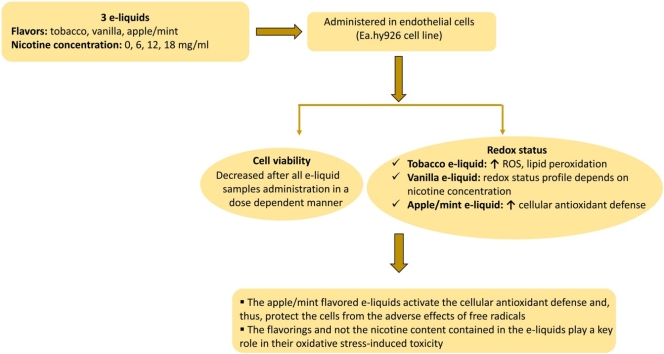
Graphical abstract
Abbreviations: CARBS, protein carbonyls; CO, carbon monoxide; DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; DMEM, Dulbecco’s modified Eagle’s medium; DNPH, 2,4-dinitrophenylhydrazine; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DPPHH, 2,2-diphenyl-1-picrylhydrazine; ENDS, electronic nicotine delivery systems; EPR, electronic paramagnetic resonance; FSC, forward light scattering; GSH, reduced form of glutathione; HCL, hydrochloric acid; HCN, hydrogen cyanide; MDA, malondialdehyde; 8-OH-dG, 8-hydroxy-deoxyguanosine; PBS, phosphate buffered saline; PG, propylene glycol; ROS, reactive oxygen species; SSC, side light scattering; TAC, total antioxidant capacity; TBA, thiobarbituric acid; TBARS, thiobarbituric acid reactive substances; TCA, trichloroacetic acid; Tris-HCl, trishydroxymethylaminomethane hydrochloride; VG, vegetable glycerin
Keywords: E-cigarettes, E-liquids, Endothelial cells, ROS, GSH, Oxidative stress
Highlights
-
•
The pattern of the effect on Ea.hy926 redox status differs among flavored e-liquids.
-
•
Tobacco flavored e-liquids increase ROS generation with concomitant increase in TBARS.
-
•
Vanilla flavored e-liquids profile depends on the nicotine content.
-
•
Apple/mint flavored e-liquids activate the cellular antioxidant defense.
-
•
Flavorings and not the nicotine content play a key role in free radical generation.
Abstract
Electronic cigarettes are constantly gaining ground as they are considered less harmful than conventional cigarettes, and there is also the perception that they may serve as a potential smoking cessation tool. Although the acute effects of electronic cigarette use have been extensively studied, the long-term potential adverse effects on human health remain largely unknown. It has been well-established that oxidative stress is involved in the development of various pathological conditions. So far, most studies on e-cigarettes concern the effects on the respiratory system while fewer have focused on the vascular system. In the present study, we attempted to reveal the effects of electronic cigarette refill liquids on the redox state of human endothelial cells (EA.hy926 cell line). For this purpose, the cytotoxic effect of three e-liquids with different flavors (tobacco, vanilla, apple/mint) and nicotine concentrations (0, 6, 12, 18 mg/ml) were initially examined for their impact on cell viability of EA.hy926 cells. Then, five redox biomarkers [reduced form of glutathione (GSH), reactive oxygen species (ROS), total antioxidant capacity (TAC), thiobarbituric acid reactive substances (TBARS) and protein carbonyls (CARBS)] were measured. The results showed a disturbance in the redox balance in favor of free radicals in tobacco flavored e-liquids while vanilla flavored e-liquids exhibited a more complex profile depending on the nicotine content. The most interesting finding of the present study concerns the apple/mint flavored e-liquids that seemed to activate the cellular antioxidant defense and, thus, to protect the cells from the adverse effects of free radicals. Conclusively, it appears that the flavorings and not the nicotine content play a key role in the oxidative stress-induced toxicity of the e-liquids.
1. Introduction
Smoking is one of the leading risk factors for cardiovascular diseases and other pathological conditions (e.g., insulin resistance, inflammation, dyslipidemia) worldwide [1,2]. Moreover, it is one of the top causes for early death [3]. The smoke generated by conventional cigarettes contains over 4,000 chemical substances including carbon monoxide (CO), hydrogen cyanide (HCN), acetaldehyde, nitrogen oxides, heavy metals and other volatile compounds with potential detrimental outcome on human health (e.g., alcohols, quinones, amines, aldehydes) [4]. The harmful effects caused by the exposure to the toxic substances of cigarette smoke depends mainly on the duration of the exposure. HCN is one of the most dangerous compounds of cigarette smoke since the long-term exposure at low doses can cause damage to lung, heart and brain [5]. Also, exposure to low doses of CO for long time periods has been associated with increased risk of heart disease. CO enters the bloodstream through the lungs and with hemoglobin prevents the transport of oxygen to the cells [6]. It also contains several major carcinogenic and mutagenic agents, such as benzo [a] pyrene and other polycyclic aromatic hydrocarbons, nitrosamines, N-heterocyclic amines and benzene [7].
The effects of cigarette smoke on human health have been extensively studied. Cigarette smoking can lead to lung diseases, diabetes, periodontitis, cancer, vascular diseases, and rheumatoid arthritis. It has also been associated with oxidative damage to macromolecules, reduced antioxidants reserves, inflammation and impaired immune status [8]. Specifically, cigarette smoke contains an abundant amount of free radicals (1015/single puff) and oxidants that can cause oxidative damage to proteins, lipids and DNA [9]. Many studies have shown that levels of isoprostanes and thiobarbituric acid–reactive substances (TBARS), both as biomarkers of lipid peroxidation, are higher in smokers compared to nonsmokers [[10], [11], [12]]. The concentrations of protein carbonyls (CARBS), as a biomarker of protein oxidation and 8-hydroxy-deoxyguanosine, as a biomarker of DNA oxidative damage, were higher in smokers than in nonsmokers [13]. A direct consequence of the exposure to oxidative stress is the depletion of the body’s circulating antioxidant micronutrients (carotenoids, vitamin C, provitamin A,) in order to encounter the oxidative damage [14,15]. Furthermore, the relationship between tobacco smoke, oxidative stress and associated endothelial dysfunction has been thoroughly investigated [16–17,18,19,20,21].
