Highlights
-
•
AQP4 and GFAP were expressed in insulin-treated C6 cells.
-
•
In C6 cells, the glutamate transport was found to be EAAC1, not GLT-1.
-
•
High dose of Aβ reduced the expressions of AQP4, GFAP, and EAAC1.
-
•
Glutamate uptake in C6 cells was inhibited by Aβ in a dose-dependent manner.
Keywords: Aquaporin 4, Amyloid β, Glutamate transporter excitatory amino acid carrier 1, Glutamate uptake, Alzheimer’s disease
Abstract
Astrocytic aquaporin 4 (AQP4) facilitates glutamate clearance via regulation of the glutamate transporter function, involved in the modulation of brain plasticity and cognitive function to prevent neurodegenerative disorders such as Alzheimer’s disease (AD). In in vitro studies, the C6 rat glioma cell line is a widely applied aging model system to investigate changes in glial cells associated with aging or AD. However, the neurotoxicity mechanism whether AQP4 mediate glutamate uptake in Aβ-stimulated C6 cell remain uncertain. In this study, we examined the effects of Aβ on the expression of AQP4, Glu transporters, Glu uptake, and cell viability in insulin-treated C6 cells. Our results showed that the expression of AQP4 mRNA and protein was significantly enhanced by insulin in older cultures (passage 45), and the expression was inhibited by Aβ at 10 μM. In addition, the cell viability and glutamate uptake in Aβ-treated C6 cells were decreased in dose-dependent manners. GFAP showed similar changes in gene and protein expression patterns as AQP4, but no significant alterations were seen in GLAST expression. In C6 cells, the glutamate transport was found to be EAAC1, not GLT-1. EAAC1 expression was decreased by the treatment of Aβ. Taken together, our findings suggest that C6 cells may have astrocytic characteristics, and the astrocytic cytotoxicity induced by Aβ was mediated by reduction of glutamate uptake through AQP4/EAAC1 pathway in C6 cells. This indicates that C6 glioma cells could be used to study the roles of AQP4 on astrocyte function in AD.
1. Introduction
Alzheimer's disease (AD) is known as senile dementia, and the formation of amyloid plaques and nerve fiber entanglement is the general cause of AD. However, to date, there are no molecular targets to prevent the onset of AD and disease progression [[1], [2], [3], [4], [5]].
Glutamate (Glu) is an excitatory neurotransmitter essential for synaptic plasticity, excitatory synaptic transmission, and neuronal development. However, excessive Glu accumulation in synaptic clefts is known to cause neurodegenerative diseases such as ischemia, epilepsy and amyotrophic lateral sclerosis [6]. Thus, regulation of Glu outside the cells is essential for excitotoxicity prevention and normal synaptic transmission. Glu transporter is the primary mechanism for removal of the released Glu. Rothstein et al. [7] revealed that both neurons and astrocytes can act as high affinity Glu transporters. Glu levels outside the astrocyte or glial cells are maintained at low concentrations by Glu transporters via activation of both neurons and glial cells. Glu transporters, such as glutamate transporter 1 (GLT-1), glutamate aspartate transporter (GLAST), and excitatory amino acid transporter 1 (EAAC1), are widely distributed throughout the CNS [[8], [9], [10]]. These transporters play important roles as glutamate, and are thought to modulate the formation and elimination of synapses as well as neuronal migration, proliferation and apoptosis [11]. In addition, Glu transporters including EAAC1, GLAST and GLT-1 in rats and rabbits are localized to the astrocytes and neurons [7,12]. GLAST and GLT-1 are expressed in the astrocyte, whereas EAAC1 and GLAST are expressed in the glial cells [[13], [14], [15]]. Also, β-amyloid (Aβ) accumulation outside the glial cells, astrocytes, and neurons is revealed the outcome of Glu transporter dysfunction [16].
Aquaporins (AQPs) are a group of water-selective membrane transport channels that allow rapid transfer of water. In the normal brain, aquaporin-1 and aquaporin-4 (AQP4) are the most studied, also AQP4 is a major isoform of AQPs in the adult brain. It is mainly expressed in astrocytes of the central nervous system, and AQP4 is well known to support the regulation of astrocytic functions. Several studies have revealed that AQP4 plays roles in synaptic plasticity, contributing to memory by regulating the Glu transporter expression. Recent studies have revealed that AQP4 knockout or downregulation exacerbates brain fluid and ion homeostasis [[17], [18], [19], [20]], is involved in cell migration and neuronal scar formation [17,21], and is associated with astrocytic functions such as neurotransmission and synaptic plastic deformation [22,23]. Furthermore, it is reported to cause a disorder resulting in the secretion of the inflammation inducer [24]. Thus, AQP4 is being investigated not only for mental disorders including depression, but also for various diseases of the nervous system [[25], [26], [27], [28]].
Glial cells are known to regulate Aβ accumulation through AQP4 expression in vivo. However, the results of current in vitro studies on the expression of AQP4 in glial cell line are unclear. Capoccia et al. [24] reported that the AQP4 inhibition in C6 cells affects cell migration and apoptosis. Contrarily, Dolman et al. [25] and Yoneda et al. [26] found that the C6 cells were unable to show the AQP4 expression.
Insulin is widely expressed in the brain, and increases astrocytic expression in cultures of glial cells. Goya et al. [28] reported that insulin treatment promotes C6 glioma cell differentiation, thereby having astrocytic properties by activating enzyme activity of glutamine synthetase, an astrocyte biomarker. Therefore, insulin had been used as a differentiation agent for realizing the properties of astrocytes in C6 glioma cells in this study.
