Abstract
Although aging is a major risk factor for intracerebral hemorrhage (ICH), there are very few studies comparing ICH pathology between young and early middle-aged mice. In this study, 8-month old mice (early middle-aged mice) were compared against 2-month old mice (young mice) in neurological and histological changes after ICH induction, such as body weight, lesion volume, astrocytic responses, and motor and cognitive functions. At day 8 after ICH, there was no significant difference in lesion volume between the two groups, and both groups did not exhibit significant cognitive decline, as assessed by spontaneous alternative Y-maze test. On the other hand, 8-month old mice showed delayed recovery from body weight loss, along with reduced astrocytic activation. Interestingly, in the two motor function tests (beam-walking test and corner turn test), 8-month old mice exhibited lower scores only in the beam-walking test, suggesting a partial disturbance in motor recovery after ICH. These results suggest that age-related differences in ICH pathology may already start to appear in early middle-aged brains.
Keywords: Intracerebral hemorrhage, middle-aged mice, motor dysfunction, astrocytes
1. Introduction
Acute spontaneous intracerebral (non-traumatic) Phemorrhage(ICH), which represents 10–20% of all types of stroke, is a life-threatening and disabling event [8]. There is no medical treatment that is effectively beneficial for ICH, so only supportive and conservative care is currently applied in clinic. The major etiology of acute spontaneous ICH is a deep perforating vasculopathy in the basal ganglia, followed by an initial mass effect to the surrounding tissue [6]. After the acute phase of pathological changes, secondary injuries in the peri-lesion, such as internal capsules formed by the corticospinal tract (CST), cause long-term neurological deficits. Therefore, along with investigating the events in the acute phase of ICH, understanding the pathological response in the peri-lesion during sub-acute phase is pivotal for future development of an effective medical treatment for ICH [14, 22].
Aging is a major risk factor of poor prognosis after both ischemic and hemorrhagic stroke [1, 5, 11, 41], and mid-life cardiovascular factors have been implied to be an independent predictive factor for later-life mild cognitive impairment and dementia [12, 16, 38]. In ICH, the rate of incident and mortality increases with age in clinic [1, 25]. Also, in pre-clinical studies, aged ICH rodents showed exacerbated ICH pathologies [9, 18, 19, 22, 40]. However, there are almost no studies comparing young mice against middle-aged mice in ICH pathology, especially in the peri-lesion area where motor fibers pass through [42]. In this study, we examined whether age-related changes in ICH pathology appear in early middle-aged brains by comparing young (2-month old) and early middle-aged (8-month) mice under the conditions of collagenase-induced ICH.
2. Materials and methods
2.1. Animals
In this study, we used nine 2-month-old and seven 8-month-old male C57BL/6J mice (Jackson Laboratory, USA) to examine whether there were differences in ICH pathology between the two cohorts (Figure 1). Mice were housed in a specific pathogen-free conditioned 12-hour light/dark cycle room (light on between 8:00 AM and 8:00 PM) with free access to food and water throughout the experiment. All experimental procedures were reviewed and approved by a Subcommittee for Research Animal Care of the Massachusetts General Hospital IACUC (Institution Animal Care and Use Committee), and we used an institutionally approved animal protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the law for the human treatment.
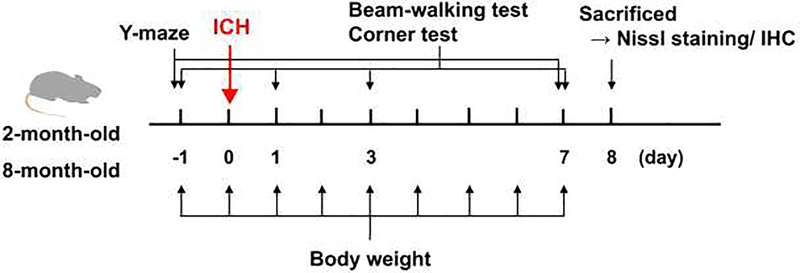
Figure 1. Experimental Scheme:
2- and 8-month-old mice were subjected to collagenase-induced intracerebral hemorrhage (ICH). Body weight was measured daily, and the two motor function tests (corner turn test and beam-walking test) were performed 1 day before and 1, 3, and 7 days after ICH. The Y-maze test was performed 1 day before and 7 days after ICH. On day 8 after ICH, all mice were sacrificed, and brain samples were used for histological examination.