Over the last decade, due to the above mentioned harmful effects of conventional cigarette, a rapid increase in the use of electronic nicotine delivery systems (ENDS) has been observed. Indeed, a continuously increasing number of individuals throughout the world tend to use electronic cigarettes and, thus vaping as a substitute for smoking [22]. According to the literature, vaping is considered safer for human health compared to tobacco smoking [23,24]. It has been reported that the liquids used for the refill of electronic cigarettes contain 4 main ingredients, namely propylene glycol (PG), vegetable glycerin (VG), nicotine and flavorings [25]. PG and VG serve as solvents and their mixtures are chemically stable. They are commercially available in a variety of ratios with 50 PG/50 V G being the most common. Different PG/VG ratios exert different effects on cloud production and throat hit. PG is responsible for the throat hit feeling, highlights the flavors of e-liquids and has reduced cloud production in relation to VG. On the other hand, VG is characterized by a more smooth texture and increased steam production, compared to PG, however it has a reduced flavor yield [26]. Both substances are organic compounds that are widely used in the food and pharmaceutical industry. Interestingly, although a number of toxic substances is produced during vaping due to thermal decomposition, such as acetone, acetaldehyde, acrolein and formaldehyde, their levels are notably lower compared to the conventional cigarette smoke [[27], [28], [29], [30]]. The vapor produced by electronic cigarette devices is considered safer than the cigarette smoke since it is claimed to contain 9–450 times fewer toxic substances [31]. in vitro experiments have shown that e-cigarette aerosols induce less cytotoxicity than cigarette smoke [32]. A study that compared the effects of tobacco smoke vs e-cigarette on the cell viability of 3T3-L1 cells has reported that only cigarette smoke decreased cell viability [33].
As mentioned above, although the role of the smoke produced by conventional cigarettes on oxidative stress is well studied and the findings are not optimistic, the available experimental data in the literature concerning the effects of e-cigarettes on blood, tissue or cell redox status and the redox-related diseases are scarce. However, it is believed that the levels of ROS, and reactive species in general, in the vapor of e-liquids are major determinants for the overall toxicity of electronic cigarettes. The production of ROS during the vaping procedure depends on specific factors, the most important of them being the brand of the cigarette, the composition of the flavorings, the voltage and the nicotine concentration of the liquid [34,35]. According to electronic paramagnetic resonance spectroscopy studies, the existence of drastic, low-half-life free radicals in the vapor of e-liquids has been confirmed [36]. A common model in which studies of the toxicological effects of electronic cigarettes focus are lung cells and tissues. Researchers have shown that aerosols induce cytotoxicity and induce oxidative stress and inflammation in both human lung cells and mice lungs [37]. Permeability of lung endothelial cells, measured by the cell-substrate impedance assay method, is also increased after exposure to electronic cigarette aerosols, regardless of nicotine concentration [38]. In addition, a study in human bronchial epithelial cells (Beas2B) and human pulmonary fibroblasts (HFL-1) has demonstrated that their exposure to standard chemicals used as flavorings in electronic cigarette refill liquids had similar detrimental effects causing inflammation and loss of epithelial function barrier [39]. Therefore, flavorings used in e-liquids may generally trigger ROS-mediated inflammatory responses, as also found in a study conducted on monocytes (MM6 and U937 cell lines) [40].
On the basis of the above, it becomes evident that the smoke generated by the conventional cigarettes is a redox altering stimuli that causes oxidative stress and several serious health problems. However, regarding electronic cigarette, although there is limited evidence in differential in vitro models pointing out that its vapor leads to free radical production, it is not yet known how they affect cell redox status. Thus, the main objective of the present study was to investigate the effects of three common liquids with ranging nicotine concentrations used as refill for electronic cigarettes on the redox status of human vascular endothelial cells. Five redox biomarkers, i.e., GSH, ROS, TAC, TBARS and CARBS levels were evaluated in order to acquire a holistic clue regarding the role of the tested e-liquids on cell redox equilibrium [41].
2. Materials and methods
2.1. The e-cigarette liquids (e-liquids)
Three e-liquid brands with different flavors and nicotine concentrations were tested (seven e-liquid samples in total). Specifically, three tobacco-flavored e-liquids (propylene glycol, vanillin, propionic acid, propenyl guaethol, linalool) with 50/50 ratio of PG/VG and nicotine concentrations equal to 0 mg/ml, 12 mg/ml and 18 mg/ml, two apple/mint-flavored e-liquids (propylene glycol, water, vanillin, menthol, mint, hexyl acetate, ethyl acetate, ethyl butyrate) with 70/30 ratio of PG/VG and nicotine concentrations equal to 12 mg/ml and 18 mg/ml and two vanilla-flavored e-liquids (propylene glycol water, vanillin, acetoin, maltol, vanilla extract, 2,3-Pentanedione) with 70/30 ratio of PG/VG and nicotine concentrations equal to 6 mg/ml and 12 mg/ml) were examined for their effects on the redox status of endothelial cells (i.e., Ea.hy926 cell line).
2.2. Cell culture
The EA.hy926 endothelial cell line examined in the present study is a hybrid cell line derived from human umbilical vein endothelial cells (HUVECs) and epithelial cells from human lung carcinoma (A549). The cells were cultured in normal Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % (v/v) fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin and 100 units/ml streptomycin in tissue culture flasks at 37 °C in 5 % CO2.
2.3. The cell viability assay
Cell viability was assessed using the XTT Cell Proliferation Assay. Endothelial cells were subcultured in 96-well plates with 1 × 104 cells per well in DMEM medium. After 24 h of incubation, the cells were treated with increasing concentrations of each e-liquid sample (0.3 %, 1 %, 2.5 %, 5 %, 10 % and 20 %) in serum-free medium for 24 h. Then, the XTT test solution was added to each well and after a 4 h-incubation the absorbance was measured at 450 nm and also at 630 nm as a reference wavelength in a Bio-Tek ELx800 microplate reader (Winooski, VT, USA). Cells incubated in DMEM serum-free medium only were used as the negative control. The absorbance of each e-liquid concentration alone in DMEM serum-free medium and XTT solution was also measured at 450 nm and 630 nm. The absorbance values of each e-liquid sample alone were subtracted from those derived from the cells treated with e-liquids. The cell viability was expressed as percentage of inhibition of cell viability, according to the following formula:
| Inhibition (%) = [(O.D.control − O.D.sample) / O.D.control] × 100, |
where O.D.control and O.D.sample indicate the optical density of the negative control and the tested compounds, respectively. All samples were measured in triplicate and at least in three independent experiments.
