Abstract
To investigate the value of the combined detection of the neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein level (CRP) in the diagnosis of COVID-19. A total of 191 patients with COVID-19 were recruited at the Third Hospital of Wuhan from 21 January 2020 to 20 February 2020. Fifty healthy volunteers were randomly selected as the control group. Age, gender, white blood cell count (WBC), CRP, lymphocyte percentage, and NLR were extracted. Quantitative clinical characteristics and laboratory values were compared between groups. Risk factors and receiver operating characteristic (ROC) curves for COVID-19 were analyzed. We found that the NLR and CRP were higher, while the lymphocyte percentage was lower in patients with COVID-19 than in healthy controls. Among patients confirmed to have COVID-19, the NLR and CRP of the moderate group were lower than those of severely ill patients (severe, critical and death groups), and the lymphocyte percentage of the moderate group was higher than that of the critical and death group. There were no significant differences in WBC among all groups. Logistic regression analysis showed that the NLR, CRP, and lymphocyte percentage were independent risk factors for COVID-19. The AUC of the combined determination of NLR and CRP was 0.863, which was higher than that of NLR, CRP, WBC, and lymphocyte percentage (AUC: 0.835, 0.775, 0.416, and 0.749, respectively).
Our results showed that the NLR and CRP were independent risk factors for COVID-19, and the combined detection of the NLR and CRP showed improved diagnostic performance for COVID-19.
Keywords: Neutrophil to lymphocyte ratio, C-reactive protein, COVID-19, risk factors, ROC curve
Introduction
Coronavirus disease 2019 (COVID-19) is a newly recognized pneumonia that has spread rapidly throughout Wuhan, Hubei Province, and to other provinces in China and around the world [1,2]. As of 21 March 2020, 81,385 confirmed cases had been reported in mainland China, causing 3255 deaths. In addition, epidemics have spread to over 100 countries around the world. COVID-19 is an emerging, rapidly evolving situation [3]. The pathogen has been identified as a novel RNA beta coronavirus that has been subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4]. Control of the spread of disease relies on rapid diagnosis and appropriate clinical management. Early and reliable detection of SARS-CoV-2 in clinical specimens will determine which patients should be immediately isolated and managed according to strict procedures of infection control. The protocols of WHO network laboratories [5] facilitated the development of the rapid diagnosis of SARS-CoV-2 using nucleic acid amplification tests (NAATs), such as RT-PCR. However, the results of NAATs could be false negatives depending on the assay, specimen, and time course of the disease [6]. Public health testing capacity is likely to become overwhelmed in areas of widespread disease activity, and turnaround times are likely to be prolonged. Decentralized testing will likely need to be made available at the hospital level.
Viral infection may produce various hematological changes. Early studies have shown that lymphocytopenia is common among patients with COVID-19 [7–9]. Accordingly, the neutrophil to lymphocyte ratio (NLR) in peripheral blood has been suggested to be useful in discriminating between types of infection [10] and predicting the outcome of infection [11]. C-reactive protein (CRP), as a classic inflammatory biomarker, is one of the most sensitive acute-phase reactants and is virtually absent from blood serum in healthy people. CRP levels can increase dramatically after bacterial and viral infections, inflammation, and severe trauma [12]. Elevated CRP levels were also observed in COVID-19 patients [9]. However, the diagnostic performance of NLR and CRP in COVID-19 remains elusive. This study is aimed at testing the combined usability of NLR and CRP as laboratory parameters, which may provide additional benefits in both the diagnosis of SARS-CoV-2 pneumonia and the early recognition of complications that may develop as a result of these clinical pictures.
Material and methods
Participants
This retrospective observational study was conducted in the Third hospital of Wuhan (Wuhan, Hubei Province, China). The study protocol was approved by the Ethics Board of the Third Hospital of Wuhan. A total of 191 consecutive patients with confirmed COVID-19 who were admitted to the Third Hospital of Wuhan from 21 January to 20 February 2020 were enrolled. All patients with COVID-19 enrolled in this study were diagnosed according to World Health Organization interim guidance [13]. Fifty healthy volunteers from Shanghai Kongjiang Hospital were selected randomly as the control group. Chest computed tomographic (CT) scans were normal in all healthy controls.
Data collection
The following clinical information and initial laboratory data upon admission of all subjects were extracted from medical records: age, gender, white blood cell count (WBC), C-reactive protein level (CRP), lymphocyte percentage, and NLR. WBC, lymphocyte percentage, neutrophil percentage and CRP were measured by an automated hematology analyzer, namely, Mindray BC-5390 (Mindray Diagnostics, Shenzhen, China), according to the manufacturer's instructions. The NLR was defined as the neutrophil percentage divided by the lymphocyte percentage. All patients confirmed to have COVID-19 were divided into moderate, severe, critical, and death groups based on the severity of their illness according to the treatment protocol for novel coronavirus-infected pneumonia (trial version 6).
