Abstract
Background
Diabetes mellitus (DM) has harmful effects on body organs, including submandibular salivary glands (SMGs). It impairs wound healing process that follow sialoadenectomy. Yet there is no complete cure to diabetes, the available medications tend to control the side effects of DM or manage insulin resistance. Herein we tried to investigate the possible effects of injectable platelet rich fibrin (i-PRF) and melatonin on wound healing in diabetic rats.
Material and methods
Surgical defects were created in SMGs of 30 rats after confirmation of DM induction. Then rats were randomly and equally allocated into three groups. Group I served as control group; group II received topically applied i-PRF, and group III received topically administrated melatonin. After 28 days all rats were euthanized, and SMGs were processed for histological and biochemical analysis.
Results
Both i-PRF and melatonin caused significant reduction of malondialdehyde (P < 0.0001) and caspase-3 (P < 0.001) and significant increase in vascular endothelial growth factors (P = 0.001,0.009 respectively) that increased SMGs regenerative capacity when compared to diabetic group. Melatonin showed superior results regarding the histomorphological structure of SMGs.
Conclusion
Melatonin and i-PRF can be possible candidates for improvement of wound healing events in SMGs of diabetic rats.
Keywords: Injectable platelet rich fibrin (i-PRF), Melatonin, Diabetes mellitus, Critical wound healing
1. Introduction
Diabetes mellitus (DM) has deleterious effects on various body systems and their functions including oral cavity, where DM contributes to a lot of complications as dry mouth and periodontal diseases. In addition to noninflammatory and non-neoplastic disorders of the salivary glands known as sialosis which is accompanied with hyposalivation and increased susceptibility to infections.1
Reactive oxygen species (ROS) which result from chronic hyperglycaemia cause permanent changes in the redox state of DNA, RNA, proteins, lipids, and carbohydrates which lead to the loss of the biological functions of the cells. Reactive aldehydes such as malondialdehyde (MDA) are byproducts of lipid peroxidation which inhibits the activity of enzymes, transporters, and receptors present in cell membranes.2
Platelet rich concentrates have been widely used in medical and dental field. Platelet rich fibrin (PRF) is a second-generation modification, developed without anticoagulant to overcome the drawbacks of the first-generation platelet rich plasma (PRP), allowing more natural and faster wound healing events. PRF fulfills the criteria of tissue engineering where a three-dimensional fibrin clot acts as a scaffold, and leukocytes are the living cells, with high concentration of growth factors,3,4 such as transforming growth factor-b (TGF-b), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and others.
To overcome the rapid degradation of PRF, injectable PRF (i-PRF) was developed via reduction of centrifugation speed and time, yielding much higher concentration of growth factor including type I collagen and osteocalcin in a more flowable/gel form that ensure longer sustained release of growth factors.5
Melatonin (N-acetyl-5-methoxytryptamine) is an indole amine mainly produced in the pineal gland in a circadian manner in a 24-h, day–night rhythm.6,7 Also, several organs and glands were reported to secret melatonin7 where mitochondria are believed to be the preferentially sites of melatonin production in peripheral tissues.8
Oral mucosa and the salivary glands have been reported to be extrapineal sources of melatonin, as the enzymes encountered in melatonin formation were found in both the oral mucosa and the glandular parenchyma.9 Melatonin is released into the blood stream then diffuses into the saliva, it has been found that it has strong antioxidant effect which protects cells against inflammatory processes and oxidative damage.10 Herein we tried to test whether melatonin or i-PRF could accelerate the process of wound healing in submandibular glands of diabetic rats. The null hypothesis was that there is no significant difference between the melatonin or i-PRF.
2. Material and methods
2.1. Study design and grouping
All experimental procedures were conducted at animal house unit, Faculty of Pharmacy, Mansoura University. All procedures were approved by ethical committee of faculty of Dentistry, Mansoura University No: M06040820.
Thirty adult male albino rats weighing from 100 to 150 gm were selected, housed in individual cage, kept in 12/12 dark and light cycle with relative 50% humidity. All rats were acclimatized for one week before the experiments and were provided with free access to standard laboratory animal diet and water.
