Abstract
The growth plate is the cartilaginous portion of long bones where the longitudinal growth of the bone takes place. Its structure comprises chondrocytes suspended in a collagen matrix that go through several stages of maturation until they finally die, and are replaced by osteoblasts, osteoclasts, and lamellar bone.
The process of endochondral ossification is coordinated by chondrocytes and a variety of humoral factors including growth hormone, parathyroid hormone, oestrogen, growth factors, cytokines, and various signalling pathways.
Chondrocytes progress from a resting state to enter the phases of proliferation and hypertrophy. Under the influence of oestrogen, the proliferation of chondrocytes decreases as the resting chondrocytes are consumed. During the terminal phase of differentiation, cartilage is replaced by blood vessels and organized bone tissue, and once chondrocytes have died, the longitudinal growth of the bone ceases and the growth plate closes.
The highly complex regulatory signals involved in this process are genetically determined, and genetic perturbations in any of the associated genes can result in abnormalities of bone growth. Hundreds of chondrodysplasias have been described, pointing to the complexity of the humoral control systems involved in endochondral ossification.
While our knowledge of the mechanisms behind the various bone growth control systems is improving, a deeper understanding of the underlying processes could aid clinicians to better understand bone health and bone growth abnormalities. This review describes the current clinical research into the physiology of the growth plate.
Cite this article: EFORT Open Rev 2020;5:498-507. DOI: 10.1302/2058-5241.5.190088
Keywords: bone growth, chondrodysplasia, growth plate, physis
Introduction
The growth plate, also known as the physis, is the cartilaginous portion at the ends of long bones where longitudinal growth of the bone takes place. This region of bone is characterized by high metabolic activity and is under the regulatory control of a wide variety of hormones and signalling compounds. Hormonal regulation of the growth plate can be affected by trauma, medications, and other extrinsic variables causing growth plate abnormalities and subsequent growth disturbances. This review article will discuss the cellular and structural composition of the growth plate, as well as its humoral regulation.
Structural composition
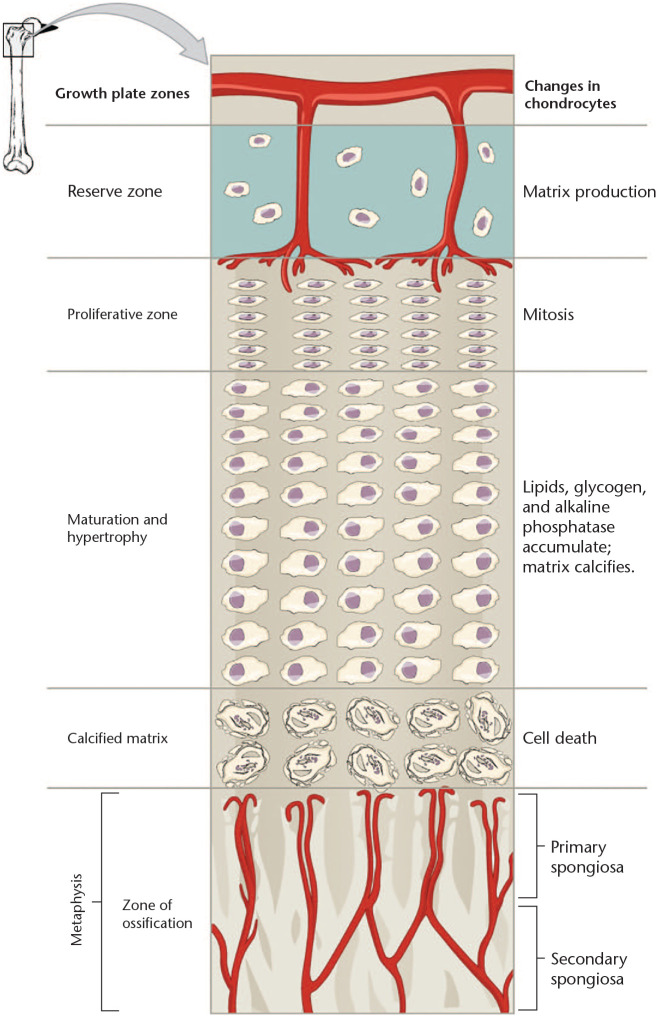
The maturation of chondrocytes in the physis is classically divided into five phases (Fig. 1).1 In a coordinated stepwise process, the chondrocytes pass through the stages of resting phase, proliferative phase, prehypertrophic phase, hypertrophic phase, and terminal phase. In the resting phase, the cells are relatively inactive in terms of mitosis and express Col2a1 genes which encode type II collagen, whereas in the proliferation phase, the chondrocytes rapidly divide and highly express Col2a1 and Acan, the gene encoding the proteoglycan aggrecan. After rapid proliferation, chondrocytes enter the prehypertrophic phase and start to express Col10a1 for type X collagen and Ihh, which encodes Indian Hedgehog. Subsequently, they increase in size and enter the hypertrophic phase, during which the chondrocytes continue to produce type X collagen, whereas the production of type II collagen ceases. After the hypertrophic phase, chondrocytes stop producing collagen altogether and die in what is called the terminal phase.1
Fig. 1.
Zones of chondrocyte maturation in growth plate.
