Abstract
Objective
To assess clinical studies that compare synthetic or enriched natural materials to autologous osseous grafts among individuals with cleft lip and palate to determine which would be the substitute to autologous bone graft for alveolar cleft repair in humans.
Materials and methods
Randomized and controlled clinical trials on alveolar clefts treated with synthetic bone substitutes and autogenous bone grafts combined with osteoinductive factors compared with autogenous bone grafts alone (with ≥4-month follow-up and reporting clinical/radiographic data) were considered eligible. MEDLINE, EMBASE, and Central databases were searched for articles published until February 2020.
Results
Of 73 eligible articles, 15 were included. Some inductive factors along with iliac crest bone decreased bone reabsorption, preserved the generated bone height/width, and reduced the required autologous bone graft volume. Bone morphogenetic protein (BMP2) as an autologous bone graft substitute, demonstrated satisfactory alveolar defect healing, by avoiding autograft use. Many materials did not yield better outcomes than did autologous grafts; however, hydroxyapatite and collagen complex, hydroxyapatite agarose composite gel, acellular dermal matrix film, fibrin glue, platelet-rich plasma, and deproteinized bovine bone showed similar bone healing outcomes, being an alternative alveolar defect treatment.
Conclusions
BMP2, as an osteoinductive factor along with a synthetic matrix, yields satisfactory bone healing and avoids the need for autologous bone grafts. However, high-quality RCTs are necessary to determine the most effective and safe concentration and protocol of BMP2 utilization as a substitute for the autologous iliac crest bone grafting.
Keywords: Biotechnology, Protein engineering, Biomedical engineering, Dental surgery, Dentistry, Oral medicine, Alveolar cleft, Cleft lip, Substitute bone, Cleft lip repair, Transplants transplantation, Alveolar bone grafting, Cleft primary palate
Biotechnology; Protein engineering; Biomedical engineering; Dental surgery; Dentistry; Oral medicine; Alveolar cleft; Cleft lip; Substitute bone; Cleft lip repair; Transplants transplantation; Alveolar bone grafting; Cleft primary palate
1. Introduction
Cleft lip and palate are one of the most frequent congenital craniofacial malformations (Rodman and Tatum, 2016; Seifeldin, 2016; Taib et al., 2015). These pathologies cause aesthetic and functional alterations, specifically in speech, feeding, hearing, craniofacial development, and oral health, which additionally affect the behavior and psycho-social well-being of affected individuals and their family (Taib et al., 2015; Sharif et al., 2016). The World Health Organization reports a global incidence of these types of malformations in 69 out of 10000 live-borns (Sharif et al., 2016).
The treatment for these pathologies is multidisciplinary and aims to re-establish aesthetics and function. Fissure repairs at an alveolar level aim to restore the osseous process continuity, favor dental eruption and alignment, achieve closure of oronasal fistulae, obtain symmetry through alar base support, and allow orthodontic movement or rehabilitation if necessary (Seifeldin, 2016; Sharif et al., 2016; Janssen et al., 2014).
Surgical closure is not enough for repairing osseous defects, as it is with soft tissues; additional materials are necessary for these types of fissures, such as bone grafts or synthetic substitutes (Sharif et al., 2016). These materials may be of natural origin, such as autografts or allografts; the latter is processed for use but some of them have problems of autoimmunity, infection transmission, poor conductivity, and limited availability (Sharif et al., 2016; Amini et al., 2012). Synthetic substitutes, such as hydroxyapatite (HA), tri-calcium phosphate (TCP), bioactive silicates (SiO2), polymers (PGA and PLA), or bio-composites built out of combinations can also be used (Sharif et al., 2016).
Autologous bone grafts harvested from the iliac crest have been considered as the gold standard for the treatment of alveolar fissures (Seifeldin, 2016; Janssen et al., 2014). However, there have been reports of complications, such as morbidity, infection, deformity, scarring, and bleeding from the donor area; additionally, there is a graft size limit, requiring an additional surgery and increasing the procedural time (Janssen et al., 2014; Amini et al., 2012). For these reasons, the replacement of these grafts by synthetic bone substitutes could overcome the previously mentioned limitations. Previous studies and systematic reviews concluded that the use of bone grafts with recombinant human bone morphogenetic protein (rhBMP2) (Wu et al., 2018; Dickinson et al., 2008) or fibrin glue (Segura-Castillo et al., 2005) is not superior to traditional bone grafts (Guo et al., 2011). In two other reviews, where clinical trials evaluated strategies to improve autologous bone grafts, such as addition of different materials to traditional grafts or their total replacement by another component, the authors could not come to a conclusion owing to insufficient evidence (Khojasteh et al., 2015) or heterogeneity of data (Janssen et al., 2014); further clinical studies with a more rigorous methodology are then warranted for the outcomes to be properly compared (Khojasteh et al., 2015; Janssen et al., 2014).
Thus, the purpose of this systematic review is to assess clinical studies that compare synthetic or enriched natural materials to autologous osseous grafts among individuals with cleft lip and palate to determine which may be the substitute to autologous bone graft for alveolar cleft repair in humans.
2. Materials and methods
The study protocol of this review was registered at the National Institute for Health Research PROSPERO (registration number 42016039040); the study was conducted in accordance with the PROSPERO Statement and the Cochrane Handbook of Systematic Reviews of Interventions (Higgins and Green, 2011).
2.1. Eligibility criteria
Both randomized clinical trials (RCTs) and controlled clinical trials with a follow-up duration of ≥4 months were considered eligible for inclusion. Studies reporting the clinical and radiographic outcomes of patients with alveolar clefts treated with synthetic bone substitutes and autogenous bone grafts associated to several osteoinductive factors compared with autogenous bone grafts alone (i.e., gold standard procedure) were included. Systematic reviews, review articles, case series or case reports, and retrospective clinical trials were excluded from the review.
2.2. Outcome measures
The primary outcome measures included as follows: changes in bone density, volume and height, decrease in the rate of bone resorption and bone formation on imaging (i.e., intraoral/panoramic radiography, cone beam computed tomography [CBCT] and computed tomography [CT]).
