes for which management guidelines exist.16 Traditionally, testing for hereditary cancer was based on a comprehensive assessment of the patient’s clinical phenotype and FH to generate a differential diagnosis from which sequential testing for specific syndromes would be initiated.17 However, the availability of NGS technologies has reduced sequencing costs, making it possible to test for multiple genes at a substantially lower cost than what it used to cost for fewer genes via Sanger sequencing methods.13 This technology enables a broad approach to testing for conditions with overlapping phenotypes and may identify hereditary cancers among patients with atypical clinical presentations or those with limited medical or family histories.18
NGS-based multi-gene panels vary, ranging from those which include only high-penetrance genes associated with breast cancer, to panels combining both high- and moderate-penetrance genes, to even some panels including genes of uncertain cancer penetrance or unproven cancer associations, as well as SNPs to generate polygenic risk scores.6 Multi-gene panels have increased detection rates of HBC syndromes, have revealed an expanded range of clinical phenotypes associated with mutations in various genes, and have enhanced our understanding of the natural history of these conditions.6,18 HBC syndromes demonstrate both variability and overlap in risks for breast and other cancers, as well as average age at onset (Table 1).19
TABLE 1.
Breast cancer-associated syndromes
| HBC Syndromes | Lifetime female breast cancer risk | Other lifetime cancer risk | Inheritance pattern | Genes associated | Breast cancer screening and management guidelines |
|---|---|---|---|---|---|
| Hereditary Breast/Ovarian cancer syndrome (HBOC)6,11 | 65% lifetime risk | Ovary (up to 44%); Primarily for BRCA2: pancreas (<5%); melanoma (<5%); male breast (6%–8%); prostate (20%–40%)53 | AD |
BRCA1 BRCA2 |
|
| Li-Fraumeni syndrome6,54 | 54% lifetime risk | Soft tissue sarcoma (15%); brain (6%); osteosarcoma (5%) | AD | TP53 |
|
| PTEN hamartoma syndrome (PHTS)/Cowden6 | 25%–50% lifetime risk | Colorectal (9%); thyroid (3%–10%); endometrium (5%–10%) | AD | PTEN |
|
| Hereditary diffuse gastric cancer syndrome (HDGC)6,55 | 39%–52% lifetime risk | Gastric (83%) | AD | CDH1 |
|
| PALB26 | 35% lifetime risk | Pancreas | AD | PALB2 |
|
Abbreviations: AD, Autosomal Dominant; HBC, Hereditary Breast Cancer; MMG, Mammogram; RRM, Risk-reduction mastectomy.
Or 5 years before the earliest known breast cancer in the family (whichever comes first).
2.1|. Framework to guide testing and management
A clinical validity framework has been developed through the Clinical Genome Resource (ClinGen) to classify genes based on the strength of evidence for association to a particular disease into definitive, strong, moderate, limited, disputed, or no reported evidence.20 High and moderate-penetrance genes are generally established to have an increased risk of HBC, whereas there are other genes with limited or uncertain data to establish an association with breast cancer and penetrance.6 Consequently, penetrance data in conjunction with clinical utility inform risk management.21 High-penetrance HBC genes may also be associated with other cancers as outlined in Table 1. Management recommendations differ depending on lifetime breast cancer risks and age distribution. Specifically, patients with pathogenic/likely pathogenic (P/LP) variants22 (also referred to as “mutation”) in high, compared to moderate-penetrance HBC genes, may be recommended to start screening at earlier ages and incorporate risk-reduction strategies, per practice guidelines developed through the National Comprehensive Cancer Network (NCCN) and updated at least annually (Table 1).6 Cancer risk management among patients with P/LP variants in moderate-penetrance genes are guided by the genetic test result, family cancer history, and other lifestyle and hormonal risk factors.23 Additionally, there are other “preliminary evidence” genes with unconfirmed or uncertain cancer risks which should not currently impact cancer risk surveillance and management, yet are included on some multi-gene cancer panels.6
2.2|. Polygenic risk scores
In addition to high- and moderate-penetrance genes, there have been an increasing number of “low penetrance” SNPs identified through genome wide association studies (GWAS) that in isolation correlate with a <twofold risk of developing breast cancer.24 However, algorithms combining multiple SNPs have been developed to generate a “polygenetic risk score,” derived based on the sum of the SNPs on overall risk of breast cancer in combination with the frequency of that SNP in the general population.24
Polygenic risk scores have shown promise for predicting an individual’s risk of breast cancer.24 Risks generated based on these algorithms, from a predominant Caucasian population, may be as high as those with high-penetrance genes.25 Several laboratories have recently incorporated their own proprietary algorithms for generating polygenic risk scores as part of the multi-gene panel testing they offer; however, these are not standardized across laboratories and are limited to Caucasian populations, representing another emerging advance that may widen the existing racial/ethnic disparity gap.26 Currently, polygenic risk scores have the potential to improve risk assessment; however, further studies are needed to validate their clinical utility, management implications, and incorporation of results into clinical practice.27 Moreover, SNPs show significant variability across racial/ethnic groups; therefore, more robust studies across diverse populations are needed to further characterize these differences and deploy them across diverse populations, such that this advance does not further exacerbate existing disparities.28
