Abstract
Adolescence is a time of major plasticity of brain systems that regulate motivated behavior and cognition, and is also the age of peak onset of nicotine use. Although there has been a decline in teen use of cigarettes in recent years, there has been a huge increase in nicotine vaping. It is therefore critically important to understand the impact of nicotine on this critical phase of brain development. Animal studies have shown that nicotine has unique effects on adolescent brain. The goal of this review is therefore to systematically evaluate age- and sex-differences in the effects of nicotine on brain and behavior. Both acute and chronic effects of nicotine on brain biochemistry and behavior, particularly drug reward, aversion, cognition and emotion, are evaluated. Gaps in our current knowledge that need to be addressed are also highlighted. This review compares and integrates human and animals findings. Although there can be no experimental studies in humans to confirm similar behavioral effects of teen nicotine exposure, an emerging observational literature suggests similarities across species. Given the substantial evidence for long-term negative impact of adolescent nicotine exposure on brain and behavior, further longitudinal assessment of health outcomes in teen and young adult e-cigarette users is warranted.
Keywords: E-cigarettes, alcohol, psychostimulants, depression, anxiety, cognition
Graphical Abstract
1. The teen vaping crisis
Whereas the use of tobacco cigarettes has declined in the United States within recent years, the use of electronic cigarettes (e-cigarettes) has increased exponentially (Cullen et al., 2019; Miech et al., 2019). Although introduced as recently as 2007 to the US market, e-cigarettes are now the most commonly used tobacco product among teenagers (Miech et al., 2017; Wang et al., 2019). According to Monitoring the Future survey data (Miech et al., 2019), 25.4% of 12th graders in 2019 used e-cigarettes within the last thirty days, an increase of 131% from two years previously (Figure 1). Use of e-cigarettes was almost as prevalent among 10th graders, and was particularly troubling among 8th graders who showed a 157% increase in 30-day e-cigarette use over the last two years. In absolute numbers, the National Youth Tobacco Survey shows that ~5 million students in grades 6–12 have tried e-cigarettes within the past 30 days, an increase from ~3.6 million in 2018 (Cullen et al., 2019). Of these, nearly 1 million are vaping daily.
Figure 1.
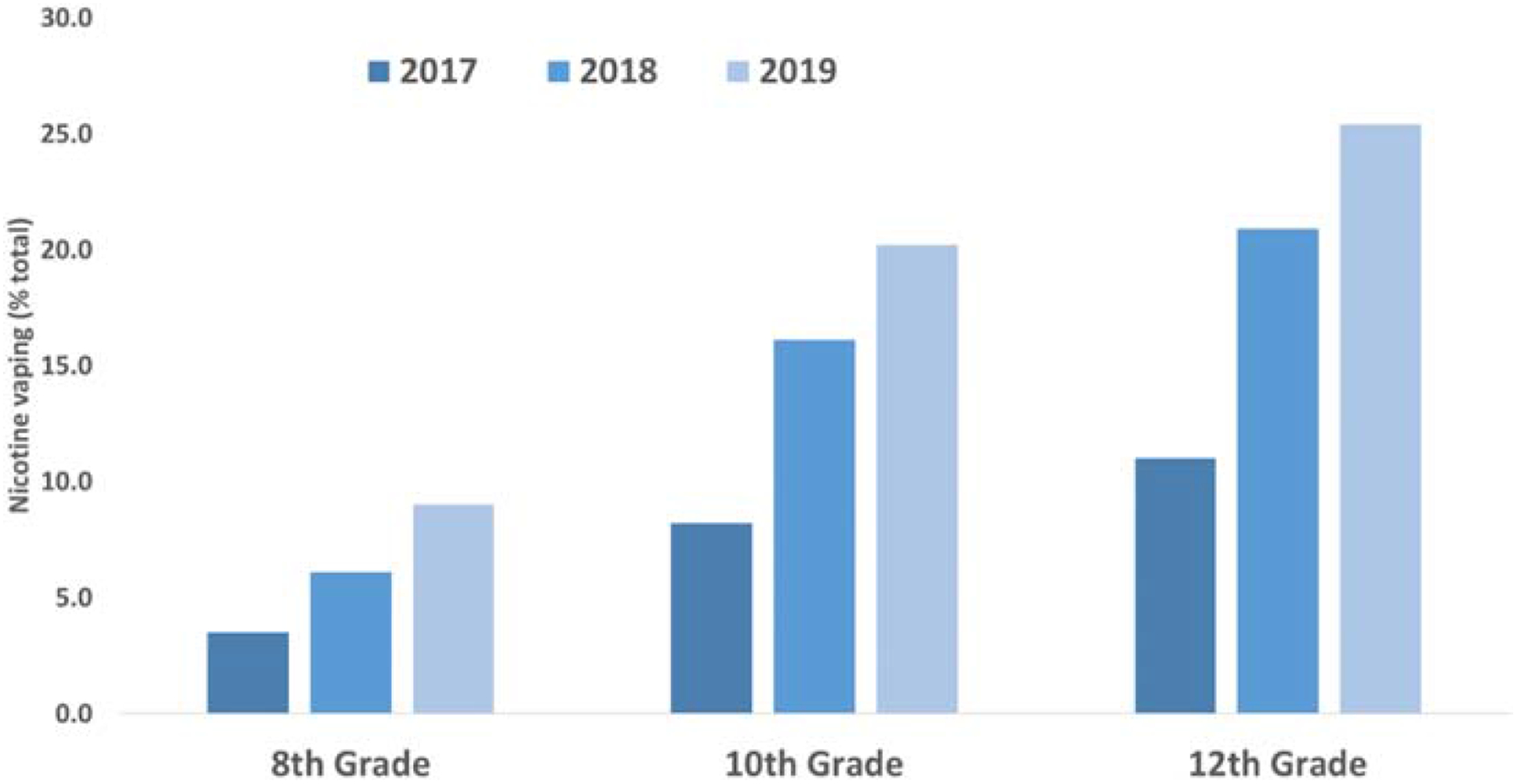
High school students vaping during the last 30 days, 2017–19 (%). From Meich et al., 2019.
There are a number of reasons for this rapid increase in e-cigarette use. Marketing of e-cigarettes to adolescents has been very effective (Marynak et al., 2018), and manufacturers offer a wide selection of flavors that appeal to youth. Flavorants can lead to different patterns of nicotine delivery and a decreased perception of risk (Voos et al., 2019; Jensenn and Boykan, 2019; Boccio et al., 2020). Adolescents who use flavorants are also frequently unaware of their nicotine exposure, with 40% of subjects with significant urinary cotinine in a recent study reporting that they used only nicotine-free products (Boykan et al., 2019b). Furthermore, adolescents who used e-cigarettes with non-traditional flavors are more likely to continue vaping and take more puffs per session six months later (Leventhal et al., 2019).
To date there has been insufficient regulation of these potentially dangerous tobacco products and, as a result, they have been readily available to minors in some states (Paradise, 2014). The perceptions of low health risk, combined with the appeal of flavors and minimal market regulations, have created a climate for the wide use of e-cigarettes among adolescents. Thus, we have a generation of teenagers who are effectively human guinea pigs for the health effects of e-cigarettes. There has been much recent concern about pulmonary injuries, particularly among those that are vaping tetrahydrocannabinol (Lozier et al., 2019). However, as has been particularly highlighted in pre-clinical studies, described below, nicotine is a neurotoxicant that can produce unique and long-lasting negative behavioral effects in adolescents.
2. Acute nicotine effects in adolescents
Nicotine mediates its effects via nicotinic cholinergic receptors (nAChRs), pentameric ligand-gated ion channels that are widely distributed throughout the brain (Dani, 2015; Zoli et al., 2015). nAChRs consist of pentameric arrays of homomeric (α7–α10) or heteromeric of α (α2–α7) and β (β2–β4) subunits, giving rise to a rich and diverse pharmacology. Whereas the endogenous transmitter, acetylcholine, induces rapid gating of Na+, K+ and/or Ca2+ ions, nicotine may either activate, inactivate or desensitize nAChRs depending on dose and subunit composition. nAChRs are expressed early in brain development and play critical maturational roles at many developmental stages, including adolescence (reviewed by Dwyer et al., 2009). Expression of nAChR binding and subunit mRNA is highly variable across development (Thorpe et al., 2020). In the ventral tegmental area (VTA), a limbic dopamine cell body region, expression of many nAChR subunits is higher in adolescents than in adults (Azam et al., 2007). Radioligand binding to α4β2* nAChRs in the VTA and in the striatum and prefrontal cortex, which are enriched in dopamine terminals, is also higher in adolescents than adults (Doura et al., 2008).