2.4. Treatment of the cells with e-liquids
The cells were seeded in 25 cm2 culture flasks for GSH and ROS determination and 75 cm2 culture flasks for the measurement of TAC, TBARS and protein carbonyl levels and were incubated for 24 h at 37 °C in 5 % CO2. Then, at a cell confluency of 70–80 %, the medium was removed and replaced with serum-free medium containing the e-liquid samples at different concentrations followed by incubation for 24 h. The untreated cells were considered as controls. Then, the cells were trypsinized, collected and centrifuged twice (300 g, 10 min, 5 °C). Each centrifugation was followed by supernatant dismissal and resuspension of cellular pellet in phosphate buffered saline (PBS). After the last centrifugation the cellular suspension was collected for the measurement of the above mentioned redox biomarkers.
2.5. Determination of GSH and ROS levels by flow cytometry (FC)
The assessment of intracellular GSH and ROS levels in the cells was carried out using flow cytometry. Mercury orange and 2' ,7'-dichlorodihydrofluorescein diacetate (DCF-DA) were used for the detection of intracellular GSH and ROS, respectively. Mercury orange is a fluorescent dye that directly binds to GSH, while DCF-DA is non-fluorescent but after entering the cell it is deacetylated by cellular esterases and is converted to its fluorescent form (DCF), which binds to intracellular ROS. A 400 μM stock solution of mercury orange was prepared using acetone as a solvent and stored in 4 °C, while a fresh 400 μM stock solution of DCF-DA in ethanol was prepared. In order to determine the GSH and ROS levels, the cells were firstly re-suspended in PBS (1 × 106 cells/ml) and were then treated with mercury orange (40 μM) or DCF-DA (10 μM) followed by a 30 min-incubation in the dark at 37 °C. Then, the cells were washed out, resuspended in PBS and submitted to the flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson, NJ, USA). The excitation and emission wavelengths for ROS were at 488 nm and 530 nm, respectively and for GSH they were at 488 and 580 nm, respectively. Also, forward light scattering (FSC) and side light scattering (SSC) that are indicators of cell size and cellular internal complexity, respectively were measured. The cells were analyzed at a flow rate of 1000 events per sec. Analyses were performed on 10,000 cells per sample and fluorescence intensities were measured. Data analysis was made using the BD Cell Quest software (Becton Dickinson). Each experiment was performed at least three times.
2.6. The protocol for the determination of TBARS levels
The determination of TBARS was performed according to Keles et al. [42] with slight modifications as described by Kerasioti et al. [43]. Four hundred microliters of the cell suspension (or 400 μl of PBS for the blank) was mixed with 500 μl of trishydroxymethylaminomethane hydrochloride (Tris-HCl) (200 mM, pH = 7.4) and 500 μl of 35 % TCA and after a 10 min-incubation at room temperature, 1 ml of 2 M Na2SO4– thiobarbituric acid (TBA) (55 mM) solution was added and the samples were incubated for 45 min at 95 °C. Then, the samples were cooled on ice for 5 min followed by addition of 1 ml of trichloroacetic acid (TCA) 70 % and vortexing. Finally, the samples were centrifuged (15.000 g, 3 min, 25 °C) and the absorbance was monitored at 530 nm. The assay requires >30 μg protein for each sample. Total protein in cellular suspension was determined using the Bradford reagent. The concentration of TBARS is expressed in terms of malondialdehyde (MDA) equivalents based on the molar extinction coefficient of MDA (155 mM−1 ∙ cm-1).
2.7. The protocol for the determination of TAC
The determination of TAC was based on the method of Janaszewska and Bartosz [44]. Briefly, 200 μl of the cell suspension was mixed with 500 μl of phosphate buffer (10 mM, pH = 7.4) and 500 μl of 2,2-diphenyl-1-picrylhydrazyl (DPPH) (0.1 mM) followed by incubation in the dark at RT for 45 min. Afterwards, the samples were centrifuged (20.000 g, 3 min, 25 °C) and the absorbance of the supernatant was monitored at 520 nm. TAC is presented as mmol of DPPH• reduced to 2,2-diphenyl-1-picrylhydrazine (DPPH:H) by the antioxidants of the samples.
2.8. The protocol for the determination of protein carbonyls
For the assessment of protein carbonyl levels, the cellular suspension was homogenized by sonication on ice. Then, the protein carbonyl concentration was measured in the homogenate spectrophotometrically according to Patsoukis et al. [45] with slight modifications as previously described by Veskoukis et al. [46]. In this assay, 200 μl of 20 % TCA was added to 200 μl of the cellular suspension and this mixture was incubated in an ice bath for 15 min and centrifuged (15,000 g, 5 min, 4 °C). The supernatant was discarded and 500 μl of 2,4-dinitrophenylhydrazine (DNPH) [in 2.5 N hydrochloric acid (HCL)] for the sample or 500 μl of 2.5 N HCL for the blank was added in the pellet. Then, the samples were centrifuged (15.000 g, 5 min, 4 °C), the supernatant was discarded and 100 μl of TCA 100 % was added. The mixture was centrifuged (15.000 g, 5 min, 4 °C), the supernatant was discarded and 1 ml of ethanol/ethyl acetate solution (1:1 v/v) was added followed by centrifugation (15.000 g, 5 min, 4 °C) in order to wash out the DNPH excess. This washing step was repeated twice. After discarding the supernatant, 1 ml of urea (5 M, pH = 2.3) was added to the samples and then they were incubated for 15 min at 37 °C. The samples were centrifuged again (15.000 g, 3 min, 4 °C) and the absorbance of the supernatant was monitored at 370 nm. The assay requires > 30 μg of protein for each sample. Total protein was assayed using the Bradford reagent. The calculation of protein carbonyl concentration was based on the molar extinction coefficient of DNPH (22 mM−1 ∙ cm-1).
