Abstract
The current rampant coronavirus infection in humans, commonly known as COVID-19, a pandemic that may cause mortality in humans, has been declared a global emergency by the World Health Organization (WHO). The morbidity and mortality rates due to the pandemic are increasing rapidly worldwide, with the USA most affected by the disease. The source COVID-19 is not absolutely clear; however, the disease may be transmitted by either by COVID-19-positive individuals or from a contaminated environment. In this review, we focused on how the COVID-19 virus is transmitted in the community. An extensive literature search was conducted using specific keywords and criteria. Based on the published report, it is concluded that COVID-19 is primarily transmitted human-to-human via oral and respiratory aerosols and droplets with the virus-contaminated environment play a lesser role in the propagation of disease. Healthcare providers and the elderly with comorbidities are especially susceptible to the infection.
Keywords: COVID-19, Transmission mode, Virus source, Virus load and shedding, Pandemic infection
1. Introduction
The first confirmed coronavirus disease 2019 (Covid-19) case was reported on November 17, 2019, in a 55-year-old resident of Hubei, Central China [1]. However, the WHO Country Office in China was only notified of the COVID-19 outbreak on December 31, 2019, which then attracted international attention to the potential pandemic. On February 1, 2020, the World Health Organization (WHO) declared that the outbreak of this respiratory illness as a Public Health Emergency of International Concern (PHEIC). Subsequently, several more cases of the infection were identified in Wuhan City, Hubei Province, China in December 2019, in patients that patronized the South China Huanan seafood wholesale and live animal marketplace in the city.
Initially, the infection was believed to be due to animal-to-human transmission without animal species association [1,2]. Later, it was realized that human-to-human transmission is, in fact, a common mode of viral spread because a growing number of patients without a history of exposure to the market had acquired the disease [3,4].
The coronavirus infection was given various names including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee of Taxonomy of Viruses (ICTV) and novel coronavirus infection (2019-nCoV or COVID-2019) by WHO. This disease is now widely known as COVID-19 [5]. The COVID-19 had spread very rapidly throughout the world [6,7] with clustering onset especially from international air travel [8,9] As of May 2020, more than 200 countries had been affected, reporting more than 4 million confirmed cases with more than 280,000 deaths. The USA alone reported more than 1.3 million cases and more than 80,000 deaths with the highest morbidity and mortality rate elderly with comorbidities and immunocompromised and organ transplantation patients, and individuals of any age with underlying chronic diseases including cancer, diabetes, kidney disease, and hypertension [10].
Coronavirus represents a large family of single-stranded, positive-sense RNA viruses found in a wide range of animals including birds, camels, cattle, cats, pigs, and bats [11], some of which are carriers of the virus. Among these animals, the rhinolophid bats are the most dangerous carriers and they do not exhibit clinical signs of infection [12]. In other animals, the virus develops severe illness such as infectious bronchitis (IB) disease in chicken, which could lead to serious economic losses to the poultry industry [13].
Coronavirus infections are not new because during the last two decades the world was shaken by several epidemics including Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) in China (2002) from civets and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Saudi Arabia (2012) from dromedary camels. The source of COVID-19 is not clear, although it was originally believed that the bats are the source of the infection [14].
The symptoms of COVID-19 infection are mild to severe with death reported in some cases [15]. The most common symptoms are lower respiratory tract infection, pneumonia, dry cough, fever, shortness of breath, dyspnea, and myalgia [1,16]. Headache, confusion, sore throat, hemoptysis, runny nose, chills, muscle and chest pain, rhinorrhea, and diarrhea with nausea and vomiting may occur but less commonly reported [16]. It is now known that patients can be infected with the virus without showing any symptoms. However, most COVID-19 cases have good prognoses with mild symptoms, and with supportive care, they recovered after 7–10 days of hospitalization [17]. Patients that developed pulmonary edema, acute respiratory distress syndrome (ARDS), multiple organ failure, may die from the infection [18].
There are several tests available for COVID-19 diagnosis, mostly based on molecular or serological methods. The molecular methods are Real-time Polymerase Chain Reaction (PCR) and E gene PCR. These tests have been effective in the detection of COVID-19 infections from respiratory secretion samples. However, confirmation of the PCR test result may be required, and this can be done by sequencing the RNA-dependent RNA polymerase (RdRp) gene [19,20]. The serological tests are used to determine the presence of antibodies to the coronavirus, in patients who recovered from COVID-19, or those that may have been acquired the disease but showing mild or no symptoms [21].
