Abstract
Identification of the underlying defects in congenital erythrocytosis has provided mechanistic insights into the regulation of erythropoiesis and oxygen homeostasis. The Hypoxia Inducible Factor (HIF) pathway plays a key role in this regard. In this pathway, an enzyme, Prolyl Hydroxylase Domain 2 (PHD2), constitutively prolyl hydroxylates HIF-2α, thereby targeting HIF-2α for degradation by the von Hippel Lindau (VHL) tumor suppressor protein. Under hypoxia, this modification is attenuated, resulting in the stabilization of HIF-2α and transcriptional activation of the erythropoietin (EPO) gene. Circulating EPO then binds to the EPO receptor (EPOR) on red cell progenitors in the bone marrow, leading to expansion of red cell mass. Loss of function mutations in PHD2 and VHL, as well as gain of function mutations in HIF-2α and EPOR, are well established causes of erythrocytosis. Here, we highlight recent developments that show that the study of this condition is still evolving. Specifically, novel mutations have been identified that either change amino acids in the zinc finger domain of PHD2 or alter splicing of the VHL gene. In addition, continued study of HIF-2α mutations has revealed a distinctive genotype-phenotype correlation. Finally, novel mutations have recently been identified in the EPO gene itself. Thus, the cascade of genes that at a molecular level leads to EPO action, namely PHD2 --> HIF2A --> VHL --> EPO --> EPOR, are all mutational targets in congenital erythrocytosis.
Keywords: Erythrocytosis, Erythropoiesis, Erythropoietin, Hypoxia Inducible Factor, Oxygen sensing, Polycythemia, Prolyl Hydroxylase Domain protein, von Hippel Lindau tumor suppressor protein, Zinc finger
1. Introduction
Red cell mass is regulated by the classical negative feedback loop in which tissue oxygenation in the kidney controls erythropoietin (EPO) production, and circulating EPO then binds to the EPO receptor (EPOR) on red cell progenitors in the bone marrow (Fig. 1). EPO triggers expansion of red cell mass, thereby improving tissue oxygenation. Within the kidney, specialized interstitial cells are the source of EPO [1–3]. These cells employ the Hypoxia Inducible Factor (HIF) pathway, a pathway that is otherwise present in essentially all cells of the body, to regulate EPO production in an oxygen-sensitive manner (Fig. 1). In the HIF pathway, in the presence of oxygen, Prolyl Hydroxylase Domain protein 2 (PHD2, also known as EGLN1) site-specifically hydroxylates the labile α sub-unit of the heterodimeric transcription HIF-2 (HIF-2α), targeting it for degradation by the von Hippel-Lindau tumor suppressor protein (VHL) [4–6]. VHL is the substrate recognition component of an E3 ubiquitin ligase complex that also includes elongin B, elongin C, cullin-2, and rbx1. Under hypoxia this modification is attenuated, HIF-2α is stabilized, binds to its stable partner ARNT (also known as HIF-β), and mediates the transcriptional activation of the EPO gene by binding to its 5’ hypoxia response element (HRE) [7–9].
Fig. 1. The feedback loop that controls red blood cell production.
The oxygen sensing mechanism which regulates EPO production in the kidney is illustrated in the top left panel. In normoxic conditions, PHD2 hydroxylates one or both of a pair of highly conserved prolines in HIF-2α in an oxygen-dependent manner, using 2-oxoglutarate and ascorbate as co-factors. This hydroxylation enables specific binding by a ubiquitin ligase complex containing VHL, elongin B (B) and elongin C (C) which leads to ubiquitination and subsequent proteasomal degradation (for simplicity, two other components of the complex, cullin-2 and rbx1, are omitted). Under hypoxia the hydroxylation of HIF-2α is attenuated, permitting it to bind to the HRE in 5’ region of the EPO gene thereby enabling enhanced EPO transcription. EPO enters the circulation and binds to EPO receptors on erythroid progenitor cells (primarily Colony Forming Unit-Erythroid cells) inducing the proliferation and differentiation of erythroid cells (top right panel). The circulating red blood cell (RBC) mass is therefore dependent on the degree of hypoxia sensed by PHD2 in the kidney, and reflects the dynamic balance between EPO-induced red cell production and subsequent loss or destruction of mature red blood cells.
Erythrocytosis is defined as an increase in red cell mass. This has traditionally required isotopic methods to determine red cell mass. In the absence of the availability of this test, hemoglobin and hematocrit levels are often employed. As one point of reference, in the 2008 WHO classification of polycythemia vera, hemoglobin levels of > 18.5 g/dl in men and > 16.5 g/dl in women were considered thresholds [10]. However, the more recent 2016 WHO classification of polycythemia vera lowered these thresholds [11], and issues surrounding the use of these values in the evaluation of erythrocytosis have been discussed [12].
Erythrocytosis has many causes that fall broadly into two groups: primary erythrocytoses, due to intrinsic defects in red cell progenitors and secondary erythrocytoses driven by EPO production. Primary erythrocytosis can be due to defects in the JAK2 or EPOR genes. Secondary erythrocytoses can be due to acquired conditions, such as hypoxia associated with chronic obstructive pulmonary disease, right-to-left cardiac shunts, exposure to high altitude, or EPO-producing tumors. Secondary erythrocytosis can also be congenital. These causes include mutations in genes that affect hemoglobin function, such as those encoding for hemoglobin α (HBA), hemoglobin β (HBB), or 2,3-bisphosphoglycerate mutase (BPGM), which synthesizes BPG, a metabolite that decreases hemoglobin affinity for oxygen. The end result of these mutations is increased hemoglobin affinity for oxygen. Another important category of congenital erythrocytosis are those affecting the HIF pathway or the EPO gene itself, which is the focus of this review. Primary erythrocytoses are typically associated with low EPO levels while secondary erythrocytoses are often accompanied by elevated EPO levels. However, it should be recognized that certain forms of secondary erythrocytosis, such as that due to PHD2 mutations, can be associated with normal EPO levels. An interpretation here is that an increased red cell mass should ordinarily lead to low EPO levels; hence, normal EPO levels in this setting are “inappropriately” normal. The evaluation of patients for erythrocytosis involves tests that include serum EPO measurements, imaging studies, oximetry, P50 measurements, and sequencing of candidate genes. Further details of the evaluation and clinical management of erythrocytosis have been the subject of recent reviews, and will not be discussed further [12–16].
