Abstract
Skin drug delivery is an emerging route in drug development, leading to an urgent need to understand the behaviour of active pharmaceutical ingredients within the skin. Given, As one of the body's first natural defences, the barrier properties of skin provide an obstacle to the successful outcome of any skin drug therapy. To elucidate the mechanisms underlying this barrier, reductionist strategies have designed several models with different levels of complexity, using non‐biological and biological components. Besides the detail of information and resemblance to human skin in vivo, offered by each in vitro model, the technical and economic efforts involved must also be considered when selecting the most suitable model. This review provides an outline of the commonly used skin models, including healthy and diseased conditions, in‐house developed and commercialized models, their advantages and limitations, and an overview of the new trends in skin‐engineered models.
Keywords: animals, bioengineering, biomimetic materials, drug development, skin artificial, humans, skin diseases
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- DPPC
dipalmitoylphosphatidylcholine
- ECM
extracellular matrix
- EPC
egg phosphatidylcholine
- FFAs
free fatty acids
- NHK
normal human keratinocytes
- PAMPA
parallel artificial membrane permeability assay
- PDMS
poly(dimethylsiloxane)
- PVPA
phospholipid vesicle‐based permeation assay
- RHE
reconstructed human epidermis
- SC
stratum corneum
- SCID
severe combined immunodeficient
1. INTRODUCTION
In the last decade, the study and development of mimetic skin models have been actively discussed, due mainly to the ethical questions raised and the establishment of new rules consequent to the prohibition of animal tests. The need of new and efficient skin mimetic models remains crucial particularly for pharmaceutical, cosmetic, and toxicological purposes. The efficacy and toxicity of new products and formulations must be assessed before human application according to the guidelines of the European Union (EU) and of the Organisation for Economic Cooperation and Development (OECD, 2004, 2015). Efficient methods for development and rationalization of drug formulations for cutaneous application demand specific skin models capable of estimating bioavailability and therapeutic properties of drug formulations.
In line with these facts, several mimetic skin models have been developed, while research continues to further improve their quality, complexity, and mimetic properties. Different biomimetic materials have been used from simple non‐lipid models to more complex cell cultures as well as different technologies to simulate the highly stratified structure of the human skin. Challenges in the development of skin substitutes involve the reproducibility within other models, capacity to better mimic the complexity of the human skin, and cost‐effective parameters (Flaten et al., 2015; Sarkiri, Fox, Fratila‐Apachitei, & Zadpoor, 2019). The focus of the present review is to summarize the mimetic models of healthy and diseased skin that have been reported, to discuss their characteristics and applications, and to identify new trends in skin engineering.
2. SKIN PENETRATION ROUTES
The skin is an attractive site for delivery of drugs and cosmetics, such as anti‐diabetic or anti‐inflammatory drugs, contraceptives, or agents for the treatment of neurodegenerative diseases. But normal skin is a serious barrier to drug absorption, which is why pharmacologists and cosmetologists have become interested in the development of new drugs, formulations, and ways of delivery.
Drugs can be administered through the skin providing a local action (dermal or topical administration) or a systemic effect, when reaches the bloodstream or lymphatic system through transdermal drug delivery (Godin & Touitou, 2012). The designations dermal and transdermal drug delivery are commonly used in skin research to distinguish these different types of drug distribution. Drug delivery through the skin can be organized depending on selected criteria. One possible classification is on the basis of the invasive or non‐invasive nature of the drug formulation (Figure 1a). Invasive routes of administration can be defined as the routes in which the drugs can enter through the skin by needle injections (subcutaneous, intramuscular, or intravenous routes) and those that consist in the implantation of a device. Considering the non‐invasive methods, there are four possible routes of drug penetration across the skin: intercellular, intrafollicular, transcellular, and polar (Pouillot, Dayan, Polla, Polla, & Polla, 2008), as depicted in Figure 1b. Intercellular and transcellular ways are considered transepidermal pathways. Sometimes, the diffusion through the skin appendages (e.g., hair follicles, sebaceous glands, and sweat glands) is classified as appendageal route (Ng & Lau, 2015).
FIGURE 1.
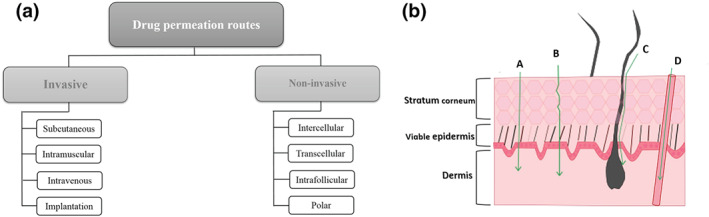
Skin drug delivery. (a) Types of drug entrance routes through the skin. (b) Pathways into the skin for transdermal drug delivery: (A) transcellular pathway (penetration through the corneocytes); (B) intercellular pathway (penetration between the corneocytes through the intercellular lipids); (C) intrafollicular pathway (penetration through the hair follicles); and (D) polar pathway (penetration through the polar pores). Adapted from Lima and Reis (2018)
3. HUMAN SKIN—STRUCTURE AND FUNCTIONS
The skin is a major organ of the human body having a surface area of ~2 m2, representing approximately 10% of the body mass for adults (Lee, Jeong, & Ahn, 2006; Ng & Lau, 2015). This physical barrier between the body and the external environment constitutes the first line of defence for the body, controlling what may enter and leave the body. Moreover, the skin is composed of a network of cells and matrix elements providing multifaceted functions such as the prevention of dehydration, protection of the body against infectious agents or UV radiation, thermoregulation, sensation and synthesis of vitamin D (Lima & Reis, 2018; Prausnitz et al., 2012).
The organization of the skin consists of three major layers: hypodermis, dermis, and epidermis. The type of structure, cellular composition, and major components of these skin layers are summarized in Figure 2, and these topics are discussed in more detail below, as a knowledge of skin structure is critical in determining the components to be included in the construction of a reliable mimetic model of skin.
FIGURE 2.
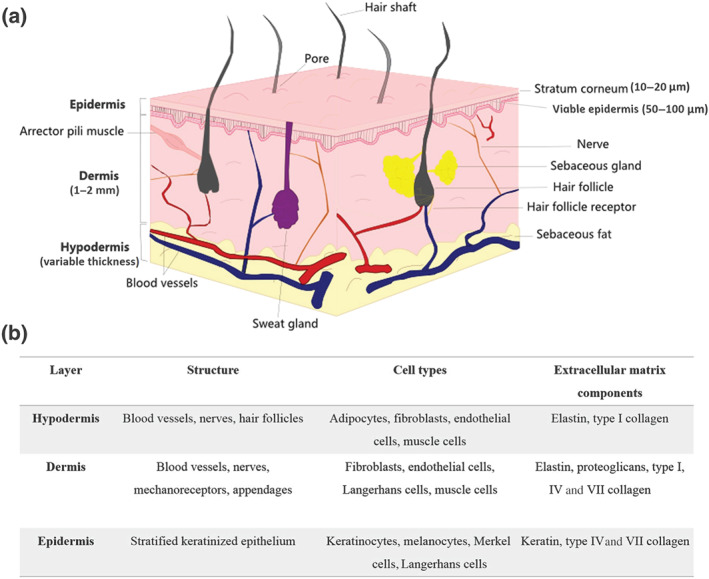
Structure of the human skin. (a) Schematic representation. (b) Characteristics of the three skin layers
3.1. Hypodermis
Hypodermis, also called the subcutaneous layer, is the innermost layer of the skin and consists of fat cells, located directly below the dermis and between the skin and the subjacent tissues of the body, such as muscles and bones. Larger lymphatic and blood vessels are found in this layer. However, this layer can be absent in some thin skin, for example, on the eyelid. The major functions of the hypodermis are insulation, mechanical integrity and support, and conductance of the vascular and neural signals of the skin (Alkilani, McCrudden, & Donnelly, 2015; Ng et al., 2015). Although it is usually considered a skin layer, another suggestion is that the hypodermis is not strictly a part of the skin, as it is connective tissue (Betts et al., 2013). Despite this discussion, and given the relevance of hypodermis for drug absorption in skin, some mimetic models also include adipocytes (Abaci, Guo, Doucet, Jacków, & Christiano, 2017), as detailed in Section 4.2.3.
3.2. Dermis
The dermis is the bigger layer of the skin and provides important physical properties, such as flexibility, elasticity, and tensile strength. It is an integrated system of fibrous and connective tissues composed of collagenous and elastin fibres, accommodating epidermally derived appendages (hair follicles, nails, sebaceous glands, and sweat glands) and sensory nerve endings, lymphatic vessels, and blood capillaries. The dermis contains resident cells, such as fibroblasts and mast cells, and cells from the immune system, such as macrophages and dermal dendritic cells. The thermal barrier, energy storage, and protection from physical injury are mainly connected with adipose tissue associated with collagen fibres found in the lower reticular dermis layer. The water content in this layer reaches 70%, favouring uptake of hydrophilic drugs. Below the reticular dermis layer, the fibrous connective tissue changes to the adipose tissue of the hypodermis (Lima & Reis, 2018). Complex skin mimetic models include some of dermis components (Sections 4.2.3 and 6).
3.3. Epidermis
Epidermis constitutes the outermost barrier between the organism and the external environment and is itself organized in four layers: the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum (SC) (Figure 2a). An additional layer, the stratum lucidum, can be found on the palm and the sole of the foot and is responsible for the thickened skin. Nevertheless, the stratum lucidum is often considered the lower part of the SC and not an individual epidermal layer. There are also hair follicles and sweat ducts that cross different skin layers (Ng et al., 2015).
The stratum basale is composed of keratinocytes, melanocytes, Merkel cells, and Langerhans cells, which participate in the immune response. The stratum spinosum is composed of keratinocytes, and the third layer, stratum granulosum, consists of several rows of keratinocytes and is a reservoir of ceramides, which are relevant lipids for the constitution of the outermost layer, the SC (Lee et al., 2006). Together, the three most intrinsic layers of the epidermis form what is called the “viable epidermis.” Keratinocytes, originating from the stratum basale, undergo progressive differentiation while migrating to the SC, a process that is necessary to maintain losses during desquamation (Ng et al., 2015). The viable epidermis is considerably hydrophilic (>50%) in contrast to the SC, which is lipophilic and contains ~10% of water (Forster, Bolzinger, Fessi, & Briancon, 2009). Due to the importance of the SC for the barrier properties of skin and for the permeation of drugs, this layer will be discussed further, in more detail.
3.3.1. Stratum corneum
The SC is composed of 10–25 layers of dead, mature, elongated, and anucleate keratinocytes, denominated as corneocytes (Ng & Lau, 2015), embedded in a lipid matrix (Walters & Roberts, 2002). The organization of the layer is usually compared with the “bricks and mortar” model where the bricks are the corneocytes and the lipids are compared with the mortar in a brick wall (Prausnitz et al., 2012; Uchida & Park, 2016). The interior of the corneocytes is filled with keratin filaments embedded in a matrix mainly consisted of filaggrin and its breakdown products.
The SC lipids are ceramides, cholesterol, cholesterol sulphate, and free fatty acids (FFAs) (Ng & Lau, 2015; Uchida & Park, 2016), which form lamellar membranes that stretch out in a horizontal direction parallel to the corneocytes. Any changes in the proportions of the lipid components can damage barrier integrity.
Concerning the functions of SC, this layer is responsible for the barrier and immune functions of skin and also provides protection of the epidermis from oxidative stress and from mechanical stress. As SC is the outermost layer and thus the first contact point between external compounds and the skin, it is one of the most relevant structures considered for the design of skin mimetic models. Some of the simplest models consist of mimicking just this layer alone (see Section 4.2.2).
4. HEALTHY SKIN MIMETIC MODELS
Despite the fact that human skin in vivo remains the gold standard skin model, its use is not always possible due to ethical concerns, regulatory issues, laboratory facilities, and the potential risk associated with the eventual toxic effects of drugs (Van Gele, Geusens, Brochez, Speeckaert, & Lambert, 2011). Moreover, results obtained by the use of human ex vivo models present significant variability because samples are usually obtained from different anatomical sites in the same donor and different donors and have unpredictable features, depending on the different subjects or different age groups (Flaten et al., 2015). These facts reinforce the need for reproducible alternative models able to better mimic normal, healthy, human skin.