Diabetes Mellitus (DM) was induced in all rats. Surgical defects were created in submandibular glands of all animals.11 Then rats were randomly allocated (using random number tables) into three groups. Group I served as control group; group II was treated with injectable platelet rich fibrin (i-PRF), while group III was treated with melatonin.
2.2. Preparation of injectable platelet rich fibrin (iPRF)
For i-PRF preparation, ten rats were anesthetized by (xylazine (5 mg/kg) and ketamine (50 mg/kg)), then 10-ml blood samples without anticoagulant were collected from the orbital sinus by a punctured tube and were rapidly centrifuged For i-PRF preparation, at 700 rpm for 3 min (60×g) at room temperature by a Duo Centrifuge (Process for PRF, Nice, France). The upper liquid layer was collected as i-PRF.4
2.3. Preparation of melatonin gel
A concentration of 1% melatonin was prepared in faculty of pharmacy, Mansoura University. To avoid the use of alcohol, Melatonin (segma Aldrich, USA) was prepared using polyethylene glycol 400 (PEG 400). 17.5 g poloxamer was soaked in 61.5 ml of water in refrigerator overnight. Melatonin (1 g) was dissolved in 20 ml PEG 400 then added to poloxamer gel, followed by homogenous mix by mechanical stirrer.12
2.4. Induction of diabetes mellitus (DM)
After overnight fasting, rats were injected with a single, intraperitoneal dose (50 mg/kg) of streptozotocin (freshly prepared in ice-cold citrate buffer) (STZ, Sigma Chemical Co., St. Louis, MO, USA).13
2.5. Surgical defect creation
After confirmation of diabetes establishment, all rats were anesthetized with intra-peritoneal injection of xylazine (12.5 mg/kg) and ketamine hydrochloride (100 mg/kg), then SMGs were surgically exposed, and a 5 mm width × 8 mm length × 1.5 mm depth custom-made tissue punch was used to create a critical size defect in SMGs of all rats. Control group were left without treatment, while both i-PRF and melatonin gel were topically applied in the defects of groups II and III respectively. After surgical closure, postoperative care was provided by intramuscular injection of Amoxicillin (Egypt-pharmacy, Egypt) (10 mg/kg) for three days for all rats.11
2.6. Biopsy collection
After 28 days, all rats were anesthetized then euthanized by overdose of halothane (Healthcare, India). The submandibular glands were surgically removed. The left halves were carefully dissected from the surrounding tissues, quickly removed, and used for biochemical analysis.
2.7. Malondialdehyde levels (MDA) measurement
SMG specimens were separated from sublingual glands, cut on dry ice, homogenized in 1.15% KCl buffer, centrifuged for approximately 10 s. The supernatant was used for MDA analysis. MDA levels in SMGs tissue homogenate were measured by a kit purchased from Biodiagnostic Company (Cairo, Egypt) according to manufacturer's instructions. Thiobarbituric acid was used to react with malondialdehyde in SMG (MDA) via formation of a thiobarbituric acid-reactive substances, the results were expressed in nanomoles per gram (nmol/g tissue).13,14
2.8. Histological and immunohistochemical staining
The right halves of SMGs were fixed in 10% buffered formalin solution, then processed for routine hematoxylin and eosin stain. Moreover, immunohistochemical staining for monoclonal anti-Cleaved Caspase-3 (Asp 175; 1:2000) and Vascular endothelial growth factor (VEGF) Polyclonal antibodies (MyBiosource, San Diego (USA)) (0.5-1μg/ml) was performed, followed by image analysis and statistical analysis.
2.9. Image analysis & statistical analysis
Ten fields were examined using thresholding technique to measure the intensity of positive immunostaining reaction in all groups. Data were coded, tabulated using Statistical Package for Social Science software computer program version 26 (SPSS, Inc., Chicago, IL, USA). The data were tested for normality using Shapiro Wick test. Quantitative parametric data were presented in mean and standard deviation (SD). One-way analysis of variance (ANOVA) followed by post-hoc Tukey tests were used. P value less than 0.05 was considered statistically significant. The statistical analysis was based on a type 1 alpha error value of 5% (a = 0.05) and a power of 0.85 sample size.