The study of erythrocytosis has informed our understanding of the HIF: EPO pathway. In pioneering work, de la Chapelle and colleagues carried out linkage analysis in a large family with known clinical and genealogical features, using a highly informative simple sequence repeat polymorphism in the 5’ region of the EPOR gene [17]. They found a highly significant linkage pointing to a mutation in the EPOR as the most probable cause of the disease phenotype in the family. Subsequently they described a heterozygous G to A transition in nucleotide 6002 of the EPOR gene in affected family members, predicting the truncation of the EPOR molecule, and loss of down-modulation normally exerted in this region of intact EPOR molecules [18]. Other EPOR mutations have recently been reviewed elsewhere [19]. This was the first disorder in humans to be ascribed to a mutation in either EPO or EPOR, and provided an impetus to uncover mutations in other genes that might be involved in erythrocytosis. Investigators turned their attention to the metazoan HIF oxygen-sensing pathway, leading to efforts to identify the specific isoforms of HIF and PHD involved in erythropoiesis.
2. Erythrocytosis caused by mutations in the oxygen sensing pathway
Erythrocytosis arising in sporadic patients and families with mutations in the genes encoding VHL, PHD2 and HIF-2α has provided important insights into their role in the hypoxia sensing pathway and as regulators of red cell mass in humans. The first erythrocytosis-associated mutation in the pathway was identified in the VHL gene [20] followed by a mutation in the PHD2 gene [21] and subsequently by a mutation of the HIF2A (also known as EPAS1) gene in a family with hereditary erythrocytosis [22].
3. Erythrocytosis caused by mutations in the PHD2 gene
The dioxygenase PHD2, encoded by the PHD2 gene acts as an oxygen sensor, and in normoxic conditions hydroxylates one or both of a pair of highly conserved prolines in the labile α subunit of HIF (Fig. 2A), using 2-oxoglutarate and ascorbate as co-factors. PHD2 is one of three paralogues in mammals. Homozygous knockout of the Phd2 gene in mice leads to embryonic lethality [23]. In contrast, mice with homozygous knockout of the Phd1 or Phd3 genes are viable. The former display improved hypoxia tolerance in skeletal muscle [24]; the latter display systemic hypotension [25]. Prolyl hydroxylation of HIF-α enables specific binding by VHL, leading to ubiquitination and subsequent proteasomal degradation. HIF-α dimerizes with ARNT and mediates a complex array of gene transcriptional responses to hypoxia in mammals through its three isoforms (HIF-1α, HIF-2α and HIF-3α).
Fig. 2. The PHD2-HIF-VHL-EPO axis and its dysregulation.
(A) Scheme depicting the distinctive oxygen-sensing mechanism that regulates EPO transcription. In the presence of oxygen, PHD2 site-specifically hydroxylates HIF-2α, thereby targeting it for degradation by VHL. Little HIF-2α remains to bind the HRE of the EPO gene, so activation is modest. ARNT is the stable subunit of HIF-2.
(B) Loss-of-function mutations in PHD2, denoted by PHD2*, cause an increase in HIF-2α and increased (or inappropriately normal) EPO gene activation, designated erythrocytosis type 3 (ECYT3) in the OMIM classification.
(C) In gain-of-function mutations in HIF-2α, denoted by HIF-2α*, hydroxylation is deceased and VHL binding is reduced, leading to increased EPO gene activation, ECYT4.
(D) Loss-of-function mutations in VHL, denoted by VHL*, reduce binding to hydroxylated HIF-2α leading to increased EPO gene activation, ECYT2.
(E) A mutation in EPO, denoted by EPO* causes a frameshift that initiates excess production of EPO from a normally noncoding EPO mRNA, ECYT5.
When the search for defects in components of the oxygen-sensing pathway that might account for erythrocytosis focused on the three PHD paralogues, a previously unrecognized cause of the condition was established. A mutation in the gene PHD2 in a family with erythrocytosis resulted in a marked decrease in PHD2 enzyme activity and segregated with phenotype [21]. Sequencing of DNA obtained from the affected family members (father and two children) revealed a heterozygous C to G change at base 950 of the coding sequence of PHD2, resulting in a predicted change of the evolutionarily preserved residue proline to arginine at codon 317 (P317R). This is in close proximity to two of the three residues in PHD2 responsible for coordinating the ferrous atom at the active site. The change was not detected in the hematologically normal mother, or in 200 normal control samples. Peptide binding, enzymatic activity and HRE reporter gene studies pointed to a marked loss of PHD2 function arising from the P317R mutation (Fig. 2B). Thus, a critical role emerged for PHD2 in the regulation of erythrocytosis. Familial erythrocytosis caused by mutations in the PHD2 gene has been designated as ECYT3 in the Mendelian Inheritance in Man classification (MIM) #: 609820.
Subsequently, it was found that mice with acute global deletion of Phd2, but not Phd1 nor Phd3 display a dramatic erythrocytosis [26, 27]. A mouse model for the P317R PHD2 mutation (P294R in the mouse) recapitulated the erythrocytosis and was consistent with haploinsufficiency as the mechanism for this particular mutation [28]. Additional studies functionally characterized a group of heterozygous loss-of-function mutations located within or near the prolyl hydroxylase domain (Fig. 3A) [21] [29–32]. These studies have also revealed interesting aspects of the original P317R PHD2 mutant, namely, that it displays no activity towards the N-terminal oxygen dependent degradation domain (which contains one of the two sites of hydroxylation, Pro-402) of HIF-1α [32], and that its activity towards the C-terminal oxygen dependent degradation domain (which contains the other site of hydroxylation, Pro-564) is substantially weaker with respect to HIF-2α as compared to HIF-1α [30]. Over twenty erythrocytosis-associated PHD2 mutations, which comprise heterozygous missense, nonsense, and frameshift mutations, have been reported [33–41]. An important clinical implication of these studies is that loss of PHD2 activity in humans can lead to erythrocytosis. Accordingly, small molecule inhibitors of the catalytic domain of PHD2 are currently in clinical trials for the treatment of anemia [42, 43].
Fig. 3. Loss of function mutations in PHD2 cause ECYT3.
(A) Diagram of PHD2 depicting the location of the zinc finger and prolyl hydroxylase domains. The sequence of the zinc finger domain across various metazoan species is shown with the areas shaded in gray denoting the zinc-binding residues. The recently reported mutations Y41C and C42R mutations cause erythrocytosis [44]. Earlier erythrocytosis-associated mutations in PHD2 reside within or near the prolyl hydroxylase domain and comprise missense (circles), nonsense (squares), and frameshift (triangles) mutations. Numbers at top and bottom indicate residue number.
(B) Model showing recruitment of PHD2 to the HSP90 complex by interaction of the PHD2 zinc finger (ZF) with a PXLE motif in p23, an HSP90 cochaperone that interacts directly with HSP90. This facilitates hydroxylation of HIF-α, a client protein of the HSP90 complex.