4.1. Ex vivo human and animal models
The main way for preclinical research of new drugs and optimization of drug formulations in skin research relies in the application of ex vivo mimetic models. The literature describes two main groups of ex vivo models obtained from human or animal organisms (Abd et al., 2016; Flaten et al., 2015). The human skin samples used in ex vivo permeation assays can be obtained from plastic surgery, amputations or cadavers and, in general, the skin samples can be collected from different sites (Schaefer, Hansen, Schneider, Contreras, & Lehr, 2008). Full‐thickness skin models obtained from excisions containing connective tissue, subcutaneous fat and all skin layers have been reported (Gaur, Mishra, & Purohit, 2013; Marimuthu, Bennet, & Kim, 2012; Sahle, Wohlrab, & Neubert, 2014).
Ex vivo epidermal membrane models also used for permeation experiments were obtained from thermal treatment (Junyaprasert, Singhsa, Suksiriworapong, & Chantasart, 2012; Kligman & Christophers, 1963) or by chemical action specifically to separate the dermal–epidermal junction (Cross, Magnusson, Winckle, Anissimov, & Roberts, 2003). Other methodologies using human dermatomed skin (Clares et al., 2014; Marepally et al., 2013) or tape stripping method were used to remove SC layers (Abd et al., 2016).
More recently, samples of abdominal skin, obtained from abdominoplasty procedures, have been used as skin models (Ternullo et al., 2018). Many studies have used human ex vivo skin models (Abd et al., 2016; Flaten et al., 2015), the study of the skin interaction with organophosphate esters (Frederiksen et al., 2018) as also the effect of nanoemulsions in skin wounds (Bonferoni et al., 2018).
The use of skin perfusion models, a surgically prepared portion of the skin including subcutaneous fat tissue with assured continuous vascular circulation, to test different drugs such as nanoparticle formulations, was reported (Ternullo, de Weerd, Flaten, Holsæter, & Škalko‐Basnet, 2017; Ternullo, de Weerd, Holsæter, Flaten, & Škalko‐Basnet, 2017). This approach is considered a promising strategy over in vitro models, as overcomes the existence of only epidermis, part of the dermis, and the lack of a vascular system.
Regarding animal ex vivo models, pig skin models are the most relevant because of the biological similarities with the human skin such as the thickness of the layer, similarity in hair follicle and blood vessel density, content of SC ceramides, dermal collagen, and elastin (Abd et al., 2016). Pig skin is easily obtained as a waste product from abbatoirs. Among the different types of pig skin, the most recommended is that from the central outside part of the ear, because its structure is closest to the human skin layers (Meyer, Schonnagel, & Fleischer, 2006). Also, permeability in different samples of pig skin varies and permeability in pig ear skin is comparable with the human skin, especially for lipophilic substances. Furthermore, the age of the animal will affect the permeability to drugs, although most reports do not specify the age of animal.
Many different drugs and formulations have been studied using ex vivo pig ear skin models, including the permeation of liposomes containing different drugs (Knudsen et al., 2012; Scognamiglio et al., 2013) and the permeation of nanoformulations (Das, Sen, Maji, Nayak, & Sen, 2017; Şenyiğit et al., 2010). Samples from other pig skin sites, specifically from abdomen (Nagelreiter, Raffeiner, Geyerhofer, Klang, & Valenta, 2013) and dorsum (Hathout et al., 2010), have also been used, but by fewer groups. Skin samples from newborn pigs have been used for evaluation of topical drug formulations (Cilurzo, Minghetti, & Sinico, 2007).
In addition to the pig skin models, several other animals are used such as primates, mice, rats, guinea pigs, rabbits, bovines (udder), and snakes (shed skin). However, these models require ethical permission. Since 2009, the use of animals for collection of toxicological data for cosmetic ingredients has been prohibited in the EU (76/768/EEC, February 2003) (Van Gele et al., 2011), mainly due to the fact that primate research is highly restricted and very expensive, and the skin of rodents (mice, rat, and guinea pigs) is sometimes considered for permeation studies, due to its high availability, small size, and quite low cost (Abd et al., 2016). Although, rat skin is most like human skin; many studies pointed out the fact that its skin is more permeable than human skin (Hughes & Edwards, 2010; van Ravenzwaay & Leibold, 2004). Additionally, shed snake skin (Kumpugdee‐Vollrath, Subongkot, & Ngawhirunpat, 2013; Wonglertnirant, Ngawhirunpat, & Kumpugdee‐Vollrath, 2012) and also udders from slaughtered cows have been used as an ex vivo animal model (Netzlaff et al., 2006). The different ex vivo animal models mainly differ in the thickness of SC, hair density, number of corneocyte layers, hydration, lipid profile, and morphology, which result in several advantages and limitations of each model (Figure 3).
FIGURE 3.
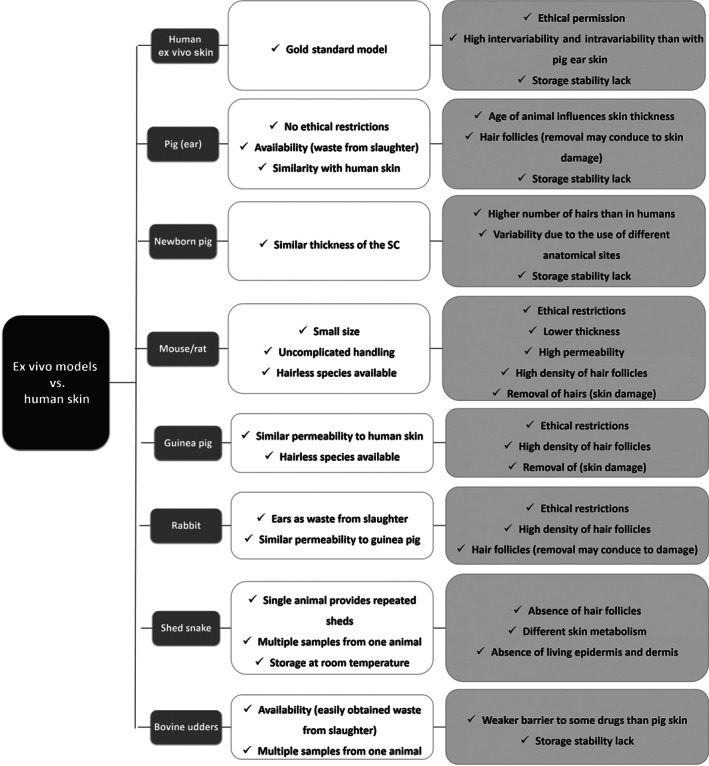
Summary of the main advantages (white boxes) and disadvantages (light grey boxes) of ex vivo skin models in relation to the human skin. SC, stratum corneum
4.2. In vitro membrane models
In order to overcome the disadvantages of ex vivo models, many other systems based on in vitro approaches have been developed. These in vitro models consist either of mimetic membranes (non‐lipid and lipid systems) or of cell cultures.
4.2.1. Non‐lipid‐based models
The first study, reported in 1970, regarding the use of non‐lipid‐based skin models, considered silicone membranes to study the release of salicylic acid (Nakano & Patel, 1970). Later, different microporous membranes, based on pure cellulose acetate, cellulose and polysulfone, were used to investigate the permeation of hydrocortisone from two commercial creams (Shah, Elkins, Lam, & Skelly, 1989). Other synthetic membranes made with polysulfone, cellulose mixed esters, polytetrafluoroethylene, and polypropylene were described and used to study nitroglycerin release from commercial ointments (Wu, Shiu, Simmons, Bronaugh, & Skelly, 1992). More recently, the interaction of many drugs and vehicles with skin has been modelled with membranes of poly(dimethylsiloxane) (PDMS) or silicone membranes (Miki et al., 2015; Oliveira, Hadgraft, & Lane, 2012; Watkinson, Guy, Oliveira, Hadgraft, & Lane, 2011).
The first parallel artificial membrane permeability assay (PAMPA) model for assessing skin penetration incorporated silicone oil and isopropyl myristate as membrane components (Ottaviani, Martel, & Carrupt, 2006). This model was used to study the permeation of a large variety of molecules (Dobričić et al., 2014; Karadzovska & Riviere, 2013). A novel substitute based on a semi‐permeable cellophane membrane and a membrane of n‐octanol in a nitrocellulose matrix was proposed by Loftsson, Konrádsdóttir, and Másson (2006) to study the permeation of different cyclodextrin formulations, and the results were similar to those found involving other biological membranes.
These skin equivalents are simple models, widely applicable, that test basic diffusion mechanisms. However, they present some disadvantages, specifically the lack of similarity with human skin and low applicability to study of the permeability of hydrophilic compounds, despite the good results obtained for lipophilic drugs (Abd et al., 2016; Miki et al., 2015).
4.2.2. Lipid‐based models
Parallel artificial membrane permeability assay
The lipid‐based skin equivalents appeared as valuable alternatives to non‐lipid‐based models, improving complexity of the model and its ability to mimic human skin, specifically, the SC layer. In 1989, a first report describing the preparation of model membranes, using SC lipids (ceramides, cholesterol, FFA, and cholesteryl sulphate), to study the permeability of drugs in the skin, was published (Abraham & Downing, 1989). The selected lipid composition was close to that found in the human SC layer. Only in 1998 more consistent studies started when Kansy and co‐workers reported the first PAMPA model based in lipids as a tool for rapid determination of passive membrane permeability of drugs (Kansy, Senner, & Gubernator, 1998). This system included a mimetic membrane from a hydrophobic filter coated with phosphatidylcholine dissolved in n‐dodecane as a membrane barrier. Even though this model was designed to assess the transcellular intestinal permeability, it was the precursor of the following models of skin.
Sinko et al. (2012) designed the skin PAMPA, which consisted of ceramide analogues called the synthetic ceramides or certramides. Certramides are cheaper alternatives having higher storage stability. This model was used in skin permeability studies and exhibited poor correlation with skin epidermis but it presented a good correlation with full‐thickness skin (Sinko et al., 2012).
More recently, a modified version of the skin PAMPA was reported (Tsinman & Sinkó, 2013) and used to evaluate the skin permeation of ibuprofen‐containing formulations. The system was able to distinguish between the different types of formulations, and the results correlated well with those found in permeation studies using human epidermis.
Several other groups reported the use of PAMPA models to investigate drug permeability of skin (Balázs et al., 2016; Köllmer et al., 2019; Wu et al., 2019; Zhang et al., 2019). Most reports of this type of mimetic models to study the interaction and permeation of many different bioactive ingredients highlights the large spectra of applicability of PAMPA approaches, in spite of the simple composition of these systems.
Phospholipid vesicle‐based permeation assay
The original phospholipid vesicle‐based permeation assay (PVPAo) was introduced as a model for screening intestinal permeability and is composed of liposomes deposited on a filter support, acting as biological barrier (Flaten, Bunjes, Luthman, & Brandl, 2006; Flaten, Dhanikula, Luthman, & Brandl, 2006). Later, by changing the lipid composition of the liposomes, a new PVPA model was developed aiming to mimic the SC (Engesland, Skar, Hansen, Skalko‐Basnet, & Flaten, 2013): Liposomes are located within the pores and on the surface of the membranes (Flaten, Bunjes, et al., 2006). Thereafter, other modified versions have been reported (Engesland et al., 2013; Engesland, Skalko‐Basnet, & Flaten, 2015; Engesland, Škalko‐Basnet, & Flaten, 2016; Ma et al., 2017; Palac et al., 2014).
Two main skin PVPA models, presenting different liposome composition, for estimating skin penetration have been described: PVPAc—egg phosphatidylcholine (EPC) and cholesterol, and PVPAs—EPC, ceramide, cholesterol, FFA, and cholesteryl sulphate (Engesland et al., 2013). Comparison of the permeability of different compounds evaluated in these PVPA models showed good agreement with permeabilities using animal models and with estimated in silico values, with few exceptions (Engesland et al., 2013).