2.3|. Expanding the number of genes on panels
As our knowledge about HBC evolves, additional moderate risk genes are being identified, and NGS has allowed for these to be added to multi-gene panels without any change in the self-pay cost of testing.29 Multi-gene panels also include genes in which breast cancer risks are not yet established or confirmed (eg, RAD50, BRIP1, XRCC2, and MRE11A, among others) and therefore remain in a category where more information is needed to confirm elevated risks and/or develop evidence-based cancer risk management. Moreover, associations may disappear as data emerge, as has been suggested with the NBN gene which was suggested to be associated with an increased breast cancer risk based on data on the Slavic truncating founder variant (657del5),30 yet more recent studies indicated no association.31 Consequently, the relationship between P/LP variants in many of these more recently identified genes and breast cancer risk is not sufficient at this time to warrant changes in medical management,32 and may evolve with time.
2.4|. Possible test results
When conducting testing for inherited cancer, the American College of Medical Genetics and Genomics (ACMG) in conjunction with the Association for Molecular Pathology have recommended five specific terms to be used for variant classification as follows: “pathogenic”, “likely pathogenic”, “uncertain significance”, “likely benign”, and “benign”.22 In practice, however, these are often lumped into 3 categories when discussing results with patients (positive, negative, and inconclusive). A positive result (ie, P/LP variant) indicates the presence of a change in the gene considered to be associated with disease. A negative result (ie, benign or likely benign) is not generally reported out by laboratories. An inconclusive result, or “variant of uncertain significance” (VUS), indicates that a gene change was found, but its impact on gene function (and thus the patient’s phenotype and risk) is uncertain at this time. When testing is conducted for an established inherited cancer gene, a positive, negative, or VUS result is associated with elevated, not elevated, or uncertain cancer risks, respectively. Given that over 90% of VUS results are downgraded to negative (benign or likely benign), VUS results should generally be managed as negative results from a clinical standpoint.33
2.5|. Laboratory considerations when conducting NGS testing
Hereditary cancer panels vary between the laboratories that perform NGS testing, not only in terms of multi-gene panel tests offered but also the genes included on those panels, testing methodologies, detection rates, and results interpretation.19 Interpretation of genetics results is not standardized across genetic testing laboratories, which can result in variable interpretation and disparate clinical utility. When performing testing through NGS, it is important to recognize that variations larger than a few base pairs in size cannot readily be recognized across all testing platforms; thus, additional techniques providing evaluation of larger, structural genomic mutations may be used to supplement this testing, with variability across testing laboratories.17 Consequently, providers of genetic testing services, as well as those who order the tests in the clinics as part of routine patient care, must be familiar with the different laboratories’ testing approaches, so that they may select the most appropriate laboratory for their patients’ genetic testing needs.17 With more genes being added to panels, the chance for detecting a VUS increases.34 Providers must be reminded when a VUS is identified, medical care should be based on the individual’s FH (similar to negative test result cases).
2.6|. Genetic counseling approaches with multi-gene panel tests
NGS has rapidly increased the utilization of multi-gene panel testing for all patients with cancer. The paradigm shift from single- to multi-gene panel testing together with increased demand for genetic services has resulted in a need to develop and refine new genetic counseling strategies to deliver genetic services and manage population needs.17 Pretest genetic counseling has evolved in the era of panel-based testing, with an increase in the number of genes tested, which includes genes of uncertain clinical utility and genes not associated with a patient’s personal or FH.35 VUS results have added an another layer of complexity to pretest genetic counseling risk assessment, given the increased likelihood of detecting a VUS as more genes are tested,33 and these uncertainties must be shared with patients as part of the process of informed consent for testing (Figure 1).17 This caveat is particularly significant when counseling minority patients, given higher rates of VUS, results in these populations.36
FIGURE 1.