3.1. Zinc finger PHD2 mutations
PHD2 is unique among the PHDs because it contains a MYND-type zinc finger (Fig. 3A). The importance of the zinc finger is of substantial interest given its strong evolutionary conservation. Recently Sinnema and colleagues [44] reported a PHD2 zinc finger mutation associated with congenital erythrocytosis in a 14 year old daughter of consanguineous parents. Whole exome sequencing using the parent-offspring trio approach revealed a homozygous missense variant in the PHD2 gene; EGLN1; Chr1 (GRCh37):g.231557511A>G; NM_022051.2: c.124T>C (p.Cys42Arg). Both parents were heterozygous for the variant, and the brother of the index case was found to be homozygous for the variant.
The MYND-type zinc finger of PHD2 binds to a Pro-Xaa-Leu-Glu motif present in components of the HSP90 pathway, such as p23 and FKBP38 (Fig. 3B). Cys-42, the residue affected by the mutation lies within the zinc finger, and is 1 of 8 evolutionarily conserved zinc-chelating residues. HIF-α is a client protein of the HSP90 pathway [45, 46] and it has been proposed that the zinc finger allows recruitment of PHD2 to the HSP90 pathway to facilitate hydroxylation of HIF-α [47].
The functional effect of the C42R PHD2 mutation on binding was examined by coexpressing wild type or C42R PHD2 (residues 1–196) with p23 in HEK293FT cells [44]. The wild-type PHD2 was found to coimmunoprecipitate with p23 but this interaction was abolished by the C42R mutation. An in vitro assay for PHD2 zinc finger function in which the PHD2 zinc finger was fused to a heterologous protein (the birA biotin ligase from E. coli) provided evidence that the zinc finger could promote recruitment to HIF-α. In HRE reporter gene assays the mutant C42R PHD2 was significantly less effective than wild-type PHD2 in suppressing hypoxia-induced HRE reporter activity gene activity. Interestingly, additional erythrocytosis-associated mutations in the zinc finger domain of PHD2 were recently reported as variants of unknown significance, but not functionally characterized [40]. Subsequent examination of one of these, Y41C, which changes a highly conserved residue was also found to abrogate PHD2 binding to p23 and was defective in its capacity to down-regulate hypoxia-induced HRE activity [44].
The genetic and functional studies are consistent with the proposition that the homozygous C42R mutation in PHD2 is the cause of the erythrocytosis in the 2 affected siblings. Characterization of knock-in mice bearing a C36S/C42S double mutation in the Phd2 allele that targets two of the predicted zinc chelating residues showed that the mice homozygous for the C36S/C42S mutation, but not heterozygous, display erythrocytosis [48]. The mouse Cys42 mutation affects the same residue as the human Cys42 mutation. The observation that 2 mutant alleles are required to produce erythrocytosis could account for its relative rarity as a cause of erythrocytosis, compared to defects affecting the catalytic site of PHD2 which are sufficient to produce a phenotype in the heterozygous state. Interestingly, the Y41C mutation is heterozygous, suggesting either that a single copy of certain zinc finger mutations is sufficient to cause the phenotype, or alternatively, that there is an as yet uncharacterized mutation in the HIF or other pathways that impact EPO production in the patient. The phenotype of the zinc finger mutations provides genetic evidence that the zinc finger has a positive regulatory function in the oxygen-sensing pathway that controls erythropoiesis.
3.2. PHD2 mutations in cancer
As with HIF2A and VHL (see below), mutations in PHD2 can be associated not only with erythrocytosis, but also with cancer. Specifically, Ladroue et al. reported on a patient with erythrocytosis and paraganglioma [49]. Sequencing of germline DNA revealed a heterozygous H374R mutation in the PHD2 gene, while sequencing of the tumor and other studies revealed the presence of the mutant allele and loss of the wild type allele (loss of heterozygosity). A P317S mutation in PHD2 that impairs activity has been identified in a melanoma tumor sample [50]. While it is difficult to draw genotype-phenotype correlations from the limited available data, it is worth noting that the H374R mutation affects an essential iron chelating residue, which in combination with loss of heterozygosity would be predicted to lead to essentially complete loss of PHD2 activity in the tumor.
4. Erythrocytosis caused by mutations in the HIF2A gene
The continuing search for additional defects in the oxygen-sensing pathway also focused on the two main HIF-α isoforms, HIF-1α and HIF-2α. Studies employing cell culture and mouse models have clearly shown that the two isoforms are not redundant. HIF-1α, for example, is the isoform responsible for the transcriptional upregulation of genes encoding for glycolytic enzymes [51]. Mice with homozygous Hif-1α deletion were found to die in utero from defective neurodevelopment and cardiovascular malformations [52, 53], whereas mice lacking both copies of Hif-2α died in utero or around birth from severe vascular defects, disturbed catecholamine homeostasis, and abnormal lung maturation [54–56]. Multiple organ pathology and mitochondrial dysfunction were noted in Hif-2α knockout mice from a mixed genetic background which survived to adulthood [57]. Deletion of Hif-2α caused pancytopenia and hypocellular bone marrow [58]. The resulting anemia was later found not to be caused by a cell-autonomous defect in erythroid precursor maturation, but actually by inadequate renal EPO production, demonstrating that Hif-2α is required for systemic EPO homeostasis in adult mice [59]. Erythrocytosis induced by conditional liver knockout of Vhl is rescued by concurrent deletion of the Hif2a, but not the Hif1a, gene, demonstrating that hepatic regulation of Epo is dependent on Hif-2α [60]. The observation that acute postnatal global ablation of Hif-2α, but not Hif-1α, results in anemia convincingly shows that Hif-2α is the main regulator of EPO synthesis in the adult mouse [61]. On the other hand, it was found that Hif-1α deficiency leads to impaired erythropoiesis during embryogenesis [62].
These findings set the stage for identifying the genes responsible for erythrocytosis in humans by studies of specific mutations in patients with inherited forms of secondary erythrocytosis. A study of the HIF2A gene in 3 generations of one family with erythrocytosis uncovered a novel G537W mutation located close to the hydroxylacceptor P531[22]. Functional characterization of the erythrocytosis-associated G537W HIF-2α mutant showed that a mutant G537W HIF-2α peptide binds less tightly to PHD2 and is less efficiently hydroxylated by PHD2 than wild-type peptide. Additional studies showed that the mutation also affected subsequent recognition of HIF-2α by VHL (Fig. 2C). A mouse model for the G537W mutation (G536W in the mouse) demonstrated erythrocytosis in mice heterozygous for the mutation that was accentuated in mice homozygous for it [63].
