Abstract
Background
Since April 2020, there have been numerous reports of children presenting with systemic inflammation, often in critical condition, and with evidence of recent infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This condition, since defined as the multisystem inflammatory syndrome in children (MIS-C), is assumed to be a delayed immune response to coronavirus disease 2019 (COVID-19), and there are frequently cardiac manifestations of ventricular dysfunction and/or coronary artery dilation.
Methods
We surveyed the inpatient MIS-C management approaches of the members of the International Kawasaki Disease Registry across 38 institutions and 11 countries.
Results
Among the respondents, 56% reported using immunomodulatory treatment for all MIS-C patients, regardless of presentation. Every respondent reported use of intravenous immunoglobulin (IVIG), including 53% administering IVIG in all patients. Steroids were most often used for patients with severe clinical presentation or lack of response to IVIG, and only a minority used steroids in all patients (14%). Acetylsalicylic acid was frequently used among respondents (91%), including anti-inflammatory and/or antiplatelet dosing. Respondents reported use of prophylactic anticoagulation, especially in patients at higher risk for venous thromboembolism, and therapeutic anticoagulation, particularly for patients with giant coronary artery aneurysms.
Conclusions
There is variation in management of MIS-C patients, with suboptimal evidence to assess superiority of the various treatments; evidence-based gaps in knowledge should be addressed through worldwide collaboration to optimize treatment strategies.
Résumé
Contexte
Depuis avril 2020, de nombreux cas d’enfants présentant une inflammation généralisée, se trouvant souvent dans un état critique et montrant des signes d’une infection récente au coronavirus du syndrome respiratoire aigu sévère 2 (SRAS-CoV-2), ont été signalés. On pense que cet état, désigné depuis sous le nom de syndrome inflammatoire multisystémique de l’enfant (SIME), pourrait être une réponse immunitaire tardive au virus de la maladie à coronavirus 2019 (COVID-19); les patients présentent souvent des manifestations cardiaques associées à une dysfonction ventriculaire ou à une dilatation des artères coronaires.
Méthodologie
Nous avons mené un sondage sur les stratégies de prise en charge du SIME en milieu hospitalier auprès des membres du registre international de la maladie de Kawasaki, qui sont rattachés à 38 établissements répartis dans 11 pays.
Résultats
Au total, 56 % des répondants ont déclaré opter pour un traitement immunomodulateur pour tous les patients présentant un SIME, quelles qu’en soient les manifestations. Tous les répondants ont déclaré avoir recours à l’administration d’immunoglobulines par voie intraveineuse, 53 % d’entre eux utilisant ce traitement chez tous les patients. Les stéroïdes étaient plus souvent utilisés chez les patients présentant des symptômes cliniques graves ou ne répondant pas aux immunoglobulines administrées par voie intraveineuse; seule une minorité de répondants ont déclaré utiliser des stéroïdes chez tous les patients (14 %). Les répondants utilisaient aussi fréquemment l’acide acétylsalicylique (91 %), à des doses anti-inflammatoires ou antiplaquettaires. Ils ont en outre déclaré avoir recours à des anticoagulants en prophylaxie, en particulier chez les patients présentant un risque élevé de thromboembolie veineuse, et à une anticoagulothérapie chez les patients présentant des anévrismes coronaires géants.
Conclusions
La prise en charge des patients présentant un SIME varie d’un médecin à l’autre, et les données permettant d’évaluer la supériorité des divers traitements employés sont insuffisantes; il conviendrait donc de mettre en place des initiatives de collaboration afin de combler les lacunes des connaissances et d’optimiser les stratégies thérapeutiques.
In April 2020, the National Health Service in the United Kingdom alerted the medical community of children presenting critically ill with findings similar to Kawasaki disease (KD) or toxic shock syndrome in the setting of recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 This syndrome was later referred to as pediatric multisystem inflammatory syndrome (PIMS) and multisystem inflammatory syndrome in children (MIS-C), with several proposed case definitions (Table 1).2, 3, 4 Since that time, there have been numerous reports of MIS-C patients across Europe and the United States.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29
Table 1.