The secondary outcome measures included as follows: changes in the operative time and absence of donor site morbidity.
2.3. Search strategy
Electronic search was conducted on MEDLINE, EMBASE, and CENTRAL databases for articles published up to February 2020, without language restrictions and limited to human subjects; the following MeSH terms and keywords based on the search strategy prepared for searching PubMed were used: 1. (alveolar clefts) AND (bone substitute) AND (humans); 2. (alveolar cleft) OR (cleft lip) AND (repair); 3. 1) OR 2); 4. (alveolar cleft) OR (cleft palate) OR (cleft lip) AND (graft) OR (repair) OR (transplants) OR (transplantation); 5. (alveolar cleft) AND (cleft lip) AND (repair) AND (transplant); 6. 4) OR 5); and 7. 3) AND 6).
Additionally, the reference list of potentially eligible articles were hand searched.
2.4. Validity assessment and data extraction
Three independent reviewers (MCG, LME, and CCO) assessed the title, abstract, and full text of the articles considered eligible for inclusion, and disagreement among them was resolved by consensus. When the three reviewers could not reach an agreement, a fourth reviewer (LC) was consulted.
The following data were extracted from each included study: 1) citation, publication year, and publication status; 2) location of the trial (private practice or university/hospital); 3) type of study (i.e., RCT or controlled clinical trial); 4) Sample; 5) types of interventions; 6) outcome measures and quality assessment; 7) conclusions; 8) source of funding; and 9) conflict of interest.
2.5. Risk of bias assessment
The methodological quality of the trials was evaluated using the Cochrane Collaboration's tool for assessing risks of bias by Higgins and Green (2011), as adapted by Chambrone et al. (2010). Briefly, the randomization and allocation methods were classified as adequate, inadequate, unclear, or not applicable, whereas the completeness of the follow-up period, blinding of examiners, selective reporting, and other sources of bias were coded as yes/no responses. Based on the answers, the risk of bias was categorized in accordance with the following classifications: 1) a low risk of bias if all criteria were met (i.e., adequate methods of randomization and allocation concealment and a yes answer to all questions on completeness of follow-up questions and masking of examiners); (2) an unclear risk of bias if one or more criteria were partly met (i.e., unclear criteria were set); or (3) a high risk of bias if one or more criteria were not met (Higgins and Green, 2011; Chambrone et al., 2010). All non randomized studies were automatically considered to be at a high risk of bias and they were not included in Table 1.
Table 1.
Risk of bias summary.
| Author/year | Randomization | Allocation | Blinding of examiners | Number of subjects at baseline and study completion reported | All patients completed the follow-up period | Selective reporting | Other sources of bias | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Iwai et al. (2015) | Unclear | Unclear | No | Yes | Yes | No | No | 3- High risk |
| Neovius et al. (2013) | Adequate | Unclear | No | Yes | Yes | No | Yes | 3- High risk |
| Pradel and Lauer (2012) | Unclear | Inadequate | No | Yes | Yes | No | Yes | 3- High risk |
| Canan et al. (2012) | Adequate | Unclear | No | Yes | Yes | No | Yes | 3- High risk |
| Marukawa et al. (2011) | Adequate | Unclear | Yes | Yes | Yes | No | Yes | 2- Unclear |
| Luaces-Rey et al. (2010) | Unclear | Unclear | Yes | Yes | Yes | No | No | 3- High risk |
| Alonso et al. (2010) | Adequate | Unclear | Yes | Yes | Yes | No | No | 2- Unclear |
| Thuaksuban et al. (2010) | Adequate | Adequate | No | Yes | No | No | No | 3- High risk |
| Lee et al. (2009) | Unclear | Unclear | No | Yes | Yes | No | No | 3- High risk |
| Dickinson et al. (2008) | Adequate | Unclear | Yes | Yes | Yes | No | Yes | 2- Unclear |
| Hegab and Shuman (2012) | Adequate | Unclear | No | Yes | Yes | No | No | 3- High risk |
| Segura-Castillo et al. (2005) | Adequate | Adequate | Yes | Yes | Yes | No | No | 1- Low risk |
| Xiao et al. (2016) | Adequate | Adequate | Unclear | Yes | Yes | No | No | 2- Unclear |
| Shawky and Seifeldin (2016) | Adequate | Adequate | Yes | Yes | Yes | No | No | 1- Low risk |
| Takemaru et al. (2016) | Adequate | Adequate | Unclear | Yes | Yes | No | No | 1- Low risk |
2.6. Data synthesis
Data were grouped into evidence tables and reported as descriptive summaries. This allowed determination of the quantity of data, as well as study variations in terms of the study characteristics and results.
3. Results
3.1. Search results and included studies
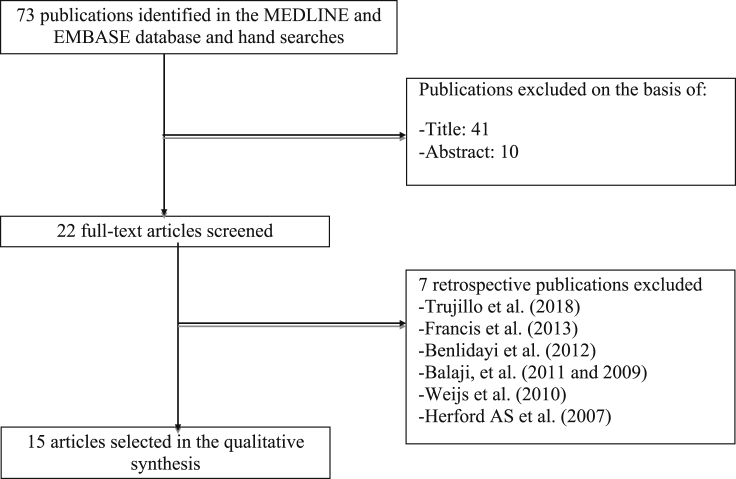
A total of 73 studies were obtained; the search results were checked on the basis of the title or abstract, yielding 22 articles. Finally, the full text of these articles was evaluated. Fifteen articles that met the selection criteria were selected (Figure 1).
Figure 1.