In vitro functional assays of three further erythrocytosis-associated mutations of HIF-2α located C-terminal to the highly conserved LXXLAP motif that contains the hyroxylacceptor proline, viz. M535V, P534L and G537R, revealed that all three mutants stabilized HIF-2α, but did so in nonidentical ways; P534L and G537R impaired binding to both PHD2 and VHL, but M535V impaired binding only to PHD2. This suggests that the M535V mutation is pathogenic mainly through effects on PHD2 binding and catalytic hydroxylation, and that impairment of the PHD2/HIF2 interaction alone is sufficient to induce erythrocytosis. This accords with the finding that mutations in PHD2 alone are a distinct cause of erythrocytosis [21] [29] [35] [64] [65]. The existence of erythrocytosis-associated VHL mutations confirms that impairment of the HIF-α/VHL interaction alone is sufficient to induce erythrocytosis.
Decreased degradation leads to stabilization of HIF-2α, a gain-of-function phenotype, and it argues against the possibility that a dominant negative mechanism involving increased mutant binding to PHD2 leading to impaired hydroxylation of wild type HIF-2α, or increased mutant binding to VHL, could be responsible for the erythrocytosis. To date no mutations in the HIF1A gene have been found to be associated with erythrocytosis in humans.
4.1. HIF2A gain-of-function mutations in erythrocytosis and neuroendocrine tumors
Familial erythrocytosis caused by a gain-of-function mutation in the HIF2A gene is an autosomal dominant disorder, and has been designated as ECYT4 in the MIM classification #: 611783. Somatic HIF-2α gain-of-function mutations were subsequently established as a cause of neuroendocrine tumors [66, 67]. Additional HIF-2α mutations associated with erythrocytosis, neuroendocrine tumors, or both have been reported [68–79]. In some cases, HIF2A somatic mosaicism was observed.
Recently Tarade and colleagues [80] examined 66 cases of HIF-2α-driven disease reported in the literature by January 1, 2018, and proposed four classes of disease: patients with pheochromocytoma and paraganglioma (PPGL) accompanied by somatostatinoma and erythrocytosis (class 1a); patients with PPGL and erythrocytosis (class 1b); patients with PPGL alone (class 1c); and patients with erythrocytosis alone (class 2), also known as ECYT4. The location of the HIF-2α mutations is shown in Fig. 4.
Fig. 4. Gain of function mutations in HIF-2α cause ECYT4 and neuroendocrine tumors.
Diagram of HIF-2α depicting the location of the oxygen dependent degradation domain (ODDD), as well as the basic helix-loop-helix (bHLH), Per-Arnt-Sim A (PAS-A), PAS-B, and C-terminal activation domain (CAD) domains. Patients who presented with PPGL combined with somatostatinoma and erythrocytosis, PPGL with erythrocytosis, PPGL only, and erythrocytosis only are shown together with the frequency of these disorders found caused by each mutation reported [80] denoted by colored circles. All HIF-2α mutations reported so far in the context of erythrocytosis are heterozygous and the majority are missense mutations. The underlined red “P” indicates Pro-531, the primary site of hydroxylation in HIF-2α. Numbers at bottom indicate residue number.
HIF-2α gain-of-function mutations in class 1a-c and ECYT4 share a common feature, an increase in HIF-2α stability, but differ in the location of the mutations. Overall, the majority of HIF-2α mutations are missense mutations located between amino acids 519 and 545. Whereas all of the ECYT4 mutations described so far lie between amino acids 533 and 540, most of the class 1a-c mutations lie between 529 and 532 (containing proline 531, the primary hydroxylation site of HIF-2α ) and another small group between 539 and 544. In agreement, Pang et al. [81] have recently reported three somatic HIF-2α mutations (p.A530V, p.P531S, and p.D539N) identified in DNA from paragangliomas (PGLs) of three patients.
To gain mechanistic insights into the phenotype-genotype relationship of HIF-2α -driven disease Tarade et al. purified the VHL-elongin B-elongin C trimeric complex and obtained a three-dimensional structure of the complex with HIF-2α (523–541) by X-ray crystallography. The majority of contacts between HIF-2α and VHL occur at the N-terminal contact area of the HIF-2α peptide through hydrogen bond and van der Waals interactions, with additional contacts being made with C-terminal residues. Class 1 mutations affect residues that make contact with VHL, whereas the majority of the ECYT4 mutations are localized to the kink region between the N-terminal and C-terminal segments. Based on temperature factor analysis residues N-terminal to the kink region (as defined by Tarade and colleagues [80], in their Figure 3) have a lower temperature factor and bind in a more rigid manner, raising the possibility that class 1 mutations would have a greater negative impact on the VHL-HIF-2α interaction than ECYT4 mutations. This was confirmed by steady-state and kinetic binding experiments which showed that class 1 mutations predominantly have a more deleterious effect on VHL affinity than ECYT4 mutations.
Overall these findings suggest that the relationship between genotype and phenotype in HIF-2α-driven disease is affected by the degree of disruption of the HIF-2α-VHL interface stability and perhaps also by the degree of disruption of the HIF-2α-PHD2 interaction. Small increases in HIF-2α caused by mild disruption are sufficient to elicit erythrocytosis, but more serious disruption, leading to higher levels of HIF-2α, can be pathogenic for neuroendocrine tumors.
5. Erythrocytosis caused by mutations in the VHL gene
The VHL tumor suppressor gene encodes a multifunctional protein that promotes degradation of HIF transcription factors by binding specifically only to the prolyl hydroxylated form of HIF-α. VHL disease is an autosomal dominant disorder with high penetrance which predisposes patients to highly vascularized malignant tumors, including clear cell renal cell carcinoma, hemangioblastoma, and pheochromocytoma/paraganglioma. VHL mutations are also associated with benign tumors [82]. The role of VHL in cancer has been recently reviewed [83, 84].
A group of homozygous VHL gene mutations cause erythrocytosis that is not associated with any malignant or benign VHL syndrome. The most celebrated of these, was discovered in citizens of Chuvashia where it is endemic, and was the first recognized congenital disorder of hypoxia-sensing [20] (Fig. 2D). Chuvash erythrocytosis is an autosomal recessive condition which results from heterozygosity for a C to T missense mutation of VHL (VHL c.598C>T, VHLR200W). Interactions between the mutated protein and HIF-1α or HIF-2α are impaired leading to increased expression of their target genes including EPO and vascular endothelial growth factor (VEGF). Chuvash erythrocytosis is associated with a high risk of both arterial and venous thrombosis in patients at sea level. In the homozygous form it augments the hypoxic response leading to elevated hematocrit. The variant VHLR200W is not associated with tumors, in contrast to patients with VHL tumor predisposition syndrome [85].
