Highlights
-
•
Electrodiagnostic testing is a valuable tool in evaluating plexopathies.
-
•
Clinical presentations depend on the etiology and time course of injury.
-
•
Management of plexopathies includes a multidisciplinary approach.
Keywords: Brachial plexus, Lumbosacral plexus, Electrodiagnosis, Imaging, Trauma, Inflammatory, Neoplastic, Radiation, Thoracic outlet syndrome
Abstract
Diseases of the brachial and lumbosacral plexus are uncommon and complex. The diagnosis of plexopathies is often challenging for the clinician, both in terms of localizing a patient’s symptoms to the plexus as well as determining the etiology. The non-specific clinical features and similar presentations to other root, nerve, and non-neurologic disorders emphasize the importance of a high clinical index of suspicion for a plexopathy and comprehensive clinical evaluation. Various diagnostic tests, including electrodiagnostic (EDX) studies, neuroimaging (including ultrasound, MRI, or PET), serologic studies, and genetic testing, may be used to confirm a plexopathy and assist in identifying the underlying etiology. EDX testing plays an important role in confirming a plexopathy defining the localization, pathophysiology, chronicity, severity, and prognosis. Given the complexity of the plexus anatomy, multiple common and uncommon NCS and an extensive needle examination is often required, and a comprehensive, individualized approach to each patient is necessary. Treatment of plexopathies often focuses on symptomatic management although, depending on the etiology, specific targeted treatments may improve outcome. This article reviews the clinical features, EDX approaches, and evaluation and treatment of brachial and lumbosacral plexopathies.
1. Introduction
The brachial and lumbosacral plexi are complex peripheral nervous system structures that serve the upper and lower limbs. While the plexi are not the direct source of the neurons serving the extremities, they contain the axons of neurons that originate in the anterior horn cells, sympathetic and parasympathetic ganglia, or sensory receptors that are connecting the central nervous system with the sensory and motor end organs. Direct trauma or diseases may injure the nerves within plexi, either in isolation or as part of involvement of the peripheral nervous system more diffusely. Disorders involving the plexi are much less common than those of other focal peripheral nervous system sites, such as radiculopathies or mononeuropathies, but the clinical presentations may have similarities. Therefore, the evaluating and treating physician must, first and foremost, maintain a high clinical suspicion for a plexopathy in any patient presenting with upper or lower limb neuropathic symptoms.
The diagnosis of plexopathies may pose challenges for the clinician, both in terms of localizing a patient’s symptoms to the plexus as well as determining the etiology. Many types of conditions can involve the brachial and lumbosacral plexus, ranging from direct penetrating or compressive trauma to immune-mediated, inflammatory, or metabolic disorders to direct or indirect effects of cancer or its treatment to other structural conditions. Various diagnostic tests, including EDX testing, neuroimaging (ultrasound, computed tomography, MRI, or PET), laboratory testing, and genetic testing, may be used to complement a comprehensive and thorough clinical assessment and assist in identifying etiologies.
This article reviews the brachial and lumbosacral plexus, including the anatomy, EDX and neuroimaging studies used in the evaluation of plexopathies, the clinical manifestations of diseases involving the plexi, and the management of brachial and lumbosacral plexopathies.
2. General pathogenesis of diseases affecting plexus
The type and degree of nerve injury within the brachial and lumbosacral plexus depends on the mechanism and severity of injury. The degree of nerve injury has been classified into three primary stages based on whether the injury involves only the myelin sheath, the axon, or the structures that support the axon (Sunderland, 1990). In mild or early lesions, such as occur with nerve compression, distortion of the myelin may be the only pathologic alteration, referred to as “neuropraxia. This pathologic change cannot be identified clinically; however NCS may show focal slowing, conduction block or increased temporal dispersion across the site of compression. Since the axons themselves are not injured, recovery may occur rapidly if the compression is removed, and the prognosis is overall favorable. Unfortunately, most injuries to the plexus are more severe and injure the axons and often the supporting structures, called “axonotmesis” or “neurotmesis”. In these cases, Wallerian degeneration of the nerve segments results in prolonged effective recovery and portends a less favorable prognosis.
3. The brachial plexus
3.1. Anatomy
The brachial plexus is the intertwining group of nerves coursing through the neck, shoulder, and axilla. The brachial plexus is derived from the distal root components in the neck and extends into the axilla. As it courses through the shoulder and upper arm, the brachial plexus divides into roots, trunks, divisions, cords, and terminal nerves (Fig. 1). The axons that course through the plexus innervate all muscles in the upper extremity and supply sensation to the entire upper limb. When evaluating patients with suspected brachial plexopathy, a solid knowledge of the anatomy of each of the components and the innervation of individual muscles by specific trunks and cords is essential for the physician to be able to reliably localize a lesion (Table 1).
Fig. 1.
The brachial plexus. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
Table 1.
Innervation of upper limb muscles by brachial plexus trunk, cord, and terminal nerve.
| Upper Trunk (C5-6) | Middle Trunk (C6-7–8) | Lower Trunk (C8-T1) | ||
|---|---|---|---|---|
| Levator scapulae (DSc) Rhomboid major (DSc) Serratus anterior (LgTh) |
Supraspinatus (SSc) Infraspinatus (SSc) |
|||
| Lateral cord | Biceps (MC) Brachialis (MC) Coracobrachialis (MC) Pectoralis major (clavicular) (LPect) |
(Coracobrachialis) (MC) Pronator teres (M) Flexor carpi radialis (M) |
||
| Posterior cord | Brachioradialis (R) Brachialis (MC,R) Supinator (R) Deltoid (Ax) Latissimus dorsi (ThD) Teres major (SubSc) Teres minor (Ax) (Triceps) (R) |
Triceps (R) Anconeus (R) Extensor carpi radialis (R) Extensor digiti communis (R) Extensor carpi ulnaris (R) Latissimus dorsi (ThD) |
Extensor indicis proprius (R) Extensor carpi ulnaris (R) Extensor pollicis longus (R) Extensor digitorum communis (R) Extensor carpi ulnaris (R) Latissimus dorsi (ThD) (Triceps) (R) |
|
| Medial cord | Abductor pollicis brevis (M) Opponens pollicis (M) First dorsal interosseous (U) Abductor digiti minimi (U) Flexor carpi ulnaris (U) Flexor digitorum profundus (M,U) Flexor pollicis longus (M) Pectoralis major (sternal) (MPect) Pronator quadratus (M) |
Ax – axillary nerve; DSc – dorsal scapular nerve; LgTh – long thoracic nerve, LPect – lateral pectoral nerve; M – median nerve; MPect – medial pectoral nerve; MC – musculocutaneous nerve; R – radial nerve; U – ulnar nerve; SSc – suprascapular nerve; SubSc – subscapular nerve; ThD – thoracodorsal nerve.
3.1.1. Roots
The brachial plexus is derived from the anterior primary rami of the C5 through T1 roots in most individuals. Approximately 5% of individuals have contributions from the C4 (“pre-fixed”) or T2 (“post-fixed”) roots. Each individual root divides into a posterior primary ramus, which does not course through the plexus but innervate the paraspinal muscles, and a ventral primary ramus, which course into the plexus. Although not technically part of the “plexus”, the roots contain the fibers connecting the spinal cord and plexus and in some disorders, such as trauma, infiltrative diseases, radiation injury, or inflammatory disorders, may be injured along with other segments of the plexus.
Since the roots are shorter, unprotected by adherent dura or epineurium, and are less “interwoven” within the nerve sheath, they are more susceptible to traction injury than the plexus. Several individual nerves branch directly off of the roots prior to the formation of trunks of the brachial plexus, including the: (1) long thoracic nerve to the serratus anterior muscle (from the C5-7 roots), (2) dorsal scapular nerve to the rhomboid muscles and levator scapulae (from the C4-5 roots), (3) the nerve to the subclavian muscle (from the C5-6 roots), and (4) phrenic nerve (from the C3-5 roots) to the diaphragm.
3.1.2. Trunks
“The anterior primary rami of the roots course distal in the neck and join to form three trunks in the supraclavicular fossa at the lateral border of the anterior and medial scalene muscles: upper (formed by the C5 and C6 roots), middle (formed predominantly by the C7 root with some contribution from C6 and C8), and lower (formed by the C8 and T1 roots) (Fig. 1). The trunks are located in a relatively superficial region in the lower anterior aspect of the posterior triangle in the neck, thereby increasing their susceptibility to traction and penetrating injuries. The suprascapular nerve (innervating the supraspinatus and infraspinatus) branches directly off of the upper trunk” (Rubin, 2008).
3.1.3. Divisions
Each of the trunks divides into two primary divisions – an anterior and posterior division – just beneath the clavicle (Fig. 1). Lesions that involve the roots and/or trunks (proximal to the divisions) are considered to be “supraclavicular” lesions, and those that involve the cords or terminal nerves (distal to the divisions) are considered to be “infraclavicular” lesions. Distinction between supraclavicular and infraclavicular lesions has important implications in etiology and potential severity and prognosis. “Supraclavicular plexopathies are more common than infraclavicular plexopathies, and are less likely to demonstrate complete recovery in severe injury than infraclavicular lesions. Although most etiologies of plexopathies can affect any region of the plexus, some disorders are more likely to affect the supraclavicular (e.g. stretch injuries, thoracic outlet syndrome, post-sternotomy surgery, neoplasms) or infraclavicular portions (e.g. humeral head fractures, penetrating injuries, radiation, neurovascular injuries)” (Rubin, 2008).
3.1.4. Cords and terminal nerves
“The divisions from the upper, middle, and lower trunks join to form three cords – the lateral, medial, and posterior cords - at the level of the proximal axilla (Fig. 1). The cords are named according to their anatomic relationship to the second portion of the axillary artery, which they surround. The cords are the longest component of the brachial plexus. Each cord terminates in one or more individual nerves” (Rubin, 2008).
The main terminal branches of the brachial plexus are the nerves serving the upper extremity. These include those arising from the medial cord (medial antebrachial cutaneous nerve, ulnar nerve, and medial portion of the median nerve), the lateral cord (musculocutaneous nerve and lateral portion of the median nerve), and posterior cord (axillary nerve, radial nerve, and thoracodorsal nerve). The medial pectoral nerve is also a branch of the medial cord, and the lateral pectoral nerve of the anterior divisions of the upper and middle trunks or lateral cord; these nerves innervate the pectoralis major and minor muscles.
3.1.5. Neighboring structures
“The brachial plexus is a vulnerable structure due to its length and relationship to surrounding structures. The lung apex, lymph nodes, bones (clavicle and ribs), and major vessels may all be sites of disease which may primarily or secondarily extend toward and involve the brachial plexus. In addition, the plexus is susceptible to injury by traction due to mobility of the neighboring shoulder joint, shoulder girdle, and neck” (Rubin, 2008).
3.2. Clinical features of brachial plexopathies
The clinical features of brachial plexopathies depend on the temporal course of the disease, site of involvement, and etiology. Furthermore, the symptoms and signs are not specific to the plexus and may be seen with other neurogenic (e.g. roots or individual nerves) and non-neurogenic disorders (Table 2).
Table 2.
Disorders that May Mimic Brachial Plexopathy.
| Brachial Plexopathy |
|---|
| Cervical radiculopathy |
| Upper extremity mononeuropathy |
| Mononeuritis multiplex |
| Multifocal motor neuropathy with conduction block |
| Amyotrophic lateral sclerosis |
| Cervical cord lesion (e.g transverse myelitis) |
| Orthopedic (shoulder) disorders (e.g. rotator cuff injury, acute calcific tendinitis, adhesive capsulitis) |
3.2.1. Timing of injury
Symptoms of brachial plexopathy can present with rapid onset (such as immediately following a high speed vehicular accident or other traumatic injury), subacutely (such as over days to weeks in neuralgic amyotrophy), or slowly (such as over months to years in neurogenic thoracic outlet syndrome or radiation-induced injury). Regardless of the timing, most patients have variable degrees of pain, sensory disturbance, and weakness.
3.2.2. Pain
Pain is a common and prominent symptom in patients with brachial plexopathies. The pain may be severe, and may be experienced as a “deep”, “aching”, or “burning” quality. The location of the pain reflects the portion of the plexus injured and often involves the shoulder or upper arm in upper trunk injuries and the distal arm or hand in lower trunk injuries, although patients may not be able to distinctly localize the pain. In some conditions, such as Parsonage Turner syndrome, pain may be worsened by movement of the arm. Unlike radiculopathies, maneuvers that increase intracranial pressure, such as Valsalva, do not typically worsen pain.
3.2.3. Sensory loss and paresthesias
Loss of sensation or positive sensory symptoms (e.g. tingling, prickling, or other sensory symptoms) is common, but is often overshadowed by pain. The distribution of sensory loss reflects the site(s) of injury to the plexus.
3.2.4. Weakness
One of the most devastating manifestations of brachial plexopathies is weakness, which may be severe and significantly impact the patient’s ability to functionally use the arm. The distribution of weakness reflects the site of plexus involvement and may be highly localized or patchy and incomplete. Injury to the upper trunk usually manifests with proximal arm weakness, whereas lower trunk lesions involve the hand. Depending on the pathophysiology, severity, and timing of the clinical examination in relationship to the onset of symptoms, atrophy may also be present.
3.2.5. Non-neuromuscular features
Other non-neuromuscular features may be present in patients with some types of brachial plexopathies, such as a Horner’s syndrome in lower trunk plexopathies or those involving the T1 root.
3.3. General evaluation of brachial plexopathies
3.3.1. Clinical history and examination
The evaluation of patients with suspected brachial plexopathies begins with a comprehensive history and examination. The patient should be asked about the timing of symptom onset, such as whether the symptoms began abruptly over minutes, subacutely over hours or days, or chronically over weeks to months. Other important historical features include a history of trauma, recent immunizations, and co-morbid medical conditions including cancer or a history of radiation to the chest or shoulder region, and the presence of systemic medical diseases. While hereditary brachial plexopathies are rare, the patient should be questioned about the presence of plexopathies (or unusual radiculopathies, which may be misdiagnosed plexopathies).
The clinical examination should include a detailed motor and sensory examination, paying special attention to the distribution of weakness, reflex changes, and sensory loss in the arm. The presence of a Horner’s syndrome, supraclavicular Tinel’s sign, or mass or fullness in the supraclavicular region may be additional clues to localization to the brachial plexus.
Since clinical symptoms and signs are not specific to brachial plexopathies and can occur with radiculopathies or single or multiple mononeuropathies, other ancillary tests, including EDX studies, are usually necessary to assist in the confirmation of a brachial plexopathy (Wilbourn, 1985). Furthermore, determining the etiology of brachial plexopathies may require additional tests, such as imaging of the plexus.
3.3.2. Electrodiagnostic testing
EDX testing utilizing a combination of nerve conduction studies (NCS) and needle electromyography (EMG) is a valuable and necessary component of the evaluation of brachial plexopathies. EDX testing helps to (1) confirm localization to the brachial plexus while excluding (or identifying) radiculopathies or mononeuropathies, (2) identify the segment(s) of the plexus involved, (3) define the pathophysiology of nerve injury (e.g. axonal or demyelinating), (4) determine the degree of axon loss, and (5) assess for evidence of reinnervation or recovery of the nerves. While in most cases EDX testing does not determine the etiology of the plexopathy, occasionally specific findings may point towards a possible cause. In evaluating a brachial plexopathy, both NCS and needle EMG provide these types of information (Ferrante, 2012a, Strakowski, 2013).
The distribution of EDX findings may be focal and precisely determine the site of involvement within the plexus, such as the lower trunk in neurogenic thoracic outlet syndrome, or patchy and variable within the plexus or its branches, such as in Parsonage Turner syndrome. Therefore, thorough and extensive EDX testing is often necessary in patients with brachial plexopathies. Table 1, Table 3 can be used as guides to selecting tests and understanding the pattern of findings based on localization (Table 1, Table 3).