2.2. Collagenase-induced intracerebral hemorrhage
Procedures for induction of ICH in mice were based on previous studies [15, 23]. Briefly, mice were anesthetized with 3.5% isoflurane and maintained on 1.5% isoflurane in 70% N2O and 30% O2, with a maintained rectal temperature between 36.5C and 37.5C. Mice were then placed in a stereotaxic frame (Kopf), and a 30-gauge needle was inserted into the right striatum (composed of caudate nucleus and putamen) through a burr hole on the skull (stereotaxic coordinates; 2.3 mm lateral to the midline, 0.2 mm anterior to the bregma and 3.5 mm depth below the skull). ICH was induced by injection of 0.025 U collagenase type VII (Sigma, St Louis, MO, USA) in 0.5 μL physiological saline, at a rate of over 0.2 μL/min. After surgical operation, mice were returned to their home cages and maintained under the same pre-operation conditions. In the current study, all mice of both groups survived up to 8 days after ICH induction, suggesting that middle-aged mice were not more susceptible to ICH than young mice.
2.3. Body weight measurement
Body weights of all animals were measured daily from 1 day before (i.e. day −1) to 7 days after (i.e. day 7) the ICH induction.
2.4. Motor function test (corner turn test and beam-walking test)
1 day before and 1, 3, and 7 days after ICH induction (day −1, 1, 3 and 7), sensory-based asymmetric activity and motor functions were evaluated using the corner test and beam-walking test, respectively. The investigator was blinded to the group allocation. In the corner test, mice were placed in the testing room in the early afternoon, at least 30 min before starting the test. Mice were led to a 30° angle corner, such that they needed to choose to turn either left or right when their whiskers contact the wall. A total of 10 trials was conducted, and a score was calculated as number of left turns/all trials×100. In the beam-walking test, mice were placed in the testing room at least 30 min before starting trials each day. A beam with a 1.1-m length, 1.27-cm width, and 0.5-m height was used as previously described [15]. All mice were trained three times per day before the ICH surgery, and in this current study, no animals were excluded due to failure in the training session. For each day of the beam-walking test, the hindlimb fault rate and walking distance were obtained as the average value from three trials. The performance score of the mice was based on an eight-point scale as previously described [24].
2.5. Cognitive function test (Y-maze test)
1 day before and 7 days after ICH induction (day −1 and 7), mice were tested for spontaneous alternation behavior with Y-maze [26]. Each mouse was placed in the arm of a symmetrical Y-maze to freely explore the maze for 8 minutes. This task was Prevideotaped,andthesequence and total number of arm entries was manually recorded in a blinded manner. Arm entry was confirmed when bilateral hind paws were placed inside the arm. Arms were cleaned with 70% Ethanol between each test. Percentage of alternation was calculated as follows: number of triads containing entries into 3 different arms/(total number of arms entered − 2) × 100.
2.6. Nissl staining and immunohistochemistry
One day after the last session of the beam-walking test (i.e. day 8 after ICH induction), mice were anesthetized and received transcardial perfusion with 40 mL 0.9% physiological saline. Then, brains were taken out, frozen with dry ice, and kept at −80C until use for Nissl staining or immunohistochemistry. Nissl staining with Cresyl Fast Violet Acetate Certified DcW-5 (Electron Microscopy Sciences, Hatfield, PA) was used for assessing lesion volume by ICH. Coronal brain sections of 20 μm thickness were obtained every 200 μm of mouse brain. The injury area in sections was measured by quantifying the Nissl staining-negative area by using ImageJ software. Injury volume was determined by integration of the injury area in each section over section depth. Immunohistochemistry was used for the purpose of assessing glial activation, where frozen sections (20 μm thickness) were fixed with 4% paraformaldehyde for 20 minutes. After being rinsed 3 times with phosphate-buffered saline (PBS) containing 0.3% Triton X-100 (PBS/T) for 5 minutes, sections were incubated in PBS/0.3% BSA solution for 1 hour at room temperature. Then, sections were incubated overnight in PBS/0.3% BSA solution containing primary antibodies at 4C. For detection of astrocytes, glial fibrillary acidic protein (GFAP) monoclonal antibody (rat) (1:200; cat# 13–0300, Thermo Fisher scientific, IL, USA) was used as the primary antibody. After rinsing 3 times with PBS/T for 5 minutes, sections were incubated with the corresponding secondary antibody (1:1000, Jackson Immunoresearch Laboratories) for 2 hours at room temperature. Finally, the sections were washed 3 times with PBS and covered with VECTASHIELD® mounting medium with DAPI (Vector Laboratories, USA). Stained sections were observed with ECLIPSE Ti-S and scanned with Retiga™ 2000R Fast 1394 Digital Camera. For evaluation of GFAP-positive area and DAPI-positive cell numbers, 2 brain sections were used, and per each section, 3 squares (400 × 400 μm2) were selected in the peri-hematomal region. Then, for each animal, the average percentage of GFAP positive area or DAPI-positive cell numbers was calculated by an operator who was blinded to the group allocation.