Statistical analysis
All statistical evaluations were performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) version 22.0. Categorical variables were presented as frequency rates and percentages. Continuous variables were evaluated for normality using the Kolmogorov–Smirnov test. Normally distributed continuous variables were expressed as the mean ± standard deviation, while non-normally distributed parameters were expressed as the median and range. To compare quantitative clinical characteristics and laboratory values between two groups, the Mann–Whitney U test was used, and the comparison between multiple groups was tested using the Kruskal-Wallis H test. The analysis of risk factors for COVID-19 was performed using logistic regression; binary logistic regression was used to combine indicators. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive effect of various markers for COVID-19 and identify a cutoff value as well as the corresponding sensitivity and specificity. A two-tailed p-value < .05 was considered statistically significant.
Results
Demographic and clinical characteristics
A total of 191 patients with confirmed SARS-CoV-2 infection were enrolled in the study. A total of 108 (56.54%) of the patients were male, and 83 (43.45%) of them were female. The mean age was 61.58 years (standard deviation: 13.61). No significant difference in gender or age was observed between healthy controls and COVID-19 patients. NLR, CRP, WBC, LY% were non-normally distributed, so the data of each group were presented as median and 25–75% tiles. The lymphocyte percentage of COVID-19 patients was identified to be significantly lower than that of healthy controls. The NLR and CRP level were significantly higher in COVID-19 patients. No significant difference was observed in WBC values (Table 1).
Table 1.
Baseline characteristics and laboratory parameters of patients infected with SARS-CoV-2 and healthy controls.
| Age (years) | Gender (male) | NLR | CRP(mg/L) | WBC(×109/L) | LY(%) | |
|---|---|---|---|---|---|---|
| COVID-19 patients (n = 191) | 61.58 ± 13.61 | 108 (56.54%) | 4.60 (7.7–9.67) | 40.32 (64.6–78.71) | 6.30 (6.9–7.52) | 16.10 (22.8–26.63) |
| Healthy controls (n = 50) | 59.04 ± 11.74 | 27 (54%) | 1.95 (1.9–2.17) | 5.00 (4.81–5.30) | 7.20 (7.0–7.46) | 29.96 (29.1–31.13) |
| P value | 0.192 | 0.747 | <0.001 | <0.001 | 0.067 | <0.001 |
LY: lymphocyte percentage.
Laboratory findings of patients with different clinical types of COVID-19 and healthy controls
For the comparison between multiple groups tested by Kruskal–Wallis H test, the values of NLR, CRP, and lymphocyte percentage were distributed differently among the healthy controls and the moderate, severe, critical, and death groups (p < .001) (Table 2). The NLR and CRP of moderate, severe, critical, and death groups were all higher than those of the healthy controls, while the lymphocyte percentage values were all lower than those of the healthy controls. For the moderate group, the NLR and CRP were lower than those of the severe, critical and death groups (p < .05), and the lymphocyte percentage was higher than that of the critical and death groups (p < .05). There was no significant difference in lymphocyte percentage, NLR or CRP among the other groups (p > .05). There was no significant difference in the WBC value among all groups (p = .423) (Table 2 and Figure 1).
Table 2.
Comparison of laboratory tests among different groups of COVID-19 patients divided according to disease severity and healthy controls.
| Group | Case number | NLR | CRP(mg/L) | WBC(×109/L) | LY(%) |
|---|---|---|---|---|---|
| Death | 45 | 6.97 (9.19–12.87)* | 113.21 (111.53–142.75)* | 6.60 (6.78–8.28) | 11.50 (16.29–24.40)* |
| Critical | 43 | 6.04 (10.86–17.43)* | 80.37 (80.85–111.55)* | 6.60 (7.01–8.58) | 13.10 (12.66–17.95)* |
| Severe | 20 | 4.94 (6.44–11.88)* | 76.45 (61.92–97.18)* | 5.80 (6.13–7.71) | 14.90 (16.69–27.97)* |
| Moderate | 83 | 3.22 (3.97–5.02)*#△O | 7.01 (19.90–34.21)*#△O | 6.10 (6.45–7.19) | 21.40 (29.60–35.58)*#△ |
| Healthy controls | 50 | 1.95 (1.96–2.17) | 5.00 (4.81–5.30) | 7.20 (7.02–7.46) | 29.96 (29.19–31.13) |
| p Value | <0.001 | <0.001 | 0.423 | <0.001 |
*: Compared with the healthy control group, p < 0.05; #: compared with the death group, p < 0.05; △: compared with the critical group, p < 0.05; O: compared with the severe group, p < 0.05.