Table 3.
Nerve conduction studies that may be abnormal based on site of brachial plexus involvement (recording site in parentheses).*
| Upper Trunk | Middle Trunk | Lower Trunk | |
|---|---|---|---|
| Suprascapular motor (SSp, InfSp) | |||
| Lateral Cord | Lateral antebrachial sensory (forearm) Median sensory (thumb) Median sensory (index finger) Musculocutaneous motor (biceps) |
Median sensory (index) Median sensory (middle) |
|
| Posterior Cord | Radial sensory (dorsal hand) Axillary motor (deltoid) |
Radial sensory (dorsal hand) Radial motor (EDC) |
Radial motor (EIP) |
| Medial Cord | Ulnar sensory (5th digit) Dorsal ulnar cutaneous sensory (dorsal-ulnar hand) Medial antebrachial sensory (forearm) Median motor (APB) Ulnar motor (ADM, FDI) |
*Abnormalities occur when significant axonal loss. Side-to-side comparison may be necessary to detect abnormality.
ADM – abductor digiti minimi; APB – abductor pollicis brevis; EIP – extensor indicis proprius, EDC – extensor digitorum communis; FDI – first dorsal interosseous; InfSp – infraspinatus; SSp – supraspinatus.
3.3.2.1. Motor nerve conduction studies
Motor NCS assess the presence and degree of axonal loss and, much less commonly, may identify focal demyelination within the plexus. Low compound muscle action potential (CMAP) amplitudes in muscles innervated by the involved nerve segments within the plexus are the typical findings, but these are not specific and may also be seen with any lower motor neuron disorder, including those involving the roots or individual peripheral nerves. An abnormality on any individual NCS may be present if that nerve branches from the portion of the plexus involved in the underlying disease process (Table 3). The most common and routinely performed motor NCS – median (recording from the thenar muscles) and ulnar (recording from the hypothenar muscles) – may demonstrate abnormalities in lower trunk or medial cord lesions, whereas less commonly performed motor NCS, including musculocutaneous, axillary, or suprascapular, may show changes in upper trunk lesions. While focal demyelination is less common in brachial plexopathies, the presence of conduction block or abnormal temporal dispersion may be seen with Erb’s point or direct root stimulation (Fig. 2).
Fig. 2.
Ulnar motor NCS with stimulation through the brachial plexus in a patient with a medial cord brachial plexopathy, demonstrating a focal conduction block between Erb’s point and the upper arm.
3.3.2.2. Sensory nerve conduction studies
The findings on sensory NCS help to distinguish a post-ganglionic (brachial plexus) lesion from a preganglionic (cervical root) lesion. In brachial plexopathies, abnormal sensory NCS responses (typically low amplitudes or absent responses) occur as a result of Wallerian degeneration of the sensory axons that have been injured or separated from the dorsal root ganglia in the neural foramen. Many different sensory NCS can be performed in the arm to help localize the site(s) of plexus involvement, including median, ulnar, radial, and medial and lateral antebrachial cutaneous (Table 3).
3.3.2.3. Needle electromyography (EMG)
Needle EMG is used in conjunction with NCS to further help localize a brachial plexopathy as well as define the severity and degree of axonal loss and reinnervation. Needle EMG abnormalities are seen in muscles supplied by the portion(s) of the plexus involved, although muscles supplied by the same segment of the plexus may be involved to different degrees. Cervical paraspinal muscles are spared in pure brachial plexopathies, but involvement of the paraspinals in the context of other findings of a brachial plexopathy may indicate a process involving both the roots and plexus (“radiculoplexus neuropathy”), such as can occur in trauma or inflammatory conditions.
Needle EMG findings include fibrillation potentials/positive sharp waves, when axonal loss without complete reinnervation has occurred, and various motor unit potential (MUP) abnormalities, depending on the time course of the disease in relation to the timing of the EMG (Daube and Rubin, 2009). In acute injuries, reduced recruitment of MUPs may be the only finding; in subacute to chronic conditions when reinnervation is in progress, increased MUP polyphasia, amplitude, and duration are seen. In very severe brachial plexopathies associated with severe loss of axons, voluntary MUPs may be absent or, if there is early, minimal reinnervation, “nascent” MUPs may be recorded (Borenstein and Desmedt, 1980). In some cases of severe, longstanding upper trunk brachial plexopathies, aberrant reinnervation by the phrenic nerve or upper cervical roots to proximal upper extremity muscles may produce a respiratory pattern of firing (sometimes referred to as a “breathing arm”) (Swift et al., 1980, Friedenberg and Hermann, 2004, Schwarz, 1965).
While the needle EMG findings do not usually assist in defining the etiology, in rare instances specific findings can provide a clue to the etiology; for example, myokymic discharges are frequently present in radiation plexopathies (Harper et al., 1989, Krarup and Crone, 2002, Lederman and Wilbourn, 1984).
3.3.3. Imaging of the brachial plexus
Depending on the clinical scenario, imaging studies are necessary to identify possible compressive structural causes, neoplastic infiltration, or abnormalities in the size or signal of the plexus structures that may be indicative of diseases. In traumatic plexopathies, imaging studies are also important to assess for root avulsion or hematoma compressing the plexus. Various imaging modalities are used to assess brachial plexopathies, including routine radiographs, computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance neurography, ultrasound, and myelography, and each has benefits and limitations.
Routine chest radiography is useful to assess for a cervical rib in patients with suspected neurogenic thoracic outlet syndrome; however, chest X-rays do not adequately visualize the plexus. Computed tomography (CT) or magnetic resonance imaging (MRI) can assess for structural lesions with in the brachial plexus. CT is most useful at identifying a hematoma or soft tissue mass, and, in conjunction with myelography, may identify a pseudomeningocoele indicating root avulsion following trauma (Fig. 3).
Fig. 3.
Myelogram and computed tomography demonstrating large pseudomeningocoele (arrows) from traumatic C7 root avulsion.
MRI is the imaging study of choice to evaluate the brachial plexus. Standard MR imaging of the plexus includes T1 and fluid sensitive sequences, along with other fat suppression techniques. MR neurography, optimizing the evaluation of the nerve structures, allows for even better assessment of the plexus (Mazal et al., 2020). A variety of findings may be seen within the brachial plexus on MRI, including increased T2 signal intensity, focal or diffuse enhancement, or enlargement or edema of nerve segments (Crim and Ingalls, 2017). Furthermore, signal abnormalities or atrophy in muscles supplied by the brachial plexus can help support a plexopathy. MRI is more sensitive than CT at identifying subtle infiltrative lesions regions or areas of enhancement. The sensitivity of MR neurography in brachial plexopathies is variable; one study of 43 patients with suspected brachial plexopathy who underwent MR neurography reviewed by two different radiologists found a sensitivity of 41–71%, with a high specificity (98–100%) for identifying brachial plexopathy (Crim and Ingalls, 2017). Other functional imaging techniques to better evaluate the brachial plexus, such as magnetoneurography, are being developed (Watanabe et al., 2019).
Neuromuscular ultrasound is evolving as a technique that may efficiently screen the brachial plexus for structural etiologies, especially at the time of the EDX evaluation. Standardized neuromuscular ultrasound approaches to evaluating the brachial plexus have been published (Baute et al., 2018). Ultrasound allows visualization of associated neural, muscular, vascular, and adjacent structures as well as allows for static imaging and dynamic assessment to determine the effect of various maneuvers and positions, including compression (Baute et al., 2018).
3.3.4. Laboratory studies
Depending on the temporal course and suspected etiology, laboratory testing may be helpful to evaluate suspected systemic medical disorders that may involve the plexus. In many instances, laboratory testing is not necessary, such as in traumatic plexopathies or those associated with radiation injury. However, inflammatory plexopathies can occur as part of a systemic metabolic disease or connective tissue disease; therefore, testing for conditions such as impaired glucose metabolism or diabetes, connective tissue diseases, systemic vasculitis, or, rarely, infectious disorders may be useful.
3.4. Specific disorders involving the brachial plexus
Many conditions may cause dysfunction the brachial plexus (Table 4) (Ferrante, 2004). The evaluation, treatment, and prognosis vary widely between the diseases as well as between patients with the same disease. Classic causes of brachial plexopathies in adults include trauma, inflammatory brachial plexitis (i.e. Parsonage-Turner syndrome or neuralgic amyotrophy), hereditary brachial plexus neuropathy due to SEPT9 mutation, neoplastic infiltration or radiation induced plexopathy, and neurogenic thoracic outlet syndrome. In the neonatal or pediatric population, Erb’s or Klumpke’s palsies are the most common causes. The clinical and EDX features of the more common causes of plexopathies will be reviewed.
Table 4.
Categories of Diseases of the Brachial or Lumbosacral Plexopathy.
| Category | Examples |
|---|---|
| Trauma | High velocity Penetrating Birth |
| Inflammatory | Neuralgic amyotrophy Hereditary neuralgic amyotrophy Diabetic lumbosacral radiculoplexus neuropathy Non-diabetic lumbosacral radiculoplexus neuropathy |
| Neoplastic | Metastatic disease Primary nerve tumors (e.g. schwannoma) |
| Radiation | |
| Structural | Neurogenic thoracic outlet syndrome Post-median sternotomy compression Psoas hematoma or abscess |
3.4.1. Trauma
3.4.1.1. Erb’s and Klumpke’s Palsies
Brachial plexopathies may occur in the neonatal period as a result of compression of the plexus in utero or at the time of complicated childbirth. Brachial plexus injuries have been classified according to the root distributions involved – C5/6 roots/upper trunk (Erb’s palsy) causing biceps and deltoid weakness and “waiter’s tip” posture of the arm; C5-6-7 roots, causing proximal arm as well as elbow and wrist extensor weakness; or C8/T1 roots/lower trunk (Klumpke’s palsy) causing hand weakness (Gilbert and Tassin, 1984). In more severe cases, the entire arm may be involved. Upper plexus (Erb’s palsy) involvement is most common while lower plexus (Klumpke’s palsy) is rare. The site of plexus involvement has important prognostic factors as approximately 90% of upper plexus involvement have complete spontaneous recovery while the recovery rate decreases substantially to <50% when the lower plexus is involved (Yang, 2014).
The diagnosis is usually clinically apparent with reduced movement of the involved arm and or hand at birth or in the early neonatal period. A careful neurologic examination assessing active and passive movements of the arms and response to noxious stimuli may help identify the pattern of weakness. EDX testing can help to determine the timing of the injury; if needle examination performed early in the neonatal period demonstrates long duration MUPs, the injury more likely occurred in utero rather than the result of birth trauma (Pitt and Vredeveld, 2005). More importantly, needle EMG testing, in conjunction with side-to-side comparison NCS, can be used to determine the degree of reinnervation and prognosis, which may be useful in considering and guiding the decision on whether to perform nerve transfer surgical procedures (Heise et al., 2004, Spires et al., 2012, Van der Looven et al., 2020). A recent systematic review of the role of EDX testing in the management of neonatal brachial plexopathy concluded that, while there is a paucity of high-quality studies in the literature, the findings from existing studies support the role of electrodiagnosis in estimating prognosis (Van der Looven et al., 2020). The presence and degree of voluntary MUPs generally correlates with clinical recovery, although in some cases may overestimate the degree of functional recovery (Sacco et al., 1962, Heise et al., 2007, Sherburn et al., 1997). Imaging of the plexus with MRI or CT myelography may be helpful to assess for root injury or avulsion, identify other structural lesions that may involve the plexus, and occasionally identify abnormalities within the plexus.
Treatment depends on the severity of injury, site of plexus involvement, and degree of reinnervation. Treatment consists of early rehabilitation with physical and occupational therapy to prevent contractures (Yang, 2014). Surgical reconstruction is utilized to improve functional movements of the arm or hand when spontaneous functional recovery does not occur. Strategies include neurolysis and nerve grafting or nerve or muscle transfers. The timing of surgery varies but is usually delayed at least 3–4 months to allow for assessment of spontaneous recovery, and is typically performed prior to 9 months (Borschel and Clarke, 2009, Bertelli and Ghizoni, 2004, Bertelli and Ghizoni, 2004, Haerle and Gilbert, 2004, Pondaag and Malessy, 2006, Yang, 2014).
3.4.1.2. Blunt and penetrating trauma
One of the most common causes of brachial plexopathies is blunt or penetrating trauma, with high velocity accidents being most frequent (Moghekar et al., 2007). Stretch injuries account for up to 50% of all traumatic plexopathies (Kim et al., 2003). Most involve forceful lateral deviation of the head away from a depressed shoulder, and most stretch injuries involve the supraclavicular portions of the nerves (roots and trunks) although infraclavicular zone injuries (involving divisions, cords, and terminal nerve branches) are not uncommon (Kim et al., 2003, Bertelli et al., 2017, Moghekar et al., 2007). While the upper trunk and middle trunks are most frequently involved, about 50% of traumatic plexopathies involve all levels of the plexus (Kim et al., 2003, Bertelli et al., 2017, Moghekar et al., 2007). Penetrating trauma frequently causes infraclavicular nerve injuries with a more even distribution of levels affected and fewer total components injured than with stretch injuries (Chuang et al., 1998, Kim et al., 2003).
In some instances, injury to the root (e.g root avulsion) may occur at the same time as injury to the plexus. Determining whether the patient has root avulsion with or without involvement of the plexus is important as it impacts the decisions on potential surgical interventions that may be offered to improve recovery or function. The presence of injury to the cervical spine bone structures or the spinal cord may raise the possibility of root avulsion.
EDX testing is used to identify whether the injury has involved the root and/or plexus, and localize the root(s) or plexus segments involved. However, EDX findings may be confounded by injury to both the roots and the plexus. Preserved sensory NCS responses in the context of sensory loss, and needle EMG abnormalities in cervical paraspinal muscles are consistent with a preganglionic root lesion, but concomitant plexus injury cannot be excluded (Robinson, 2015, Bunnell and Kao, 2018). Abnormal sensory responses and the absence of needle EMG findings in paraspinals suggest injury only to the plexus. EDX testing is not only performed to localize the injury to the plexus and/or roots but also useful in the evaluation of patients for surgical intervention. The EDX findings help to determine severity and prognosis and whether the nerve appears to be intact, based on the degree of denervation and the presence of any voluntary MUP firing in a muscle. In patients who may be candidates for surgical intervention, such as nerve transfer procedures, EDX testing helps to identify appropriate donor nerves for transfer.
The timing of the performance of the initial EDX study following trauma is important. The study should be conducted at least three weeks following the injury to allow for the development of EDX abnormalities, such as the emergence of fibrillation potentials/positive sharp waves and alterations in NCS. Early after an axonal injury (e.g. approximately 7–10 days) the CMAP amplitude decreases proportional to the degree of axonal loss and, thus, can be an estimate of the severity of the injury and prognosis. Low-amplitude or unobtainable CMAPs indicate more severe distal Wallerian degeneration and likely indicate progressively poor prognoses (Robinson, 2015, Bunnell and Kao, 2018). After approximately 3 months, however, reinnervation may result in an increase in the CMAP amplitude, which may overestimate the proportion of preserved axons. The presence and severity of fibrillation potentials/positive sharp waves indicates denervation but is of limited prognostic value. The presence of voluntary motor unit activation and relative preservation of recruitment indicates that the nerve is in continuity with the muscle and also estimates axon preservation. Normal or near normal recruitment patterns indicate better prognosis whereas discrete recruitment or a lack of voluntary activation indicates poor prognosis (Impastato et al., 2019).