2.7. Statistical analysis
Statistical analysis was performed with Prism 8(Graphpad,La Jolla, CA) and conducted by Student’s t-test or two-way repeated-measures analysis of variance, followed by Sidak multiple comparisons test. To analyze the relationship between body weight loss and the beam-walking test results, Pearson’s correlation analysis was used. Differences with P < 0.05 were considered statistically significant, and data were expressed as mean plus and/or minus SD.
3. Results
3.1. Recovery from body weight loss after ICH
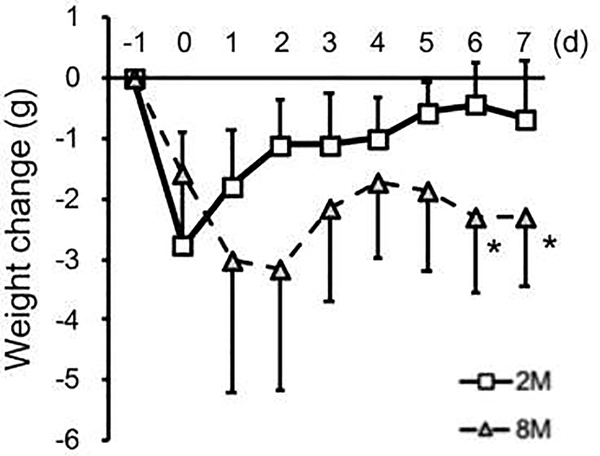
To confirm the homogeneous severity of stroke insults [28], body weight changes after ICH were compared between 2-month old and 8-month old mice. In both groups, the body weight decreased in the acute phase after ICH, presumably due to (i) ICH-caused brain damage, which results in the inability to reduce food intake, and (ii) general post-surgery damage, which causes temporal anorexia. After this, 2-month old mice gradually recovered from body weight loss, but the decrease in body weight of 8-month old mice was sustained (Figure 2).
Figure 2. Recovery from body weight loss after ICH:
A significant difference was observed in Age x Time Prcourseinteractions(F (8, 112) = 5.55, P < 0.001). The post-hoc analysis showed that there were significant differences between 2-month and 8-month old groups at multiple time points after ICH (*P < 0.05, Sidak multiple comparison test). Data expressed as mean ± SD. N = 9 for 2 M, and N = 7 for 8 M.
3.2. Histological changes after ICH
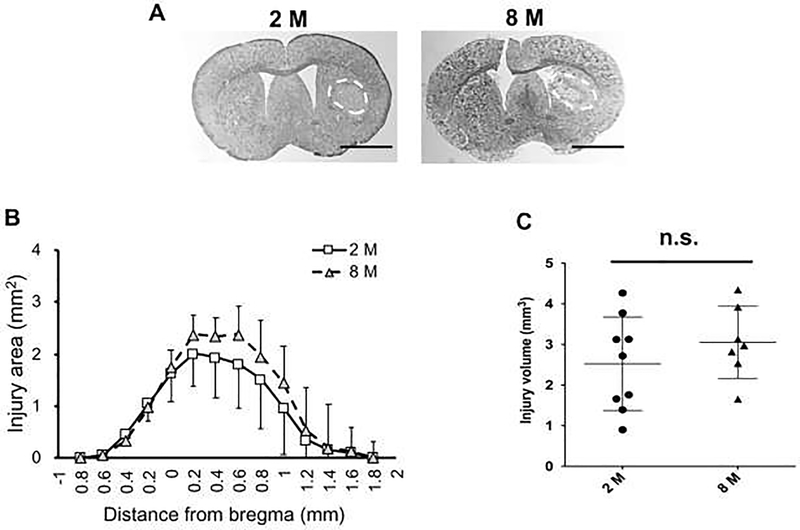
To determine whether the collagenase-injected mice developed any hemorrhagic lesion in the striatum, Nissl staining was performed at day 8 after ICH induction, using mouse coronal brain sections ranging from −0.6 to 1.6 mm to the bregma (Figure 3a). As expected, the collagenase injection into striatum caused cerebral hemorrhage in both 2-month and 8-month old groups (Figure 3a), and there were no significant differences in lesion area (Figure 3b) or volume (Figure 3c). However, in the lateral peri-lesioned area (Figure 4a) at day 8 after ICH, astrocyte activation (assessed by GFAP positive areas) of 8-month old mice was lower compared to that of 2-month old mice (Figure 4b). This difference was not due to a change in total cell number in the peri-lesioned area because there was no difference in DAPI-positive number between the two groups (Figure 4c).