3. Results
3.1. Determination of MDA levels in salivary homogenate
Malondialdehyde is one of lipid peroxidation byproducts, that represents a sequala of oxidative cell injury. The level of MDA was significantly higher in diabetic group when compared to i-PFR and melatonin treated groups. Both iPFR and melatonin significantly reduced the MDA levels (P < 0.0001) Table 1
Table 1.
Levels of MDA in salivary homogenate in all groups analyzed by One-way ANOVA, followed by post-hoc Tukey.
Data expressed as Mean ± SD.
P:Probability *:significance <0.05.
Represents significant decrease compared to (DM) control diabetic group and b: significant difference between i-PRF & Melatonin.
3.2. Routine H&E stain results
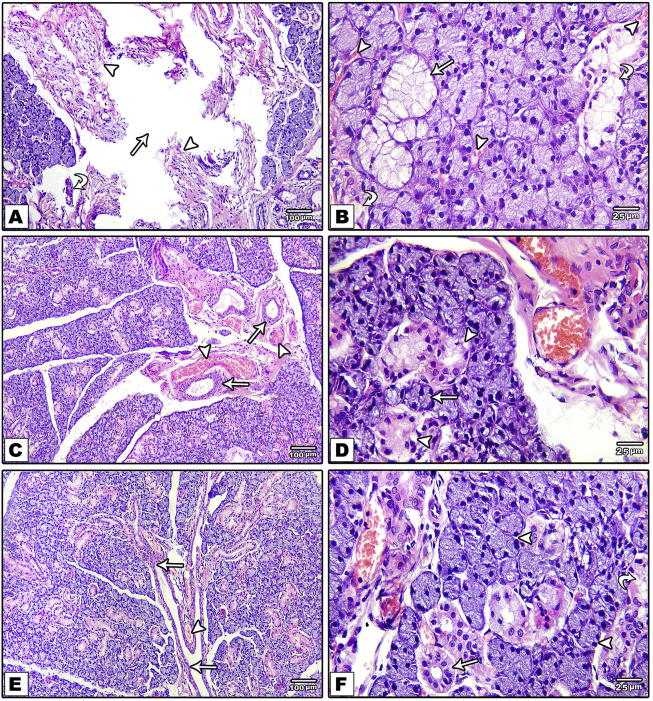
H&E stained sections of control diabetic group showed altered structure and impaired healing capacity of diabetic SMGs tissue. This was reflected by relatively large interacinar space representing the defect site, occupied with relatively scanty fibrous granulation tissue with small blood vessels and small duct and acinar like structures (Fig. 1; A&B). Parenchymal tissues showed mucous changes, vacuolation and distorted ductal structure (Fig. 1, B). These changes were reduced by both i-PRF (Fig. 1, C) and melatonin (Fig. 1, E) local application. Reduction of defect size, formation of numerous blood vessels and almost normal acinar and ductal configuration of pyramidal cells bordering a narrow lumen and basely located nuclei were observed in i-PRF (Fig. 1, D) and Melatonin (Fig. 1, F) treated groups.
Fig. 1.
Photomicrograph of H&E stained sections of control group shows the defect site (arrow), small blood vessels (arrowhead) and small duct and acinar like structures (curved arrow) (A). Mucous changes in acini (arrow), vacuolation of and loss of normal duct structure (curved arrow), and extravasated blood around acini and ducts (arrowhead) (B). i-PRF group shows engorged blood vessels (arrowhead), newly formed ducts (arrows) (C) and reduction of vacuolation both in acinar (arrow) and ductal (arrow head) structures (D). Melatonin group shows duct system (excretory duct connected to interlobular ducts) (arrow) and closely backed lobules, blood vessels (arrow head) (E&F). (H&E ×100: A,C,E; ×400:B,D,F).