In astrocytes, decreasing level of GFAP leads to the activation of caspase-3 and cell damage, which are common features in AD [29,30]. In addition, Varmazyari et al. [31] revealed that GFAP determination of cortex and cerebellum in rats inhibited by neuronal toxicity. Treatment with Aβ peptides results in apoptosis of astrocytes or neurons, with the subsequent activation of cleaved caspase-3. The activation of cleaved caspase-3 caused by apoptosis of Aβ peptides-treated astrocyte or neuron. Zhang et al. [32] reported that activation of Bax and inhibition of bcl-2 induces Cytochrome C which, in turn, commits the cell to apoptosis by activating the release of caspase related proteins.
In this study, we investigate the expression of AQP4 in response to Aβ in the presence of insulin as a differentiation promoting agent. In the insulin-treated C6 cells, effects of Aβ peptide on the expression of AQP4, Glu transporters, Glu uptake, and cell viability were examined to observe the changes in astrocytic responses in Aβ-stimulated C6 glioma cells.
2. Materials and methods
2.1. Reagents
MTT (3-(4,5-dimethylthiazol-2-yl)2-,5-diphenyltetrazolium bromide), insulin, wortmannin were purchased from Sigma-Aldrich (St. Louis, MO). Antibody against AQP4 (sc-32739) was purchased from Santa Cruz Biotechnology (California, USA), cleaved caspase-3 (#9664), GLT-1 (#3838), GLAST (#5684), EAAC1 (#14501), GFAP (#80788), and horseradish peroxidase-linked anti-mouse secondary antibody (#7076), horseradish peroxidase-linked anti-rabbit secondary antibody (#7074) were purchased from Cell Signaling Technology (Beverly, MA, USA). The β-amyloid25-35 (Aβ25-35) and β-amyloid1-42 (Aβ1-42) were purchased from Abcam Biotechnology (Cambridge, UK). TGN-020 was purchased from Tocris Bioscience (Bristol, United Kingdom). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM) were purchased from the American Type Culture Collection (Manassas, VA, USA).
2.2. Cell culture
C6 cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM supplemented with penicillin (120 units/mL), streptomycin (120 units/mL), and 10 % FBS in a 5 % CO2 atmosphere at 37 ℃.
2.3. Reverse transcription polymerase chain reaction (RT-PCR)
C6 cells were seeded into a 6-well cell culture plate (1 × 105 cells per well, passage number 45) for 24 h. And the aggregated Aβ1-42 or Aβ25-35 (0.1, 0.5, 1, 5 or 10 μM) with insulin (10 ng/mL) treated with C6 cells for 48 h. Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA purity and concentration was measured by spectrometry at 260, 280 nm. Total RNA was reverse transcribed into cDNA using a SuperScript® II Reverse Transcriptase (18064-014, Thermo Fisher Scientific, Inc., Waltham, USA) according to the manufacturer's protocol. The amplification conditions were as follows: AQP4, 45 s at 98 ℃ followed by 30 cycles of 10 s at 98 ℃, 30 s at 57 ℃ and 60 s at 72 ℃; GLT-1, 45 s at 98 ℃ followed by 30 cycles of 10 s at 98 ℃, 30 s at 55 ℃ and 60 s at 72 °C; GLAST, 45 s at 98 ℃ followed by 30 cycles of 10 s at 98 ℃, 30 s at 60 ℃ and 60 s at 72 ℃; EAAC1, 45 s at 98 ℃ followed by 30 cycles of 10 s at 98 ℃, 30 s at 54 ℃ and 60 s at 72 ℃; GFAP, 45 s at 98 °C followed by 30 cycles of 10 s at 98 ℃, 30 s at 60 ℃ and 60 s at 72 ℃; β-actin, 45 s at 98 °C followed by 30 cycles of 10 s at 98 ℃, 30 s at 58 ℃ and 60 s at 72 ℃. Primers sequences were as follows: AQP4 forward, 5'GC ATG AAT CCA GCT CGA TCC TTT GG-3′ and reverse, 5'-AA TGG GTG GCA GGA AAT CTG AGG C3′ (product, 315 bp); GLT-1 forward, 5′-TAC AGC CCT TTA CGA AGC C-3′ and reverse, 5′-TGA TAG ACA ATC CCA GCC C-3′ (product, 242 bp); GLAST forward, 5′-CTA CTCA CCG TCA GCG CTG T-3′ and reverse, 5′-AGC ACA AAT CTG GTG ATG CG-3′ (product, 1012 bp); EAAC1 forward, 5‘-TGT TAG TTG TGA GCA TCA AGC-3′ and reverse, 5′-CCT TTT TCT CCC ATT TTT CC-3′ (product, 324 bp); GFAP forward, 5′-GGT GTC CAG GCT GGT TTC TC-3′ and reverse, 5′-CAA GCC AGA CCT CAC AGC G-3′ (product, 515 bp); βactin forward, 5'-GA GGC ATC CTG ACC CTG AAG-3′ and reverse, 5'CA TCA CAA TGC CAG TGG TAC G-3′ (product, 433 bp). Differential expression was calculated by analysis of the target gene amplification following normalization to the rat β-actin endogenous reference.