Case definition of inflammatory syndrome in children
| Royal College of Paediatrics and Child Health, United Kingdom, May 1, 2020 | Child presenting with:
|
| Centers for Disease Control and Prevention, United States, May 14, 2020 | Individual aged < 21 y presenting with:
|
| World Health Organization, May 15, 2020 | Individual aged ≤ 19 y with fever ≥ 3 d AND ≥ 2 of the following:
|
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Patients with MIS-C are most commonly aged 8-11 years, with a slight predominance of boys, and are normally previously healthy.7,24,25,28,29 There is typically a delay in MIS-C presentation after the peak in coronavirus disease 2019 (COVID-19) cases in the local population, by 2-6 weeks, with the current hypothesis that MIS-C is a delayed immune response to a recent SARS-CoV-2 infection rather than direct viral injury.16,21,24,25 Patients most commonly present with fever, gastrointestinal symptoms, and KD-like symptoms, such as rash and conjunctival injection, along with laboratory evidence of significant inflammation. Presentation varies by age, with younger patients presenting more often with KD-like features, and older patients presenting with more myocarditis-like symptoms, including 73% with myocarditis in the cohort aged 13-20 years, compared to 39% in the cohort aged 0-5 years, in a recent large case series.24 Cardiac involvement is common, particularly in those with severe presentations, with markedly elevated brain natriuretic peptide levels and mild–moderate troponin elevation.
There have been numerous descriptive case series over the past few months describing clinical presentations of MIS-C. However, data are scarce regarding specific indications for treatment in MIS-C patients, with variation among series. Thus, we surveyed the members of the International Kawasaki Disease Registry (IKDR) to improve our understanding of treatment practices among a large group of pediatric cardiologists.
Materials and Methods
The IKDR was established in 2013, with the primary objective at inception to study the prevalence of coronary artery aneurysms after KD, along with the clinical and management factors associated with outcomes.30 Current members of the IKDR include mainly pediatric cardiologists from 65 participating hospitals representing Canada and the United States primarily, with additional sites in Taiwan, Chile, Brazil, Argentina, Mexico, Italy, Australia, Israel, and the United Kingdom. Since the onset of the current pandemic and the emergence of MIS-C, the focus of the IKDR has been directed toward characterizing MIS-C, given the important cardiac manifestations noted in children and the similarities to KD.
We performed an online survey to assess management decisions during hospitalizations for MIS-C. The physicians were asked to identify criteria used for administration of different immunomodulatory and antithrombotic therapies. The 12-question survey was approved by the institutional review board of The Children’s Hospital of Philadelphia with exempt determination. The survey was reviewed independently by 3 cardiologists (ME, AD, BM) and underwent a pilot test trial prior to distribution to the IKDR. Responses were anonymous and voluntary, and IKDR members were approached via e-mail and routine virtual meetings with request for completion (Supplemental Table S1). Only one IKDR member per institution was asked to complete the survey, and answers were collected via the web-based application Research Electronic Data Capture (REDCap) hosted at The Children’s Hospital of Philadelphia.
Results
Among the IKDR membership, members from 36 of 65 institutions completed the survey, with 2 additional members commenting that they have not encountered MIS-C yet, for an overall response rate of 58%.
The most frequently reported specialties treating inpatient and outpatient MIS-C included cardiology (94% inpatient and outpatient), infectious disease (86% inpatient, 47% outpatient), and rheumatology (83% inpatient, 69% outpatient; Supplemental Figure S1). Additional inpatient providers included general pediatrics (78%), followed by hematology (42%) and immunology (17%).
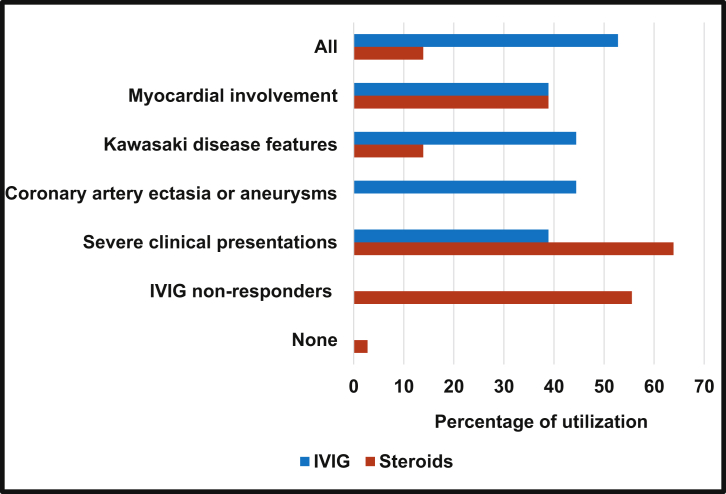
Among the respondents, 56% reported using immunomodulatory treatment for all MIS-C patients, regardless of presentation. All respondents reported that intravenous immunoglobulin (IVIG) was indicated for MIS-C patients, but the circumstances varied and included the presence of clinical features of KD (44%), coronary artery involvement (44%), myocardial involvement (39%), and severe clinical presentation, such as intensive care admission or presentation with shock (39%). Most often, the IVIG dose of 2 g/kg was reported (86%). For 53% of respondents, IVIG was indicated for all MIS-C patients regardless of the presence of additional features (Fig. 1).