Later, the PVPAs model was examined with different liposomes containing diclofenac. The results showed a rising permeation ranking of diclofenac from liposomal formulations correlating with the physicochemical parameters of the vehicles (Palac et al., 2014). The PVPA model was further optimized with a complex skin PVPA containing all the classes of lipids found in the SC, and the penetration‐enhancing effect of menthol was investigated for a set of active compounds (Ma et al., 2017).
PVPA skin models were studied in comparison with a reconstructed human skin model (EpiSkin®). The permeability results indicate that the PVPA model can distinguish between the liposomal formulations and drug solutions, as opposed to EpiSkin. Moreover, PVPA barriers were straightforward to prepare, effective, economical, and exhibited long storage properties (Engesland et al., 2015).
Other lipid‐based models have been reported as skin mimetic systems and were used for the study of the effect of a set of synthetic surfactants on the skin. These models contain dipalmitoylphosphatidylcholine (DPPC) and cholesterol or a mixture of ceramide, stearic acid, and cholesterol (Jurek, Góral, Mierzyńska, Moniuszko‐Szajwaj, & Wojciechowski, 2019). Recently, our research group developed a cheaper and simpler alternative that simulates SC (Shakel, Nunes, Costa Lima, & Reis, 2019) and can allow the screening of drug candidates. The design was based on PVPA approaches and had a lipid composition, which resembled more closely that of human SC, particularly in the prevalence percentage of ceramides. This barrier presents some advantages as it can be stored at −20°C and applied with several co‐solvents, without losing integrity.
Other lipid‐based models
A different type of skin mimetic model, Strat‐M®, was designed and commercialized to mimic the SC and is composed of a synthetic membrane comprising multiple layers of different materials, as porous polyether sulfone and polyolefin, enclosed by a combination of lipids (ceramides, cholesterol, and FFA) and other components, forming a three‐dimensional (3D) structure. In contrast to other 3D models (Section 4.2.3), this is not a cell‐based system. Strat‐M was used to evaluate the permeation efficiency of hydrophilic molecules (Haq, Goodyear, Ameen, Joshi, & Michniak‐Kohn, 2018). Even though this model lacks the capacity to mimic the complex architecture of full human skin, it represents a valuable alternative, as its composition closely resembles that of the SC layer and the results obtained using Strat‐M are highly reproducible due to the simple nature and standardized construction of this model (Yun, Jung, Choi, Choi, & Cho, 2018).
4.2.3. Cell‐based skin models
The first studies of the development of in vitro skin equivalents in mid‐1970s included the use of normal human keratinocytes (NHKs), as a model for skin irritancy (Rheinwald, 1989). The cell culture of NHKs begins with a small piece of the human skin obtained from surgery. It allows the use of a large number of cells, leading to the opportunity for testing many substances. This model shows good ability for testing hydrophilic compounds, although it is less suited to evaluate poorly water‐soluble compounds and complex formulations (Ponec, 1992). The model was improved with its application in membranes, which support the NHKs during growth, forming the so‐called reconstructed human epidermis. More complex models mimicking the full‐thickness skin consist of the fibroblast‐populated collagen matrices (dermis equivalent) and an epidermal cover composed of NHKs (Van Gele et al., 2011).
The complexity of the skin mimetic models has been increasing to obtain models comprising simple or more complex cell cultures. Single‐cell or multicell‐type two‐dimensional (2D) cultures, in which cells are grown as a monolayer on solid flat surfaces, such as polystyrene or glass, are some of the most often used, cell‐based approaches due to their relative simplicity and cost‐effectiveness (Randall, Jüngel, Rimann, & Wuertz‐Kozak, 2018).
The first studies regarding 2D skin cell mimetics described the growth of monolayers of human keratinocytes (obtained from newborn foreskin), deposited on culture plates (Rheinwald & Green, 1977). As a further advance, the authors included a primary fibroblast cell line in the keratinocyte cultures to closely resemble the skin. Furthermore, fibroblasts are important for the growth of keratinocyte cultures, specifically by the secretion of extracellular matrix (ECM) components, such as collagen (Rheinwald & Green, 1975). The 2D models have been successfully used (Amelian, Wasilewska, Megias, & Winnicka, 2017; Ponec, 1992). Of note, an epidermal monolayer using human keratinocytes (HaCaT cells) was differentiated in a high calcium concentration medium to assess drug delivery systems interactions (Silva, Barreiros, Segundo, Costa Lima, & Reis, 2017).
Despite the good performance of 2D models, 3D alternatives represent more precisely the reconstructed skin models, which constitute artificially fabricated skin mimetics. 3D (single‐cell or multicell type) reconstructed skin models are made by the deposition of different layers of human cells in culture on a polymeric matrix. More complex 3D systems usually include fibroblast cultures embedded in a matrix to mimic the dermis layer and keratinocytes seeded on top of the latter layer to simulate the epidermis. An air–liquid interface is established to allow the differentiation of keratinocytes and formation of the different epidermal sublayers, thus producing a more complex and interactive system. The artificial matrix may include collagen or fibrin fibres or even alginate and chitosan or different synthetic polymers (Sarkiri et al., 2019). The incorporation of melanocytes (Min et al., 2018), adipocytes (Klar, Zimoch, & Biedermann, 2017), and endothelial cells (Dai et al., 2018) in 3D models has already been reported. The main differences between 2D and 3D skin models are summarised in Figure 4.
FIGURE 4.
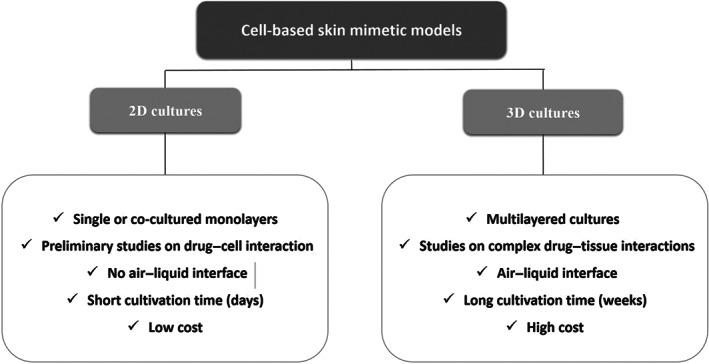
Main characteristics of two‐dimensional (2D) and three‐dimensional (3D) cell‐based skin mimetic models
3D skin equivalents have been described as a model composed of fibroblasts and keratinocytes grafted on a viscose rayon support, which was created to test potential skin irritants (Canton et al., 2010). More recently, a new in‐house reconstructed human epidermis (RHE) model for in vitro skin irritation assays has been developed presenting high similarity to human epidermis (Pedrosa et al., 2017). This system was further compared with a full‐thickness skin mimetic model to evaluate the performance in animal tests for skin irritants, and both models revealed capacity to correctly classify the tested compounds according to their corrosive nature (Catarino et al., 2018). Other models comprises sucrose co‐polymers and fibroblasts, leading to the formation of a macromolecular assembly, which potentiates collagen deposition (Benny, Badowski, Lane, & Raghunath, 2016). A 3D human skin model containing vitrified collagen that supported the culture of dendritic cells, keratinocytes, and fibroblasts (Uchino, Takezawa, & Ikarashi, 2009) was developed. Other three‐layered constructs featuring a hypodermis‐like layer have been reported as full‐thickness in vitro models of the human skin (Monfort, Soriano‐Navarro, García‐Verdugo, & Izeta, 2013; Trottier, Marceau‐Fortier, Germain, Vincent, & Fradette, 2008).
Skin‐engineered substitutes may be used not only as alternatives to ex vivo and in vitro non‐cell‐based models for testing pharmaceutical or cosmetic ingredients, in both healthy or pathological conditions, but they can also be applied in patients for regeneration of damaged skin, especially in the treatment of burn injuries and skin wounds (reviewed in Sarkiri et al., 2019; Yu et al., 2019).
According to the layers represented in each mimetic system, two main types of reconstructed skin models have been described; the RHE models that mimic the epidermis, and the full‐thickness reconstructed human skin equivalents that aim to simulate full human skin (Flaten et al., 2015; Randall et al., 2018). Some RHE skin models are produced in the laboratory, for research purposes, however, other models are already commercially available. Amongst others, the RHE systems, such as EpiSkin, SkinEthic®, and EpiDerm®, and full‐thickness reconstructed human skin models, so‐called living skin equivalents (e.g., GraftSkin®, EpiDermFT®, and Pheninon®) models, are available (Abd et al., 2016; Yun et al., 2018). Some of these models have been validated according to rules implementing the EU and OECD guidelines for testing dangerous ingredients for the skin (OECD, 2015). SkinEthic, EpiDerm, and EpiSkin are probably the most used models, and their use was approved by the EU Reference Laboratory for alternatives to animal testing (OECD, 2011). The SkinEthic and EpiDerm consist of epidermal keratinocytes cultured on polycarbonate membrane, whereas EpiSkin is composed of stratified human keratinocytes cultured on a collagen‐based matrix (Yun et al., 2018).
A comparison of the permeation of several compounds in human epidermal membranes, pig skin, and three RHE models (SkinEthic, EpiDerm, and EpiSkin) was reported by Schafer‐Korting and collaborators. Interestingly, the results showed that the RHE models, mostly SkinEthic, were significantly more permeable than human epidermis and pig skin. However, the permeation of the compounds through pig skin and the RHEs resembled that obtained in human epidermis. Interestingly, the expected improvement in reproducibility with the RHEs compared with the ex vivo skin was not observed (Schafer‐Korting et al., 2008).
These commercially available models have been used for several purposes, such as the evaluation of the permeability of drugs, as well as for irritation and toxicological studies (Alépée, Grandidier, Tornier, & Cotovio, 2015). Some studies have compared full‐thickness reconstructed human skin systems with animal and human skin models, and the results demonstrated their applicability as skin mimetic systems (Schafer‐Korting et al., 2008; Schreiber et al., 2005). Despite the fact that cell‐based models are closer to human skin, due to the presence of human cell in their composition, these models present some disadvantages, mainly due to the lack of skin appendages, their high cost of production, and the extremely short shelf‐life (Flaten et al., 2015). The main advantages and disadvantages of the in vitro models are summarised in Figure 5.
FIGURE 5.
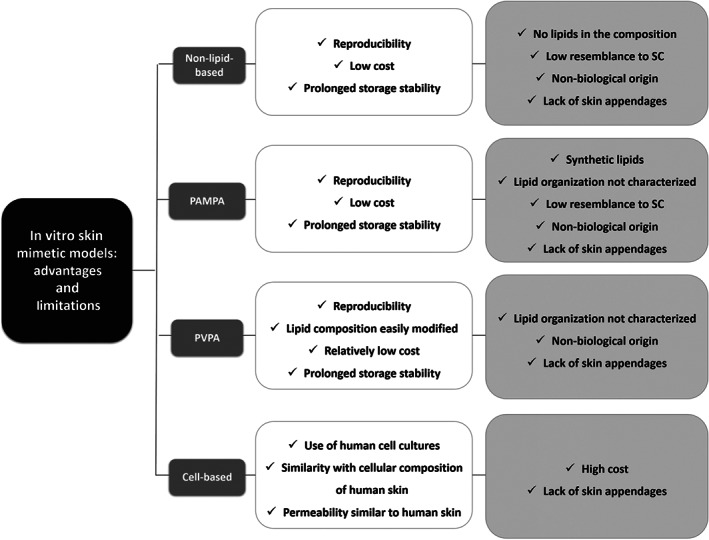
Summary of the main advantages (white boxes) and disadvantages (light grey boxes) of non‐lipid model membranes, parallel artificial membrane permeability assay (PAMPA), phospholipid vesicle‐based permeation assay (PVPA), and cell‐based skin equivalent models. SC, stratum corneum
5. MODELS OF SKIN DISEASE
The skin is an obvious site for the application of treatments for skin diseases. The assessment of drug effects on skin disease is clearly essential but the availability of excised human diseased skin is limited, mainly due to the increasing regulatory restrictions on the use of animals and humans.