Flow diagram of study inclusion (Balaji, 2009; Balaji, 2011; Benlidayi et al., 2012; Francis et al., 2013; Herford et al., 2007; Trujillo et al., 2018).
4. Methodological quality of the included studies
Of the 15 studies included in this systematic review, three were considered to have a low risk of bias; four, unclear risk; and eight, high risk. The principal sources of bias were allocation concealment and blinding of examiners (Table 1).
4.1. Effects of interventions
Given the substantial degree of heterogeneity found in terms of the studies' methodology, the most transparent approach was to present the data without meta-analysis. Therefore, the effects of interventions were assessed using the outcomes of the individual studies exclusively. The selected studies were separated according to two different alveolar repair strategies: 1) studies comparing autologous bone grafts associated to osteoinductive factors with autologous bone grafts (Table 2) and 2) studies comparing autologous bone graft substitutes like synthetic materials or heterografts with autologous bone grafts (Table 3) for alveolar cleft repair.
Table 2.
Characteristics of the studies evaluated of group 1, Autologous bone grafts combined with osteoconductive factors.
| Author/year [ref.] | n. |
Study type | Experimental group biomaterials | Control group | Follow-up | Type of radiographical assessment | Conclusion | Bias risk | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||||
| Takemaru et al. (2016) | 5 | 10 | RCT | Bioabsorbable HA/Col with iliac bone |
Iliac bone | Immediately and at 1, 6, and 12 months postoperatively | CT scans after 1, 6 and 12 months | HA/Col can be effectively used in combination with an iliac graft and can reduce the amount of autogenos iliac graft. | 1- Low risk |
| Xiao et al. (2016). | 30 | 30 | RCT | Acellular dermal matrix film combined with alveolar bone grafting | Iliac bone | 1 week and 3 months postoperatively | CT scans after 1 week and 3 months | The mixture can reduce bone resorption and result in better osteogenesis. | 2- Unclear |
| Shawky and Seifeldin (2016). | 12 | 12 | RCT | Platelet-rich fibrin with iliac bone | Iliac bone | 6 months postoperatively | CT scans after 6 months | Platelet-rich fibrin in combination with autogenous bone improves the volume, but does not enhance the bone density. | 1- Low risk |
| Iwai et al. (2015) | 10 | 10 | PCT | Mixture of HAp gel and iliac bone | 9: Iliac bone 1: Autologous jawbone |
1, 3, and 6 months postoperatively | Oclussal radiography after 1, 3 and 6 months |
HAp gel with autologous bone is as effective as autologous bone alone and can reduce the volume of the autologous bone required. | 3- High risk |
| Hegab and Shuman (2012) | 10 | 10 | PCT | Autogenous iliac bone graft with PRP | Iliac bone | 1, 6, and 12 months postoperatively | Digital panoramic radiography after 1, 6, and 12 months |
The application of PRP is more favorable to that of alveolar bone grafts. | 3- High risk |
| Marukawa et al. (2011) | 14 | 6 | RCT | Iliac bone graft with PRP | Iliac bone | 1 and 6 months and 1 year postoperatively | CT scans after 1, 6 and 12 months and panoramic radiography after 1 week, 1,6 and 12 months | The added PRP reduced the resorption of the regenerated bone. | 2- Unclear risk |
| Luaces-Rey et al. (2010) | 10 | 10 | PCT | Iliac bone graft with PRP | Iliac bone | Immediate postoperative and at 3 and 6 months postoperatively | Panoramic radiography after 3 and 6 months | The use of PRP is not justified in the treatment of alveolar clefts. | 3- High risk |
| Thuaksuban et al. (2010) | 15 | 15 | RCT | Iliac bone with DBB | Iliac bone | 3 days and 1, 3, 6, 12, 18, and 24 months postoperatively | Intraoral and occlusal radiography after 3 days and 1, 3, 6, 12, 18, and 24 months |
The added DBB reduced the amount of the autogenous bone required, patient morbidity, and hospitalization. | 3- High risk |
| Lee et al. (2009) | 30 | 30 | PCT | Iliac bone graft with PRP | Iliac bone | 1 week and 1, 3, 6, and 12 months postoperatively | 1 week and 1, 3, 6, and 12 months alter operation | Combining grafting with PRP seems to be insufficient as a countermeasure against bone resorption. | 3- High risk |
| Segura-Castillo (2005) | 13 | 14 | RCT | Fibrin glue with iliac crest | Iliac bone | 3 months postoperatively | CT | Fibrin glue significantly diminished bone resorption and improved graft integration. | 1- Low risk |
HA/Col: hydroxyapatite and collagen.
PRP: platelet-rich plasma.
DBB: deproteinized bovine bone.
HAp: hydroxyapatite agarose composite.
CT: computed tomography.
CBCT: cone beam computed tomography.
PCT: prospective controlled trial.
RCT: randomized controlled trial.
Table 3.
Characteristics of the studies evaluated of group 2, Autologous bone graft substitute.
| Author/year [ref.] | n. |
Study type | Experimental group biomaterials | Graft type | Control group | Follow-up (months) | Radiographical assessment type | Conclusion | Bias risk | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Experimental group | |||||||||
| Neovius et al. (2013) | 4 | 3 | RCT | BMP2 in hydrogel | Synthetic | Iliac bone | 6 months postoperatively | CT san after 6 months. | BMP2 causes severe postoperative swelling. The use of hydrogel as a BMP2 delivery vehicle may reduce the amount of growth factors needed to achieve therapeutic repair. |
3- High risk |
| Pradel and Lauer (2012) | 4 | 4 | PCT | Autogenous osteoblasts cultured on the demineralized bone matrix Osteovit | Heterograft | Iliac bone | 6 months postoperatively | CT scan after 6 months | The use of autogenous osteoblasts cultured on Osteovit is a promising alternative in alveolar cleft repair. No disadvantages in comparison to traditional bone grafts were observed. | 3- High risk |
| Canan et al. (2012) | 12 | 6 | RCT | rhBMP2 in collagen sponges | Synthetic | Iliac bone | 3, 6, and 12 months postoperativel | CT scan after 3, 6 and 12 months | There was no difference among the groups analyzed. | 3- High risk |
| Alonso et al. (2010) | 8 | 8 | RCT | Resorbable collagen matrix with rhBMP2 | Synthetic | Iliac bone | 6 and 12 months postoperatively | CT scan after 6 and 12 months | The use of rhBMP2 for repairing alveolar cleft yielded satisfactory bone healing and avoided donor site morbidity. | 2- Unclear |
| Dickinson et al. (2008) | 9 | 12 | RCT | Collagen matrix/BMP2 construct | Synthetic | Iliac bone | 6 months postoperatively | CT, Panorex, periapical views after 6 months | BMP2 improved bone healing and reduced morbidity compared with traditional iliac bone grafts. | 2- Unclear |
PCT: prospective controlled trial.