3.3. IHC of cleaved caspase-3 (cas-3) and vascular endothelial growth factor (VEGF)
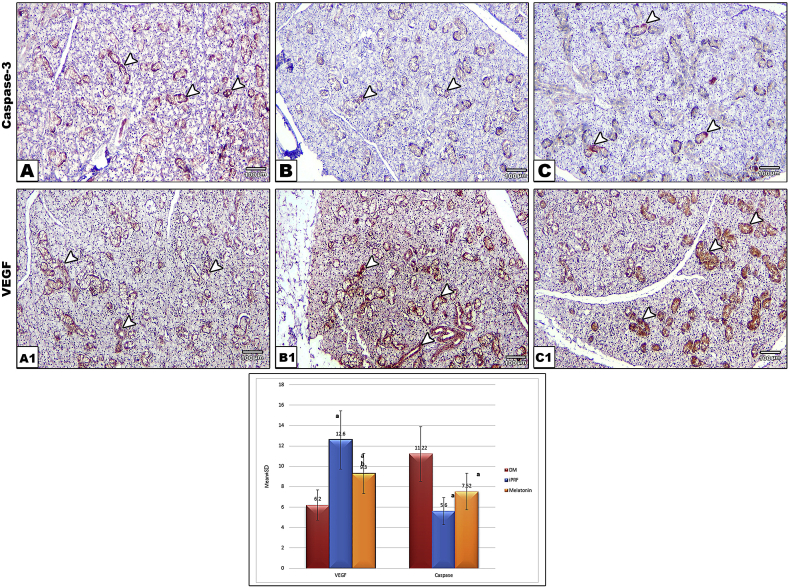
Specific binding of antibodies looked like fine-brown granular staining of cell cytoplasm. There was no significant difference between i-PRF and melatonin in Cas-3 expression (P = 0.1), while there was a significant reduction of Cas-3 in both groups compared to control group (P < 0.001). I-PRF group showed marked increase in VEGF expression (P = 0.001,0.006) compared to both control and melatonin respectively. Where melatonin (P = 0.009) showed significantly higher VEGF expression when compared to control group (Fig. 2).
Fig. 2.
Photomicrograph of cas-3 stained sections showing variable intensity of ductal reaction being intense in (A) in control group and moderate to mild reaction in iPRF (B) and melatonin (C) groups. While VEGF showed highest intensity in (B1) in i-PRF group and moderate to mild reaction in melatonin (C1) and control (B1) groups, respectively. (IHC ×100) The chart bar shows levels of expression of VEGF and Cas-3 expression in all groups analyzed by One-way ANOVA. Where a: represents significant difference compared to (DM) control diabetic group and b: significant difference between i-PRF & Melatonin.
4. Discussion
Hyperglycemia induced by STZ leads to changes in the morphology, secretory function and acyl fatty acid quantity in rat salivary glands.15 In this study the used dose for diabetes induction was a well-documented dose of 50 mg/kg of STZ to make sure of diabetes induction with minimal mortality rate.13
In our study MDA levels in salivary homogenate was significantly higher in diabetic group, this finding was in accordance with Erel et al., 16who reported that DM causes lipid peroxidation as an early sign of oxidative damage through oxidative stress and production of free radicals. Also, Knaś et al.13 stated that hyperglycemia leads to increased endothelial permeability, which causes the passage of oxidation products from vessels to salivary glands.
In the present study groups II and III showed significant decrease in MDA levels, these results were in agreement with Sudnikovich et al. who proved that melatonin prevents the increase in nitric oxide (NO) levels in blood plasma during STZ-induced diabetes, and acts as a NO scavenger and carrier.17
Parenchymal histopathological changes reported in H&E sections of control group were similar to those presented by Buyuk et al. who found that diabetes cause seromucous acinar changes and altered ductal structures.18 I-PRF treated group II, showed reduction in histopathological changes compared to the control group. Along with reduction of defect size, i-PRF treated group revealed a higher regenerative capacity of SMG with the formation of numerous blood vessels and ducts.