Figure 1.
IVIG and steroid use in MIS-C among IKDR members. Answer to IKDR survey question regarding indications for IVIG and steroid use in MIS-C patients, based on percentage of responses. Note that survey asked about “coronary artery ectasia or aneurysms” only for IVIG, and about “IVIG non-responders” only for steroids. IKDR, International Kawasaki Disease Registry; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
Respondents reported steroid use most frequently in patients with severe clinical presentation (64%), followed by those who do not respond to IVIG (ongoing fevers and/or inflammation; 56%; Fig. 1). Only a minority of respondents indicated that they would use steroids for all patients (14%). Other individual responses regarding indications for steroids included the presence of valve dysfunction noted on echocardiogram even without ventricular dysfunction, patients deemed to be at a higher risk of developing coronary artery aneurysms (for example, the very young), and patients with macrophage activation syndrome.
Respondents indicated that they would use adjunct immunomodulatory medications, particularly in severe or refractory cases of MIS-C, including anakinra (58%), infliximab (28%), and tocilizumab (8%). Two additional respondents commented that although they have not encountered a patient with refractory disease yet, they would consider using anakinra or infliximab in that setting.
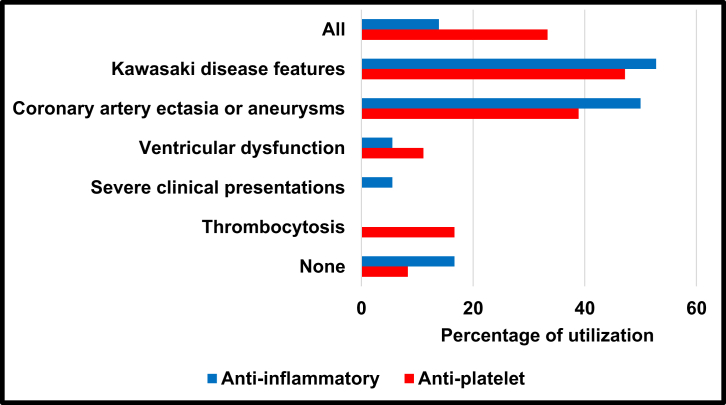
Acetylsalicylic acid was felt to be indicated by 91% of respondents. Respondents reported anti-inflammatory dosing at 30-50 mg/kg per day (56%), rather than 80-100 mg/kg per day (25%), more frequently, with 17% reporting that they did not use anti-inflammatory dosing. Respondents indicated that anti-inflammatory dosing would be used most commonly for those patients with KD features (53%) and/or coronary artery ectasia or aneurysms (50%; Fig. 2). Antiplatelet dosing (3-5 mg/kg per day) would be used most commonly in those with KD features (47%) and coronary artery involvement (39%), but sometimes for all MIS-C patients (33%).
Figure 2.
ASA use in MIS-C among IKDR members. Answer to IKDR survey question regarding indications for ASA use in MIS-C patients, based on percentage of responses. Note that survey asked about “severe clinical presentations” only for the anti-inflammatory dosing (30-50 mg/kg per day or 80-100 mg/kg per day), and about “thrombocytosis” only for antiplatelet dosing (3-5 mg/kg per day). ASA, acetylsalicylic acid; IKDR, International Kawasaki Disease Registry; MIS-C, multisystem inflammatory syndrome in children.
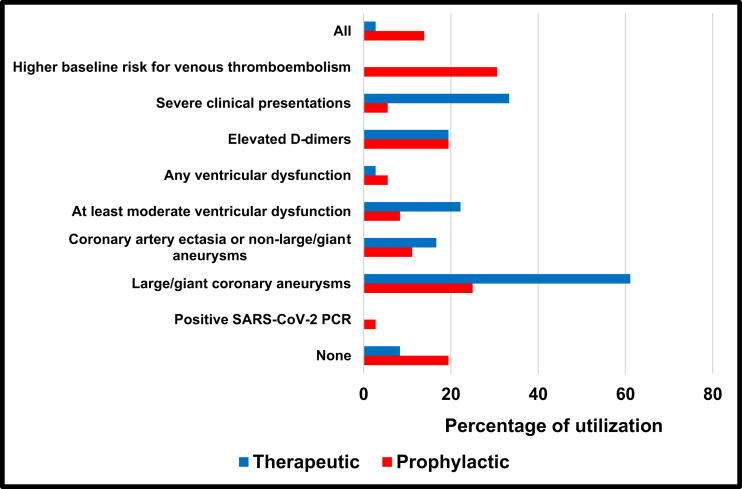
Respondents indicated that they would use prophylactic anticoagulation most commonly for patients felt to have a higher baseline risk for venous thromboembolism, such as the presence of altered mobility and obesity (31% of respondents), in addition to giant coronary artery aneurysms (25%) and elevated D-dimer levels (19%). Therapeutic anticoagulation dosing would be used for patients with giant coronary artery aneurysms (61%) and those with a severe clinical presentation (33%; Fig. 3).