2.1. Acute neurochemical effects on monoamine systems.
Functional assays confirm the immaturity of nAChRs in adolescence. In vitro rubidium efflux assays have shown α4β2* nAChRs to have higher functional activity in striatum and other brain regions in adolescents than adults (Britton et al., 2007; Kota et al., 2007). Nicotine-stimulated dopamine release from ventral striatal slices shows complex age- and sex-dependence, being highest in male rats during early adolescence (postnatal day (P)30), with females showing no significant age differences (Azam et al., 2007). In vivo microdialysis has confirmed that nicotine induces greater release of dopamine in the nucleus accumbens of adolescents than adults (Corongiu et al., 2020). Nicotine-induced neuronal activation, as measured by immediate early gene expression, is also enhanced during adolescence in several limbic regions, including nucleus accumbens shell, basolateral amygdala, VTA and medial prefrontal cortex (Schochet et al., 2005; Shram et al., 2007; Dao et al., 2011). Furthermore, electrophysiological studies have shown that VTA dopamine neurons are more sensitive to nicotine-induced synaptic plasticity in adolescents than adults (Placzek et al., 2009). Serotonin systems are also uniquely sensitive to nicotine during adolescence (Slotkin & Seidler, 2009; Bang & Commons, 2011; Dao et al., 2011). Following acute injection of a low dose of nicotine, there is broader regional activation of serotonin cells within the ascending projections of the raphe in adolescents than in adults (Bang and Commons, 2011). Brief treatment with low-dose nicotine during early adolescence (postnatal days (P) 28–31) but not adulthood, also increases presynaptic markers of serotonin function in the forebrain (Dao et al., 2011). Although presynaptic dopamine markers are not impacted by this nicotine treatment, there is an increase in the efficacy of striatal D2 dopamine receptors (Mojica et al., 2014; Linker et al., 2020).
2.2. Short-term behavioral effects.
Although nicotine is more rapidly metabolized in rodents during pre-adolescence (P23–25) than in adults, leading to lower blood levels, no pharmacokinetic differences have been seen during the adolescent period starting at P28 (Portugal et al., 2012; Ahsan et al., 2014; Craig et al., 2014; Arany et al., 2017). Thus, differences in nicotine behavioral effects between adolescents and adults cannot be accounted for by differences in drug clearance rate. Whereas most studies have shown age differences in the acute in vivo effects of nicotine, there is no consistent pattern to these findings. Some studies have found adolescent rodents to be less sensitive than adults to the behavioral effects of nicotine. Miller et al. (2019) found an age-related increase in nicotine-induced hypothermia in male mice, with adult mice being the most sensitive. Strain differences were observed in which early-adolescent mice of the DBA/2J strain were insensitive to nicotine-induced hypothermia, whereas C57BL/ 6J were sensitive at this age. However, both strains showed increased sensitivity to nicotine as development progressed. Acute nicotine is also less effective in adolescents than adults at enhancing the context pre-exposure facilitation effect, a hippocampal-dependent learning paradigm (Kutlu et al., 2016). Whereas adolescent mice only showed learning enhancement at the highest nicotine dose, adult mice exhibited behavioral effects at lower nicotine doses. Age differences have also been seen in the aversive effects of nicotine. Whereas adults of both sexes experience aversive effects of high doses of nicotine, adolescents tolerate the drug well (Adriani et al., 2002; Shram et al., 2006; Torres et al., 2008, 2009; Lenoir et al., 2015). This may reflect late maturation of the medial habenula to lateral dorsal tegmental pathway that underlies nicotine aversion (Wolfman et al., 2018). Adult female rats show less aversion to nicotine than do adult males or oviarectomized females, suggesting that ovarian hormones protect against this effect (Torres et al, 2009).
In contrast, acute nicotine treatment induces enhanced or unique effects in adolescents in other behavioral paradigms. Whereas nicotine inhibits locomotor activity in late adolescence and adulthood, it either has no effect (Lopez et al., 2003; Belluzzi et al., 2004; Miller et al., 2019) or stimulates motor activity in early adolescence (Adriani et al., 2002; Cao et al., 2010). These age differences in nicotine-induced locomotor behavior are independent of sex. Although animals in late adolescence exhibit the same inhibitory locomotor response to acute nicotine as adults, they show greater locomotor sensitization to repeated nicotine treatment (Belluzzi et al., 2004). Adolescent males have been shown to have a strong anxiolytic response to nicotine treatment which diminishes with age, whereas adolescent females have much lower anxiolytic or outright anxiogenic responses depending on the behavioral test (Elliott et al., 2004; Cao et al., 2010). It is possible that the age-dependent increase in the anxiogenic effect of nicotine reflects an activation of the stress response in adults, which is greater in females than males (Cao et al., 2010). This is consistent with a substantial clinical and pre-clinical literature that indicates that female adults are more sensitive to stressors, including nicotine, than males (Torres & O’Dell, 2016).
Adolescents of both sexes are more sensitive than adults to the rewarding effects of nicotine, as shown in conditioned place preference tests (Vastola et al., 2002; Shram et al., 2006; Kota et al., 2007; Torres et al., 2008; Ahsan et al., 2014; Lenoir et al., 2015), with some studies demonstrating reward after a single pairing of drug and context (Belluzzi et al., 2004; Brielmaier et al., 2007). Although one group has argued against a biological vulnerability of adolescents to nicotine (Shram et al., 2008), the majority of studies have shown that adolescent rats readily acquire intravenous nicotine self-administration and have higher drug intake than adults (Chen et al., 2007; Levin et al. 2003, 2007; Natividad et al., 2013: Ahsan et al., 2014; Gellner et al., 2016). Some of the increased drug intake in adolescents in intravenous self-administration tests may reflect elevated motor activity and non-specific increases in responding (Gellner et al., 2016). However, when this is controlled for, adolescent males are seen to be sensitive to lower doses of nicotine than adults at low schedules of reinforcement. This increased sensitivity to lower nicotine doses is also seen in many of the conditioned place preference studies described above. Using drinking behavior to measure the rewarding effect of nicotine, mice have also been shown to have a peculiar vulnerability to oral self-administration of nicotine during early adolescence (Adriani et al., 2002). Whereas Adriani and colleagues found no sex differences in this effect, most other studies have not compared sex and age differences within the same study to allow a direct comparison of males and females. However, in one recent study in which reward was measured by reduction in brain reward thresholds, there was no sex difference in the rewarding effects of nicotine in adults, whereas adolescent females (P40–59) were significantly more sensitive to the rewarding effect of nicotine than their male counterparts (Xue et al., 2020). This finding is consistent with an earlier study in which self-administration of nicotine with the tobacco constituent, acetaldehyde, was compared across sex and age (Park et al., 2007). Whereas both sexes found the drug combination to be highly rewarding during early adolescence (P27), this initial reinforcing effect had significantly diminished in males at later ages (P34 and adult), whereas it remained high in females. Although these findings may suggest that adolescent females find nicotine and tobacco more rewarding than males, recent data on middle and high school students suggest few sex differences in rates of tobacco product use, with males showing slightly elevated consumption (Wang et al., 2019).
Smoking and drinking are two of the earliest drug use behaviors in human teens, and many reports indicate a positive interaction of smoking and drinking in this age group (Dani & Harris, 2005; Kristjansson et al., 2015). Our animal studies have also shown a unique interaction of nicotine and alcohol in adolescent male rats (Lárraga et al., 2017). Using an intravenous self-administration paradigm, we have shown that nicotine combined with alcohol is self-administered at much higher rates in male adolescents than adults, and with much greater alcohol intake in adolescents than that of alcohol alone (Figure 2A). This was not observed in females. Mechanistic studies showed that the decreased response to the drug combination in adults reflected the late maturation of an inhibitory kappa opioid receptor modulation. When adult males were pretreated with the kappa opioid receptor antagonist, norbinaltorphimine, to block this they self-administered the drug combination at the same high rate as adolescents (Lárraga et al., 2017).
Figure 2.