2.9. Statistical analysis
The results were analyzed by one-way ANOVA followed by Tukey's test multiple comparisons test. The results are expressed as mean ± SEM. The level of statistical significance was set at p < 0.05. All statistical analyses were performed using the SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Determination of the cytotoxic effects of e-liquids
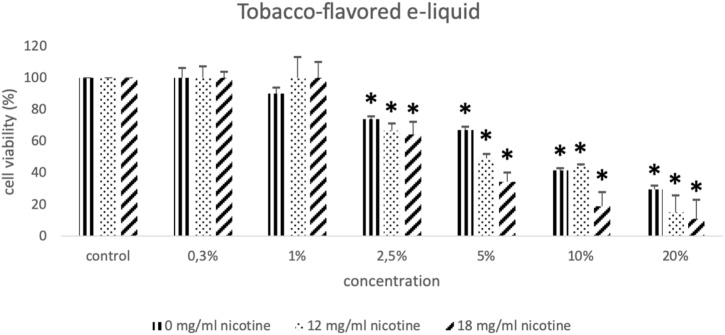
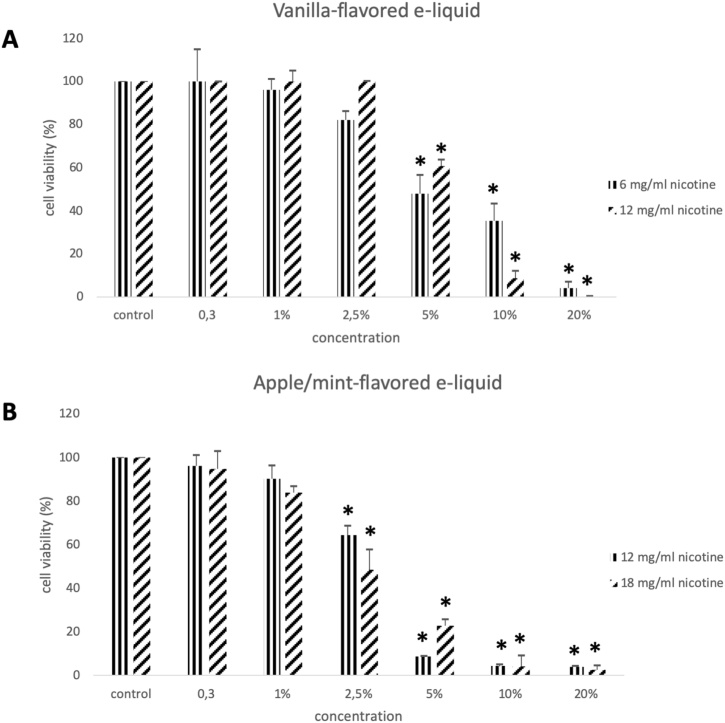
The results from the cell viability assay show that all e-liquids exhibited toxic action on EA.hy926 endothelial cells. Specifically, all three tobacco-flavored e-liquids with nicotine levels equal to 0, 12, 18 mg/ml exerted cytotoxic effect at 2.5 %, 5 %, 10 % and 20 % (Fig. 1A, B, C). The two vanilla-flavored e-liquids with nicotine levels at 6 and 12 mg/ml showed cytotoxic effect at concentrations equal to 5 %, 10 % and 20 % (Fig. 2A, B). Finally, the two examined apple/mint-flavored e-liquids (nicotine levels: 12 and 18 mg/ml) showed cytotoxic effect at concentrations equal to 2.5 %, 5 %, 10 % and 20 % (Fig. 2D, E). Subsequently, a cytotoxic concentration of each e-liquid sample was used for the evaluation of their effects on GSH, ROS, TBARS, TAC and CARBS levels.
Fig. 1.
The effects of the three tobacco-flavored e-liquid samples with nicotine concentrations equal to 0 mg/ml, 12 mg/ml and 18 mg/ml on the viability of the EA.hy926 cells presented as % of control (untreated sample). *: Statistically significant compared to the control (P < 0.05).
Fig. 2.
The effects of the two vanilla-flavored e-liquid samples with nicotine concentrations equal to 6 mg/ml and 12 mg/ml (A) and the two apple/mint-flavored e-liquid samples with nicotine concentrations equal to 12 mg/ml and 18 mg/ml (B) on the viability of the EA.hy926 cells presented as % of control (untreated sample). *: Statistically significant compared to the control (P < 0.05).
3.2. The effects of the e-liquid samples on GSH levels
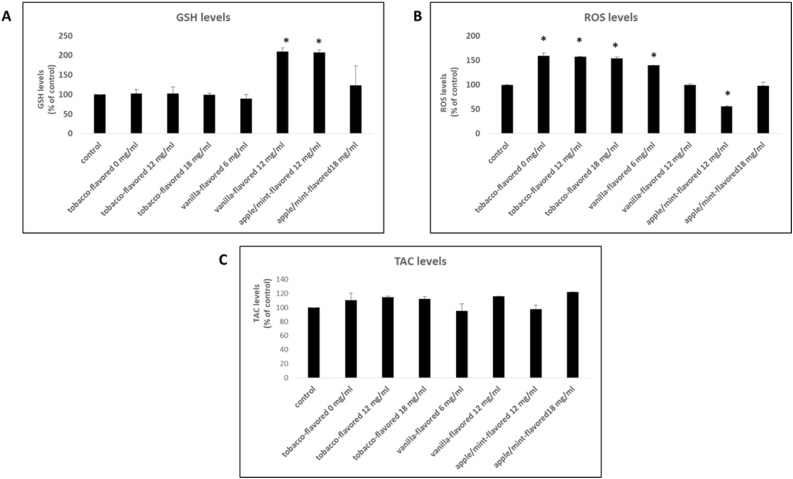
The vanilla-flavored sample with nicotine concentration equal to 12 mg/ml increased GSH levels of the cells by 110 % at the cytotoxic concentration compared to the control (Fig. 3A). The apple/mint-flavored sample with nicotine concentration equal to 12 mg/ml increased the levels of GSH by 107 % at the cytotoxic concentration. (Fig. 3A).
Fig. 3.
The effects of the tested e-liquid samples on GSH (A), ROS (B) and TAC (C) levels at the cytotoxic concentration. *: Statistically significant compared to the control (untreated sample) (P < 0.05).