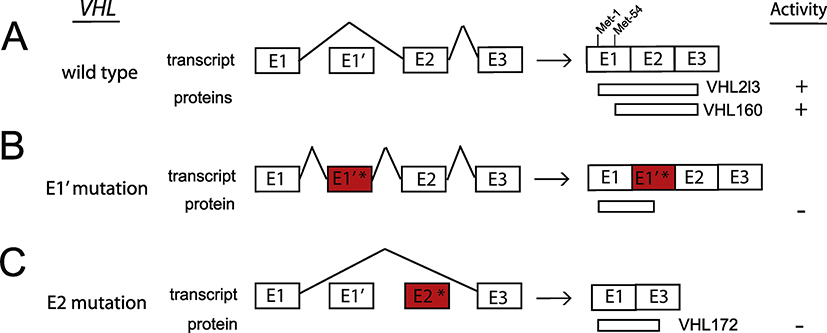
Two other homozygous VHL mutations, H191D and P138L, and several compound heterozygotes--three in combination with the R200W mutation, have been reported to cause congenital erythrocytosis but not cancer [86–91]. VHL contains 3 canonical exons (Fig. 5A) which encode a 213 amino acid (aa) protein, VHL213, and a shorter isoform, VHL160, which is initiated from an in-frame internal translation start site at Met-54 [92]. A naturally occurring splice variant formed from direct splicing of E1 and E3 is translated into VHL172 (VHLXE2). Both VHL213 and VHL160 are involved in the regulation of the oxygen sensing pathway and mutations would appear to have the potential to lead to the development of erythrocytosis or tumors. That being stated, there are individuals with homozygous M54I mutations that abolishes the internal translation start site (and hence the production of VHL160) who display erythrocytosis, extremely high EPO levels, but no tumors, suggesting that VHL213 and VHL160 do not have identical functions [93].
Fig. 5. Loss of function mutations in VHL cause ECYT2 and can be due to aberrant splicing.
(A) Wild type VHL contains 3 canonical exons (E1, E2, E3) that encode the functionally active 213 aa protein, VHL213 (also known as p30). VHL E1 contains an internal translation initiation codon, Met-54, that causes the production of a truncated form, VHL160 (also known as p19). Lenglet and colleagues (2018) reported a new spliced isoform that contains a cryptic exon E1’. Exons are not drawn to scale.
(B) Mutations in E1’ (denoted by E1’*) result in inclusion of E1’. The isoform containing E1 spliced with E1’ may theoretically encode a protein of 193 aa, of which 114 aa are encoded by E1, and 79 aa by E1’ and is functionally inactive.
(C) Mutations in E2 (denoted by E2*) result in splicing of E1 to E3. The isoform containing E1 spliced with E3 encodes VHL172 which lacks E2 and is inactive.
5.1. Splicing mutations in the VHL gene
Particular mutations of VHL are generally linked to either erythrocytosis or the development of tumors. The mechanisms that govern the phenotype-genotype relationship of VHL-driven disease have been characterized for some [94, 95] but not all mutations, so it is of interest that Lenglet and colleagues [96] have recently identified a novel cryptic exon in intron 1 of the VHL gene (E1’) and synonymous mutations in VHL exon 2 that affected splicing in both congenital erythrocytosis and typical VHL disease.
They investigated a patient with erythrocytosis with a known synonymous VHL c.429C>T p.Asp143Asp (D143D) mutation in the heterozygous state, and no other mutations in the 3 VHL canonical exons. RT-PCR, using primers in E1 and E3 showed a strong decrease in the E1E2E3 isoform, and some minor extra fragments of larger size in the patient’s and mother’s samples. Sequencing of these fragments revealed new VHL transcripts containing intronic sequences. This intronic sequence, termed the E1’ cryptic exon was spliced to E1 at its 5’ end and to either E2 or E3 at its 3’ end (Fig. 5B). Theoretically its translation may lead to a protein of 193 aa containing the first 114 aa encoded by E1, plus 79 aa of unknown function encoded by E1’. Sequencing of the new cryptic exon identified a previously unreported variant c.340+770T>C.
Investigation of six additional families with hereditary erythrocytosis revealed the presence of more E1’ mutations. In five of the families, the affected family members were compound heterozygotes in which one VHL allele contained the E1’ mutation while the other VHL allele contained a coding sequence mutation. In the sixth family, the affected individual was homozygous for the E1’ mutation.
E1’ mutations were also detected in typical VHL disease lacking alteration in the canonical VHL coding sequence exons. Sequencing of the new E1’ cryptic exon in a large family with hereditary hemangioblastoma, clear cell renal cell carcinoma and pheochromocytoma identified 2 heterozygous variants, a previously unknown variant, c.340+617C>G and a c.340+648T>C variant originally reported as a rare polymorphism. These variants cosegregated in 6 patients who developed VHL disease, but were absent in the four healthy descendants tested, indicating their presence on a single disease-associated allele. All of the new E1’ mutations caused complex dysregulation of VHL splicing with excessive retention of E1’, and significantly, were associated with downregulation of VHL protein expression.
In addition, they demonstrated a pathogenic role for synonymous mutations in VHL Exon 2 that altered splicing through E2-skipping in a group of 5 families with erythrocytosis or VHL disease (Fig. 5C). The complex regulation of VHL splicing may contribute to the elucidation of genotype/phenotype relationships, in VHL-related syndromes. In particular, the synonymous variants D143D and P138P should be considered as pathogenic mutations. The evidence suggests that a specific region in E2 acts as a splicing regulatory domain. Depending on the mutation in this region, the impact could be moderate (D143D, G144R and P138L) or severe (P138P) correlating with the severity of the disease, vis-à-vis congenital erythrocytosis and cancer. Insufficient VHL levels rather than reduced HIF binding may lead to an impairment of HIF degradation. Mutations identified in E1’ in association with erythropoiesis had less severe impact on splicing than the mutations associated with cancer, confirming that erythrocytosis was associated with VHL hypomorphic mutations.
6. Erythrocytosis caused by mutations in the EPO gene
Recently Zmajkovic and colleagues [97] identified a mutation in the EPO gene that cosegregated with the disorder in a large family with autosomal dominant erythrocytosis (Fig. 2E). Genomewide linkage analysis identified a cosegregating region on chromosome 7q22.1 with a LOD score of 3.3, and targeted sequencing of the constituent 215 genes revealed a heterozygous single base deletion in exon 2 of EPO located on chromosome 7: 100,319,199 GG→G as the only candidate gene mutation. This c.32delG mutation, denoted ΔG, was present in all of the affected family members. The deletion causes a frameshift that truncates the EPO signal peptide and generates a novel peptide which is terminated after an additional 51 amino acids (see Fig. 6). This would be predicted to produce a loss of EPO function, which was unexpected, and inconsistent with an erythrocytosis phenotype.
Fig. 6. Gain of function mutation in EPO cause ECYT5 and are due to a single nucleotide deletion in Exon 2.