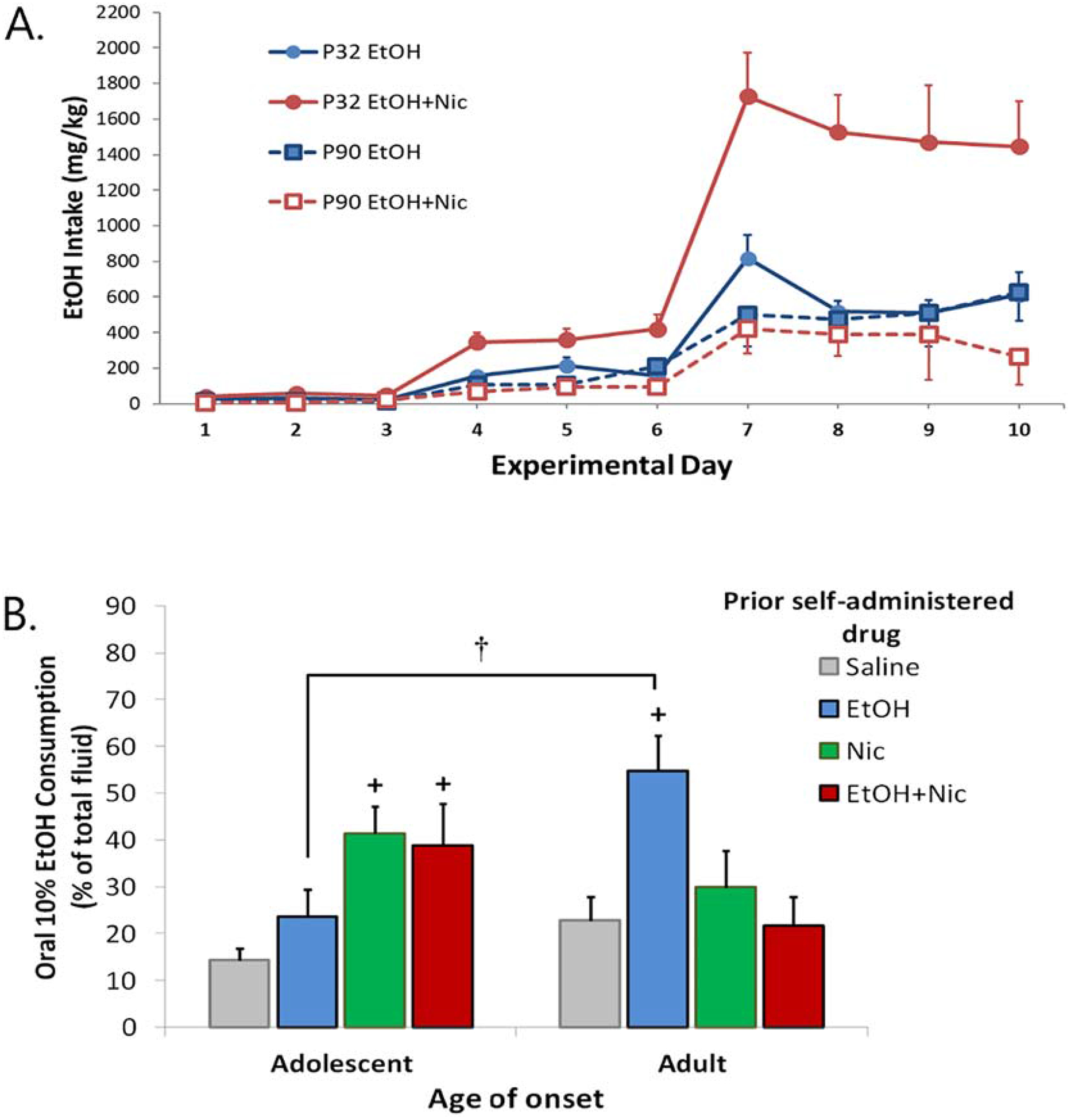
Acute and long term effects of co-administration of nicotine (Nic) + ethanol (EtOH). A. Self-administration of EtoH alone or EtOH + Nic in adolescent (P32) or adult (P90) male rats. B. Animals from A. were subsequently placed in a 2-bottle drinking in the dark paradigm and EtOH intake (% of total) measured. + significantly different from Saline, p<0.05. † significant age difference, p<0.05. From Lárraga et al., 2017.
3. Chronic effects of adolescent nicotine treatment
3.1. Withdrawal syndrome.
Abrupt cessation of tobacco use in dependent smokers results in withdrawal symptoms that include both somatic and affective components. Somatic symptoms include bradycardia, insomnia, and gastrointestinal discomfort, whereas negative affective symptoms include anger, anxiety, craving, depression, difficulty concentrating, impatience, insomnia, and restlessness (Hughes, 2007a). There is limited research on withdrawal from e-cigarettes, but recent analysis of the US Population Assessment of Tobacco and Health Survey and a clinical trial by the same investgators indicated that withdrawal symptoms were mild in never smokers (Hughes & Callas, 2019; Hughes et al., 2020), whereas another study of self-reported dependence symptoms suggested there were similarities to tobacco dependence but with some unique indicators (Soule et al., 2020). Some clinical studies of cessation of tobacco use in teenagers indicate that they may be especially sensitive to withdrawal, exhibiting symptoms of dependence soon after smoking initiation and before the establishment of daily smoking habits (DiFranza et al., 2007; Dierker & Mermelstein, 2010; Zhan et al., 2012). A recent study has reported that teen e-cigarette users are more likely to report dependence signs and be daily users if they use high nicotine content pods, such as Juul (Boykan et al., 2019a).
Nicotine withdrawal following chronic passive administration in animals results in a syndrome with both somatic and affective components, including disrupted operant performance, avoidance behavior, weight gain, anxiety-like behaviors, decreased reward sensitivity, and opioid-like withdrawal behaviors (Malin, 2001; Hughes, 2007b). Although these withdrawal signs are routinely seen in adult rodents, this is not the case for adolescents. In most studies, male adolescents have been found to exhibit less prominent somatic withdrawal symptoms than adults (O’Dell et al., 2006; Kota et al., 2007; Shram et al., 2008; Keeley et al., 2019), although female adolescent mice have been reported to show increased physical withdrawal signs as compared to adults (Kota et al., 2008). Strain differences have also been noted in adolescent physical withdrawal signs (Hamilton et al., 2010); whereas Sprague Dawley rats of both sexes were found to show significant withdrawal signs for two days after nicotine removal, Long Evans rats exhibit shortened (males) or non-existent (females) withdrawal. Affective signs of withdrawal, as measured by anxiety-like behaviors on the elevated plus maze and open field, increased brain reward threshold, conditioned place avoidance, and depressive-like behaviors in the forced swim test, are also generally found to be lower in adolescent rats and mice of both sexes than adults (O’Dell et al., 2006, 2007; Kota et al., 2007, 2008; Shram et al., 2008; Iñiguez et al., 2009; Torres et al., 2013). This overall reduction in withdrawal signs in adolescent rodents as compared to adults may reflect a resistance to decreased mesolimbic dopamine function (Natividad et al., 2010, 2012). There is a notable discrepancy between human studies, in which teens are thought to be more sensitive to withdrawal than adults, and preclinical studies where the opposite is observed. One possible reason is that route and timing of nicotine administration may make a difference, as has been shown in one prior rodent study (Kota et al., 2008). Two studies in which an inhalation method for tobacco smoke or nicotine was used did note substantial physical withdrawal in adolescents, although adults were not included for comparison (de la Pena, 2015; Kallupi et al., 2019). Alternatively, tobacco constituents, which have been shown to enhance reinstatement of nicotine seeking behavior (Costello et al., 2014; Cross et al., 2020), may increase withdrawal signs. Further studies are needed to address this issue.
3.2. Drug reward.
Many studies have shown long term consequences of adolescent nicotine exposure on drug reward. Adolescent nicotine treatment increases subsequent nicotine reward and decreases aversion in adults (Adriani et al., 2003; Torres et al., 2008; Kallupi et al., 2019). This may reflect an age-dependent increase in α5, α6 and β2 nAChR subunits in the ventral midbrain (Adriani et al., 2003). These findings are also consistent with clinical data which show that teen e-cigarette use is associated with greater risk for subsequent initiation and continuation of cigarette smoking (Soneji et al, 2017; Kinnunen et al., 2019).
Adolescent nicotine also leads to increased rewarding effects of other abused drugs. Human studies indicate that smoking and e-cigarette use is associated with greater alcohol consumption in adolescents (Kristjansson et al., 2015). Adult dependent smokers are also much more likely to be alcoholics than non-smokers, and the great majority of alcoholics smoke (DiFranza & Guerrera, 1990; Falk et al., 2006). Furthermore, there is a strong relationship between early onset of smoking and alcohol dependence and problem use (Grant & Dawson, 1997). Since most smokers begin before the age of 18 (Hanna et al., 2001; SAMHSA, 2011), it is critical that animal studies model the impact of adolescent nicotine exposure on alcohol intake and evaluate underlying mechanisms.
As shown in Figure 2B, self-administration of nicotine or nicotine + ethanol during adolescence leads to increased oral consumption of ethanol in young adult males (Lárraga et al., 2017). This is quite different from animals that self-administered these drugs as adults, where intravenous ethanol self-administration increased subsequent ethanol intake. Passive exposure to nicotine during adolescence, but not adulthood, also leads to a subsequent increase in ethanol intake in adults (Thomas et al., 2018). This latter study also explored mechanisms of enhanced ethanol self-administration following adolescent nicotine exposure, and found that it resulted from altered GABA signaling within the VTA. Adolescent, but not adult, nicotine downregulated the chloride channel, KCC2, causing a depolarizing shift in EGABA and resulting inhibition of ethanol-stimulated dopamine release. This elevated drinking following adolescent nicotine exposure could be prevented by co-administration of the KCC2 agonist, CLP290, offering a possible therapeutic strategy to reduce excessive drinking in smokers. It is important to note that although sex differences were found in the acute reinforcing effects of nicotine-ethanol combinations (Lárraga et al., 2017), sex has not been examined as a variable in these long-term studies. This is an important area for further research given clinical findings of a stronger association of smoking and binge drinking in women than men (Wilsnack et al., 2018).