Source. This figure is licensed under the Creative Commons Attribution-Share Alike 4.0 International license from Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, 19 June 2013.
This seemingly linear process is coordinated by a wide variety of signalling cascades that interact with genes, receptors, and transcription factors. This complexity allows for fine-tuning of the process based on environmental and temporal influences. The regulatory signals reach chondrocyte transcription factors such as Sox9, RUNX2, and Osterix, triggering a precisely coordinated cascade of events that results in healthy osteogenesis.2 There are many pathways, signals, receptors, mediators, agonists, and antagonists that interact with considerable ‘combinatory mixing and matching’,1 making it possible for bone tissue to develop within the cartilaginous template formed during foetal development (Fig. 2). This complex system is vulnerable to a wide variety of genetic abnormalities, and to date nearly 500 genetic skeletal disorders have been described. This article focuses on the best-studied signalling pathways and factors involved in the regulatory mechanisms of osteogenesis.
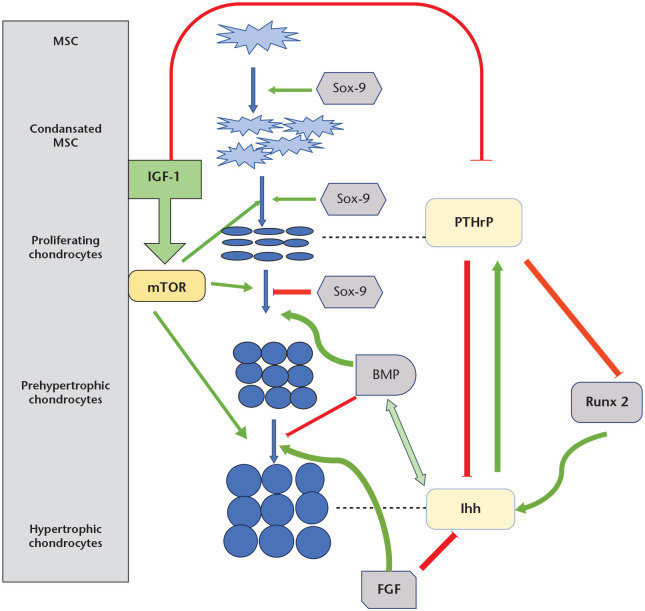
Fig. 2.
Paracrine control of growth plate.
Notes. Ihh (Indian hedgehog) enhances the proliferation and maturation of chondrocytes and induces the expression of parathyroid hormone-related protein (PTHrP) in the periarticular region. PTHrP prevents premature hypertrophic differentiation. The negative feedback loop between Ihh and PTHrP keeps chondrocytes in the proliferating state, controls chondrocyte proliferation, and maintains the lengths of columns.
Ihh and bone morphogenic protein (BMP) are in a positive feedback loop with each other and up-regulate chondrocyte proliferation together. In addition, BMP inhibits the development of terminally differentiated chondrocytes.
Fibroblast growth factor (FGF) signalling is antagonistic to BMP activity. FGF expression down-regulates chondrocyte proliferation and hypertrophy by inhibiting Ihh and promotes chondrocyte differentiation.
Runt-related transcription factor 2 (RUNX2) positively regulates Ihh expression and promotes chondrocyte proliferation, but it is inhibited by PTHrP, which is induced by Ihh.
Sox-9 signalling contributes to chondrogenesis in different steps. Sox-9 up-regulates chondrogenic mesenchymal condensation, chondrocyte differentiation, and normal chondrocyte proliferation and inhibits the transition of proliferating chondrocytes to hypertrophy.
Insulin-like growth factor 1 (IGF-1) signalling modulates chondrogenesis by both suppressing PTHrP production and inducing the mammalian target of rapamycin (mTOR) signalling activity, which plays a role in all stages of chondrocyte maturation.
During childhood, the width of the growth plate gradually decreases, together with the proliferative zone in response to the regulatory influence of estrogen.3 Eventually all chondrocytes die and cartilage is replaced by mature bone, leaving behind a line called the epiphyseal scar. The closure of the physis marks the end of a bone’s longitudinal growth.4 This process leads to the formation of a long bone within the template shape established and maintained by the physis.5
Longitudinal growth of the bone in the physis is orchestrated by the chondrocytes, which create and maintain their own environment.6 Chondrocytes divide and grow in a more or less columnar arrangement, secreting extracellular matrix (ECM) and enzymes that control the mineralization of the ECM. As the chondrocytes age, they coordinate their own death and secrete peptides that stimulate the ingrowth of blood vessels and the migration of osteoblasts and osteoclasts.7 Eventually, linear growth ceases and the process of ossification continues until all ECM is replaced by bone.
