Abstract
Liver fibrosis is a hepatic wound-healing response caused by chronic liver diseases that include viral hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis, and cholestatic liver disease. Liver fibrosis eventually progresses to cirrhosis that is histologically characterized by an abnormal liver architecture that includes distortion of liver parenchyma, formation of regenerative nodules, and a massive accumulation of extracellular matrix (ECM). Despite intensive investigations into the underlying mechanisms of liver fibrosis, developments of anti-fibrotic therapies for liver fibrosis are still unsatisfactory. Recent novel experimental approaches, such as single-cell RNA sequencing and proteomics, have revealed the heterogeneity of ECM-producing cells (mesenchymal cells) and ECM-regulating cells (immune cells and endothelial cells). These approaches have accelerated the identification of fibrosis-specific subpopulations among these cell types. The ECM also consists of heterogenous components. Their production, degradation, deposition, and remodeling are dynamically regulated in liver fibrosis, further affecting the functions of cells responsible for fibrosis. These cellular and ECM elements cooperatively form a unique microenvironment: a fibrotic niche. Understanding the complex interplay between these elements could lead to a better understanding of underlying fibrosis mechanisms and to the development of effective therapies.
1. Introduction
Liver fibrosis is a consequence of chronic liver injury from chronic viral infections (e.g., hepatitis B and C), alcohol abuse, metabolic disorders (e.g., NASH; non-alcoholic steatohepatitis), cholestasis, parasite infections, hepatotoxin exposure, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson’s disease. The pathological hallmark of liver fibrosis is the excessive accumulation of extracellular matrix (ECM) due to its production exceeding its degradation in the liver parenchyma. Insufficient treatment of underlying liver disease causes progressive liver fibrosis and ultimately leads to cirrhosis. Patients with cirrhosis have a poor prognosis due to liver dysfunction, ascites, portal hypertension, and hepatocellular carcinoma. Cirrhosis is the 11th leading cause of death worldwide (1), with 1.16 million annual deaths. The recent advancement of anti-viral therapies has significantly reduced the proportion of viral hepatitis, but NASH has emerged as the predominant etiology of underlying liver disease for liver fibrosis, especially in Western countries. Notably, fibrosis is an independent risk factor for survival in patients with NASH even before progressing to cirrhosis (2). Liver fibrosis also increases the risk of cardiovascular events: the leading cause of death for NASH patients. Unfortunately, liver transplantation is the only curative therapy for liver fibrosis to date, and the development of effective anti-fibrotics is a significant unmet medical need.
In recent decades intensive investigations have elucidated the fundamental molecular mechanisms of liver fibrosis (3). It is associated with complex interactions between multiple types of cells in the liver. Damage to liver parenchymal cells (hepatocytes and biliary epithelial cells) can often be the initial trigger for the fibrotic response. These damaged cells release damage-associated molecular patterns (DAMPs) including nuclear proteins (e.g., HMGB-1), cytokines (e.g., IL-1α, IL-33, and S100A8/9), intracellular molecules (e.g., Hsp70), and mitochondrial components (mitochondrial DNA) (3, 4). In advanced liver disease, intestinal bacterial products (e.g., LPS; lipopolysaccharide) can translocate to the liver via the portal vein as pathogen-associated molecular patterns (PAMPs) due to increased intestinal permeability. Injured hepatocyte-derived DAMPs and intestine-derived PAMPs cooperatively promote inflammatory and fibrogenic responses in the liver. During fibrosis progression, hepatic mesenchymal cells, including hepatic stellate cells (HSCs), transdifferentiate into ECM-producing myofibroblasts. Immune and vascular system cells also contribute to ECM accumulation by regulating HSC transdifferentiation and modulating ECM production and degradation (also defined as ‘ECM remodeling’). These non-parenchymal cells (e.g., mesenchymal cells, immune cells, and endothelial cells) are the key cellular components of the fibrosis microenvironment.
Mesenchymal cells, immune cells, and endothelial cells in the fibrotic liver have subpopulations with different origins and functions. The ECM also consists of heterogeneous components that are dynamically regulated in response to liver injury. In turn, the altered composition of ECM components affects the functions of surrounding cells. These diverse subpopulations of cells and ECM components interact with each other to make a dynamic and unique liver fibrosis microenvironment.
This review discusses the interplay among fibrosis-specific subpopulations of mesenchymal cells, immune cells, and endothelial cells and ECM components which cooperatively form fibrosis-specific microenvironment (i.e., a fibrotic niche) (Figure 1). In addition to a summary of well-established liver fibrosis mechanisms, current molecular mechanisms of liver fibrosis are also discussed.
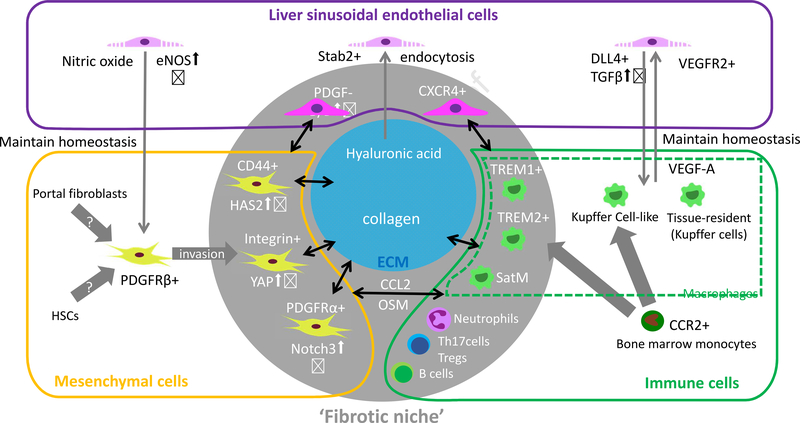
Figure 1. Fibrosis-specific subpopulations of mesenchymal, immune, and endothelial cells cooperate with the ECM to comprise the liver fibrosis niche.
Graphical summary of the key components of the unique microenvironment in the liver. Liver mesenchymal cells, immune cells, endothelial cells, and extracellular matrix (ECM) have heterogenous subpopulations with different ontogenies and functions. Accumulating evidence has identified fibrosis-specific subpopulations of macrophages. Other immune cells (e.g. Neutrophils, B cells, Th17 cells, and regulatory T cells) also contribute to fibrosis. Their contributions to the underlying mechanisms of fibrosis are less investigated compared to macrophages. The interplay between these components maintains homeostasis in the healthy liver. In the diseased liver, this homeostasis breaks down, and pathological components emerge that cooperate to promote liver fibrosis.
2. Extracellular matrix
The ECM is a three-dimensional network of proteins and non-protein components that provide the structural and biochemical scaffolds for surrounding cells in tissues and organs. It is composed of two major classes of biomolecules, fibrous proteins and glycosaminoglycans (5, 6). The fibrous proteins (e.g., collagen, elastin, fibronectin, and laminin) provide tensile strength to the tissue and support cell adhesion, proliferation, apoptosis, survival, and differentiation. The glycosaminoglycans are a family of large polymers containing repeated disaccharide elements including heparan sulfate, derman sulfate, keratan sulfate, and hyaluronic acid (also known as hyaluronan; HA), most of which (with the exception of HA) attach to core proteins to form proteoglycans. Glycosaminoglycans sequester and release bioactive molecules locally, such as growth factors and cytokines (7). Therefore, ECM plays critical roles in maintaining tissue and organ homeostasis, and also contribute to adaption to injuries. Liver ECM is heterogeneous, including collagens, nidogen, laminin, fibronectin, biglycan, mimecan, versican, decorin, lumican, elastin, and glycosaminoglycans (7, 8) (Table 1). Constant and appropriate regulation of both ECM synthesis and degradation is critical for maintaining tissue and organ homeostasis in the healthy liver. In chronic liver injury, ECM is over-produced and aberrantly deposited in liver parenchyma, with ECM biosynthesis often exceeding its degradation and leading to ECM and tissue remodeling - fibrosis. Importantly, ECM remodeling and fibrosis not only alter the quantity of ECM but also its composition (9, 10). Many studies have investigated the regulatory mechanisms and functional roles of different ECM components in liver fibrosis (Table 2).
Table 1.
Dynamic changes in ECM components in the healthy and fibrotic liver.
| ECM Components | Liver fibrosis etiologies Experimental models | Changes in ECM components | References |
|---|---|---|---|
| Fibronectin, tenascin, elastin | Rat, BDL model | Tenascin and fibronectin expressed around portal vein soon after injury. Type I, III, IV collagen, elastin are progressively deposited from day 2 to 7 after BDL. Laminin pre-exists in normal liver. |
(11) |
| Fibronectin, Type IV collagen, laminin | Rat, chronic CCL4 model | Fibronectin deposits after 2 weeks of chronic injury. Type I collagen deposit from 4 weeks of injury around the central vein. Type IV and laminin deposition detected after 3–4 weeks of injury. |
(12) |
| Biglycan, Decorin | Human, healthy and fibrotic liver | Biglycan and Decorin observed in the space of Disse and around the small bile duct and vessel wall of healthy livers, with strong immunoreactivity in fibrotic areas of the chronically injured liver. | (229) |
| Hyaluronic acid | Human, NASH liver | Hyaluronic acid is increasingly deposited in the fibrotic liver. | (52) |
| Hyaluronic acid | Mouse, CCl4, TAA, BDL, CDHFD model Rat, DMN-induced cirrhosis | Hyaluronic acid increasingly deposited concomitant with fibrosis progression. | (52) (53) |
| Type IV collagen | Human, liver cirrhosis | Type IV collagen is highly upregulated compared to the healthy liver with a 14-fold increase in liver cirrhosis. | (14, 28) |
| Type IV collagen, laminin | Human, liver fibrosis | Type IV collagen along with laminin increasingly deposited in the space of Disse, resulting in the formation of a perisinusoidal basement membrane in the fibrotic liver. | (37) |
| Nidogen-1 | Mouse, partial hepatectomy model | Nidogen-1 expression is detected in the portal tract but is only focally present in the periportal parenchyma. Its expression is enhanced in the regenerating liver. | (230) |
| Laminin | Rat, partial hepatectomy model | Laminin α 1 chain is transiently expressed in sinusoid during regeneration. | (230) |
| Perlecan | Rat, chronic CCl4 model | Perlecan is localized in the basement membranes of portal biliary and LSEC in a healthy liver. In the fibrotic liver, it is highly expressed in the perisinusoidal space of Disse and the matrix of fibrous septa. | (231) |
| Perlecan | Human, chronic cholestatic disease | Perlecan is distributed in association with a ductular reaction in the periportal parenchyma. | (232, 233) |
| Elastin | Human, fibrotic liver | Elastin accumulates in the internodular fibrous septa and enlarged portal area, especially in advanced fibrosis. | (41, 234) |
Table 2.