2.4. Western blot
C6 cells were seeded into a 6-well cell culture plate (1 × 105 cells per well, passage number 45) for 24 h, and then treated with aggregated Aβ1-42 or Aβ25-35 (0.1, 0.5, 1, 5 or 10 μM) or TGN-020 (AQP4 inhibitor, 1 μM) or wortmannin (EAAC1 inhibitor, 1 μM) with insulin (10 ng/mL) for 48 h. C6 cells were lysed in membrane protein extraction kit (Thermo Fisher Scientific, Inc., Waltham, USA). Membrane protein concentrations were measured using a BCA protein assay (Thermo Fisher Scientific, Waltham, USA). Membrane proteins (100 μg) were separated by 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to PVDF membranes (Whatman GmbH, Dassel, Germany). The transferred proteins blocked with 5% skimmed milk in Tris-buffered saline containing 0.1 % Tween-20 for 1 h and then incubated with AQP4 (1:200 dilution), cleaved caspase-3 (1:500 dilution), GLT-1 (1:200 dilution), GLAST (1:200 dilution), EAAC1 (1:200 dilution), GFAP (1:200 dilution), β-actin (1:1,000 dilution) for overnight at 4 ℃. After three washes in Tris-buffered saline containing 0.1 % Tween-20, membranes were incubated with horseradish peroxidase-linked anti-mouse secondary antibody for 1 h. Proteins were detected by enhanced chemiluminescence and visualized using image software (UVP Vision Works® LS Image Acquisition & Analysis Software, Upland, CA).
2.5. Cell viability assay
The Aβ-treated C6 cell viabilities were measured using MTT colorimetric assay. Briefly, C6 cells (5 × 104 cells per well, passage number 45) were seeded in a 24-well cell culture plate. And the aggregated Aβ1-42 or Aβ25-35 (0.1, 0.5, 1, 5 or 10 μM) with insulin (10 ng/mL) were treated with seeded C6 cells for 48 h. The produced formazan crystals were dissolved in 500 μL of DMSO, and absorbances were measured by spectrophotometry using a microplate reader (Bio-Tek Instruments, Winoosk, VT, USA) at 550 nm.
2.6. Glutamate uptake
Glu uptake was studied in C6 cells seeded at 1 × 105 cells per 6-well cell culture plate (passage number 45). After 24 h incubation, the cells were treated with various concentrations of aggregated Aβ1-42 or Aβ25-35 (0.1, 0.5, 1, 5, 10 μM) with insulin (10 ng/mL). After 48 h incubation, the cells lysates were prepared and luciferase activity was determined using the Glutamate-Glo™ assay (TM495, Promega Corporation, Madison, Wisconsin, USA) and luminescence determined using a luminometer (GM2000, Promega Corporation, Madison, Wisconsin, USA).
2.7. Statistical analysis
Results are presented as means ± standard deviations ellipse (SDE) values of three experiments. A significant difference from the respective control for each experimental test condition was assessed using Student’s t-test for each paired experiment, and significant difference is considered at p < 0.05, p < 0.01 and p < 0.001.
3. Results
3.1. Effects on AQP4 levels in insulin and Aβ and insulin-treated C6 cells
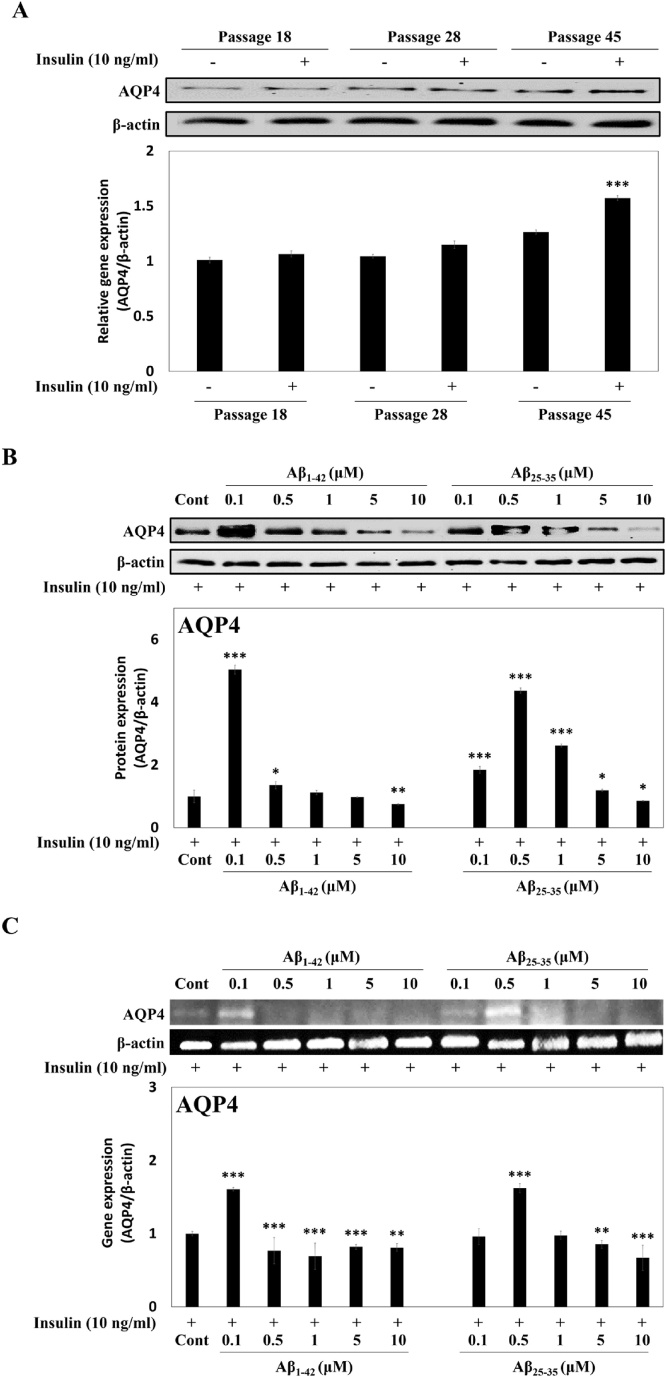
Changes in AQP4 levels were investigated in insulin-treated C6 cells (passage 15, 28, 45). The normal early (passage 18, 28) or elder (passage 45) C6 cells showed weak AQP4 protein expression (Fig. 1A, lane 1, 3, 5). However, exposure of the elder C6 cells to insulin enhanced the AQP4 protein expression about 1.6-fold (Fig. 1A, lane 5, 6). Aβ1-42 or Aβ25-35 of lower concentration increased the AQP4 levels (5.04-fold at 0.1 μM, 4.37-fold at 0.5 μM, respectively); however, suppression of AQP4 expression was observed after exposure to high concentration (10 μM) of Aβ1-42 (0.75-fold) and Aβ25-35 (0.86-fold) (Fig. 1B). In addition, the AQP4 mRNA expression in Aβ1-42 (0.1 μM) or Aβ25-35 (0.5 μM)-treated C6 cells was up-regulated about 1.6-fold compared to the control cells (Fig. 1C), but at 10 μM Aβ peptides, the AQP4 mRNA expressions was significantly decreased.