In addition to EDX testing, imaging is necessary to assess the root and plexus structures, and to confirm root avulsion. Computed tomographic (CT) myelography has been a standard imaging modality used to detect root avulsion, and has the advantage of not only detecting preganglionic lesions, but also identifying cervical spine bone abnormalities such as fractures (Fig. 3). Because CT myelography is an invasive procedure and requires contrast administration and exposure to radiation, MRI imaging has become a commonly used modality to assess for avulsion. MRI has the advantage of assessing the neural structures in the brachial plexus in addition to the roots distal to the neural foramen, and can also identify hematomas and other soft tissue or muscle injuries. In comparing CT myelography with MRI, early studies found that CT myelography was more accurate than MRI (85% vs 52%) at identifying intradural root avulsions (Carvalho et al., 1997). In a systematic review comparing the diagnostic accuracy of the two modalities, high diagnostic sensitivities (82–91%) and specificities (92–100%) of MRI in detecting root integrity or pseudomeningoceles were found, but the high or uncertain risk of bias in the studies limited the comparison of MRI with CT myelography (Fuzari et al., 2018). Another recent systematic review and meta-analysis of MRI compared to surgical exploration in detecting root avulsion in traumatic adult brachial plexopathies found a mean sensitivity of 93% (95% confidence interval of 77–98%) and mean specificity of 72% (95% confidence interval of 42–90%), concluding that MRI offers “modest” diagnostic accuracy for traumatic brachial plexus root avulsion (Wade et al., 2019). Ultrasound is a simple, non-invasive imaging tool that can assess peripheral nervous system structures. Studies comparing the sensitivity and specificity of ultrasound compared to surgical exploration in traumatic brachial plexus injuries were systematically reviewed and found high sensitivities (93–95%) in injuries involving the C5-C7 roots, but lower sensitivities (56–71%) in the C8 and T1 root injuries (Chin et al., 2018).
Treatment of traumatic brachial plexopathies is challenging. Treatment includes a multi-disciplinary team approach and includes pain management specialists to assist with pain control and physical and occupational therapists to guide therapy and prevent contractures. In severe traumatic plexopathies where substantial motor weakness persists following the injury or where the nerve is not in continuity, surgical intervention may improve function. Primary repair of the nerves may be performed within days after the injury, while secondary repair may be performed at a later date after the injury (Martin et al., 2019, Spinner and Kline, 2000). The timing of surgical intervention is debated; there have been no randomized controlled trials addressing this question and no consensus on optimal timing of surgery. A recent systematic review of studies assessing outcome related to the timing of surgery found significantly better motor outcomes, pain, and quality of life when surgery was performed within 6 months of injury (Martin et al., 2019). While a higher percentage of patients undergoing surgery within 3 months were found to have higher strength scoring than those operated between 3 and 6 months, it was concluded that early surgery must be balanced with the potential for spontaneous recovery. Other factors may impact outcome, including age over 30–40 years (which portents a poorer outcome than younger patients) and site of injury, with upper (C5-6) plexus injuries having a more favorable outcome than lower (C8-T1) plexus injuries (Coulet et al., 2010, Kim et al., 2003, Kline, 2009, Terzis and Barbitsioti, 2012, Terzis et al., 1999).
“Immediate primary repair is usually recommended when there has been a clean laceration of the nerve by a sharp object and where the nerve endings are not injured by crush or stretch. Secondary early surgical repair is generally recommended for blunt injuries or injuries with extensive soft tissue damage where the nerve injury appears to be complete or very severe (Bunnell and Kao, 2018, Martin et al., 2019). Surgical options include internal neurolysis, resection and reanastomosis, or resection and grafting. In those cases where the nerve injury is so severe that primary repair or grafting are impossible, then neurotization with anastomosis of one nerve to another, may be another option. Finally, if the above procedures fail or if longer periods of time (over 6 months) have elapsed since the injury, other secondary surgeries can be performed, including tendon or muscle transfers and arthrodesis” (Rubin, 2008).
Surgical series confirm better recovery following repair of injuries of the C5-7 roots, upper and middle trunks, lateral cord to the musculocutaneous nerve, and medial and posterior cords to the axillary and radial nerves (i.e, nerves serving more proximal arm muscles) (Kim et al., 2003). The determination of the presence of nerve root avulsion is also prognostic, as muscles innervated by avulsed roots do not recover and performing nerve repair distal to the root has no beneficial effect.
3.4.1.3. Burner and stinger syndrome
“Burner or stinger syndrome refers to a transient stretch injury to the plexus that typically follows sudden, forceful trauma to the shoulder, typically during contact sporting activities” (Rubin, 2008, Ahearn et al., 2019). While this can occur with any form of trauma to the head or shoulder, the term typically refers to symptoms that occur in athletes. The incidence of burner/stinger syndrome is higher in athletes involved in sports that place the head and neck and increased risk of direct blows at high velocity, such as American football, rugby, or wrestling. Incidence studies have found that up to 34% of rugby players and 65% of American football players had experienced a burner/stinger syndrome during their career and the risk of recurrence ranged from 20 to 80% (Aval et al., 2007, Green et al., 2017, Kawasaki et al., 2015, Levitz et al., 1997).
In burner/stinger syndrome, the C5-C6 roots or upper portion of the plexus are the sites most likely to be stretched from traction due to rapid separation of the head and shoulder. In a large study of 276 athletes referred for EDX testing for upper extremity nerve injuries, 40 patients (predominantly male and primarily American football players and wrestlers) had clinical manifestations of burner syndrome and EDX findings were consistent with a C5-C6 root or upper trunk brachial plexus injury (Krivickas and Wilbourn, 2000). The typical presentation is that of sudden onset of pain and paresthesias or sensory loss, with or without weakness, in the upper and distal arm. The symptoms are usually transient, lasting for several minutes; however, they may be prolonged and last more than 6 weeks (Thomas et al., 1999).
The evaluation of patients with burner/stinger syndrome begins with assessment at the time of injury, primarily with immobilization and stabilization of the cervical spine until assessment for spine injury can be adequately accomplished. Once the cervical spine is stable, a careful neurologic evaluation to assess for weakness and sensory loss is important. EDX testing and imaging of the cervical spine and brachial plexus is not necessary if the symptoms are transient and completely resolve; however, persistent symptoms should be further evaluated using the modalities described previously with other types of trauma.
Management of burner/stinger syndrome is primarily conservative, including rest and pain control. If weakness is persistent or severe, physical therapy may be necessary to improve recovery. There are no standard consensus guidelines on when it is safe for the athlete to return to play (Ahearn et al., 2019, Vaccaro et al., 2002). Athletes who experience only brief transient symptoms with complete resolution after a few minutes are generally thought to be able to safely return to play, but those with persistent symptoms or weakness should be further evaluated to assess the degree of nerve injury, and decisions made based on the degree and timing of recovery (Ahearn et al., 2019). To reduce the chance of recurrence, preventative measures such as appropriate education on tackling techniques and strengthening and conditioning of core muscle groups may be beneficial (Cramer, 1999).
3.4.1.4. Rucksack or backpack palsy
Traction injury to the upper portion of the plexus may occur following wearing a heavy backpack or rucksack (termed “rucksack palsy”). In a study of 17 military personnel who suffered brachial plexus injuries while wearing an “airborne rucksack” during the Vietnam conflict, the majority suffered from weakness and sensory loss in an upper trunk distribution (Daube, 1969). In a recent review of 63 Dutch military personnel with backpack palsies, the mean age was 23 years and 84% had marched with their backpack within 24 h of developing symptoms (Dorhout Mees et al., 2020). Approximately 90% of patients experienced proximal arm weakness and 4% experienced pain.
“Rucksack palsy” can also occur in military personnel as well as individuals who wear other carrying devices, such as backpacks or child-carrying packs (Dorhout Mees et al., 2020, Rose et al., 2016). Factors that may play a role in the development of rucksack palsy include the weight of the load, duration of pressure on the shoulder, and characteristics of the device (Daube, 1969). Furthermore, structural factors such as an aberrant anterior scalene muscle, fibrous bands, or bony abnormalities may pose a predisposition to developing the palsy (Daube, 1969).
Symptoms include sensory loss, pain, and transient weakness following use of the device. The symptoms are transient and improve or resolve following discontinuation of use of the device. The prognosis is overall good with complete resolution expected, although the rate of recovery is inversely proportional to the initial severity (Daube, 1969, Dorhout Mees et al., 2020). However, in the Dutch study, 90% of patients had incomplete recovery with some residual weakness, although details about the timing of reevaluation of the patients were lacking (Dorhout Mees et al., 2020).
3.4.2. Neuralgic amyotrophy (“Parsonage Turner syndrome”)
Neuralgic amyotrophy is an immune-mediated plexopathy of unknown cause (Parsonage and Aldren Turner, 1948). A variety of names have been used to describe this condition, including “Parsonage-Turner syndrome” (after the physicians who detailed the syndrome), “idiopathic brachial plexopathy”, “acute brachial plexitis”, “shoulder-girdle neuritis”, “acute multiple brachial neuropathy”, “cryptogenic brachial plexus neuropathy”, “paralytic brachial neuritis”, and “brachial plexus neuropathy” (Seror, 2017). This condition affects individuals of all ages and, while considered to be a rare entity, a recent study suggested an incidence as high as 1/1000 (Rotondo et al., 2020, van Alfen et al., 2015).
3.4.2.1. Etiology
While often “idiopathic,” neuralgic amyotrophy has been associated with various conditions, including infections, immunizations, connective tissue diseases, trauma, surgical operations, and pregnancy (Parsonage and Aldren Turner, 1948, Rubin, 2001, Seror, 2017) (Table 5). A precipitating cause is identified in only approximately 50% of patients (Seror, 2017, van Alfen and van Engelen, 2006). In patients who develop symptoms following immunizations, the onset of symptoms occurs between 3 and 21 days following the injections and may involve either the injected or non-injected limb (Tsairis et al., 1972). Symptom onset has also described between 3 and 14 days following minor procedures in the post-operative period.
Table 5.
Conditions Associated with Neuralgic Amyotrophy.
| Infectious Diseases (Viral, Bacterial) |
| Immunizations and Injections |
| Connective tissue diseases |
| Pregnancy and post-partum |
| Surgery or Post-operative |
| Strenuous Exercise |
“Neuralgic amyotrophy has been attributed to inflammation of the brachial plexus. However, isolated or unequal involvement of individual nerves arising off of the plexus or of nerves that do not technically arise from the brachial plexus but branch directly off of the cervical roots (e.g. long thoracic and phrenic nerves) suggest that this disorder is not technically a true ‘plexopathy’. The etiology of neuralgic amyotrophy is unknown, although the most common theory suggests immune-mediated mechanisms. The most supportive evidence of an inflammatory process stems from biopsy of the brachial plexus in four patients, demonstrating mononuclear, T-lymphocytic inflammatory infiltrates surrounding the epineural and endometrial vessels of the nerves” (Suarez et al., 1996, Rubin, 2008).
3.4.2.2. Clinical manifestations
Patients typically manifest with sudden onset of severe shoulder or arm pain, which occurs at night in 61% of patients (Tsairis et al., 1972). Pain may be experienced in any region of the upper limb, including the proximal and distal limb, and is commonly aggravated by movement of the limb. As a result, patients support the affected limb in a characteristic “elbow flexion-shoulder adduction” position. The pain may last for hours to weeks before subsiding, although may persist for over 2 months in about 10% of patients (Parsonage and Aldren Turner, 1948). While older studies suggested that pain resolves in nearly 90% of patients, others have found that pain may persist for months or years in up to 70% of patients, although the pain may be neuropathic or due to non-neuropathic generators (van Alfen and van Engelen, 2006). Since arm and neck pain can be seen in cervical radiculopathies, which are much more common than brachial plexopathies, awareness of the clinical features and pattern of neuralgic amyotrophy is important in order to expedite making the correct diagnosis, prevent unnecessary testing, and guide therapy.
Muscle weakness and atrophy is usually delayed by days to weeks following the onset of pain. In most cases, weakness begins within two weeks after the onset of pain and worsens as the pain subsides, but may occur as early as 1 day in 34% of patients, 1–7 days in 39%, and 1–4 weeks in 27% (Seror, 2017, Tsairis et al., 1972). Weakness may involve any distribution of the brachial plexus and is confined to a single nerve distribution in 6–46% of patients (Ferrante and Wilbourn, 2017, Seror, 2017, van Alfen and van Engelen, 2006). Isolated or combined involvement of pure motor nerves, including the suprascapular, long thoracic, terminal motor nerve branches of mixed nerves, and anterior interosseous nerves, are most commonly involved, followed by nerves that are predominantly motor, such as the axillary (Feinberg et al., 2017, Ferrante and Wilbourn, 2017). Involvement of purely sensory nerves is rare, most commonly involving the lateral antebrachial cutaneous sensory nerve (Feinberg et al., 2017, Ferrante and Wilbourn, 2017). The fact that terminal motor branches and even nerves outside of the brachial plexus (e.g. phrenic or recurrent laryngeal nerve, or nerve roots) are commonly involved suggests that this syndrome is more similar to a “mononeuropathy multiplex” rather than a pure “brachial plexopathy” (Feinberg et al., 2017, Ferrante and Wilbourn, 2017, van Alfen and van Engelen, 2006). Sensory loss occurs in up to 66% of patients but is often not readily recognized due to the focus on the severe degree of pain and weakness (Tsairis et al., 1972).
Bilateral brachial plexus involvement occurs in up to 29% of patients and is usually asymmetric (Tsairis et al., 1972). The interval between symptoms on each side is usually within 24 h but may develop after several months. In some cases, pain may be unilateral but weakness and atrophy bilateral, and vice-versa.
3.4.2.3. Evaluation
EDX testing is an important step in the evaluation of neuralgic amyotrophy but may be complicated by findings involving different segments of the plexus to different degrees or involving individual nerves, including nerves that are derived proximal to the plexus or within the plexus (Ferrante and Wilbourn, 2017). The EDX evaluation is often extensive and requires performance of multiple NCS and examination of many muscles, including less commonly examined muscles, to determine the portions of the plexus and nerves involved. Additionally, needle examination of the contralateral limb may be useful to identify subclinical involvement in the other limb.
In some instances, isolated unilateral or bilateral phrenic neuropathies, anterior interosseous neuropathy (indicating more proximal fascicular involvement in the plexus), long thoracic neuropathy, or suprascapular neuropathy may be found (Ferrante and Wilbourn, 2017, Tsao et al., 2006, van Alfen et al., 2018).
MRI of the plexus may be normal or demonstrate abnormal T2 hyperintensities in the involved segments of the plexus or individual extra-plexus nerves (Lieba-Samal et al., 2016, Sarikaya et al., 2005, Scalf et al., 2007, van Alfen and van Engelen, 2006, Zara et al., 2012). Furthermore, MRI and ultrasonography have demonstrated discreet hourglass-like constrictions of nerves in the terminal branches of the plexus (Qi et al., 2013, Sneag et al., 2018, Sneag et al., 2017) (Fig. 4). In some cases with anterior interosseous nerve involvement, MR neurography has identified high signal in fascicles of the median nerve in the upper arm, supporting extra-plexus involvement of the nerves (Pham et al., 2014). In a recent study, 24/27 patients with Parsonage Turner syndrome demonstrated normal MRI findings in the plexus but a few patients demonstrated T2 hyperintensities in the axillary and suprascapular nerves; however, 32 of 38 involved nerves branching from the plexus demonstrated intrinsic constrictions of the nerves (Sneag et al., 2018, Sneag et al., 2017). MRI of the cervical spine is often performed and is useful to exclude other disorders such as a Pancoast tumor or cervical radiculopathy.
Fig. 4.

MRI of the brachial plexus demonstrating increased signal and multifocal constrictions (arrows) in the right lower trunk in a patient with neuralgic amyotrophy.
“Laboratory studies are usually normal unless the disorder is associated with a systemic infection or connective tissue disease. Cerebrospinal fluid studies may reveal a mild elevation in protein without abnormal pleocytosis, but is usually normal” (Rubin, 2001, Rubin, 2008).