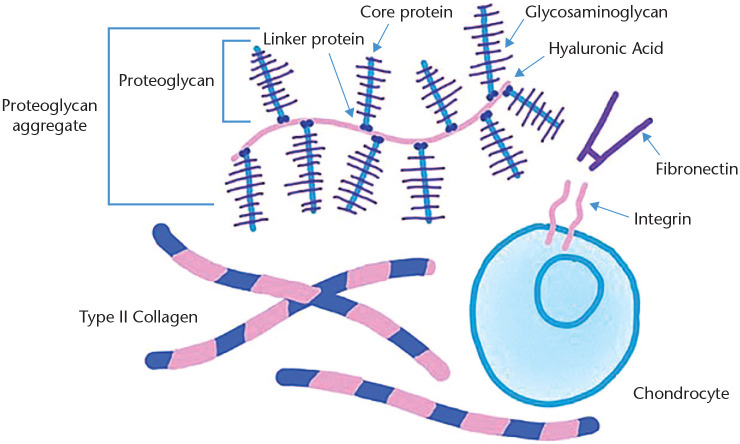
The ECM created by the chondrocytes is both strong and flexible and is made up of a complex network of collagens and proteoglycans (Fig. 3). As the mitotic chondrocytes in the proliferative zone transform into non-mitotic hypertrophic cells and eventually undergo cell death, they continue to produce large quantities of ECM that becomes calcified.6 The major structural collagen of the growth plate cartilage is type II collagen, a strong fibrous complex of three identical strands interwoven for strength. Type IX collagen is made up of three different types of interwoven strands and sits on the surface of types II and XI collagen, forming a strong fibrous sheath. Type XI collagen is composed of three identical strands and typically runs through the core of type II collagen fibrils. This gives each collagen fibre a three-layer structure consisting of type XI collagen in the centre of the fibre, which is surrounded by a thick layer of type II collagen, and an outer layer of type IX collagen.8 This layered structure has high tensile strength and flexibility. Type X collagen is shorter and creates hexamers as it binds together to form non-fibrillar cartilage that is unique to hypertrophic chondrocytes.9
Fig. 3.
Components of the extracellular matrix (ECM).
Source. This figure is is licensed under the Creative Commons Attribution-Share Alike 4.0 International license from Kassidy Veasaw.
In addition to collagen, a variety of glycoproteins are also present in the ECM secreted by chondrocytes. These include aggrecan, a large chondroitin sulphate proteoglycan that helps retain water in the cartilage due to its high negative ionic charge.10 Matrillins help hold aggrecan clumps in place,11 whereas perlecan, a large heparin sulphate/chondroitin sulphate complex, helps provide structural strength and promotes angiogenesis.12 Cartilage oligomeric matrix protein, also known as COMP5 or thrombospondin5, provides homeostatic support for the chondrocytes themselves and catalyses the polymerization of type II collagen.13
Humoral control of physeal growth and fusion
While chondrocytes in the growth plate control the local processes of bone growth, they are in turn controlled by numerous systemic regulatory factors that regulate their rate of proliferation, maturation, and ultimately the strength of the bone. Growth hormone (GH) initiates and maintains the process of growth through the stimulation of insulin-like growth factor 1 (IGF1), a growth factor that leads to chondrocyte hypertrophy and the production of ECM.14,15 Local factors in the perichondrium also affect bone growth, contributing to chondrocyte hypertrophy and osteoblast invasion. Bone morphogenetic proteins (BMPs),16 fibroblast growth factors (FGFs),17 and wingless/Int-1 (Wnt) signalling18 are all involved in the communication between perichondral cells and cartilage chondrocytes.
Other regulators include parathyroid hormone-related protein (PTHrP), a member of the parathyroid hormone family that supports chondrocytes and maintains the width of the growth plate.19 The protein Indian hedgehog (Ihh) helps coordinate chondrocyte differentiation, calcification, and ossification in a feedback loop with PTHrP.20 Levels of both of these paracrine regulators decrease towards the end of puberty. Mutations in the genes responsible for the production of these proteins result in a premature closure of the growth plate, which in turn leads to short stature.
The differentiation of mesenchymal stem cells into osteoblasts is partly mediated by the transcription factor RUNX2 (runt-related transcription factor 2). RUNX2 is also involved in regulating chondrocyte maturation and is itself controlled in part by PTHrP.21 The effect of RUNX2 seems to be strongest during the proliferative phase of chondrocytes. RUNX2 works through the transforming growth factor beta (TGF-β) superfamily of peptides to help control the maturation of chondrocytes while inhibiting their terminal stages.