Clinical data have shown a link between teen smoking and use of other drugs, particularly psychostimulants. For example, one study showed that those who used tobacco before age 15 were more than 80x more likely to use illegal drugs than those who did not, with cocaine being the most widely used drug among teen cigarette smokers (Lai et al., 2000). Other studies have shown that 90% of cocaine users smoked before they began drug use, and that cocaine dependence is highest among users who initiated cocaine use after tobacco use (Levine et al., 2011). Furthermore, those who were regular smokers were more likely to ‘like’ or ‘want’ cocaine when they first tried it, responses that are significant predictors of dependence and lifetime cocaine use (Lambert et al., 2006). Thus, teen smoking is associated with greater psychostimulant use and poorer treatment outcomes (Weinberger & Sofluoglu, 2009; Ren & Lotfipour, 2019).
Preclinical studies have confirmed a positive interaction of nicotine with psychostimulants, particularly following adolescent nicotine exposure. Animals treated with nicotine during early adolescence, but not late adolescence or adulthood, have been found to have enhanced rewarding effects of cocaine, methamphetamine and morphine, as measured by conditioned place preference later in adulthood (McMillen et al., 2005; Alajaji et al., 2016; Kota et al., 2018; Cadoni et al., 2019), but see (Kelley & Middaugh, 1999; Kelley & Rowan, 2004). Nicotine treatment during early adolescence also increases subsequent cocaine-induced locomotor sensitization (Kelley & Rowan, 2004; McQuown et al., 2009; Alajaji et al., 2016; Reed and Izenwasser, 2017). This effect may be specific to males, however, since adolescent nicotine-induced enhancement of cocaine- and amphetamine-induced locomotor sensitization was not seen in female mice (Collins & Izenwasser, 2004; Collins et al., 2004).
Other studies, using the self-administration procedure, have shown that nicotine pretreatment of adolescents, but not adults, increases the subsequent reinforcing effects of cocaine (McQuown et al., 2007; Dao et al., 2011; Reed & Izenwasser, 2017; Dickson et al., 2014; Linker et al., 2020), but see (Levin et al., 2011; Pomfrey et al., 2015; Schassburger et al., 2016). The enhancement of cocaine intake is seen immediately following nicotine pretreatment and persists through adulthood, but is not seen for sucrose. Increased cocaine intake is observed in both males and females, and results from an upward shift of the dose-response curve. A similar increase in methamphetamine and ethanol intake following adolescent nicotine treatment has also been seen (Dao et al., 2011; Pipkin et al., 2014). Whereas initial self-administration is enhanced by adolescent nicotine treatment, there is not a similar increase in drug-primed reinstatement (Mojica et al., 2014; Pipkin et al., 2014), although one study of preadolescent animals found an increase in cue-induced drug seeking behavior in a high sucrose preferring rat strain (Anker & Carroll, 2011).
Many long-term neurochemical and morphological changes have been shown to result from adolescent treatment with nicotine that are distinct from that in adult (Ehlinger et al., 2016; Cadoni et al., 2019). However, few of these have been linked to behavioral changes. One study which compared Lewis (addiction prone) and Fischer (addiction resistant) rats has shown that only Lewis rats show enhancement of cocaine conditioned place preference and a concomitant potentiation of dopamine response in the nucleus accumbens shell following adolescent nicotine treatment (Cadoni et al., 2019). This finding highlights the importance of genetic background in mediating the effects of adolescent nicotine and may explain some of the discrepant findings reported in the literature. To further examine underlying mechanisms we have used a paradigm in which Sprague Dawley rats are treated once daily for four days with a low dose of nicotine (0.06 mg/kg, i.v.), intended to mimic the early phase of teen smoking, and have shown this enhances subsequent reinforcing effects of cocaine, methamphetamine and alcohol (McQown et al., 2007; Dao et al., 2011) in adolescents but not adults of both sexes. The long-lasting enhancement of cocaine intake can be blocked by co-administration of a serotonin 5HT1A or dopamine D2 receptor antagonist during nicotine pretreatment (Dao et al., 2011; Linker et al., 2020). These behavioral findings are consistent with biochemical studies that show regional forebrain increases in serotonin, SERT or 5HIAA levels in adolescents but not adults. Although presynaptic dopamine markers are not impacted by adolescent nicotine pretreatment, D2 receptor protein and G protein coupling in the nucleus accumbens are increased (Mojica et al., 2014; Linker et al., 2020). Furthermore, there is a long-lasting increase in locomotion induced by the D2 receptor agoinst, quinpirole, which is blocked by co-administration of the 5HT1A antagonist, WAY 100 635, with nicotine. Thus, both 5HT1A and D2 receptors are critical modulators of adolescent nicotine effects on reward pathways. These enhancing effects of nicotine are restricted to early adolescence and are not seen at later ages.
In a recent study, we have further examined the mechanism by which adolescent nicotine increases cocaine self-administration, and have demonstrated a critical role for microglia (Linker et al., 2020). Whereas nicotine inhibits microglial activation in adults, it has the opposite effect in adolescents and induces reactive microglia. Microglial activation then selectively prunes glutamate synapses in the nucleus accumbens and basolateral amygdala, and results in increased cocaine self-administration. Nicotine enhancement of cocaine self-administration is blocked by minocycline, which inhibits microglial actvation, or by the selective CSF1R inhibitor, PLX3397, which clears microglia from the brain. This immune response in adolescents is induced by nicotine via activation of D2 receptors, and a resulting neuronal release of the fractalkine signaling ligand, CXRCL1, which binds to microglial CX3CR1 receptors to regulate synaptic pruning (Figure 3; Luo et al., 2019). The possible role of 5HT1A receptors in this pathway has not yet been examined.
Figure 3.
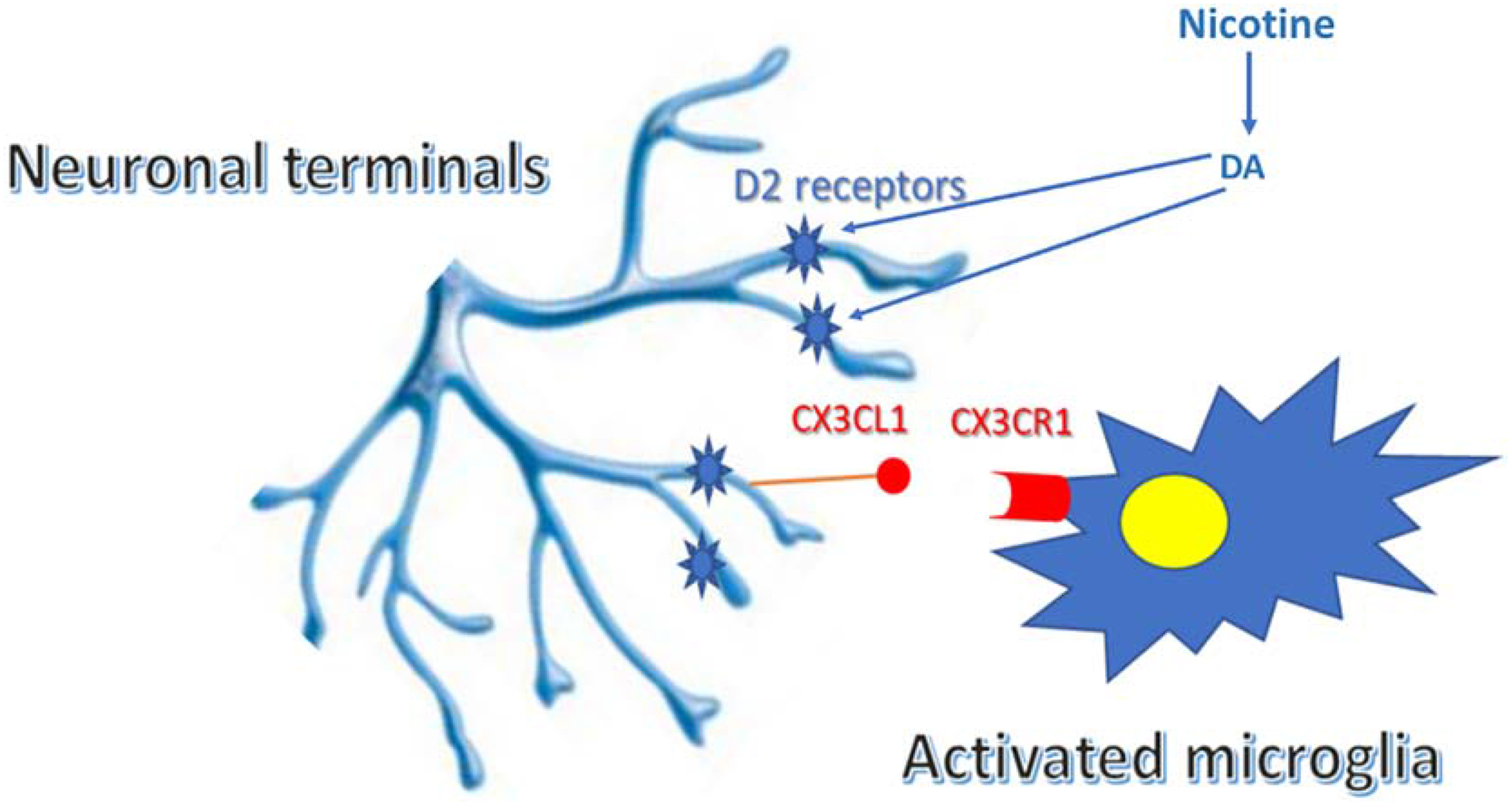
Schematic of mechanism of adolescent nicotine-induced increase in cocaine self-administration. Nicotine activates the fractalkine signaling pathway in the nucleus accumbens via D2 dopamine (DA) receptors. CX3CL1 released from neurons binds to the CX3CR1 receptor on microglia to induce an inflammatory response and subsequent pruning of neuronal terminals. From Linker et al., 2020.