Mechanistic roles of key EMC components during liver fibrosis.
| ECM component | Receptors Mechanisms | Effects on cell function and fibrosis progression | Possible therapeutic target in liver fibrosis |
|---|---|---|---|
| Type I collagen | Integrin αv/β1/DDR1/DDR2 | αv signals activate latent TGFβ (235). β1 induces PAK/YAP signaling leading to HSC activation and liver fibrosis (73). DDR2 promotes HSC proliferation and invasion via MMP2 (71). |
Blocking αv attenuates liver fibrosis (69). PAK inhibition attenuates liver fibrosis (73). DDR2-deficient mice showed exacerbated hepatotoxin-induced liver fibrosis (70). |
| Arrestine, Tumstatin (fragments from Type IV collagen) | Integrin αv/β3 | suppresses endothelial cell proliferation, migration, angiogenesis (66, 67). | Tumstatin suppresses tumor growth (66). |
| Fibronectin | Integrin αvβ6 | Cholangiocytes proliferate, local TGFβ1 activation (236). | αvβ6 antagonist EMD527040 attenuates bile ductular proliferation and peribiliary collagen deposition (236). |
| Anastellin (fragments from fibronectin) | Binds to full-length fibronectin to form polymerized fibronectin multimers and inhibits cell migration (237). | Administration of polymeric fibronectin inhibits tumor growth, angiogenesis, and metastasis (238). | |
| Hyaluronic acid | CD44/TLR4/stab2 | Induces invasive phenotype of HSC via HA/CD44/Notch1 pathway. | Blocking HA production by 4-MU or HAS2 depletion prevents liver fibrosis (52). |
| laminin | Integrin | Supports proliferation and differentiation of hepatic progenitor cells in the fibrotic liver (38, 39). | Promotes progenitor cell differentiation of biliary phenotype (39). |
| elastin | Cross-linking of fibers. Elastin-derived peptide chemoattracts monocytes, fibroblasts, endothelial cells (45). | Relates to the irreversibility of liver fibrosis (44). | |
| Decorin | cMET (239) Binding to cytokines(240) |
Activates macrophages by interfering with TGFβ binding to macrophages (241). | Decorin acts as antifibrotic by binding TGF-β and controlling its bioactivity(242), and tumor repressor by binding PDGFRα (243). |
| Biglycan | TLR2/4 (244) Binding to cytokines(245) |
Proinflammatory activation of macrophages (244). |
2.1. Fibrous proteins (collagen, laminin, and elastin)
The collagens are the most well-investigated ECM components of liver fibrosis. Types I and III collagens are increasingly produced and predominantly deposited in human and rodent fibrotic livers (11–15). Many studies of liver fibrosis have focused on the regulatory mechanisms for types I and III collagens. Most collagens are produced by activated myofibroblasts, and this production is promoted by environmental stimuli such as cytokines, DAMPs, hypoxia, and mechanical stress (discussed in Section 3 and summarized in Table 3). Collagens can be degraded by matrix metalloproteinases (MMPs), a family of zinc-dependent endoproteinases produced by various hepatic cells, including macrophages (6, 16), and MMP activity can be inhibited by tissue inhibitor of metalloproteinase 1 (TIMP1) from myofibroblasts and macrophages (16). In addition, post-transcriptional and post-translational regulation of collagens can affect their deposition. Lysyl-oxidase (LOX) and lysyl-oxidase like protein 2 (LOXL2) are both upregulated in response to the hypoxic environment that accompanies liver fibrosis. LOX and LOXL2 oxidize the lysine residues of collagens, inducing the crosslinking of fibrous proteins and promoting the resistance to ECM degradation which contributes to ECM deposition, remodeling, and fibrosis (17–19). In liver fibrosis, collagen deposition is regulated by a balance between production, cross-linking, and degradation. As a consequence, types I and III collagens are increasingly deposited in the chronically injured liver (9, 20), but clinical evidence suggests that collagen deposition is reversible once the underlying liver injury is eliminated (9, 21–25). Monocyte/macrophage-derived MMP-12 and MMP-13 both contribute to fibrosis regression by degrading the ECM (26, 27). The deposition of types I and III collagens is often assessed to validate the efficacy of therapeutic interventions for liver fibrosis.
Table 3.
Key mechanistic roles of non-parenchymal cells in liver fibrosis.
| Cell types | Key roles | Mechanisms | Possible therapeutic targets in liver fibrosis |
|---|---|---|---|
| HSCs and myofibroblasts | Collagen production | Activated by TGFβ (138), HSP47 (93, 94) OSM (139, 140), Galectin (246, 247), |
Antibodies or inhibitors for LOXL2 (collagen cross-linking) (19, 99, 248) PPARs agonists (deactivation of HSCs) (95, 96) FXR agonists (97, 98) ASK1 inhibitors (inflammation, apoptosis) (91, 92) HSP47 inhibition (HSC apoptosis, collagen secretion) (93, 94) Galectin 3 inhibitor (HSC activation) (246, 247) |
| Hyaluronan production | HAS2 upregulation by WT1 | 4-MU (suppression of HA) | |
| Migration/invasion | PDGF (103, 105, 111, 249), YAP | PDGF inhibitor (101) YAP inhibitor |
|
| Macrophages | HSC activation via pro-fibrogenic cytokine production | TGFβ, PDGF, OSM(139, 140), TNF (141–143), IL-1β | CCR2/ CCR5 dual inhibitor (250, 251) |
| ECM degradation | Production of MMPs, liver regenerating factors | Adoptive transfer of monocytes/macrophages | |
| LSECs | Maintains quiescent HSCs (213), Kupffer cells (152) | VEGF-mediated NO production (213) KLF2 upregulation in LSECs promotes HSC deactivation in a paracrine manner (215). | sCG activator (217) Statin upregulates KLF2 in LSECs, reverses dysfunction of LSECs and fibrosis (214). |
| Promotes infiltration of neutrophils (219) and monocytes. | Mechanical stress induces CXCL1 via Notch (219). | ||
| Produces angiocrine factors for liver regeneration and fibrosis in injured livers (152, 213, 225). | The balance of two CXCL12 receptors (CXCR4/7) controls the transcriptional factor ID1 (213, 225). | A small molecule inhibitor for CXCR4 does not improve fibrosis (227, 228). |
Type IV collagen is the prevalent collagen in a healthy liver, with type I and type III collagen being in lower abundance. Type IV collagen is a component of the basement membrane around large vessels and bile ducts, but sinusoidal endothelial cells (LSECs) in a healthy liver lack a typical basement membrane (28, 29). This unique feature of hepatic sinusoids allows LSECs to maintain ‘fenestrae’ (30), cytoplasmic pores that enable the efficient flow of molecules from sinusoidal lumens to hepatocytes, and vice versa (31). In the fibrotic liver, type IV collagen deposition is also increased between hepatic sinusoids and hepatocytes in response to injury (10, 32). The appearance of the perisinusoidal basement membrane in the fibrotic liver (28) may affect liver function through decreased LSEC fenestrae and impaired flow between hepatic sinusoids and hepatocytes (30). This change in the basement membrane also affects HSC function (33) and that of epithelial cells (hepatocytes and cholangiocytes) by regulating their cellular polarities (34, 35). Even though the serum concentration of type IV collagen is related to the severity of liver fibrosis (36), the impact of type IV collagen on the liver microenvironment, and its regulation during liver fibrosis, have not been as well-investigated as those of types I and III collagens. Further investigations are needed.
Laminin is also increasingly deposited in liver fibrosis and is another basement membrane component together with type IV collagen (12, 37). It supports the proliferation and differentiation of hepatic progenitor cells in liver fibrosis (38–40).
The deposition of elastin is observed especially in advanced liver fibrosis (41, 42), contributes to liver stiffness, and is related to the irreversibility of fibrosis (43, 44). Elastin-derived peptides are bioactive and act as chemoattractants for monocytes, fibroblasts, and endothelial cells (45).
2.2. Hyaluronic acid
HA is a major glycosaminoglycan produced and deposited in liver fibrosis, and its serum level has been used as a biomarker for diagnosing the stages of liver fibrosis in clinical practice (46–48), with higher levels indicating poorer prognoses in patients with chronic liver injury (49–51). Large deposits of HA are seen in fibrotic areas of diseased livers, while no or minimal HA deposition is observed in the normal liver (52, 53). In the healthy liver, the majority of HA is trapped in LSECs and degraded internally in lysosomes (54). In conditions of liver inflammation and fibrosis, LSECs are dysfunctional and unable to capture and breakdown HA (47). Increased HA in the fibrotic liver has often been explained by the impaired uptake of HA by LSECs, but recent studies have indicated the critical role of increased HA production in organ fibrosis, including the liver (52, 55–58). Activated HSCs are the main producers of HA, with HA synthase-2 (HAS2) being the critical enzyme for HA assembly in these cells (52). Importantly, mice with either a deletion or overexpression of HAS2 specifically in HSCs showed remarkable reductions or exacerbations, respectively, of HA production, HSC activation, and liver fibrosis. HA activates Notch1 signaling in HSCs through its receptors, Toll-like receptor (TLR) 4 and CD44, leading to further HSC activation and fibrosis exacerbation. In HSCs, HAS2 upregulation is transcriptionally regulated by TGFβ signaling, mediated through the induction of a transcriptional factor Wilms tumor 1 (WT1). Targeting the TGFβ/WT1/HAS2/HA axis in HSCs could be a promising therapeutic approach for liver fibrosis. Indeed, suppression of HA production by 4-methylumbelliferone, an inhibitor of HA synthesis, decreased liver fibrosis (52, 59).
2.3. Dynamic regulation of the ECM
Most previous studies have examined a single ECM protein or glycosaminoglycan that is abundant in the fibrotic liver (e.g., type I collagen, elastin, and HA), but the ECM contains many diverse components. In addition, each component has its own bioactivity, depending on modifications such as crosslinking and fragmentation. It is likely that multiple ECM components with varying modifications exert pleiotropic functions in the context of different types of injury, locations, and stages of fibrosis. Recent proteomics-based analyses of the ECM ‘the matrisome’ are leading to a comprehensive understanding of the dynamic regulation of ECM components in normal and diseased organs, including the liver (10, 60, 61).
In the liver matrisome study, liver samples were processed through a series of increasingly rigorous extraction buffers to separate proteins by solubility (fractionation) after chronic alcohol and/or lipopolysaccharide (LPS) exposure in mice (10). Extracted proteins were identified using liquid chromatography/tandem mass spectrometry (LC-MS/MS). As a reflection of protein structure, as well as degradation, cross-linking, and enzymatic activation, each matrix protein showed unique solubility and distribution in each fraction. Cross-linked protein abundance was greater in carbon tetrachloride-induced fibrotic livers compared to non-fibrotic livers (treated with lipopolysaccharide [LPS] or/and ethanol), consistent with the idea that LOX and LOXL2 increase fibrous-protein crosslinking (e.g., collagen and elastin) during fibrosis progression (17–19). Interestingly, both ethanol and LPS administration increased the amounts of liver matrix proteins compared to untreated liver, with unique matrix component changes, indicating that the ECM found in diseased livers is remodeled according to stimulus-specific mechanisms. Interestingly, administration of LPS and ethanol generally do not induce fibrosis in mice. Instead, these stimuli can be considered pre-fibrotic and suggest that compositional changes in matrix proteins start soon after injury and before fibrosis. These early changes in ECM components may influence the future pro-fibrotic milieu in the context of ongoing liver injury.
Other proteomics-based ECM analyses of mouse chronic liver injury and human liver disease (62, 63) are also illuminating. The mouse study used a carbon tetrachloride model to induce pericentral fibrosis, and a diet containing 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet (DDC) to induce periportal fibrosis. The results demonstrated that ECM alterations were not homogenous and differed between periportal and pericentral areas, suggesting differential regulatory mechanisms for ECM production in the context of injury location (62). In the human study, analyses of 57 liver biopsy samples from HCV hepatitis patients with stages F1–F4 fibrosis demonstrated unique ECM compositions according to fibrosis stage, suggesting that ECM remodeling occurs throughout the development of fibrosis (63). Taken together, these studies suggest that ECM is dynamically remodeled both spatially and temporally: a unique feature of liver fibrosis pathogenesis.
The proteomics-based approaches can detect ECM proteins including fibrous proteins and protein components of proteoglycan. However, it is still challenging to analyze carbohydrate structure of the glycosaminoglycan which is also a major component of ECM. The glycosaminoglycans consist of large linear polysaccharides with many branches, which make numerous possible configurations. The regulatory mechanism of synthesis and degradation of glycosaminoglycan is complex. The emerging techniques in the glycobiology field may resolve this issue. Even though proteomics- and glycobiology-based studies can delineate the previously unappreciated involvement of ECM components during fibrosis progression, the biological functions of ECM components are still unclear. Future studies should validate how ECM components affect cellular functions and how those functions impact pathogenesis, including fibrosis, inflammation, angiogenesis, regeneration, and carcinogenesis. A comprehensive understanding of ECM production, degradation, deposition, and remodeling dynamics during fibrosis progression and regression could uncover potential therapeutic targets and diagnostic markers in patients with liver fibrosis.