3.4.2.4. Treatment and prognosis
“No specific treatments have been systematically proven to be helpful in reducing the degree of neurologic impairment or improving the prognosis in neuralgic amyotrophy. There have been no controlled studies of corticosteroids, intravenous immunoglobulin, or other immunosuppressants. Corticosteroids, if administered in the acute, painful phase of the disease, may reduce the degree of pain but has not been clearly demonstrated to alter the course of the disease (Tsairis et al., 1972, van Eijk et al., 2009). Analgesic medication, such as narcotics, may be necessary early in the course of the disease, but are often ineffective in reducing the degree of pain. Physical therapy and regular range of motion exercises have also been advocated to prevent secondary complication, such as shoulder immobility” (Rubin, 2008).
The prognosis of neuralgic amyotrophy is favorable in most patients, with improvement occurring in 36% within one year, 75% by the end of the second year, and 89% of patients after three years (Beghi et al., 1985, Cruz-Martínez et al., 2002, Feinberg et al., 2017, Ferrante and Wilbourn, 2017, Tsairis et al., 1972). Recovery begins with improvement in pain, usually several weeks after onset. Depending on the severity of weakness and atrophy, the degree and temporal course of recovery varies. Upper plexus involvement (i.e., suprascapular nerve) recovers more rapidly than more distal involvement (i.e., anterior interosseous nerve). Likewise, the initial severity of denervation correlates with time to initial reinnervation; 50% of muscles with 2 + fibrillation potentials/positive sharp waves will achieve initial reinnervation by approximately 3 months but only 25% of muscles with 3 + fibrillation potentials/positive sharp waves begin reinnervating by about 8 months (Feinberg et al., 2017).
“More recent evidence has suggested that the prognosis may be less favorable than had previously been considered, and a relatively high percentage of patients demonstrate persistent deficits and a moderate degree of pain for more than 3 years following the attack (van Alfen and van Engelen, 2006). Recurrent attacks are uncommon, but have been reported in 1–5% of patients (Tsairis et al., 1972). The occurrence of multiple episodes over time, especially in younger patients, should raise the possibility of hereditary neuralgic amyotrophy” (Rubin, 2008).
3.4.3. Hereditary neuralgic amyotrophy (HNA)
“Hereditary neuralgic amyotrophy” (HNA), an autosomal dominant inherited disease, is much less common than sporadic neuralgic amyotrophy. HNA begins in the second or third decades or even earlier in childhood (van Alfen, 2005). Approximately 42% of patients experience their first attack in childhood. Patients manifest with recurrent episodes of symptoms attributable to brachial plexus dysfunction, features that are indistinguishable from sporadic neuralgic amyotrophy. Patients with HNA may have associated phenotypic features including hypotelorism, cleft palate, epicanthal folds, redundant cervical skin, dysmorphic ears, short stature, or widely spaced teeth (Dunn et al., 2008, van Alfen, 2011). The weakness in HNA is typically worse than in sporadic neuralgic amyotrophy, as patients with HNA more often experience complete paresis of involved muscles (van Alfen, 2005, van Alfen and van Engelen, 2006). Patients with HNA have a higher incidence of recurrent episodes of involvement, often experiencing three or more attacks. Given the higher number of attacks, which are often associated with incomplete recovery, the prognosis is worse in terms of pain, weakness, and disability (van Alfen and van Engelen, 2006).
The EDX findings in HNA are indistinguishable from those in sporadic PTS. Genetic studies have mapped the gene defect to a mutation in the Septin-9 (SPT9) protein on chromosome 17q25 in some kindreds (Watts et al., 2001).
3.4.4. Neurogenic thoracic outlet syndrome
“Thoracic outlet syndrome (TOS) is a clinical syndrome characterized by arm pain and numbness due to presumed transient compromise of the subclavian vasculature from compression or narrowing of the vessels as they course through the thoracic outlet” (Rubin, 2008) (Fig. 5) (Ferrante and Ferrante, 2017). In most cases, there is no sustained injury to the nerve structures and, thus, no neurologic deficits. However, in some cases, the lower trunk of the brachial plexus is compressed by a cervical rib or cervical band (referred to as ‘true” or “classic” neurogenic TOS). True neurogenic TOS was first described by Gilliatt in 1970 (Gilliatt et al., 1970). Studies to determine the prevalence of true neurogenic TOS are confounded by the inclusion criteria and the definition used for TOS. In studies that exclude “disputed” TOS, the prevalence of true neurogenic TOS is approximately 1/1,000,000 (Franklin et al., 2000, Gilliatt et al., 1970). True neurogenic TOS affects young to middle aged adults and females account for 94% of patients (Ferrante and Ferrante, 2017, Tsao et al., 2014).
Fig. 5.

The thoracic outlet. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
In true TOS, a fibrous band extends from a cervical rib or elongated C7 transverse process to the first rib stretches C8-T1 fibers in the lower trunk of the plexus as it courses through the thoracic outlet (Fig. 6). These structures deform and compress the lower supraclavicular brachial plexus, most commonly the distal T1 and C8 nerve roots or less commonly the proximal lower trunk (Ferrante, 2012b). While a C7 spine abnormality is common, approximately 20% of patients will not demonstrate any bony abnormality of the C7 vertebrae (Tsao et al., 2014). In most cases, the T1 fibers are more affected than the C8 fibers (Ferrante and Ferrante, 2017, Ferrante and Wilbourn, 1995).
Fig. 6.

A cervical rib compressing the brachial plexus in thoracic outlet syndrome. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
3.4.4.1. Clinical features
The clinical features of neurogenic TOS include pain, weakness, and numbness. Disturbance of motor function predominates, with weakness and atrophy of median nerve innervated thenar muscles (due to primary innervation by the T1 root) more than ulnar, radial, or other median nerve innervated muscles. Numbness and sensory loss is seen in the medial forearm more than the medial hand.
3.4.4.2. Evaluation
The characteristic EDX findings of true neurogenic TOS are those of a lower trunk brachial plexopathy. NCS demonstrate low median and ulnar CMAP and ulnar and/ medial antebrachial sensory amplitudes. The medial antebrachial cutaneous sensory and median motor responses are the NCS most likely to demonstrate abnormalities (Ferrante and Wilbourn, 1995, Levin et al., 1998, Seror, 2004). In some patients with true neurogenic TOS, relative reductions of the CMAP or SNAP amplitudes compared to the unaffected may be present despite the absolute amplitudes falling within normal range, emphasizing the importance of side-to-side NCS comparisons (Tsao et al., 2014). Needle examination demonstrates abnormalities that may include fibrillation potentials/positive sharp waves and long duration MUPs with reduced recruitment in lower trunk innervated muscles. The abductor pollicis brevis (APB) muscle is preferentially innervated by the T1 root and is most commonly affected in neurogenic TOS, with changes indicating chronic axonal loss with reinnervation seen in the APB in about 50% of cases (Ferrante, 2012a, Tsao et al., 2014). Abnormalities are less prominent in muscles primarily innervated by the C8 root (e.g, triceps brachii) compared to the T1 root.
Radiographic studies are important to identify a structural process such as a cervical rib, elongated C7 transverse process, or fibrous band. Routine cervical spine radiographs, CT imaging, and MRI can identify bony abnormalities, but none of the imaging modalities are sensitive enough to identify a fibrous band. Improved imaging techniques, including MR neurography and advanced MR protocols are evolving which may help identify displacement of nerve structures (Magill et al., 2015, Yldzgören et al., 2014).
3.4.4.3. Treatment and prognosis
“Surgical resection of the band or removal of a cervical rib leads to improvement or resolution of symptoms in most patients with true neurogenic TOS. In a series of thirty-three surgically treated patients over a 25-year period at a single institution, pain, weakness, and sensory loss improved in the majority of patients although motor recovery was rarely complete (Tender et al., 2004). This is in contrast to patients with non-neurogenic TOS, in which retrospective studies have shown no significant benefit of surgery compared to conservative management (Franklin et al., 2000). Surgical complications are rare in experienced centers, but may include pleural tear, pneumothorax, hematoma, vascular insult, and the development of a more severe brachial plexopathy following transaxillary resection of the first rib due to traumatic injury to the plexus” (Franklin et al., 2000, Tender et al., 2004, Rubin, 2008).
3.4.5. Post-median sternotomy brachial plexopathy
A similar condition to neurogenic TOS may occur with cardiothoracic surgeries involving median sternotomy where retraction of the chest wall or fracture of the first rib causes traction on the C8 rami or lower trunk of the brachial plexus due to posterior clavicular displacement and compression of the plexus (Levin et al., 1998). The incidence of post-median sternotomy plexopathies ranges from 0.5 to 38% of cases (Healey et al., 2013, Unlu et al., 2006). Limited retraction, caudal placement of the retractor, and limited cardiopulmonary bypass time may be protective (Healey et al., 2013). The clinical and EDX features are similar to those that occur in neurogenic TOS.
3.4.6. Compression from compartment syndromes
“The infraclavicular portion of the brachial plexus (cords) travels from the clavicle to the axilla where the individual terminal nerves are formed. The medial brachial fascial compartment is a section of the upper arm formed by the medial intermuscular septum where the axillary vessels and terminal nerves of the brachial plexus travel. Lesions located in this compartment, such as hematomas following axillary arteriography, humerus fracture, or axillary artery aneurysms may lead to an increase in the intracompartmental pressure and secondary compression and ischemia to the nerves within the compartment. Any of the terminal nerves (median, ulnar, radial, axillary, musculocutaneous) may be affected to different degrees, although the median and ulnar are typically affected more often than other nerves (Tsao and Wilbourn, 2003). Treatment with surgical evacuation and urgent decompression of the compartment within hours of symptom onset is important to optimize recovery” (Rubin, 2008).
3.4.7. Neoplastic brachial plexopathy
The brachial plexus lies in close proximity to the lung, breast, and lymphatic system. Therefore, neoplastic invasion can result in brachial plexus dysfunction. In patients admitted to a large cancer center in the 1970s, 0.43% were found to have a brachial plexopathy (Kori et al., 1981). The frequency is higher in patients with breast cancer, where up to 4.9% experience symptoms of a brachial plexopathy up to 5 years following treatment.
Neoplastic plexopathies are most commonly due to local spread or metastatic disease. Lung and breast tumors, lymphoma, and sarcoma, account for nearly 80% of all neoplasms (Kori et al., 1981). Metastatic infiltration of peripheral nerves is uncommon in patients with lymphoma, although in one autopsy series, 40% of patients with lymphoma had involvement of peripheral nerves (Bourque et al., 2018). Rare cases of metastatic infiltration of the brachial plexus, associated with focal conduction block that mimicked chronic inflammatory demyelinating polyradiculopathy, have been described (Bourque et al., 2018). Neoplastic spread by direct extension can also be erosive and aggressive and can involve nerve roots and even the spinal cord, in addition to the plexus.
Primary peripheral nerve tumors, such as schwannomas and neurofibromas are rarely located in the brachial plexus (Fig. 7). When present, they are slow growing and patients present with progressive paresthesias, without significant pain.
Fig. 7.

MRI demonstrating a left brachial plexus schwannoma (arrow).
3.4.7.1. Clinical features
Most patients with neoplastic brachial plexopathies present with pain, which is usually severe in quality (Jaeckle, 2010, Kim et al., 2019; Kori et al., 1981). The location of symptoms and distribution of clinical findings depends on the site of brachial plexus involvement; the most common site of involvement is the lower trunk/medial cord or the entire plexus (Harper et al., 1989, Kori et al., 1981). When the lower trunk is involved, the patient may experience pain in the medial arm or forearm and medial hand, although any portion of the arm may be affected. Progressive weakness and atrophy and sensory loss follows the onset of pain. Horner’s syndrome was found in half of the patients in one series and in a recent review of 44 patients with brachial plexopathies from metastatic breast cancer, 66% demonstrated malignant lymphedema (Kim et al., 2019; Harper et al., 1989, Kori et al., 1981, Krarup and Crone, 2002, Lederman and Wilbourn, 1984).
When a brachial plexopathy occurs as a result of infiltration or compression from a mass in the lung apex (Pancoast tumor), the lower trunk is primarily affected and the clinical features are those of a lower trunk plexopathy. This may be associated with a Horner’s syndrome given the involvement of the T1 root or cervical sympathetic ganglion.
3.4.7.2. Evaluation
The EDX findings in neoplastic plexopathies reflect the distribution, severity, and temporal course of plexus involvement, and frequently include low motor and sensory NCS and fibrillation potentials and long duration, high amplitude MUP with reduced recruitment on needle EMG of affected muscles (Seror, 2001). EDX studies are also useful to support localization to the plexus rather than the root, which can help guide appropriate imaging studies.
The diagnosis of neoplastic invasion of the brachial plexus is confirmed by imaging studies. MRI with contrast is the primary imaging modality used and may demonstrate several findings, including a mass compressing or infiltrating the brachial plexus, T2 hyperintensity, fascicular disorganization, or nodular enhancement or thickening of the plexus (Chhabra et al., 2011, Thawait et al., 2011). MRI characteristically demonstrates high T2 signal abnormality within the plexus. While a similar finding can be seen in radiation induced plexopathy, high T2 signal is more common in neoplastic than radiation plexopathy (van Es et al., 1997). In primary nerve tumors of the brachial plexus, MRI shows enhancing solitary lesions within the plexus. 18FDG-PET may demonstrate increased metabolic activity within the plexus, which may be helpful to suggest tumor infiltration rather than radiation injury (Luthra et al., 2006, Weiler-Sagie et al., 2010). In cases where neoplastic involvement of the plexus is uncertain, surgical exploration and pathologic confirmation may be necessary.
3.4.7.3. Treatment and prognosis
Treatment of neoplastic brachial plexopathies is focused on treatment of the malignancy with localized radiation or chemotherapy. Improvement in pain and neurologic deficits following radiation is variable; although between 46 and 86% of patients (many with breast cancer) have been reported to have partial or complete remission of pain or neurologic deficits following radiation therapy, often with adjuvant chemotherapy or hormonal therapy (Kamenova et al., 2009, Kori et al., 1981). Symptomatic treatment for pain control includes neuropathic and other pain medications, regional nerve blocks, and occasionally infusion pumps.
3.4.8. Radiation-induced brachial plexopathy
Radiation-induced brachial plexopathy is a rare manifestation of radiation therapy. While this is seen most commonly in patients who have been treated with radiation to the region of the plexus for breast cancer, it may also occur following treatment for other head and neck, lung, or metastatic cancer from distant neoplasms to lymph nodes in the shoulder (Yan et al., 2019). Less than 5–9% of patients treated with radiation develop brachial plexopathy following radiation (Emami et al., 1991, Mondrup et al., 1990). Several factors have been associated with an increased risk of development of brachial plexopathy, the most important being the dose of radiation. A meta-analysis of studies assessing the risk of radiation brachial plexopathy relative to the radiation dose found that the radiation dose was ≤6000 cGy in 62.5% of studies that reported <5% incidence of radiation plexopathy, and ≤6600 cGy in 75% of the studies with the same incidence (Yan et al., 2019). Thus, maximum radiation doses of <6000–6600 cGy appear to have a low risk of development of radiation plexopathy and there is an increased risk with each 1000 cGy of radiation greater than 6000 cGy (Yan et al., 2019). Other factors that may increase the likelihood of developing radiation plexopathy include increased number of ports of radiation administration, the administration of adjunctive chemotherapy, and the extent of axillary node dissection (Emami et al., 1991). The region of the brachial plexus involved following radiation is variable, with some studies specifying a predilection for the upper plexus and others the lower plexus or entire plexus (Harper et al., 1989, Kori et al., 1981, Krarup and Crone, 2002, Lederman and Wilbourn, 1984).
The underlying mechanism and pathophysiology of nerve injury is unknown. “Pathologic studies have demonstrated loss of myelin, fibrosis and thickening of the neurolemma sheath, and hyalinization and obliteration of the vaso-nervorum to the brachial plexus, suggesting either focal compression of the plexus by fibrosis or chronic nerve ischemia as possible underlying mechanisms” (Rubin, 2008).