Models representing healthy skin cannot reflect the altered morphological and physiological characteristics caused by disease. Accordingly, the development of skin disease models represents a serious challenge and the modification of the existent mimetic models for healthy skin can be considered a possible strategy towards a design embracing the required disease characteristics. Models have been created concerning alterations of skin pigmentation, photodamage (photodermatitis), inflammatory disorders (psoriasis and atopic dermatitis), cutaneous wounds, and skin cancer (melanoma) (Abd et al., 2016; Amelian et al., 2017; Randall et al., 2018; Yun et al., 2018).
Few examples of lipid‐based models for diseases are found in the literature. In particular, the lipid systems such asthe modified skin PVPA membranes in which different degrees of leakiness were considered to represent distinct degrees of compromised skin (Engesland et al., 2013). Later, the permeation of a set of drugs was investigated in the modified PVPA membranes (Engesland et al., 2016). More complex skin disease models have been reported comprising in vitro cell‐based and in vivo animal models, mainly based on modified mouse or guinea pig organisms (Randall et al., 2018; Sarkiri et al., 2019). in vivo and in vitro models developed for psoriasis, atopic dermatitis, dermatophytosis, and skin cancer will be assessed in the following sections.
5.1. Psoriatic models
A substantial number of genetically engineered mice were developed to be used as skin disease models, and in particular, some of them have been studied as in vivo models of psoriasis (Bocheńska, Smolińska, Moskot, Jakóbkiewicz‐Banecka, & Gabig‐Cimińska, 2017). For example, the development of the epidermal VEGF receptor‐knockout mice is considered a psoriasis model, and used to identify a specific role for epidermal VEGF in the maintenance of permeability‐barrier homeostasis (Elias et al., 2008). Knockout mice for proteins exhibiting skin with characteristics of psoriasis have been developed, since these factors are important for the differentiation of epithelial cells (Szabowski et al., 2000). Additionally, over‐expression of IL‐1α in the murine epidermis leads to a increased proinflammatory condition and, thus, these mice can be considered an interesting model (Groves, Mizutani, Kieffer, & Kupper, 1995). Mice with deletion of the IL‐1 receptor antagonist exhibited the development of an inflammatory response, similar to those observed in human psoriatic skin , and has been considered a useful psoriatic model (Shepherd, Little, & Nicklin, 2004).
Cell‐based in vitro systems have been described to simulate compromised skin, and in general, they are developed in‐house. Most models have been designed to mimic skin inflammatory diseases, such as like the approaches developed to simulate psoriasis, as extensively reviewed in Bocheńska et al. (2017) and Yun et al. (2018). An example is the work published by Chiricozzi et al. (2014) in which a full‐thickness skin model closely resembling in vivo epidermal architecture was used to identify cytokine‐responsive genes in psoriasis and the effect of cytokine antagonists. Another study described the design of a human psoriatic skin equivalent used to study cytokine‐induced gene expression and the effect of different drugs in the disease context (Tjabringa et al., 2008).
5.2. Atopic dermatitis models
Regarding atopic dermatitis, one of the most frequently used in vivo model is the NC/Nga mouse (Vestergaard et al., 1999). These animals spontaneously develop skin lesions when housed under conventional conditions, which closely resemble those found in humans. Another in vivo atopic dermatitis model is the flaky tail (ma/maFlgft/ft) mouse, which expresses mutations in the genes involved in the development of the atopic‐like skin lesions (Lane, 1972; Moniaga et al., 2010). In addition, knockout mice for histamine H4 receptors were developed to be used as a model for atopic dermatitis, and the results obtained in studies using this model identified the importance of this receptor as a potential therapeutic target for atopic dermatitis. However, some of these models exhibited profiles of atopic dermatitis distinct from those of the disease in humans (Löwa, Jevtić, Gorreja, & Hedtrich, 2018). Recently, a report described the use of oxazolone‐induced hairless mice for the study of new treatments for atopic dermatitis (Moner et al., 2018).
Additionally, in vitro atopic dermatitis mimetic models were described (Huet et al., 2018; Randall et al., 2018) such as the 3D RHE model used to investigate filaggrin expression in the epidermis of atopic patients (Pendaries et al., 2014). Another compromised reconstructed epidermis model has been designed to mimic atopic dermatitis (Rouaud‐Tinguely et al., 2015). A multicell‐type 3D model to mimic atopic dermatitis, which includes human foreskin fibroblasts, human keratinocytes, memory‐effector CD45 (RO+) T cells, collagen type I, and fibronectin, was also reported (Engelhart, El Hindi, Biesalski, & Pfitzner, 2005). To study the effect of the exposure to UV light to the formation of wrinkles and discoloration process, a full‐thickness skin model that mimics photodermatitis disease has been used (Kuchler et al., 2011).
5.3. Dermatophytosis models
Many experimental models were designed to study dermatophytosis and to evaluate the efficacy of potential antifungal treatments, as reviewed in Faway, Lambert de Rouvroit, and Poumay (2018). Few in vivo animal alternatives have been explored using different species (guinea pig;‐Baldo et al., 2010; Li, Guo, Dawuti, & Aibai, 2015 or mouse; Baltazar et al., 2015; Cambier et al., 2014). This approach presents some disadvantages, specifically regarding the different degree of severity of lesions and the extent of inflammatory responses, which can significantly differ according to the host and the pathogen. Despite these limitations, a skin disease model for dermatophytosis was reported by Cambier et al. (2014) using an experimental mouse model for the study of the different fungal infections in the skin. ex vivo mimetic models obtained from isolated explants from various species, including humans, have been considered (Peres et al., 2016). Although these alternatives are valuable approaches, they present some disadvantages specially limited availability and high variability between different donors.
For these reasons, in vitro models have been described as advantageous alternatives to study cells' responses in the presence of dermatophytes, such as systems comprising keratinocytes cultured in monolayers (Firat et al., 2014). In addition, RHE models of dermatophytosis have been reported: Two alternatives have been described and designed by modifying the commercially available systems EpiDerm (Achterman et al., 2015) and Episkin (Liang et al., 2016). Using arthroconidia and reconstructed feline epidermis, another in vitro model of dermatophytosis was developed to investigate the efficacy of a set of antifungal molecules (Tabart, Baldo, Vermout, Losson, & Mignon, 2008). Other studies considered the use of RHE models to the investigation of antifungal agents: the effect of terbinafine in a reconstructed tissue (Rashid, Edward, & Richardson, 1995) and another study regarding Candida albicans (Green et al., 2004). Thus, RHE has demonstrated the potential of this type of models for the control of dermatophytosis. More recently, an RHE model of dermatophytosis was developed and used to study infections caused by arthroconidia caused by different fungal species (Faway, Cambier, Mignon, Poumay, & Lambert de Rouvroit, 2016). This alternative allowed the simulation of a typical in vivo skin fungal infection and aims to improve the knowledge on the interaction between the dermatophytes and epidermis, as well as the effect of antifungal drugs.
Overall, the currently developed models of dermatophytosis present a useful approach to increase insight into the progression and treatment of several fungal infections in the skin. However, further improvements remain crucial, particularly in the development of more realistic models to better represent the conditions in humans during in vivo infection.
5.4. Skin cancer models
Skin models in mice have been used to mimic melanoma and other common skin cancers, such as squamous cell carcinoma and basal cell carcinoma. An investigation described a squamous cell carcinoma mouse model in which the skin of SKH1 hairless mice is exposed to UVB irradiation and used to study the potential activity of anti‐cancer drugs (Burns et al., 2013). This mouse model was further used to investigate the effect of different drugs in the treatment of squamous cell carcinoma (Singh et al., 2015; Wang et al., 2013). Other mouse models were developed to assess the effect of anti‐tumor signalling inhibitors for the pathways of basal cell carcinoma (Filocamo et al., 2016; Tang et al., 2011), while xenograft models were applied in the evaluation of the activity of potential anti‐melanoma drugs (Chen et al., 2012; Schroder, Komljenovic, Hecker, & Korff, 2016; Yu et al., 2016), and a hairless mouse model that spontaneously develops cutaneous malignant melanoma has been reported (Thang et al., 2012).
Chimeric models in which living human skin is transplanted onto the skin of severe combined immunodeficient (SCID) mice have been used to study of the effect of drugs in living human skin. This approach developed a human psoriatic model to study therapeutic options (Kundu‐Raychaudhuri, Chen, Wulff, & Raychaudhuri, 2014). Likewise, SCID mouse–human melanoma models were described for the analysis of different cancer targets and therapies (Salton et al., 2015; Yue et al., 2015).
Design of in vitro skin cancer mimetic models may be complex as it includes the incorporation of various tumour entities in a 3D skin system to resemble cell–cell and cell–ECM interactions (Marconi, Quadri, Saltari, & Pincelli, 2018). As representative examples, a 3D human skin reconstructed model, included cultured melanocytes (Li, Fukunaga‐Kalabis, & Herlyn, 2011), while melanoma cells (A375), normal human‐derived epidermal keratinocytes, normal human‐derived dermal fibroblasts, and collagen type I were assembled to simulate a metastatic melanoma (Mohapatra, Coppola, Riker, & Pledger, 2007). Later, a skin squamous carcinoma mimetic model was proposed with squamous carcinoma cell lines (SCC12B2 and SCC13 cell lines) and normal human‐derived epidermal keratinocytes, normal human‐derived dermal fibroblasts, and collagen type I (Commandeur, van Drongelen, de Gruijl, & El Ghalbzouri, 2012). Moreover, a human 3D melanoma model, which includes primary keratinocytes and fibroblasts embedded into a collagen I scaffold and different types of cancer cell lines such as SBCL2 (RGP) and WM‐115 (VGP) 451‐LU (MM) cells, was designed to mimic in vivo tumour environment and showed in vivo‐like responses (Vorsmann et al., 2013).
5.5. Commercially available skin disease models
In addition to in‐house developed systems, some disease models are commercially available for the screening of new drugs, such as MelanoDerm®, Melanoma®, Psoriasis®, and “Psoriasis‐Like” products (Amelian et al., 2017).
MelanoDerm (www.mattek.com/products/melanoderm/, accessed 09/08/2019) includes normal human‐derived epidermal keratinocytes and normal human melanocytes and has been used for the screening of agents to prevent UVB‐induced DNA damage (Li et al., 2011; Passeron, Namiki, Passeron, Le Pape, & Hearing, 2009). Melanoma (www.mattek.com/products/melanoma/, accessed 09/08/2019) represents a full‐thickness skin cancer model consisting of human malignant melanoma cells, normal human‐derived epidermal keratinocytes, and normal human‐derived dermal fibroblasts, and the use of this model has been described for the investigation of some potential active anti‐melanoma drugs (Li et al., 2011; Ma et al., 2008). The composition of the commercially available Psoriasis model (www.mattek.com/products/psoriasis/, accessed 09/08/2019) includes normal human‐derived epidermal keratinocytes and psoriatic dermal fibroblasts, expressing psoriasis‐specific markers and releasing psoriasis‐specific proinflammatory cytokines. This model allows the study of the biology of psoriasis and the screening of anti‐psoriasis drugs (www.mattek.com/application/anti-psoriasis-drug-screening/, accessed 09/08/2019). The “Psoriasis‐like” system consists of normal human‐derived epidermal keratinocytes cultured in a special medium to induce a diseased psoriatic phenotype, mainly via the destabilization of the epidermis (www.ateralabs.com, accessed 09/08/2019).
These commercially available models can represent a valuable alternative to in‐house developed systems and could provide more reproducible results. However, the cost and the low shelf storage time are two serious disadvantages of these approaches. Nevertheless, these alternatives may be useful in the understanding of the role of several skin diseases as well in the evaluation of new targets and potential treatments for some skin disorders.