Figure 3. Histological changes after ICH - hemorrhagic brain damage:
(a) Representative images of Nissl-stained coronal sections obtained at day 8 after ICH induction in 2-month (top left) and 8-month (top right) old mice. (b) Quantitative result of lesion area for individual axial sections at 8 days after ICH induction. Two-way ANOVA did not show any significant differences between the two groups (Interaction for Distance to bregma x Age; F (13, 182) = 0.92, P = 0.53. Age; F(1, 14) = 1.04, P = 0.33. Distance to bregma; F (2.00, 28.1) = 52.2, P < 0.001). (c) Quantitative result of the integrated total lesion volume, confirming no significant difference between 2- and 8-month old mice (P = 0.32, Unpaired t-test with Welch’s correction). Data expressed as mean plus and/or minus SD. N = 9 for 2 M, and N = 7 for 8 M.
Figure 4. Histological changes after ICH - astrocyte activation:
(a) Representative images of GFAP immunohistochemistry. The rectangles indicate the areas used for quantification of GFAP immunoreactivity. Scale bars = 100 μm. (b) Quantitative result of GFAP-positive area at day 8 after ICH induction showing significant difference between 2- and 8-month old mice (P = 0.0051, Student’s t-test). Data expressed as mean ± SD. N = 9 for 2 M, and N = 7 for 8 M. (c) Quantitative result of DAPI-positive cell numbers at day 8 after ICH induction showing no significant difference between 2- and 8-month old mice (P = 0.14, Student’s t-test). Data expressed as mean ± SD. N = 9 for 2 M, and N = 7 for 8 M.
3.3. Neurological deficits after ICH
To examine whether 8-month old mice show different neurological deficits after ICH, 2 types of behavioral tests were performed; one was the corner turn test to evaluate sensory-motor asymmetry, and the other was the beam-walking test to evaluate general motor functions. In the corner test, both groups showed symmetric behavior before ICH induction (e.g. day −1), and at day 1 after ICH induction, the laterality ratio increased in both groups (Figure 5a). Asymmetric behavior then eased over the next several days without significant differences between Pre-p2-month and 8-month old mice (Figure 5a). In contrast, in the beam-walking test, the motor function recovery in 8-month old mice was slower, despite the similarity in deficits in the acute phase between 2-month and 8-month old mice (Figure 5b for the fault rate, and Figure 5c for the score of beam-walking).
Figure 5. Neurological deficits after ICH:
(a) In laterality index of the corner test, two-way ANOVA did not show any significant differences between the two groups (Interactions Age x Time course; F (3, 42) = 0.47, P = 0.71. Age; F (1, 14) = 0.32, P = 0.58. Time course; F (3, 42) = 47.04, P < 0.001). (b) In the fault rate of the beam-walking test, significant differences between the two groups were observed (Two-way repeated-measures ANOVA, Age x Time course interactions; F (3, 42) = 4.78, P = 0.006. Age; F (1, 14) = 12.1, P = 0.004. Time course; F (2.73, 38.2) = 196.9, P < 0.001), and the post-hoc analysis showed that the fault rate of 8-month-old mice was significantly greater at day 7 (*P < 0.05, Sidak multiple comparison test). (c) In the score of beam-walking test, there were trends in the difference of both Age x Time course interactions (F (3, 42) = 2.71, P = 0.057; Two-way repeated-measures ANOVA) and Age (F (1, 14) = 3.61, P = 0.078), and therefore, we conducted a post-hoc analysis. 8-month old mice showed a lower score at day 7 (*P < 0.05, Sidak multiple comparison test). Data expressed as mean ± SD. N = 9 for 2 M, and N = 7 for 8 M.