3.3. Cognition.
The hippocampus is a brain region that is critical for memory formation and retrieval, and plays an important role in processing contextual information and spatial learning (Opitz, 2014). Contextual fear learning is a hippocampal-dependent task in which performance is impacted by adolescent nicotine treatment (Holliday and Gould, 2016; Holliday et al., 2016). Animals treated with nicotine as adolescents that were tested as adults showed decreased context-induced freezing, and were less sensitive than saline-treated controls to nicotine enhancement of performance, effects that were not seen in adults treated with nicotine. Similar performance decrements were observed in another contextual conditioning task following adolescent nicotine treatment (Spaeth et al., 2010). Whereas sex differences were not observed for adolescent nicotine effects on contextual conditioning (Spaeth et al., 2010), these have been observed for hippocampal-dependent spatial learning tasks. Adult performance in an object learning task and a novel object test were decreased following adolescent nicotine treatment of females, but not males (Mateos et al., 2011). A sex-specific decrease in nAChR binding in prefrontal cortex was also observed in adult females following adolescent nicotine treatment, although this has not been established as a mechanism for their performance deficit.
deBry & Tiffany (2008) proposed a Tobacco-Induced Neurotoxicity of Adolescent Cognitive Development (TINACD) theory which postulates that smoking during early adolescence, a period of major neurodevelopment of brain structures regulating inhibitory control, leads to increased impulsivity and inattention. In particular, the prefrontal cortex, which plays a critical role in integrating emotional and motivational states to regulate top-down attentional processes, is still actively maturing during adolescence and is a target for aberrant nicotine effects. Prefrontal cortical activity, working memory and attentional performance are all reduced in teen smokers during withdrawal (Jacobsen et al., 2005; 2007), although male smokers are significantly more impaired during tests of selective and divided attention than female smokers and nonsmokers. Cognitive performance has also been shown to be influenced negatively by adolescent nicotine exposure in animal studies (Schochet et al., 2005; 2008; Counotte et al., 2011). Nicotine treatment of male adolescent rats resulted in increased impulsive behavior and decreased attention in adulthood, but had no effect on animals that are treated as adults (Counotte et al., 2011). Although adolescent nicotine increased prefrontal cortex levels of mGluR2 immediately after drug treatment, there was a significant decrease five weeks later when these behavioral studies were conducted. This change in mGluR2 levels was adolescent-specific and not seen in adults. Electrophysiological data showed that adolescent nicotine treatment leads to decreased synaptic mGluR2 signaling and reduced short-term depression of prefrontal cortex layer V pyramidal neurons, which may compromise their ability to filter out irrelevant signals (Goriounova & Mansvelder, 2012). A critical mechanistic role for this reduction in mGluR2 function has been confirmed by pharmacological evidence that attentional deficits in animals treated with nicotine as adolescents are reversed by prefrontal cortex infusion of the mGluR2 agonist, LY379268. Given findings of sex differences in human studies of smoking effects on cognition (Jacobsen et al., 2005; 2007), these animal studies need replication that includes females.
3.4. Emotional regulation.
There is a strong association between teen use of tobacco products and anxiety and depression (Patton et al., 1998; Jamner et al., 2003; McKenzie et al., 2010; Leventhal et al., 2016; Cho et al., 2018). Whereas many studies suggest that tobacco use is preceded by emotional dysregulation, and may be a form of self-medication (Gehricke et al., 2007; Weinstein et al., 2008), there is substantial evidence for a bidirectional association between smoking/vaping and depression (Chaiton et al., 2009; Lechner et al., 2017; Esmaeelzadeh, 2018). Other predictors of teen and young adult smoking include anhedonia, aggression, low hedonic capacity and lower distress tolerance (Roberts et al., 2010; Audrain-McGovern et al., 2012; Trujillo et al., 2016; Stone et al., 2017; Trucco et al., 2018).
Many animal studies have also shown a correlation between chronic adolescent nicotine exposure and long term increases in anxiety and depression. Nicotine treatment during adolescence, but not adulthood, increases anxiety in later life, as shown by elevated plus maze, time spent in the light side of a box, or time spent in the center zone of an open field (Slawecki et al., 2003; Smith et al., 2006; Iñiguez et al., 2009; Holliday et al., 2016; Jobson et al., 2019). Several studies have also shown an age-specific increase in long-term depressive symptoms and anhedonia, as measured by immobility in the forced swim test and decreased sucrose preference (Iñiguez et al., 2009; Holliday et al., 2016; Jobson et al., 2019). One group has also found that adolescent, but not adult nicotine, induces long-term social withdrawal and deficits in social cognition (Jobson et al., 2019). Thus, adolescent nicotine exposure may produce a long-term vulnerability to the adverse effects of stress, resulting in a negative emotional state. Whereas it has been argued that these preclinical studies, which use fairly high doses of nicotine, do not adequately model the use of tobacco products in teenagers (Pushkin et al., 2019), it should be noted that Juul and other vape pod systems result in adolescent exposure to high doses of nicotine and subsequent dependence (Goniewicz et al., 2018; Boykan et al., 2019). It is also notable that most of the studies conducted to date have used only male mice or rats. Thus, there is a gap in the preclinical literature on the impact of adolescent nicotine on emotional responding in females. This is an important area for further research, given human findings of sex differences in the relationship between smoking urges and mood states (Delfino et al., 2001).
Mechanistic analyses have shown that adolescent nicotine induces profound and long-lasting neuronal and molecular alterations in regions that are critical for emotional regulation (Slawecki et al., 2005; Holliday et al., 2016; Jobson et al., 2019). In particular, the behavioral alterations are accompanied by increased firing frequency and bursting of neurons in the VTA and prefrontal cortex (Jobson et al., 2019). A selective downregulation of dopamine D1 receptor expression in the prefrontal cortex has also been observed, which may have may have occurred in response to elevated sub-cortical dopaminergic firing and bursting. Clinical studies have previously shown that striatal levels of the D1 receptor are negatively associated with major depression, particularly in those with anger attacks (Dougherty et al., 2006; Cannon et al., 2009). Although the Jobson study did not suggest possible therapeutic approaches to treatment of adolescent nicotine-induced mood disorders, an earlier study has shown that both nicotine and the antidepressants, fluoxetine and buproprion, could reduce the associated negative emotional states (Iñiguez et al., 2009).
4. Conclusions
Adolescence is a time of major plasticity of those brain systems that regulate motivated behavior and cognition (Yuan et al., 2015). It is also the age of peak onset of nicotine use (Miech et al., 2017). Although there has been a decline in teen use of cigarettes in recent years, there has been a huge increase in nicotine vaping (Cullen et al., 2019; Miech et al., 2019). As a result, it is critically important to understand the impact of nicotine on this phase of brain development. Given ethical constraints on conducting experimental drug studies on human teens, this necessitates the use of animal studies.
As outlined in this review, preclinical studies have consistently shown unique effects of nicotine on adolescent brain. There is an increased number and activity of nAChRs in brain regions that are important for reward (Doura et al., 2007; Kota et al., 2007) , and an increase in nicotine-induced DA release in limbic regions of adolescent brain (Azam et al., 2007; Corongiu et al., 2020). There are significant age differences in many of the acute behavioral effects of nicotine. Some behavioral effects, such as nicotine reward, are greater in adolescents than adults (Adriani et al., 2003; Torres et al., 2008; Gellner et al., 2016). In contrast, other behavioral effects, such as aversion, are greater in adults (O’Dell et al., 2006, Shram et al., 2006; Torres et al., 2008).