Fig. 1.
AQP4 expressions in C6 cells under various conditions, (A) effects of cell passages and insulin on the AQP4 protein expression, (B) the AQP4 protein expression in Aβ1-42 or Aβ25-35-treated C6 cells in the presence of insulin, (C) the AQP4 gene expression in Aβ1-42 or Aβ25-35-treated C6 cells in the presence of insulin. β-Actin was used as the internal control for Western blot and RT-PCR analysis. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. insulin alone (n = 3).
3.2. Effect of Aβ1-42 or Aβ25-35 on the cytotoxicity and glutamate uptake in insulin-treated C6 cells
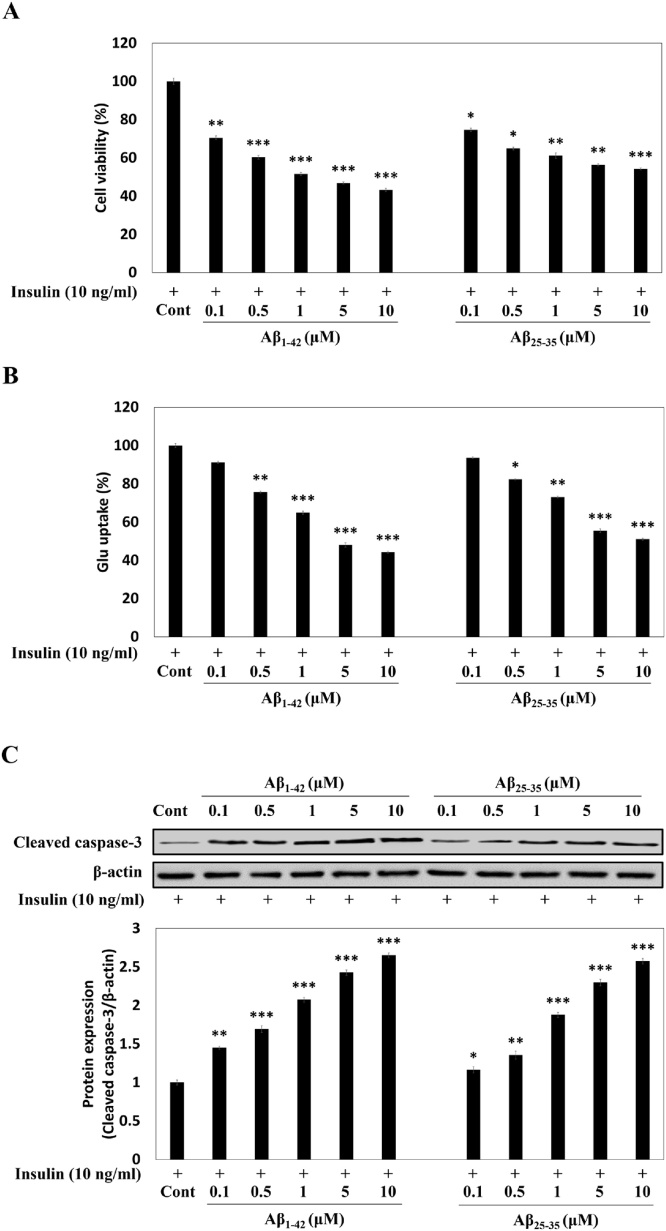
Aβ1-42 or Aβ25-35 reduced the cell viability up to 48.4 % or 55.1 % at the concentration of 10 μM in insulin-treated C6 cells (passage number 45) (Fig. 2A). Under the same condition, Glu uptake in the cells (passage number 45) was also inhibited by the Aβ peptides in a dose-dependent manner (Fig. 2B), suggesting that Aβ induced accumulation of Glu and is assumed to cause cell death.
Fig. 2.
Effects of Aβ peptides on the cell viability (A), Glu uptake (B), and caspase-3 protein expression (C), in insulin-treated C6 cells (passage number 45). TGN-020 was used as an AQP4 inhibitor, and wortmannin used as an EAAC1 inhibitor in the insulin-treated C6 cells. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. insulin alone (n = 3).
Caspase-3 is considered to be a phenotype of apoptosis, and is responsible for the maturation of procaspase-3. Cleavages of caspase-3 substrates are reported to play a vital role in neuronal apoptosis [25]. We therefore performed Western blot analysis to examine the effect of Aβ peptide treatment on caspase-3 protein expression in insulin-treated C6 cells (passage number 45). Exposure of insulin-treated C6 cells to Aβ1-42 (0.1−10 μM) dose-dependently upregulated the cleaved caspase-3 by approximately 1.45-, 1.69-, 2.08-, 2.43-, and 2.65-fold, respectively, as compared to the insulin-treated C6 cells (Fig. 2C). Moreover, Aβ25-35 exposure (0.1−10 μM) also promoted the dose-dependent increase of cleaved caspase-3 protein expression by 1.16-, 1.36-, 1.88-, 2.30-, and 2.57-fold, respectively, as compared to insulin-treated C6 cells. Our data therefore confirms that apoptotic cell death is the outcome of Aβ accumulation or targeted Aβ sequence part in C6 cells.