3.4. Cognitive function after ICH
On day −1 and 7 after ICH induction, the spontaneous Y-maze test was performed to evaluate cognitive function. In addition, along with the percent of alternation (an index of working memory), the number of arm entries was counted for each mouse to assess exploratory activity. Before ICH induction, there was a difference in the number of arm entries between 2-month and 8-month old mice (Figure 6a), indicating an age-related decline in exploratory activity. However, for the working memory itself, there were no significant differences between the two groups at both points, i.e. before ICH induction and 8 days after ICH induction (Figure 6b).
Fig. 6. Cognitive function after ICH:
(a) Number of arm entries in Y-maze test at day −1 and 7. Two-way repeated-measures ANOVA showed significant differences between the two groups (Interactions Age x Time course; F (1, 14) = 9.49, P < 0.01. Age; F (1, 14) = 12.0, P < 0.01. Time course; F (1, 14) = 2.86, P=0.11). The post-hoc analysis showed a significant decrease in number of arm entries between 2-month-old mice and 8-month-old mice at day −1 (*P < 0.05, Sidak multiple comparison test). (b) Change in spontaneous alternation in Y-maze test at day-1 and 7. No significant difference was observed between two groups (Two-way repeated-measures ANOVA, Age x Time course interactions; F (1, 14) = 0.09, P = 0.77). Data expressed as mean + SD. N = 9 for 2 M, and N = 7 for 8 M.
4. Discussion
In this study, we compared young mice (2-month old) against early middle-aged mice (8-month old) and demonstrated that (i) early middle-aged mice showed reduced glial activation at day 8 after ICH induction despite no difference in hemorrhagic lesion volume between the two cohorts, (ii) early middle-aged mice could not recover from body weight loss after ICH, and (iii) early middle-aged mice showed slower recovery from neurological deficits in some behavioral tests. Acute spontaneous ICH, which mainly occurs in the basal ganglia, is a devastating type of stroke that often causes death or prolonged neurological deficits, such as long-term hemiparesis. Previous studies identified multiple factors that beneficially or adversely affect ICH pathology [1, 5, 21], and our current findings are consistent with and may expand the existing literatures by confirming that aging is indeed an important negative factor for ICH pathology.
Aging is an independent predictor of ICH outcome in clinic [11, 13, 37]. However, some studies showed inconsistent results about the relationship between aging and ICH outcomes [2], and therefore, an experimental study may be necessary to explore the underlying mechanisms by which aging exacerbates ICH pathology. In our study, some of the age-related changes in ICH pathology appeared even in early middle-aged mice, i.e. 8-month old mice showed prolonged body weight loss after ICH while young mice showed recovery from body weight loss. Notably, in our system, early middle-aged mice exhibited slower recovery in the beam-walking Pre-protest,but there was no significant difference in behavioral recovery in the corner turn test between the two groups. These two behavior tests were both reported to be capable of distinguishing the ratio of behavior recovery after ICH between young and aged mice [9], suggesting that the compensatory responses after ICH in early middle-aged mice may have been partially disturbed.
Another important finding of this study is that despite similar lesion volumes in both young and early middle-aged mice, astrocytic responses after ICH differed. Recent advances in proteomic and transcriptomic technology have now revealed the complex heterogeneity of astrocyte function [3, 4], and indeed, astrocytes play both detrimental and beneficial roles after brain injury [30, 33]. For example, activated astrocytes (e.g. astrogliosis) lead to the formation of glial scars, which isolate and protect the non-injured tissue from exposure to toxic elements, contributing to the suppression of inflammation [17, 43]. On the other hand, glial scars create physical and biochemical barriers that inhibit axonal regeneration, resulting in delayed functional recovery. Furthermore, reactive astrocytes produce both cyto-toxic factors (e.g. pro-inflammatory cytokines) and pro-survival factors (e.g. growth factors), depending on context after brain injury, including ICH [29, 32, 34–36]. Our current study showed that middle-aged mice exhibited less astrocyte activation in the peri-lesioned area at day 8 after ICH. Considering that astrocyte activation is related to inflammatory responses after brain injury [7, 27, 39] and that some drugs are reported to ameliorate motor and sensorimotor dysfunction by suppressing inflammation or promoting anti-inflammation without affecting hematoma resolution [15, 20], it is possible that reduced astrocyte activation in middle-aged mice may be a reason for slower recovery after ICH.