RCT: randomized controlled trial.
CT: computed tomography.
4.2. Autologous bone grafts associated to osteoinductive factors
The studies evaluated reported an average age between 8 to 21 years old.
Ten studies conducted on autologous bone grafts from the iliac crest combined with different biomaterials were found. These materials were as follows: HA (Takemaru et al., 2016; Iwai et al., 2015), acellular dermal matrix film (Xiao et al., 2016), platelet-rich plasma (PRP) (Shawky and Seifeldin, 2016; Hegab and Shuman, 2012; Marukawa et al., 2011; Luaces-Rey et al., 2010; Lee et al., 2009), deproteinized bovine bone (DBB) (Thuaksuban et al., 2010), and fibrin glue (Segura-Castillo et al., 2005). The control group in these studies was treated with bone grafts from the iliac crest or mandibular bone.
Among the analyzed articles, four were prospective controlled studies (PCTs) (Iwai et al., 2015; Hegab and Shuman, 2012; Luaces-Rey et al., 2010; Lee et al., 2009), and six were RCTs (Segura-Castillo et al., 2005; Takemaru et al., 2016; Xiao et al., 2016; Shawky and Seifeldin, 2016; Marukawa et al., 2011; Thuaksuban et al., 2010).
Two studies evaluated the effect of HA associated to autologous bone graft: Iwai et al. (2015), found that the combination of HA agarose composite (HAp) gel and bone graft is equally effective as an autologous graft alone for treating bone defects in cleft lip and alveolar cleft; additionally, the gel could be further beneficial by reducing the required graft volume. Osteogenesis and bone density were evaluated using dental and occlusal radiographic films obtained preoperatively and 1, 3, and 6 months postoperatively (Iwai et al., 2015). To determine osteogenesis radiographically, the bone density on the radiographic films was measured using ImageJ. Conversely, Takemaru et al. (2016), evaluated the utility and efficacy of bioabsorbable HA and collagen complex (HA/Col) for secondary bone grafting in unilateral alveolar cleft. To determine the alveolar cleft and bone volumes, CT scans were obtained immediately before surgery and 1, 6, and 12 months after surgery. It was observed that the autogenous bone was gradually absorbed, while the HA/Col was absorbed and replaced by the autogenous bone. Thus, the authors conclude that HA/Col can be used as an iliac graft in alveolar bone grafting to reduce the amount of autogenous bone required (Takemaru et al., 2016).
Xiao et al. (2016), evaluated the osteogenic effect of guided bone regeneration (GBR) compared with conventional alveolar bone grafting alone for alveolar cleft defects. GBR was performed using an acellular dermal matrix film combined with alveolar bone grafting using iliac crest bone grafts. The bone graft volume was measured using three-dimensional CBCT. CBCT was performed for all patients 1 week and 3 months after alveolar bone grafting. The bone resorption rate was significantly higher in the non-GBR group (36.50%) than in the GBR group (31.69%). The results suggest that the application of an autogenous iliac bone combined with the GBR technique for alveolar bone grafting of alveolar cleft can reduce bone resorption and result in better osteogénesis (Xiao et al., 2016).
Five of the selected articles used PRP as an additional factor to autologous bone graft. Shawky and Seifeldin (2016), found a mean bone formation rate of 82.6% ± 3.9% in the PRP group compared with 68.38% ± 6.67% in the control group 6 months after the intervention. Although the quality (bone density) was greater in the control group than in the experimental group, they did not find significant differences between them (Shawky and Seifeldin, 2016).
Marukawa et al. (2011), reported that the addition of PRP reduces postoperative bone reabsorption, preserving the width and height of the graft better than the control intervention. One year after surgery, a 26.5%±-0.71% bone loss rate was detected among the PRP group and 35.5%±-2.12% among the control group under radiographic parameters (occlusal or panoramic radiography and computerized tomography) (Marukawa et al., 2011).
However, Lee et al. (2009), did not find any significant differences in the reabsorption rate upon comparison of autologous grafts with and without PRP. Additionally, Luaces-Rey et al. (2010), did not find any significant differences in the bone formation among the groups; they also did not justify the use of PRP in the treatment of alveolar fissures, although they reported that a combination between PRP and bone graft facilitates handling during the surgical procedure.
Segura-Castillo et al. (2005), studied the application of fibrin glue to autologous bone grafts as an alternative to the reduction of postoperative bone resorption. The pre- and postoperative graft volumes, bone densities, and bone qualities were evaluated between their experimental group treated with fibrin glue and control group treated with bone graft alone. The bone volume was significantly higher in the experimental group than in the control group; they also found lower resorption rates in the experimental group (29.72%) than in the control group (62.26%). They concluded that fibrin glue can decrease bone resorption and increase bone formation and graft integration (Segura-Castillo et al., 2005).
Thuaksuban et al. (2010), compared the bone height and density using occlusal radiography between two groups: a group treated with autologous bone graft alone and another group treated with bone graft with DBB; they concluded that although there were no significant differences between the groups, there was a reduction in the required autologous bone volume as well as postoperative morbidity.
4.3. Autologous bone graft substitutes
The studies evaluated reported an average age between 8 and 16 years old.
There were five articles in which different scaffolds with cell lines or osteoinductive factors, such as autogenic osteoblasts (Pradel and Lauer, 2012), and BMP2 (Dickinson et al., 2008; Neovius et al., 2013; Alonso et al., 2010; Canan et al., 2012), were evaluated, which can replace autologous bone grafts as a treatment for alveolar fissures. Among them, four were RCTs (Dickinson et al., 2008; Neovius et al., 2013; Alonso et al., 2010; Canan et al., 2012), and one was a PCT (Pradel and Lauer, 2012).