Finally, it is clear that all these skin disease models have contributed importantly to the study of skin disorders and in related drug development. However, some limitations hamper the wide application of these alternatives, particularly their reproducibility and reliability in the representation of pathological conditions, the extensive time and high cost of production and large‐scale development. Particularly for the study of more complex skin disorders, such as cancer, standardized systems are essential to guarantee reliable results regarding the effects of a drug in the evolution of a disease. For these reasons, automated manual approaches, such as 3D bioprinting (discussed in Section 6), may represent promising strategies, in comparison with traditional fabrication methods. Significant progress has been achieved by different fabrication methods, but the currently available skin disease models still remain to be further improved.
6. NEW TRENDS IN SKIN MODEL ENGINEERING
Despite the great developments in lipid‐ or cell‐based models in vitro, the demand for new and more realistic human skin models remains. Thus, and following the most recent advances in 3D bioengineering technology, the production of bioprinted skin has been reported. Several tissue and organ models have been fabricated, and the skin is not an exception (Randall et al., 2018; Yun et al., 2018). Bioprinting is now considered a promising method to produce skin equivalents as it allows the preparation of multilayered and multicellular systems. These new approaches comprise a computer‐controlled deposition of skin cells and matrix polymers following spatially controlled patterns, thus controlling the architecture of the skin model with high reproducibility (Randall et al., 2018).
Complex human skin models could be biofabricated through different 3D printing techniques such as electrospinning, micro‐extrusion, inkjet printing, and laser‐assisted bioprinting (Randall et al., 2018; Yu et al., 2019; Yun et al., 2018). The selection of the most adequate printing technique is usually determined by the type of the biomaterials chosen for the mimetic model. The variety of available printing technologies has provided many options to fine‐tune the structure of the model according to the desired application.
In the electrospinning technique, different voltages are applied to the polymer solution to generate filaments that are then deposited on to a surface. In micro‐extrusion printing, the polymer solution passes through a needle, is deposited layer by layer on the platform and can be assembled into multiple layers by controlling the needle movements. Alternatively, inkjet printing methods allow the dropwise deposition of the bio‐ink, and a variety of droplets can be generated, using temperature or pressure variations. Laser‐assisted bioprinting uses a laser beam, which is pulsed on top of the donor layer containing the desired bio‐ink formulation and thus leading to the creation of bio‐ink droplets that are then deposited in the acceptor surface. The fine tuning of the laser position allows the construction of a model with the desired pattern (Yu et al., 2019).
In recent years, many studies have been reported such as a direct cell printing method which produced multilayered models containing fibroblasts, keratinocytes, and a collagen‐based hydrogel (Lee et al., 2014). In another study, a mixture of collagen/fibroblasts was used as the bio‐ink, and melanocytes and keratinocytes were deposited to obtain functional skin constructs (Min et al., 2018). A model in which keratinocytes and fibroblasts were embedded in a collagen/Matrigel® matrix has also been reported, and results have shown that cells were able to express connexins, pan‐cadherin, and laminin (Koch et al., 2012).
In addition to bioprinting technics, other complex next‐generation skin models involve microfluidic technology, to provide the so‐called skin‐on‐a‐chip devices (Sriram et al., 2018; Zhang et al., 2018). Skin‐on‐a‐chip systems comprise the growing of different cells in a microscale environment, using a microfluidic culture device in which a dynamic perfusion and controlled ventilation are possible, thus presenting advantages specifically in epidermal morphogenesis and differentiation. However, due to the high costs and technical requirements associated with microfluidic devices, their use is still limited and thus further investigations are needed to optimize these methods.
In summary, the production of readily accessible and reproducible constructs for use in research laboratories, with high durability and at a low price, is still to be achieved. Some studies have already reported the first steps in meeting these challenges (Abaci et al., 2017). However, it is expected that further studies in this field of research can solve the existent drawback of the available models.
7. CONCLUSIONS
As a result of the strict legislation regarding human and animal tests and, particularly, to meet the urgent need for efficient skin mimetic models, many different alternatives have been described. The numerous examples mentioned in the present review highlighted the differences between those models and their field of application. To a lesser extent, some skin disease models have been designed to mimic the characteristics of compromised skin.
Several improvements in the quality and complexity and mimetic properties of the skin mimetic models have been achieved, along with the use of different technologies of production. Nevertheless, some important challenges persist such as (a) reproducibility, (b) human skin mimicking capacity, (c) applicability of use of drugs exhibiting distinct physicochemical properties, (d) storage conditions and shelf time, and (e) cost‐effective ratio. The most recent advances have been made mainly using advanced bioengineering technologies to incorporate a range of components of skin components in the models, such as vascularization, innervation, pigments and other complex structures such as hairs, and some of those systems are already commercially available.
In the future, it is expected that the models can evolve towards the “perfect” skin mimetic model, overcoming the current drawbacks and allowing the efficient screening of drugs and cosmetics, in healthy or diseased conditions. However, the selection of the most useful and adequate model(s) will probably be always a crucial point in the design of a study, as the nature and complexity of the developed systems can be significantly different. In the selection process of the best skin mimetic model, several aspects may be considered, for example, the aims of the study, the properties of the drugs, the biological effect expected for the compounds, amongst other important factors, specifically those regarding the characteristics of the existent mimetic skin models such as their availability in the market, quality, complexity, stability, price, and mimetic properties. In some investigations, for a fully consistent study, perhaps the concomitant use of different skin mimetic models can gather more information than a single mimetic model. This literature review in human skin models, from healthy to disease models highlights the aspects to consider when selecting and using a mimetic model, in the context of drug development.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia, Ministério da Ciência, Tecnologia e Ensino Superior) through Grant UID/QUI/50006/2020 and from the European Union (European Regional Development Fund—FEDER funds through COMPETE POCI‐01‐0145‐FEDER‐030834) and national funds (FCT, Fundação para a Ciência e Tecnologia) through Project PTDC/QUI‐COL/30834/2017. T.M. thanks her funding from this project. S.A.C.L. thanks funding from FCT/MEC (CEECIND/01620/2017) financed by national funds. The authors are greatly indebted to all the financing sources. Administrative and technical support from Ms Manuela Barros is also acknowledged.
Moniz T, Costa Lima SA, Reis S. Human skin models: From healthy to disease‐mimetic systems; characteristics and applications. Br J Pharmacol. 2020;177:4314–4329. 10.1111/bph.15184
REFERENCES
- Abaci, H. , Guo, Z. , Doucet, Y. , Jacków, J. , & Christiano, A. (2017). Next generation human skin constructs as advanced tools for drug development. Experimental Biology and Medicine, 242(17), 1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd, E. , Yousef, S. A. , Pastore, M. N. , Telaprolu, K. , Mohammed, Y. H. , Namjoshi, S. , … Roberts, M. S. (2016). Skin models for the testing of transdermal drugs. Clinical Pharmacology, 8, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham, W. , & Downing, D. T. (1989). Preparation of model membranes for skin permeability studies using stratum corneum lipids. The Journal of Investigative Dermatology, 93(6), 809–813. [DOI] [PubMed] [Google Scholar]
- Achterman, R. R. , Moyes, D. L. , Thavaraj, S. , Smith, A. R. , Blair, K. M. , White, T. C. , & Naglik, J. R. (2015). Dermatophytes activate skin keratinocytes via mitogen‐activated protein kinase signaling and induce immune responses. Infection and Immunity, 83(4), 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alépée, N. , Grandidier, M.‐H. , Tornier, C. , & Cotovio, J. (2015). An integrated testing strategy for in vitro skin corrosion and irritation assessment using SkinEthic™ Reconstructed Human Epidermis. Toxicology in Vitro, 29(7), 1779–1792. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani, A. Z. , McCrudden, M. T. , & Donnelly, R. F. (2015). Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics, 7(4), 438–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelian, A. , Wasilewska, K. , Megias, D. , & Winnicka, K. (2017). Application of standard cell cultures and 3D in vitro tissue models as an effective tool in drug design and development. Pharmacol Reports, 69(5), 861–870. [DOI] [PubMed] [Google Scholar]
- Balázs, B. V. , Gábor, Berkó, S. , Budai‐Szűcs, M. , Kelemen, A. , Sinkó, B. , … Csányi, E. (2016). Investigation of the efficacy of transdermal penetration enhancers through the use of human skin and a skin mimic artificial membrane. Journal of Pharmaceutical Sciences, 105(3), 1134–1140. [DOI] [PubMed] [Google Scholar]
- Baldo, A. , Mathy, A. , Tabart, J. , Camponova, P. , Vermout, S. , Massart, L. , … Mignon, B. (2010). Secreted subtilisin Sub3 from Microsporum canis is required for adherence to but not for invasion of the epidermis. The British Journal of Dermatology, 162(5), 990–997. [DOI] [PubMed] [Google Scholar]
- Baltazar, L. M. , Werneck, S. M. , Carneiro, H. C. , Gouveia, L. F. , de Paula, T. P. , Byrro, R. M. , … Santos, D. A. (2015). Photodynamic therapy efficiently controls dermatophytosis caused by Trichophyton rubrum in a murine model. The British Journal of Dermatology, 172(3), 801–804. [DOI] [PubMed] [Google Scholar]
- Benny, P. , Badowski, C. , Lane, E. B. , & Raghunath, M. (2016). Improving 2D and 3D skin in vitro models using macromolecular crowding. JoVE, 114, e53642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts, J. G. , Young, K. A. , Poe, J. A. W. E. J. B. , Kruse, D. H. , Korol, O. , Johnson, J. E. , … DeSaix, P. (2013). The integumentary system In OpenStax . (Ed.), Anatomy and physiology. Houston, Texas: OpenStax. [Google Scholar]