At present, there is no proven antiviral medication available for COVID-19. The current treatment method for infected patients is mainly supportive care [1,22]. However, it was reported that patients at Jinyintan Hospital in Wuhan, China treated with oral doses of oseltamivir, lopinavir, and ritonavir tablets and intravenous injection of ganciclovir showed faster recovery rate [18,23]. On the other hand, dexamethasone lowered 4 weeks of mortality among hospitalized patients that received either invasive mechanical ventilation or oxygen alone at randomization but not among those received no respiratory support [24]. Additionally, alpha-interferon, a combination of Hydroxychloroquine and azithromycin, arbidol, favipiravir were given some critical recovery rate in various countries [25]. In contrast, remdesivir touted as the savior drug for COVID-19 patients but failed in the first clinical trial [26].
Risk factors for transmission of COVID-19 are largely uncharacterized [27]. However, to avoid the spread of COVID-19, the CDC and WHO have instituted a preparedness and prevention agenda for the infection to be used by the public, first-responders, and healthcare providers and professionals [28,29]. Although this agenda is somewhat practiced by hospitals with confirmed COVID-19 patients, the general population is not completely compliant, thus, putting them at extremely high risk of exposure to the infections from colleagues, household members, and the community [27,30].
Clusters of local transmission must be identified [31,32] to improve control of spread through preparedness, readiness, and response actions [33]. Control of the spread of COVID-19 also requires precise information on human mobility, epidemiological, and genetic data at local, regional, and global levels. This information will ensure success in the deployment of resources in the mitigation of COVID-19 transmission [30,34].
Treatment and control of viral disease are complicated by the propensity of the virus to mutate, making development and deployment anti-viral vaccines very challenging [35,36]. In this regard, a recent study reported the initial analysis of APOBEC and ADAR deaminase-mediated mutation signature patterns in complete COVID-19 genomes from informative locations and times in China, USA and Spain identified a unique set of new putative coordinated Riboswitches in COVID-19 genomes and variants in common strain Haplotypes. The results reveal that COVID-19 diversifies using switching of RNA Haplotypes with minimal alteration to protein structure [37]. Thus, vaccine designs that assume that the main viral protein antigens will be the only putative protective targets could fail to produce effective and protective immunity. Therefore, the challenges for targeted drug and vaccine development for COVID-19 needs understanding COVID-19 adaptation and survival strategy and identifying the host Haplotype, and which vaccine(s) is effective for each Haplotype group [37].
However, there are other alternative means to control COVID-19 infection and preventing transmission. This can be done implementing containment, which includes isolation of known cases, quarantine of persons believed at high risk of exposure, especially during the initial phase of the outbreak, and closing regional and country borders and restricting movement and travel and screening travelers for the infection [2,38,39]. As the last resort and if necessary, the community could be placed on complete lockdown [40].
The exact source of the COVID-19 virus remains elusive [41]. To ensure that the pandemic can be curtailed, the source and mode of transmission of the infection must be precisely determined. Thus, in this review, we focused on the causative agent, the main modes of transmission, and sources of COVID-19.
2. Methodology
2.1. Document identification on COVID-19
Several pertinent online databases including PubMed, Google Scholar, Scopus, bioRxiv, medRxiv, and chemRxiv were searched to collect relevant data. Information from some articles published online by the Chinese Center for Disease Prevention and Control, and the WHO was also included in the review.
2.2. Inclusion criteria
All publications available in peer-reviewed journals, review, practical and technical articles addressing the main routes, modes, and sources of COVID-19 virus transmission in humans were selected, inclusive of full review articles, practical, and technical papers.
2.3. Exclusion criteria
Case reports, editorials, letters, letters to the editor, comments/commentaries, short/brief communications, rapid reviews, short/rapid reports, opinions, news, seminars, features, correspondences, exercises, protocols, guidance, highlights, forums, images, interviews, insights, panels, symposia, abstracts, personal views, perspectives, preprints, podiums, policies, consensus documents, and practices were excluded from the review.