Figure 3.
Anticoagulation use in MIS-C among IKDR members. Answer to IKDR survey question regarding indications for anticoagulation use in MIS-C patients, based on percentage of responses. Note that survey asked about “higher baseline risk of venous thromboembolism” only for prophylactic anticoagulation. IKDR, International Kawasaki Disease Registry; MIS-C, multisystem inflammatory syndrome in children; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Discussion
In this survey, we found important variation regarding the management approaches for patients with MIS-C. Approximately half of respondents would treat all MIS-C patients with immunomodulatory therapies, regardless of presentation, whereas others recommended treatment in only severe cases. IVIG was most often indicated, and steroids were typically reserved for more critical cases. Biologic therapies, such as an anakinra, were also commonly reported. Respondents frequently used antiplatelet therapy and prophylactic anticoagulation, reserving therapeutic anticoagulation for patients with giant coronary artery aneurysms or severe clinical presentations.
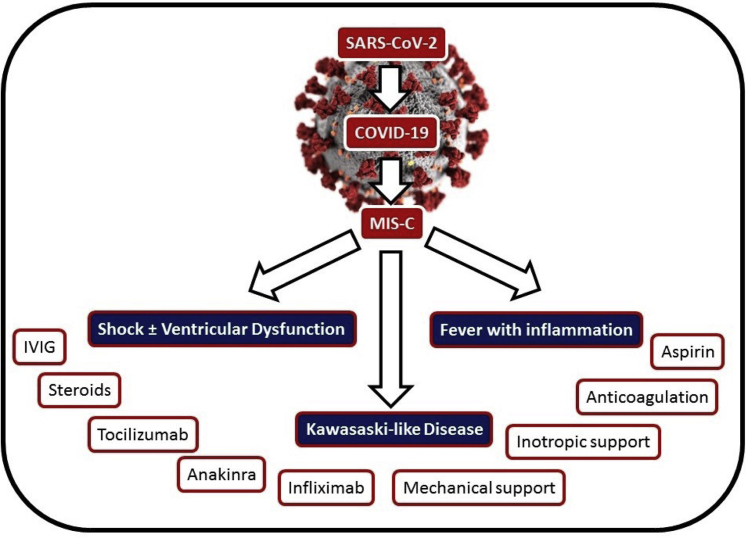
There have been several phenotypes reported in the literature for MIS-C, including (i) severe presentation with shock with or without ventricular dysfunction, (ii) KD-like presentation with evidence of systemic inflammation, and (iii) fever with evidence of inflammation not requiring intensive care support (Fig. 4). The degree of shock and hypotension are often out of proportion to the degree of ventricular dysfunction. Some patients have presented with severe ventricular dysfunction with a myocarditis-like presentation; others have had milder ventricular dysfunction without evidence of shock. Ventricular systolic dysfunction is common among MIS-C patients, reported in 33%-55% of patients.14,24,25,29 Left-ventricular systolic dysfunction has been most commonly in the mild-to-moderately diminished range, although several studies have reported patients with severely diminished ventricular function.7,25 The presence of coronary artery involvement is less common than ventricular dysfunction, typically found in 8%-19%, but as high as 36%, of cases.7,14,24,25,28,29 Although the majority of studies have described mild coronary artery ectasia/aneurysms, the presence of giant coronary artery aneurysms has been reported.5,14,27 Arrhythmias are not common in MIS-C patients, but they can occur,5,7,11,13,14,19,23,25 as reported in 12 of 186 patients in one large series.25 These arrhythmias have included atrial arrhythmias (premature atrial contractions and atrial fibrillation), ventricular arrhythmias (premature ventricular contractions, nonsustained ventricular tachycardia, and one patient with a wide complex tachycardia requiring extracorporeal membrane oxygenation [ECMO]), and atrioventricular block (first and second degree without further details on second degree).14,25 The cardiac presentation of patients with MIS-C is summarized in Table 2.