In the hypertrophic zone of the growth plate, the chondrocytes die as a result of apoptosis or other processes, leaving behind a calcified matrix that consists of deteriorating collagen fibres intermingled with large clumps of aggrecan and the glycosaminoglycan hyaluronan. At this point, blood vessels enter the milieu, bringing with them osteoblasts, osteoclasts, and bone marrow components. Osteoclasts remove this calcified collagen matrix, allowing the ingrowth of blood vessels and the introduction of osteoblasts.6 An important mediator of this process is vascular endothelial growth factor (VEGF), which regulates osteoblast differentiation. The production of VEGF is stimulated by oestrogen in both males and females, and is up-regulated in puberty. VEGF serves to promote angiogenesis, allowing the entry of osteoblasts and osteoclasts, which then form mature trabecular bone. VEGF could also be important in physeal closure regulation.4
Vitamin D is involved in the differentiation of chondrocytes, and the metabolites of vitamin D are produced locally in the growth plate, where they function to decrease the proliferation of chondrocytes through PTHrP and Ihh. Vitamin D is well-recognized for its role in the deposition of calcium in bones; however, its effect on the growth plate is not well understood. Its mechanism of action may involve Ihh and PTHrP.22
A class of proteins known as bone morphogenetic proteins (BMPs) aid in the growth and differentiation of chondrocytes and are in turn themselves produced by chondrocytes in a positive feedback loop with Ihh. According to research conducted using rat models of bone growth, BMPs appear to have a significant signalling role in the differentiation of chondrocytes.16
The Wnt signalling pathway is also important in chondrocyte development, stimulating their evolution into hypertrophic chondrocytes while inhibiting the transformation of progenitor cells into chondrocytes in favour of osteoblasts.18 The Notch signalling pathway is situated downstream from Ihh, BMP, and PTHrP pathways, and acts to inhibit the differentiation of chondrocytes. Overexpression of Notch signalling results in the stunted growth of long bones.2
Important in the regulation of chondrocyte growth and differentiation is the fibroblast growth factor (FGF) group of signalling proteins. This pathway inhibits the proliferation of chondrocytes, thereby limiting the longitudinal growth of bones.12,17 There have been nearly two dozen subtypes of FGF described, with FGF18 being involved in chondrocyte maturation.23
Retinoic acid signalling
Retinoic acid (RA), a derivative of vitamin A, is also important in growth plate regulation. When RA binds to its receptors, it directly regulates gene transcription, blocking the transcription of certain genes that inhibit cartilage growth and cartilage differentiation. RA is thought to regulate the directional growth of limbs within the limb buds.24 RA is involved in both stimulating25 and repressing26 actions, making its analysis challenging. It does appear to be important in limb bud development and growth plate homeostasis, controlling other signalling pathways including FGF, Wnt, BMP, and sonic hedgehog protein (Shh). Excessive RA, such as in pharmacologic treatment with retinoids, can result in skeletal abnormalities as well.27
mTOR signalling
The mammalian target of rapamycin (mTOR) pathway is a critical regulator of cellular metabolism and cartilage growth. mTOR is involved in the growth and homeostasis of metabolic systems throughout the body. The mTOR system comprises several intracellular and extracellular signals and is activated by a variety of extrinsic humoral factors such as the epidermal growth factor, IGF, glucose, and oxygen. mTOR is controlled by adenosine monophosphate-activated protein kinase (AMPK) when cellular nutrients are scarce. Genetic mutations that result in the absence or deficiency of mTOR are associated with impaired embryonic skeletal growth and mutations.28 Many of these mutations are lethal, underscoring the importance of mTOR signalling in multiple tissue types. In the growth plate, mTOR signalling coordinates chondrocyte proliferation and hypertrophy, and the absence of mTOR signalling results in the shortening or absence of the limb bud, chondrodysplasia, and dwarfism.
Insulin-like growth factor (IGF) signalling
Insulin-like growth factor is involved in endochondral ossification. Its two ligands (IGF1 and IGF2) bind to two receptors (IGFR1 and IGFR2), thereby stimulating the production of peptides and signals that control the growth and survival of chondrocytes by signalling the mTOR pathway.14 These ligands and the peptides regulated by them act as both intracellular signalling peptides and extracellular hormones. Absence of IGF leads to skeletal abnormalities and dwarfism. IGF appears to be involved in controlling the proliferation, hypertrophy, survival, and maturation of chondrocytes. In humans, homozygous deletions of IGF1 result in severe growth retardation with severe osteopenia.29 Other mutations have resulted in microcephaly, deafness, and mental retardation.
The circadian clock
The ossification of the growth plate appears to be affected by the circadian clock. This endogenous time-keeping system regulates gene expression and physiology on a 24-hour cycle. Circadian rhythms control positive and negative feedback loops throughout the body, stimulated by external factors such as sun exposure. These factors trigger the transcription of various regulatory peptides, including those controlling skeletal growth and homeostasis. For example, Col2a1 and Col10a1 are expressed with a diurnal rhythm.30 Evidence is mounting that there is a cartilage-specific circadian clock that controls processes within the growth plate.31 Endogenous hormone cycles related to the circadian clock, such as the hypophyseal-pituitary-adrenal axis, catecholamines, and glucocorticoids, all exert positive and negative influences on osteoblasts.32 Similarly, PTH is well known to show diurnal variations, and experimental studies with bioluminescence showed that PTH directly influenced physeal cartilage growth cycles.33 These findings add weight to the age-old advice of mothers to their children to get their sleep if they want to grow up big and tall.
Novel transcriptional co-factors
All of these signalling pathways affect a final common pathway of a multi-faceted transcriptions network. The transcription factors Sox9, RUNX2 and Osterix appear to respond to the above signalling pathways and in turn control the transcription of co-factors that control growth plate formation. Other peptides that control these transcriptional factors include β-catenin, core binding factor subunit beta (CBFβ), c-Jun activation domain-binding protein-1 (JAB1), and yes-associated protein 1 (YAP1). Together, these factors and co-factors regulate chondrocyte growth and maturation, subsequently controlling the growth of the bone. Specific examples of these co-factors include the constitutive photomorphogenesis 9 (COP9) signalosome and YAP1.
A summary of the stages of endochondral ossification as well as the relevant signalling peptides at each stage is shown in Table 1.
Table 1.