2.4. Data collection
Gathering of publications from websites was done with the Medical Subject Heading (MeSH) using terms such as new Coronavirus, novel Coronavirus, COVID-19, 2019-nCov, SARS-Coronavirus-2, SARS-CoV-2, MERS-Coronavirus-2, and MERS-CoV-2. Keywords from the title of the subject matter, such as COVID-19 mode of transmission, route of transmission of COVID-19, and source of COVID-19, COVID-19 viral load, and shedding were also used. To ensure that all relevant researches are including, the following terms were used in combination with COVID-19: transmission, spread, source, viral load, viral shed, infection, respiratory illness, pneumonia, outbreak, pandemic, quarantine, and lockdown. The titles, author list, and abstracts of these publications were evaluated to avoid data duplication.
2.5. Extraction of the data
Data from selected publications were extracted and documented in the excel spreadsheet.
3. Results and discussion
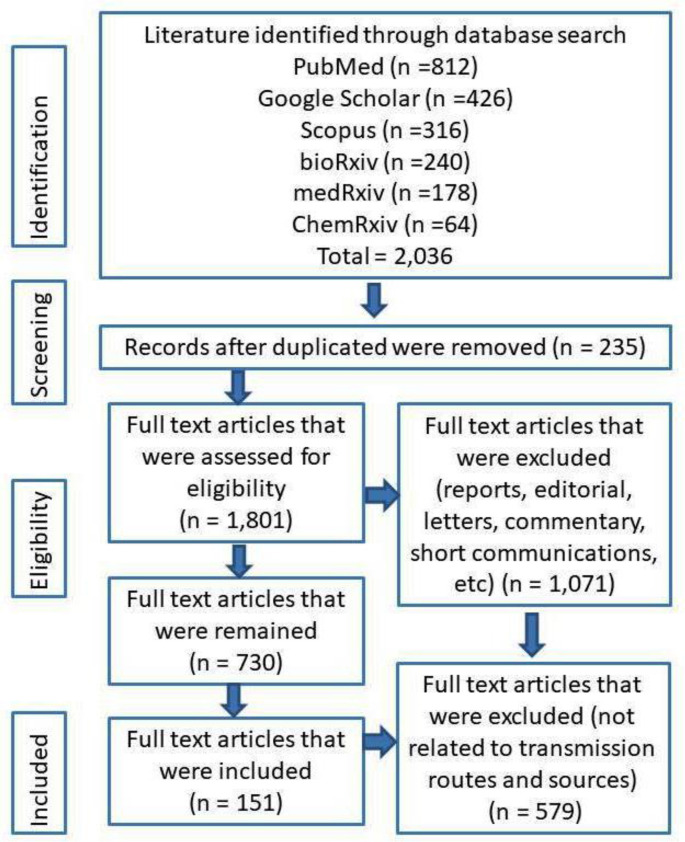
A total number of 2036 of publications were identified. Among these publications, 235 were duplicated studies and excluded. The remaining 1801 publications were scrutinized based on exclusion criteria, and as a result, 1071 publications were removed from the study. The remaining 730 articles were again screened and finally, only 151 publications were found relevant included in the review (Fig. 1 ).
Fig. 1.
PRISMA flow diagram for the current systematic review process indicating the study selection stages and analyzed results.
3.1. Emergence of coronavirus
After the report of an emerging coronavirus disease epidemic in Wuhan City, China in December 2019, early in January 2020, the Chinese Center of Disease Control and Prevention of CDC China conducted an epidemiology investigation by collecting 585 collected environmental samples from the Huanan Seafood Market in Wuhan, Hubei Province, China on two occasions on January 1 and 12 2020. They discovered that 33 samples were positive for COVID-19 and suggested that wild animals sold at the market were the origin of the virus [42]. Earlier in December 2019, in the province, among lung fluid, blood, and throat swab samples collected from 41 pneumonia cases of unknown etiology, 15 were positive for virus-specific nucleic acid sequence. Upon isolation, the ultrastructure of the virus was typical of human coronavirus species [43]. The virus is related to some β-coronaviruses genera in bats [[43], [44], [45]].