3.3. Effects of Aβ1-42 or Aβ25-35 on the expressions of Glu receptors and GFAP in insulin-treated C6 cells
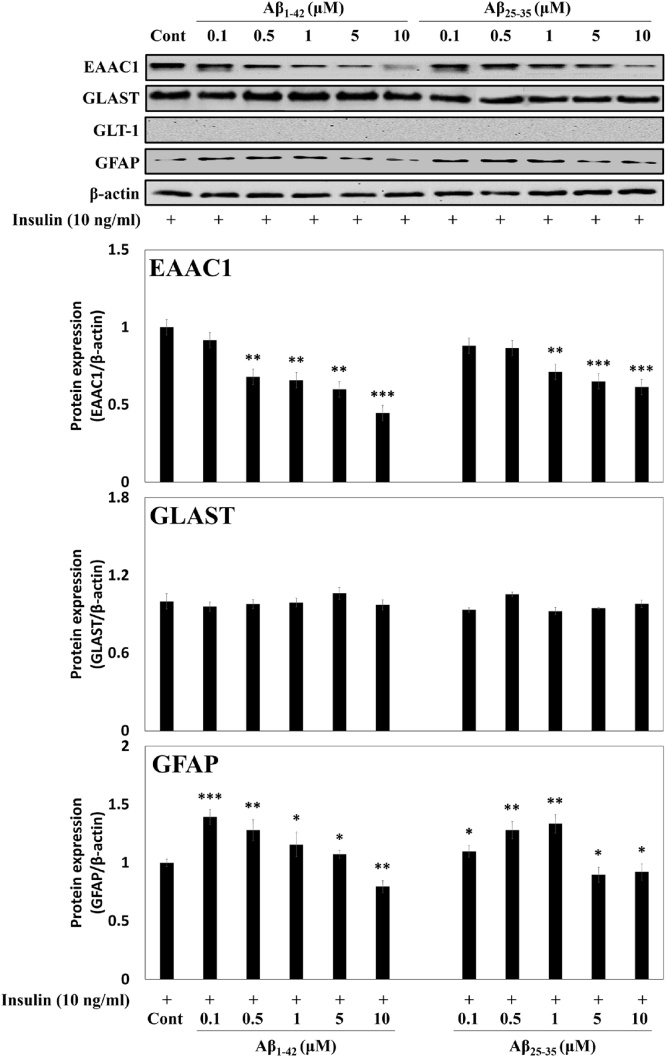
We then checked the changes in protein expressions of Glu receptors and GFAP upon exposure to Aβ by Western blot. Since the expression of GLT-1 mRNA and protein was not detected in insulin-treated C6 cells (passage number 45), it was not examined further. Exposure to Aβ1-42 or Aβ25-35 suppressed the EAAC1 protein expression about 0.4-fold or 0.6-fold, respectively, at 10 μM compared with the control, suggesting that the inhibition of Glu uptake in Aβ-treated C6 cells might be mediated by the suppression of the EAAC1 expression (Fig. 3). GFAP expression was also significantly reduced about 0.8 and 0.9-fold at 10 μM of Aβ, respectively.
Fig. 3.
Effects of Aβ peptides on Glu receptors and GFAP protein expressions in insulin-treated C6 cells (passage number 45). β-Actin was used as the internal control for Western blot analysis. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. insulin alone (n = 3).
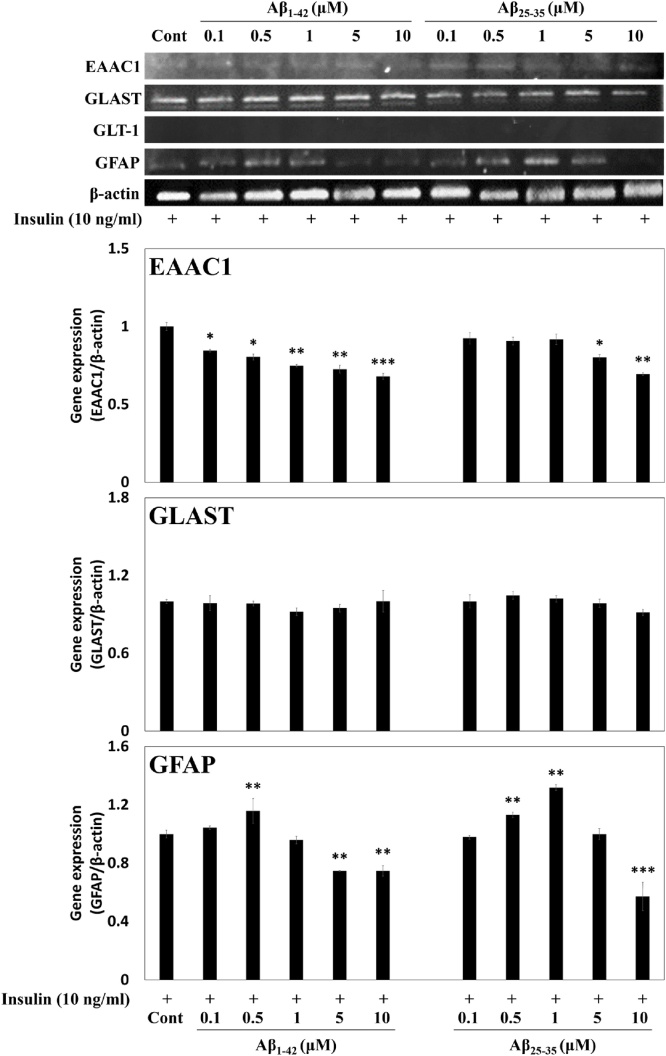
In addition, the gene expressions of the Glu receptors and GFAP in Aβ1-42 or Aβ25-35 with insulin-treated C6 cells (passage number 45) were measured using RT-PCR. As with the protein expressions, mRNA expression of EAAC1 was reduced by 0.7-fold and 0.6-fold at 10 μM, respectively, with the treatment of Aβ1-42 or Aβ25-35, while GLAST showed no change in the expression. On the other hand, GFAP expression was also significantly reduced about 0.7 and 0.6-fold at 10 μM of Aβ, respectively (Fig. 4). From these results, it was confirmed that insulin treatment upregulated the expression of AQP4 in insulin-treated C6 cells, which was inhibited by exposure to Aβ peptides. Overall, both Aβ peptides exhibited similar effects, but in particular, Aβ1-42 showed significant inhibitory effects on the cell viability, Glu uptake, and the expressions of Glu transporters and GFAP at concentration of 10 μM.