Stages of endochondral ossification and relevant signalling peptides
| Stage | Events | Signalling peptides |
|---|---|---|
| Resting | Home to stem-like cells Production of collagen types II, IX, and XI and the proteoglycan aggrecan |
Ihh stimulates chondrocytes to produce PTHrP causing chondrocytes to divide Vitamin D receptors expressed BMP antagonists expressed |
| Proliferation | Cells undergo rapid mitosis Marked increase in production of type II collagen, aggrecan, perlecan |
Stimulated by GH through the action of IGF Ihh maintains chondrocytes in proliferative phase via PTHrP Chondrocytes produce more Ihh BMP agonists expressed which promote mitosis FGF expressed to control proliferation Stimulated by Wnt signalling and β-catenin Down-regulated by Notch signals Down-regulated by oestrogen Stimulated by mTOR signalling |
| Hypertrophic | Chondrocytes cease dividing Cells increase in size Production of non-fibrillar collagen type X Remodelling of surrounding ECM, possibly from pressure exerted by expanding chondrocytes and hyaluronan-aggrecan complexes Mineralization of ECM Cells express MMPs Chondrocytes begin secreting VEGF |
Triiodothyronine stimulates transition from proliferative to hypertrophic Vitamin D enhances hypertrophy Chondrocytes produce more Ihh which promotes their transition from proliferative to hypertrophic PTHrP signalling falls off BMP antagonists (Smad6/7) expressed which stop mitosis Inhibited by FGF Stimulated by β-catenin and canonical Wnt signals Inhibited by Notch, stimulated by mTOR Regulated by RUNX2 |
| Calcification | Chondrocytes stop producing collagen type X Terminal differentiation of chondrocytes by unknown process: apoptosis, autophagy, transdifferentiation, hypoxia |
Production of VEGF Mineralization of cartilage |
| Ossification | Invasion by blood vessels Invasion by osteoclasts, osteoblasts, hematopoietic components Production of lamellar bone |
VEGF stimulates angiogenesis Osteoblasts, osteoclasts under systemic control by calcitonin, PTH, thyroid hormone, oestrogen |
Note. BMP, bone morphogenic protein; ECM, extracellular matrix; FGF, fibroblast growth factor; GH, growth hormone; IGF, insulin-like growth factor ; Ihh, Indian hedgehog; MMPs, matrix metalloproteinases; mTOR, mammalian target of rapamycin; PTH, parathyroid hormone; PTHrP, parathyroid hormone receptor protein; RUNX2, runt-related transcription factor 2; VEGF, vascular endothelial growth factor; Wnt, wingless/Int-1
Hormones and the physis
In addition to local growth factors and signalling compounds, systemic hormones act on the growth plate, providing overarching control of the process of growth. Oestrogen causes maturation of the growth plate, accelerating skeletal maturation and the accumulation of minerals into the cartilage. Oestrogen also promotes the closure of the physis, stopping the axial growth of the bone. Decreases in total oestrogen or oestrogen receptor sensitivity results in longer bones and tall stature.3
Growth hormone is produced by the anterior pituitary and is released in a pulsatile manner into the bloodstream. Most of the growth hormone is secreted during sleep, especially during the REM phase. Growth hormone affects multiple tissues in the body, increasing the growth and differentiation of chondrocytes as well as the deposition of calcium in bones. Growth hormone supplementation has been advocated in constitutionally short stature and is not allowed in athletes because of its anabolic steroid effects.15
Adolescence is characterized by rapid bone growth in normal children. There are a multitude of hormonal changes that occur during this crucial time in humans, including increases in growth hormone, testosterone, and oestrogen among others. Growth hormone is thought to represent the major factor driving rapid increases in growth of the epiphysis,34 and testosterone and other androgens increase the longitudinal growth of bones. This effect appears to work through oestrogen receptors. Higher testosterone levels in humans are associated with taller stature.3
While growth hormone, oestrogen, and testosterone all impact growth, their mechanisms of action are not clear. It has been proposed that the hormones stimulate the production of some of the signalling peptides described above, whereas other evidence suggests that the hormones may directly stimulate the receptors of the signalling peptides.6 Pharmacological manipulation of hormones can have significant effects on prepubertal growth.
Growth plate closure
As puberty nears its end, the growth plate undergoes a process of senescence. There is a gradual decrease in the height of the growth plate starting with the proliferative and hypertrophic zones. The size of the chondrocytes in the hypertrophic zone decreases as they produce less ECM. This seems to be associated with endocrine and apocrine changes rather than changes to the chondrocytes themselves. Once senescence reaches a certain point, the growth plate fuses, leaving behind a visible ‘scar’.6 Another possible explanation for senescence is a decrease in the proliferative capacity of the stem cells in the resting zone.
The process of growth plate senescence intensifies in response to oestrogen. This hormone increases the rate of transformation from the resting state to the proliferative phase, essentially exhausting the supply of these cells.35 The final step leading to the loss of active chondrocytes is a matter of significant scientific controversy but may involve apoptosis, autophagy, transdifferentiation into osteoblasts, or hypoxia.