3.2. Genetic analysis of the virus concerning transmission
After examination of approximately 169 genomes, the COVID-19 virus can be classified into two major genotypes, namely, Type I (A and B) and II. Based on the phylogenetic investigation, Type IA almost appeared like the ancestral COVID-19 virus. Type II, which evolved from Type I, is a predominant and highly contagious form of the virus, and this virus is suggested to be one responsible for the epidemic that began in the wet-market of Wuhan City, China. People with COVID-19 that did not have direct contact with the Wuhan marketplace may have been infected with the Type I virus [46].
3.3. Modes and sources of coronavirus transmission
The character and trends of current COVID-19 transmission can be used as indicators of how the COVID-19 pandemic will behave throughout the rest of 2020 and in the following years [7].
3.4. Animal-to-human transmission
The transmission of COVID-19 in humans is no clear [47], although early in the pandemic, it was declared that the infection is zoonotic [48], that is, from animals to human [43,49,50].
The bat coronavirus, Beta-CoV/bat/Yunnan/RaTG13/2013 (bat/RaTG13), is similar to the human COVID-19 virus [51], suggesting bats to be the reservoir for acute infection in humans [52]. In fact, at the whole genome level, the human nCoV-2019 is 96% identical to a bat coronavirus [53]. However, in another report, Ji et al. (2020) described the homologous recombination within the spike glycoprotein of COVID-19 that favors cross-species transmission. It was also suggested snake as another probable virus reservoir for human infections because of the resampling similarity codon usage bias of COVID-19 is similar to that of Bungarus multicinctus snake [54]. On the other hand, recent studies had implicated the pangolins as the missing link in COVID-19 infection to humans because they provided a partial spike gene to COVID-19. The critical functional sites in the spike protein of COVID-19 in humans are nearly identical (91.02% at the whole genome level) to a virus isolated from a pangolin. Based on this finding, it was also suggested that the pangolins are a natural reservoir of the COVID-19 virus and play a major role in the transmission of the disease to humans [[55], [56], [57]].
Recently, many patients without a history of exposure to the marketplace in Wuhan, China, or animals elsewhere and medical professionals had contracted the disease, which indicates that the infection is not merely animal-to-human transmission, instead, it may occur between humans [16,[58], [59], [60]]. This mode of transmission of the disease appears to be similar to SARS and MERS, known to infect and spread among humans through close contact and respiratory droplets from coughs and sneezes [11]. Whether COVID-19 behavior is similar to SARS or MERS needs further investigations.
3.5. Human-to-human transmission
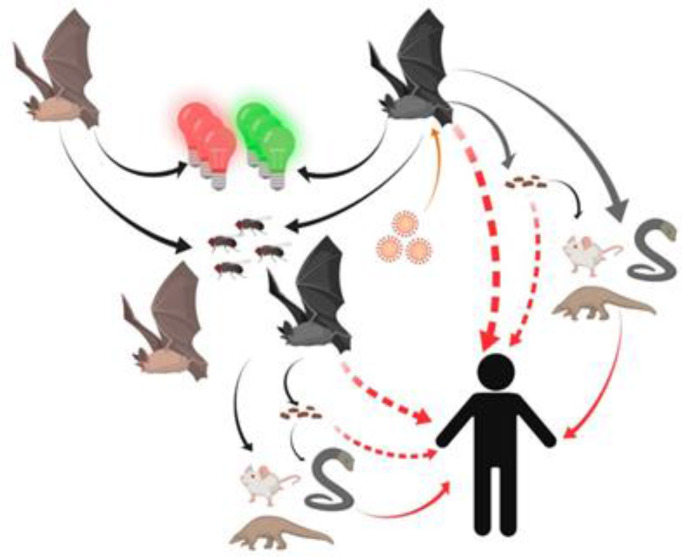
Current data showed that there is the human-to-human transmission of COVID-19 [61], suggesting that to be the main mode of transmission of the disease in the current pandemic [62]. Thus, there is a need for rigorous surveillance and testing to prevent further expansion of the pandemic [[63], [64], [65]]. Generally, patients showing symptoms of COVID-19 will spread the disease to those in close contact [66,67]. However, many COVID-19 patients are asymptomatic and can serve as carriers and unknowingly transmit the virus [[68], [69], [70]]. This may be the reason for the number of COVID-19 cases increased drastically and rapidly in some societies that lax in the practice of isolation and social distancing [59,71,72], and within families with asymptomatic infected individuals [73] (Fig. 2 ).