These results were like that of Dohan et al. who reported that PRF consists of growth factors which play crucial role during the healing stages, also fibronectin, which acts as cell proliferation and migration guide, potentiates the stimulative effects of PDGF-BB. It also acts as an autogenous “antibiotic”, as it contains plenty of leukocytes, thereby reducing the risk of infection.3
Melatonin treated group III revealed almost normal acinar and ductal structures with enhanced regeneration of SMG tissues. Concomitantly, a study conducted by Ashour on SMG, melatonin was found to raise cellular activity and decrease the process of degeneration in the secretory acini in aged rats.19
According to Ozler et al. melatonin when administered topically or systemically, it stimulates fibroblasts to produce collagen fibers and stimulates growth factors that organizes the wound healing process and cell proliferation in dose- and time-dependent manner20
In immunohistochemical results, control group showed moderate reaction to caspase 3 which was consistent with Fukuoka et al. results, where STZ induced diabetes increased expression rate of caspase 3 in ducts of SMG, with increased glucose oxidation and mitochondrial generation of ROS, that leads to strand breaks in nuclear DNA and accelerated apoptosis.21
In this study the expression of caspase 3 was decreased in both i-PRF and melatonin treated groups which was in accordance with kargarpour et al. who found that PRF reduced the expression levels of pro-apoptotic Bax and caspase-3 along with the antiapoptotic marker gene B cell lymphoma-2 (BCL2L1). Additionally, Kunduzova et al. reported that melatonin prevents renal post-reperfusion apoptotic cell death due to blockage of caspase-3 activity 22 23
Our study showed increased expression of VEGF in control group these findings were along with Perrotti et al. who proved that there was increase in VEGF expression in ducts of salivary gland of diabetic rats. Where diabetes resembles, a state of chronic hypoxia most enzymes, such as NOS and VEGF, are stimulated to act as a homeostatic defense mechanism.24,25
In study groups strongest expression of VEGF was in the i-PRF group. It is proposed that i-PRF promotes healing process and neo-revascularization by releasing pro-wound healing growth factors including PDGF, VEGF, TGF-β and production of collagen.5VEGF showed moderate expression in melatonin treated group, as melatonin was found to increase VEGF and to have a significant pro-angiogenic activity in tissues.26
5. Conclusion
The early wound healing changes was not reported in this study, only after 28th were the possible regenerative changes assessed. Within the limitation of the present results, melatonin and I-prf are proposed to put the diabetic gland in a postnatal mode helping the regeneration via decreasing the cleaved caspase-3 levels and paving the road for neovascularization that was observed with increased levels of VEGF in both groups, with some privilege to melatonin as regard histological results and the antioxidant effect of melatonin against lipid peroxidation caused by diabetes. Further studies should be conducted to investigate the superiority of melatonin in histomorphological improvement on ultrastructure levels and the possible contributions and differences of both i-PRF and Melatonin in early wound healing stages. Regenerative potential of both i-PRF and Melatonin was not only reported in soft tissue but also in bone regeneration both in human and experimental levels. A combination of both i-PRF and melatonin for diabetic wound healing over different time points and preservation of alveolar bone after teeth extraction would be of interest for forthcoming interests both in clinical and experimental levels.
References
- 1.Mascarenhas P., Fatela B., Barahona I. Effect of diabetes mellitus type 2 on salivary glucose--a systematic review and meta-analysis of observational studies. PloS One. 2014;9 doi: 10.1371/journal.pone.0101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosicka-Maciąg E. [Biological consequences of oxidative stress induced by pesticides] Postępy Higieny Medycyny Doświadczalnej. 2011;65:357–366. doi: 10.5604/17322693.948816. [DOI] [PubMed] [Google Scholar]
- 3.Dohan D.M., Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Miron R.J., Fujioka-Kobayashi M., Hernandez M. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Invest. 2017;21:2619–2627. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 5.Varela H.A., Souza J.C.M., Nascimento R.M. Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin Oral Invest. 2019;23:1309–1318. doi: 10.1007/s00784-018-2555-2. [DOI] [PubMed] [Google Scholar]