Pradel and Lauer (2012), compared between their experimental group of children treated with autogenous osteoblasts cultured on demineralized bone matrix Osteovit and control group treated with autologous bone grafts harvested from the iliac crest using CBCT. Preoperatively, the mean volume of the cleft defects was similar in both groups. Six months postoperatively, the mean volume was 0.55 cm3 ± 0.24 cm3 in the experimental group and 0.59 cm3 ± 0.23 cm3 in the control group. The group treated with autogenic osteoblasts presented a cleft ossification rate of 40.9% compared with 36.6% in the group treated with autologous bone grafts. They concluded that the use of this technique is a promising alternative to autologous grafts (Pradel and Lauer, 2012).
Four articles studied the use of BMP2 or rhBMP2. Dickinson et al. (2008), reported that their experimental group treated with a matrix of BMP2 collagen presented a higher bone formation rate in the alveolar cleft with a 95% of filled with bone new than did the control group treated with traditional iliac graft in their volumetric analysis. The values had a significant difference (p < 0.01). Additionally, the experimental group had better bone healing and enhanced mineralization. Alonso et al. (2010), evaluated two groups using CT: one group treated with rhBMP2 in re-absorbable collagen matrices and another group treated with conventional bone grafts found after 12 months a bone consolidation, and an average residual cleft defect and a percentage alveolar defect filled, similar in both groups. Canan et al. (2012), did not find significant differences in the residual bone defect volume between patients treated with rhBMP2 in collagen sponges and autologous bone grafts, with an average bone formation rate of 75.1% and 78.8%, respectively (p = 0.937), after 12 months of follow-up.
Nevertheless, Neovius et al. (2013), showed how high doses of BMP2 induce severe gingival inflammation. This discovery was associated with the hydrogel's concentration increment (from 50 μg to 250 μg) and concludes that the outcomes depend not only on the dose but also on the age of the recipient and placement of the implant. Therefore, the use of this factor must be carefully evaluated for certain applications. Despite this, some authors agree that the use of BMP2 as an autologous bone graft substitute reduces morbidity of the donor area and the need for additional surgery (Alonso et al., 2010; Dickinson et al., 2008).
5. Discussion
5.1. Summary of the main results
This systematic review included 15 articles, which were divided according to two strategies: 1) autologous bone grafts associated to osteoinductive factors and 2) autologous bone graft substitutes.
The main results of the included studies suggest that alveolar bone grafts supplemented with PRP could be an effective alternative to autologous grafts alone (Hegab and Shuman, 2012; Marukawa et al., 2011). The results reported by Hegab and Shuman (2012), also suggest that it is possible to achieve a more favorable result with the application of PRP to alveolar bone grafts. However, some authors insure that its use is not justifiable because it does not improve the results in terms of the quality and quantity of the bone formed compared with the use of autologous bones alone (Shawky and Seifeldin, 2016; Luaces-Rey et al., 2010; Lee et al., 2009).
The use of HA/Col, HAp gel, or DBB in combination with iliac crest bone grafting reduces the volume of the autologous bone graft required (Takemaru et al., 2016; Iwai et al., 2015; Thuaksuban et al., 2010); this suggests that such methods could have favorable results in the treatment of alveolar bone clefts, as they reduce the morbidity of the donor site (Iwai et al., 2015).
Some authors report that fibrin glue or acellular dermal matrix film with alveolar bone graft harvested from the iliac crest reduces bone resorption, improving graft integration and bone formation (Xiao et al., 2016; Segura-Castillo et al., 2005).
Autologous bone graft substitutes, such as BMP2 in collagen matrices, demonstrate satisfactory healing, making them a promising alternative for repair of alveolar grafts, as they avoid the use of autologous bone grafts, reducing donor morbidity (Canan et al., 2012; Alonso et al., 2010; Dickinson et al., 2008). Although an increase in localized inflammation associated with the use of BMP2 has been reported, the release of this factor through hydrogels may decrease this adverse effect (Neovius et al., 2013). Osteovit is considered an alternative for alveolar repair, as it yields advantages similar to those of a conventional graft (Pradel and Lauer, 2012).
5.2. Quality of the evidence
The risk assessment using the Cochrane tool, adapted by Chambrone et al. (2010), showed that only three of the 15 articles on alternatives for repair of alveolar defects presented a low risk of bias (Table 1). Most of the articles evaluated had a high risk of bias owing to problems in randomization, allocation, and/or blinding of examiners, which affects the quality of the studies and analysis of the results. The other sources of bias detected in the studies were the inappropriate sample size, conflicts of interest, and uninformed funding sources.
The Cochrane Risk of Bias Tool (Chambrone et al., 2010) used in our review does not account for “sample size”. However, estimate the sample size is important in designing RCT and necessary to answer the research question. Most of the articles reviewed did not calculate the sample size, even some authors suspect that the no significant difference between the groups analyzed was due to the sample size (Hegab and Shuman., 2012). Nevertheless, Takemaru et al. (2016), report that despite a small sample size they have results that show HA/Col can be effectively used in combination with an iliac graft and can reduce the amount of autogenous iliac graft. Others studies that used BMP2 protein attribute the small sample size to the high cost of the protein (Canan et al., 2012).
5.3. Limitations and potential biases in the review process
In the present review, only articles in English were included, which could generate a significant loss of results. Moreover, the heterogeneity of the data did not allow data to be pooled into a meta-analysis, which might have an impact on the overall results obtained. For example, the sample size greatly varied among the studies (Pradel and Lauer, 2012; Lee et al., 2009); analyses were performed from four patients per group up to 60 individuals, depending on the case.