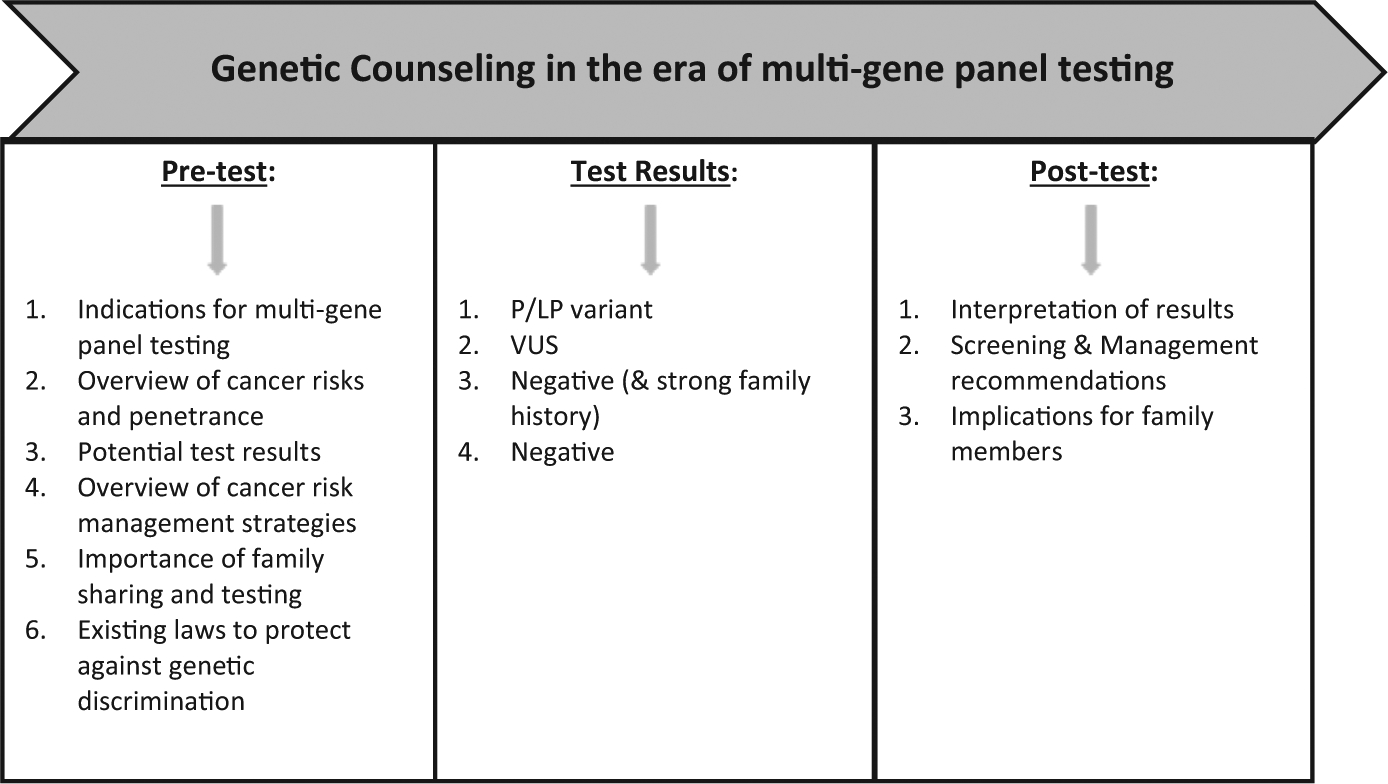
Components of Genetic Counseling and Testing Services
Although multi-gene panels make it easier to order comprehensive testing and reduce the chance of missing an underlying hereditary cancer syndrome, providers must maintain the patient’s autonomy and ability to make an informed decision, without overwhelming them with options and information.17,35 The traditional pretest genetic counseling model continues to be refined for effective integration into clinical practice.35 Post-test genetic counseling is recommended for all patients found to have a P/LP variant, VUS, or a negative result despite a strong FH to put the diagnosis into the appropriate clinical context.37
3|. TUMOR-BASED SCREENING AND TESTING
Tumor sequencing is increasingly used, particularly among patients with advanced stage cancer, to identify molecular targets to guide cancer treatment.38 However, this testing may uncover inherited (often unexpected) cancer susceptibility in 4%–12%,39,40 including those who may not meet clinical criteria for germline testing based on personal and family cancer history.41,42 Tumor testing approaches generally encompass tumor-only testing or tumor-normal paired testing. For tumor-normal paired testing, “normal” tissue may either be normal DNA extracted from tissue adjacent to the tumor or from germline DNA extracted from blood or saliva. With use of adjacent normal tissue, somatic mutations can be missed due to a “field effect” in which mutations present in the tumor are found in the adjacent normal tissue.43
Tumor-only testing is more common likely due to the lower cost, but has a higher false-positive rate, thus may lead to the administration of inappropriate cancer treatment.44 In contrast, tumor-normal paired testing subtracts normal/germline variants which detect so-matic-only variants. Consequently, it reduces the false-positive rate, given as many as one-third of actionable mutations in tumor-only analyses may incorrectly classify germline mutations as somatic mutations. However, germline pathogenic variants may be masked, unless there is a specific process to report these results to the clinician for patient disclosure.