3.3. The effects of the e-liquid samples on ROS levels
The tobacco-flavored sample with nicotine concentration equal to 0 mg/ml, 12 mg/ml and 18 mg/ml and the vanilla-flavored sample (nicotine concentration: 6 mg/ml) increased ROS levels at the cytotoxic concentration by 59 %, 57 %, 54 % and 40 %, respectively compared to the control (Fig. 3B). In contrast, the apple/mint-flavored sample with nicotine concentration equal to 12 mg/ml decreased ROS levels at the cytotoxic concentration by 44 % compared to the control (Fig. 3B).
3.4. The effects of the e-liquid samples on TAC levels
As it is shown in Fig. 3C, no statistically significant alterations in TAC levels were observed after incubation of the cells with the e-liquid samples.
3.5. The effects of the e-liquid samples on TBARS levels
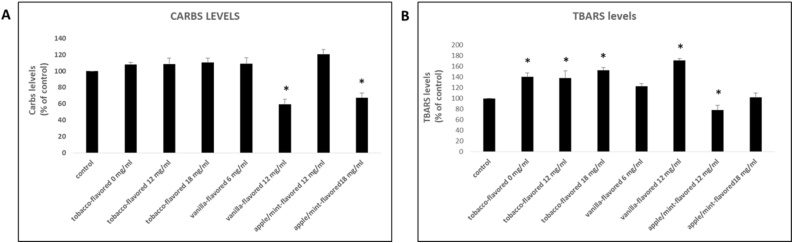
The tobacco-flavored samples with nicotine concentrations equal to 0 mg/ml, 12 mg/ml and 18 mg/ml increased TBARS levels at cytotoxic concentration by 40 %, 38 % and 53 % respectively compared to the control (Fig. 4B). The vanilla-flavored sample with nicotine concentrations equal 12 mg/ml increased TBARS levels at the cytotoxic concentration by 71 % compared to the control. Finally, the 12 mg/ml- apple/mint-flavored sample decreased TBARS levels at the cytotoxic concentration by 22 % compared to the control (Fig. 4B).
Fig. 4.
The effects of the tested e-liquid samples on protein carbonyls (A) and TBARS (B) levels at the cytotoxic concentration. *: Statistically significant compared to the control (untreated sample) (P < 0.05).
3.6. The effects of the e-liquid samples on protein carbonyls levels
The vanilla-flavored sample with nicotine concentration 12 mg/ml decreased protein carbonyls levels at the cytotoxic concentration by 41 % compared to the control (Fig. 4A). The 18 mg/ml- apple/mint-flavored sample decreased protein carbonyls levels at the cytotoxic concentration by 32 % compared to the control (Fig. 4A).
4. Discussion
The present study examined the role of three flavored electronic cigarette refill liquids on the redox state of human endothelial cells. Regarding the cytotoxicity of the e-liquids on the Ea.hy926 cell line, we have observed a dose-dependent action since increasing e-liquid concentrations led to decreased cell viability. Specifically, we report that tobacco-flavored and apple/mint-flavored e-liquids started exhibiting cytotoxic action at the concentration of 2.5 % while vanilla-flavored e-liquid started inhibiting cell viability at the concentration of 5 %, indicating that tobacco-flavored and apple/mint-flavored e-liquids elicit more toxic effects on endothelial cells. Rowell et al. [47] have found that 4 out of the 13 different flavors tested exhibited more harmful effects on the viability of CALU3 airway epithelial cell line. In addition, while comparing apple/mint-flavored and vanilla-flavored at the same nicotine concentration (12 mg/ml) and the same solvent ratio (50 % PG/VG), it became evident that the observed difference at the starting cytotoxic concentration is attributed to the different flavoring compounds of each product. Another observation is that different nicotine concentrations between the same e-liquid had no effect on the starting cytotoxic concentration. In detail, tobacco-flavored e-liquid at nicotine concentrations of 0, 12 and 18 mg/ml started inhibiting cell viability at the concentration of 2.5 %. Vanilla-flavored e-liquid at nicotine concentrations of 6 and 12 mg/ml started inhibiting cell viability at the concentration of 5 %. Finally, apple/mint-flavored e-liquid at nicotine concentrations of 12 and 18 mg/ml started inhibiting cell viability at the concentration of 2.5 %. From the above, it is also noted that the absence of nicotine, in the tested e-liquids, does not affect the cytotoxic action as it is shown from the results in tobacco-flavored e-liquid. It can be concluded that, the three specific flavors of e-liquids used in this study, contribute to the observed differences in cytotoxic action rather than nicotine concentration and solvent ratio. Similarly, the treatment of human MG-63 and Saos-2 osteoblast-like cells with e-liquids have revealed a decrease of cell viability in a dose dependent-manner and a flavor-dependent degree of osteotoxicity independently of nicotine [48].
It has been shown that aerosols derived from electronic cigarette refill liquids exhibit similar toxicity profiles with non-vaporized refill liquids, rendering direct exposure of cells to e-liquids reliable for the determination of the relative toxicity [47]. In fact, a specific framework has been proposed for the toxicological assessment of the effect of electronic cigarette refill liquids for in vitro systems [49]. Based on the above, the overall redox status of the endothelial cells was evaluated after exposure to the e-liquids by measuring biomarkers of oxidative damage and antioxidant molecules [41]. Concerning tobacco-flavored e-liquid, it was found that independently of nicotine concentration, ROS levels were increased at the cytotoxic concentration. This effect probably led to damage in cell membranes, which was confirmed by the increased TBARS (a biomarker of lipid peroxidation) levels. The vanilla-flavored e-liquid, with nicotine concentration equal to 6 mg/ml at the cytotoxic concentration induces the production of ROS and exerts a trend to increase TBARS levels. When it contains 12 mg/ml nicotine, vanilla-flavored e-liquid increases GSH levels accompanied by a concurrent increase of TBARS and a decrease of protein carbonyls levels both at the cytotoxic concentration. Apple/mint-flavored e-liquid with nicotine concentration equal to 12 mg/ml increased GSH levels and this subsequently led to decreased ROS levels and lipid peroxidation. As for apple/mint-flavored e-liquid with nicotine concentration equal to 18 mg/ml, a tendency for increase in GSH and a protection in favor of proteins (reduced protein carbonyl levels) were observed. From the above, it is concluded that the pattern of the effect on the 5 redox biomarkers is different among e-liquids. Specifically, tobacco-flavored e-liquid promotes ROS production and lipid peroxidation while apple/mint-flavored e-liquid enhances GSH production, which can lead to decreased ROS levels and protects against lipid and protein oxidation. Concerning vanilla-flavored e-liquid a different effect was observed as it increased GSH, ROS and TBARS levels while protected from protein oxidation. In general, the two e-liquids (tobacco-flavored and vanilla-flavored) increase ROS production and promote lipid peroxidation, except for apple/mint-flavored e-liquid as it protects endothelial cell membranes from oxidative stress.