Animal studies have also shown that adolescent nicotine exposure can induce long-term changes in brain and behavior. The behavioral changes, which last until adulthood, include increased rewarding effects of other abused drugs (see Yuan et al., 2015 for review), decreased attention (Counotte et al., 2011) and mood changes (Slawecki et al., 2003; Smith et al., 2006; Iñiguez et al., 2009; Holliday et al., 2016; Jobson et al., 2019). Increasingly, these animal studies are assessing molecular mechanisms underlying these long-term behavioral changes, which may provide insight into possible therapeutic strategies to reduce long-term adverse effects of teen nicotine use. These preclinical findings suggest that teen nicotine vaping may result in similar long-term deleterious effects, and are consistent with recent observations of linkages between vaping and substance use, mental health problems and impulsivity (Grant et al., 2019; Roys et al., 2019). Thus, further longitudinal assessment of health outcomes in teen and young adult e-cigarette users is warranted.
Highlights.
Animal studies have shown that nicotine has unique effects on adolescent brain.
Nicotine exposure during adolescence has long-lasting effects that are seen in adulthood, but are not seen in animals that are treated with nicotine as adults.
Lasting effects include increased rewarding effects of abused drugs, deficits in cognitive function and emotional dysregulation.
Acknowledements
I would like to thank Michelle Cano, Yasamin Heydari and Raveena Parmar for their help in preparing this manuscript.
Funding: This work was supported by National Institutes of Health grant DA040440.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no competing interests,
References
- Adriani W, Macri S, Pacifici R and Laviola G, 2002. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology, 27(2), pp.212–224. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB and Piazza PV, 2003. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. Journal of Neuroscience, 23(11), pp.4712–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan HM, de la Pena JBI, Botanas CJ, Kim HJ, Yu GY and Cheong JH, 2014. Conditioned place preference and self-administration induced by nicotine in adolescent and adult rats. Biomolecules & therapeutics, 22(5), p.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji M, Kota D, Wise LE, Younis RM, Carroll FI, Levine A, Selley DE, Sim-Selley LJ and Damaj MI, 2016. Early adolescent nicotine exposure affects later-life cocaine reward in mice. Neuropharmacology, 105, pp.308–317. [DOI] [PubMed] [Google Scholar]
- Anker JJ and Carroll ME, 2011. Adolescent nicotine exposure sensitizes cue-induced reinstatement of cocaine seeking in rats bred for high and low saccharin intake. Drug and alcohol dependence, 118(1), pp.68–72. [DOI] [PubMed] [Google Scholar]
- Arany I, Hall S and Dixit M, 2017. Age-dependent sensitivity of the mouse kidney to chronic nicotine exposure. Pediatric research, 82(5), pp.822–828. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K and Sass J, 2012. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine & Tobacco Research, 14(10), pp.1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y and Leslie FM, 2007. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience, 144(4), pp.1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ and Commons KG, 2011. Age-dependent effects of initial exposure to nicotine on serotonin neurons. Neuroscience, 179, pp.1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS and Leslie FM, 2004. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology, 174(3), pp.389–395. [DOI] [PubMed] [Google Scholar]
- Boccio CM, Jackson DB and Leal WE, 2020. Nicotine and marijuana attitudes among flavor-only vaping youth: New evidence from Monitoring the Future. Addictive behaviors, 102, p.106186. [DOI] [PubMed] [Google Scholar]
- Boykan R, Goniewicz ML and Messina CR, 2019a. Evidence of nicotine dependence in adolescents who use Juul and similar pod devices. International journal of environmental research and public health, 16(12), p.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykan R, Messina CR, Chateau G, Eliscu A, Tolentino J and Goniewicz ML, 2019b. Self-reported use of tobacco, e-cigarettes, and marijuana versus urinary biomarkers. Pediatrics, 143(5), p.e20183531. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG and Smith RF, 2007. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicology and teratology, 29(1), pp.74–80. [DOI] [PubMed] [Google Scholar]
- Britton AF, Vann RE and Robinson SE, 2007. Perinatal nicotine exposure eliminates peak in nicotinic acetylcholine receptor response in adolescent rats. Journal of Pharmacology and Experimental Therapeutics, 320(2), pp.871–876. [DOI] [PubMed] [Google Scholar]
- Cadoni C, De Felice M, Corongiu S, Dessi C, Espa E, Melis M and Fenu S, 2019. Role of genetic background in the effects of adolescent nicotine exposure on mesolimbic dopamine transmission. Addiction biology, p.e12803. [DOI] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y and Leslie FM, 2010. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacology Biochemistry and Behavior, 96(1), pp.82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton MO, Cohen JE, O’Loughlin J and Rehm J, 2009. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC public health, 9(1), p.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matta SG and Sharp BM, 2007. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology, 32(3), pp.700–709. [DOI] [PubMed] [Google Scholar]
- Cho J, Goldenson NI, Stone MD, McConnell R, Barrington-Trimis JL, Chou CP, Sussman SY, Riggs NR and Leventhal AM, 2018. Characterizing polytobacco use trajectories and their associations with substance use and mental health across mid-adolescence. Nicotine and Tobacco Research, 20(suppl_1), pp.S31–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL and Izenwasser S, 2004. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology, 46(3), pp.349–362. [DOI] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J and Izenwasser S, 2004. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. European journal of pharmacology, 502(1–2), pp.75–85. [DOI] [PubMed] [Google Scholar]
- Corongiu S, Dessì C and Cadoni C, 2020. Adolescence versus adulthood: differences in basal mesolimbic and nigrostriatal dopamine transmission and response to drugs of abuse. Addiction biology, 25(1), p.e12721. [DOI] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD and Leslie FM, 2014. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology, 39(8), pp.1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, Van Der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T and Spijker S, 2011. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nature neuroscience, 14(4), p.417. [DOI] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, Novalen M, Miksys S and Tyndale RF, 2014. Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug Metabolism and Disposition, 42(9), pp.1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Reynaga DD, Cano M, Belluzzi JD, Zaveri NT and Leslie FM, 2020. Differences in mechanisms underlying reinstatement of cigarette smoke extract-and nicotine-seeking behavior in rats. Neuropharmacology, 162, p.107846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Liu ST, Bernat JK, Slavit WI, Tynan MA, King BA and Neff LJ, 2019. Flavored tobacco product use among middle and high school students—United States, 2014–2018. Morbidity and Mortality Weekly Report, 68(39), p.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, 2015. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine In International review of neurobiology (Vol. 124, pp. 3–19). Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA and Harris RA, 2005. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature neuroscience, 8(11), pp.1465–1470. [DOI] [PubMed] [Google Scholar]
- Dao JM, McQuown SC, Loughlin SE, Belluzzi JD and Leslie FM, 2011. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology, 36(7), pp.1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña JB, Ahsan HM, Tampus R, Botanas CJ, dela Peña IJ, Kim HJ, Sohn A, dela Peña I, Shin CY, Ryu JH and Cheong JH, 2015. Cigarette smoke exposure during adolescence enhances sensitivity to the rewarding effects of nicotine in adulthood, even after a long period of abstinence. Neuropharmacology, 99, pp.9–14. [DOI] [PubMed] [Google Scholar]
- deBry SC and Tiffany ST, 2008. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine & Tobacco Research, 10(1), pp.11–25. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Jamner LD and Whalen CK, 2001. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine & Tobacco Research, 3(3), pp.235–248. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Miller MM, Rogers TD, Blaha CD and Mittleman G, 2014. Effects of adolescent nicotine exposure and withdrawal on intravenous cocaine self- administration during adulthood in male C57BL/6J mice. Addiction biology, 19(1), pp.37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR and Guerrera MP, 1990. Alcoholism and smoking. Journal of studies on alcohol, 51(2), pp.130–135. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB and Perry DC, 2008. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain research, 1215, pp.40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC and Leslie FM, 2009. The dynamic effects of nicotine on the developing brain. Pharmacology & therapeutics, 122(2), pp.125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG and Smith RF, 2016. Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Structure and Function, 221(1), pp.133–145. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM and Grunberg NE, 2004. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacology biochemistry and behavior, 77(1), pp.21–28. [DOI] [PubMed] [Google Scholar]
- Esmaeelzadeh S, Moraros J, Thorpe L and Bird Y, 2018. Examining the association and directionality between mental health disorders and substance use among adolescents and young adults in the US and Canada—a systematic review and meta-analysis. Journal of clinical medicine, 7(12), p.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY and Hiller-Sturmhöfel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health, 29(3), p.162. [PMC free article] [PubMed] [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, Belluzzi JD and Leslie FM, 2007. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine & Tobacco Research, 9(Suppl_4), pp.S523–S536. [DOI] [PubMed] [Google Scholar]