In addition, the quantitative assessment method recommended for alveolar bone grafts is tomography scan (Honma et al., 1999; Rosentein et al., 1997). Diagnostic images were used in some of the studies analyzed in this review; however, other authors used occlusal radiographs (Iwai et al., 2015; Thuaksuban et al., 2010), Panorex (Hegab and Shuman, 2012; Luaces-Rey et al., 2010), and periapical radiographs (Iwai et al., 2015; Lee et al., 2009). This type of two-dimensional images has not been proven as a reliable tool for volumetric analysis for different degrees of magnification and does not provide clear results on the transverse plane (Feichtinger et al., 2007; Weijs et al., 2010).
5.4. Agreements and disagreements with other studies and systematic reviews
Tissue engineering aims to replace traditional bone grafts, which require a second surgical site, with another material that yields similar or better results than those obtained with autologous grafts alone, in terms of bone graft healing, volume and bone density, and bone reabsorption reduction.
In the literature, there are six systematic reviews conducted on this topic since 2011; Janssen et al. (2014), suggest that the use of an osteoinductive factor (DBB, b-TCP, or fibrin glue) with an autologous bone may increase the size of the graft when it is insufficient or decrease the amount of autologous bones needed. In the present review, similar results were found for factors, such as HA/Col, DBB, fibrin glue, and PRP.
Wu et al. (2018), evaluated the efficacy of bone substitute materials, iliac cancellous bone graft supplementary material, and autogenous bone graft and concluded that BMP2 bound to absorbable collagen sponge shares a similar cleft repair efficacy with iliac grafts. An iliac graft covering with an acellular dermis matrix membrane or mixing iliac graft with PRP may increase bone retention depending on the patients' age. A mandibular graft is more effective than an iliac graft, whereas cranial and rib grafts are less effective for alveolar cleft reconstruction. The authors reported enormous heterogeneity in the selection of patients, interventions, and outcomes assessed, similar to what was found in this systematic review (Wu et al., 2018).
Different authors who focus their systematic reviews on analyzing the use of BMP2 in the alveolar defects treatment support findings such as the use of BMP2 as the most favorable technique for bone formation; however, adverse effects, such as localized inflammation, caused by its application must be considered (Khojasteh et al., 2015; van Hout et al., 2011).
In the systematic review developed by Li et al. (2019), where a meta-analysis with results of Alonso et al. (2010) and Canan et al. (2012), was conducted; the author concludes that rhBMP-2 treatment groups seem to score lower than the control groups on the parameters of bone formation rate and increase of bone volume. However, when analyzing the results of these studies, we found that Alonso et al. (2010), conclude that rhBMP2 therapy resulted in satisfactory bone healing and reduced morbidity compared with traditional iliac crest bone grafting and Canan et al. (2012) showed that rhBMP2 use is more effective than periosteoplasty and as effective as autologous iliac crest bone grafting, which makes it promising in craniofacial reconstruction. However, In another recent meta-analysis where they evaluated the same studies in our review (Canan et al., 2012; Alonso et al., 2010; Dickinson et al., 2008), they conclude that the absence of statistical significance when comparing groups treated with BMP2 versus conventional bone grafts could support the effectiveness of the methods evaluated (Scalzone et al., 2019). On another hand Guo et al. (2011), analyzing one of the their selected studies where compare an artificial material plus rhBMP-2 with traditional iliac grafting refer that they can't conclude which one is superior due to high risk of bias even with significant differences found. In addition, it is important to highlight that despite the methodological discrepancies and limitations identified within all the included studies, those per se do not seem to be enough to decrease the impact and importance of their individual outcomes.
5.5. Other outcome measures not included in the objectives, dental eruption
An indispensable factor in the alveolar clefts treatment is to facilitate dental eruption (Shawky and Seifeldin, 2016), however that aspect was not evaluated in many of the studies reviewed. The purpose of the current study was focused to compare the bone healing (Neovius et al., 2013; Dickinson et al., 2008), ossification in the cleft area (Pradel and Lauer, 2012), alveolar defect volume, formed bone volume (Takemaru et al., 2016; Shawky and Seifeldin, 2016; Canan et al., 2012), bone formation rate (Canan et al., 2012), bone density (Shawky and Seifeldin, 2016; Iwai et al., 2015; Canan et al., 2012; Marukawa et al., 2011; Thuaksuban et al., 2010; Lee et al., 2009; Segura-Castillo et al., 2005), osteogenesis effect (Xiao et al., 2016; Iwai et al., 2015), resorption rate (Xiao et al., 2016; Hegab and Shuman, 2012; Marukawa et al., 2011; Lee et al., 2009), bone regeneration (Luaces-Rey et al., 2010), and bone quality (Segura-Castillo et al., 2005), between groups evaluated.
Few studies took into account dental eruption within the main objectives some authors refer it in their studies. Neovius et al. (2013), showed normal root development and dental eruption in two patients treated with BMP 250 ug/ml −1 however the results of the other groups were not presented. Pradel and Lauer (2012), don't have results about teeth eruption on the cleft area in the study analyzed in this review, however they report results of a case report where the canine tooth had erupted spontaneously in a patient treated with autogenous osteoblasts cultured on demineralized bone matrix (Pradel et al., 2008).
Some authors refer eruption was not affected but they don't make a difference by groups (Xiao et al., 2016; Shawky and Seifeldin, 2016; Canan et al., 2012). Dickinson et al. (2008) conclude that more studies are needed to evaluate the dental eruption with BMP2 technique. Thuaksuban et al. (2010), demonstrated by occlusal radiographs spontaneous or orthodontic dental eruption through the graft areas of both groups treated with autogenous bone and DBB composite or autologous bone graft alone. Dickinson et al. (2008), and Marukawa et al. (2011), evaluated skeletally mature patients, that could be the reason was not evaluated the canine eruption.
6. Conclusions
6.1. Autogenous bone grafts combined with osteoinductive factors
There is no consensus on the benefits of using PRP combined with autologous bone grafts for the management of alveolar clefts. HA used as an inductive factor can reduce the volume of autologous bones required. Conversely, the rate of bone resorption can be diminished using autologous bone grafts in combination with acellular dermal matrix film or fibrin glue.