Surrounding the testing processes are ethical issues concerning the consent process, which should include informing the patient of the potential to identify germline-inherited cancer predisposing conditions, particularly among patients consented for tumor-normal paired testing. Consent models vary and may incorporate an opt-in (testing is only done if the patient specifically consents to it) versus opt-out (testing is done unless the patient specifically indicates they do not want it). Although the opt-out model may pose some ethical challenges, the opt-in model poses additional complexities given there are now therapies available based on germline testing for inherited cancer.10 The opt-in model may also vary in the extent of inherited genes evaluated, which may include the 59 genes recommended by the American College of Medical Genetics (ACMG) for reporting following exome testing,45 or alternatively a broad versus narrower phenotype-driven inherited multi-gene cancer panel. As testing is increasingly incorporated into the workflow in the context of a cancer diagnosis, the workflow for consent must be considered simultaneously.
Should tumor-normal paired testing identify a germline P/LP variant, current practices are to recommend confirmation in a laboratory experienced in conducting germline inherited cancer testing. In contrast, a negative result on tumor-normal paired testing is not helpful given that sensitivity of most tumor tests is lower than most germline-focused tests, particularly for intermediate-sized deletions and duplications. Furthermore, the filtering of tumor sequencing data may differ between tumor and germline testing laboratories. Consequently, among patients who meet testing guidelines, germline testing is clinically indicated regardless of the results of tumor sequencing. However, as testing practices continue to evolve, it is important to assess whether tumor-normal paired testing will impact insurance coverage for subsequent germline-only testing.
Finally, the interpretation of sequencing results may differ between tumor and germline testing, which adds an additional layer of complexity. This is because tumor testing results are focused on therapeutic drug targets as the basis for identifying “positive” results, versus in the germline, where the focus is on identifying a nonfunctional transcript of the gene which raises cancer risks. Consequently, a variant that is known to be P/LP in the germline may be interpreted as normal in tumor testing reports (thus not reported) or as a VUS because it does not have clear treatment implications.
Given the complexities involved in tumor sequencing, the American Society of Clinical Oncology (ASCO) policy update recommends that patients provide informed consent before proceeding directly to tumor sequencing.35 Providers must communicate the potential for incidental and secondary germline information to patients before conducting tumor sequencing, ascertain patient preferences regarding the receipt of germline information, and allow patients to decline receipt of germline information.35
4|. IMPROVING IDENTIFICATION OF HEREDITARY CANCER PREDISPOSITION THROUGH FAMILY SHARING OF RESULTS
Once an individual is identified to have a mutation in a high-penetrance inherited cancer predisposition gene, it becomes very important that this information be shared with at-risk relatives so that they too can be proactive with cancer risk management (especially if they are confirmed to have the identified familial mutation).14 Most HBC syndromes are inherited in an autosomal dominant pattern, meaning that first- and second-degree relatives of a known mutation carrier have a 50% and 25% chance, respectively, to also carry the mutation.46,47 The first individual within a family found to harbor a mutation is generally responsible for sharing their positive result with relatives. Improving family sharing and motivating relatives of mutation carriers to access clinical cancer genetic services is a priority for maximizing health outcomes and translating genetics into preventative care delivery.