2.4. ECM-cellular interactions
In contrast to mechanisms of ECM production, little attention has been paid to the biological effects of ECM on cellular functions. The ECM can affect cellular phenotypes through ECM-cellular interactions mediated by specific receptors such as the integrins (64, 65), and some ECM molecules are degraded by proteinases to generate and release bioactive fragments (66, 67). The roles of key EMC components on cellular functions during liver fibrosis are summarized in Table 2. In liver fibrosis, the ECM regulates HSC phenotype through at least two types of collagen receptors: integrins (comprised of α and β subunits) and discoidin domain receptors (DDR) (68–71). Highlighting the importance of the integrin α subunit in liver fibrosis, mice with a specific deletion of integrin αv in PDGFRβ-expressing cells (HSCs) showed attenuated liver fibrosis induced by carbon tetrachloride (69) and pharmacological blockade of αv-containing integrins by CWHM-12 also attenuated carbon tetrachloride-induced liver fibrosis (69). One possible mechanism for this is that αv-containing integrins are known to be involved in the conversion of the latent form of TGFβ to its active form that activates HSCs and promotes liver fibrosis (72). Integrin β1 can also regulate the profibrogenic phenotype of activated HSCs (73). Mechanistically, a serine/threonine-protein kinase p21-activated kinase (PAK) and yes-associated protein (YAP) are the key contributors for the integrin β1-mediated profibrotic actions (73). Thus, integrin ECM receptors promote HSC activation and fibrosis through activation of pro-fibrotic pathways, and may be therapeutic targets for liver fibrosis.
DDR is a receptor tyrosine kinase whose ligands are fibrillar collagens rather than peptide-like growth factors (74). Expression of DDR2 increased in human cirrhotic livers (75) and in experimental models of hepatotoxin- (70) and alcohol-induced liver fibrosis (76). The DDR2 receptor expressed in HSCs interacts with collagen and promoted HSC proliferation and an invasive phenotype in vitro with increased MMP2 expression and activity (71). In carbon tetrachloride-induced liver fibrosis, DDR2-deficient mice showed exacerbated fibrosis and inflammation, suggesting that the type I collagen/DDR2-dependent signaling is a negative regulator for pro-fibrotic HSC phenotypic change (70). In the human cirrhotic liver, however, increased DDR2 expression was observed especially around bile ducts in cholestatic liver injuries (e.g., primary biliary cholangitis and primary sclerosing cholangitis), but less expression was seen in fibrotic livers with other etiologies (75). Thus, the impact of DDR2 on fibrogenesis in cholestatic liver injury may be different from hepatocytic injury-induced fibrosis, such as carbon tetrachloride-induced fibrosis.
Receptors for HA, such as CD44, TLR4, TLR2, and Receptor for Hyaluronan Mediated Motility (RHAMM), are expressed in various types of liver cells. HSCs express CD44 and TLR4, through which HA activates HSCs and promotes fibrosis (52). CD44 is also expressed in LSECs (47) and in neutrophils (77). CD44 receptors in LSECs could be important for trapping HA, and CD44 receptors in neutrophils might contribute to neutrophil chemotaxis, suggesting that HA may exert multiple roles via HA-specific receptors in different liver cells. Importantly, the molecular size of HA is crucial for determining its biological influence (78–80). High molecular weight (HMW)-HA is generally thought to be protective against tissue injury while low molecular weight (LMW)-HA is pro-inflammatory and pro-fibrogenic (52, 81, 82). HA is synthesized by cell-membrane bound hyaluronan synthases (HAS1, HAS2, and HAS3) and initially generated as the HMW form on the cell surface and then released (83). In the setting of liver fibrosis, the pro-fibrogenic function of HA is mainly exerted by the LMW form (52). The mechanism of conversion from the HMW-HA form to the LMW form during liver fibrosis is still to be elucidated. One recent study demonstrated the different molecular mechanisms used by HMW- and LMW-HA through the CD44 receptor on the aggressiveness of breast cancer cells (79). HMW-HA induced CD44 clustering on these cancer-cell membranes and promoted MST1/2 activation, inhibiting oncogenic YAP activation. In contrast, the LMW form did not induce CD44 clustering and instead promoted the formation of the intracellular PAR1b/MST complex that resulted in YAP nuclear translocation and the promotion of cell migration and an aggressive phenotype. Thus, changes in HA molecular weight around cancer cells could play a critical role in phenotype switching via CD44 and Hippo/YAP signaling pathways. A similar mechanism might be involved in the HA-induced invasive phenotype of HSCs (discussed below).
In addition to ECM-cellular interactions via specific receptors, ECM components such as collagen and proteoglycan can also regulate cellular functions by capturing and releasing ECM-binding soluble proteins, such as growth factors, cytokines, chemokines, and enzymes (7, 84). For example, PDGF has been shown to bind to, and be released from, fibrous collagen (85). TGFβ also binds to collagen (86) and the bioavailability of TGFβ is regulated by a complex interaction with the ECM (discussed extensively in reviews 87, 88). Some ECM components (e.g., decorin and biglycan) also interfere with TGFβ activity by neutralizing or inhibiting TGFβ signaling (87, 88).
In summary, the dynamic processes of production, degradation, deposition, and remodeling of ECM in the chronically injured liver regulate cellular phenotypes and functions, and play critical roles in fibrosis pathogenesis. These ECM-liver cell interactions may be unique and important therapeutic targets for liver fibrosis. Future studies will better define these mechanisms in this underappreciated field of liver fibrosis research.
3. Hepatic stellate cells and Myofibroblasts
3.1. Activation of hepatic stellate cells and transdifferentiation into myofibroblasts
HSCs are a population of mesenchymal-type cells in the liver. In the normal liver, HSCs reside the space between hepatocytes and sinusoids (i.e., the space of Disse) with shared features of pericytes and vitamin A-storing cells, and are defined as quiescent HSCs. In this state, quiescent HSCs do not produce detrimental collagen. Upon chronic liver injury, HSCs become activated and transdifferentiate into the principal cells producing the ECM: myofibroblasts (89, 90). Activated HSC-derived myofibroblasts are contractile with the up-regulation of alpha-smooth muscle actin (α-SMA), and produce both collagen and TIMP1. Due to TIMP1 suppression of MMP activity that degrades ECM, increased TIMP1 production is likely to result in increased accumulation of ECM. Thus, activated HSCs skew the balance of ECM production and degradation that leads to fibrosis progression. HSCs can be activated by various fibrotic mediators, including cytokines, chemokines, DAMPs, PAMPs, hypoxia, and mechanical stress. These factors are extensively reviewed elsewhere (3). Currently, various novel anti-fibrotic strategies targeting HSC activation are in Phase I, II, and III clinical trials especially for NAFLD fibrosis. Current targets include activation of apoptosis signal-regulating kinase 1 (ASK1) (91, 92), HSP47 (93, 94), PPARs (95, 96), FXR (97, 98), and LOXL (19, 99) (Table 3). Unfortunately, most have shown minimal influence on advanced fibrosis. Establishing an antifibrotic therapy is still challenging partly due to the multiple roles of HSCs in the contexts of etiology, location, interacting cellular partners, and stages of fibrosis. The following sections discuss unresolved topics in liver fibrosis.
3.2. Migration and invasive HSC phenotypes
Increased ECM production is the predominant feature of myofibroblasts in liver fibrosis, but HSC migration and invasiveness are also critical features of myofibroblast behavior in lung (55) and liver (52) fibrosis. During liver fibrosis progression, activated HSCs migrate and accumulate in the injured area, but little is known about the mechanisms of migration and invasive phenotypes of myofibroblasts.
The terms ‘migration’ and ‘invasion’ are often used to describe myofibroblast and fibroblast phenotypes without clear definitions. In liver fibrosis research, the translocation of myofibroblasts from a normal location in intact tissue to a fibrotic area is often referred to as ‘migration’. The term ‘invasive phenotype’ is used in lung fibrosis and cancer research. Cell invasion is often considered to be related to cell migration, but invading cells do more than simply migrate. When myofibroblasts move into a fibrous scar area, ECM degradation and proteolysis is required, so invasive myofibroblasts should have the capacity for both ECM degradation and migration. The invasive phenotype of fibroblasts surrounding cancer cells can regulate vascular remodeling and contribute to cancer development (100). It is likely that similar mechanisms are involved in fibrosis progression because the vascular system also has a critical role in liver fibrosis (discussed in Section 7).
However, in liver fibrosis research, the migration and degradation phenotypes of myofibroblasts are still considered separately. How these phenotypes are coordinated to induce the invasive myofibroblast phenotype has not been studied well, but below we discuss possible effectors for inducing the invasive HSC phenotype during liver fibrosis progression based on currently available knowledge.
PDGF signaling
PDGF is a pro-fibrogenic cytokine and an attractive therapeutic target for liver fibrosis (101, 102). The differential impact of PDGF isoforms (PDGF-AA/AB/BB/CC/DD) and their receptors (PDGFR-αα, PDGFR-αβ, and PDGFR-ββ) have been investigated using genetically modified mouse models and through observational studies of human liver fibrosis (103–109). Both PDGFRβ (103–107) and PDGFRα (108–110) expressed in HSCs contribute to the development of liver fibrosis. PDGF is a potent mitogen that primes HSC proliferation to myofibroblasts. In addition to that, PDGF also has a critical role in the migration of myofibroblasts in liver fibrosis (103, 111). The intracellular signaling pathways related to PDGF-induced cell migration during development have been well-investigated (112). Both PDGFRα and PDGFRβ induce various signaling pathways (e.g., Ras-MAPK and PI3K) that are associated with cytoskeleton remodeling and cell migration (113). The activated Ras pathway leads to the activation of downstream Raf-1 and MAPK cascades that induce gene transcription related to cell migration, growth, and differentiation. PDGF-induced PI3K activation promotes actin reorganization, directs cell movement, stimulates cell growth, and inhibits apoptosis (114). PDGFRβ also exerts modulatory roles on Rho-associated protein kinase 1 (ROCK1) to mediate the migration of vascular smooth muscle cells (115). Compared to migration, fewer studies have examined whether PDGF is associated with the ECM degradation capacity of HSCs necessary for the invasive phenotype. Transgenic mice with Pdgf-b overexpression display progressive liver fibrosis with increased MMP2 and MMP9 enzyme activities (106). As MMPs are produced by activated HSCs (116, 117), PDGF-mediated MMP expression enhances the ECM degradation capacity of HSCs as well as their migration phenotype, both of which are required for the myofibroblast invasive phenotype. In summary, PDGF plays a critical role in fibrosis progression, and could also be crucial for the induction of the invasive phenotype of HSCs. Further investigations are required for understanding the role of PDGF in the HSC invasive phenotype and ECM degradation and remodeling.
Hippo/YAP signaling
YAP is a transcriptional regulator that contributes to cell proliferation, mechanics, and migration following its nuclear translocation in organ morphogenesis (e.g., lung branching and endothelial-cell sprouting) and cancer invasiveness (118, 119). YAP nuclear translocation is induced by various signals, including metabolic changes in lipids, Wnt, G protein-coupled receptor signaling as well as cellular sensing of increased matrix stiffness (120). Hippo/YAP signaling can also be activated by ECM-cellular interactions. In mesenchymal stem cells, sensing of ECM stiffness and cell spreading promotes the activation of Hippo/YAP signaling, which enhances the assembly of focal adhesion complexes by inducing the transcription of integrins and focal adhesion-docking proteins, which determines cell shape, migration, and the strength of cell adhesion to the ECM (119). In cancer-associated fibroblasts, cellular sensing of ECM stiffness further promotes ECM remodeling via YAP activation (121). During liver fibrosis progression, increased ECM production and dynamic ECM remodeling enhances ECM stiffness. Myofibroblasts likely sense and respond to altered ECM stiffness via YAP activation. In the fibrotic liver, YAP is expressed in activated HSCs, and inhibiting it reduced fibrosis in a rodent model (122), indicating that YAP activation contributes to liver fibrosis progression. In activated HSCs, YAP and PAK are activated as core downstream signaling molecules of integrin β1 (73), a collagen receptor. YAP activation also promotes the sustained expression of integrin β1, suggesting that an autoregulatory loop of the integrin β1-YAP-ECM axis perpetuates the fibrogenic phenotype of myofibroblasts. Integrin deletion reduced HSC migration and collagen production in vitro and pharmacological inhibition of either integrin β1 or YAP signaling attenuated liver fibrosis in vivo. Thus, the integrin β1-YAP-ECM axis plays a role in HSC activation and migration.
Taken together, deposited collagen induces HSC migration as well as further HSC activation and fibrosis via the integrin-YAP pathway. It is still unknown whether YAP signaling also contributes to the invasive phenotype of HSCs (with capacities for both migration and ECM degradation).
Hyaluronic acid
HA is associated with inducing the invasive myofibroblast phenotype in lung and liver fibrosis (52, 55, 56), and an HA-induced invasive phenotype is also described in breast cancer (79, 123). HAS2 overexpression in (myo)fibroblasts induced an invasive phenotype and fibrosis progression in the lung and the liver, while HAS2 depletion abrogated the invasive phenotype of (myo)fibroblasts (52). This invasive phenotype is CD44-dependent, and mediated through HA production by the following proposed mechanism.