6.2. Autogenous bone graft substitutes
BMP2, as an osteoinductive factor, in combination with a synthetic matrix, yields satisfactory bone healing and avoids the need for the use of autologous bone grafts and morbidity. Finally, based on the results observed, we can conclude that the significant non-difference observed in the studies could favor the use of BMP2 as a substitute for autologous grafting in the treatment of alveolar clefts. However, in addition to the high risk of bias presented by the studies, factors such as variability in the doses of BMP2 used and the methods of protein release that can produce adverse effects located in the graft area (inflammation), prevent us from suggesting a specific technique. For that reason is necessary to determine the most effective and safe concentration of BMP by performing RCTs with adequate sample size, low level of bias and long-term follow-up.
6.3. Implications for practice
The use of bone substitutes might be considered as an alternative treatment for alveolar defects, as it avoids the need for a second surgical site, reduces donor morbidity, and allows the use of great amounts of material.
6.4. Implications for research
Future studies should follow the CONSORT statement (2010) and include immediate and at least 6–12 months of follow-up assessments based on CBCT or CT images to allow volumetric analyses of alveolar grafts.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Alonso N., Tanikawa D.Y., Freitas Rda S., Canan L., Jr., Ozawa T.O., Rocha D.L. Evaluation of maxillary alveolar reconstruction using a resorbable collagen sponge with recombinant human bone morphogenetic protein-2 in cleft lip and palate patients. Tissue Eng. Part C Methods. 2010;16:1183–1189. doi: 10.1089/ten.TEC.2009.0824. [DOI] [PubMed] [Google Scholar]
- Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S.M. Alveolar cleft defect closure with iliac bone graft, rhBMP-2 and rhBMP-2 with zygoma shavings: comparative study. Ann. Maxillofac. Surg. 2011;1:8–13. doi: 10.4103/2231-0746.83144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S.M. Use of recombinant human bone morphogenetic protein (rhBMP-2) in reconstruction of maxillary alveolar clefts. J. Maxillofac. Oral Surg. 2009;8:211–217. doi: 10.1007/s12663-009-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlidayi M.E., Tatli U., Kurkcu M., Uzel A., Oztunc H. Comparison of bovine-derived hydroxyapatite and autogenous bone for secondary alveolar bone grafting in patients with alveolar clefts. J. Oral Maxillofac. Surg. 2012;1:e95–e102. doi: 10.1016/j.joms.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Canan L.W., Jr., da Silva Freitas R., Alonso N., Tanikawa D.Y., Rocha D.L., Coelho J.C. Human bone morphogenetic protein-2 use for maxillary reconstruction in cleft lip and palate patients. J. Craniofac. Surg. 2012;23:1627–1633. doi: 10.1097/SCS.0b013e31825c75ba. [DOI] [PubMed] [Google Scholar]
- Chambrone L., Chambrone L.A., Lima L.A. Effects of occlusal overload on peri-implant tissue health: a systematic review of animal-model studies. J. Periodontol. 2010;81:1367–1378. doi: 10.1902/jop.2010.100176. [DOI] [PubMed] [Google Scholar]
- Dickinson B.P., Ashley R.K., Wasson K.L., O'Hara C., Gabbay J., Heller J.B., Bradley J.P. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast. Reconstr. Surg. 2008;121:209–217. doi: 10.1097/01.prs.0000293870.64781.12. [DOI] [PubMed] [Google Scholar]
- Feichtinger M., Mossbock R., Karcher H. Assessment of bone resorption after secondary alveolar bone grafting using three-dimensional computed tomography: a three-year study. Cleft Palate Craniofac J. 2007;44:142–148. doi: 10.1597/06-047.1. [DOI] [PubMed] [Google Scholar]
- Francis C.S., Mobin S.S., Lypka M.A. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast. Reconstr. Surg. 2013;131:1107–1115. doi: 10.1097/PRS.0b013e3182865dfb. [DOI] [PubMed] [Google Scholar]
- Guo J., Li C., Zhang Q. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database Syst. Rev. 2011;15:CD008050. doi: 10.1002/14651858.CD008050.pub2. [DOI] [PubMed] [Google Scholar]
- Hegab A.F., Shuman M.A. Efficacy of platelet-rich plasma in reduction of the resorption of the alveolar cleft bone graft. A comparative study. Dentistry. 2012;2 2161-1122. [Google Scholar]
- Herford A.S., Boyne P.J., Rawson R., Williams R.P. Bone morphogenetic protein-induced repair of the premaxillary cleft. J. Oral Maxillofac. Surg. 2007;65:2136–2141. doi: 10.1016/j.joms.2007.06.670. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Green S. Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. [Google Scholar]
- Honma K., Kobayashi T., Nakajima T., Hayasi T. Computed tomographic evaluation formation after secondary bone grafting of alveolar clefts. J. Oral Maxillofac. Surg. 1999;57:1209–1213. doi: 10.1016/s0278-2391(99)90488-3. [DOI] [PubMed] [Google Scholar]
- Iwai S., Shimizu H., Suzawa Y., Akashi M., Yura Y. Hydroxyapatite agarose composite gels as a biochemical material for the repair of alveolar bone defects due to cleft lip and palate. J. Oral Maxillofac. Surg. 2015;27:637–644. [Google Scholar]
- Janssen N.G., Weijs W.L., Koole R., Rosenberg A.J., Meijer G.J. Tissue engineering strategies for alveolar cleft reconstruction: a systematic review of the literature. Clin. Oral Invest. 2014;18:219–226. doi: 10.1007/s00784-013-0947-x. [DOI] [PubMed] [Google Scholar]