(A) Wild type EPO mRNA, transcribed from promoter P1, contains 5 exons (E1-E5) that encode a protein of 194 aa consisting of a 27 aa signal peptide and the functionally active 167 aa mature protein. The sequence of the first 14 amino acids of the signal peptide is shown. An alternative promoter located in intron 1, P2, produces a transcript that contains 4 exons which does not make functional EPO. Single underlined green AUG = AUG1, double underlined blue AUG = AUG2. Exons are not drawn to scale.
(B) Zmajkovic et al (2018) [97] found that a single-nucleotide deletion introduces a frameshift in E2 (denoted by E2*) that interrupts translation of the normal EPO mRNA transcribed from P1 (producing a short non-functional peptide terminating in exon 3), but instead initiates excess functionally active EPO from the normally noncoding transcript from the alternative P2 promoter utilizing AUG2. The location of the G that is deleted in Exon 2 is shown in red and by triangle in panel A and the sequence of the first 9 amino acids is shown. In essence, the P2 transcripts produce polypeptides arising from the alternative translation initiation site in Exon 2 that are now in the proper reading frame with respect to the remainder of the EPO coding sequence and are responsible for the overproduction of EPO leading to erythrocytosis. The signal peptide encoded by the E2 mutant is 22 aa, 5 aa shorter than wild type EPO signal peptide.
To investigate how ΔG, an apparent loss-of-function variant of EPO, produces EPO the authors searched for alternative EPO mRNAs. In addition to the expected transcripts derived from the canonical promoter P1 upstream of exon 1 they discovered unexpected EPO mRNA transcripts which originate from an alternative promoter (P2) in intron 1 (Fig. 6A). Interestingly both transcripts were detected in mRNA from normal kidney and liver.
To determine which transcripts are capable of producing EPO cDNAs corresponding to P1 or P2, with or without the ΔG mutation, the cDNAs were transfected into HEK293 cells, and EPO was measured in the culture supernatants. Cells transfected with ΔG P2 produced more EPO than those transfected with wild-type P1 cDNA and the EPO was biologically active. Overall the results indicate that the EPO ΔG mutation terminates translation of EPO P1 transcripts prematurely, but paradoxically alters the normally non-coding P2 transcripts so that AUG2, which ordinarily is out of frame with respect to the canonical EPO reading frame, initiates translation of the EPO coding sequence due to the frameshift, and produces an excess of biologically active EPO. A comparison of translated protein in this situation with that produced under normal circumstances, as well as the proposed mechanism for EPO overproduction by the ΔG single-nucleotide deletion in Exon 2 of EPO is depicted in Fig. 6B.
Further evidence to support this interpretation was derived from a recent report of a father and daughter with a single C nucleotide deletion (ΔC) of unknown significance located in EPO exon 2 just 13 nucleotides upstream of the ΔG mutation just described (c.19delC; chromosome 7: 100, 319, 185 TC→T) [39]. To test whether EPO ΔC has the same effect as ΔG Zmajkovic and colleagues transfected HEK293 cells with ΔC P2 cDNA constructs [97]. The P2 transcripts with the ΔC mutation produced high amounts of biologically active EPO, demonstrating that the EPO ΔC causes erythrocytosis by the same mechanism as the EPO ΔG mutation. Familial erythrocytosis caused by mutations in the EPO gene has now been designated as ECYT5 in the MIM classification #: 617907.
7. Conclusions and Future Considerations
Collectively, these studies have genetically defined the core genes of the pathway that regulates red cell mass in an oxygen-sensitive manner in humans. The recent studies have, moreover, provided insight into the fascinating biology of the genes and their gene products. It should also be noted that the majority of congenital cases of erythrocytosis still have no identifiable cause, suggesting that there may be additional genes and/or mechanisms. As one example of this, whole genome sequencing identified a variant in the 5’UTR of the EPO gene (NM_000799.2:c.−136G>A) in two independent families with erythrocytosis that cosegregated with the phenotype [98]. This variant is distinct from the aforementioned coding sequence mutation in the EPO gene, raising the possibility that it may cause erythrocytosis by a different mechanism. It is likely that continuing study of congenital erythrocytosis will provide additional insights into how erythropoiesis is regulated by oxygen.
8. Practice points.
Erythrocytosis can be classified as primary and secondary. Primary erythrocytosis is due to defects in erythroid progenitors. The most common primary erythrocytos is is polycythemia vera. Secondary erythrocytosis is driven by EPO.
Primary erythrocytosis is characteristically associated with low EPO levels while secondary erythrocytosis is typically accompanied by elevated or normal EPO levels.
Secondary erythrocytosis has many different causes. Included among these are mutations in genes of the HIF: EPO pathway, including PHD2, HIF2A, VHL, and EPO.
Genetic lesions in the HIF: EPO pathway should be particularly considered as a cause of erythrocytosis in patients with normal to elevated EPO levels, a family history of erythrocytosis, or in situations in which other secondary causes of erythrocytosis, such as high affinity hemoglobin, EPO-producing tumors, or right to left cardiac shunts, have been ruled out.
9. Research agenda.
Gain further insight into genotype: phenotype correlations in erythrocytosis-associated mutations in genes of the HIF: EPO pathway.
Characterize the biological function of the zinc finger of PHD2.
Understand the role of splicing in VHL function.
Determine the normal role of alternate EPO transcripts.
Identify new mechanisms of erythrocytosis.
Acknowledgments
Work on the HIF pathway in the authors’ laboratories is supported by NIH grants R01-DK104796 (FSL) and R33-HL120751 (FSL and TRL).
Footnotes
Conflict of interest
No conflicts to disclose.