In our current study, there was no difference in hemorrhagic lesion volume between young and early middle-aged mice, but the recovery speed was different. These findings may provide a clinically relevant indication that the middle-aged population may suffer from a longer rehabilitation duration after ICH compared to the younger population, even if the injury volume was similar. Nevertheless, there are some limitations and caveats in this study, which motivate us to conduct further studies to understand the age-related differences of ICH pathology. Firstly, our current study assessed the lesion volume and astrocyte activation at only one time point of the sub-acute phase. However, at the acute phase of ICH pathology (3 days after ICH induction), astrocyte activation was reported to be more prevalent in aged SD rats (22-month old) compared to young rats (2-month old) [40]. Therefore, it would be possible that a glial activation pattern after ICH may differ depending Pre-proofnotonlyontime-course afterinjury,butalsoon aging processes. To further our understanding of age-related changes in ICH pathology, we need to assess lesion volume Journalandglialresponsesatmultipletimepointsin future studies. Second, we showed that early middle-aged mice showed worse neurological deficits in the beam-walking test along with slower recovery from body weight loss. In our study, there was a correlation between worse results in the beam-walking test and body weight loss in ICH mice (Δ Body weight loss vs Fault rate at day 7 after ICH: R2 = 0.43, P = 0.0058); however, we did not provide any direct evidence that the body weight loss caused the neurological deficits, and as far as we know, there are no reports that have examined how body weight loss may cause neurological deficits in the beam-walking test after ICH induction in mice. If body weight loss does not cause any neurological deficits in ICH mice, we may use values of body weight changes as an index to reflect neurological function. Therefore, it is worthwhile to pursue whether body weight loss by ICH is the main reason for neurological deficits in mice in the research field of pre-clinical ICH study. Third, we conducted one single cognitive function test (e.g. Y-maze test) up to only 8 days after ICH induction, and in our system, no significant cognitive decline was observed in both 2-month and 8-month old mice. However, past studies reported that cognitive function was affected by ICH in mice [10, 31], and therefore, future studies are warranted to study long-term effects in learning and memory function after ICH with multiple cognitive tests. Finally, to understand the mechanisms of age-related changes of ICH pathology more deeply, we may need to clarify what kind of compensatory responses would be susceptible to aging. In particular, as our current study indicates that ICH-induced glial responses in the peri-lesion area are changed by aging, investigation into glial heterogeneity with next gene sequencing approaches is an initial step for this research direction.
5. Conclusions
In summary, we provide a novel insight into the mechanisms of age-related changes in ICH pathology by comparing 2-month-old young mice against 8-month-old middle-aged mice. It has been widely accepted that aged mice exhibit worse outcomes after ICH, and our current study suggests that some of these differences may start to appear even in early middle-aged mice.
Highlights.
8-month old mice were compared with 2-month old mice after intracerebral hemorrhage
There was no significant difference in lesion volume at day 8 between the two groups
8-month old mice showed delayed recovery along with reduced astrocytic activation
Acknowledgments
The authors thank Drs. Eng H. Lo, Yuki Kurauchi, Akihiro Hisatsune and Takahiro Seki for their support and advice on this study. The authors thank Ms. Nozomi Suzuki for technical assistance. Supported in part by NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ariesen MJ, Claus SP, Rinkel GJ, Algra A, Risk factors for intracerebral hemorrhage in the general population: a systematic review, Stroke 34 (2003) 2060–2065. [DOI] [PubMed] [Google Scholar]
- [2].Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G, Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality, Stroke 24 (1993) 987–993. [DOI] [PubMed] [Google Scholar]