- Bocheńska, K. , Smolińska, E. , Moskot, M. , Jakóbkiewicz‐Banecka, J. , & Gabig‐Cimińska, M. (2017). Models in the research process of psoriasis. International Journal of Molecular Sciences, 18(12), 2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonferoni, M. C. , Riva, F. , Invernizzi, A. , Dellera, E. , Sandri, G. , Rossi, S. , … Ferrari, F. (2018). Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. European Journal of Pharmaceutics and Biopharmaceutics, 123, 31–41. [DOI] [PubMed] [Google Scholar]
- Burns, E. M. , Tober, K. L. , Riggenbach, J. A. , Schick, J. S. , Lamping, K. N. , Kusewitt, D. F. , … Oberyszyn, T. M. (2013). Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh‐1 mice in a model of UVB‐induced cutaneous squamous cell carcinoma. Carcinogenesis, 34(2), 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier, L. , Weatherspoon, A. , Defaweux, V. , Bagut, E. T. , Heinen, M. P. , Antoine, N. , & Mignon, B. (2014). Assessment of the cutaneous immune response during Arthroderma benhamiae and A. vanbreuseghemii infection using an experimental mouse model. The British Journal of Dermatology, 170(3), 625–633. [DOI] [PubMed] [Google Scholar]
- Canton, I. , Cole, D. M. , Kemp, E. H. , Watson, P. F. , Chunthapong, J. , Ryan, A. J. , … Haycock, J. W. (2010). Development of a 3D human in vitro skin co‐culture model for detecting irritants in real‐time. Biotechnology and Bioengineering, 106(5), 794–803. [DOI] [PubMed] [Google Scholar]
- Catarino, C. M. , do Nascimento Pedrosa, T. , Pennacchi, P. C. , de Assis, S. R. , Gimenes, F. , Consolaro, M. E. L. , … Maria‐Engler, S. S. (2018). Skin corrosion test: A comparison between reconstructed human epidermis and full thickness skin models. European Journal of Pharmaceutics and Biopharmaceutics, 125, 51–57. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Wu, Q. , Zhang, Z. , Yuan, L. , Liu, X. , & Zhou, L. (2012). Preparation of curcumin‐loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules, 17(5), 5972–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi, A. , Nograles, K. E. , Johnson‐Huang, L. M. , Fuentes‐Duculan, J. , Cardinale, I. , Bonifacio, K. M. , … Krueger, J. G. (2014). IL‐17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE, 9(2), e90284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilurzo, F. , Minghetti, P. , & Sinico, C. (2007). Newborn pig skin as model membrane in in vitro drug permeation studies: A technical note. AAPS PharmSciTech, 8(4), 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clares, B. , Calpena, A. C. , Parra, A. , Abrego, G. , Alvarado, H. , Fangueiro, J. F. , & Souto, E. B. (2014). Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. International Journal of Pharmaceutics, 473(1‐2), 591–598. [DOI] [PubMed] [Google Scholar]
- Commandeur, S. , van Drongelen, V. , de Gruijl, F. R. , & El Ghalbzouri, A. (2012). Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma. Cancer Science, 103(12), 2120–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, S. E. , Magnusson, B. M. , Winckle, G. , Anissimov, Y. , & Roberts, M. S. (2003). Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. The Journal of Investigative Dermatology, 120(5), 759–764. [DOI] [PubMed] [Google Scholar]
- Dai, N. T. , Huang, W. S. , Chang, F. W. , Wei, L. G. , Huang, T. C. , Li, J. K. , … Wang, Y. (2018). Development of a novel pre‐vascularized three‐dimensional skin substitute using blood plasma gel. Cell Transplantation, 27(10), 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, B. , Sen, S. O. , Maji, R. , Nayak, A. K. , & Sen, K. K. (2017). Transferosomal gel for transdermal delivery of risperidone: Formulation optimization and ex vivo permeation. JJournal of Drug Delivery Science and Technology, 38, 59–71. [Google Scholar]
- Dobričić, V. , Marković, B. , Nikolic, K. , Savić, V. , Vladimirov, S. , & Čudina, O. (2014). 17β‐carboxamide steroids—In vitro prediction of human skin permeability and retention using PAMPA technique. European Journal of Pharmaceutical Sciences, 52, 95–108. [DOI] [PubMed] [Google Scholar]
- Elias, P. M. , Arbiser, J. , Brown, B. E. , Rossiter, H. , Man, M.‐Q. , Cerimele, F. , … Feingold, K. R. (2008). Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: Implications for the pathogenesis of psoriasis. The American Journal of Pathology, 173(3), 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart, K. , El Hindi, T. , Biesalski, H. K. , & Pfitzner, I. (2005). In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Archives of Dermatological Research, 297(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Engesland, A. , Skalko‐Basnet, N. , & Flaten, G. E. (2015). Phospholipid vesicle‐based permeation assay and EpiSkin® in assessment of drug therapies destined for skin administration. Journal of Pharmaceutical Sciences, 104(3), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Engesland, A. , Škalko‐Basnet, N. , & Flaten, G. E. (2016). In vitro models to estimate drug penetration through the compromised stratum corneum barrier. Drug Development and Industrial Pharmacy, 42(11), 1742–1751. [DOI] [PubMed] [Google Scholar]
- Engesland, A. , Skar, M. , Hansen, T. , Skalko‐Basnet, N. , & Flaten, G. E. (2013). New applications of phospholipid vesicle‐based permeation assay: Permeation model mimicking skin barrier. Journal of Pharmaceutical Sciences, 102(5), 1588–1600. [DOI] [PubMed] [Google Scholar]
- Faway, É. , Cambier, L. , Mignon, B. , Poumay, Y. , & Lambert de Rouvroit, C. (2016). Modeling dermatophytosis in reconstructed human epidermis: A new tool to study infection mechanisms and to test antifungal agents. Medical Mycology, 55(5), 485–494. [DOI] [PubMed] [Google Scholar]
- Faway, É. , Lambert de Rouvroit, C. , & Poumay, Y. (2018). In vitro models of dermatophyte infection to investigate epidermal barrier alterations. Experimental Dermatology, 27(8), 915–922. [DOI] [PubMed] [Google Scholar]
- Filocamo, G. , Brunetti, M. , Colaceci, F. , Sasso, R. , Tanori, M. , Pasquali, E. , … Pazzaglia, S. (2016). MK‐4101, a potent inhibitor of the hedgehog pathway, is highly active against medulloblastoma and basal cell carcinoma. Molecular Cancer Therapeutics, 15(6), 1177–1189. [DOI] [PubMed] [Google Scholar]
- Firat, Y. H. , Simanski, M. , Rademacher, F. , Schroder, L. , Brasch, J. , & Harder, J. (2014). Infection of keratinocytes with Trichophytum rubrum induces epidermal growth factor‐dependent RNase 7 and human beta‐defensin‐3 expression. PLoS ONE, 9(4), e93941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten, G. E. , Bunjes, H. , Luthman, K. , & Brandl, M. (2006). Drug permeability across a phospholipid vesicle‐based barrier: 2. Characterization of barrier structure, storage stability and stability towards pH changes. European Journal of Pharmaceutical Sciences, 28(4), 336–343. [DOI] [PubMed] [Google Scholar]
- Flaten, G. E. , Dhanikula, A. B. , Luthman, K. , & Brandl, M. (2006). Drug permeability across a phospholipid vesicle based barrier: A novel approach for studying passive diffusion. European Journal of Pharmaceutical Sciences, 27(1), 80–90. [DOI] [PubMed] [Google Scholar]
- Flaten, G. E. , Palac, Z. , Engesland, A. , Filipovic‐Grcic, J. , Vanic, Z. , & Skalko‐Basnet, N. (2015). In vitro skin models as a tool in optimization of drug formulation. European Journal of Pharmaceutical Sciences, 75, 10–24. [DOI] [PubMed] [Google Scholar]
- Forster, M. , Bolzinger, M. A. , Fessi, H. , & Briancon, S. (2009). Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. European Journal of Dermatology, 19(4), 309–323. [DOI] [PubMed] [Google Scholar]
- Frederiksen, M. , Stapleton, H. M. , Vorkamp, K. , Webster, T. F. , Jensen, N. M. , Sørensen, J. A. , … Nielsen, J. B. (2018). Dermal uptake and percutaneous penetration of organophosphate esters in a human skin ex vivo model. Chemosphere, 197, 185–192. [DOI] [PubMed] [Google Scholar]
- Gaur, P. K. , Mishra, S. , & Purohit, S. (2013). Solid lipid nanoparticles of guggul lipid as drug carrier for transdermal drug delivery. BioMed Research International, 2013, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin, B. , & Touitou, E. (2012). Dermal and transdermal delivery In Bhushan B. (Ed.), Encyclopedia of nanotechnology (pp. 517–526). Dordrecht: Springer Netherlands. [Google Scholar]
- Green, C. B. , Cheng, G. , Chandra, J. , Mukherjee, P. , Ghannoum, M. A. , & Hoyer, L. L. (2004). RT‐PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology, 150(2), 267–275. [DOI] [PubMed] [Google Scholar]
- Groves, R. W. , Mizutani, H. , Kieffer, J. D. , & Kupper, T. S. (1995). Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. PNAS, 92(25), 11,874–11,878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq, A. , Goodyear, B. , Ameen, D. , Joshi, V. , & Michniak‐Kohn, B. (2018). Strat‐M® synthetic membrane: Permeability comparison to human cadaver skin. International Journal of Pharmaceutics, 547(1), 432–437. [DOI] [PubMed] [Google Scholar]
- Hathout, R. M. , Woodman, T. J. , Mansour, S. , Mortada, N. D. , Geneidi, A. S. , & Guy, R. H. (2010). Microemulsion formulations for the transdermal delivery of testosterone. European Journal of Pharmaceutical Sciences, 40(3), 188–196. [DOI] [PubMed] [Google Scholar]
- Huet, F. , Severino‐Freire, M. , Chéret, J. , Gouin, O. , Praneuf, J. , Pierre, O. , … Le Gall‐Ianotto, C. (2018). Reconstructed human epidermis for in vitro studies on atopic dermatitis: A review. Journal of Dermatological Science, 89(3), 213–218. [DOI] [PubMed] [Google Scholar]
- Hughes, M. F. , & Edwards, B. C. (2010). In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicology and Applied Pharmacology, 246(1‐2), 29–37. [DOI] [PubMed] [Google Scholar]
- Junyaprasert, V. B. , Singhsa, P. , Suksiriworapong, J. , & Chantasart, D. (2012). Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. International Journal of Pharmaceutics, 423(2), 303–311. [DOI] [PubMed] [Google Scholar]
- Jurek, I. , Góral, I. , Mierzyńska, Z. , Moniuszko‐Szajwaj, B. , & Wojciechowski, K. (2019). Effect of synthetic surfactants and soapwort (Saponaria officinalis L.) extract on skin‐mimetic model lipid monolayers. Biochimica et Biophysica Acta, 1861(3), 556–564. [DOI] [PubMed] [Google Scholar]
- Kansy, M. , Senner, F. , & Gubernator, K. (1998). Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. Journal of Medicinal Chemistry, 41(7), 1007–1010. [DOI] [PubMed] [Google Scholar]
- Karadzovska, D. , & Riviere, J. E. (2013). Assessing vehicle effects on skin absorption using artificial membrane assays. European Journal of Pharmaceutical Sciences, 50(5), 569–576. [DOI] [PubMed] [Google Scholar]
- Klar, A. S. , Zimoch, J. , & Biedermann, T. (2017). Skin tissue engineering: Application of adipose‐derived stem cells. BioMed Research International, 2017, 9747010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman, A. M. , & Christophers, E. (1963). Preparation of isolated sheets of human stratum corneum. Archives of Dermatology, 88, 702–705. [DOI] [PubMed] [Google Scholar]
- Knudsen, N. Ø. , Rønholt, S. , Salte, R. D. , Jorgensen, L. , Thormann, T. , Basse, L. H. , … Foged, C. (2012). Calcipotriol delivery into the skin with PEGylated liposomes. European Journal of Pharmaceutics and Biopharmaceutics, 81(3), 532–539. [DOI] [PubMed] [Google Scholar]
- Koch, L. , Deiwick, A. , Schlie, S. , Michael, S. , Gruene, M. , Coger, V. , … Chichkov, B. (2012). Skin tissue generation by laser cell printing. Biotechnology and Bioengineering, 109(7), 1855–1863. [DOI] [PubMed] [Google Scholar]
- Köllmer, M. , Mossahebi, P. , Sacharow, E. , Gorissen, S. , Gräfe, N. , Evers, D.‐H. , & Herbig, M. E. (2019). Investigation of the compatibility of the skin PAMPA model with topical formulation and acceptor media additives using different assay setups. AAPS PharmSciTech, 20(2), 89. [DOI] [PubMed] [Google Scholar]