From the above results it can be deduced that the specific flavorings appear to play an important role in free radical generation, as some flavored e-liquids seem to induce ROS production and others to inhibit it, which is also confirmed by the literature. Specifically, Bitzer et al. [50] have examined the effect of 49 different flavoring chemicals in the production of free radicals in e-cigarette aerosols. They resulted that flavoring substances play a significant role either in enhancing or in inhibiting free radical production. They also related the production of free radicals to the ability to oxidize biologically relevant lipids, as tested by the TBARS method. In addition, findings from free radical determination have shown enhanced release of ROS from vaporized e-liquids in both cellular and non-cellular systems, which is associated with an increase in lipid peroxidation as determined by measuring TBARS levels in lung homogenates from mice that were exposed to e-cigarette vapor [51]. It has also been shown that aerosols produced by electronic cigarette refill liquids induce ROS production, DNA destruction and cell death in vascular endothelial cells [52]. In an attempt to assess the vascular safety of electronic cigarettes, a randomized single-blind cross-over study was conducted by Carnevale et al. [53] that involved 40 healthy adults (smokers and non-smokers) and the effects of conventional cigarettes and electronic cigarettes on oxidative stress and endothelial function were studied and compared. After the measurement of redox biomarkers, both conventional and electronic cigarettes have been found to have adverse effects with respect to oxidative stress and endothelial function, with the electronic cigarette causing less damage.
Regarding the cytotoxicity and the biological roles of e liquids, several studies have appeared in the literature during the last decade. To begin with, a relevant study reported the capacity of electronic cigarette to be approximately 10 fold less toxic in an in vivo model than conventional cigarettes [54], whereas it has also been demonstrated that the electronic cigarettes are less mutagenic that conventional ones [55]. So far, many different flavoring substances with different flavor composition and concentration are used in e-liquids [56], as it has also been analysed with chormatographic tools [57]. Some of these flavoring substances, have already been reported to be harmful when inhaled [58]. Specifically, when citronellol, a natural acyclic monoterpenoid, is removed from the e-liquid mixture, the cytotoxic effects were decreased [59]. Another study examined the potential of cinnamon flavored e-liquids to induce oxidative stress and demonstrated that both unvaped and aerosolized cinnamon-flavored e-liquids increased the production of ROS, whereas flavorless e-liquids did not significantly alter the levels of ROS [60]. In general, conventional smoking induces several detrimental effects on human organism [61] and the use of antioxidants as potential therapeutic agents has been proposed [62]. The impact of most of these flavoring substances, alone or in a mixture, is still unclear, and additional experimental evidence should be generated to support the regulatory framework. E-liquid manufacturers are currently able to diminish the toxicological hazards of their products by identifying potential harmful flavoring substances or combinations of them as well as their toxic doses. Therefore, the know-how on toxicological data from the use of such e-liquids in in vitro and in vivo models is of utmost importance for tobacco industry.
The present study aspires to shed light on how three specific flavored e-liquids interact with endothelial cells by affecting their redox status. At present, there is limited literature evidence regarding the effects of the e-liquids on the redox status of endothelial cells. We report herein that the role of three specific e-liquids (tobacco, vanilla, apple/mint) on cell redox status depends highly on their flavor and, therefore, their chemical composition and not their nicotine content. The overall toxicity resulting from the use of e-liquids in electronic cigarettes is a multi-factorial phenomenon, in which many variables are involved and therefore this study may at present merely give indications about the effect of three specific flavored e-liquids on the redox state of the endothelial cells. It is therefore necessary to further research on this subject to elucidate the mechanisms governing the toxicological effects of refill liquids.
CRediT authorship contribution statement
Efthalia Kerasioti: Writing - original draft, Methodology. Aristidis S. Veskoukis: Writing - review & editing. Zoi Skaperda: Writing - review & editing. Apostolis Zacharias: Investigation. Konstantinos Poulas: Resources. Demetrios Kouretas: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Not applicable.
Contributor Information
Efthalia Kerasioti, Email: e-f-thalia@hotmail.com.
Aristidis S. Veskoukis, Email: veskoukis@gmail.com.
Zoi Skaperda, Email: zoiskap94@gmail.com.
Apostolis Zacharias, Email: apostoliszacharias@gmail.com.
Konstantinos Poulas, Email: kpoulas@upatras.gr.
George Lazopoulos, Email: g.lazopoulos@med.uoc.gr.
Demetrios Kouretas, Email: dkouret@uth.gr.