First, the invasive phenotype of lung fibroblasts is accompanied by the upregulation of MMP2, MMP9, and MMP12 and the downregulation of Timp3 and ADAMTS1, suggesting that enhanced ECM degradation is involved (55). However, it is still unclear whether these molecules are directly induced by a CD44 signal or if induction is mediated through other mechanisms, such as by growth factors PDGF and TGFβ. Second, HA can induce Ccl2 expression in HSCs and macrophages. As HSCs also express CCR2 receptors, it is possible that the CCL2-CCR2 interaction further promotes the migration of myofibroblasts in an autocrine manner. Third, HA can modify the activation of Hippo/YAP signaling through CD44, which is associated with cell migration as discussed above. Fourth, Notch signaling is activated by HA, which potentially enhances cell migration. Notch-mediated cell-cell communication and cell-fate restriction is critical for organ development (124, 125). For example, Notch expression is necessary for coordinating cell migration induced by FGF during brain development (126). Also, Notch signaling contributes to the organ fibrosis. In lung fibrosis, Notch1 promotes pericyte proliferation and transdifferentiation to myofibroblasts via the PDGF/ROCK pathway (127). Inhibition of Notch signaling by a γ-secretase inhibitor attenuated hepatic fibrosis in rats (128). Our recent study demonstrated that Notch1 signaling contributed to the HSC invasive phenotype and that overexpressed Jagged1 and Notch1 promoted liver fibrosis in mice (52). Notch3 is also upregulated in diseased human livers (129) and in animal models (128). In a single-cell RNA-sequencing analysis of human fibrotic livers, Notch3 was expressed on scar-associated mesenchymal cells (110). The knockdown of NOTCH3 expression in primary human HSCs resulted in the reduced expression of type I and type III collagens, but the role of Notch3 in the migration/invasive phenotype is still unknown. Taken together, HA contributes to the induction of an invasive phenotype of myofibroblasts through multiple mechanisms.
3.3. Diverse origins of myofibroblasts
Another current myofibroblast topic concerns their origin. To date, five possible origins are proposed: 1) HSCs, 2) portal fibroblasts, 3) bone marrow-derived fibrocytes, 4) bone marrow mesenchymal stem cells, and 5) mesothelial cell-originated cells (130–133). HSCs have long been identified as primary precursors of collagen-producing myofibroblasts. Recent fate-tracing experiments using mice with genetic labeling of specific cell types have strongly suggested that activated HSCs are the principal source of collagen in experimental toxin-induced and cholestatic liver injury (134), while portal fibroblasts have also been reported as responsible for collagen-producing cells by other studies (135, 136).
For HSC investigations into phenotype and function, density-gradient centrifugation has long been used to isolate HSCs (137) based on their feature of carrying vitamin A-containing lipid droplets. Therefore, myofibroblast precursors that do not contain lipid droplets are likely to be lost using this approach. The lack of specific cell-surface markers for HSCs also makes it challenging to identify possible myofibroblast precursors. The previous study addressed this issue by using an in vivo lineage-tracing system where Cre recombinase was driven using the promoter of an HSC-specific vitamin A metabolism-associated enzyme, lecithin-retinol acyltransferase (Lrat) (134). Reporter mice demonstrated that most of the α-SMA+ collagen-producing cells (myofibroblasts) were derived from HSCs in fibrosis models induced either by hepatotoxin-induced parenchymal injury or by bile duct ligation-induced cholestatic injury, indicating that HSCs were the primary source of myofibroblasts in those liver fibrosis models (134).
Another study focused on the contribution of portal fibroblasts to cholestatic liver fibrosis induced by bile-duct ligation. Using a collagen α1(I) promoter-driven GFP transgenic (Col-GFP) mouse, the study showed that the GFP-positive (collagen-producing) vitamin A-negative cell population contributed to the αSMA-expressing activated myofibroblast pool, especially in early-stage fibrosis induced by bile-duct ligation. In that study, portal fibroblasts were defined as the non-HSC collagen-producing cell population (135) and contributed to fibrosis progression. From currently available knowledge, the conclusion is that HSCs are the primary source of activated hepatic myofibroblasts that contribute to aberrant ECM production and liver fibrosis. Other cell populations, such as portal fibroblasts, may also play a role in the specific etiology of liver fibrosis, such as cholestatic liver fibrosis. The suggestion is that HSCs, portal fibroblasts, and other precursors of myofibroblasts, may all share various markers and therefore make it challenging to identify, differentiate, and isolate myofibroblasts derived from different origins.
Single-cell transcriptomic analysis has brought new insight into the field of liver fibrosis research. Single-cell RNA sequencing analysis of human fibrotic liver has revealed the heterogeneity of mesenchymal cell populations (110). This analysis clustered myofibroblasts into four subpopulations based on transcriptomic patterns. Of these, scar-associated mesenchymal cells were identified as the population with high expressions of PDGFRA, COLL1A1, and COLL3A1 genes that accumulated in the scar area. Other populations were identified as vascular smooth muscle cells with MYH11 expression, quiescent HSCs with RSG5 (but less collagen) expression, and a population expressing mesothelial markers. The scar-associated mesenchymal cells were further divided into two subpopulations, one of which expressed OSR1 and genes associated with portal fibroblasts. Another scar-associated mesenchymal subpopulation was identified as cells derived from HSCs with RSG5 expression using pseudotemporal ordering and RNA velocity analyses. These data corroborated the previously proposed concept that myofibroblasts are derived from both HSCs and portal fibroblasts. There are still ongoing debates as to the origin and functional roles of myofibroblast precursors in liver fibrosis. In addition to identifying their possible origins, their precursor-to-myofibroblast induction mechanisms also need to be elucidated. Each myofibroblast subpopulation with a different origin may be induced and regulated by unique environmental stimuli in the context of liver-injury type, stage of fibrosis, and fibrosis-response location. Further investigations may help to develop both precision and personalized medicine for treating liver fibrosis by targeting these subpopulations of myofibroblasts.
4. Hepatic macrophages
The liver contains a large number of resident macrophages, known as Kupffer cells (KCs), that reside within sinusoidal lumens. KCs maintain liver immune homeostasis physiologically as well as pathologically. KCs can trap and take up gut-derived PAMPs in the portal circulation as well as blood-borne pathogens in the systemic circulation. However, in a healthy liver, KCs are tolerogenic, which prevents unnecessary immune responses to PAMPs and food-derived products and toxins that are constantly delivered from the gut via portal blood.
Importantly, once liver injury and inflammation occurs, circulating monocytes quickly infiltrate the injured site and differentiate into macrophages. These infiltrating macrophages are more sensitive to environmental stimuli, such as DAMPs, PAMPs, and cytokines/chemokines, than resident KCs. These environmental factors can lead to activated macrophages that produce various cytokines/chemokines, including TGFβ (138), PDGF (103–105, 111), OSM (139, 140), TNFα (141–143), and IL-1β. These cytokines can then activate HSCs and induce a pro-fibrogenic response (Table 3). The relative contributions of KCs and infiltrating macrophages in liver fibrosis have not been fully determined. The following sections discuss different types of hepatic macrophages based on the recent advances in hepatic macrophage research.
4.1. Ontogeny of hepatic macrophages: tissue-resident KCs and infiltrating macrophages
After organ injury, monocytes either from peripheral blood or from bone marrow infiltrate the injured site and differentiate into macrophages (144), further contributing to inflammation and the subsequent wound healing response (fibrosis) via cytokine production. Without injury, organs also have a pool of resident macrophages (145). Lineage-tracing studies using rodent models have shown that tissue-resident macrophages of embryonic origin are distributed before the bone marrow-mediated adult style hematopoiesis begins (146–150). Liver-resident tissue macrophages of KCs have the capacity for self-renewal independent of the bone marrow-derived monocyte/macrophage pool (146, 148–150). Most notions about KC homeostasis are based on studies using mouse models with healthy livers, and a cellular basis for KC homeostasis after liver injury has been unclear until recently. A recent study demonstrated that, upon liver injury, some of the original resident macrophages (KCs) disappeared, and infiltrating monocytes/macrophages acquired a resident macrophage (KC)-like phenotype (151–153). Functionally, KC-like cells acquired the ability to metabolize iron, a functional feature of KCs (154). This evidence suggests that these bone marrow-derived KC-like cells can contribute to the tissue-resident macrophage pool after liver injury. However, the contributions of these KC-like cells to cytokine production, liver inflammation, and fibrosis are still unclear. Bone marrow-derived KC-like cells may be less tolerogenic compared to the original, embryo-derived, KCs. In summary, for chronic liver injury, hepatic macrophage populations can be classified into at least three groups based on different ontogenies and functions: 1) cytokine-producing bone marrow-derived infiltrating macrophages, 2) embryo-derived original tissue-resident macrophages (KCs), and 3) bone-marrow-derived tissue-resident macrophage-like cells. In addition, GATA6+ peritoneal macrophages can also contribute to the hepatic macrophage pool (155). Currently, the impact of these hepatic macrophage subpopulations on fibrosis, maturation and functioning of tissue-resident macrophage-like cells, and on the longevity of tissue-resident macrophage-like cells is still unclear. Further investigations may address these points.
4.2. Functional classification of macrophages: identification of fibrosis-associated macrophages
In contrast to an ontogeny-based classification, a function-based classification is an alternative approach for understanding the contribution of hepatic macrophages to liver fibrosis. Macrophage function is often classified as either pro-inflammatory or anti-inflammatory (156, 157). The pro-inflammatory phenotype has long been identified in various disease models, and is induced in response to environmental stimuli such as DAMPs, PAMPs, and cytokines (158). This phenotype is also known as ‘classically activated’ or ‘M1’, and these macrophages have the ability to produce large amounts of pro-inflammatory cytokines (e.g., TNFα, IL-1β, and IL-6) that promote liver inflammation (159). In contrast, the Th2-type cytokines IL-4 and IL-13 promoted the proliferation of a host-protective macrophage phenotype against parasite infection (160). This macrophage phenotype is not accompanied by classical inflammation, so its activation is considered to be an alternative form ‘M2’. M1/M2 macrophages are often classified mirroring the Th1/Th2 classification of CD4+ T cells, and M2 macrophages can counteract M1 macrophages by producing anti-inflammatory cytokines such as IL-10 and TGFβ (161–164). IFNγ and LPS are commonly used for classically inducing the activation of M1 macrophages in vitro, and both IL-4 and IL-13 are used in the same way to activate the M2 phenotype (165). Another macrophage subpopulation (anti-inflammatory, profibrogenic, and TGFβ-mediated) contributes to the wound-healing response and fibrosis (166), and are sometimes classified as M2-like. In the spectrum of monocyte-derived macrophage activation states, M1 and M2 designations represent the extremes, but they are useful for understanding the signaling pathways underlying the macrophage plasticity (156). However, in chronic liver injury mixed phenotypes, and the coexistence of macrophages in different activation states, are often observed (157). Several explanations for this are possible. First, macrophage phenotyping is not simple, and it is hard to differentiate the actual states of macrophages as either M1 or M2. Second, different ontogenies can affect phenotype skewing even with the same environmental stimuli. Indeed, resident KCs are less responsive than bone marrow-derived KCs (167). Moreover, M2-designated macrophages derived from circulating monocytes and those from tissue macrophages are phenotypically and functionally distinct. Third, many studies have defined macrophage phenotypes in bulk using FACS or RNA expression rather than at the single-cell level. Macrophage phenotypes might also differ between macrophages residing in a fibrotic area compared to those in intact liver tissue. These distinct macrophage phenotypes might be induced by different fibrotic responses associated with zonal scar distributions in liver fibrosis. When different hepatic macrophage populations are mixed and bulk analyzed, macrophage phenotypes are confusing, and different from the straight-forward in vitro phenotyping of monocyte-derived macrophages.
A recent study has also identified more subtypes of monocytes/macrophages involved in fibrosis. In the lung, Ceacam1+Msr1+Ly6C-F4/80-Mac1+ monocytes contributed to the fibrosis disease progression (168). This monocyte subset was derived from Ly6C-FcεRI+ granulocyte/macrophage progenitors and shared segregated-nucleus-containing atypical monocytes (SatMs) characteristics based on unique morphology. In addition to this lung-fibrosis model, SatMs also contributed to the development of liver fibrosis in a high-fat diet model, in which Cebpb played a role (168).