3.4.8.1. Clinical features
The mean age of patients developing radiation brachial plexopathy reported in a large meta-analysis was 56.9 years (Yan et al., 2019). Patients present with slowly progressive paresthesias, sensory loss, pain, weakness, and atrophy. Compared to neoplastic plexopathies, radiation-induced plexopathies more commonly present with sensory loss or paresthesia rather than pain. Symptom onset ranges from one month to eighteen years following radiation exposure, with a median time of 7 months (Harper et al., 1989, Kori et al., 1981, Yan et al., 2019).
3.4.8.2. Evaluation
EDX testing is important to identify brachial plexus involvement and can be used, in some cases, to support radiation injury as the cause of the plexopathy. EDX features include low motor and sensory NCS amplitudes and long duration, high amplitude MUP. Conduction block across the plexus in the ulnar nerve following stimulation at Erb’s point is more common in radiation-induced than in neoplastic plexopathy, but this finding is uncommon and not diagnostic (Harper et al., 1989). On needle EMG, myokymic discharges are recorded in up to 63% of patients and 24% of muscles; in contrast, myokymic discharges are rarely encountered in neoplastic plexopathy (Harper et al., 1989). MRI of the brachial plexus may be normal or show increased or decreased T2 signal and fibrosis (van Es et al., 1997) (Fig. 8).
Fig. 8.

MRI demonstrating increased signal and thickening of the entire left brachial plexus (arrow) in a patient with radiation-induced brachial plexopathy.
3.4.8.3. Treatment and prognosis
“The course of radiation plexopathy is typically one of steady progression or stabilization in 90% of patients, although cases of improvement have rarely been reported (Killer and Hess, 1990). There is no established treatment to reverse or improve the nerve injury, although surgical interventions such as neurolysis or neurolysis with omental grafting, have been performed in some patients with variable improvement in symptoms. A report of resolution of conduction block following anticoagulation therapy suggested that ischemic nerve injury may contribute to the pathogenesis of radiation-induced nerve damage and strategies to improve nerve perfusion may be effective (Soto, 2005)” (Rubin, 2008). While a few anecdotal cases have reported improvement with hyperbaric oxygen therapy, no improvement in functional outcome was found in a randomized, phase II trial (Pritchard et al., 2001, Stowe et al., 2020). The treatment, therefore, remains supportive with pain control, and physical and occupational therapy.
4. The lumbosacral plexus
4.1. Anatomy
The lumbosacral plexus is a complex structure in the pelvis that arises from the anterior rami of the T12-S4 nerve roots. In contrast to the brachial plexus, the lumbosacral plexus has less “merging” of nerve fascicles or formation of trunks or cords. However, the structure is complex in the many nerves that branch directly from the plexus (Table 6). The lumbosacral plexus can be considered as two adjacent plexi - the lumbar and the sacral (Fig. 9, Fig. 10).
Table 6.
Nerve branches and muscles innervated through the lumbar and sacral plexus.
| Nerve | Muscles | Sensory Distribution | |
|---|---|---|---|
| Lumbar Plexus | Iliohypogastric (L1-2) | – | Inferior abdominal wall |
| Ilioinguinal (L1-2) | – | Medial groin | |
| Genitofemoral (L1-2) | – | – | |
| Lateral femoral cutaneous (L3-4) | – | Anterolateral thigh | |
| Obturator (L2,3,4) | Adductor longus Adductor magnus Gracilis |
– | |
| Femoral (L2,3,4) | Quadriceps | – | |
| Saphenous (L2,3,4) | – | Medial leg and foot | |
| Sacral Plexus | Superior gluteal (L4-5) | Gluteus medius Tensor fascia lata |
– |
| Inferior gluteal (L4-S1) | Gluteus maximus | – | |
| Sciatic (L4-S2) | Anterior tibialis Peroneus longus Gastrocnemius Soleus Foot muscles |
Foot Lateral leg |
|
| Pudendal (S2,3,4) | External anal sphincter | Perineal | |
Fig. 9.
The lumbosacral plexus and branches in coronal view. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
Fig. 10.
The lumbar and sacral plexus in lateral view. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
The lumbar plexus is derived from the anterior rami of the L1 – L4 roots, which join to form several branches within the psoas muscle (Fig. 11) (Table 6). Relatively minor branches include the iliohypogastric (from T12-L1 roots), ilioinguinal (from the L1 root), and genitofemoral (from the L1-2 roots) nerves, which contain mostly sensory fibers from the abdominal wall and medial groin. The lateral femoral cutaneous nerve is a pure sensory nerve that branches from the upper to mid lumbar plexus (L2-3 roots) and supplies the sensation to the anterolateral thigh. Isolated compression of this nerve is commonly, resulting in “meralgia paresthetica”, but sensory disturbance in the distribution of that nerve can also occur in lumbar plexopathies. The major branches (which contain motor and sensory fibers) of the lumbar plexus are the obturator nerve and the femoral nerve, which supply most muscles in the anterior and medial thigh (Table 6). While these nerves supply sensation to the anterior and medial thigh, the femoral nerve also gives off a distal sensory nerve, the saphenous nerve, which has a more distal sensory innervation to the anteromedial lower leg and foot. The lumbosacral trunk derives from the L4-5 nerve roots before continuing as a contributor to the sciatic nerve (L4-S3).
Fig. 11.
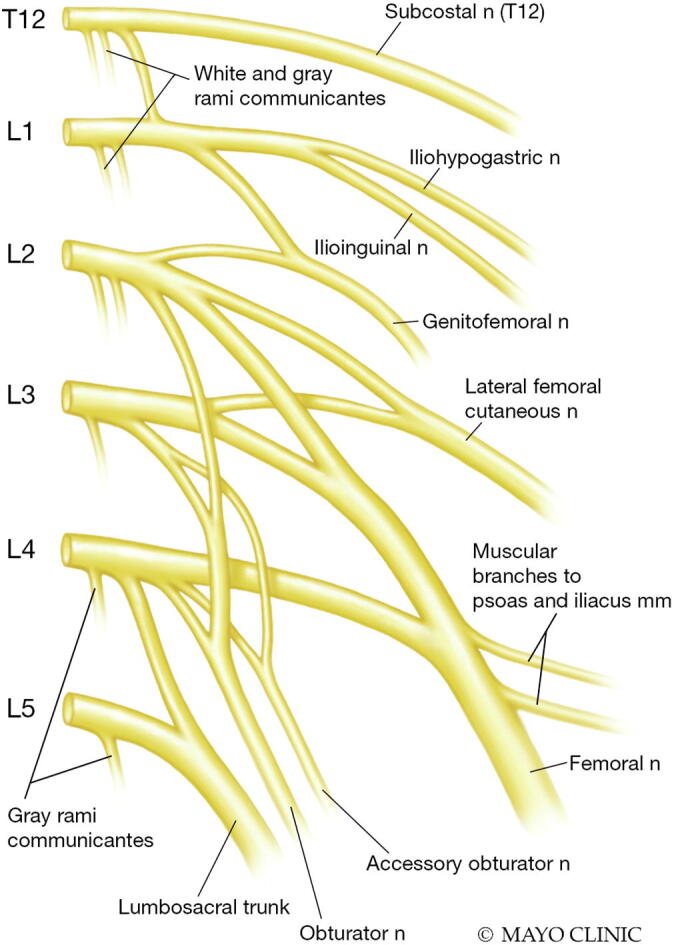
Branches of the lumbar plexus. (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.)
The sacral plexus is formed from the L5 - S4 roots and has several major branches (Fig. 9, Fig. 10) (Table 6). The major branches of the sacral plexus include the superior gluteal nerve (L4-S1), inferior gluteal nerve (L5-S2), sciatic nerve, posterior femoral nerve (S1-3), and pudendal nerve (S1-S4).
While the lumbosacral plexus is a deep structure in the pelvis and is therefore not as susceptible to direct trauma, its proximity to other muscle, vascular, and intestinal structures predisposes it to injury from disorders that involve these areas.
4.2. General evaluation of lumbosacral plexopathies
4.2.1. Clinical history and examination
Similar to the evaluation of brachial plexopathies, a comprehensive history and examination is the first step in evaluating patients with suspected lumbosacral plexopathies. The history should include the timing of onset of symptoms and course of progression, recent trauma, and general medical conditions (e.g. diabetes or cancer). The distribution of clinical findings, including weakness and sensory loss, helps to localize the injury to the lumbar or sacral plexus, or both. In lumbar plexopathies, pain and sensory loss is primarily localized to the anterolateral and medial thigh region and weakness involves in the quadriceps, hip flexors, and hip adductors. In sacral plexopathies, sensory loss and weakness involves the posterior thigh and the leg and foot. The presence of other associated features, such as enlarged inguinal lymph nodes (suggestive of possible cancer) or a pulsatile femoral mass (suggestive of possible aneurysm), may provide clues to the etiology. Since other conditions can mimic lumbosacral plexopathies, additional testing is often required to confirm the localization and assess for etiology (Table 7).
Table 7.
Lumbosacral Plexopathy Mimickers.
| Lumbosacral radiculopathy |
| Lower extremity mononeuropathy (femoral, peroneal, tibial) |
| Mononeuritis multiplex |
| Orthopedic disorders (e.g. hip fracture, hip osteoarthritis, avascular necrosis) |
4.2.2. Electrodiagnostic testing
The goals of performing EDX testing in lumbosacral plexopathies are similar to those for brachial plexopathies, and include helping to localize the disorder to the lumbosacral plexus, exclude more common lumbosacral radiculopathies, and define the pathophysiology and severity of nerve injury. While there are fewer NCS that can be performed to reliably evaluate the lumbosacral plexus compared to the brachial plexus, the same EDX concepts apply – abnormal sensory NCS and the absence of needle EMG findings in the lumbosacral paraspinals are supportive of a plexopathy. Sensory NCS studies (e.g. sural, superficial fibular, medial and lateral plantar, and saphenous) are less reliable as they are technically difficult and some may be of low amplitude or absent normally, especially in older individuals; thus, side-to-side comparison for significant asymmetry of the amplitudes is important in confirming that a lower limb sensory response is truly abnormal.
For assessment of the lumbar plexus, the femoral motor NCS and lateral femoral cutaneous or saphenous sensory NCS can be performed but are technically challenging. Needle examination of the iliopsoas, adductor longus, and quadriceps muscles may be abnormal. In sacral plexopathies, the fibular and tibial motor NCS, and sural and superficial fibular and plantar sensory NCS may be abnormal. Needle EMG may demonstrate abnormalities in distal fibular (tibialis anterior, peroneus longus) and tibial (gastrocnemius, tibialis posterior) innervated muscles as well as more proximal sciatic (biceps femoris short or long head, semitendinosus, semimembranosus). The presence of needle EMG abnormalities in proximal muscles that are innervated by nerves derived directly from the plexus, such as inferior gluteal (gluteus maximus) or superior gluteal (gluteus medius, tensor fasciae latae) nerves helps to distinguish a sacral plexopathy from a sciatic neuropathy.
4.2.3. Imaging of the lumbosacral plexus
Similar to imaging of the brachial plexus, MRI is the imaging study of choice to evaluate the lumbosacral plexus. Abnormal MRI findings in lumbosacral plexopathies include increased T2 signal intensity, focal or diffuse enhancement, or enlargement or edema of nerve segments. MRI is more sensitive than CT at identifying subtle infiltrative lesions, although CT may be useful to assess for psoas hematoma.
4.3. Specific diseases of the lumbosacral plexus (Table 4)
4.3.1. Trauma
Because of its deep location in the pelvis, the lumbosacral plexus is relatively shielded from direct injury and is less prone to stretch injuries than the brachial plexus. As a result, trauma accounts for only a small percentage of lumbosacral plexopathies. Most are the result of penetrating trauma (e.g. gunshot or puncture wounds) or high velocity injuries (Chiou-Tan et al., 2001, Kutsy et al., 2000). Traumatic lumbosacral plexopathies are frequently associated with pelvic or hip joint injuries, such as fractures. Over a 5-year period at a single institution, of 2794 patients treated for a pelvic or acetabulum fracture, only 22 patients (incidence 0.7%) suffered a lumbosacral plexopathy as a complication (Kutsy et al., 2000). The frequency was significantly higher (2.03%) in patients suffering sacral fractures or sacroiliac joint dislocations, likely due to the fact that the plexus is in close proximity to the sacral bone and sacroiliac joint. Another retrospective study of 4123 patients referred for EDX testing over a 5 year period identified 29 patients, primarily men, with lumbosacral plexopathy as a result of a motor vehicle accident (n = 10) or gunshot wound (n = 19) (Chiou-Tan et al., 2001). Of those suffering injuries from gunshot wounds, most occurred in the abdomen rather than the hip or leg. Furthermore, injury to the upper plexus was preferentially involved in patients with gunshot wounds, but the upper plexus and lower plexus were affected to a similar degree in patients with motor vehicle accidents. EDX testing demonstrates typical findings of lumbosacral plexopathy, with sensory NCS abnormalities and fibrillation potentials/positive sharp waves seen in muscles supplied by the fibular division more than that tibial division muscles (Kutsy et al., 2000).
4.3.2. Diabetic and non-diabetic lumbosacral radiculoplexus neuropathy
The lumbosacral plexus may be involved in isolation or along with additional roots and/or nerves as part of an immune-mediated or inflammatory disorder (Laughlin and Dyck, 2014). This most commonly occurs in patients with diabetes, although can also occur in the absence of diabetes. When associated with diabetes, this condition has been referred to by a variety of names, including “diabetic amyotrophy”, “Bruns-Garland syndrome”, “proximal diabetic neuropathy”, and “ diabetic lumbosacral radiculoplexus neuropathy” (LSRPN) (Dyck and Windebank, 2002). In a recent population based study from 2000 to 2015 in Olmsted County, Minnesota, the overall incidence of LSRPN was 4.16/100,000, with an incidence of 2.79/100,000 in diabetics and 1.27/100,000 in non-diabetics (Ng et al., 2019). Diabetic LSRPN occurs in middle or older age patients with type 2 diabetes. In the Olmsted County group of 59 patients, the mean patient age was 70 years (range 24–88). The syndrome usually occurs during a period of relatively well-controlled diabetes and patients often do not exhibit other long-term diabetic complications.
4.3.2.1. Clinical features
Diabetic and non-diabetic LSRPN have similar clinical, EDX, and pathological features (Dyck, 2001, Dyck and Windebank, 2002). The syndrome initially begins with acute (days) to subacute (weeks) onset and progression of pain in the thigh, leg, buttock, and/or back, which is predominantly unilateral but may spread to involve the contralateral limb. The pain may be of an achy, sharp, or burning quality (Dyck, 2001). Pain often subsides spontaneously. Weakness and atrophy occurs shortly following the onset of pain and is usually the most prominent and debilitating symptom. Neurological deficits may be focal or patchy, but often involve the proximal and distal leg, and may extend to involve the other leg (Dyck and Thaisetthawatkul, 2014, Laughlin and Dyck, 2014). The median time of symptom onset to diagnosis is 2 months (range 1–72 months) and the median time to bilateral disease is approximately 3 months (Dyck and Windebank, 2002, Ng et al., 2019). In one series, half of patients were wheelchair bound during their illness due to weakness (Dyck and Windebank, 2002).
In both forms, patients often experience additional symptoms, including prominent weight loss and autonomic features (Dyck and Windebank, 2002). Weight loss is a common feature and averages 15 lb in the non-diabetic and 30 lb in the diabetic forms, although some patients in both groups experience much more profound weight loss (Dyck, 2001). Autonomic features include orthostatic symptoms, urinary and sexual dysfunction, and constipation.