Figure 4.
Clinical presentations of MIS-C. MIS-C patients typically present with shock with or without ventricular dysfunction, Kawasaki-like disease, or fever with inflammation. There are a variety of treatment options, with practice variability. COVID-19, coronavirus disease 2019; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Cardiac manifestations in multisystem inflammatory syndrome in children
| Belhadjer, et al.7 n = 35 France, Switzerland |
Whittaker, et al.14 n = 58 United Kingdom |
Dufort, et al.24 n = 99 New York, United States |
Feldstein, et al.25 n = 186 United States (excluding New York) |
|
|---|---|---|---|---|
| Age (range), y | 10 (2-16) | 9 (5.7-14) | NP | 8.3 (3.3-12.5) |
| Male gender | 18 (51) | 25 (43) | 53 (54) | 115 (62) |
| Race | ||||
| Black | NP | 22 (38) | 31/78 (40) | 46 (25) |
| Asian | NP | 18 (31) | 4/78 (5) | NP |
| White | NP | 12 (21) | 29/78 (37) | 35 (19) |
| Other | NP | 6 (10) | 14/78 (18) | 107 (58) |
| Comorbidities | 10 (29) | 7 (12) | 36 (36) | 51 (27) |
| Obese | 6 (17), overweight | NP | 29 (29) | 45/153 (29) |
| Elevated BNP | 28/28 (100) | NP | NP | 94/128 (73) |
| Elevated NT-proBNP |
5/5 (100) | 24/29 (83) | 74/82 (90) | NP |
| Elevated troponin | 35/35 (100) | 34/50 (68) | 63/89 (71) | 77/153 (50) |
| Echocardiogram findings | n = 35 | n = 55 | n = 93 | n = 170 |
| LV systolic dysfunction | 35 (100) | 18 (33) | 51 (55) | 65 (38) |
| LVEF 30%-55% | 25 (71) | NP | NP | 56 (33) |
| LVEF <30% | 10 (29) | NP | NP | 9 (5) |
| Pericardial effusion | 3 (9) | NP | 32 (34) | 44 (26) |
| Coronary artery abnormalities | ||||
| Dilation (z score ≥ 2, < 2.5) | 6 (17) | 1 (2) | NP | NP |
| Aneurysm (z score ≥ 2.5) | 0 (0) | 7 (13) | 9 (10) | 15 (9) |
| Giant aneurysm (z score ≥ 10) | 0 (0) | 2 (4) | NP | NP |
| Arrhythmias | 1 (3) | 4 (7) | NP | 12 (6) |
| Treatment | ||||
| IVIG | 25 (71) | 41 (71) | 69 (70) | 144 (77) |
| Steroids | 12 (34) | 37 (64) | 63 (64) | 91 (49) |
| Inotropic support | 28 (80) | 27 (47) | 61 (62) | 90 (48) |
| ECMO | 10 (29) | 3 (5) | 4 (4) | 8 (4) |
| Mortality | 0 (0) | 1 (2) | 2 (2) | 4 (2) |
Data are n (%), n/N (%), or median (interquartile range), unless otherwise stated. BNP, B-type natriuretic peptide; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; LV, left ventricle; LVEF, left-ventricular ejection fraction; NP, not provided; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Naturally, given the clinical presentation similar to KD and KD shock syndrome, authors have questioned whether MIS-C is SARS-CoV-2–triggered KD or a distinct entity.31, 32, 33, 34, 35 In Bergamo, Italy, authors compared 19 KD patients prior to the pandemic since 2015—to 10 patients with KD-like presentations in the current era.6 In addition to the higher incidence, recent patients have been older, with more frequent cardiac involvement, higher serum ferritin levels, higher neutrophils, lower lymphocytes, lower platelets, and lower sodium levels. Similarly, when comparing 16 MIS-C patients across 7 hospitals in France to patients with KD, MIS-C patients were again older, with similar laboratory differences, increased prevalence of myocarditis, and increased prevalence of IVIG resistance.17 Whittaker et al.14 compared the laboratory features for 58 MIS-C children to those from children with KD and KD shock syndrome, based on prior database records from California. The MIS-C patients had consistently different laboratory levels, in addition to older age: higher white blood cells, higher neutrophils, lower lymphocytes, lower hemoglobin, lower platelets, higher C-reactive protein, lower albumin, higher ferritin, higher troponin, and higher D-dimer.14 Additionally, racial and ethnic backgrounds differ between MIS-C and KD patients. Several studies have commented on the relatively high proportion of MIS-C patients of African-American or Afro-Caribbean descent.5,11,14,18 There is typically a higher incidence of KD in Asian populations, but authors from South Korea have reported no cases of MIS-C.36 Lastly, although about 5% of patients with KD present with shock, MIS-C patients present with shock at least 35%-50% of the time.25,29 Further research, particularly immunologic profiling, is necessary before any conclusions are reached to determine if indeed KD and MIS-C are distinct entities despite their overlapping features.