References
- 1.Salahuddin S., Prabhakaran D., Roy A. Pathophysiological mechanisms of tobacco-related CVD. Global Heart. 2012;7:113–120. doi: 10.1093/eurheartj/ehw106. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M., Wang H., Lozano R., Davis A., Liang X., Zhou M., Vollset S.T., Ozgoren A.A., Abdalla S., Abd-Allah F., Aziz M.I.A., Abera S.F., Aboyans V., Abraham B., Abraham J.P., Abuabara K.E., Abubakar I., Abu-Raddad L.J., Abu-Rmeileh N.M.E., Tom Achoki T. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G.A., Forouzanfar M.H., Moran A.E., Barber R., Nguyen G., Feigin V.L., Naghavi M., Mensah G.A., Christopher J.L., Murray C.J.L. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann D., Hoffmann I., El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 5.Mahernia S., Amanlou A., Kiaee G., Massoud Amanlou M. Determination of hydrogen cyanide concentration in mainstream smoke of tobacco products by polarography. J. Environ. Health Sci. Eng. 2015;13:57. doi: 10.1186/s40201-015-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zevin S., Saunders S., Gourlay S.G., Peyton Jacob P., I.I.I., Benowitz N.L. Cardiovascular effects of carbon monoxide and cigarette smoking. J. Am. Coll. Cardiol. 2001;38:1633–1638. doi: 10.1016/S0735-1097(01)01616-3. [DOI] [PubMed] [Google Scholar]
- 7.Talhout R., Schulz T., Florek E., van Benthem J., Wester P., Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 2011;8(2):613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnie R.J., Stratton K., Kwan L.Y. National Academies Press (US); Washington (DC): 2015. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. [DOI] [PubMed] [Google Scholar]
- 9.Pryor W.A., Stone K. Oxidants in cigarette smoke: radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 10.Bloomer R.J., Solis A.D., Fisher-Wellman K.H., Smith W.A. Postprandial oxidative stress is exacerbated in cigarette smokers. Br. J. Nutr. 2008;99:1055–1060. doi: 10.1017/S0007114507844370. [DOI] [PubMed] [Google Scholar]
- 11.Kocyigit A., Selek S., Celik H., Dikilitas M. Mononuclear leukocyte DNA damage and oxidative stress: the association with smoking of hand-rolled and filter cigarettes. Mutat. Res. 2011;721:136–141. doi: 10.1016/j.mrgentox.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Seet R.C.S., Lee C.Y., Loke W.M., Huang S.H., Huang H., Looi W.F., Chew E.S., Quek A.M., Lim E.C., Halliwell B. Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011;50:1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Padmavathi P., Reddy V.D., Kavitha G., Paramahamsa M., Varadacharyulu N. Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide. 2010;23:181–186. doi: 10.1016/j.niox.2010.05.287. [DOI] [PubMed] [Google Scholar]
- 14.Evans P., Halliwell B. Micronutrients: oxidant/antioxidant status. Br. J. Nutr. 2001;85:S67–S74. doi: 10.1079/bjn2000296. [DOI] [PubMed] [Google Scholar]
- 15.Alberg A.J. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–137. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease. J. Am. Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Pappas R.S. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3:1181–1198. doi: 10.1039/c1mt00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A., Dey N., Ghosh A., Das S., Chattopadhyay D.J., Chatterjee I.B. Molecular and cellular mechanisms of cigarette smoke-induced myocardial injury: prevention by vitamin C. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karademirci M.M., Kutlu R., Kilinc I. Relationship between smoking and and total antioxidant status, total oxidant status, oxidative stress index, vitamin C, vitamin E. Clin. Respir. J. 2018;12:2006–2012. doi: 10.1111/crj.12757. [DOI] [PubMed] [Google Scholar]
- 20.Talukder M.A.H., Johnson W.M., Varadharaj S., Lian J., Kearns P.N., El-Mahdy M.A., Liu X., Zweier J.L. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H388–H396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos P.P., Oliveira F., Ferreira V.C.M.P., Polegato B.F., Roscani M.G., Fernandes A.A., Modesto P., Rafacho B.P.M., Zanati S.G., Di Lorenzo A., Matsubara L.S., Paiva S.A.R., Zornoff L.A.M., Marcos F., Minicucci M.F., Azevedo P.S. The role of lipotoxicity in smoke cardiomyopathy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stimson G.V., Thom B., Costall P. Disruptive innovations: the rise of the electronic cigarette. Int. J. Drug Policy. 2014;25(4):653–655. doi: 10.1016/j.drugpo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 23.McNeill A., Brose L.S., Calder R., Hitchman S.C. Public Health England; 2015. E-Cigarettes: an Evidence Update. August. [Google Scholar]
- 24.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., Graham I., Michael Stephen Hall M.S., Hobbs F.D.R., Løchen M.L., Löllgen H., Marques-Vidal P., Perk J., Prescott E., Redon J., Richter D.J., Sattar N., Smulders Y., Tiberi M., van der Worp H.B., van Dis I., Verschuren W.M.M., Binno S. European guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of The European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 Societies and by invited experts) Developed with The special contribution of The European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton D.L., Kwan L.Y., Stratton K. National Academies Press (US); Washington (DC): 2018. Public Health Consequences of E-Cigarettes. [DOI] [PubMed] [Google Scholar]
- 26.Harvanko A., Kryscio R., Martin C., Kelly T. Stimulus effects of propylene glycol and vegetable glycerin in electronic cigarette liquids. Drug Alcohol Depend. 2019 doi: 10.1016/j.drugalcdep.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farsalinos K.E., Voudris V. Do flavouring compounds contribute to aldehyde emissions in e-cigarettes? Food Chem. Toxicol. 2018;115:212–217. doi: 10.1016/j.fct.2018.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Jensen R.P., Strongin R.M., Peyton D.H. Solvent chemistry in the electronic cigarette reaction vessel. Sci. Rep. 2017;7(November 2016):1–11. doi: 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klager S., Vallarino J., MacNaughton P., Christiani D.C., Lu Q., Allen J.G. Flavoring chemicals and aldehydes in E-cigarette emissions. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.7b02205. [DOI] [PubMed] [Google Scholar]