Fig. 4.
Effects of Aβ peptides on Glu receptors and GFAP gene expressions in insulin-treated C6 cells (passage number 45). β-Actin was used as the internal control for RT-PCR analysis. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. insulin alone (n = 3).
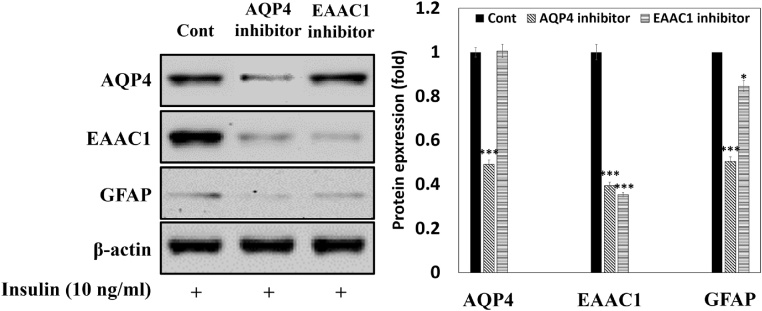
3.4. Effect of relationship between AQP4, GFAP, EAAC1 in insulin-treated C6 cells
AQP4 is co-expressed with glutamate transporters, and acts as a selective transmembrane pore for water transport [14]. In addition, the accumulation of Aβ peptides in astrocytes results in structural destruction of the astrocyte [16]. In this study, we evaluated protein expressions of AQP4, GFAP, and EAAC1 after exposure to TGN-020 (AQP4 inhibitor, 10 μM) or wortmannin (EAAC1 inhibitor, 0.1 μM), in insulin-treated C6 cells (passage number 45). Inhibition of AQP4 was observed to suppress the protein expressions of AQP4, EAAC1, and GFAP by 0.49-, 0.39-, and 0.50-fold, respectively, as compared to the control insulin-treated C6 cells (Fig. 5). However, inhibition of EAAC1 decreased EAAC1 and GFAP protein expressions by about 0.35- and 0.85-fold, respectively, but not AQP4 protein expression, when compared to the insulin-treated C6 cells. Our data indicates that AQP4 expression is upstream of EAAC1 and GFAP protein expressions.
Fig. 5.
Relationship between AQP4, EAAC1, and GFAP protein expressions in insulin-treated C6 cells (passage number 45). TGN-020 and wortmannin were used as AQP4 inhibitor and EAAC1 inhibitor, respectively, in insulin-treated C6 cells. Results are presented as the means ± SDE of percentages calculated with respect to control levels of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. insulin alone (n = 3).
4. Discussion
AQP4, which is present in astrocytes, has been shown to play an important role in the regulation of memory and cognitive function by mediating the clearance of amyloid to reduce cytotoxicity and thereby protecting neurons. Several studies have reported that deficiency of AQP4 is associated with memory impairment [27]. It was found that the brain in long term of AQP4 gene knockout mice increased brain Aβ plaque deposit compared to normal mice, and also, the behavioral study of AQP4 knockout mice showed impairment in memory [33]. Moreover, astrocytes and microglia play central roles as regulators for Aβ clearance and degradation [[34], [35], [36]]. However, the relationship between AQP4 and Aβ-induced toxicity in C6 cells, an astrocytic cell line, has not been explored previously. In the present study, we investigated whether the expressions of AQP4 and Glu transporter together with cytotoxicity and Glu accumulation in C6 cells are regulated by Aβ peptides for the first time. AQP4 protein was expressed in C6 cells, but the level was very low and it was confirmed that the expression increased as the number of cell passage increased (Fig. 1A). Our results indicate that the later cell passage shows more astrocytic properties, which is consistent with the previous reports [28,37].
To study the role of astrocytic AQP4 in C6 cells, optimum conditions for its expression showing astrocytic properties were examined. Our data revealed that insulin promoted AQP4 expression in C6 cells, as previously suggesting insulin as a differentiating agent for astrocytic cells from dendrocytic C6 cells.
The expression of AQP4 with Aβ treatment (Aβ1-42 and Aβ25-35) were investigated in astrocytic C6 cells in this study. Recent study using cultured cortical neurons demonstrated that Aβ25-35 or Aβ1-42-induces cell damage by increasing the Glu release [38]. Lower concentration of Aβ1-42 or Aβ25-35 (up to 0.1 μM and 0.5 μM, respectively) results in over-expression of AQP4, but Aβ1-42 or Aβ25-35 treatments at higher concentration (5 μM and 10 μM, respectively), results in suppression of AQP4. In addition, our data confirmed that the change in expression of AQP4 by treatment with the Aβ1-42 peptide was more sensitive than the Aβ25-35 peptide (Fig. 1B). Yang et al. [29] tested the effect of AQP4 on astrocyte damage after incubating with various concentrations of Aβ1–42 (0.1, 1 and 10 μM); these data revealed that exposure to AQP4 decreases the astrocyte damage induced by Aβ1-42 or Aβ25-35. In our data, Aβ peptides, which decreased AQP4 expression, induced cell death, suggesting that AQP4 may regulate the Aβ-induced toxicity in C6 cells to protect astrocytic function.