- Gellner CA, Belluzzi JD and Leslie FM, 2016. Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats. Neuropharmacology, 109, pp.247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A and King BA, 2019. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. Morbidity and Mortality Weekly Report, 68(6), p.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, Wang L, Christensen C, Ambrose B, Borek N and van Bemmel D, 2018. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA network open, 1(8), pp.e185937–e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA and Mansvelder HD, 2012. Short-and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harbor perspectives in medicine, 2(12), p.a012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF and Dawson DA, 1997. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse, 9, pp.103–110. [DOI] [PubMed] [Google Scholar]
- Grant JE, Lust K, Fridberg DJ, King AC and Chamberlain SR, 2019. E-cigarette use (vaping) is associated with illicit drug use, mental health problems, and impulsivity in university students. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists, 31(1), pp.27 – 35. [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Perry ME, Berger SS and Grunberg NE, 2010. Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine & Tobacco Research, 12(12), pp.1236–1245. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Yi HY, Dufour MC and Whitmore CC, 2001. The relationship of early-onset regular smoking to alcohol use, depression, illicit drug use, and other risky behaviors during early adolescence: results from the youth supplement to the third national health and nutrition examination survey. Journal of substance abuse, 13(3), pp.265–282. [DOI] [PubMed] [Google Scholar]
- Holliday ED and Gould TJ, 2016. Chronic nicotine treatment during adolescence attenuates the effects of acute nicotine in adult contextual fear learning. Nicotine & Tobacco Research, 19(1), pp.87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday ED, Nucero P, Kutlu MG, Oliver C, Connelly KL, Gould TJ and Unterwald EM, 2016. Long- term effects of chronic nicotine on emotional and cognitive behaviors and hippocampus cell morphology in mice: comparisons of adult and adolescent nicotine exposure. European Journal of Neuroscience, 44(10), pp.2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, 2007a. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research, 9(3), pp.315–327. [DOI] [PubMed] [Google Scholar]
- Hughes JR, 2007b. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine & Tobacco Research, 9(3), pp.329–339. [DOI] [PubMed] [Google Scholar]
- Hughes JR and Callas PW, 2019. Prevalence of withdrawal symptoms from electronic cigarette cessation: A cross-sectional analysis of the US Population Assessment of Tobacco and Health. Addictive behaviors, 91, pp.234–237. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Peters EN, Callas PW, Peasley-Miklus C, Oga E, Etter JF and Morley N, 2020. Withdrawal Symptoms From E-Cigarette Abstinence Among Adult Never-Smokers: A Pilot Experimental Study. Nicotine and Tobacco Research, 22(5), pp.740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z and Bolanos-Guzmán CA, 2009. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology, 34(6), pp.1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ and Pugh KR, 2005. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological psychiatry, 57(1), pp.56–66. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M and Mencl WE, 2007. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological psychiatry, 61(1), pp.31–40. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Whalen CK, Loughlin SE, Mermelstein R, Audrain-McGovern J, Krishnan-Sarin S, Worden JK and Leslie FM, 2003. Tobacco use across the formative years: A road map to developmental vulnerabilities. Nicotine & Tobacco Research, 5(Suppl_1), pp. S71–S87. [DOI] [PubMed] [Google Scholar]
- Jenssen BP and Boykan R, 2019. Electronic cigarettes and youth in the United States: A call to action (at the local, national and global levels). Children, 6(2), p.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobson CL, Renard J, Szkudlarek H, Rosen LG, Pereira B, Wright DJ, Rushlow W and Laviolette SR, 2019. Adolescent nicotine exposure induces dysregulation of mesocorticolimbic activity states and depressive and anxiety-like prefrontal cortical molecular phenotypes persisting into adulthood. Cerebral Cortex, 29(7), pp.3140–3153. [DOI] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Larrosa E and George O, 2019. Exposure to passive nicotine vapor in male adolescent rats produces a withdrawal-like state and facilitates nicotine self-administration during adulthood. European Neuropsychopharmacology, 29(11), pp.1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley RJ, Mayer TE, Hsu LM, Lu H, Yang Y and Stein EA, 2019. Differential expression of nicotine withdrawal as a function of developmental age in the rat. Pharmacology Biochemistry and Behavior, 187, p.172802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BM and Middaugh LD, 1999. Periadolescent nicotine exposure reduces cocaine reward in adult mice. Journal of addictive diseases, 18(3), pp.27–39. [DOI] [PubMed] [Google Scholar]
- Kelley BM and Rowan JD, 2004. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. International journal of developmental neuroscience, 22(5–6), pp.339–348. [DOI] [PubMed] [Google Scholar]
- Kinnunen JM, Ollila H, Minkkinen J, Lindfors PL, Timberlake DS and Rimpelä AH, 2019. Nicotine matters in predicting subsequent smoking after e-cigarette experimentation: a longitudinal study among Finnish adolescents. Drug and alcohol dependence, 201, pp.182–187. [DOI] [PubMed] [Google Scholar]
- Kota D, Alajaji M, Bagdas D, Selley DE, Sim-Selley LJ and Damaj MI, 2018. Early adolescent nicotine exposure affects later-life hippocampal mu-opioid receptors activity and morphine reward but not physical dependence in male mice. Pharmacology Biochemistry and Behavior, 173, pp.58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR and Damaj MI, 2008. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology, 198(2), pp.201–210. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE and Damaj MI, 2007. Nicotine dependence and reward differ between adolescent and adult male mice. Journal of Pharmacology and Experimental Therapeutics, 322(1), pp.399–407. [DOI] [PubMed] [Google Scholar]
- Kristjansson AL, Mann MJ and Sigfusdottir ID, 2015. Licit and illicit substance use by adolescent e-cigarette users compared with conventional cigarette smokers, dual users, and nonusers. Journal of Adolescent Health, 57(5), pp.562–564. [DOI] [PubMed] [Google Scholar]
- Kutlu MG and Gould TJ, 2016. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiology & behavior, 155, pp.162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB and McCoy CB, 2000. The association between cigarette smoking and drug abuse in the United States. Journal of addictive diseases, 19(4), pp.11–24. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M and Schenk S, 2006. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction, 101(5), pp.713–725. [DOI] [PubMed] [Google Scholar]
- Lárraga A, Belluzzi JD and Leslie FM, 2017. Nicotine increases alcohol intake in adolescent male rats. Frontiers in behavioral neuroscience, 11, p.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Janssen T, Kahler CW, Audrain-McGovern J and Leventhal AM, 2017. Bi-directional associations of electronic and combustible cigarette use onset patterns with depressive symptoms in adolescents. Preventive medicine, 96, pp.73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B and Izenwasser S, 2015. Sex differences in conditioned nicotine reward are age-specific. Pharmacology Biochemistry and Behavior, 132, pp.56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Cho J, Kirkpatrick MG, McConnell RS, Stone MD, Pang RD, Audrain-McGovern J and Barrington-Trimis JL, 2019. Flavored E-cigarette Use and Progression of Vaping in Adolescents. Pediatrics, 144(5) e20190789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Sussman S, Kirkpatrick MG, Unger JB, Barrington-Trimis JL and Audrain-McGovern J, 2016. Psychiatric comorbidity in adolescent electronic and conventional cigarette use. Journal of psychiatric research, 73, pp.71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ and Slotkin TA, 2007. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicology and teratology, 29(4), pp.458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE and Swartzwelder HS, 2003. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology, 169(2), pp.141–149. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K and Rezvani AH, 2011. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behavioural brain research, 225(2), pp.473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB and Kandel ER, 2011. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Science translational medicine, 3(107), pp.107ra109–107ra109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker KE, Elabd MG, Tawadrous P, Cano M, Green KN, Wood MA and Leslie FM, 2020. Microglial activation increases cocaine self-administration following adolescent nicotine exposure. Nature Communications, 11(1), pp.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Simpson D, White N and Randall C, 2003. Age- and sex- related differences in alcohol and nicotine effects in C57BL/6J mice. Addiction biology, 8(4), pp.419–427. [DOI] [PubMed] [Google Scholar]
- Lozier MJ, Wallace B, Anderson K, Ellington S, Jones CM, Rose D, Baldwin G, King BA, Briss P, Mikosz CA and Force ST, 2019. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injuries—United States, December 2019. Morbidity and Mortality Weekly Report, 68(49), p.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Chu SF, Zhang Z, Xia CY and Chen NH, 2019. Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain research bulletin, 146, pp.12–21. [DOI] [PubMed] [Google Scholar]