Another recent study revealed that macrophages expressing triggering-receptor expressed on myeloid cells 1 (TREM1), an amplifier of inflammation, contributed to fibrosis progression that was induced by the hepatotoxin, carbon tetrachloride (140). The TREM1-expressing macrophages also produced oncostatin M (OSM), an IL-6 family cytokine that can induce liver fibrosis by promoting interactions between macrophages and HSCs (139).
More recent studies using single-cell RNA-sequencing analyses demonstrated the heterogeneity of liver macrophages in both the healthy liver (169) and the fibrotic liver (110). In the human cirrhotic liver, single-cell RNA-sequencing analysis identified TREM2+CD9+ macrophages as the scar-associated subpopulation. Interestingly, an RNA trajectory analysis suggested that the scar-associated macrophage subpopulation originated from circulating monocytes and that this infiltrating macrophage population contributed to fibrosis progression.
All of these studies identified fibrosis-associated macrophage subpopulations independently, so any relationships between these cell populations are unclear. In addition, the mechanisms by which these subpopulations induced HSC activation and fibrosis are still undefined. Further studies of the specific fibrogenic mediators produced by these macrophage subpopulations is needed.
5. Neutrophils
Neutrophils are innate immunity cells that can capture invading pathogens via phagocytosis and neutrophil extracellular traps (NETs) (170). Neutrophils are generally known to exacerbate inflammation, and thereby contribute to fibrosis progression (171–173). However, neutrophils also have the ability to alleviate liver inflammation and fibrosis. In a NASH mouse model, the anti-inflammatory role of neutrophils was mediated through miR-223 (174). This finding supports research indicating that neutrophils are crucial contributors to the resolution of inflammation in various tissues, including heart, skin, and joints (175–178). In addition, an anti-fibrotic influence of neutrophils was mediated through MMP production in both carbon tetrachloride- and bile-duct ligation-induced liver fibrosis (179, 180). The heterogeneity of neutrophils and the distinct roles of different neutrophil subpopulations in inflammation and fibrosis can explain the opposing actions of neutrophils.
There is an emerging concept that neutrophils can be functionally divided as either N1 or N2, mirroring the M1/M2 and Th1/Th2 classifications (181, 182). A consensus for this N1/N2 concept is growing, especially in the field of cancer research, where N1 and N2 cells have anti- and pro-tumorigenic functions, respectively (181). The presence of TGFβ in a tumor microenvironment prevents the generation of anti-tumorigenic, N1 cells (183). This is also compatible with the idea that TGFβ is a potent fibrosis promoter, an environment generally considered to be pro-tumorigenic. In addition, N2 neutrophils can secrete pro-fibrogenic factors, including OSM (139, 184), but the precise mechanisms for N1/N2 induction, and their roles in liver fibrosis, are still unknown.
6. The adaptive immune system
Histologically, T and B cells are both abundant in chronically injured liver. These cells are also closely associated with HSCs, suggesting an interaction between these cellular components in liver fibrosis (185). The critical role of adaptive immunity has long been identified in chronic liver infections, including viral hepatitis and parasitic infections (186). Although the adaptive immune system plays an anti-microbial role, the contribution of adaptive immunity to fibrosis has also been investigated. B cells have been identified as a pro-fibrogenic cellular component in liver fibrosis, and mice deficient in B cells showed attenuated liver fibrosis induced by carbon tetrachloride treatment (187). B cell-derived soluble factors are thought to activate HSCs in an antibody- and T cell-independent manner. After liver injury, HSC-derived retinoic acid activated B cells via MyD88 and led to chemoattraction of inflammatory monocytes (188), so B cells may regulate early-stage fibrosis by regulating HSC-monocyte interactions.
The contribution of T cells has long been explained by the Th1/Th2 theory, where Th1 and Th2 cells are anti- and pro-fibrogenic, respectively (189). For example, Th2 cells produce fibrogenic cytokines, including IL-4 and IL-13. IL-13 can activate HSCs through IL-13 receptors, but independently of TGFβ (190). The Th2-mediated fibrotic response also seems to be important in parasitic infection-induced liver fibrosis (190). In contrast, Th1 cell-related cytokines (e.g., IFNγ and IL-12) are anti-fibrotic (191, 192). This classical Th1/Th2 theory has been revisited by recent findings that another T cell subset plays a critical role in liver fibrosis. Th17 cells are increased in chronically injured liver (193) and induced in a IL-6- and TGFβ-dependent manner (194, 195). The fibrogenic role of Th17 cells was delineated using IL-17 receptor-deficient mice. IL-17 produced from Th17 cells activated HSCs via STAT3 (197). IL-17 receptor was also expressed on KCs (196) and cholangiocytes, suggesting that IL-17 has pleotropic functions in liver fibrosis. Th17 cells also participated in the production of IL-22 that has a regenerative role in hepatocytes after liver injury (197, 198). These myriad roles of Th17 cells in liver fibrosis are not fully understood.
Regulatory T cells (Tregs) are also involved in the pathogenesis of liver fibrosis. Tregs negatively regulate bile-duct ligation-induced fibrosis (199) and parasitic infection-induced fibrotic granuloma formation (200). In contrast, IL-8 producing Tregs promote liver fibrosis in chronic hepatitis C (201). These controversial observations suggest that the role of Tregs in liver fibrosis can be diverse and context-dependent. Interestingly, Treg phenotypes are regulated by ECM components, including HA. HMW-HA induces FOXP3 and IL-10 induction in T cells via CD44 clustering, leading to the induction of Tregs (202, 203). A profibrogenic role for cytotoxic CD8+ T cells has also been suggested, and this influence was antagonized by IL-10 treatment (204).
In addition, other non-conventional T cell subsets, including NKT cells (205, 206), γδT cells (207) also have roles in liver fibrosis. The roles played by T cells in liver fibrosis are still not fully understood, and each subset’s role might depend on the specific etiology and staging of fibrosis.
7. Liver sinusoidal endothelial cells
Liver sinusoidal endothelial cells (LSECs) are another critical component that affects liver fibrosis (Table 3). LSECs are specialized endothelial cells that form the hepatic sinusoids, where blood flow comes from both the portal vein and the hepatic artery, and outflows are to the central vein. The morphologically unique features of LSECs are the presence of fenestrae (non-diaphragmed pores that traverse the cytoplasm), and the lack of a basement membrane. These features allow solutes to be exchanged between the sinusoid lumen and the space of Disse. During fibrosis progression, LSEC fenestrae are diminished, through a process called ‘capillarization’, preventing the exchange of substances and oxygen between liver parenchyma and sinusoidal blood. Another functional feature of LSECs is a high capacity for endocytosis (208), which can eliminate ECM components that include HA, chondroitin sulfate, and both type I and type III pro-collagens via scavenger receptors (e.g., stabilin-1 and stabilin-2) (47, 209–211). However, LSECs lose the capacity for endocytosis in the fibrotic liver, resulting in detrimental ECM accumulation there (47) and in other organs such as the kidney (212).
7.1. LSEC interactions with immune cells
The anatomical proximity of sinusoidal cells - LSECs, HSCs (residing in the space of Disse), and KCs (attached to LSECs in the sinusoid lumen) - allows for interactions among these cells.
LSECs in the healthy liver prevent HSC activation via nitric oxide (NO) production. In contrast, the defenestrated and capillarized LSECs of the fibrotic liver lose their ability to suppress HSC activation (213). The transcription factor KLF2 in LSECs plays a role in preventing the dysfunctional suppression of HSC activation (214, 215). NO-mediated inhibition of HSC activation might mediated through the soluble guanylate cyclase (sGC) / cyclic guanosine monophosphate (cGMP) / protein kinase G pathway (216). In support of this, the administration of Bay60–2770 (an activator of sCG) promoted the reversal of LSEC capillarization, HSC quiescence, and fibrosis regression (217).
LSECs also have the unique function of modulating KC phenotype in the liver. In the normal liver, the depletion of tissue-resident KCs allows liver-infiltrating monocytes to differentiate into macrophages with a KC-like phenotype (151). During this process, LSEC-derived DLL4, a Notch ligand, and TGFβ contribute to the maintenance of KC identity (152). Thus, signals from LSECs are critical for maintaining KC function in the liver. The contribution of LSEC-KC interactions to the pathogenesis of liver fibrosis is still to be elucidated. LSECs also contribute to fibrosis by regulating immune responses via the attachment, migration, and infiltration of both neutrophils and lymphocytes (218).
In a congenital liver disease model induced by partial central-vein ligation, the mechanical stretch of LSECs induced CXCL1 expression via the Notch and Peazo channel pathways of LSECs, and a CXCL1-dependent recruitment of neutrophils led to fibrosis progression (219). Whether these mechanisms are common in other fibrosis models needs to be investigated.
LSECs express CXCL16, a cell membrane-bound ligand for CXCR6, and CXCR6 regulates the number of NKT cells that patrol as part of intravascular immune surveillance in hepatic sinusoids (206). LSECs also regulate the adaptive immune system via antigen presentation and leukocyte recruitment (220).
7.2. The role of vascular endothelial growth factor (VEGF)
The LSEC phenotype in a healthy liver is maintained by vascular endothelial growth factor (VEGF)-A produced by various cells, including HSCs, hepatocytes (221), and macrophages (222). There are paradoxical features for the role of VEGF in the fibrotic liver. VEGF promotes HSC proliferation and activation (223), but VEGF derived from macrophages may also be required for fibrosis resolution (222, 224). The genetic ablation of VEGF in macrophages, and the pharmacological inhibition of VEGFR2 signaling prevented the angiogenic response, and the resolution of liver fibrosis, induced by carbon tetrachloride treatment and bile-duct ligation (222). VEGF inhibition also reduced LSEC permeability and recruitment of scar-associated macrophages that contribute to fibrosis resolution via CXCL9 and MMP13 (224).
7.3. The fibrosis-specific phenotype of LSECs
A subpopulation of LSECs, the fibrosis-specific phenotype is a key regulator of the fibrotic response in injured liver. After liver injury, LSECs secrete angiogenic factors (e.g., hepatocyte growth factor and Wnt2) that promote liver regeneration. These angiogenic factors are produced via inhibitor of DNA-binding protein 1 (ID1) in a CXCR7 (a CXCL12 receptor)-dependent manner (225). During chronic liver injury, the sustained activation of FGFR1 in LSECs increases the expression of CXCR4 (another CXCL12 receptor) and interferes with protective and regenerative CXCR7 signaling; shifting to an overall fibrogenic response (225). Indeed, LSEC-specific ablation of either FGFR1 or CXCR4 in mice restored pro-regenerative pathways. Thus, the balance between two CXCL12 receptors (CXCR4 and CXCR7) on LSECs regulated the regenerative and fibrogenic responses (225). However, genetic ablation (226) or administration of an inhibitor of CXCR4 (227, 228) in a fibrosis mouse model not only failed to improve, but even worsened fibrosis. Considering that endothelial cell-specific deletion of CXCR4 improved fibrosis (225), cell-specific blockade of the CXCR4 pathway is warranted as an approach for treating liver fibrosis. Single-cell RNA-sequencing of human fibrotic livers identified an expanded fibrosis-specific endothelial cell population in cirrhotic livers restricted to the scar area (110). In this population, CD34+PLVAP+VWA1+ endothelial cells showed increased expression of pro-fibrogenic genes, including PDGFD, PDGFB, and CD34+PLVAP+ACKR1+ endothelial cells enhanced leukocyte transmigration.
In summary, LSEC functions are unique for maintaining liver homeostasis: endocytosis of ECM, maintenance of HSC quiescence, and KC-mediated immune tolerance. Disruption of their homeostatic functions in chronic injury promotes fibrosis progression. In addition, a fibrosis-specific LSEC phenotype is observed around scars, aggressively promoting fibrosis via HSC activation and immune cell recruitment. Compared to myofibroblasts and immune cells, little is known about the fibrosis-specific role of LSECs. Modulation of the LSEC fibrosis-specific phenotype could be an effective anti-fibrotic approach.