Author Agreement 17–5-19
I agree that the following statement concerning authorship contributions to our manuscript “Update on mutations in the HIF: EPO pathway and their role in erythrocytosis” is correct.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wenger RH, Hoogewijs D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol. 2010;298:F1287–96. [DOI] [PubMed] [Google Scholar]
- [2].Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Suzuki N, Yamamoto M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016;468:3–12. [DOI] [PubMed] [Google Scholar]
- [4].Kaelin WG Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. [DOI] [PubMed] [Google Scholar]
- [5].Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. [DOI] [PubMed] [Google Scholar]
- [7].Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A. 1991;88:8725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Storti F, Santambrogio S, Crowther LM, Otto T, Abreu-Rodriguez I, Kaufmann M, et al. A novel distal upstream hypoxia response element regulating oxygen-dependent erythropoietin gene expression. Haematologica. 2014;99:e45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hirano I, Suzuki N, Yamazaki S, Sekine H, Minegishi N, Shimizu R, et al. Renal Anemia Model Mouse Established by Transgenic Rescue with an Erythropoietin Gene Lacking Kidney-Specific Regulatory Elements. Mol Cell Biol. 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. [DOI] [PubMed] [Google Scholar]
- [11].Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- [12].McMullin MF. Investigation and Management of Erythrocytosis. Curr Hematol Malig Rep. 2016;11:342–7. [DOI] [PubMed] [Google Scholar]
- [13].Bento C, McMullin MF, Percy M, Cario H. Primary Familial and Congenital Polycythemia 2016 Nov 10. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395975/ [PubMed] [Google Scholar]
- [14].Patnaik MM, Tefferi A. The complete evaluation of erythrocytosis: congenital and acquired. Leukemia. 2009;23:834–44. [DOI] [PubMed] [Google Scholar]
- [15].Hong WJ, Gotlib J. Hereditary erythrocytosis, thrombocytosis and neutrophilia. Best Pract Res Clin Haematol. 2014;27:95–106. [DOI] [PubMed] [Google Scholar]
- [16].Oliveira JL. Algorithmic evaluation of hereditary erythrocytosis: Pathways and caveats. Int J Lab Hematol. 2019;41 Suppl 1:89–94. [DOI] [PubMed] [Google Scholar]
- [17].de la Chapelle A, Sistonen P, Lehvaslaiho H, Ikkala E, Juvonen E. Familial erythrocytosis genetically linked to erythropoietin receptor gene. Lancet. 1993;341:82–4. [DOI] [PubMed] [Google Scholar]
- [18].de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993;90:4495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vocanec D, Prijatelj T, Debeljak N, Kunej T. Genetic variants of erythropoietin (EPO) and EPO receptor genes in familial erythrocytosis. Int J Lab Hematol. 2019;41:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–21. [DOI] [PubMed] [Google Scholar]
- [21].Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–80. [DOI] [PubMed] [Google Scholar]
- [25].Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28:3386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG, Jr. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arsenault PR, Pei F, Lee R, Kerestes H, Percy MJ, Keith B, et al. A knock-in mouse model of human PHD2 gene-associated erythrocytosis establishes a haploinsufficiency mechanism. J Biol Chem. 2013;288:33571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110:2193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pappalardi MB, Martin JD, Jiang Y, Burns MC, Zhao H, Ho T, et al. Biochemical characterization of human prolyl hydroxylase domain protein 2 variants associated with erythrocytosis. Biochemistry. 2008;47:11165–7. [DOI] [PubMed] [Google Scholar]
- [31].Ladroue C, Hoogewijs D, Gad S, Carcenac R, Storti F, Barrois M, et al. Distinct deregulation of the hypoxia inducible factor by PHD2 mutants identified in germline DNA of patients with polycythemia. Haematologica. 2012;97:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chowdhury R, Leung IK, Tian YM, Abboud MI, Ge W, Domene C, et al. Structural basis for oxygen degradation domain selectivity of the HIF prolyl hydroxylases. Nat Commun. 2016;7:12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Albiero E, Ruggeri M, Fortuna S, Finotto S, Bernardi M, Madeo D, et al. Isolated erythrocytosis: study of 67 patients and identification of three novel germ-line mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Haematologica. 2012;97:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bento C, Almeida H, Maia TM, Relvas L, Oliveira AC, Rossi C, et al. Molecular study of congenital erythrocytosis in 70 unrelated patients revealed a potential causal mutation in less than half of the cases (Where is/are the missing gene(s)?). Eur J Haematol. 2013;91:361–8. [DOI] [PubMed] [Google Scholar]
- [35].Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008;40:160–5. [DOI] [PubMed] [Google Scholar]
- [36].Jang JH, Seo JY, Jang J, Jung CW, Lee KO, Kim SH, et al. Hereditary gene mutations in Korean patients with isolated erythrocytosis. Ann Hematol. 2014;93:931–5. [DOI] [PubMed] [Google Scholar]
- [37].Bento C, Percy MJ, Gardie B, Maia TM, van Wijk R, Perrotta S, et al. Genetic basis of congenital erythrocytosis: mutation update and online databases. Hum Mutat. 2014;35:15–26. [DOI] [PubMed] [Google Scholar]
- [38].Gardie B, Percy MJ, Hoogewijs D, Chowdhury R, Bento C, Arsenault PR, et al. The role of PHD2 mutations in the pathogenesis of erythrocytosis. Hypoxia (Auckl). 2014;2:71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Camps C, Petousi N, Bento C, Cario H, Copley RR, McMullin MF, et al. Gene panel sequencing improves the diagnostic work-up of patients with idiopathic erythrocytosis and identifies new mutations. Haematologica. 2016;101:1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oliveira JL, Coon LM, Frederick LA, Hein M, Swanson KC, Savedra ME, et al. Genotype-Phenotype Correlation of Hereditary Erythrocytosis Mutations, a single center experience. Am J Hematol. 2018. [DOI] [PubMed] [Google Scholar]
- [41].Barradas J, Rodrigues CD, Ferreira G, Rocha P, Constanco C, Andrade MR, et al. Congenital erythrocytosis - discover of a new mutation in the EGLN1 gene. Clin Case Rep. 2018;6:1109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koury MJ, Haase VH. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hasegawa S, Tanaka T, Nangaku M. Hypoxia-inducible factor stabilizers for treating anemia of chronic kidney disease. Curr Opin Nephrol Hypertens. 2018;27:331–8. [DOI] [PubMed] [Google Scholar]
- [44].Sinnema M, Song D, Guan W, Janssen JWH, van Wijk R, Navalsky BE, et al. Loss-of-function zinc finger mutation in the EGLN1 gene associated with erythrocytosis. Blood. 2018;132:1455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–44. [DOI] [PubMed] [Google Scholar]
- [46].Katschinski DM, Le L, Heinrich D, Wagner KF, Hofer T, Schindler SG, et al. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J Biol Chem. 2002;277:9262–7. [DOI] [PubMed] [Google Scholar]
- [47].Song D, Li LS, Heaton-Johnson KJ, Arsenault PR, Master SR, Lee FS. Prolyl hydroxylase domain protein 2 (PHD2) binds a Pro-Xaa-Leu-Glu motif, linking it to the heat shock protein 90 pathway. J Biol Chem. 2013;288:9662–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Arsenault PR, Song D, Chung YJ, Khurana TS, Lee FS. The Zinc Finger of Prolyl Hydroxylase Domain Protein 2 Is Essential for Efficient Hydroxylation of Hypoxia-Inducible Factor alpha. Mol Cell Biol. 2016;36:2328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–92. [DOI] [PubMed] [Google Scholar]