- 6.Reiter R.J., Rosales-Corral S.A., Liu X.Y., Acuna-Castroviejo D., Escames G., Tan D.X. Melatonin in the oral cavity: physiological and pathological implications. J Periodontal Res. 2015;50:9–17. doi: 10.1111/jre.12176. [DOI] [PubMed] [Google Scholar]
- 7.Acuña-Castroviejo D., Escames G., Venegas C. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci : CM. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suofu Y., Li W., Jean-Alphonse F.G. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E7997–e8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel Moneim A.E., Guerra-Librero A., Florido J. Oral mucositis: melatonin gel an effective new treatment. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellavía S.L., Sanz E.G., Gallará R.V., Carpentieri A., Vermouth N.T. Effect of sympathetic denervation of the pineal gland on maternal co-ordination of the circadian rhythm of alpha-amylase in parotid gland from young rats. Arch Oral Biol. 1993;38:1121–1125. doi: 10.1016/0003-9969(93)90175-l. [DOI] [PubMed] [Google Scholar]
- 11.Abd El-Latif N., Abdulrahman M., Helal M., Grawish M.E. Regenerative capacity of allogenic gingival margin- derived stem cells with fibrin glue on albino rats' partially dissected submandibular salivary glands. Arch Oral Biol. 2017;82:302–309. doi: 10.1016/j.archoralbio.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Yousuf D.A., Afify O.M., El Soudany K.S., Ghoniem S.M. The effect of local application of melatonin gel on the healing of periodontal osseous defects in experimentally induced diabetes in rabbits. Tanta Dental Journal. 2013;10:48–57. [Google Scholar]
- 13.Knaś M., Maciejczyk M., Daniszewska I. Oxidative damage to the salivary glands of rats with streptozotocin-induced diabetes-temporal study: oxidative stress and diabetic salivary glands. Journal of diabetes research. 2016;2016 doi: 10.1155/2016/4583742. 4583742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 15.Mahay S., Adeghate E., Lindley M.Z., Rolph C.E., Singh J. Streptozotocin-induced type 1 diabetes mellitus alters the morphology, secretory function and acyl lipid contents in the isolated rat parotid salivary gland. Mol Cell Biochem. 2004;261:175–181. doi: 10.1023/b:mcbi.0000028753.33225.68. [DOI] [PubMed] [Google Scholar]
- 16.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Sudnikovich E.J., Maksimchik Y.Z., Zabrodskaya S.V. Melatonin attenuates metabolic disorders due to streptozotocin-induced diabetes in rats. Eur J Pharmacol. 2007;569:180–187. doi: 10.1016/j.ejphar.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Buyuk B., Parlak S.N., Keles O.N. Effects of diabetes on post-menopausal rat submandibular glands: a histopathological and stereological examination. The Eurasian journal of medicine. 2015;47:199–207. doi: 10.5152/eurasianjmed.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashour M.A. Long-term effect of melatonin on submandibular salivary glands in old rats. 1998;4:324–331. [Google Scholar]
- 20.Ozler M., Simsek K., Ozkan C. Comparison of the effect of topical and systemic melatonin administration on delayed wound healing in rats that underwent pinealectomy. Scand J Clin Lab Investig. 2010;70:447–452. doi: 10.3109/00365513.2010.506926. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka C.Y., Simões A., Uchiyama T. The effects of low-power laser irradiation on inflammation and apoptosis in submandibular glands of diabetes-induced rats. PloS One. 2017;12 doi: 10.1371/journal.pone.0169443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kargarpour Z., Nasirzade J., Strauss F.J. Platelet-rich fibrin suppresses in vitro osteoclastogenesis. J Periodontol. 2020;91:413–421. doi: 10.1002/JPER.19-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunduzova O.R., Escourrou G., Seguelas M.H. Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. Faseb J. 2003;17:872–874. doi: 10.1096/fj.02-0504fje. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PubMed] [Google Scholar]
- 24.Perrotti V., Piattelli A., Piccirilli M., Bianchi G., Di Giulio C., Artese L. Vascular endothelial growth factor expression (VEGF) in salivary glands of diabetic rats. Int J Immunopathol Pharmacol. 2007;20:55–60. doi: 10.1177/039463200702001s12. [DOI] [PubMed] [Google Scholar]
- 25.Felaco M., Grilli A., Gorbunov N. Endothelial NOS expression and ischemia-reperfusion in isolated working rat heart from hypoxic and hyperoxic conditions. Biochim Biophys Acta. 2000;1524:203–211. doi: 10.1016/s0304-4165(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 26.Ramírez-Fernández M.P., Calvo-Guirado J.L., de-Val J.E. Melatonin promotes angiogenesis during repair of bone defects: a radiological and histomorphometric study in rabbit tibiae. Clin Oral Invest. 2013;17:147–158. doi: 10.1007/s00784-012-0684-6. [DOI] [PubMed] [Google Scholar]