In rare cases, pain in not a prominent feature and a painless, subacute motor neuropathy may occur in diabetics (Garces-Sanchez et al., 2011). In 23 patients reported with this phenotype, the mean age was 62 years (range 36–78) and the clinical features (including prominent weight loss) were similar to painful diabetic LSRPN apart from more symmetric and severe involvement, some upper limb involvement, and slower progression. Nerve biopsies demonstrated features of microvasculitis and ischemic injury, similar to diabetic LSRPN.
4.3.2.2. Evaluation
Laboratory studies usually demonstrate markers of diabetes or impaired glucose metabolism, but are otherwise unremarkable; the erythrocyte sedimentation rate may rarely be elevated (Dyck, 2001). Elevated cerebrospinal fluid protein without pleocytosis may be seen in both forms, but is higher in the diabetic form (Dyck, 2001).
The EDX features are those of a patchy, axonal neurogenic process and include low amplitude compound muscle action potential and sensory nerve action potential amplitudes with only mildly slowed distal latencies and conduction velocities (Bastron and Thomas, 1981, Dyck, 2001, Dyck and Thaisetthawatkul, 2014, Laughlin and Dyck, 2013). Since the process is patchy, in some instances sensory axons to the foot may be spared and routinely tested SNAPs may be normal, giving the impression of a polyradiculopathy rather than a radiculoplexus neuropathy (Dyck, 2001, Laughlin and Dyck, 2013). Depending on the timing of the EDX study, needle examination findings include fibrillation potentials/positive sharp waves (often prominent) and reduced recruitment and long duration MUPs. The distribution of needle EMG findings is often patchy throughout the leg, but commonly involved muscles innervated through the lumbar plexus (e.g. iliopsoas, adductor longus, quadriceps). Needle EMG abnormalities are commonly found in the lumbar paraspinal muscles, indicating involvement of the roots in addition to post-ganglionic nerve segments and supporting the term “radiculoplexus neuropathy” (Dyck and Thaisetthawatkul, 2014, Laughlin and Dyck, 2013).
Imaging studies are often unnecessary in patients with a classic presentation; however, in patients without a typical presentation or with a slowly progressive course, MRI may be useful to exclude other infiltrative or compressive causes. MR neurography has anecdotally demonstrated thickening and high signal in individual nerves (e.g. femoral, obturator, sciatic) (Filosto et al., 2013, McCormack et al., 2018).
Histopathologic studies from nerve biopsies in both diabetic and non-diabetic LSRPN demonstrate focal or multifocal nerve fiber degeneration, perineurial thickening, epineurial neovascularization, segmental demyelination and axonal degeneration, indicating ischemic nerve injury from a microvasculitis as the underlying mechanism (Dyck et al., 2000, Dyck and Windebank, 2002).
4.3.2.3. Treatment and prognosis
Since the pathophysiology is one of inflammation and microvasculitis, immunosuppressive treatment may be useful; however, there are no large randomized controlled trials that have shown definite benefit (Dyck and Windebank, 2002). Reports of improvement with intravenous methylprednisolone, immunoglobulin, or plasma exchange suggest that immunotherapies may be beneficial, particularly at reducing the degree and duration of pain (Pascoe et al., 1997, Dyck et al., 2000, Dyck et al., 2002, Triggs et al., 1997). In a recent Cochrane systematic review of immunotherapy trials for DLSRPN, only one placebo-controlled trial of methylprednisolone was identified as published in abstract form, leading to a conclusion that there is no evidence to support a positive or negative effect of immunotherapy (Chan et al., 2017). That multicenter, prospective treatment trial using 1 g intravenous methylprednisolone weekly over a 12 week period did not find a statistically significant improvement in the neuropathy impairment score in the treated versus non-treated patients, but secondary outcome measures of pain were improved in the treated patients (Dyck et al., 2006).
The syndrome is usually monophasic; improvement occurs in the majority of patients, although recovery is usually incomplete (Dyck, 2001, Dyck and Windebank, 2002). Long-term outcome is similar in diabetic and non-diabetic LSRPN, with morbidity associated with incomplete recovery of weakness. In 42 patients with non-diabetic LSRPN, recovery was delayed and incomplete at a median follow-up time of 35 months (Dyck, 2001). In the majority of patients with persistent weakness, the most common distribution involved the distal leg, including foot drop (Dyck, 2001). Two patients eventually developed diabetes and seven experienced a relapse of symptoms on the same or contralateral side.
4.3.3. Neoplastic lumbosacral plexopathy
Infiltration of the lumbosacral plexus by neoplasms is rare in cancer patients, with a frequency of 0.71%; however, since lumbosacral plexopathies are also rare, neoplastic invasion is one of the more common etiologies (Jaeckle et al., 1985). Lumbosacral plexopathies develop from local invasion or direct extension from neighboring tumors. Colorectal, urogenital, prostate, lymphoma, and retroperitoneal and pelvic sarcomas account for over 80% of the tumors (Jaeckle et al., 1985). Perineural spread of pelvic malignancies, such as prostate, bladder, rectal, and cervical can spread from the primary organ to the plexus through the splanchnic nerves (Capek et al., 2015, Ladha et al., 2006). Metastases from extra-abdominal tumors such as breast or lung occur in approximately 25% (Jaeckle et al., 1985). Most patients with neoplastic plexopathies have known underlying malignancies; however plexopathy may be the initial manifestation of cancer in up to 15% of patients (Jaeckle, 2010).
4.3.3.1. Clinical features
The clinical manifestations and findings in neoplastic lumbosacral plexopathies depend on the site of plexus involvement. In a review of 85 cancer patients with lumbosacral plexopathy, the lower plexus was involved in 51%, upper plexus in 31%, and entire plexus in 18% (Jaeckle et al., 1985). Pain is common and the initial symptom in approximately 70% of patients. The pain is often dull and achy and may be imprecisely localized, but may be “radicular” in nature, mimicking a radiculopathy. Weakness or paresthesias is the presenting symptom in 15% of patients. If the iliopsoas muscle is involved by tumor infiltration, patients may have difficulty lying flat with their leg straight. On clinical examination, weakness and sensory loss in the leg are present in 86% and 73% of patients, respectively (Jaeckle et al., 1985). In patients with sacral plexus involvement, symptoms and signs may involve the foot or lower leg, and may mimic and L5 or S1 radiculopathy. Additional clinical features may include abdominal or rectal mass, leg edema, and hydronephrosis. Bilateral, usually asymmetric plexus involvement occurs in approximately 2/3 of patients.
4.3.3.2. Evaluation
EDX testing may demonstrate low motor and sensory amplitudes on NCS and fibrillation potentials and large MUP on needle EMG. The diagnosis of neoplastic involvement is made by careful imaging studies. Magnetic resonance imaging (MRI) is more sensitive than CT in showing a mass compressing the plexus or thickening or enlargement of the plexus (Taylor et al., 1997). FDG-PET imaging may also increase the sensitivity in detecting tumor in the lumbosacral plexus.
4.3.3.3. Treatment and prognosis
Similar to neoplastic brachial plexopathies, treatment is focused of treating the malignancy, usually involving radiation to the lumbosacral plexus, with or without chemotherapy. Subjective improvement in symptoms, lasting a mean of 4 months, has been reported in 85% of patients, with objective improvement in 48% (Thomas et al., 1985). Symptomatic treatment is similar to that for neoplastic brachial plexopathy.
4.3.4. Radiation-induced lumbosacral plexopathy
Radiation injury may involve lumbar and sacral plexus, or both. Symptoms typically follow radiation treatment for malignancies in the pelvic or lower abdominal regions, including lymphomas, testicular cancer, or gynecologic cancers. The onset of neurologic symptoms may begin months or years following the radiation exposure (Aho and Sainio, 1983). Clinical manifestations include progressive weakness, sensory loss, and pain. Myokymic discharges are found in approximately 60% of patients on needle EMG, which can help to support radiation injury as the cause of the plexopathy (Aho and Sainio, 1983, Thomas et al., 1985).
4.3.5. Hematoma, abscess, and aneurysm
The lumbosacral plexus may rarely be compressed by large retroperitoneal hematomas, abscesses, or large aneurysms. Factors such as anticoagulation usage or bleeding disorders may predispose a patient to the development of an iatrogenic hemorrhage (e.g following procedures in the groin such as puncture for vascular access or abdominal or pelvic trauma or surgery), thereby increasing the risk for psoas hematoma formation. While retroperitoneal or groin hemorrhages accounted for only 11% of all complications of femoral artery catheterizations in one study, when a hemorrhage into the iliacus or psoas muscle results in a compartment syndrome, injury to the lumbar plexus or, less commonly, femoral and/or obturator nerves, may develop (Lumsden et al., 1994, Abel et al., 2018).
“Other masses that may develop in structures neighboring the lumbosacral plexus include retroperitoneal abscesses or femoral artery aneurysms. Isolated aneurysms of the common iliac artery are rare, but lumbosacral plexopathies have been described in several patients (Gardiner et al., 2006). In these cases, pain in the low back, buttock, hip, or thigh may occur over weeks or months, although expansion of the aneurysm may produce acute worsening of symptoms” (Rubin, 2008).
Patients with masses or hematomas involving the lumbosacral plexus present with pain and weakness, particularly in the thigh region. Symptom onset and progression may be acute and rapid, such as following trauma or vascular procedures, or gradual, such as with abscess or aneurysm formation. Imaging studies including ultrasound, CT, or MRI are diagnostic.
5. Summary
Diseases of the brachial and lumbosacral plexus pose a challenge for neurologists and other specialists who evaluate and treat patients with spine and limb symptoms. The non-specific clinical features and similar presentations to other root, nerve, and non-neurologic disorders emphasize the importance of a high clinical index of suspicion for a plexopathy. EDX testing is important to confirm plexopathies, however the types of individual NCS and the extensive needle examination require a comprehensive, individualized, and technically cautious approach to each patient. While the etiologies of plexopathies are usually not determined with EDX testing, supplemental imaging and other testing, along with a through clinical history, can be used. Treatment of plexopathies most often focus on symptomatic management, although, depending on the etiology, specific targeted treatments may improve outcome.
6. Declarations of interest
None.
References
- Abel N.A., Januszewski J., Vivas A.C., Uribe J.S. Femoral nerve and lumbar plexus injury after minimally invasive lateral retroperitoneal transpsoas approach: electrodiagnostic prognostic indicators and a roadmap to recovery. Neurosurg. Rev. 2018;41(2):457–464. doi: 10.1007/s10143-017-0863-7. [DOI] [PubMed] [Google Scholar]
- Ahearn B.M., Starr H.M., Seiler J.G. Traumatic brachial plexopathy in athletes. J. Am. Acad. Orthop. Surg. 2019;27(18):677–684. doi: 10.5435/JAAOS-D-17-00746. [DOI] [PubMed] [Google Scholar]
- Aho K., Sainio K. Late irradiation-induced lesions of the lumbosacral plexus. Neurology. 1983;33(7):953–955. doi: 10.1212/wnl.33.7.953. [DOI] [PubMed] [Google Scholar]
- Aval S.M., Durand P., Shankwiler J.A. Neurovascular injuries to the athlete’s shoulder: Part I. J. Am. Acad. Orthop. Surg. 2007;15(4):249–256. doi: 10.5435/00124635-200704000-00008. [DOI] [PubMed] [Google Scholar]
- Bastron J.A., Thomas J.E. Diabetic polyradiculopathy: clinical and electromyographic findings in 105 patients. Mayo Clin. Proc. 1981;56(12):725–732. [PubMed] [Google Scholar]
- Baute V., Strakowski J.A., Reynolds J.W., Karvelas K.R., Ehlers P., Brenzy K.J. Neuromuscular ultrasound of the brachial plexus: a standardized approach. Muscle Nerve. 2018;58(5):618–624. doi: 10.1002/mus.26144. [DOI] [PubMed] [Google Scholar]
- Beghi E., Kurland L.T., Mulder D.W., Nicolosi A. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970–1981. Ann. Neurol. 1985;18(3):320–323. doi: 10.1002/ana.410180308. [DOI] [PubMed] [Google Scholar]
- Bertelli J.A., Ghizoni M.F. The Towel Test: A useful technique for the clinical and electromyographic evaluation of obstetric brachial plexus palsy. J Hand Surgery. 2004;29(2):155–158. doi: 10.1016/j.jhsb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Bertelli J.A., Ghizoni M.F., Soldado F. Patterns of brachial plexus stretch palsy in a prospective series of 565 surgically treated patients. J Hand Surg. 2017;42(6):443–456. doi: 10.1016/j.jhsa.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Borenstein S., Desmedt J.E. Range of variations in motor unit potentials during reinnervation after traumatic nerve lesions in humans. Ann. Neurol. 1980;8(5):460–467. doi: 10.1002/ana.410080503. [DOI] [PubMed] [Google Scholar]
- Borschel G.H., Clarke H.M. Obstetrical brachial plexus palsy. Plast. Reconstr. Surg. 2009;124(Supplement):144e–155e. doi: 10.1097/PRS.0b013e3181a80798. [DOI] [PubMed] [Google Scholar]
- Bourque P.R., Warman Chardon J., Bryanton M., Toupin M., Burns B.F., Torres C. Neurolymphomatosis of the brachial plexus and its branches: case series andlLiterature review. Can. J. Neurol. Sci. 2018;45(2):137–143. doi: 10.1017/cjn.2017.282. [DOI] [PubMed] [Google Scholar]
- Bunnell A.E., Kao D.S. Planning interventions to treat brachial plexopathies. Phys. Med. Rehabil. Clin. N. Am. 2018;29(4):689–700. doi: 10.1016/j.pmr.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Capek S., Howe B.M., Amrami K.K., Spinner R.J. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg. Focus. 2015;39(3):E14. doi: 10.3171/2015.7.FOCUS15209. [DOI] [PubMed] [Google Scholar]
- Carvalho G.A., Nikkhah G., Matthies C., Penkert G., Samii M. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J. Neurosurg. 1997;86(1):69–76. doi: 10.3171/jns.1997.86.1.0069. [DOI] [PubMed] [Google Scholar]
- Chan, Y.C., Lo, Y.L., Chan, E.S. Immunotherapy for diabetic amyotrophy. Cochrane Database Syst. Rev. 2017;7(7):CD006521. Published 2017 Jul 26. doi:10.1002/14651858.CD006521.pub4. [DOI] [PMC free article] [PubMed]
- Chhabra A., Andreisek G., Soldatos T., Wang K.C., Flammang A.J., Belzberg A.J. MR neurography: past, present, and future. Am J Roentgenology. 2011;197(3):583–591. doi: 10.2214/AJR.10.6012. [DOI] [PubMed] [Google Scholar]
- Chin B., Ramji M., Farrokhyar F., Bain J.R. Efficient imaging: examining the value of ultrasound in the diagnosis of traumatic adult brachial plexus injuries, a systematic review. Neurosurgery. 2018;83(3):323–332. doi: 10.1093/neuros/nyx483. [DOI] [PubMed] [Google Scholar]
- Chiou-Tan F.Y., Kemp K., Elfenbaum M., Chan K.-T., Song J. Lumbosacral plexopathy in gunshot wounds and motor vehicle accidents. Am. J. Phys. Med. Rehabil. 2001;80(4):280–285. doi: 10.1097/00002060-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Chuang T.-Y., Chiou-Tan F.Y., Vennix M.J. Brachial plexopathy in gunshot wounds and motor vehicle accidents: comparison of electrophysiologic findings. Arch. Phys. Med. Rehabil. 1998;79(2):201–204. doi: 10.1016/s0003-9993(98)90300-8. [DOI] [PubMed] [Google Scholar]
- Coulet B., Boretto J.G., Lazerges C., Chammas M. A comparison of intercostal and partial ulnar nerve transfers in restoring elbow flexion following upper brachial plexus injury (C5–C6±C7) J. Hand Surg. 2010;35(8):1297–1303. doi: 10.1016/j.jhsa.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Cramer C.R. A reconditioning program to lower the recurrence rate of brachial plexus neurapraxia in collegiate football players. J. Athl. Train. 1999;34(4):390–396. [PMC free article] [PubMed] [Google Scholar]