We examined that the expression of Glu receptors in insulin and Aβ-treated C6 cells. EAAC1 expressions were down-regulated by Aβ1-42 or Aβ25-35 treatment, which induced decrease in Glu uptake, suggesting that EAAC1 is a major Glu transporter in C6 cells. Likewise, Dall’Igna et al. [39] revealed that the C6 cells express EAAC1, which in turn regulates Glu uptake. On the other hand, GLT-1 is not expressed in glial cells, but is expressed only in astrocytes, which are inhibited by Aβ through MAPK pathway and oxidative stress [[13], [14], [15], [16], [17]].
GFAP is an intermediate microfibrous protein of astrocytes, and expression of GFAP is used as a marker of astrogliosis regulation. Accumulation of Aβ plaques from astrocytes is accompanied with a decrease in GFAP expression [29]. From our data, GFAP and AQP4 expressions in C6 cells are suppressed by Aβ treatment at higher concentration, suggesting that Aβ-induced cell damage is due to astrocytes dysfunction through the inhibition of AQP4. In other words, it seems that Aβ clearance is mediated by the expression of AQP4 and Glu transporter in C6 cells as revealed in astrocytes [40,41].
In this study, we confirmed the altered expressions of AQP4 and EAAC1, and the mechanism of the AQP4/EAAC1 pathway protecting C6 cells from damage by Aβ. In order to establish an AD model in a C6 cell, the influence of cell passage and Aβ peptides concentration were investigated, and an in vitro model with astrocyte characteristics was implemented by inducing the differentiation of C6 cells by insulin treatment. This study is expected to be able to study the association of AQP4 expression to natural products such as Panax ginseng [42] or Scrophularia buergeriana extract [43] reported as AD prophylactic agents using C6 cells with astrocyte properties [44]. We believe that our data will facilitate further studies on the astrocytic functions targeting AQP4 in AD.
CRediT authorship contribution statement
Se-Ho Park: Methodology, Investigation, Validation, Data curation, Writing - original draft, Visualization. Jae-Yeul Lee: Software, Resources, Formal analysis. Kwang-Hwan Jhee: Writing - review & editing. Seun-Ah Yang: Conceptualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1A02048746).
References
- 1.De Strooper B., Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 2.Heneka M.T., Carson M.J., Khoury J.El, Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborn L.M., Kamphuis W., Wadman W.J., Hol E.M. Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Serrano-Poze A., Muzikansky A., Gόmez-Isla T., Growdon J.H., Betensky R.A., Frosch M.P., Hyman B.T. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 2013;72:462–471. doi: 10.1097/NEN.0b013e3182933788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verkhratsky A., Parpura V., Pekna M., Pekny M., Sofroniew M. Glia in the pathogenesis of neurodegenerative disease. Biochem. Soc. Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 6.Mayer M.L., Westbrook G.L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog. Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Rothstein J.D., Martin L., Levey A.L., Dykes-Hoberg M., Jin L., Wu D., Nash N., Kuncl R.W. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 8.Kanai Y., Hediger M.A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 9.Pines G., Danbolt N.C., Bjørås M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathisen J., Seeberg E., Kanner B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 10.Storck T., Schulte S., Hofmann K., Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullensvang K., Lehre K.P., Storm-Mathisen J., Danbolt N.C. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur. J. Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 12.Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transporter in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte N., Hermans E. Glutamate-induced glioma cell proliferation is prevented by functional expression of the glutamate transporter GLT-1. FEBS Lett. 2008;582:1847–1852. doi: 10.1016/j.febslet.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X.N., Sun X.L., Gao L., Fan Y., Ding J.H., Hu G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocyte. Mol. Cell. Neurosci. 2007;34:34–39. doi: 10.1016/j.mcn.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi M.G., Gazzola G.C., Tognazzi L., Bussolati O. C6 glioma cells differentiated by retinoic acid overexpress the glutamate transporter excitatory amino acid carrier 1 (EAAC1) Neuroscience. 2008;151:1042–1052. doi: 10.1016/j.neuroscience.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 16.Matos M., Augusto E., Oliveira C.R., Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocyte: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Kong H., Fan Y., Xie J., Ding J., Sha L., Shi X., Sun X., Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell. Sci. 2008;12:4029–4036. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- 18.Thrane A.S., Rappold P.M., Fujita T., Torres A., Bekar L.K., Takano T., Peng W., Wang F., Thrane V.R., Enger R., Haj-Yasein N.N., Skare Ø., Holen T., Klungland A., Ottersen O.P., Nedergaard M., Nagelhus E.A. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. U. S. A. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Gao J., Ding J., Hu G., Xiao M. Aquaporin-4 expression contributes to decreases in brain water content during mouse postnatal development. Brain Res. Bull. 2013;94:49–55. doi: 10.1016/j.brainresbull.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X.N., Xie L.L., Liang R., Sun X.L., Fan Y., Hu G. AQP4 knockout aggravates ischemia/reperfusion injury in mice. CNS Neurosci. Ther. 2012;18:388–394. doi: 10.1111/j.1755-5949.2012.