Management strategies reported in the literature fall into 3 categories: (i) treatment of inflammation, (ii) treatment of shock, and (iii) thromboprophylaxis. However, there is currently suboptimal evidence supporting these treatments among primarily descriptive case series. Fortunately, the vast majority of MIS-C patients have recovered with the current empiric treatment approaches rather quickly, and among the largest studies across the United States,29 France,16 and the United Kingdom,28 there have been only 13 deaths. These generally positive outcomes increase the difficulty in determining which treatment strategy is the most efficacious.
Management practices for anti-inflammatory treatment for MIS-C have been extrapolated from treatment of KD, toxic shock syndrome, and cytokine storm in COVID-19. Given the overlap of clinical features among KD, myocarditis, and MIS-C, many clinicians have treated MIS-C with IVIG. IVIG has been the mainstay of KD treatment since the 1980s,37 as recommended by the American Heart Association guidelines,38 and has sometimes been used in the treatment of myocarditis. Among the largest case series, 70%-81% of patients have received IVIG, with variation in the dose of IVIG used (1 g/kg vs 2 g/kg).7,24,25,28,29 The use of IVIG varies by presentation, with one series reporting 100% use for those with KD-like presentations, 72% for those with shock, and 61% for those with fever and inflammation alone, although the effect on outcome cannot be determined from these data.14
Steroid use, most often methylprednisolone, has been frequently reported in the literature for MIS-C, with a range of 49%-73% of patients receiving steroids across large case series.24,25,28,29 The rationale for using steroids are its anti-inflammatory properties and frequent use in KD, particularly in high-risk KD patients,39,40 and other inflammatory disorders. Additional therapies used less commonly in the literature for treatment of inflammation include interleukin (IL)-6 inhibitor tocilizumab,8,10,17,20,21,23,25,27,28 IL-1 inhibitor anakinra,7,8,10,12,14,20,21,23,25,26,28 and infliximab.14,20,21,28 Many institutions have used these medications as a second-line agent if there has been no improvement after initial treatment with IVIG, but others have used them for primary treatment, particularly tocilizumab in the presence of cytokine storm.8
Patients frequently present with shock, often requiring inotropic support, including epinephrine, norepinephrine, dopamine, dobutamine, and milrinone.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 One particular study commented on the goal of avoiding milrinone due to concern of peripheral vasodilation.18 The use of ECMO varied considerably as well, with the most prevalent use in France, where 10 of 35 patients with ventricular dysfunction received ECMO support.7