- Crim J., Ingalls K. Accuracy of MR neurography in the diagnosis of brachial plexopathy. Eur. J. Radiol. 2017;95:24–27. doi: 10.1016/j.ejrad.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Cruz-Martínez A., Barrio M., Arpa J. Neuralgic amyotrophy: variable expression in 40 patients. J. Peripher. Nerv. Syst. 2002;7(3):198–204. doi: 10.1046/j.1529-8027.2002.02025.x. [DOI] [PubMed] [Google Scholar]
- Daube J.R. Rucksack paralysis. JAMA. 1969;208(13):2447–2452. [PubMed] [Google Scholar]
- Daube J.R., Rubin D.I. Needle electromyography. Muscle Nerve. 2009;39(2):244–270. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]
- Dorhout Mees S.M., Faals N.L., Alfen N. Backpack palsy and other brachial plexus neuropathies in the military population. J. Peripher Nerv. Syst. 2020;25(1):27–31. doi: 10.1111/jns.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn H.G., Daube J.R., Gomez M.R. Heredofamilial brachial plexus neuropathy (hereditary neuralgic amyotrophy with brachial predilection) in childhood. Dev. Med. Child Neurol. 2008;20(1):28–46. doi: 10.1111/j.1469-8749.1978.tb15179.x. [DOI] [PubMed] [Google Scholar]
- Dyck P., O'Brien P., Bosch E., Grant I., Burns T., Windebank A. The multi-center double-blind controlled trial of IV methylprednisolone in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 2006;66(5):A191. [Google Scholar]
- Dyck P.J.B. Non-diabetic lumbosacral radiculoplexus neuropathy: natural history, outcome and comparison with the diabetic variety. Brain. 2001;124(6):1197–1207. doi: 10.1093/brain/124.6.1197. [DOI] [PubMed] [Google Scholar]
- Dyck P.J.B., Engelstad J., Norell J., Dyck P.J. Microvasculitis in non-diabetic lumbosacral radiculoplexus neuropathy (LSRPN): similarity to the diabetic variety (DLSRPN) J. Neuropathol. Exp. Neurol. 2000;59(6):525–538. doi: 10.1093/jnen/59.6.525. [DOI] [PubMed] [Google Scholar]
- Dyck P.J.B., Norell J.E., Dyck P.J. Methylprednisolone may improve lumbosacral radiculoplexus neuropathy. J. Peripher. Nerv. Syst. 2002;7(1):65–66. [Google Scholar]
- Dyck P.J.B., Thaisetthawatkul P. Lumbosacral plexopathy. Continuum. 2014;20:1343–1358. doi: 10.1212/01.CON.0000455877.60932.d3. [DOI] [PubMed] [Google Scholar]
- Dyck P.J.B., Windebank A.J. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–491. doi: 10.1002/mus.10080. [DOI] [PubMed] [Google Scholar]
- Emami B., Lyman J., Brown A., Cola L., Goitein M., Munzenrider J.E. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- Feinberg J.H., Nguyen E.T., Boachie-Adjei K., Gribbin C., Lee S.K., Daluiski A. The electrodiagnostic natural history of parsonage–turner syndrome. Muscle Nerve. 2017;56(4):737–743. doi: 10.1002/mus.25558. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547–568. doi: 10.1002/mus.20131. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A. Electrodiagnostic assessment of the brachial plexus. Neurol. Clinics. 2012;30(2):551–580. doi: 10.1016/j.ncl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A. The thoracic outlet syndromes. Muscle Nerve. 2012;45(6):780–795. doi: 10.1002/mus.23235. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A., Ferrante N.D. The thoracic outlet syndromes: Part 1. Overview of the thoracic outlet syndromes and review of true neurogenic thoracic outlet syndrome. Muscle Nerve. 2017;55(6):782–793. doi: 10.1002/mus.25536. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A., Wilbourn A.J. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879–889. doi: 10.1002/mus.880180813. [DOI] [PubMed] [Google Scholar]
- Ferrante M.A., Wilbourn A.J. Lesion distribution among 281 patients with sporadic neuralgic amyotrophy. Muscle Nerve. 2017;55(6):858–861. doi: 10.1002/mus.25422. [DOI] [PubMed] [Google Scholar]
- Filosto M., Pari E., Cotelli M., Todeschini A., Vielmi V., Rinaldi F. MR Neurography in diagnosing nondiabetic lumbosacral radiculoplexus neuropathy. J. Neuroimaging. 2013;23(4):543–544. doi: 10.1111/jon.12039. [DOI] [PubMed] [Google Scholar]
- Franklin G.M., Fulton-Kehoe D., Bradley C., Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers' compensation. Neurology. 2000;54(6):1252–1258. doi: 10.1212/wnl.54.6.1252. [DOI] [PubMed] [Google Scholar]
- Friedenberg S., Hermann R. The breathing hand: obstetric brachial plexopathy reinnervation from thoracic roots? J. Neurol. Neurosurg. Psychiatry. 2004;75(1):158–160. [PMC free article] [PubMed] [Google Scholar]
- Fuzari H.K.B., Dornelas de Andrade A., Vilar C.F., Sayão L.B., Diniz P.R.B., Souza F.H. Diagnostic accuracy of magnetic resonance imaging in post-traumatic brachial plexus injuries: a systematic review. Clin. Neurol. Neurosurg. 2018;164:5–10. doi: 10.1016/j.clineuro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Garces-Sanchez M., Laughlin R.S., Dyck P.J., Engelstad J.K., Norell J.E., Dyck P.J.B. Painless diabetic motor neuropathy: a variant of diabetic lumbosacral radiculoplexus neuropathy? Ann. Neurol. 2011;69(6):1043–1054. doi: 10.1002/ana.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M.D., Mangwani J., Williams W.W. Aneurysm of the common iliac artery presenting as a lumbosacral plexopathy. J. Bone Joint Surg. Br. 2006;88-B(11):1524–1526. doi: 10.1302/0301-620X.88B11.17745. [DOI] [PubMed] [Google Scholar]
- Gilbert A., Tassin J. Surgical repair of the brachial plexus in obstetric paralysis. Chirurgie. 1984;110(1):70–75. [PubMed] [Google Scholar]
- Gilliatt R.W., Quesne P.M.L., Logue V., Sumner A.J. Wasting of the hand associated with a cervical rib or band. J. Neurol. Neurosurg. Psychiatry. 1970;33(5):615–624. doi: 10.1136/jnnp.33.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Zuckerman S.L., Dalton S.L., Djoko A., Folger D., Kerr Z.Y. A 6-year surveillance study of “Stingers” in NCAA American football. Res. Sports Med. 2017;25(1):26–36. doi: 10.1080/15438627.2016.1258642. [DOI] [PubMed] [Google Scholar]
- Haerle M., Gilbert A. Management of complete obstetric brachial plexus lesions. J. Pediatr. Orthop. 2004;24(2):194–200. doi: 10.1097/00004694-200403000-00012. [DOI] [PubMed] [Google Scholar]
- Harper C.M., Thomas J.E., Cascino T.L., Litchy W.J. Distinction between neoplastic and radiation-induced brachial plexopathy, with emphasis on the role of EMG. Neurology. 1989;39(4):502–506. doi: 10.1212/wnl.39.4.502. [DOI] [PubMed] [Google Scholar]
- Healey S., O'Neill B., Bilal H., Waterworth P. Does retraction of the sternum during median sternotomy result in brachial plexus injuries? Interact. Cardiovasc. Thorac. Surg. 2013;17(1):151–157. doi: 10.1093/icvts/ivs565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C.O., Lorenzetti L., Marchese A.J.T., Gherpelli J.L.D. Motor conduction studies for prognostic assessment of obstetrical plexopathy. Muscle Nerve. 2004;30(4):451–455. doi: 10.1002/mus.20121. [DOI] [PubMed] [Google Scholar]
- Heise C.O., Siqueira M.G., Martins R.S., Gherpelli J.L.D. Clinical-electromyography correlation in infants with obstetric brachial plexopathy. J. Hand Surg. 2007;32(7):999–1004. doi: 10.1016/j.jhsa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Impastato D.M., Impastato K.A., Dabestani P., Ko J.H., Bunnell A.E. Prognostic value of needle electromyography in traumatic brachial plexus injury. Muscle Nerve. 2019;60(5):595–597. doi: 10.1002/mus.26684. [DOI] [PubMed] [Google Scholar]
- Jaeckle K. Neurologic Manifestations of neoplastic and radiation-induced plexopathies. Semin. Neurol. 2010;30(03):254–262. doi: 10.1055/s-0030-1255219. [DOI] [PubMed] [Google Scholar]
- Jaeckle K.A., Young D.F., Foley K.M. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35(1):8–15. doi: 10.1212/wnl.35.1.8. [DOI] [PubMed] [Google Scholar]
- Kamenova B., Braverman A.S., Schwartz M., Sohn C., Lange C., Efiom-Ekaha D. Effective treatment of the brachial plexus syndrome in breast cancer patients by early detection and control of loco-regional metastases with radiation or systemic therapy. Int. J. Clin. Oncol. 2009;14(3):219–224. doi: 10.1007/s10147-008-0838-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Ota C., Yoneda T., Maki N., Urayama S., Nagao M. Incidence of stingers in young rugby players. Am. J. Sports Med. 2015;43(11):2809–2815. doi: 10.1177/0363546515597678. [DOI] [PubMed] [Google Scholar]
- Killer H.E., Hess K. Natural history of radiation-induced brachial plexopathy compared with surgically treated patients. J. Neurol. 1990;237(4):247–250. doi: 10.1007/BF00314628. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Cho Y.-J., Tiel R.L., Kline D.G. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J. Neurosurg. 2003;98(5):1005–1016. doi: 10.3171/jns.2003.98.5.1005. [DOI] [PubMed] [Google Scholar]
- Kim J., Jeon J.Y., Choi Y.J., Choi J.K., Kim S.-B., Jung K.H. Characteristics of metastatic brachial plexopathy in patients with breast cancer. Support. Care Cancer. 2019;28(4):1913–1918. doi: 10.1007/s00520-019-04997-6. [DOI] [PubMed] [Google Scholar]
- Kline D.G. Timing for brachial plexus injury: a personal experience. Neurosurg. Clin. N. Am. 2009;20(1):24–26. doi: 10.1016/j.nec.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Kori S.H., Foley K.M., Posner J.B. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31(1):45–50. doi: 10.1212/wnl.31.1.45. [DOI] [PubMed] [Google Scholar]
- Krarup C., Crone C. Neurophysiological studies in malignant disease with particular reference to involvement of peripheral nerves. J. Neurol. 2002;249(6):651–661. doi: 10.1007/s00415-002-0688-2. [DOI] [PubMed] [Google Scholar]
- Krivickas L.S., Wilbourn A.J. Peripheral nerve injuries in athletes: a case series of over 200 injuries. Semin. Neurol. 2000;20(2):225–232. doi: 10.1055/s-2000-9832. [DOI] [PubMed] [Google Scholar]
- Kutsy R.L., Robinson L.R., Routt M.L. Lumbosacral plexopathy in pelvic trauma. Muscle Nerve. 2000;23(11):1757–1760. doi: 10.1002/1097-4598(200011)23:11<1757::aid-mus13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ladha S.S., Spinner R.J., Suarez G.A., Amrami K.K., Dyck P.J.B. Neoplastic lumbosacral radiculoplexopathy in prostate cancer by direct perineural spread: an unusual entity. Muscle Nerve. 2006;34(5):659–665. doi: 10.1002/mus.20597. [DOI] [PubMed] [Google Scholar]
- Laughlin R.S., Dyck P.J.B. Electrodiagnostic testing in lumbosacral lexopathies. Phys. Med. Rehabil. Clin. N. Am. 2013;24(1):93–105. doi: 10.1016/j.pmr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Laughlin R.S., Dyck P.J.B. Elsevier; 2014. Diabetic Radiculoplexus Neuropathies. Diabetes and the Nervous System; pp. 45–52. [DOI] [PubMed] [Google Scholar]
- Lederman R.J., Wilbourn A.J. Brachial plexopathy: recurrent cancer or radiation? Neurology. 1984;34(10):1331–1335. doi: 10.1212/wnl.34.10.1331. [DOI] [PubMed] [Google Scholar]
- Levin K.H., Wilbourn A.J., Maggiano H.J. Cervical rib and median sternotomy-related brachial plexopathies: a reassessment. Neurology. 1998;50(5):1407–1413. doi: 10.1212/wnl.50.5.1407. [DOI] [PubMed] [Google Scholar]
- Levitz C.L., Reilly P.J., Torg J.S. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia. Am. J. Sports Med. 1997;25(1):73–76. doi: 10.1177/036354659702500114. [DOI] [PubMed] [Google Scholar]
- Lieba-Samal D., Jengojan S., Kasprian G., Wöber C., Bodner G. Neuroimaging of classic neuralgic amyotrophy. Muscle Nerve. 2016;54(6):1079–1085. doi: 10.1002/mus.25147. [DOI] [PubMed] [Google Scholar]
- Lumsden A.B., Miller J., Kosinski A., Allen R., Dodson T., Salam A. A prospective evaluation of surgically treated groin complications following percutaneous cardiac procedures. Am. Surgeon. 1994;60(2):132–137. [PubMed] [Google Scholar]
- Luthra K., Shah S., Purandare N., Medhi S., Rangarajan V., Samuel A.M. F-18 FDG PET-CT appearance of metastatic brachial plexopathy in a case of carcinoma of the breast. Clin. Nucl. Med. 2006;31(7):432–434. doi: 10.1097/01.rlu.0000223116.30927.48. [DOI] [PubMed] [Google Scholar]
- Magill S.T., Brus-Ramer M., Weinstein P.R., Chin C.T., Jacques L. Neurogenic thoracic outlet syndrome: current diagnostic criteria and advances in MRI diagnostics. Neurosurg. Focus. 2015;39(3):E7. doi: 10.3171/2015.6.FOCUS15219. [DOI] [PubMed] [Google Scholar]
- Martin E., Senders J.T., DiRisio A.C., Smith T.R., Broekman M.L.D. Timing of surgery in traumatic brachial plexus injury: a systematic review. J. Neurosurg. 2019;130(4):1333–1345. doi: 10.3171/2018.1.JNS172068. [DOI] [PubMed] [Google Scholar]
- Mazal A.T., Faramarzalian A., Samet J.D., Gill K., Cheng J., Chhabra A. MR neurography of the brachial plexus in adult and pediatric age groups: evolution, recent advances, and future directions. Expert Rev. Med. Devices. 2020;17(2):111–122. doi: 10.1080/17434440.2020.1719830. [DOI] [PubMed] [Google Scholar]
- McCormack E.P., Alam M., Erickson N.J., Cherrick A.A., Powell E., Sherman J.H. Use of MRI in diabetic lumbosacral radiculoplexus neuropathy: case report and review of the literature. Acta Neurochir. 2018;160(11):2225–2227. doi: 10.1007/s00701-018-3664-z. [DOI] [PubMed] [Google Scholar]
- Moghekar A.R., Moghekar A.R., Karli N., Chaudhry V. Brachial plexopathies: etiology, frequency, and electrodiagnostic localization. J. Clin. Neuromusc. Dis. 2007;9(1):243–247. doi: 10.1097/CND.0b013e3181450f7a. [DOI] [PubMed] [Google Scholar]
- Mondrup K., Olsen N.K., Pfeiffer P., Rose C. Clinical and electrodiagnostic findings in breast cancer patients with radiation-induced brachial plexus neuropathy. Acta Neurol. Scand. 1990;81(2):153–158. doi: 10.1111/j.1600-0404.1990.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Ng P.S., Dyck P.J., Laughlin R.S., Thapa P., Pinto M.V., Dyck P.J.B. Lumbosacral radiculoplexus neuropathy: incidence and the association with diabetes mellitus. Neurology. 2019;92(11):e1188–e1194. doi: 10.1212/WNL.0000000000007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage M.J., Aldren Turner J.W. Neuralgic amyotrophy: the shoulder-girdle syndrome. Lancet. 1948;251(6513):973–978. doi: 10.1016/s0140-6736(48)90611-4. [DOI] [PubMed] [Google Scholar]
- Pascoe M.K., Low P.A., Windebank A.J., Litchy W.J. Subacute diabetic proximal neuropathy. Mayo Clin. Proc. 1997;72(12):1123–1132. doi: 10.4065/72.12.1123. [DOI] [PubMed] [Google Scholar]
- Pham M., Baumer P., Meinck H.M., Schiefer J., Weiler M., Bendszus M. Anterior interosseous nerve syndrome: fascicular motor lesions of median nerve trunk. Neurology. 2014;82(7):598–606. doi: 10.1212/WNL.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt M., Vredeveld J.-W. The role of electromyography in the management of the brachial plexus palsy of the newborn. Clin. Neurophys. 2005;116(8):1756–1761. doi: 10.1016/j.clinph.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Pondaag W., Malessy M.J.A. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J. Neurosurg. Pediatr. 2006;105(1):33–40. doi: 10.3171/ped.2006.105.1.33. [DOI] [PubMed] [Google Scholar]
- Pritchard J., Anand P., Broome J., Davis C., Gothard L., Hall E. Double-blind randomized phase II study of hyperbaric oxygen in patients with radiation-induced brachial plexopathy. Radiother. Oncol. 2001;58(3):279–286. doi: 10.1016/s0167-8140(00)00319-4. [DOI] [PubMed] [Google Scholar]
- Qi H.T., Wang X.M., Li S.Y., Wang G.B., Wang D.H., Wang Z.T. The role of ultrasonography and MRI in patients with non-traumatic nerve fascicle torsion of the upper extremity. Clin. Radiol. 2013;68(9):e479–e483. doi: 10.1016/j.crad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Robinson L.R. How electrodiagnosis predicts clinical outcome of focal peripheral nerve lesions. Muscle Nerve. 2015;52(3):321–333. doi: 10.1002/mus.24709. [DOI] [PubMed] [Google Scholar]
- Rose K., Davies A., Pitt M., Ratnasinghe D., D'Argenzio L. Backpack palsy: a rare complication of backpack use in children and young adults – a new case report. Eur. J. Paediatr. Neurol. 2016;20(5):750–753. doi: 10.1016/j.ejpn.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Rotondo E., Pellegrino N., Di Battista C., Graziosi A., Di Stefano V., Striano P. Clinico-diagnostic features of neuralgic amyotrophy in childhood. Neurol. Sci. 2020;41(7):1735–1740. doi: 10.1007/s10072-020-04314-8. [DOI] [PubMed] [Google Scholar]
- Rubin D.I. Neuralgic amyotrophy: clinical features and diagnostic evaluation. Neurologist. 2001;7(6):350–356. doi: 10.1097/00127893-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Rubin DI. Diseases of the plexus. in Spinal Cord, Root, and Plexus Disorders. Continuum 2008; 14: 156-179. doi: 10.1212/01.CON.0000324129.85559.74.