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auguste K.I., Jin S., Uchida K., Yan D., Manley G.T., Papadopoulos C., Verkman A.S. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 22.Scharfman H.E., Binder D.K. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem. Int. 2013;63:702–711. doi: 10.1016/j.neuint.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulos M.C., Verkman A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capoccia E., Cirillo C., Marchetto A., Tiberi S., Sawikr Y., Pesce M., D’Alessandro A., Scuderi C., Sarnelli G., Cuomo R., Steardo L., Esposito G. S100β-p53 disengagement by pentamidine promotes apoptosis and inhibits cellular migration via aquaporin-4 and metalloproteinase-2 inhibition in C6 glioma cells. Oncol. Lett. 2015;9:2864–2870. doi: 10.3892/ol.2015.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolman D., Drndarski S., Abbott N.J., Rattray M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J. Neurochem. 2005;93:825–833. doi: 10.1111/j.1471-4159.2005.03111.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda K., Yamamoto N., Asai K., Sobue K., Fujita Y., Fujita M., Mase M., Yamada K., Nakanishi M., Tada T., Miura Y., Kato T. Regulation of aquaporin-4 expression in astrocytes. Mol. Brain Res. 2001;89:94–102. doi: 10.1016/S0169-328X(01)00067-5. [DOI] [PubMed] [Google Scholar]
- 27.Lan Y.L., Zhao J., Ma T., Li S. The potential roles of aquaporin 4 in Alzheimer’s disease. Mol. Neurobiol. 2016;53:5300–5309. doi: 10.1007/s12035-015-9446-1. [DOI] [PubMed] [Google Scholar]
- 28.Goya L., Feng P.T., Aliabadi S., Timiras P.S. Effect of growth factors on the in vitro growth and differentiation of early and late passage C6 glioma cells. Int. J. Dev. Neurosci. 1996;14:409–417. doi: 10.1016/0736-5748(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Yang W., Wu Q., Yuan C., Gao J., Xiao M., Gu M., Ding J., Hu G. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol. Cell. Neurosci. 2012;49:406–414. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Salam A.M., Al Hemaid W.A., Afifi A.A., Othman A.I., Farrag A.R.H., Zeitoun M.M. Consolidating probiotic with dandelion, coriander and data palm seeds extracts against mercury neurotoxicity and for maintaining normal testosterone levels in male rats. Toxicol. Rep. 2018;5:1069–1077. doi: 10.1016/j.toxrep.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varmazyari A., Taghizadehghalehjoughi A., Sevim C., Baris O., Eser G., Yildirim S., Hacimuftuoglu A., Buha A., Wallace D.R., Tsatsakis A., Aschner M., Mezhuev Y. Cadmium sulfide-induced toxicity in the cortex and cerebellum: in vitro and in vivo studies. Toxicol. Rep. 2020;7:637–648. doi: 10.1016/j.toxrep.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Zhao X., Lin S., Liu F., Ma J., Han Z., Jia F., Xie W., Zhang Q., Li X. Neuroprotective effect of ent-Kaur-15-en-17-al-18-oic acid on amyloid beta peptide-induced oxidative apoptosis in Alzheimer’s disease. Molecules. 2020;25:142. doi: 10.3390/molecules25010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z., Xiao N., Chen Y., Huang H., Marshall C., Gao J., Cai Z., Wu T., Hu G., Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang C., Huang X., Huang X., Mai H., Li J., Jiang T., Wang X., Lü T. Aquaporin-4 and Alzheimer’s disease. J. Alzheimers Dis. 2016;52:391–402. doi: 10.3233/JAD-150949. [DOI] [PubMed] [Google Scholar]
- 35.Ries M., Sastre M. Mechanisms of Aβ clearance and degradation by glial cells. Front. Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan Y.L., Chen J.J., Hu G., Xu J., Xiao M., Li S. Aquaporin 4 in astrocytes is a target for therapy in Alzheimer’s disease. Curr. Pharm. Design. 2017;23:4948–4957. doi: 10.2174/1381612823666170714144844. [DOI] [PubMed] [Google Scholar]
- 37.Galland F., Seady M., Taday J., Smaili S.S., Gonçalves C.A., Leite M.C. Astrocyte culture models: molecular and function characterization of primary culture, immortalized astrocytes and C6 glioma cells. Neurochem. Int. 2019;131 doi: 10.1016/j.neuint.2019.104538. [DOI] [PubMed] [Google Scholar]
- 38.Feng Z., Zhang J.T. Protective effect of melatonin on β-amyloid-induced apoptosis in rat astroglioma c6 cells and its mechanism. Free Radic. Biol. Med. 2004;37:1790–1801. doi: 10.1016/j.freeradbiomed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Dall’Igna O.P., Bobermin L.D., Souza D.O., Quincozes-Santos A. Riluzole increased glutamate uptake by cultured C6 astroglial cells. Int. J. Dev. Neurosci. 2013;31:482–486. doi: 10.1016/j.ijdevneu.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K., Hayashi M., Nakano H., Shimazaki M., Sugimori K., Koshino Y. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer’s disease. J. Alzheimers Dis. 2004;6:623–632. doi: 10.3233/JAD-2004-6606. [DOI] [PubMed] [Google Scholar]
- 41.Mouser P., Head E., Ha K.H., Rohn T.T. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am. J. Pathol. 2006;168:936–946. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., Tsatsakis A., Spandidos D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease. Mol. Med. Rep. 2019;19:2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H.J., Spandidos D.A., Tsatsakis A., Margina D., Izotov B.N., Yang S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019;43:2144–2152. doi: 10.3892/ijmm.2019.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aloizou A.M., Siokas V., Vogiatzi C., Peristeri E., Docea A.O., Petrakis D., Provatas A., Folia V., Chalkia C., Vinceti M., Wilks M., Izotov B.N., Tsatsakis A., Bogdanos D.P., Dardiotis E. Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. doi: 10.1016/j.toxlet.2020.03.005. [DOI] [PubMed] [Google Scholar]