8. Conclusions
Fibrosis is not a fixed and inactive ‘scar’, but a dynamically active response regulated by many factors. Myofibroblasts, LSECs, and immune cells cooperatively produce ECM during fibrosis progression (Figure 1). The ECM that is produced and deposited in turn modulates the fibrogenic phenotypes of those cells. Recent single-cell RNA sequencing and proteomic technologies are facilitating a more comprehensive understanding of the heterogeneity and identity of fibrosis-specific subpopulations of mesenchymal cells, endothelial cells, immune cells, and ECM. Those new findings may lead to novel and unique concepts around the idea that fibrosis-specific types of cells and various ECM components interact closely to create a unique profibrotic microenvironment around fibrotic scars (a fibrotic niche). To fully understand the cellular communications of each subpopulation in situ, information not only about cell types and gene or protein expressions is necessary, but also specific location information for these cellular components within the liver is important. It is still challenging to obtain positional information using current single-cell RNA-sequencing and proteomics approaches. A combination of these novel technologies with classical histological analyses is one of the attractive approaches to tackle this issue in future studies. Another important approach to improve our understanding of fibrosis niche is carbohydrate analysis. The cellular communication between cells and their environment is modulated by the complex carbohydrate structures of glycosaminoglycan, which would not be indicated from RNA-sequencing or proteomics approaches. Emerging techniques in the glycobiology field could add the previously unknown regulatory mechanism of fibrosis niche. Further understanding of this fibrotic niche and the identification of its induction and persistence mechanisms may lead to novel and effective anti-fibrotic therapies.
Highlights.
Despite significant unmet medical needs for liver fibrosis, its molecular mechanisms are not fully understood.
Mesenchymal, immune, and endothelial cells cooperate to regulate the production, degradation, deposition, and remodeling of the extracellular matrix (ECM), leading to the development of liver fibrosis.
The dynamic processes of ECM production, degradation, deposition, and remodeling in turn regulate the immune and vascular systems, and activation of mesenchymal cells in liver fibrosis.
The cellular and ECM components of chronically injured liver are heterogenous and contain fibrosis-specific subpopulations.
The interplay between fibrosis components creates a unique ‘fibrotic niche’ microenvironment; a promising target for treating liver fibrosis.
Acknowledgements
Financial Support:
This work is supported by NIH grants R01DK085252, R01AA027036, P01CA233452 and Cedars-Sinai Medical Center (Cedars-Sinai Cancer-Center for Integrated Research in Cancer and Lifestyle Award).
Abbreviations
- ADAMTS1
ADAM metallopeptidase with thrombospondin type 1 motif 1
- α-SMA
alpha smooth muscle actin
- ECM
extracellular matrix
- eNOS
endothelial NO synthetase
- DAMPs
damage-associated molecular patterns
- DDRs
discoidin domain receptors
- GPCRs
G-protein coupled receptors
- HA
Hyaluronic acid
- HAS-2
Hyaluronic acid synthase-2
- HMGB-1
High mobility group box 1
- HMW-HA
high molecular weight hyaluronic acid
- HSCs
hepatic stellate cells
- KC
Kupffer cell
- LPS
lipopolysaccharide
- LSEC
liver sinusoidal endothelial cells
- LMW-HA
low molecular weight hyaluronic acid
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinases
- NASH
non-alcoholic steatohepatitis
- NETs
neutrophil extracellular traps
- NO
nitric oxide
- OSM
Oncostatin M
- OSR1
Odd-Skipped Related Transcription Factor 1
- PAK
p21-activated kinase
- PAMPs
pathogen-associated molecular patterns
- PDGF
platelet derived growth factor
- ROCK1
Rho-associated protein kinase 1
- RSG5
Regulator of G-protein signaling 5
- sGC
soluble guanylate cyclase
- TLR
toll-like receptor
- TREM1
triggering receptor expressed on myeloid cells 1
- WT1
Wilms tumor 1
- YAP
yes-associated protein
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asrani SK, Devarbhavi H, Eaton J, and Kamath PS. Burden of liver diseases in the world. Journal of hepatology. 2019;70(1):151–71. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataller R, and Brenner DA. Liver fibrosis. The Journal of clinical investigation. 2005;115(2):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki E, and Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology (Baltimore, Md). 2015;61(3):1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantz C, Stewart KM, and Weaver VM. The extracellular matrix at a glance. Journal of cell science. 2010;123(Pt 24):4195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnans C, Chou J, and Werb Z. Remodelling the extracellular matrix in development and disease. Nature reviews Molecular cell biology. 2014;15(12):786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JM, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. American journal of physiology Gastrointestinal and liver physiology. 2015;308(10):G807–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gressner AM, and Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. Journal of cellular and molecular medicine. 2006;10(1):76–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benyon RC, and Iredale JP. Is liver fibrosis reversible? Gut. 2000;46(4):443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massey VL, Dolin CE, Poole LG, Hudson SV, Siow DL, Brock GN, et al. The hepatic “matrisome” responds dynamically to injury: Characterization of transitional changes to the extracellular matrix in mice. Hepatology (Baltimore, Md). 2017;65(3):969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmouliere A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Laboratory investigation; a journal of technical methods and pathology. 1997;76(6):765–78. [PubMed] [Google Scholar]
- 12.Martinez-Hernandez A The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Laboratory investigation; a journal of technical methods and pathology. 1985;53(2):166–86. [PubMed] [Google Scholar]
- 13.Rojkind M, Giambrone MA, and Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76(4):710–9. [PubMed] [Google Scholar]
- 14.Rojkind M, and Ponce-Noyola P. The extracellular matrix of the liver. Collagen and related research. 1982;2(2):151–75. [DOI] [PubMed] [Google Scholar]
- 15.Aycock RS, and Seyer JM. Collagens of normal and cirrhotic human liver. Connective tissue research. 1989;23(1):19–31. [DOI] [PubMed] [Google Scholar]
- 16.Hemmann S, Graf J, Roderfeld M, and Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. Journal of hepatology. 2007;46(5):955–75. [DOI] [PubMed] [Google Scholar]
- 17.Kagan HM, and Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. Journal of cellular biochemistry. 2003;88(4):660–72. [DOI] [PubMed] [Google Scholar]
- 18.Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, et al. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. Journal of hepatology. 2005;43(3):499–507. [DOI] [PubMed] [Google Scholar]
- 19.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nature medicine. 2010;16(9):1009–17. [DOI] [PubMed] [Google Scholar]
- 20.Burt AD, Griffiths MR, Schuppan D, Voss B, and MacSween RN. Ultrastructural localization of extracellular matrix proteins in liver biopsies using ultracryomicrotomy and immuno-gold labelling. Histopathology. 1990;16(1):53–8. [DOI] [PubMed] [Google Scholar]
- 21.Dolmazashvili E, Abutidze A, Chkhartishvili N, Karchava M, Sharvadze L, and Tsertsvadze T. Regression of liver fibrosis over a 24-week period after completing direct- acting antiviral therapy in patients with chronic hepatitis C receiving care within the national hepatitis C elimination program in Georgia: results of hepatology clinic HEPA experience. European journal of gastroenterology & hepatology. 2017;29(11):1223–30. [DOI] [PubMed] [Google Scholar]
- 22.Pares A, Caballeria J, Bruguera M, Torres M, and Rodes J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. Journal of hepatology. 1986;2(1):33–42. [DOI] [PubMed] [Google Scholar]
- 23.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105–17. [DOI] [PubMed] [Google Scholar]
- 24.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122(5):1303–13. [DOI] [PubMed] [Google Scholar]
- 25.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Flejou JF, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. The New England journal of medicine. 2001;344(6):418–23. [DOI] [PubMed] [Google Scholar]
- 26.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. Journal of immunology (Baltimore, Md : 1950). 2007;178(8):5288–95. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):E3186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mak KM, and Mei R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anatomical record (Hoboken, NJ : 2007). 2017;300(8):1371–90. [DOI] [PubMed] [Google Scholar]
- 29.Hahn E, Wick G, Pencev D, and Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire RF, Bissell DM, Boyles J, and Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology (Baltimore, Md). 1992;15(6):989–97. [DOI] [PubMed] [Google Scholar]
- 31.Wisse E, De Zanger RB, Charels K, Van Der Smissen P, and McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology (Baltimore, Md). 1985;5(4):683–92. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Aziz G, Lebeau G, Rescan PY, Clement B, Rissel M, Deugnier Y, et al. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. The American journal of pathology. 1990;137(6):1333–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman SL, Roll FJ, Boyles J, Arenson DM, and Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. The Journal of biological chemistry. 1989;264(18):10756–62. [PubMed] [Google Scholar]
- 34.Bissell DM, Caron JM, Babiss LE, and Friedman JM. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Role of basement-membrane matrix. Molecular biology & medicine. 1990;7(2):187–97. [PubMed] [Google Scholar]
- 35.Bissell DM, Arenson DM, Maher JJ, and Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. The Journal of clinical investigation. 1987;79(3):801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirayama C, Suzuki H, Takada A, Fujisawa K, Tanikawa K, and Igarashi S. Serum type IV collagen in various liver diseases in comparison with serum 7S collagen, laminin, and type III procollagen peptide. Journal of gastroenterology. 1996;31(2):242–8. [DOI] [PubMed] [Google Scholar]
- 37.Mak KM, Chen LL, and Lee TF. Codistribution of collagen type IV and laminin in liver fibrosis of elderly cadavers: immunohistochemical marker of perisinusoidal basement membrane formation. Anatomical record (Hoboken, NJ : 2007). 2013;296(6):953–64. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzini S, Bird TG, Boulter L, Bellamy C, Samuel K, Aucott R, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59(5):645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64(12):1949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura Y, Matsui S, Miyata N, Harada K, Kikkawa Y, Ohmuraya M, et al. Differential expression of Lutheran/BCAM regulates biliary tissue remodeling in ductular reaction during liver regeneration. eLife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall TJ, Dolman GE, Duff CM, Paish EC, Zaitoun A, Irving W, et al. Hepatic elastin content is predictive of adverse outcome in advanced fibrotic liver disease. Histopathology. 2018;73(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao W, Yang A, Chen W, Wang P, Liu T, Cong M, et al. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochimica et biophysica acta Molecular basis of disease. 2018;1864(4 Pt A):1129–37. [DOI] [PubMed] [Google Scholar]
- 43.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126(7):1795–808. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Yan X, Xu A, Sun Y, Wang B, Huang T, et al. Dynamics of elastin in liver fibrosis: Accumulates late during progression and degrades slowly in regression. Journal of cellular physiology. 2019;234(12):22613–22. [DOI] [PubMed] [Google Scholar]
- 45.Duca L, Floquet N, Alix AJ, Haye B, and Debelle L. Elastin as a matrikine. Critical reviews in oncology/hematology. 2004;49(3):235–44. [DOI] [PubMed] [Google Scholar]
- 46.Neuman MG, Cohen LB, and Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clinical biochemistry. 2016;49(3):302–15. [DOI] [PubMed] [Google Scholar]
- 47.Tamaki S, Ueno T, Torimura T, Sata M, and Tanikawa K. Evaluation of hyaluronic acid binding ability of hepatic sinusoidal endothelial cells in rats with liver cirrhosis. Gastroenterology. 1996;111(4):1049–57. [DOI] [PubMed] [Google Scholar]
- 48.Guechot J, Loria A, Serfaty L, Giral P, Giboudeau J, and Poupon R. Serum hyaluronan as a marker of liver fibrosis in chronic viral hepatitis C: effect of alpha-interferon therapy. Journal of hepatology. 1995;22(1):22–6. [DOI] [PubMed] [Google Scholar]
- 49.Peters L, Mocroft A, Soriano V, Rockstroh J, Rauch A, Karlsson A, et al. Hyaluronic acid levels predict risk of hepatic encephalopathy and liver-related death in HIV/viral hepatitis coinfected patients. PloS one. 2013;8(5):e64283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen JF, Christiansen KM, Staugaard B, Moessner BK, Lillevang S, Krag A, et al. Combining liver stiffness with hyaluronic acid provides superior prognostic performance in chronic hepatitis C. PloS one. 2019;14(2):e0212036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korner T, Kropf J, and Gressner AM. Serum laminin and hyaluronan in liver cirrhosis: markers of progression with high prognostic value. Journal of hepatology. 1996;25(5):684–8. [DOI] [PubMed] [Google Scholar]
- 52.Yang YM, Noureddin M, Liu C, Ohashi K, Kim SY, Ramnath D, et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Science translational medicine. 2019;11(496). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satoh T, Ichida T, Matsuda Y, Sugiyama M, Yonekura K, Ishikawa T, et al. Interaction between hyaluronan and CD44 in the development of dimethylnitrosamine-induced liver cirrhosis. Journal of gastroenterology and hepatology. 2000;15(4):402–11. [DOI] [PubMed] [Google Scholar]