Children with MIS-C have laboratory features suggestive of a pro-coagulable state, and the use of antiplatelet and/or anticoagulation therapies has been reported frequently. Atlhough many studies did not report any acetylsalicylic acid use, others have reported using anti-inflammatory dosing or more commonly antiplatelet dosing.5,6,9,11,13,15,17,18,20,21,23,28,29 There have only been scattered reports of thrombosis with MIS-C, including 2 patients in the 78-patient United Kingdom study.28 Several series have discussed anticoagulation,5,7,12,13,20,21,23,25,28,29 most commonly prophylactic dosing rather than therapeutic dosing. Indications for prophylactic anticoagulation typically were not provided, although one study commented that anticoagulation was indicated for patients with elevated D-dimer or fibrinogen levels, left-ventricular dysfunction, electrocardiographic changes, or any coronary artery abnormality.21
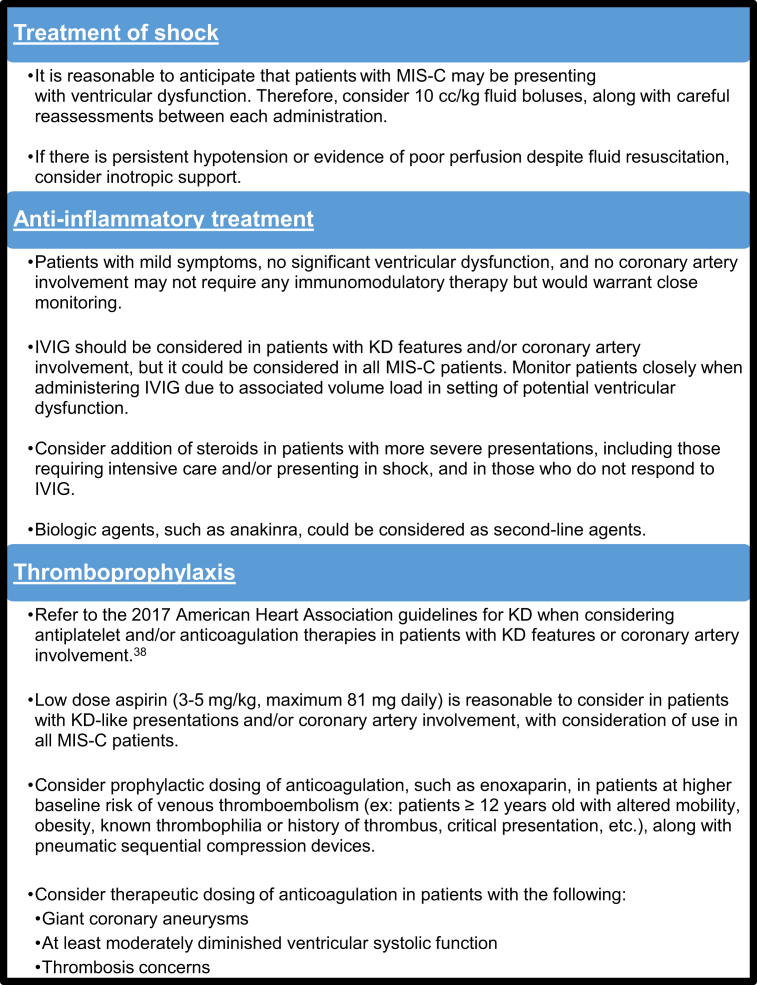
The management of MIS-C has been individualized at institutions with various clinical pathways and management algorithms. Recent studies have summarized and sometimes proposed specific therapeutic approaches,41, 42, 43, 44, 45, 46 but there has not been any study comparing these treatment strategies to assess outcomes. Based on the IKDR survey and review of the literature, we propose best practices for management of MIS-C, which warrants a multidisciplinary approach (Fig. 5).
Figure 5.
Best practices for the management of MIS-C, based on literature review and IKDR survey responses. IKDR, International Kawasaki Disease Registry; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; MIS-C, multisystem inflammatory syndrome in children.
The IKDR survey regarding management of MIS-C has limitations. We may not have detailed all of the many possible nuances in MIS-C management, but our goal was to assess generally accepted management strategies. The survey may not be reflective of actual practice. The questions asked members about current institutional practices, but members may have responded with approaches they might use in patient scenarios, rather than those actually used. Bias is also possible given that the IKDR members are nearly all pediatric cardiologists, and although practice variation certainly may exist among institutions, there may be variation relative to other providers and IKDR members within the individual institutions as well.
Conclusions
Treatment of patients with MIS-C often includes management of shock, use of immunomodulatory therapies, and use of thromboprophylaxis agents. Using these approaches, the vast majority of reported patients fortunately have recovered quickly. However, there is wide variation in management, and evidence to assess efficacy and superiority of the various treatment algorithms is suboptimal. Gaps in knowledge should be addressed through prospective trials and registries. Due to the rarity of the condition, multicenter collaboration is critical to understanding of this disease, with the potential for generating increased knowledge regarding KD in the process.
Acknowledgements
We thank and acknowledge the members of the International Kawasaki Disease Registry for their voluntary participation in the survey. We thank all healthcare providers and essential workers providing care for MIS-C patients during this pandemic, and certainly the patients and their families.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported adhered to the relevant ethical guidelines.
See page 639 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.09.004.