Figure 1.
Distribution of NLR, CRP, WBC, and lymphocyte percentage levels for the different patient-groups and healthy controls. *: p < .05.
Analytical results of risk factors of SARS-CoV-2 infection
Logistic regression analysis was performed using NLR, CRP, WBC, lymphocyte percentage, gender, and age as independent variables and SARS-CoV-2 infection as the dependent variable. The results are detailed in Table 3. The results showed that NLR, CRP, and lymphocyte percentage were possible risk factors for SARS-CoV-2 infection, among which NLR was the most strongly associated with COVID-19 (OR = 21.517, 95% CI: 5.912–78.317, p < .001).
Table 3.
Logistic regression analysis of factors that affect SARS-CoV-2 infection.
| β value | SE | Wald | p Value | OR (95% CI) | |
|---|---|---|---|---|---|
| NLR | 3.069 | 0.659 | 21.676 | <.001 | 21.517 (5.912–78.317) |
| CRP | 0.105 | 0.051 | 4.161 | .041 | 1.111 (1.004–1.229) |
| WBC | −0.164 | 0.163 | 1.013 | .314 | 0.848 (0.616–1.169) |
| LY% | 0.211 | 0.058 | 14.430 | <.001 | 1.248 (1.113–1.399) |
| Gender | 0.861 | 0.607 | 2.015 | .156 | 2.366 (0.720–7.770) |
| Age | 0.033 | 0.021 | 2.496 | .114 | 1.034 (0.992–1.078) |
The diagnostic performance of NLR, CRP and the combined detection of the NLR and CRP for COVID-19
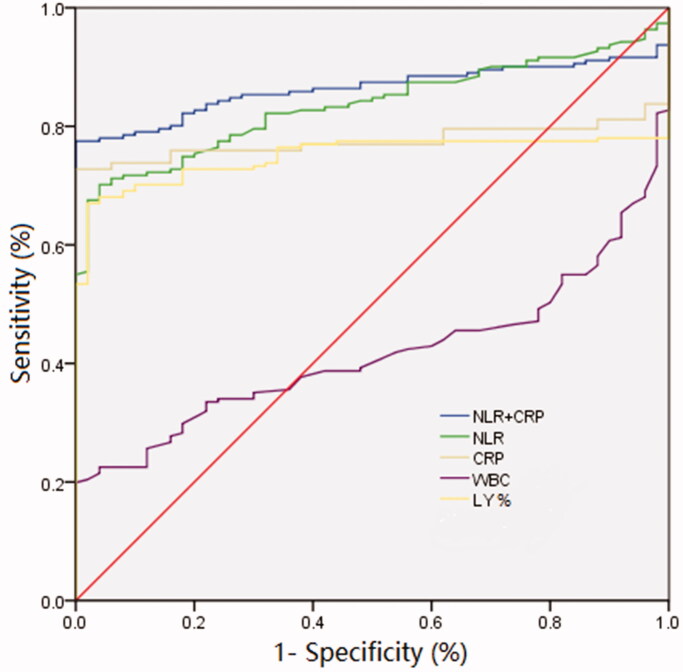
The ROC curve analysis showed that the area under the curve (AUC) of NLR, CRP, WBC, and lymphocyte percentage diagnosis of COVID-19 were 0.835, 0.775, 0.416, and 0.749, respectively. Moreover, we included the two best-performing indicators, CRP and NLR, in the binary logistic regression analysis, and the equation was NLR + 0.1478 × CRP. We found that when we combined CRP with NLR, the diagnostic accuracy was even higher (AUC = 0.863, 95% CI: 0.818–0.909), suggesting that the combined detection of NLR and CRP can improve the diagnostic performance compared to the detection of only one of these markers (Figure 2 and Table 4).
Figure 2.
ROC curves indicating the diagnostic performance of WBC, lymphocyte percentage, CRP, NLR, and combined CRP and NLR in COVID-19.
Table 4.
Comparison of the diagnostic efficacy of WBC, lymphocyte percentage, CRP, NLR, and combined CRP and NLR for COVID-19.
| AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) | Cut-off value | |
|---|---|---|---|---|---|
| NLR | 0.835 (0.786–0.884) | 0.662 | 70.2 | 96.0 | 3.17 |
| CRP | 0.775 (0.717–0.832) | 0.668 | 72.8 | 94.0 | 8.55 |
| WBC | 0.416 (0.347–0.485) | 0.184 | 20.4 | 68.0 | 9.85 |
| LY% | 0.749 (0.690–0.809) | 0.650 | 67.0 | 98.0 | 21.34 |
| NLR and CRP | 0.863 (0.818–0.909) | 0.755 | 77.5 | 98.0 | 0.71 |
Discussion
The present study of 191 cases of COVID-19 patients showed that the lymphocyte percentage of COVID-19 patients was lower than that of healthy controls. Although an association between viral infection and lymphocytosis has long been established [10], lymphopenia occurring in COVID-19 patients has been reported by previous studies [7–9], and this phenomenon was also observed in SARS [14]. Direct invasion by SARS-CoV viral particles damages the cytoplasmic component of the lymphocyte and causes its destruction [15]; indirect mechanisms such as vascular cell adhesion molecule-1 (sVCAM-1), soluble Fas ligand (sFasL) or intense cytokine storms can induce apoptosis in lymphocytes [16]. Recently, the NLR has been proposed as a novel predictor of mortality in various diseases, such as heart failure and several types of cancer [17,18]; however, the use of the NLR in the differential diagnosis of pneumonia is rare [19]. In this study, we found that the neutrophil to lymphocyte ratio was significantly higher in COVID-19 patients than in healthy controls. To the best of our knowledge, this is the first study to investigate the NLR in SARS-CoV-2 infections. Moreover, our study found that patients with higher severity of pneumonia may have lower lymphocyte percentages and higher NLR, and logistic regression analysis showed that both lymphocyte percentage (OR = 1.248, 95% CI: 1.113–1.399, p < .001) and NLR (OR = 21.517, 95% CI: 5.912–78.317, p < .001) were independent risk factors predicting SARS-CoV-2 infection.
The CRP was significantly higher in patients infected with SARS-CoV-2, and we found that the CRP in the severely ill groups (severe, critical, and death group) was significantly higher than that in the moderate group or healthy controls, which was consistent with the findings of a previous study [9]. Higher blood CRP levels, as a non-specific inflammation marker, play an instructive role in the acquired immune response as an innate recognition lectin [20], and elevated CRP levels have also been associated with acute dyspnea due to pneumonia and bronchitis [21]. Our study showed that CRP (OR = 1.111, 95% CI: 1.004–1.229, p = .041) was an independent risk factor predicting COVID-19. Subsequently, we analyzed the diagnostic performance of the parameters mentioned above. ROC curves showed that lymphocyte percentage, NLR, and CRP had good diagnostic efficiency (AUC: 0.749, 0.835, 0.775). Moreover, when the NLR and CRP were combined, the AUC increased to 0.863, with a sensitivity of 77.5% and a specificity of 98%.
In conclusion, the outbreak of pneumonia infected with SARS-CoV-2 has had extensive influence around the world, which requires scientists, clinicians and governments around the world to work swiftly to combat COVID-19. In concert with recent studies [9], the similarities between the clinical features of COVID-19 and those of previous beta coronavirus infections have been noted. In our study, we found that the lymphopenia and elevated CRP found in COVID-19 patients mimicked those of patients with SARS-CoV infection. This could be caused by the phylogenetic homogeneity between SARS-CoV-2 and other beta coronaviruses. In addition, our study further suggested that the NLR was an obvious independent risk factor predicting SARS-CoV-2 infection, and when combined with CRP, the diagnostic efficiency for SARS-CoV-2 infection improved. Given that this study was limited by its sample size, more comprehensive studies are required to help establish the role of these parameters in predicting COVID-19.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019. (COVID-19) situation report 29. Jan 31, 2020. [cited 2020 Feb 29]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200218-sitrep-29-covid-19.pdf?sfvrsn=6262de9e_2
- 4.Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020[R]. Geneva: World Health Organization; 2020. [Google Scholar]
- 6.Wang P, Anderson N, Pan Y, et al. The SARS-CoV-2 outbreak: diagnosis, infection prevention, and public perception. Clin Chem. 2020;66(5):644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W, Ni Z, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naess A, Nilssen SS, Mo R, et al. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017;45(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozbay M, Ugur M, Uyarel H, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in infective endocarditis: in-hospital and long-term clinical results. J Heart Valve Dis. 2014;23(5):617–623. [PubMed] [Google Scholar]
- 12.Fischbach F. A manual of laboratory and diagnostic tests. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 13.WHO: clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected . https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 14.Wang JT, Sheng WH, Fang CT, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10(5):818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PK, Chen GG.. Mechanisms of lymphocyte loss in SARS coronavirus infection. Hong Kong Med J. 2008;14(Suppl 4):21–26. [PubMed] [Google Scholar]
- 17.Durmus E, Kivrak T, Gerin F, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105(6):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolan RD, Lim J, McSorley ST, et al. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7(10):e46561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fearon DT, Locksley RM.. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50–53. [DOI] [PubMed] [Google Scholar]
- 21.Chang HL, Chen KT, Lai SK, et al. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105(6):439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]