- 54.Fraser JR, Laurent TC, and Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. Journal of internal medicine. 1997;242(1):27–33. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. The Journal of experimental medicine. 2011;208(7):1459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nature medicine. 2016;22(11):1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, et al. Hyaluronan facilitates transforming growth factor-beta1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. The Journal of biological chemistry. 2011;286(20):17618–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorski DJ, Petz A, Reichert C, Twarock S, Grandoch M, and Fischer JW. Cardiac fibroblast activation and hyaluronan synthesis in response to hyperglycemia and diet-induced insulin resistance. Scientific reports. 2019;9(1):1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andreichenko IN, Tsitrina AA, Fokin AV, Gabdulkhakova AI, Maltsev DI, Perelman GS, et al. 4-methylumbelliferone Prevents Liver Fibrosis by Affecting Hyaluronan Deposition, FSTL1 Expression and Cell Localization. International journal of molecular sciences. 2019;20(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, and Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix biology : journal of the International Society for Matrix Biology. 2016;49:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Decaris ML, Gatmaitan M, FlorCruz S, Luo F, Li K, Holmes WE, et al. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Molecular & cellular proteomics : MCP. 2014;13(7):1741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klaas M, Kangur T, Viil J, Maemets-Allas K, Minajeva A, Vadi K, et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Scientific reports. 2016;6:27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baiocchini A, Montaldo C, Conigliaro A, Grimaldi A, Correani V, Mura F, et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PloS one. 2016;11(3):e0151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 65.Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY). 2009;326(5957):1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer cell. 2003;3(6):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mundel TM, and Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvascular research. 2007;74(2–3):85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carloni V, Romanelli RG, Pinzani M, Laffi G, and Gentilini P. Expression and function of integrin receptors for collagen and laminin in cultured human hepatic stellate cells. Gastroenterology. 1996;110(4):1127–36. [DOI] [PubMed] [Google Scholar]
- 69.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nature medicine. 2013;19(12):1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olaso E, Arteta B, Benedicto A, Crende O, and Friedman SL. Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. The American journal of pathology. 2011;179(6):2894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. The Journal of clinical investigation. 2001;108(9):1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. [DOI] [PubMed] [Google Scholar]
- 73.Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nature communications. 2016;7:12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, et al. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. The Journal of biological chemistry. 2002;277(21):19206–12. [DOI] [PubMed] [Google Scholar]
- 75.Mao TK, Kimura Y, Kenny TP, Branchi A, Gishi RG, Van de Water J, et al. Elevated expression of tyrosine kinase DDR2 in primary biliary cirrhosis. Autoimmunity. 2002;35(8):521–9. [DOI] [PubMed] [Google Scholar]
- 76.Zhang XH, Yan M, Liu L, Wu TJ, Ma LL, and Wang LX. Expression of discoidin domain receptors (DDR2) in alcoholic liver fibrosis in rats. Archives of medical research. 2010;41(8):586–92. [DOI] [PubMed] [Google Scholar]
- 77.McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. The Journal of experimental medicine. 2008;205(4):915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, et al. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. The Journal of biological chemistry. 2010;285(39):30170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ooki T, Murata-Kamiya N, Takahashi-Kanemitsu A, Wu W, and Hatakeyama M. High-Molecular-Weight Hyaluronan Is a Hippo Pathway Ligand Directing Cell Density-Dependent Growth Inhibition via PAR1b. Developmental cell. 2019;49(4):590–604.e9. [DOI] [PubMed] [Google Scholar]
- 80.Wu M, Cao M, He Y, Liu Y, Yang C, Du Y, et al. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(4):1290–8. [DOI] [PubMed] [Google Scholar]
- 81.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, et al. Resolution of lung inflammation by CD44. Science (New York, NY). 2002;296(5565):155–8. [DOI] [PubMed] [Google Scholar]
- 82.Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, et al. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. American journal of respiratory cell and molecular biology. 2004;30(1):51–60. [DOI] [PubMed] [Google Scholar]
- 83.Stern R, Kogan G, Jedrzejas MJ, and Soltes L. The many ways to cleave hyaluronan. Biotechnology advances. 2007;25(6):537–57. [DOI] [PubMed] [Google Scholar]
- 84.Schuppan D, Ruehl M, Somasundaram R, and Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Seminars in liver disease. 2001;21(3):351–72. [DOI] [PubMed] [Google Scholar]
- 85.Somasundaram R, and Schuppan D. Type I, II, III, IV, V, and VI collagens serve as extracellular ligands for the isoforms of platelet-derived growth factor (AA, BB, and AB). The Journal of biological chemistry. 1996;271(43):26884–91. [DOI] [PubMed] [Google Scholar]
- 86.Paralkar VM, Vukicevic S, and Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Developmental biology. 1991;143(2):303–8. [DOI] [PubMed] [Google Scholar]
- 87.Genovese F, Barascuk N, Larsen L, Larsen MR, Nawrocki A, Li Y, et al. Biglycan fragmentation in pathologies associated with extracellular matrix remodeling by matrix metalloproteinases. Fibrogenesis & tissue repair. 2013;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamaguchi Y, Mann DM, and Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–4. [DOI] [PubMed] [Google Scholar]
- 89.Friedman SL, Roll FJ, Boyles J, and Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(24):8681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gabele E, Brenner DA, and Rippe RA. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Frontiers in bioscience : a journal and virtual library. 2003;8:d69–77. [DOI] [PubMed] [Google Scholar]
- 91.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology (Baltimore, Md). 2018;67(2):549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nature medicine. 2017;23(4):439–49. [DOI] [PubMed] [Google Scholar]
- 93.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nature biotechnology. 2008;26(4):431–42. [DOI] [PubMed] [Google Scholar]
- 94.Kawasaki K, Ushioda R, Ito S, Ikeda K, Masago Y, and Nagata K. Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. The Journal of biological chemistry. 2015;290(6):3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(21):E1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–59.e5. [DOI] [PubMed] [Google Scholar]
- 97.Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Scientific reports. 2016;6:33453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet (London, England). 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(4):1599–609. [DOI] [PubMed] [Google Scholar]
- 100.Lin N, Chen Z, Lu Y, Li Y, Hu K, and Xu R. Role of activated hepatic stellate cells in proliferation and metastasis of hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology. 2015;45(3):326–36. [DOI] [PubMed] [Google Scholar]
- 101.Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y, Zhang SZ, et al. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Molecular medicine reports. 2017;16(6):7879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borkham-Kamphorst E, and Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine & growth factor reviews. 2016;28:53–61. [DOI] [PubMed] [Google Scholar]
- 103.Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. The American journal of pathology. 1996;148(3):785–800. [PMC free article] [PubMed] [Google Scholar]
- 104.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine & growth factor reviews. 2004;15(4):255–73. [DOI] [PubMed] [Google Scholar]
- 105.Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, and Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors alpha and beta in activated rat hepatic stellate cells (HSC). Cytokine. 2005;31(5):349–57. [DOI] [PubMed] [Google Scholar]
- 106.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. Journal of hepatology. 2006;45(3):419–28. [DOI] [PubMed] [Google Scholar]
- 107.Kocabayoglu P, Lade A, Lee YA, Dragomir AC, Sun X, Fiel MI, et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. Journal of hepatology. 2015;63(1):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler N, Bomble M, Ostendorf T, et al. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Laboratory investigation; a journal of technical methods and pathology. 2008;88(10):1090–100. [DOI] [PubMed] [Google Scholar]
- 109.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, and Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. Journal of hepatology. 2007;46(6):1064–74. [DOI] [PubMed] [Google Scholar]
- 110.Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinzani M, Gesualdo L, Sabbah GM, and Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. The Journal of clinical investigation. 1989;84(6):1786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heldin CH, and Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological reviews. 1999;79(4):1283–316. [DOI] [PubMed] [Google Scholar]
- 113.Andrae J, Gallini R, and Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes & development. 2008;22(10):1276–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu Q, Klippel A, Muslin AJ, Fantl WJ, and Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science (New York, NY). 1995;268(5207):100–2. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y, Lv M, Lin H, Hong Y, Yang F, Sun Y, et al. ROCK1 induces ERK nuclear translocation in PDGF-BB-stimulated migration of rat vascular smooth muscle cells. IUBMB life. 2012;64(2):194–202. [DOI] [PubMed] [Google Scholar]
- 116.Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, and Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology (Baltimore, Md). 1999;29(6):1760–7. [DOI] [PubMed] [Google Scholar]
- 117.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, and Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. Journal of hepatology. 1999;30(1):48–60. [DOI] [PubMed] [Google Scholar]
- 118.Totaro A, Panciera T, and Piccolo S. YAP/TAZ upstream signals and downstream responses. Nature cell biology. 2018;20(8):888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skladal P, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nature communications. 2017;8:15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. [DOI] [PubMed] [Google Scholar]
- 121.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15(6):637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. Journal of hepatology. 2015;63(3):679–88. [DOI] [PubMed] [Google Scholar]
- 123.Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, and McCarthy JB. Hyaluronan, Inflammation, and Breast Cancer Progression. Frontiers in immunology. 2015;6:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cau E, and Blader P. Notch activity in the nervous system: to switch or not switch? Neural development. 2009;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li XY, Zhai WJ, and Teng CB. Notch Signaling in Pancreatic Development. International journal of molecular sciences. 2015;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei L, Al Oustah A, Blader P, and Roussigne M. Notch signaling restricts FGF pathway activation in parapineal cells to promote their collective migration. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang YC, Chen Q, Luo JM, Nie J, Meng QH, Shuai W, et al. Notch1 promotes the pericyte-myofibroblast transition in idiopathic pulmonary fibrosis through the PDGFR/ROCK1 signal pathway. Experimental & molecular medicine. 2019;51(3):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y, Zheng S, Qi D, Zheng S, Guo J, Zhang S, et al. Inhibition of Notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PloS one. 2012;7(10):e46512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nijjar SS, Crosby HA, Wallace L, Hubscher SG, and Strain AJ. Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularization. Hepatology (Baltimore, Md). 2001;34(6):1184–92. [DOI] [PubMed] [Google Scholar]
- 130.Li Y, Wang J, and Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(6):2324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Laboratory investigation; a journal of technical methods and pathology. 2003;83(2):163–73. [DOI] [PubMed] [Google Scholar]
- 132.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. Journal of hepatology. 2006;45(3):429–38. [DOI] [PubMed] [Google Scholar]
- 133.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell stem cell. 2015;16(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature communications. 2013;4:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(32):E3297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katsumata LW, Miyajima A, and Itoh T. Portal fibroblasts marked by the surface antigen Thy1 contribute to fibrosis in mouse models of cholestatic liver injury. Hepatology communications. 2017;1(3):198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mederacke I, Dapito DH, Affo S, Uchinami H, and Schwabe RF. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nature protocols. 2015;10(2):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dooley S, and ten Dijke P. TGF-beta in progression of liver disease. Cell and tissue research. 2012;347(1):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsuda M, Tsurusaki S, Miyata N, Saijou E, Okochi H, Miyajima A, et al. Oncostatin M causes liver fibrosis by regulating cooperation between hepatic stellate cells and macrophages in mice. Hepatology (Baltimore, Md). 2018;67(1):296–312. [DOI] [PubMed] [Google Scholar]
- 140.Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Arbab AS, Dzutsev A, et al. The innate immune receptor TREM-1 promotes liver injury and fibrosis. The Journal of clinical investigation. 2018;128(11):4870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55(3):415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, and Kozawa O. Tumor necrosis factor-alpha promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PloS one. 2013;8(6):e65251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tarrats N, Moles A, Morales A, Garcia-Ruiz C, Fernandez-Checa JC, and Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology (Baltimore, Md). 2011;54(1):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science (New York, NY). 2006;311(5757):83–7. [DOI] [PubMed] [Google Scholar]
- 145.Ginhoux F, and Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–49. [DOI] [PubMed] [Google Scholar]
- 146.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (New York, NY). 2012;336(6077):86–90. [DOI] [PubMed] [Google Scholar]
- 147.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nature communications. 2016;7:10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, et al. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity. 2019;51(4):655–70.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A, et al. Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cellular immunology. 2017;322:74–83. [DOI] [PubMed] [Google Scholar]
- 154.Scott CL, and Guilliams M. The role of Kupffer cells in hepatic iron and lipid metabolism. Journal of hepatology. 2018;69(5):1197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J, and Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165(3):668–78. [DOI] [PubMed] [Google Scholar]