- Kuchler, S. , Henkes, D. , Eckl, K. M. , Ackermann, K. , Plendl, J. , Korting, H. C. , … Schafer‐Korting, M. (2011). Hallmarks of atopic skin mimicked in vitro by means of a skin disease model based on FLG knock‐down. Alternatives to Laboratory Animals, 39(5), 471–480. [DOI] [PubMed] [Google Scholar]
- Kumpugdee‐Vollrath, M. , Subongkot, T. , & Ngawhirunpat, T. (2013). Model membrane from shed snake skins. International Journal of Pharmacy and Pharmaceutical Sciences, 7(10), 669–676. [Google Scholar]
- Kundu‐Raychaudhuri, S. , Chen, Y. J. , Wulff, H. , & Raychaudhuri, S. P. (2014). Kv1.3 in psoriatic disease: PAP‐1, a small molecule inhibitor of Kv1.3 is effective in the SCID mouse psoriasis—Xenograft model. Journal of Autoimmunity, 55, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, P. W. (1972). Two new mutations in linkage group XVI of the house mouse: Flaky tail and varitint‐waddler‐J. The Journal of Heredity, 63(3), 135–140. [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , Jeong, S. K. , & Ahn, S. K. (2006). An update of the defensive barrier function of skin. Yonsei Medical Journal, 47(3), 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, V. , Singh, G. , Trasatti, J. P. , Bjornsson, C. , Xu, X. , Tran, T. N. , … Karande, P. (2014). Design and fabrication of human skin by three‐dimensional bioprinting. Tissue Engineering. Part C, Methods, 20(6), 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Fukunaga‐Kalabis, M. , & Herlyn, M. (2011). The three‐dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. Journal of Visualized Experiments, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. J. , Guo, X. , Dawuti, G. , & Aibai, S. (2015). Antifungal activity of ellagic acid in vitro and in vivo . Phytotherapy Research, 29(7), 1019–1025. [DOI] [PubMed] [Google Scholar]
- Liang, P. P. , Huang, X. Z. , Yi, J. L. , Chen, Z. R. , Ma, H. , Ye, C. X. , … Chen, J. (2016). A Trichophyton rubrum infection model based on the reconstructed human epidermis—Episkin®. Chinese Medical Journal, 129(1), 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, S. A. C. , & Reis, S. (2018). Nanotechnological approaches in drug absorption through skin topical delivery In Publishing J. S. (Ed.), Nanoparticles in life sciences and biomedicine. New York. [Google Scholar]
- Loftsson, T. , Konrádsdóttir, F. , & Másson, M. (2006). Development and evaluation of an artificial membrane for determination of drug availability. International Journal of Pharmaceutics, 326(1), 60–68. [DOI] [PubMed] [Google Scholar]
- Löwa, A. , Jevtić, M. , Gorreja, F. , & Hedtrich, S. (2018). Alternatives to animal testing in basic and preclinical research of atopic dermatitis. Experimental Dermatology, 27(5), 476–483. [DOI] [PubMed] [Google Scholar]
- Ma, M. , Di, H.‐J. , Zhang, H. , Yao, J.‐H. , Dong, J. , Yan, G.‐J. , … Chen, J. (2017). Development of phospholipid vesicle‐based permeation assay models capable of evaluating percutaneous penetration enhancing effect. Drug Development and Industrial Pharmacy, 43(12), 2055–2063. [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Kurtyka, C. A. , Boyapalle, S. , Sung, S.‐S. , Lawrence, H. , Guida, W. , & Cress, W. D. (2008). A small‐molecule E2F inhibitor blocks growth in a melanoma culture model. Cancer Research, 68(15), 6292–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi, A. , Quadri, M. , Saltari, A. , & Pincelli, C. (2018). Progress in melanoma modelling in vitro. Experimental Dermatology, 27(5), 578–586. [DOI] [PubMed] [Google Scholar]
- Marepally, S. , Boakye, C. H. A. , Shah, P. P. , Etukala, J. R. , Vemuri, A. , & Singh, M. (2013). Design, synthesis of novel lipids as chemical permeation enhancers and development of nanoparticle system for transdermal drug delivery. PLoS ONE, 8(12), e82581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimuthu, M. , Bennet, D. , & Kim, S. (2012). Self‐assembled nanoparticles of PLGA‐conjugated glucosamine as a sustained transdermal drug delivery vehicle. Polymer Journal, 45, 202. [Google Scholar]
- Meyer, W. , Schonnagel, B. , & Fleischer, L. G. (2006). A note on integumental (1→3)(1→6)β‐d‐glucan permeation, using the porcine ear skin model. Journal of Cosmetic Dermatology, 5(2), 130–134. [DOI] [PubMed] [Google Scholar]
- Miki, R. , Ichitsuka, Y. , Yamada, T. , Kimura, S. , Egawa, Y. , Seki, T. , … Morimoto, Y. (2015). Development of a membrane impregnated with a poly(dimethylsiloxane)/poly(ethylene glycol) copolymer for a high‐throughput screening of the permeability of drugs, cosmetics, and other chemicals across the human skin. European Journal of Pharmaceutical Sciences, 66, 41–49. [DOI] [PubMed] [Google Scholar]
- Min, D. , Lee, W. , Bae, I. H. , Lee, T. R. , Croce, P. , & Yoo, S. S. (2018). Bioprinting of biomimetic skin containing melanocytes. Experimental Dermatology, 27(5), 453–459. [DOI] [PubMed] [Google Scholar]
- Mohapatra, S. , Coppola, D. , Riker, A. I. , & Pledger, W. J. (2007). Roscovitine inhibits differentiation and invasion in a three‐dimensional skin reconstruction model of metastatic melanoma. Molecular Cancer Research, 5(2), 145–151. [DOI] [PubMed] [Google Scholar]
- Moner, V. , Fernández, E. , Calpena, A. C. , Garcia‐Herrera, A. , Cócera, M. , & López, O. (2018). A lamellar body mimetic system for the treatment of oxazolone‐induced atopic dermatitis in hairless mice. Journal of Dermatological Science, 90(2), 172–179. [DOI] [PubMed] [Google Scholar]
- Monfort, A. , Soriano‐Navarro, M. , García‐Verdugo, J. M. , & Izeta, A. (2013). Production of human tissue‐engineered skin trilayer on a plasma‐based hypodermis. Journal of Tissue Engineering and Regenerative Medicine, 7(6), 479–490. [DOI] [PubMed] [Google Scholar]
- Moniaga, C. S. , Egawa, G. , Kawasaki, H. , Hara‐Chikuma, M. , Honda, T. , Tanizaki, H. , … Kabashima, K. (2010). Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. The American Journal of Pathology, 176(5), 2385–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelreiter, C. , Raffeiner, S. , Geyerhofer, C. , Klang, V. , & Valenta, C. (2013). Influence of drug content, type of semi‐solid vehicle and rheological properties on the skin penetration of the model drug fludrocortisone acetate. International Journal of Pharmaceutics, 448(1), 305–312. [DOI] [PubMed] [Google Scholar]
- Nakano, M. , & Patel, N. K. (1970). Release, uptake, and permeation behavior of salicylic acid in ointment bases. Journal of Pharmaceutical Sciences, 59(7), 985–988. [DOI] [PubMed] [Google Scholar]
- Netzlaff, F. , Schaefer, U. F. , Lehr, C. M. , Meiers, P. , Stahl, J. , Kietzmann, M. , & Niedorf, F. (2006). Comparison of bovine udder skin with human and porcine skin in percutaneous permeation experiments. Alternatives to Laboratory Animals, 34(5), 499–513. [PubMed] [Google Scholar]
- Ng, K. W. , & Lau, W. M. (2015). Skin deep: The basics of human skin structure and drug penetration In Dragicevic N. & Maibach H. I. (Eds.), Percutaneous penetration enhancers chemical methods in penetration enhancement: Drug manipulation strategies and vehicle effects (pp. 3–11). Berlin Heidelberg: Springer. [Google Scholar]
- Organisation for Economic Cooperation and Development . (2004). Test No. 428: Skin absorption: in vitro method.
- Organisation for Economic Cooperation and Development . (2011). Test No. 156: Guidance notes on dermal absorption.
- Organisation for Economic Cooperation and Development . (2015). Test No. 439: in vitro skin irritation: Reconstructed human epidermis test method.
- Oliveira, G. , Hadgraft, J. , & Lane, M. E. (2012). The role of vehicle interactions on permeation of an active through model membranes and human skin. International Journal of Cosmetic Science, 34(6), 536–545. [DOI] [PubMed] [Google Scholar]
- Ottaviani, G. , Martel, S. , & Carrupt, P.‐A. (2006). Parallel artificial membrane permeability assay: A new membrane for the fast prediction of passive human skin permeability. Journal of Medicinal Chemistry, 49(13), 3948–3954. [DOI] [PubMed] [Google Scholar]
- Palac, Z. , Engesland, A. , Flaten, G. E. , Skalko‐Basnet, N. , Filipovic‐Grcic, J. , & Vanic, Z. (2014). Liposomes for (trans)dermal drug delivery: The skin‐PVPA as a novel in vitro stratum corneum model in formulation development. Journal of Liposome Research, 24(4), 313–322. [DOI] [PubMed] [Google Scholar]
- Passeron, T. , Namiki, T. , Passeron, H. J. , Le Pape, E. , & Hearing, V. J. (2009). Forskolin protects keratinocytes from UVB‐induced apoptosis and increases DNA repair independent of its effects on melanogenesis. The Journal of Investigative Dermatology, 129(1), 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa, T. D. N. , Catarino, C. M. , Pennacchi, P. C. , Assis, S. R. , Gimenes, F. , Consolaro, M. E. L. , … Maria‐Engler, S. S. (2017). A new reconstructed human epidermis for in vitro skin irritation testing. Toxicology in Vitro, 42, 31–37. [DOI] [PubMed] [Google Scholar]
- Pendaries, V. , Malaisse, J. , Pellerin, L. , Le Lamer, M. , Nachat, R. , Kezic, S. , … Simon, M. (2014). Knockdown of filaggrin in a three‐dimensional reconstructed human epidermis impairs keratinocyte differentiation. The Journal of Investigative Dermatology, 134(12), 2938–2946. [DOI] [PubMed] [Google Scholar]
- Peres, N. T. , Silva, L. G. , Santos Rda, S. , Jacob, T. R. , Persinoti, G. F. , Rocha, L. B. , … Martinez‐Rossi, N. M. (2016). In vitro and ex vivo infection models help assess the molecular aspects of the interaction of Trichophyton rubrum with the host milieu. Medical Mycology, 54(4), 420–427. [DOI] [PubMed] [Google Scholar]
- Ponec, M. (1992). In vitro cultured human skin cells as alternatives to animals for skin irritancy screening. International Journal of Cosmetic Science, 14(6), 245–264. [DOI] [PubMed] [Google Scholar]
- Pouillot, A. , Dayan, N. , Polla, A. S. , Polla, L. L. , & Polla, B. S. (2008). The stratum corneum: A double paradox. Journal of Cosmetic Dermatology, 7(2), 143–148. [DOI] [PubMed] [Google Scholar]
- Prausnitz, M. R. , Elias, P. M. , Franz, T. J. , Schmuth, M. , Tsai, J. C. , Menon, G. K. , … Feingold, K. R. (2012). Skin barrier and transdermal drug delivery In Bolognia J., Jorizzo J. L., & Schaffer J. V. (Eds.), Dermatology (pp. 2065–2073). Philadelphia: Elsevier Saunders. [Google Scholar]
- Randall, M. J. , Jüngel, A. , Rimann, M. , & Wuertz‐Kozak, K. (2018). Advances in the biofabrication of 3D skin in vitro: Healthy and pathological models. Frontiers in Bioengineering and Biotechnology, 6(154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid, A. , Edward, M. , & Richardson, M. D. (1995). Activity of terbinafine on Trichophyton mentagrophytes in a human living skin equivalent model. Medical Mycology, 33(4), 229–233. [PubMed] [Google Scholar]
- Rheinwald, J. G. (1989). Methods for clonal growth and serial cultivation of normal human epidermal keratinocytes and mesothelial cells In Cell growth and division. Oxford: IRL. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J. G. , & Green, H. (1975). Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell, 6(3), 331–343. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J. G. , & Green, H. (1977). Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature, 265(5593), 421–424. [DOI] [PubMed] [Google Scholar]