Supplementary Material
References
- 1.Pediatric Critical Care Society PICS Statement: Increased number of reported cases of novel presentation of multisystem inflammatory disease. https://pccsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf Available at:
- 2.World Health Organization Multisystem inflammatory syndrome in children and adolescents with COVID-19: Scientific Brief. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at:
- 3.Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf Available at: [DOI] [PubMed]
- 4.Centers for Disease Control and Prevention Health Alert Network (HAN) Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at:
- 5.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 8.Waltuch T., Gill P., Zinns L.E., et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department [e-pub ahead of print]. Am J Emerg Med 10.1016/j.ajem.2020.05.058, accessed June 4, 2020. [DOI] [PMC free article] [PubMed]
- 9.Chiotos K., Bassiri H., Behrens E. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimaud M., Starck J., Levy M. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller J., Cantor A., Zachariah P. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung E.W., Zachariah P., Gorelik M. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blondiaux E., Parisot P., Redheuil A., et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series [e-pub ahead of print]. Radiology 10.1148/radiol.2020202288, accessed June 14, 2020. [DOI] [PMC free article] [PubMed]
- 16.Belot A., Antona D., Renolleau S. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pouletty M., Borocco C., Ouldali N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort [e-pub ahead of print] Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramcharan T., Nolan O., Lai C.Y. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfler A., Mannarino S., Giacomet V., Camporesi A., Zuccotti G. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health. 2020;4:e26–e27. doi: 10.1016/S2352-4642(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushik S., Aydin S.I., Derespina K.R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capone C.A., Subramony A., Sweberg T. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood associated with severe acute respiratory syndrome coronavirus 2 infecton. Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hameed S., Elbaaly H., Reid C.E.L., et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 [e-pub ahead of print]. Radiology 10.1148/radiol.2020202543, accessed June 28, 2020. [DOI] [PMC free article] [PubMed]
- 23.Riollano-Cruz M., Akkoyun E., Briceno-Brito E., et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience [e-pub ahead of print]. J Med Virol 10.1002/jmv.26224, accessed June 28, 2020. [DOI] [PMC free article] [PubMed]
- 24.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 383:347-358. [DOI] [PMC free article] [PubMed]
- 25.Feldstein L.R., Rose E.B., Horwitz S.M. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334=46. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children [e-pub ahead of print]. JAMA Neurol 10.1001/jamaneurol.2020.2687, accessed July 9, 2020. [DOI] [PMC free article] [PubMed]
- 27.Ouldali N., Pouletty M., Mariani P. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies P., Evans C., Kanthimathinathan H.K. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godfred-Cato S., Bryant B., Leung J. COVID-19-associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manlhiot C., Newburger J.W., Low T. Low-molecular-weight heparin vs warfarin for thromboprophylaxis in children with coronary artery aneurysms after Kawasaki disease: a pragmatic registry trial. Can J Cardiol. 2020;36:1598–1607. doi: 10.1016/j.cjca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Shulman S.T. Pediatric coronavirus disease-2019-associated multi-system inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:285–286. doi: 10.1093/jpids/piaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin M. Childhood multisystem inflammatory syndrome—a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S., Chen M., Weng J. COVID-19 and Kawasaki disease in children. Pharmacol Res. 2020;159:104951. doi: 10.1016/j.phrs.2020.104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCrindle B.W., Manlhiot C., SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? [e-pub ahead of print]. JAMA 2020;324:246-248. [DOI] [PubMed]
- 35.Loke Y.-H., Berul C.I., Harahsheh A.S., Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? [e-pub ahead of print]. Trends Cardiovasc Med 2020;30:389-396. [DOI] [PMC free article] [PubMed]
- 36.Kim Y.J., Park H., Choi Y.Y. Defining association between COVID-19 and the multisystem inflammatory syndrome in children through the pandemic. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newburger J.W., Takahashi M., Burns J.C. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 38.McCrindle B.W., Rowley A.H., Newburger J.W. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T., Saji T., Otani T. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen S., Dong Y., Kiuchi M.G. Coronary artery complication in Kawasaki disease and the importance of early intervention: a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1156–1163. doi: 10.1001/jamapediatrics.2016.2055. [DOI] [PubMed] [Google Scholar]
- 41.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for pediatric patients with multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19 [e-pub ahead of print]. Arthritis Rheumatol 10.1002/art.41454, accessed July 31, 2020. [DOI] [PMC free article] [PubMed]
- 42.Hennon T.R., Penque M.D., Abdul-Aziz R., et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach [e-pub ahead of print]. Prog Pediatr Cardiol 10.1016/j.ppedcard.2020.101232, accessed July 31, 2020. [DOI] [PMC free article] [PubMed]
- 43.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents [e-pub ahead of print]. Lancet Infect Dis 10.1016/S1473-3099(20)30651-4, accessed August 22, 2020. [DOI] [PMC free article] [PubMed]
- 44.Sperotto F., Friedman K.G., Son M.B.F., et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 10.1007/s00431-020-03766-6, accessed August 19, 2020. [DOI] [PMC free article] [PubMed]
- 45.Abrams J.Y., Godfred-Cato S.E., Oster M.E. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.