Genetic testing is underutilized by first-degree relatives, with 36% or fewer undergoing testing after a family member is identified with hereditary cancer.48 Furthermore, sharing of test results with distant relatives occurs less frequently, and those relatives do not have the opportunity for testing and cancer prevention.49 Other factors that influence family sharing include recognition of at-risk relatives, relationship type, physical or emotional closeness, perceptions of whether relatives want to know, anticipation of relatives’ reactions, and personal emotions.49–51 Health benefits from genetic testing can be magnified through development and implementation of strategies that encourage communication of genetic results, especially positive results, with at-risk relatives.50 It will be important to develop and test interventions to improve the identification of HBC within families given the important public health implications and opportunity to reduce overall cancer morbidity and mortality.
5|. CONCLUSION
The increasing use of multi-gene panel testing has provided an efficient and comprehensive testing approach for hereditary cancer predisposition. Despite advantages of ordering multi-gene panel testing for HBC, challenges arise in choosing among the various panels from different laboratories that include varying types and numbers of genes. Moreover, multi-gene panel testing must be paired with updated workflow and informed consent processes such that these services may be scaled up. Further research is needed in this complex area to better standardize testing methodologies and results interpretation across laboratories.52 Additional opportunities and challenges will arise as centers perform tumor sequencing given the emerging indications of informing treatment and prognosis for advanced stage cancers. Tumor-based testing requires development of its own workflow, and consideration of consent models with necessary information to be disclosed prior to testing, particularly with tumor-normal paired testing. Other efforts to improve identification of HBC includes enhancing resultsharing with family members once an individual is diagnosed with HBC. Ultimately, regardless of how advanced our technologies for identifying patients with hereditary cancer risk become, the need for a thorough history and examination (with emphasis on FH), appropriate risk counseling, screening, and management guidance by health care providers proficient in genetics will remain critical to optimize patient care and realize the promise of precision oncology across all populations.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Felix GES, Zheng Y, Olopade OI. Mutations in context: implications of BRCA testing in diverse populations. Fam Cancer. 2018;17:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippini SE, Vega A. Breast cancer genes: beyond BRCA1 and BRCA2. Front Biosci (Landmark Ed). 2013;18:1358–1372. [DOI] [PubMed] [Google Scholar]
- 4.Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet. 2008;40:17–22. [DOI] [PubMed] [Google Scholar]
- 5.Piccinin C, Panchal S, Watkins N, Kim RH. An update on genetic risk assessment and prevention: the role of genetic testing panels in breast cancer. Expert Rev Anticancer Ther. 2019;19:787–801. [DOI] [PubMed] [Google Scholar]
- 6.Network NCC. Genetic/Familial high-risk assessment: Breast and Ovarian. (Version3.2019) [Accessed October 20, 2019]. Available from: https://www-nccn-org.proxy.library.vanderbilt.edu/2019
- 7.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The breast cancer linkage consortium. Am J Hum Genet. 1998;62:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellsworth RE, Decewicz DJ, Shriver CD, Ellsworth DL. Breast cancer in the personal genomics era. Curr Genomics. 2010;11:146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor PM, Lindström S, Behrens S, et al. Assessment of interactions between 205 breast cancer susceptibility loci and 13 established risk factors in relation to breast cancer risk, in the Breast Cancer Association Consortium. Int J Epidemiol. 2020;49(1):216–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thavaneswaran S, Rath E, Tucker K, et al. Therapeutic implications of germline genetic findings in cancer. Nat Rev Clin Oncol. 2019;16:386–396. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. [DOI] [PubMed] [Google Scholar]
- 13.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA Oncol. 2010;304:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cragun D, Kinney AY, Pal T. Care delivery considerations for widespread and equitable implementation of inherited cancer predisposition testing. Expert Rev Mol Diagn. 2017;17:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35(34):3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fecteau H, Pal T. Clinical Considerations in the Conduct of Cancer Next-Generation Sequencing Testing and Genetic Counseling In: Wu W, Choudhry H, eds. Next Generation Sequencing in Cancer Research, Volume 2: From Basepairs to Bedsides. 2 Springer International Publishing; 2015:81–101. [Google Scholar]
- 18.Fountzilas C, Kaklamani VG. Multi-gene panel testing in breast cancer management In: Gradishar WJ, ed. Optimizing Breast Cancer Management. Cham, Switzerland: Springer International Publishing; 2018:121–140. [DOI] [PubMed] [Google Scholar]