Disorders of the growth plate
Given the complexity of the physiology of the normal growth plate, it is not surprising that over 300 congenital abnormalities of skeletal growth have been defined.36 Hereditary multiple osteochondromas (HMO) and achondroplasia are two common disorders caused by disturbances in regulatory growth plate pathways that are understood at the molecular level. HMO, also known as hereditary multiple exostoses, is caused by autosomal dominant mutations in the exostosin-1 and -2 (Ext-1 and Ext-2) genes. These genes are responsible for encoding glycosyltransferases for heparan sulphate (HS) synthesis.37,38 Abnormalities in HS have been suggested to effect chondrogenesis with respect to several aspects, such as signalling HS-dependent proteins, including Indian Hedgehog (Ihh), parathyroid hormone-related protein (PTHrP), bone morphogenetic protein (BMP), and wingless/Int-1 (Wnt), disturbances in the Ihh/PTHrP pathway or downstream of the fibroblast growth factor (FGF)/BMP pathway. All these effects result in defects in chondrocyte differentiation and eventually lead to exostoses formation along the border of the growth plates.39,40
Achondroplasia is an autosomal dominant disorder characterized by small stature (dwarfism), rhizomelic shortening of the long bones, thoracolumbar kyphosis, craniofacial disorders, including macrocephaly and prominent forehead, and hypermobile knees and hips.41
Achondroplasia has been linked to mutations in gene expressing fibroblast growth factor receptor (FGFR3).42 Overexpression of FGFR3 signalling inhibits another important physiological regulator of chondrocyte differentiation, Ihh, and this process involves an increase in downstream regulation of chondrogenesis by promoting apoptosis, causing a decrease in chondrocyte proliferation, and suppression of chondrocyte hypertrophy, resulting in dwarfism with a decrease in bone elongation. These mutations may also lead to other chondrodysplasic syndromes, such as thanatophoric dysplasia (TD), severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN), and hypochondroplasia.
Some of the other well-defined disorders are listed in Table 2.
Table 2.
Representative disorders of the growth plate
| Syndrome | Defect | Zone involved | Manifestations |
|---|---|---|---|
| Campomelic dysplasia | SOX9 gene defect | Proliferative | 95% mortality in neonatal period; scoliosis, kyphosis, short limbs |
| Achondroplasia | Fibroblast growth factor receptor defect | Proliferative | Dwarfism |
| Spondyloepiphyseal dysplasia (SED) | COL2A1 gene, produces type II collagen | Proliferative | Dwarfism, short trunk and neck, severe near-sightedness |
| Achondrogenesis type II | COL2A1 gene, produces type II collagen | Proliferative | Long bones do not ossify; generally fatal before puberty |
| Hypochondrogenesis | COL2A1 gene, produces type II collagen | Proliferative | Similar to achondrogenesis type II; spinal deformities less severe |
| Kniest dysplasia | COL2A1 gene, produces type II collagen | Proliferative | Dwarfism; kyphoscoliosis, arthritis, blindness |
| Stickler syndrome | Mutations in the COL11A1, COL11A2, COL2A1 genes, produce type II and XI collagen | Proliferative | Mild to severe, short stature, atypical facies, arthritis |
| Pseudoachondroplasia | Mutation in the gene encoding cartilage oligomeric matrix protein (COMP) | Prehypertrophic | Growth retardation starting age 2–3; short limbs; gait abnormalities |
| Multiple epiphyseal dysplasia (MED) (Fairbank’s disease) | Mutation in genes encoding COL9A1, COL9A2, COL9A3, COMP, MATN3 | Prehypertrophic | Fatigue, waddling gait, very small ossification centres, short stature |
| Brachydactyly type A1 | Numerous subtypes; mutation in Ihh gene | Prehypertrophic | Short fingers; some have absent ulna |
| Acrocapitofemoral dysplasia | Mutation in Ihh gene | Prehypertrophic | Short stature of variable degrees with short limbs, brachydactyly, narrow thorax |
| Cleidocranial dysplasia (CCD) | Mutation in RUNX2 gene | Hypertrophic | Growth retardation before, defective growth of skull bones; complete or partial absence of clavicles, thumbs |
Note. Ihh, Indian hedgehog.
Systemic causes of growth plate disorders
Some medications and diseases have been implicated as the cause of short stature in children. Stimulant medications for attention deficit hyperactivity disorder, glucocorticoids for the treatment of asthma and severe dermatitis, and certain antibiotics have been cited as causes of reversible stunting of growth, although those patients typically display ‘catch-up growth’ once the medication is discontinued. In a 1979 study,43 children on neurostimulants showed decreases in the ECM of the growth plate. Subsequent studies have, however, refuted these assertions. The study led by Derfoul demonstrated that corticosteroids were necessary for the differentiation from mesenchymal stem cells to chondrocytes,44 whereas Silvestrini et al found that corticosteroid administration increased apoptosis in the terminal differentiation of chondrocytes in the zone of calcification and impaired the transition of chondrocytes from the resting zone to the zone of proliferation, slowing growth.45 Ciprofloxacin has also been shown to cause growth retardation in children,46 although its effects may not be clinically significant.47 Diabetes,48 hypothyroidism,49 and renal failure15 have been investigated as acquired causes of growth retardation that impact growth through their effects on systemic hormones, ECM, and chondrocytes.