- Marynak K, Gentzke A, Wang TW, Neff L and King BA, 2018. Exposure to electronic cigarette advertising among middle and high school students—United States, 2014–2016. Morbidity and Mortality Weekly Report, 67(10), p.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP and Viveros MP, 2011. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. Journal of psychopharmacology, 25(12), pp.1676–1690. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Olsson CA, Jorm AF, Romaniuk H and Patton GC, 2010. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10- year longitudinal study. Addiction, 105(9), pp.1652–1659. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Davis BJ, Williams HL and Soderstrom K, 2005. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. European journal of pharmacology, 509(2–3), pp.161–164. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD and Leslie FM, 2007. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicology and teratology, 29(1), pp.66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Dao JM, Belluzzi JD and Leslie FM, 2009. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology, 207(1), p.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, 2001. Nicotine dependence: studies with a laboratory model. Pharmacology Biochemistry and Behavior, 70(4), pp.551–559. [DOI] [PubMed] [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Bachman JG and Patrick ME, 2019. Adolescent vaping and nicotine use in 2017–2018—US national estimates. New England Journal of Medicine, 380(2), pp.192–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Patrick ME, O’malley PM and Johnston LD, 2017. E-cigarette use as a predictor of cigarette smoking: results from a 1-year follow-up of a national sample of 12th grade students. Tobacco control, 26(e2), pp.e106–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Caruso MJ and Kamens HM, 2019. Early-adolescent male C57BL/6J and DBA/2J mice display reduced sensitivity to acute nicotine administration. Neuroscience letters, 690, pp.151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica CY, Belluzzi JD and Leslie FM, 2014. Age-dependent alterations in reward-seeking behavior after brief nicotine exposure. Psychopharmacology, 231(8), pp.1763–1773. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV and O’Dell LE, 2012. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. Journal of neurochemistry, 123(4), pp.578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV and O’dell LE, 2010. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse, 64(2), pp.136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC and O’Dell LE, 2013. Adolescence is a period of development characterized by short-and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behavioural brain research, 257, pp.275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF and Markou A, 2006. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology, 186(4), pp.612–619. [DOI] [PubMed] [Google Scholar]
- Opitz B, 2014. Memory function and the hippocampus In The hippocampus in clinical neuroscience (Vol. 34, pp. 51–59). Karger Publishers. [DOI] [PubMed] [Google Scholar]
- Paradise J, 2014. Electronic cigarettes: smoke-free laws, sale restrictions, and the public health. American journal of public health, 104(6), pp.e17–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han SH, Cao J and Leslie FM, 2007. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacology Biochemistry and Behavior, 86(2), pp.297–305. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M and Bowes G, 1998. Depression, anxiety, and smoking initiation: a prospective study over 3 years. American journal of public health, 88(10), pp.1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin JA, Kaplan GJ, Plant CP, Eaton SE, Gil SM, Zavala AR and Crawford CA, 2014. Nicotine exposure beginning in adolescence enhances the acquisition of methamphetamine self-administration, but not methamphetamine-primed reinstatement in male rats. Drug and alcohol dependence, 142, pp.341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA and Dani JA, 2009. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochemical pharmacology, 78(7), pp.686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomfrey RL, Bostwick TA, Wetzell BB and Riley AL, 2015. Adolescent nicotine exposure fails to impact cocaine reward, aversion and self-administration in adult male rats. Pharmacology Biochemistry and Behavior, 137, pp.30–37. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA and Gould TJ, 2012. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiology of learning and memory, 97(4), pp.482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin AN, Eugene AJ, Lallai V, Torres-Mendoza A, Fowler JP, Chen E and Fowler CD, 2019. Cannabinoid and nicotine exposure during adolescence induces sex-specific effects on anxiety-and reward-related behaviors during adulthood. PloS one, 14(1), e0211346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC and Izenwasser S, 2017. Nicotine produces long-term increases in cocaine reinforcement in adolescent but not adult rats. Brain research, 1654, pp.165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M and Lotfipour S, 2019. Nicotine Gateway Effects on Adolescent Substance Use. Western Journal of Emergency Medicine, 20(5), pp.696 – 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Glod CA, Kim R and Hounchell J, 2010. Relationships between aggression, depression, and alcohol, tobacco: Implications for healthcare providers in student health. Journal of the American Academy of Nurse Practitioners, 22(7), pp.369–375. [DOI] [PubMed] [Google Scholar]
- Roys MR, Peltier MR, Stewart SA, Waters AF, Waldo KM and Copeland AL, 2019. The association between problematic alcohol use, risk perceptions, and e-cigarette use. The American journal of drug and alcohol abuse, pp.1–8. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings Rockville, MD, USA: Substance Abuse & Mental Health Services Administration; Report nr HHS Publication No. (SMA) 11–4658. [Google Scholar]
- Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC and Sved AF, 2016. Adolescent rats self-administer less nicotine than adults at low doses. Nicotine & Tobacco Research, 18(9), pp.1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Bremer QZ, Brownfield MS, Kelley AE and Landry CF, 2008. The dendritically targeted protein Dendrin is induced by acute nicotine in cortical regions of adolescent rat brain. European Journal of Neuroscience, 28(10), pp.1967–1979. [DOI] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE and Landry CF, 2005. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience, 135(1), pp.285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z and Lê AD, 2006. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacol. Berl. 186, 201e208. Neurosci Biobehav Rev, 24, p.417463Spear. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z and Lê AD, 2007. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neuroscience letters, 418(3), pp.286–291. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF and Lê AD, 2008. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology, 198(2), pp.181–190. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J and Ehlers CL, 2003. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology Biochemistry and Behavior, 75(2), pp.355–361. [DOI] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ, 2009. Nicotine exposure in adolescence alters the response of serotonin systems to nicotine administered subsequently in adulthood. Developmental neuroscience, 31(1–2), pp.58–70. [DOI] [PubMed] [Google Scholar]
- Smith LN, McDonald CG, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Falco AM and Smith RF, 2006. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacology Biochemistry and Behavior, 85(1), pp.91–97. [DOI] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA and Spindle TR, 2017. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA pediatrics, 171(8), pp.788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule EK, Lee JG, Egan KL, Bode KM, Desrosiers AC, Guy MC, Breland A and Fagan P, 2020. “I cannot live without my vape”: Electronic cigarette user-identified indicators of vaping dependence. Drug and Alcohol Dependence, 209, p.107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS and Burk JA, 2010. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacology Biochemistry and Behavior, 96(4), pp.501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MD, Audrain-McGovern J and Leventhal AM, 2017. Association of anhedonia with adolescent smoking susceptibility and initiation. Nicotine & Tobacco Research, 19(6), pp.738–742. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T and Dani JA, 2018. Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration. Cell reports, 23(1), pp.68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe HH, Hamidullah S, Jenkins BW and Khokhar JY, 2020. Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacology & Therapeutics, 206, p.107431. [DOI] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM and O’Dell LE, 2013. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Frontiers in psychiatry, 4, p.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA and O’Dell LE, 2009. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology, 206(2), pp.303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV and O’Dell LE, 2016. Stress is a principal factor that promotes tobacco use in females. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 65, pp.260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA and O’Dell LE, 2008. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology Biochemistry and Behavior, 90(4), pp.658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Hussong A, Burmeister M and Zucker RA, 2018. Biological underpinnings of an internalizing pathway to alcohol, cigarette, and marijuana use. Journal of abnormal psychology, 127(1), p.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo Á, Obando D and Trujillo CA, 2016. Family dynamics and alcohol and marijuana use among adolescents: The mediating role of negative emotional symptoms and sensation seeking. Addictive behaviors, 62, pp.99–107. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI and Spear LP, 2002. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & behavior, 77(1), pp.107–114. [DOI] [PubMed] [Google Scholar]
- Voos N, Smith D, Kaiser L, Mahoney MC, Bradizza CM, Kozlowski LT, Benowitz NL, O’Connor RJ and Goniewicz ML, 2019. Effect of e-cigarette flavors on nicotine delivery and puffing topography: results from a randomized clinical trial of daily smokers. Psychopharmacology, pp.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Gentzke AS, Creamer MR, Cullen KA, Holder-Hayes E, Sawdey MD, Anic GM, Portnoy DB, Hu S, Homa DM and Jamal A, 2019. Tobacco product use and associated factors among middle and high school students—United States, 2019. MMWR Surveillance Summaries, 68(12), p.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH and Sofuoglu M, 2009. The impact of cigarette smoking on stimulant addiction. The American journal of drug and alcohol abuse, 35(1), pp.12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein R, Shiffman S and Flay B, 2008. Mood variability and cigarette smoking escalation among adolescents. Psychology of Addictive Behaviors, 22(4), p.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack RW, Wilsnack SC, Gmel G and Kantor LW, 2018. Gender differences in binge drinking: Prevalence, predictors, and consequences. Alcohol research: current reviews, 39(1), p.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, Bruchas MR and McGehee DS, 2018. Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nature communications, 9(1), pp.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Behnood-Rod A, Wilson R, Wilks I, Tan S and Bruijnzeel AW, 2020. Rewarding effects of nicotine in adolescent and adult male and female rats as measured using intracranial self-stimulation. Nicotine and Tobacco Research, 22(2), pp.172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Pistillo F and Gotti C, 2015. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology, 96, pp.302–311. [DOI] [PubMed] [Google Scholar]