- 19.Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw. 2014;12:1339–1346. [DOI] [PubMed] [Google Scholar]
- 20.Lee K, Seifert BA, Shimelis H, et al. Clinical validity assessment of genes frequently tested on hereditary breast and ovarian cancer susceptibility sequencing panels. Genet Med. 2019;21:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cybulski C, Wokołorczyk D, Jakubowska A, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29:3747–3752. [DOI] [PubMed] [Google Scholar]
- 24.Willoughby A, Andreassen PR, Toland AE. Genetic testing to guide risk-stratified screens for breast cancer. J Pers Med. 2019;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes E, Judkins T, Wagner S, et al. Abstract PD1–08: Development and validation of a combined residual risk score to predict breast cancer risk in unaffected women negative for mutations on a multi-gene hereditary cancer panel. Cancer Res. 2018;78:PD1–08–PD01–08. [Google Scholar]
- 27.Yadav S, Couch FJ. Germline genetic testing for breast cancer risk: the past, present, and future. Am Soc Clin Oncol Educ Book. 2019;61–74. [DOI] [PubMed] [Google Scholar]
- 28.Huo D, Hu H, Rhie SK, et al. Comparison of breast cancer molecular features and survival by African and European ancestry in the cancer genome Atlas. JAMA Oncol. 2017;3:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary E, Iacoboni D, Holle J, et al. Expanded gene panel use for women with breast cancer: identification and intervention beyond breast cancer risk. Ann Surg Oncol. 2017;24:3060–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss A, Garber JE, King T. Breast cancer surgical risk reduction for patients with inherited mutations in moderate penetrance genes. JAMA Surg. 2018;153:1145–1146. [DOI] [PubMed] [Google Scholar]
- 33.Mersch J, Brown N, Pirzadeh-Miller S, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA. 2018;320:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurian AW, Hughes E, Handorf EA, et al. Breast and ovarian cancer penetrance estimates derived from Germline multiple-gene sequencing results in women. JCO Precis Oncol. 2017;1–12. [DOI] [PubMed] [Google Scholar]
- 35.Robson ME, Bradbury AR, Arun B, et al. American society of clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. [DOI] [PubMed] [Google Scholar]
- 36.Caswell-Jin JL, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234–239. [DOI] [PubMed] [Google Scholar]
- 37.Rainville IR, Rana HQ. Next-generation sequencing for inherited breast cancer risk: counseling through the complexity. Curr Oncol Rep. 2014;16:371. [DOI] [PubMed] [Google Scholar]
- 38.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a Germline BRCA mutation. N Engl J Med. 2018;379:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrader KA, Cheng DT, Joseph V, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meric-Bernstam F, Brusco L, Daniels M, et al. Incidental germline variants in 1000 advanced cancers on a prospective somatic genomic profiling protocol. Ann Oncol. 2016;27:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15:465–473. [DOI] [PubMed] [Google Scholar]
- 42.Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331–337. [PubMed] [Google Scholar]
- 44.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7:283ra253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalia SS, Adelman K, Bale SJ, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19(2):249–255. [DOI] [PubMed] [Google Scholar]
- 46.Cheung EL, Olson AD, Yu TM, Han PZ, Beattie MS. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev. 2010;19:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton JG, Peshkin BN, Mays D, DeMarco TA, Patenaude AF, Tercyak KP. Maternal perceptions of BRCA genetic counseling communication processes about disclosing cancer risk information to children and adult relatives. Psychooncology. 2018;27:1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landsbergen K, Verhaak C, Kraaimaat F, Hoogerbrugge N. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4:115–119. [DOI] [PubMed] [Google Scholar]
- 49.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chopra I, Kelly KM. Cancer risk information sharing: the experience of individuals receiving genetic counseling for BRCA1/2 mutations. J Health Commun. 2017;22:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elrick A, Ashida S, Ivanovich J, et al. Psychosocial and clinical factors associated with family communication of cancer genetic test results among women diagnosed with breast cancer at a young age. J Genet Couns. 2017;26:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niraula S Tumor genomic sequencing as an impetus to screen for germline mutations: primum non nocere. J Oncol Pract. 2019;15:474–475. [DOI] [PubMed] [Google Scholar]
- 53.Lecarpentier J, Silvestri V, Kuchenbaecker KB, et al. Prediction of breast and prostate cancer risks in male BRCA1 and BRCA2 mutation carriers using polygenic risk scores. J Clin Oncol. 2017;35:2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mai PL, Best AF, Peters JA, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122:3673–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Network NCC. Genetic/Familial high-risk assessment: Gastric Cancer (Version2.2019) [Accessed October 20, 2019]. Available from: https://www-nccn-org.proxy.library.vanderbilt.edu/2019