- Khojasteh A., Kheiri L., Motamedian S.R., Nadjmi N. Regenerative medicine in the treatment of alveolar cleft defect: a systematic review of the literature. J. Cranio-Maxillo-Fac. Surg. 2015;43:1608–1613. doi: 10.1016/j.jcms.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Lee C., Nishihara K., Okawachi T., Iwashita Y., Majima H.J., Nakamura N. A quantitative radiological assessment of outcomes of autogenous bone graft combined with platelet-rich plasma in the alveolar cleft. Int. J. Oral Maxillofac. Surg. 2009;38:117–125. doi: 10.1016/j.ijom.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Li F., Yu F., Liao X., Wu C., Wang Y., Li C., Lou F., Li B., Yin B., Wang C., Ye L. Efficacy of recombinant human BMP2 and PDGF-BB in orofacial bone regeneration: a systematic review and meta-analysis. Sci. Rep. 2019;9(1):8073. doi: 10.1038/s41598-019-44368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luaces-Rey R., Arenaz-Búa J., Lopez-Cedrún-Cembranos J.L. Is PRP useful in alveolar cleft reconstruction? Platelet-rich plasma in secondary alveoloplasty. Med. Oral Patol. Oral Cir. Bucal. 2010;1:e619–e623. doi: 10.4317/medoral.15.e619. [DOI] [PubMed] [Google Scholar]
- Marukawa E., Oshina H., Iino G., Morita K., Omura K. Reduction of bone resorption by the application of platelet-rich plasma (PRP) in bone grafting of the alveolar cleft. J. Cranio-Maxillo-Fac. Surg. 2011;39:278–283. doi: 10.1016/j.jcms.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Neovius E., Lemberger M., Docherty Skogh A.C., Hilborn J., Engstrand T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J. Plast. Reconstr. Aesthetic Surg. 2013;66:37–42. doi: 10.1016/j.bjps.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Pradel W, Tausche E, Gollogly J, Lauer G. Spontaneous tooth eruption after alveolar cleft osteoplasty using tissue-engineered bone: a case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;105:440–444. doi: 10.1016/j.tripleo.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Pradel W., Lauer G. Tissue-engineered bone grafts for osteoplasty in patients with cleft alveolus. Ann. Anat. 2012;194:545–548. doi: 10.1016/j.aanat.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Rodman R.E., Tatum S. Controversies in the management of patients with cleft lip and palate. Facial Plast Surg Clin North AM. 2016;24:255–264. doi: 10.1016/j.fsc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Rosentein S.W., Long R.E., Dado D.V., Vinson B., Alder M.E. Comparison of 2-D calculations from periapical and occlusal radiographs versus 3D calculations from CAT scans in determining bone support for cleft-adjacent teeth following early alveolar bone grafts. Cleft Palate Craniofac. J. 1997;34:199–205. doi: 10.1597/1545-1569_1997_034_0199_codcfp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Scalzone A, Flores-Mir C, Carozza D, d’Apuzzo F, Grassia V, Perillo L. Secondary alveolar bone grafting using autologous versus alloplastic material in the treatment of cleft lip and palate patients: systematic review and meta-analysis. Prog. Orthod. 2019;20(1):6. doi: 10.1186/s40510-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Castillo J.L., Aguirre-Camacho H., González-Ojeda A., Michel-Perez J. Reduction of bone resorption by the application of fibrin glue in the reconstruction of the alveolar cleft. J. Craniofac. Surg. 2005;16:105–112. doi: 10.1097/00001665-200501000-00020. [DOI] [PubMed] [Google Scholar]
- Seifeldin S.A. Is alveolar cleft reconstruction still controversial? (Review of literature) Saudi Dent. J. 2016;28:3–11. doi: 10.1016/j.sdentj.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif F., Ur Rehman I., Muhammad N., Macneil S. Dental materials for cleft palate repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;1:1018–1028. doi: 10.1016/j.msec.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Shawky H., Seifeldin S.A. Does platelet-rich fibrin enhance bone quality and quantity of alveolar cleft reconstruction? Cleft Palate Craniofac. J. 2016;53:597–606. doi: 10.1597/14-290. [DOI] [PubMed] [Google Scholar]
- Taib B.G., Taib A.G., Swift A.C., van Eeden S. Cleft lip and palate: diagnosis and management. Br. J. Hosp. Med. (Lond). 2015;76:584–585. doi: 10.12968/hmed.2015.76.10.584. [DOI] [PubMed] [Google Scholar]
- Takemaru M., Sakamoto Y., Sakamoto T., Kishi K. Assessment of bioabsorbable hydroxyapatite for secondary bone grafting in unilateral alveolar cleft. J. Plast. Reconstr. Aesthetic Surg. 2016;69:493–496. doi: 10.1016/j.bjps.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Thuaksuban N., Nuntanaranont T., Pripatnanont P. A comparison of autogenous bone graft combined with deproteinized bovine bone and autogenous bone graft alone for treatment of alveolar cleft. Int. J. Oral Maxillofac. Surg. 2010;39:1175–1180. doi: 10.1016/j.ijom.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Trujillo R.L., Kadioglu O., Currier G.F., Smith K.S., Yetkiner E. Volumetric cleft changes in treatment with bone morphogenic protein/β-tricalcium phosphate versus grafts from the iliac crest or symphysis. J. Oral Maxillofac. Surg. 2018;76:1991–1997. doi: 10.1016/j.joms.2018.03.009. [DOI] [PubMed] [Google Scholar]
- van Hout W.M., Mink van der Molen A.B., Breugem C.C., Koole R., Van Cann E.M. Reconstruction of the alveolar cleft: can growth factor-aided tissue engineering replace autologous bone grafting? A literature review and systematic review of results obtained with bone morphogenetic protein-2. Clin. Oral Invest. 2011;15:297–303. doi: 10.1007/s00784-011-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijs W.L., Siebers T.J., Kuijpers-Jagtman A.M., Bergé S.J., Meijer G.J., Borstlap W.A. Early secondary closure of alveolar clefts with mandibular symphyseal bone grafts and b-tri calcium phosphate (b-TCP) Int. J. Oral Maxillofac. Surg. 2010;39:424–429. doi: 10.1016/j.ijom.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Wu C., Pan W., Feng C., Su Z., Duan Z., Zheng Q., Hua C., Li C. Grafting materials for alveolar cleft reconstruction: a systematic review and best-evidence synthesis. Int. J. Oral Maxillofac. Surg. 2018;47:345–356. doi: 10.1016/j.ijom.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Xiao W.L., Zhang D.Z., Chen X.J., Yuan C., Xue L.F. Osteogenesis effect of guided bone regeneration combined with alveolar cleft grafting: assessment by cone beam computed tomography. Int. J. Oral Maxillofac. Surg. 2016;45:683–687. doi: 10.1016/j.ijom.2016.01.013. [DOI] [PubMed] [Google Scholar]