Chemotherapy and radiation therapy have both been implicated in causing growth abnormalities. Anti-cancer drugs that exert inhibitory effects on angiogenesis may interfere with the ossification of bone, a step that requires the invasion of blood vessels into the calcified ECM.50 Radiotherapy that includes the growth plate can cause growth abnormalities in that limb by damaging the stem cells and chondrocytes.51
Trauma to the growth plate has long been known to possibly interfere with growth at that site. In addition to the obvious explanation of avascular necrosis in poorly healed Salter fractures, fractures through the growth plate initiate a cascade of inflammation with the release of destructive cytokines. These cytokines and macrophages damage the evolving chondrocytes, impairing their ability to produce ECM and upsetting the normal orderly architecture of the growth plate.52 Orthopaedic care provided in a timely fashion can limit that damage, but growth abnormalities are common after growth plate injuries and may even result in nonunions.53
Conclusions
Axial elongation of long bones via endochondral ossification is a highly complex and structured process that is tightly controlled by systemic hormones, local growth factors, signalling cytokines, and cellular differentiation. The most important regulator of this process is the chondrocyte as it transitions through differentiation, growth, and ultimately apoptosis while recruiting blood vessel growth and the influx of osteoblasts and osteoclasts. The end product is lamellar bone, laid down within the template established by the cartilage of the growth plate. Numerous genetic defects, diseases, therapeutics, and trauma have been shown to interfere with growth. Improved knowledge of normal growth plate physiology could help clinicians identify treatable disorders and promote normal stature and growth. Future research should further clarify the signalling mechanisms controlling growth plate physiology in an effort to identify and intervene in congenital growth retardation at an earlier stage.
Footnotes
ICMJE Conflict of interest statement: The author was granted a scholarship by the The Scientific and Technological Research Council of Turkey (TUBİTAK) for travel and registration expenses for participation with an accepted oral presentation in the 38th SICOT Orthopaedic World Congress that was held in Cape Town, not related to the submitted work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Samsa WE, Zhou X, Zhou G. Signaling pathways regulating cartilage growth plate formation and activity. Semin Cell Dev Biol 2017;62:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohn A, Rutkowski TP, Liu Z, et al. Notch signaling controls chondrocyte hypertrophy via indirect regulation of Sox9. Bone Res 2015;3:15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res 2005;64:157–165. [DOI] [PubMed] [Google Scholar]
- 4. Shim KS. Pubertal growth and epiphyseal fusion. Ann Pediatr Endocrinol Metab 2015;20:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaroszewicz J, Kosowska A, Hutmacher D, Swieszkowski W, Moskalewski S.Insight into characteristic features of cartilage growth plate as a physiological template for bone formation. J Biomed Mater Res A 2016;104:357–366. [DOI] [PubMed] [Google Scholar]
- 6. Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr 2011;75:383–391. [DOI] [PubMed] [Google Scholar]
- 7. Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol 2011;211:109–121. [DOI] [PubMed] [Google Scholar]
- 8. Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: type XI collagen is restricted to thin fibrils. J Histochem Cytochem 1995;43:967–979. [DOI] [PubMed] [Google Scholar]
- 9. van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev 2003;24:782–801. [DOI] [PubMed] [Google Scholar]
- 10. Villemure I, Stokes IA. Growth plate mechanics and mechanobiology: a survey of present understanding. J Biomech 2009;42:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muttigi MS, Han I, Park HK, Park H, Lee SH. Matrilin-3 role in cartilage development and osteoarthritis. Int J Mol Sci. 2016;17:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith SM, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol 2007;26:175–184. [DOI] [PubMed] [Google Scholar]
- 13. Posey KL, Hecht JT. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets 2008;9:869–877. [DOI] [PubMed] [Google Scholar]
- 14. Isgaard J, Möller C, Isaksson OG, Nilsson A, Mathews LS, Norstedt G. Regulation of insulin-like growth factor messenger ribonucleic acid in rat growth plate by growth hormone. Endocrinology 1988;122:1515–1520. [DOI] [PubMed] [Google Scholar]
- 15. Paschou SA, Kanaka-Gantenbein C, Chrousos GP, Vryonidou A. Growth hormone axis in patients with chronic kidney disease. Hormones (Athens) 2019;18:71–73. [DOI] [PubMed] [Google Scholar]
- 16. Yoon BS, Pogue R, Ovchinnikov DA, et al. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 2006;133:4667–4678. [DOI] [PubMed] [Google Scholar]
- 17. Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev 2015;29:1463–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Usami Y, Gunawardena AT, Iwamoto M, Enomoto-Iwamoto M. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest 2016;96:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin TJ. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol Rev 2016;96:831–871. [DOI] [PubMed] [Google Scholar]
- 20. Amano K, Densmore MJ, Lanske B. Conditional deletion of Indian hedgehog in limb mesenchyme results in complete loss of growth plate formation but allows mature osteoblast differentiation. J Bone Miner Res 2015;30:2262–2272. [DOI] [PubMed] [Google Scholar]