- [3].Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS, Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence, Neuron 95 (2017) 531–549 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA, Normal aging induces A1-like astrocyte reactivity, Proc Natl Acad Sci U S A 115 (2018) E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, C. American Heart Association Stroke, R. Council on Cardiovascular, Intervention, N. Council on Cardiovascular, S. Council on Cardiovascular, Anesthesia, C. Council on Clinical, Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association, Stroke 43 (2012) 1711–1737. [DOI] [PubMed] [Google Scholar]
- [6].Cordonnier C, Demchuk A, Ziai W, Anderson CS, Intracerebral haemorrhage: current approaches to acute management, Lancet 392 (2018) 1257–1268. [DOI] [PubMed] [Google Scholar]
- [7].Emsley HC, Tyrrell PJ, Inflammation and infection in clinical stroke, J Cereb Blood Flow Metab 22 (2002) 1399–1419. [DOI] [PubMed] [Google Scholar]
- [8].Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V, Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review, Lancet Neurol 8 (2009) 355–369. [DOI] [PubMed] [Google Scholar]
- [9].Gong Y, Hua Y, Keep RF, Hoff JT, Xi G, Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits, Stroke 35 (2004) 2571–2575. [DOI] [PubMed] [Google Scholar]
- [10].Hartman R, Lekic T, Rojas H, Tang J, Zhang JH, Assessing functional outcomes following intracerebral hemorrhage in rats, Brain Res 1280 (2009) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC, The ICH score: a simple, reliable grading scale for intracerebral hemorrhage, Stroke 32 (2001) 891–897. [DOI] [PubMed] [Google Scholar]
- [12].Horder H, Johansson L, Guo X, Grimby G, Kern S, Ostling S, Skoog I, Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women, Neurology 90 (2018) e1298–e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Juvela S, Risk factors for impaired outcome after spontaneous intracerebral hemorrhage, Arch Neurol 52 (1995) 1193–1200. [DOI] [PubMed] [Google Scholar]
- [14].Keep RF, Hua Y, Xi G, Intracerebral haemorrhage: mechanisms of injury and therapeutic targets, Lancet Neurol 11 (2012) 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kinoshita K, Matsumoto K, Kurauchi Y, Hisatsune A, Seki T, Katsuki H, A Nurr1 agonist amodiaquine attenuates inflammatory events and neurological deficits in a mouse model of intracerebral hemorrhage, J Neuroimmunol 330 (2019) 48–54. [DOI] [PubMed] [Google Scholar]
- [16].Knopman DS, Haeberlein SB, Carrillo MC, Hendrix JA, Kerchner G, Margolin R, Maruff P, Miller DS, Tong G, Tome MB, Murray ME, Nelson PT, Sano M, Mattsson N, Sultzer DL, Montine TJ, Jack CR Jr., Kolb H, Petersen RC, Vemuri P, Canniere MZ, Schneider JA, Resnick SM, Romano G, van Harten AC, Wolk DA, Bain LJ, Siemers E, The National Institute on Aging and the Alzheimer's Association Research Framework for Alzheimer's disease: Perspectives from the Research Roundtable, Alzheimers Dement 14 (2018) 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laird MD, Vender JR, Dhandapani KM, Opposing roles for reactive astrocytes following traumatic brain injury, Neurosignals 16 (2008) 154–164. [DOI] [PubMed] [Google Scholar]
- [18].Leclerc JL, Li C, Jean S, Lampert AS, Amador CL, Diller MA, Tolosano E, Dore S, Temporal and age-dependent effects of haptoglobin Pre-proofdeletiononintracerebralhemorrhage-induced brain damage and neurobehavioral outcomes, Exp Neurol 317 (2019) 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee JC, Cho GS, Choi BO, Kim HC, Kim WK, Aging exacerbates intracerebral hemorrhage-induced brain injury, J Neurotrauma 26 (2009) 1567–1576. [DOI] [PubMed] [Google Scholar]
- [20].Li Q, Wan J, Lan X, Han X, Wang Z, Wang J, Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice, J Cereb Blood Flow Metab 37 (2017) 3110–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lim TC, Mandeville E, Weng D, Wang LS, Kurisawa M, Leite-Morris K, Selim MH, Lo EH, Spector M, Hydrogel-Based Therapy for Brain Repair After Intracerebral Hemorrhage, Transl Stroke Res (2019). [DOI] [PubMed] [Google Scholar]