- [50].Liu S, Zhang G, Guo J, Chen X, Lei J, Ze K, et al. Loss of Phd2 cooperates with BRAF(V600E) to drive melanomagenesis. Nat Commun. 2018;9:5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A. 2000;97:8386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–10. [DOI] [PubMed] [Google Scholar]
- [57].Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–40. [DOI] [PubMed] [Google Scholar]
- [58].Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–40. [DOI] [PubMed] [Google Scholar]
- [59].Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–40. [DOI] [PubMed] [Google Scholar]
- [60].Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, et al. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–11. [DOI] [PubMed] [Google Scholar]
- [63].Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, et al. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J Biol Chem. 2013;288:17134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Percy MJ, Lee FS. Familial erythrocytosis: molecular links to red blood cell control. Haematologica. 2008;93:963–7. [DOI] [PubMed] [Google Scholar]
- [65].Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood Rev. 2008;22:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Darr R, Nambuba J, Del Rivero J, Janssen I, Merino M, Todorovic M, et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer. 2016;23:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gale DP, Harten SK, Reid CD, Tuddenham EG, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood. 2008;112:919–21. [DOI] [PubMed] [Google Scholar]
- [69].Martini M, Teofili L, Cenci T, Giona F, Torti L, Rea M, et al. A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica. 2008;93:1068–71. [DOI] [PubMed] [Google Scholar]
- [70].Percy MJ, Chung YJ, Harrison C, Mercieca J, Hoffbrand AV, Dinardo CL, et al. Two new mutations in the HIF2A gene associated with erythrocytosis. Am J Hematol. 2012;87:439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].van Wijk R, Sutherland S, Van Wesel AC, Huizinga EG, Percy MJ, Bierings M, et al. Erythrocytosis associated with a novel missense mutation in the HIF2A gene. Haematologica. 2010;95:829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Comino-Mendez I, de Cubas AA, Bernal C, Alvarez-Escola C, Sanchez-Malo C, Ramirez-Tortosa CL, et al. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum Mol Genet. 2013;22:2169–76. [DOI] [PubMed] [Google Scholar]
- [73].Toledo RA, Qin Y, Srikantan S, Morales NP, Li Q, Deng Y, et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2013;20:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang C, Sun MG, Matro J, Huynh TT, Rahimpour S, Prchal JT, et al. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood. 2013;121:2563–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Buffet A, Smati S, Mansuy L, Menara M, Lebras M, Heymann MF, et al. Mosaicism in HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2014;99:E369–73. [DOI] [PubMed] [Google Scholar]
- [76].Taieb D, Yang C, Delenne B, Zhuang Z, Barlier A, Sebag F, et al. First report of bilateral pheochromocytoma in the clinical spectrum of HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2013;98:E908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lorenzo FR, Yang C, Ng Tang Fui M, Vankayalapati H, Zhuang Z, Huynh T, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl). 2013;91:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Perrotta S, Stiehl DP, Punzo F, Scianguetta S, Borriello A, Bencivenga D, et al. Congenital erythrocytosis associated with gain-of-function HIF2A gene mutations and erythropoietin levels in the normal range. Haematologica. 2013;98:1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Welander J, Andreasson A, Juhlin CC, Wiseman RW, Backdahl M, Hoog A, et al. Rare germline mutations identified by targeted next-generation sequencing of susceptibility genes in pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2014;99:E1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tarade D, Robinson CM, Lee JE, Ohh M. HIF-2alpha-pVHL complex reveals broad genotype-phenotype correlations in HIF-2alpha-driven disease. Nat Commun. 2018;9:3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pang Y, Gupta G, Jha A, Yue X, Wang H, Huynh TT, et al. Nonmosaic somatic HIF2A mutations associated with late onset polycythemia-paraganglioma syndrome: Newly recognized subclass of polycythemia-paraganglioma syndrome. Cancer. 2019;125:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64. [DOI] [PubMed] [Google Scholar]
- [84].Kaelin WG Jr. The VHL Tumor Suppressor Gene: Insights into Oxygen Sensing and Cancer. Trans Am Clin Climatol Assoc. 2017;128:298–307. [PMC free article] [PubMed] [Google Scholar]
- [85].Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104:653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bento MC, Chang KT, Guan Y, Liu E, Caldas G, Gatti RA, et al. Congenital polycythemia with homozygous and heterozygous mutations of von Hippel-Lindau gene: five new Caucasian patients. Haematologica. 2005;90:128–9. [PubMed] [Google Scholar]
- [87].Bond J, Gale DP, Connor T, Adams S, de Boer J, Gascoyne DM, et al. Dysregulation of the HIF pathway due to VHL mutation causing severe erythrocytosis and pulmonary arterial hypertension. Blood. 2011;117:3699–701. [DOI] [PubMed] [Google Scholar]
- [88].Lanikova L, Lorenzo F, Yang C, Vankayalapati H, Drachtman R, Divoky V, et al. Novel homozygous VHL mutation in exon 2 is associated with congenital polycythemia but not with cancer. Blood. 2013;121:3918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pastore Y, Jedlickova K, Guan Y, Liu E, Fahner J, Hasle H, et al. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am J Hum Genet. 2003;73:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pastore YD, Jelinek J, Ang S, Guan Y, Liu E, Jedlickova K, et al. Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003;101:1591–5. [DOI] [PubMed] [Google Scholar]
- [91].Tomasic NL, Piterkova L, Huff C, Bilic E, Yoon D, Miasnikova GY, et al. The phenotype of polycythemia due to Croatian homozygous VHL (571C>G:H191D) mutation is different from that of Chuvash polycythemia (VHL 598C>T:R200W). Haematologica. 2013;98:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schoenfeld A, Davidowitz EJ, Burk RD. A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci U S A. 1998;95:8817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bartels M, van der Zalm MM, van Oirschot BA, Lee FS, Giles RH, Kruip MJ, et al. Novel Homozygous Mutation of the Internal Translation Initiation Start Site of VHL is Exclusively Associated with Erythrocytosis: Indications for Distinct Functional Roles of von Hippel-Lindau Tumor Suppressor Isoforms. Hum Mutat. 2015;36:1039–42. [DOI] [PubMed] [Google Scholar]
- [94].Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG Jr. von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet. 2001;10:1019–27. [DOI] [PubMed] [Google Scholar]
- [95].Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, et al. Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet. 2001;10:1029–38. [DOI] [PubMed] [Google Scholar]
- [96].Lenglet M, Robriquet F, Schwarz K, Camps C, Couturier A, Hoogewijs D, et al. Identification of a new VHL exon and complex splicing alterations in familial erythrocytosis or von Hippel-Lindau disease. Blood. 2018;132:469–83. [DOI] [PubMed] [Google Scholar]
- [97].Zmajkovic J, Lundberg P, Nienhold R, Torgersen ML, Sundan A, Waage A, et al. A Gain-of-Function Mutation in EPO in Familial Erythrocytosis. N Engl J Med. 2018;378:924–30. [DOI] [PubMed] [Google Scholar]
- [98].Taylor JC, Martin HC, Lise S, Broxholme J, Cazier JB, Rimmer A, et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat Genet. 2015;47:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]