- 21. Liao L, Jiang H, Fan Y, et al. Runx2 is required for postnatal intervertebral disc tissue growth and development. J Cell Physiol 2019;234:6679–6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab 2015;29:621–631. [DOI] [PubMed] [Google Scholar]
- 23. Hung IH, Schoenwolf GC, Lewandoski M, Ornitz DM. A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development. Dev Biol 2016;411:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sekiya I, Tsuji K, Koopman P, et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem 2000;275:10738–10744. [DOI] [PubMed] [Google Scholar]
- 25. Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol 2002;157:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Luca F, Uyeda JA, Mericq V, et al. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology 2000;141:346–353. [DOI] [PubMed] [Google Scholar]
- 27. Steineck A, MacKenzie JD, Twist CJ. Premature physeal closure following 13-cis-retinoic acid and prolonged fenretinide administration in neuroblastoma. Pediatr Blood Cancer 2016;63:2050–2053. [DOI] [PubMed] [Google Scholar]
- 28. Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 2012;18:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agrogiannis GD, Sifakis S, Patsouris ES, Konstantinidou AE. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol Med Rep 2014;10:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doody KM, Bottini N. Chondrocyte clocks make cartilage time-sensitive material. J Clin Invest 2016;126:38–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takarada T, Kodama A, Hotta S, et al. Clock genes influence gene expression in growth plate and endochondral ossification in mice. J Biol Chem 2012;287:36081–36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kondo H, Togari A. Circadian regulation of bone metabolism by β-adrenergic signaling, glucocorticoids, and clock genes. J Oral Biosci 2015;57:9–13. [Google Scholar]
- 33. Okubo N, Fujiwara H, Minami Y, et al. Parathyroid hormone resets the cartilage circadian clock of the organ-cultured murine femur. Acta Orthop 2015;86:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Juul A. The effects of oestrogens on linear bone growth. Hum Reprod Update 2001;7:303–313. [DOI] [PubMed] [Google Scholar]
- 35. Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A 2001;98:6871–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phornphutkul C, Gruppuso PA. Disorders of the growth plate. Curr Opin Endocrinol Diabetes Obes 2009;16:430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jamsheer A, Socha M, Sowińska-Seidler A, Telega K, Trzeciak T, Latos-Bieleńska A. Mutational screening of EXT1 and EXT2 genes in Polish patients with hereditary multiple exostoses. J Appl Genet 2014;55:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCormick C, Duncan G, Goutsos KT, Tufaro F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci U S A 2000;97:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones KB, Pacifici M, Hilton MJ. Multiple hereditary exostoses (MHE): elucidating the pathogenesis of a rare skeletal disorder through interdisciplinary research. Connect Tissue Res 2014;55:80–88. [DOI] [PubMed] [Google Scholar]
- 40. Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 2005;132:5055–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet 2007;370:162–172. [DOI] [PubMed] [Google Scholar]
- 42. Di Rocco F, Biosse Duplan M, Heuzé Y, et al. FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum Mol Genet 2014;23:2914–2925. [DOI] [PubMed] [Google Scholar]
- 43. Kilgore BS, Dickinson LC, Burnett CR, Lee J, Schedewie HK, Elders MJ. Alterations in cartilage metabolism by neurostimulant drugs. J Pediatr 1979;94:542–545. [DOI] [PubMed] [Google Scholar]
- 44. Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells 2006;24:1487–1495. [DOI] [PubMed] [Google Scholar]
- 45. Silvestrini G, Ballanti P, Patacchioli FR, et al. Evaluation of apoptosis and the glucocorticoid receptor in the cartilage growth plate and metaphyseal bone cells of rats after high-dose treatment with corticosterone. Bone 2000;26:33–42. [DOI] [PubMed] [Google Scholar]
- 46. Aziz J, Morgan M. The possible protective role of magnesium on Ciprofloxacin Induced chondrotoxicity on the epiphyseal plate growth of juvenile albino rat: a histological and morphometric study. Acad Anat Int 2015;1. [Google Scholar]
- 47. Patel K, Goldman JL. Safety concerns surrounding quinolone use in children. J Clin Pharmacol 2016;56:1060–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kashef S, Karamizadeh Z. Hypercalciuria, hyperphosphaturia and growth retardation in children with diabetes mellitus. Iran J Med Sci 2002;27:11–14. [Google Scholar]
- 49. Lewinson D, Harel Z, Shenzer P, Silbermann M, Hochberg Z. Effect of thyroid hormone and growth hormone on recovery from hypothyroidism of epiphyseal growth plate cartilage and its adjacent bone. Endocrinology 1989;124:937–945. [DOI] [PubMed] [Google Scholar]
- 50. Voss SD, Glade-Bender J, Spunt SL, et al. Growth plate abnormalities in pediatric cancer patients undergoing phase 1 anti-angiogenic therapy: a report from the Children’s Oncology Group Phase I Consortium. Pediatr Blood Cancer 2015;62:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hess CB, Thompson HM, Benedict SH, et al. Exposure risks among children undergoing radiation therapy: considerations in the era of image guided radiation therapy. Int J Radiat Oncol Biol Phys 2016;94:978–992. [DOI] [PubMed] [Google Scholar]
- 52. Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci 2013;14:15767–15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pedrazzini A, Bastia P, Bertoni N, et al. Distal radius nonunion after epiphyseal plate fracture in a 15 years old young rider. Acta Biomed 2018;90:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]