- Rouaud‐Tinguely, P. , Boudier, D. , Marchand, L. , Barruche, V. , Bordes, S. , Coppin, H. , … Closs, B. (2015). From the morphological to the transcriptomic characterization of a compromised three‐dimensional in vitro model mimicking atopic dermatitis. The British Journal of Dermatology, 173(4), 1006–1014. [DOI] [PubMed] [Google Scholar]
- Sahle, F. F. , Wohlrab, J. , & Neubert, R. H. H. (2014). Controlled penetration of ceramides into and across the stratum corneum using various types of microemulsions and formulation associated toxicity studies. European Journal of Pharmaceutics and Biopharmaceutics, 86(2), 244–250. [DOI] [PubMed] [Google Scholar]
- Salton, M. , Kasprzak, W. K. , Voss, T. , Shapiro, B. A. , Poulikakos, P. I. , & Misteli, T. (2015). Inhibition of vemurafenib‐resistant melanoma by interference with pre‐mRNA splicing. Nature Communications, 6, 7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkiri, M. , Fox, S. C. , Fratila‐Apachitei, L. E. , & Zadpoor, A. A. (2019). Bioengineered skin intended for skin disease modeling. International Journal of Molecular Sciences, 20(6), 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, U. F. , Hansen, S. , Schneider, M. , Contreras, J. L. , & Lehr, C. M. (2008). Models for skin absorption and skin toxicity testing In Drug absorption studies (Vol. 7) (pp. 3–33). [Google Scholar]
- Schafer‐Korting, M. , Bock, U. , Diembeck, W. , Dusing, H. J. , Gamer, A. , Haltner‐Ukomadu, E. , … Weimer, M. (2008). The use of reconstructed human epidermis for skin absorption testing: Results of the validation study. Alternatives to Laboratory Animals, 36(2), 161–187. [DOI] [PubMed] [Google Scholar]
- Schreiber, S. , Mahmoud, A. , Vuia, A. , Rubbelke, M. K. , Schmidt, E. , Schaller, M. , … Schafer‐Korting, M. (2005). Reconstructed epidermis versus human and animal skin in skin absorption studies. Toxicology in Vitro, 19(6), 813–822. [DOI] [PubMed] [Google Scholar]
- Schroder, H. , Komljenovic, D. , Hecker, M. , & Korff, T. (2016). Transdermal drug targeting and functional imaging of tumor blood vessels in the mouse auricle. FASEB, 30(2), 923–932. [DOI] [PubMed] [Google Scholar]
- Scognamiglio, I. , De stefano, D. , Campani, V. , Mayol, L. , Carnuccio, R. , Fabbrocini, G. , … De Rosa, G. (2013). Nanocarriers for topical administration of resveratrol: A comparative study. International Journal of Pharmaceutics, 440(2), 179–187. [DOI] [PubMed] [Google Scholar]
- Şenyiğit, T. , Sonvico, F. , Barbieri, S. , Özer, Ö. , Santi, P. , & Colombo, P. (2010). Lecithin/chitosan nanoparticles of clobetasol‐17‐propionate capable of accumulation in pig skin. Journal of Controlled Release, 142(3), 368–373. [DOI] [PubMed] [Google Scholar]
- Shah, V. P. , Elkins, J. , Lam, S.‐Y. , & Skelly, J. P. (1989). Determination of in vitro drug release from hydrocortisone creams. International Journal of Pharmaceutics, 53(1), 53–59. [Google Scholar]
- Shakel, Z. , Nunes, C. , Costa Lima, S. A. , & Reis, S. (2019). Development of a novel human stratum corneum model, as a tool in the optimization of drug formulations. International Journal of Pharmaceutics, 569, 118571. [DOI] [PubMed] [Google Scholar]
- Shepherd, J. , Little, M. C. , & Nicklin, M. J. H. (2004). Psoriasis‐like cutaneous inflammation in mice lacking interleukin‐1 receptor antagonist. The Journal of Investigative Dermatology, 122(3), 665–669. [DOI] [PubMed] [Google Scholar]
- Silva, E. , Barreiros, L. , Segundo, M. A. , Costa Lima, S. A. , & Reis, S. (2017). Cellular interactions of a lipid‐based nanocarrier model with human keratinocytes: Unravelling transport mechanisms. Acta Biomaterialia, 53, 439–449. [DOI] [PubMed] [Google Scholar]
- Singh, A. , Singh, A. , Sand, J. M. , Bauer, S. J. , Hafeez, B. B. , Meske, L. , & Verma, A. K. (2015). Topically applied Hsp90 inhibitor 17AAG inhibits UVR‐induced cutaneous squamous cell carcinomas. The Journal of Investigative Dermatology, 135(4), 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinko, B. , Garrigues, T. M. , Balogh, G. T. , Nagy, Z. K. , Tsinman, O. , Avdeef, A. , & Takacs‐Novak, K. (2012). Skin–PAMPA: A new method for fast prediction of skin penetration. European Journal of Pharmaceutical Sciences, 45(5), 698–707. [DOI] [PubMed] [Google Scholar]
- Sriram, G. , Alberti, M. , Dancik, Y. , Wu, B. , Wu, R. , Feng, Z. , … Wang, Z. (2018). Full‐thickness human skin‐on‐chip with enhanced epidermal morphogenesis and barrier function. Materials Today, 21(4), 326–340. [Google Scholar]
- Szabowski, A. , Maas‐Szabowski, N. , Andrecht, S. , Kolbus, A. , Schorpp‐Kistner, M. , Fusenig, N. E. , & Angel, P. (2000). c‐Jun and JunB antagonistically control cytokine‐regulated mesenchymal–epidermal interaction in skin. Cell, 103(5), 745–755. [DOI] [PubMed] [Google Scholar]
- Tabart, J. , Baldo, A. , Vermout, S. , Losson, B. , & Mignon, B. (2008). Reconstructed interfollicular feline epidermis as a model for the screening of antifungal drugs against Microsporum canis . Veterinary Dermatology, 19(3), 130–133. [DOI] [PubMed] [Google Scholar]
- Tang, T. , Tang, J. Y. , Li, D. , Reich, M. , Callahan, C. A. , Fu, L. , … de Sauvage, F. J. (2011). Targeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of smoothened. Clinical Cancer Research, 17(10), 3378–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternullo, S. , Basnet, P. , Holsæter, A. M. , Flaten, G. E. , de Weerd, L. , & Škalko‐Basnet, N. (2018). Deformable liposomes for skin therapy with human epidermal growth factor: The effect of liposomal surface charge. European Journal of Pharmaceutical Sciences, 125, 163–171. [DOI] [PubMed] [Google Scholar]
- Ternullo, S. , de Weerd, L. , Flaten, G. E. , Holsæter, A. M. , & Škalko‐Basnet, N. (2017). The isolated perfused human skin flap model: A missing link in skin penetration studies? European Journal of Pharmaceutical Sciences, 96, 334–341. [DOI] [PubMed] [Google Scholar]
- Ternullo, S. , de Weerd, L. , Holsæter, A. M. , Flaten, G. E. , & Škalko‐Basnet, N. (2017). Going skin deep: A direct comparison of penetration potential of lipid‐based nanovesicles on the isolated perfused human skin flap model. European Journal of Pharmaceutics and Biopharmaceutics, 121, 14–23. [DOI] [PubMed] [Google Scholar]
- Thang, N. D. , Yajima, I. , Nakagawa, K. , Tsuzuki, T. , Kumasaka, M. Y. , Ohgami, N. , … Kato, M. (2012). A novel hairless mouse model for malignant melanoma. Journal of Dermatological Science, 65(3), 207–212. [DOI] [PubMed] [Google Scholar]
- Tjabringa, G. , Bergers, M. , van Rens, D. , de Boer, R. , Lamme, E. , & Schalkwijk, J. (2008). Development and validation of human psoriatic skin equivalents. The American Journal of Pathology, 173(3), 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier, V. , Marceau‐Fortier, G. , Germain, L. , Vincent, C. , & Fradette, J. (2008). IFATS collection: Using human adipose‐derived stem/stromal cells for the production of new skin substitutes. Stem Cells, 26(10), 2713–2723. [DOI] [PubMed] [Google Scholar]
- Tsinman, K. , & Sinkó, B. (2013). A high throughput method to predict skin penetration and screen topical formulations. Cosmet Toiletries, 128(3), 192–199. [Google Scholar]
- Uchida, Y. , & Park, K. (2016). Stratum corneum In Kabashima K. (Ed.), Immunology of the skin: Basic and clinical sciences in skin immune responses (pp. 15–30). Tokyo: Springer Japan. [Google Scholar]
- Uchino, T. , Takezawa, T. , & Ikarashi, Y. (2009). Reconstruction of three‐dimensional human skin model composed of dendritic cells, keratinocytes and fibroblasts utilizing a handy scaffold of collagen vitrigel membrane. Toxicology in Vitro, 23(2), 333–337. [DOI] [PubMed] [Google Scholar]
- Van Gele, M. , Geusens, B. , Brochez, L. , Speeckaert, R. , & Lambert, J. (2011). Three‐dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opinion on Drug Delivery, 8(6), 705–720. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaay, B. , & Leibold, E. (2004). A comparison between in vitro rat and human and in vivo rat skin absorption studies. Human & Experimental Toxicology, 23(9), 421–430. [DOI] [PubMed] [Google Scholar]
- Vestergaard, C. , Yoneyama, H. , Murai, M. , Nakamura, K. , Tamaki, K. , Terashima, Y. , … Matsushima, K. (1999). Overproduction of Th2‐specific chemokines in NC/Nga mice exhibiting atopic dermatitis‐like lesions. The Journal of Clinical Investigation, 104(8), 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorsmann, H. , Groeber, F. , Walles, H. , Busch, S. , Beissert, S. , Walczak, H. , & Kulms, D. (2013). Development of a human three‐dimensional organotypic skin‐melanoma spheroid model for in vitro drug testing. Cell Death & Disease, 4, e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, K. A. , & Roberts, M. S. (2002). The structure and function of skin In Dermatological and transdermal formulations (pp. 1–39). CRC Press. [Google Scholar]
- Wang, H. , Li, J. , Lv, T. , Tu, Q. , Huang, Z. , & Wang, X. (2013). Therapeutic and immune effects of 5‐aminolevulinic acid photodynamic therapy on UVB‐induced squamous cell carcinomas in hairless mice. Experimental Dermatology, 22(5), 362–363. [DOI] [PubMed] [Google Scholar]
- Watkinson, R. M. , Guy, R. H. , Oliveira, G. , Hadgraft, J. , & Lane, M. E. (2011). Optimisation of cosolvent concentration for topical drug delivery III—Influence of lipophilic vehicles on ibuprofen permeation. Skin Pharmacology and Physiology, 24(1), 22–26. [DOI] [PubMed] [Google Scholar]
- Wonglertnirant, N. , Ngawhirunpat, T. , & Kumpugdee‐Vollrath, M. (2012). Evaluation of the mechanism of skin enhancing surfactants on the biomembrane of shed snake skin. Biological & Pharmaceutical Bulletin, 35(4), 523–531. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Deng, Z. , Zhou, B. , Qi, M. , Hong, M. , & Ren, G. (2019). Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. Journal of Molecular Liquids, 283, 399–409. [Google Scholar]
- Wu, S. T. , Shiu, G. K. , Simmons, J. E. , Bronaugh, R. L. , & Skelly, J. P. (1992). In vitro release of nitroglycerin from topical products by use of artificial membranes. Journal of Pharmaceutical Sciences, 81(12), 1153–1156. [DOI] [PubMed] [Google Scholar]
- Yu, J. R. , Navarro, J. , Coburn, J. C. , Mahadik, B. , Molnar, J. , Holmes, J. H. IV , … Fisher, J. P. (2019). Current and future perspectives on skin tissue engineering: Key features of biomedical research, translational assessment, and clinical application. Advanced Healthcare Materials, 8(5), 1801471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Du, L. , Zhu, L. , Liu, X. , Zhang, B. , Fu, G. , & Jin, Y. (2016). Melanoma therapy with transdermal mitoxantrone cubic phases. Drug Delivery, 23(5), 1565–1570. [DOI] [PubMed] [Google Scholar]
- Yue, L. , Huang, Z. M. , Fong, S. , Leong, S. , Jakowatz, J. G. , Charruyer‐Reinwald, A. , … Ghadially, R. (2015). Targeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanoma. Melanoma Research, 25(2), 138–148. [DOI] [PubMed] [Google Scholar]
- Yun, Y. E. , Jung, Y. J. , Choi, Y. J. , Choi, J. S. , & Cho, Y. W. (2018). Artificial skin models for animal‐free testing. Journal of Pharmaceutical Investigation, 48(2), 215–223. [Google Scholar]
- Zhang, Q. , Sito, L. , Mao, M. , He, J. , Zhang, Y. S. , & Zhao, X. (2018). Current advances in skin‐on‐a‐chip models for drug testing. Microphysiol Sys, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Lane, M. E. , Hadgraft, J. , Heinrich, M. , Chen, T. , Lian, G. , & Sinko, B. (2019). A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. International Journal of Pharmaceutics, 556, 142–149. [DOI] [PubMed] [Google Scholar]