- 156.Sica A, Invernizzi P, and Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology (Baltimore, Md). 2014;59(5):2034–42. [DOI] [PubMed] [Google Scholar]
- 157.Sica A, and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122(3):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang X, and Mosser DM. Macrophage activation by endogenous danger signals. The Journal of pathology. 2008;214(2):161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, and Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. Journal of leukocyte biology. 2006;79(2):285–93. [DOI] [PubMed] [Google Scholar]
- 160.Gordon S, and Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. [DOI] [PubMed] [Google Scholar]
- 161.Lang R, Patel D, Morris JJ, Rutschman RL, and Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. Journal of immunology (Baltimore, Md : 1950). 2002;169(5):2253–63. [DOI] [PubMed] [Google Scholar]
- 162.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nature medicine. 2010;16(4):452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40(5):720–33. [DOI] [PubMed] [Google Scholar]
- 165.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86. [DOI] [PubMed] [Google Scholar]
- 166.Wynn TA, and Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123(20):e110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541(7635):96–101. [DOI] [PubMed] [Google Scholar]
- 169.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nature communications. 2018;9(1):4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (New York, NY). 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- 171.Xu R, Huang H, Zhang Z, and Wang FS. The role of neutrophils in the development of liver diseases. Cellular & molecular immunology. 2014;11(3):224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roh YS, Zhang B, Loomba R, and Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. American journal of physiology Gastrointestinal and liver physiology. 2015;309(1):G30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou Z, Xu MJ, Cai Y, Wang W, Jiang JX, Varga ZV, et al. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cellular and molecular gastroenterology and hepatology. 2018;5(3):399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Calvente CJ, Tameda M, Johnson CD, Del Pilar H, Lin YC, Adronikou N, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. The Journal of clinical investigation. 2019;130:4091–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Science translational medicine. 2015;7(315):315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cumpelik A, Ankli B, Zecher D, and Schifferli JA. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Annals of the rheumatic diseases. 2016;75(6):1236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nishio N, Okawa Y, Sakurai H, and Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordrecht, Netherlands). 2008;30(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. European heart journal. 2017;38(3):187–97. [DOI] [PubMed] [Google Scholar]
- 179.Harty MW, Muratore CS, Papa EF, Gart MS, Ramm GA, Gregory SH, et al. Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers. The American journal of pathology. 2010;176(3):1271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saijou E, Enomoto Y, Matsuda M, Yuet-Yin Kok C, Akira S, Tanaka M, et al. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatology communications. 2018;2(6):703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang X, Qiu L, Li Z, Wang XY, and Yi H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Frontiers in immunology. 2018;9:2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Silvestre-Roig C, Hidalgo A, and Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127(18):2173–81. [DOI] [PubMed] [Google Scholar]
- 183.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16(3):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, and Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer research. 2005;65(19):8896–904. [DOI] [PubMed] [Google Scholar]
- 185.Muhanna N, Horani A, Doron S, and Safadi R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clinical and experimental immunology. 2007;148(2):338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Protzer U, Maini MK, and Knolle PA. Living in the liver: hepatic infections. Nature reviews Immunology. 2012;12(3):201–13. [DOI] [PubMed] [Google Scholar]
- 187.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. The Journal of clinical investigation. 2005;115(11):3072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thapa M, Chinnadurai R, Velazquez VM, Tedesco D, Elrod E, Han JH, et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology (Baltimore, Md). 2015;61(6):2067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature reviews Immunology. 2004;4(8):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chiaramonte MG, Donaldson DD, Cheever AW, and Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. The Journal of clinical investigation. 1999;104(6):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, et al. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology (Baltimore, Md). 2008;48(3):963–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376(6541):594–6. [DOI] [PubMed] [Google Scholar]
- 193.Hammerich L, Heymann F, and Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clinical & developmental immunology. 2011;2011:345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8(9):950–7. [DOI] [PubMed] [Google Scholar]
- 195.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, and Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. [DOI] [PubMed] [Google Scholar]
- 196.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(3):765–76.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Radaeva S, Sun R, Pan HN, Hong F, and Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology (Baltimore, Md). 2004;39(5):1332–42. [DOI] [PubMed] [Google Scholar]
- 198.Hepatoprotective Gao B. and anti-inflammatory cytokines in alcoholic liver disease. Journal of gastroenterology and hepatology. 2012;27 Suppl 2:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. Journal of immunology (Baltimore, Md : 1950). 2011;187(3):1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Turner JD, Jenkins GR, Hogg KG, Aynsley SA, Paveley RA, Cook PC, et al. CD4+CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS neglected tropical diseases. 2011;5(8):e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Langhans B, Kramer B, Louis M, Nischalke HD, Huneburg R, Staratschek-Jox A, et al. Intrahepatic IL-8 producing Foxp3(+)CD4(+) regulatory T cells and fibrogenesis in chronic hepatitis C. Journal of hepatology. 2013;59(2):229–35. [DOI] [PubMed] [Google Scholar]
- 202.Nagy N, Kaber G, Johnson PY, Gebe JA, Preisinger A, Falk BA, et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. The Journal of clinical investigation. 2015;125(10):3928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, and Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. Journal of leukocyte biology. 2009;86(3):567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127(3):870–82. [DOI] [PubMed] [Google Scholar]
- 205.Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. Journal of immunology (Baltimore, Md : 1950). 2013;190(10):5226–36. [DOI] [PubMed] [Google Scholar]
- 206.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS biology. 2005;3(4):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology (Baltimore, Md). 2014;59(2):630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology (Baltimore, Md). 2015;61(5):1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hansen B, Longati P, Elvevold K, Nedredal GI, Schledzewski K, Olsen R, et al. Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Experimental cell research. 2005;303(1):160–73. [DOI] [PubMed] [Google Scholar]
- 210.McCourt PA, Smedsrod BH, Melkko J, and Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology (Baltimore, Md). 1999;30(5):1276–86. [DOI] [PubMed] [Google Scholar]
- 211.Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, et al. Stabilin-1 and −2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. The Biochemical journal. 2002;362(Pt 1):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schledzewski K, Geraud C, Arnold B, Wang S, Grone HJ, Kempf T, et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and −2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. The Journal of clinical investigation. 2011;121(2):703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Deleve LD, Wang X, and Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology (Baltimore, Md). 2008;48(3):920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Marrone G, Maeso-Diaz R, Garcia-Cardena G, Abraldes JG, Garcia-Pagan JC, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64(9):1434–43. [DOI] [PubMed] [Google Scholar]
- 215.Marrone G, Russo L, Rosado E, Hide D, Garcia-Cardena G, Garcia-Pagan JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. Journal of hepatology. 2013;58(1):98–103. [DOI] [PubMed] [Google Scholar]
- 216.Griffith TM. Studies of endothelium-derived relaxant factor (EDRF), its nature and mode of action. European heart journal. 1985;6(1):37–49. [DOI] [PubMed] [Google Scholar]
- 217.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142(4):918–27.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shetty S, Lalor PF, and Adams DH. Lymphocyte recruitment to the liver: molecular insights into the pathogenesis of liver injury and hepatitis. Toxicology. 2008;254(3):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019;157(1):193–209.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shetty S, Lalor PF, and Adams DH. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nature reviews Gastroenterology & hepatology. 2018;15(9):555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.DeLeve LD, Wang X, Hu L, McCuskey MK, and McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. American journal of physiology Gastrointestinal and liver physiology. 2004;287(4):G757–63. [DOI] [PubMed] [Google Scholar]
- 222.Kantari-Mimoun C, Castells M, Klose R, Meinecke AK, Lemberger UJ, Rautou PE, et al. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology (Baltimore, Md). 2015;61(6):2042–55. [DOI] [PubMed] [Google Scholar]
- 223.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52(9):1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146(5):1339–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505(7481):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tsuchiya A, Imai M, Kamimura H, Takamura M, Yamagiwa S, Sugiyama T, et al. Increased susceptibility to severe chronic liver damage in CXCR4 conditional knock-out mice. Digestive diseases and sciences. 2012;57(11):2892–900. [DOI] [PubMed] [Google Scholar]
- 227.Saiman Y, Jiao J, Fiel MI, Friedman SL, Aloman C, and Bansal MB. Inhibition of the CXCL12/CXCR4 chemokine axis with AMD3100, a CXCR4 small molecule inhibitor, worsens murine hepatic injury. Hepatology research : the official journal of the Japan Society of Hepatology. 2015;45(7):794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chow LN, Schreiner P, Ng BY, Lo B, Hughes MR, Scott RW, et al. Impact of a CXCL12/CXCR4 Antagonist in Bleomycin (BLM) Induced Pulmonary Fibrosis and Carbon Tetrachloride (CCl4) Induced Hepatic Fibrosis in Mice. PloS one. 2016;11(3):e0151765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hogemann B, Edel G, Schwarz K, Krech R, and Kresse H. Expression of biglycan, decorin and proteoglycan-100/CSF-1 in normal and fibrotic human liver. Pathology, research and practice. 1997;193(11–12):747–51. [DOI] [PubMed] [Google Scholar]
- 230.Kikkawa Y, Mochizuki Y, Miner JH, and Mitaka T. Transient expression of laminin alpha1 chain in regenerating murine liver: restricted localization of laminin chains and nidogen-1. Experimental cell research. 2005;305(1):99–109. [DOI] [PubMed] [Google Scholar]
- 231.Gallai M, Kovalszky I, Knittel T, Neubauer K, Armbrust T, and Ramadori G. Expression of extracellular matrix proteoglycans perlecan and decorin in carbon-tetrachloride-injured rat liver and in isolated liver cells. The American journal of pathology. 1996;148(5):1463–71. [PMC free article] [PubMed] [Google Scholar]
- 232.Roskams T, Moshage H, De Vos R, Guido D, Yap P, and Desmet V. Heparan sulfate proteoglycan expression in normal human liver. Hepatology (Baltimore, Md). 1995;21(4):950–8. [PubMed] [Google Scholar]
- 233.Roskams T, Rosenbaum J, De Vos R, David G, and Desmet V. Heparan sulfate proteoglycan expression in chronic cholestatic human liver diseases. Hepatology (Baltimore, Md). 1996;24(3):524–32. [DOI] [PubMed] [Google Scholar]
- 234.Kanta J Elastin in the Liver. Frontiers in physiology. 2016;7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Henderson NC, and Sheppard D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochimica et biophysica acta. 2013;1832(7):891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, and Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135(2):660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Morla A, Zhang Z, and Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367(6459):193–6. [DOI] [PubMed] [Google Scholar]
- 238.Yi M, and Ruoslahti E. A fibronectin fragment inhibits tumor growth, angiogenesis, and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. The Journal of cell biology. 2009;185(4):743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. The Biochemical journal. 1994;302 ( Pt 2):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Comalada M, Cardo M, Xaus J, Valledor AF, Lloberas J, Ventura F, et al. Decorin reverses the repressive effect of autocrine-produced TGF-beta on mouse macrophage activation. Journal of immunology (Baltimore, Md : 1950). 2003;170(9):4450–6. [DOI] [PubMed] [Google Scholar]
- 242.Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S, et al. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Laboratory investigation; a journal of technical methods and pathology. 2011;91(3):439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Baghy K, Horvath Z, Regos E, Kiss K, Schaff Z, Iozzo RV, et al. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. The FEBS journal. 2013;280(10):2150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. The Journal of clinical investigation. 2005;115(8):2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nastase MV, Young MF, and Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012;60(12):963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PloS one. 2013;8(10):e75361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155(4):1140–53. [DOI] [PubMed] [Google Scholar]
- 249.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124(1):147–59. [DOI] [PubMed] [Google Scholar]
- 250.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, et al. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemporary clinical trials. 2016;47:356–65. [DOI] [PubMed] [Google Scholar]
- 251.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PloS one. 2016;11(6):e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]