- [22].Lively S, Schlichter LC, Age-related comparisons of evolution of the inflammatory response after intracerebral hemorrhage in rats, Transl Stroke Res 3 (2012) 132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matsumoto K, Kinoshita K, Yoshimizu A, Kurauchi Y, Hisatsune A, Seki T, Katsuki H, Laquinimod and 3,3'-diindolylemethane alleviate neuropathological events and neurological deficits in a mouse model of intracerebral hemorrhage, J Neuroimmunol 342 (2020) 577195.. [DOI] [PubMed] [Google Scholar]
- [24].Matsushita H, Hijioka M, Hisatsune A, Isohama Y, Shudo K, Katsuki H, A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice, J Cereb Blood Flow Metab 31 (2011) 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nilsson OG, Lindgren A, Stahl N, Brandt L, Saveland H, Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden, J Neurol Neurosurg Psychiatry 69 (2000) 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ohtomo R, Kinoshita K, Ohtomo G, Takase H, Hamanaka G, Washida K, Islam MR, Wrann CD, Katsuki H, Iwata A, Lok J, Lo EH, Arai K, Treadmill Exercise Suppresses Cognitive Decline and Increases White Matter Oligodendrocyte Precursor Cells in a Mouse Model of Prolonged Cerebral Hypoperfusion, Transl Stroke Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Panickar KS, Norenberg MD, Astrocytes in cerebral ischemic injury: morphological and general considerations, Glia 50 (2005) 287–298. [DOI] [PubMed] [Google Scholar]
- [28].Park SY, Marasini S, Kim GH, Ku T, Choi C, Park MY, Kim EH, Lee YD, Suh-Kim H, Kim SS, A method for generating a mouse model of stroke: evaluation of parameters for blood flow, behavior, and survival [corrected], Exp Neurobiol 23 (2014) 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pearson VL, Rothwell NJ, Toulmond S, Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: Pre-proofcorrelation with the progression of cell death, Glia 25 (1999) 311–323. [PubMed] [Google Scholar]
- [30].Pekny M, Wilhelmsson U, Pekna M, The dual role of astrocyte activation and reactive gliosis, Neurosci Lett 565 (2014) 30–38. [DOI] [PubMed] [Google Scholar]
- [31].Shi E, Shi K, Qiu S, Sheth KN, Lawton MT, Ducruet AF, Chronic inflammation, cognitive impairment, and distal brain region alteration following intracerebral hemorrhage, FASEB J 33 (2019) 9616–9626. [DOI] [PubMed] [Google Scholar]
- [32].Shi SX, Li YJ, Shi K, Wood K, Ducruet AF, Liu Q, IL (Interleukin)-15 Bridges Astrocyte-Microglia Crosstalk and Exacerbates Brain Injury Following Intracerebral Hemorrhage, Stroke 51 (2020) 967–974. [DOI] [PubMed] [Google Scholar]
- [33].Sofroniew MV, Vinters HV, Astrocytes: biology and pathology, Acta Neuropathol 119(2010)7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Swanson RA, Ying W, Kauppinen TM, Astrocyte influences on ischemic neuronal death,Curr Mol Med 4 (2004) 193–205. [DOI] [PubMed] [Google Scholar]
- [35].Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo EH, Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage, J Cereb Blood Flow Metab 27 (2007) 460–468. [DOI] [PubMed] [Google Scholar]
- [36].Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ, Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets, J Stroke 22 (2020) 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, Kase CS, Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival, Ann Neurol 29 (1991) 658–663. [DOI] [PubMed] [Google Scholar]
- [38].Virta JJ, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, Kaprio J,Midlife cardiovascular risk factors and late cognitive impairment, Eur J Epidemiol 28 (2013) 405–416. [DOI] [PubMed] [Google Scholar]
- [39].Wang J, Dore S, Inflammation after intracerebral hemorrhage, J Cereb Blood Flow Metab 27 (2007) 894–908. [DOI] [PubMed] [Google Scholar]
- [40].Wasserman JK, Yang H, Schlichter LC, Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats, Eur J Neurosci 28 (2008) 1316–1328. [DOI] [PubMed] [Google Scholar]
- [41].Yousufuddin M, Young N, Aging and ischemic stroke, Aging (Albany NY) 11 (2019)2542–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu Z, Tang L, Chen L, Li J, Wu W, Hu C, Erythropoietin reduces brain injury after intracerebral hemorrhagic stroke in rats, Mol Med Rep 8 (2013) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [43].Zhong JH, Zhou HJ, Tang T, Cui HJ, Yang AL, Zhang QM, Zhou JH, Zhang Q, Gong X, Zhang ZH, Mei ZG, Activation of the Notch-1 signaling pathway may be involved in intracerebral hemorrhage-induced reactive astrogliosis in rats, J Neurosurg 129 (2018) 732–739. [DOI] [PubMed] [Google Scholar]