- Sacco G., Buchthal F., Rosenfalck P. Motor unit potentials at different ages. Arch. Neurol. 1962;6(5):366–373. doi: 10.1001/archneur.1962.00450230028004. [DOI] [PubMed] [Google Scholar]
- Sarikaya S., Sumer M., Özdolap Ş., Erdem C.Z. Magnetic resonance neurography diagnosed brachial plexitis: a case report. Arch. Phys. Med. Rehabil. 2005;86(5):1058–1059. doi: 10.1016/j.apmr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Scalf R.E., Wenger D.E., Frick M.A., Mandrekar J.N., Adkins M.C. MRI findings of 26 patients with Parsonage-Turner syndrome. Am. J. Roentgenol. 2007;189(1):W39–W44. doi: 10.2214/AJR.06.1136. [DOI] [PubMed] [Google Scholar]
- Schwarz G.A. Posttraumatic respiratory-biceps brachii synkinesis. Arch. Neurol. 1965;13(4):427–431. doi: 10.1001/archneur.1965.00470040093015. [DOI] [PubMed] [Google Scholar]
- Seror P. Brachial plexus neoplastic lesions assessed by conduction study of medial antebrachial cutaneous nerve. Muscle Nerve. 2001;24(8):1068–1070. doi: 10.1002/mus.1111. [DOI] [PubMed] [Google Scholar]
- Seror P. Medial antebrachial cutaneous nerve conduction study, a new tool to demonstrate mild lower brachial plexus lesions. A report of 16 cases. Clin. Neurophys. 2004;115(10):2316–2322. doi: 10.1016/j.clinph.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Seror P. Neuralgic amyotrophy. An update. Joint Bone Spine. 2017;84(2):153–158. doi: 10.1016/j.jbspin.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Sherburn E.W., Kaplan S.S., Kaufman B.A., Noetzel M.J., Park T.S. Outcome of surgically treated birth-related brachial plexus injuries in twenty cases. Pediatr. Neurosurg. 1997;27(1):19–27. doi: 10.1159/000121220. [DOI] [PubMed] [Google Scholar]
- Sneag D.B., Rancy S.K., Wolfe S.W., Lee S.C., Kalia V., Lee S.K. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome. Muscle Nerve. 2018;58(3):359–366. doi: 10.1002/mus.26108. [DOI] [PubMed] [Google Scholar]
- Sneag D.B., Saltzman E.B., Meister D.W., Feinberg J.H., Lee S.K., Wolfe S.W. MRI bullseye sign: an indicator of peripheral nerve constriction in parsonage-turner syndrome. Muscle Nerve. 2017;56(1):99–106. doi: 10.1002/mus.25480. [DOI] [PubMed] [Google Scholar]
- Soto O. Radiation-induced conduction block: resolution following anticoagulant therapy. Muscle Nerve. 2005;31(5):642–645. doi: 10.1002/mus.20273. [DOI] [PubMed] [Google Scholar]
- Spinner R.J., Kline D.G. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23(5):680–695. doi: 10.1002/(sici)1097-4598(200005)23:5<680::aid-mus4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Spires M.C., Leonard J.A., Wolfe J. Practical Management of Pediatric and Adult Brachial Plexus Palsies. Elsevier; 2012. The role of electrodiagnosis in infants with brachial plexus palsies; pp. 68–74. [Google Scholar]
- Stowe H.B., Mullins B.T., Chera B.S. Hyperbaric oxygen therapy for radiation-induced brachial plexopathy, a case report and literature review. Rep. Pract. Oncol. Radiother. 2020;25(1):23–27. doi: 10.1016/j.rpor.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski J.A. Electrodiagnosis of plexopathy. PMR. 2013;5:S50–S55. doi: 10.1016/j.pmrj.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Suarez G.A., Giannini C., Bosch E.P., Barohn R.J., Wodak J., Ebeling P. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis. Neurology. 1996;46(2):559–561. doi: 10.1212/wnl.46.2.559. [DOI] [PubMed] [Google Scholar]
- Sunderland S.S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13(9):771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- Swift T.R., Leshner R.T., Gross J.A. Arm-diaphragm synkinesis: electrodiagnostic studies of aberrant regeneration of phrenic motor neurons. Neurology. 1980;30(4):339–344. doi: 10.1212/wnl.30.4.339. [DOI] [PubMed] [Google Scholar]
- Taylor B.V., Kimmel D.W., Krecke K.N., Cascino T.L. Magnetic resonance imaging in cancer-related lumbosacral plexopathy. Mayo Clin. Proc. 1997;72(9):823–829. doi: 10.4065/72.9.823. [DOI] [PubMed] [Google Scholar]
- Tender G.C., Thomas A.J., Thomas N., Kline D.G. Gilliatt-Sumner hand revisited: a 25-year experience. Neurosurgery. 2004;55(4):883–890. doi: 10.1227/01.neu.0000137889.51323.0d. [DOI] [PubMed] [Google Scholar]
- Terzis J.K., Barbitsioti A. Primary restoration of elbow flexion in adult post-traumatic plexopathy patients. J. Plast. Reconstr. Aesthet. Surg. 2012;65(1):72–84. doi: 10.1016/j.bjps.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Terzis J.K., Vekris M.D., Soucacos P.N. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast. Reconstr. Surg. 1999;104(5):1221–1240. doi: 10.1097/00006534-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Thawait S., Chaudhry V., Thawait G., Wang K., Belzberg A., Carrino J. High-resolution MR neurography of diffuse peripheral nerve lesions. Am. J. Neuroradiol. 2011;32(8):1365–1372. doi: 10.3174/ajnr.A2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.E., McCullen G.M., Yuan H.A. Cervical spine injuries in football players. J. Am. Acad. Orthop. Surg. 1999;7(5):338–347. doi: 10.5435/00124635-199909000-00006. [DOI] [PubMed] [Google Scholar]
- Thomas J.E., Cascino T.L., Earle J.D. Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology. 1985;35(1):1–7. doi: 10.1212/wnl.35.1.1. [DOI] [PubMed] [Google Scholar]
- Triggs W.J., Young M.S., Eskin T., Valenstein E. Treatment of idiopathic lumbosacral plexopathy with intravenous immunoglobulin. Muscle Nerve. 1997;20(2):244–246. doi: 10.1002/(sici)1097-4598(199702)20:2<244::aid-mus20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Tsairis P., Dyck P.J., Mulder D.W. Natural history of brachial plexus neuropathy: report on 99 patients. Arch. Neurol. 1972;27(2):109–117. doi: 10.1001/archneur.1972.00490140013004. [DOI] [PubMed] [Google Scholar]
- Tsao B.E., Ferrante M.A., Wilbourn A.J., Shields R.W. Electrodiagnostic features of true neurogenic thoracic outlet syndrome. Muscle Nerve. 2014;49(5):724–727. doi: 10.1002/mus.24066. [DOI] [PubMed] [Google Scholar]
- Tsao B.E., Ostrovskiy D.A., Wilbourn A.J., Shields R.W. Phrenic neuropathy due to neuralgic amyotrophy. Neurology. 2006;66(10):1582–1584. doi: 10.1212/01.wnl.0000216140.25497.40. [DOI] [PubMed] [Google Scholar]
- Tsao B.E., Wilbourn A.J. The medial brachial fascial compartment syndrome following axillary arteriography. Neurology. 2003;61(8):1037–1041. doi: 10.1212/01.wnl.0000089488.35632.08. [DOI] [PubMed] [Google Scholar]
- Unlu Y., Velioglu Y., Kocak H., Becit N., Ceviz M. Brachial plexus injury following median sternotomy. Interact. Cardiovasc. Thorac. Surg. 2006;6(2):235–237. doi: 10.1510/icvts.2006.137380. [DOI] [PubMed] [Google Scholar]
- Vaccaro A.R., Klein G.R., Ciccoti M., Pfaff W.L., Moulton M.J.R., Hilibrand A.J. Return to play criteria for the athlete with cervical spine injuries resulting in stinger and transient quadriplegia/paresis. Spine J. 2002;2(5):351–356. doi: 10.1016/s1529-9430(02)00202-4. [DOI] [PubMed] [Google Scholar]
- van Alfen N. Histology of hereditary neuralgic amyotrophy. J. Neurol. Neurosurg. Psychiatry. 2005;76(3):445–447. doi: 10.1136/jnnp.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat. Rev. Neurol. 2011;7(6):315–322. doi: 10.1038/nrneurol.2011.62. [DOI] [PubMed] [Google Scholar]
- van Alfen N., Doorduin J., van Rosmalen M.H.J., van Eijk J.J.J., Heijdra Y., Boon A.J. Phrenic neuropathy and diaphragm dysfunction in neuralgic amyotrophy. Neurology. 2018;91(9):e843–e849. doi: 10.1212/WNL.0000000000006076. [DOI] [PubMed] [Google Scholar]
- van Alfen N., van Eijk J.J.J., Ennik T., Flynn S.O., Nobacht I.E.G., Groothuis J.T. Incidence of neuralgic amyotrophy (Parsonage Turner Syndrome) in a primary care setting - a prospective cohort study. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0128361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alfen N., van Engelen B.G.M. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(2):438–450. doi: 10.1093/brain/awh722. [DOI] [PubMed] [Google Scholar]
- Van der Looven R., Le Roy L., Tanghe E., Van den Broeck C., De Muynck M., Vingerhoets G. Early electrodiagnosis in the management of neonatal brachial plexus palsy: a systematic review. Muscle Nerve. 2020;61(5):557–566. doi: 10.1002/mus.26762. [DOI] [PubMed] [Google Scholar]
- van Eijk J.J.J., van Alfen N., Berrevoets M., van der Wilt G.J., Pillen S., van Engelen B.G.M. Evaluation of prednisolone treatment in the acute phase of neuralgic amyotrophy: an observational study. J. Neurol. Neurosurg. Psychiatry. 2009;80(10):1120–1124. doi: 10.1136/jnnp.2008.163386. [DOI] [PubMed] [Google Scholar]
- van Es H.W., Engelen A.M., Witkamp T.D., Ramos L.M.P., Feldberg M.A.M. Radiation-induced brachial plexopathy: MR imaging. Skeletal Radiol. 1997;26(5):284–288. doi: 10.1007/s002560050236. [DOI] [PubMed] [Google Scholar]
- Wade R.G., Takwoingi Y., Wormald J.C.R., Ridgway J.P., Tanner S., Rankine J.J. MRI for detecting root avulsions in traumatic adult brachial plexus injuries: a systematic review and meta-analysis of diagnostic accuracy. Radiology. 2019;293(1):125–133. doi: 10.1148/radiol.2019190218. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kawabata S., Hoshino Y., Ushio S., Sasaki T. Novel function imaging technique for the brachail plexus based on magnetoneurography. Clin. Neurophysiol. 2019;130:2114–2123. doi: 10.1016/j.clinph.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Watts G.D.J., O'Briant K.C., Borreson T.E., Windebank A.J., Chance P.F. Evidence for genetic heterogeneity in hereditary neuralgic amyotrophy. Neurology. 2001;56(5):675–678. doi: 10.1212/wnl.56.5.675. [DOI] [PubMed] [Google Scholar]
- Weiler-Sagie M., Bushelev O., Epelbaum R., Dann E.J., Haim N., Avivi I. 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. J. Nucl. Med. 2010;51(1):25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- Wilbourn A.J. Electrodiagnosis of plexopathies. Neurol. Clin. 1985;3(3):511–529. [PubMed] [Google Scholar]
- Yan M., Kong W., Kerr A., Brundage M. The radiation dose tolerance of the brachial plexus: a systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2019;18:23–31. doi: 10.1016/j.ctro.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.J.S. Neonatal brachial plexus palsy—Management and prognostic factors. Semin. Perinatol. 2014;38(4):222–234. doi: 10.1053/j.semperi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Yldzgören M.T., Ekiz T., Kara M., Yörübulut M., Özçakar L. Magnetic resonance imaging of a fibrous band causing true neurogenic thoracic outlet syndrome. Am. J. Phys. Med. Rehabil. 2014;93(8):732–733. doi: 10.1097/PHM.0000000000000090. [DOI] [PubMed] [Google Scholar]
- Zara G., Gasparotti R., Manara R. MR imaging of peripheral nervous system involvement: Parsonage-Turner Syndrome. J. Neurol. Sci. 2012;315(1–2):170–171. doi: 10.1016/j.jns.2011.11.020. [DOI] [PubMed] [Google Scholar]