- 30.Sleiman M., Logue J.M., Montesinos V.N., Russell M.L., Litter M.I., Gundel L.A., Destaillats H. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016;50(17):9644–9651. doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- 31.Goniewicz M.L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., Prokopowicz A., Jablonska-Czapla M., Rosik-Dulewska C., Havel C., Jacob P., 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azzopardi D., Patel K., Jaunky T., Santopietro S., Camacho O.M., McAughey J., Gaça M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods. 2016;26:477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zagoriti Z., El Mubarak M.A., Farsalinos K., Topouzis S. Effects of exposure to tobacco cigarette, electronic cigarette and heated tobacco product on adipocyte survival and differentiation in vitro. Toxics. 2020;8:9. doi: 10.3390/toxics8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leigh N.J., Lawton R.I., Hershberger P.A., Goniewicz M.L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tobacco Control. 2016;25:ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J., Zhang Y., Sisler J.D., Shaffer J., Leonard S.S., Morris A.M., Demokritou P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018;344:549–557. doi: 10.1016/j.jhazmat.2017.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel R., Durand E., Trushin N., Prokopczyk B., Foulds J., Elias R.J., Richie J.P. Highly reactive free radicals in electronic cigarette aerosols. Chem. Res. Toxicol. 2015;28(9):1675–1677. doi: 10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., Robinson R., Rahman I. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):1–26. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweitzer K.S., Chen S.X., Law S., Van Demark M., Poirier C., Justice M.J., Hubbard W.C., Kim E.S., Lai X., Wang M., Kranz W.D., Carroll C.J., Ray B.D., Bittman R., Goodpaster J., Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2015;309(2):L175–L187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerloff J., Sundar I.K., Freter R., Sekera E.R., Friedman A.E., Robinson R., Pagano T., Rahman I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography–mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl. In Vitro Toxicol. 2017;3(1):28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthumalage T., Prinz M., Ansah K.O., Gerloff J., Sundar I.K., Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2018;8(JAN):1–13. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veskoukis A., Kerasioti E., Priftis A., Kouka P., Spanidis Y., Makri S., Kouretas D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: the biomarker issue. Curr. Opin. Toxicol. 2019;13:99–109. [Google Scholar]
- 42.Keles M.S., Taysi S., Sen N., Aksoy H., Akcay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001;28:141–143. doi: 10.1017/s0317167100052823. [DOI] [PubMed] [Google Scholar]
- 43.Kerasioti E., Stagos D., Priftis A., Aivazidis S., Tsatsakis A.M., Hayes A.W., Kouretas D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014;155:271–278. doi: 10.1016/j.foodchem.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 44.Janaszewska A., Bartosz G. Assay of total antioxidant capacity: comparisonof four methods as applied to human blood plasma. Scand. J. Clin. Lab. Invest. 2002;62:231–236. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- 45.Patsoukis N., Zervoudakis G., Panagopoulos N.T., Georgiou C.D., Angelatou F., Matsokis N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004;357:83–86. doi: 10.1016/j.neulet.2003.10.080. [DOI] [PubMed] [Google Scholar]
- 46.Veskoukis A.S., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers. 2016;21:208–217. doi: 10.3109/1354750X.2015.1126648. [DOI] [PubMed] [Google Scholar]
- 47.Rowell T.R., Reeber S.L., Lee S.L., Harris R.A., Nethery R.C., Herring A.H., Glish G.L., Tarran R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2017;313(1):L52–L66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otero C.E., Noeker J.A., Brown M.M., Wavreil F.D.M., Harvey W.A., Mitchell K.A., Heggland S.J. Electronic cigarette liquid exposure induces flavor-dependent osteotoxicity and increases expression of a key bone marker, collagen type. J. Appl. Toxicol. 2019;39:888–898. doi: 10.1002/jat.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iskandar A.R., Gonzalez-Suarez I., Majeed S., Marescotti D., Sewer A., Xiang Y., Leroy P., Guedj E., Mathis C., Schaller J.P., Vanscheeuwijck P., Stefan Frentzel S., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol. Mech. Methods. 2016;26(6):389–413. doi: 10.3109/15376516.2016.1170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bitzer Z.T., Goel R., Reilly S.M., Elias R.J., Silakov A., Foulds J., Muscat J., Richie J.P. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 2018;120(November 2017):72–79. doi: 10.1016/j.freeradbiomed.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sussan T.E., Gajghate S., Thimmulappa R.K., Ma J., Kim J.H., Sudini K., Consolini N., Cormier S.A., Lomnicki S., Farhana Hasan F., Pekosz A., Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):1–15. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson C., Majeste A., Hanus J., Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. 2016 doi: 10.1093/TOXSCI/KFW166. [DOI] [PubMed] [Google Scholar]
- 53.Carnevale R., Sciarretta S., Violi F., Nocella C., Loffredo L., Perri L., Mariangela Peruzzi M., Marullo A.G.M., De Falco E., Chimenti I., Valentina Valenti V., Biondi-Zoccai G., Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Parker T.T., Rayburn J. A comparison of electronic and traditional cigarette butt leachate on the development of Xenopus laevis embryos. Toxicol. Rep. 2017;4:77–82. doi: 10.1016/j.toxrep.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Godec T., Crooks I., Scott K., Meredith C. In vitro mutagenicity of gas-vapour phase extracts from flavoured and unflavoured heated tobacco products. Toxicol. Rep. 2019;7(6):1155–1163. doi: 10.1016/j.toxrep.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu S.H., Sun J.Y., Bonnevie E., Cummins S.E., Gamst A., Yin L., Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control. 2014;23(Suppl 3) doi: 10.1136/tobaccocontrol-2014-051670iii3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavvalakis M.P., Stivaktakis P.D., Tzatzarakis M.N., Kouretas D., Liesivuori J., Alegakis A.K., Vynias D., Tsatsakis A.M. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J. Anal. Toxicol. 2015;39(4):262–269. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 58.Farsalinos K.E., Kistler K.A., Gillman G., Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob. Res. 2015;17(2):168–174. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marescotti D., Mathis C., Belcastro V., Leroy P., Acali S., Martin F., Dulize R., Bornand D., Peric D., Guedj E., Torres L.O., Biasioli M., Fuhrimann M., Fernandes E., Frauendorfer F., Suarez I.G., Sciuscio D., Ivanov N.V., Peitsch M.C., Hoeng J. Systems toxicology assessment of a representative e-liquid formulation using human primary bronchial epithelial cells. Toxicol. Rep. 2019;25(7):67–80. doi: 10.1016/j.toxrep.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wavreil F.D.M., Heggland S.J. Cinnamon-flavored electronic cigarette liquids and aerosols induce oxidative stress in human osteoblast-like MG-63 cells. Toxicol. Rep. 2019;29(7):23–29. doi: 10.1016/j.toxrep.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flouris A.D., Poulianiti K.P., Chorti M.S., Jamurtas A.Z., Kouretas D., Owolabi E.O., Tzatzarakis M.N., Tsatsakis A.M., Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. Epub 2012 Jul 31. [DOI] [PubMed] [Google Scholar]
- 62.Hamza P.Z., El-Shenawy N.S. Anti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicol. Rep. 2017;4:399–407. doi: 10.1016/j.toxrep.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]