Fig. 2.
Main routes of transmission for COVID-19 to humans [71].
The COVID-19 is a fast expanding pandemic, which caught many countries off-guard. In many countries, the control of the infection is hindered by inadequate emergency settings, suboptimal logistics, and scarcity of personal protective equipment (PPE) [74]. This had endangered front liners and healthcare providers that were directly involved in the handling of COVID-19 cases. This situation suggests the need for other treatment means, like telemedicine, which is ideal for medical practitioners to evaluate, diagnose and treat patients from a distance, thus, while avoiding contact with and exposure to infected patients [66,67]. Telemedicine is especially ideal because [75,76] physicians can simultaneously attend to patients at several distant locations. In pandemics, where the medical practitioners are stretched to provide services, telemedicine will serve to provide the means to obtain the much need medical attention to a large number of patients [77].
Currently, it is difficult to determine whether a COVID-19 case is imported, secondary, and the result of tertiary transmission [78,79]. However, there are confirmed cases of secondary and tertiary transmission of COVID-19 [80,81], and these cases are believed to be the leading causes for rapid cycles of disease transmission from one generation to the next [35]. There are now many pieces of evidence of chains of at least four generations (highest level of first-generation) infections, which unequivocal suggest the existence of sustained human-to-human transmission [63,[82], [83], [84]].
This type of human-to-human COVID-19 transmission includes:
3.5.1. Horizontal Transmission
Based on the latest guidelines from Chinese health authorities, there are 3 main transmission routes for the COVID-19 virus in humans [[85], [86], [87]], namely direct contact, aerosol, and droplets.
3.5.1.1. Direct Contact Transmission
Direct contact transmission may occur through direct contact with virus-contaminated objects or surfaces and infecting people through the mouth, nose, or eyes [88,89]. Healthcare providers attending COVID-19 patients are especially at risk of being infected via this mode of disease transmission [84], one reason there are numerous nosocomial infections [54,86]. In direct contact transmission, the fomites are suspected to be the main source of infectious particles [90,91]. The transmission of COVID-19 can be minimized by frequent washing of hands with an alcohol-based hand rub or soap and water and avoiding touching eyes, nose, and mouth with contaminated hands [92].
3.5.1.2. Aerosol Transmission
Although the COVID-19 virus is not principally an airborne virus [93], the aerosols from expired air coughs, and sneezes that contaminate the immediate environment [88,89] are among media for virus spread [[94], [95], [96]]. Aerosol transmission is not just from people with symptoms of the disease, even asymptomatic COVID-19-positive people can be the source of infection [69,97]. In close environments, the virus-containing aerosol may persist in the air for long periods and at high concentrations, further increasing the rate of transmission [82]. The virus remains viable for at least 3 h in aerosols and 48–72 h on stainless steel and plastic surfaces [98].
Nosocomial transmission of the COVID-19 [99] is a serious threat to healthcare providers. Nosocomial infections through aerosols can occur when they perform procedures on the respiratory pathway, perform dental care [94] and hemodialysis [100], and manage of intensive care unit (ICU) [101]. Management of respiratory diseases and disorders often requires the use of high-flow nasal oxygen or endoscopy, procedures that may generate COVID-19 virus-laden aerosols into the environment, and infecting inadequately protected people. To minimize the risk of infection among healthcare providers, the CDC had formulated recommendations on the use of PPE including gowns, gloves, N95 respirators, face-shield, or goggles. In extreme cases, a powered, air-purifying respirator (PAPR) should be used to aid respiration healthcare providers. These precautions should be concurrent with the practice of social distancing [[102], [103], [104]].
3.5.1.3. Droplet Transmission
Respiratory air normally contains an abundance of droplets of sizes less than 5 μm in diameter. Coughing and sneezing cause increased expulsion of droplets from the oral cavity and respiratory tract. In COVID-19 patients these droplets contain a virus that if inhaled or ingested or landing on the mucous membranes will cause disease in people [98,105]. This mode transmission is the most dangerous form of COVID-19 spread among healthcare providers [106]. The use of PPE with efficient barriers to the droplets and maintenance of personal and environmental hygiene will limit the rate of infections [107].
COVID-19 viral transmission may also occur via feces, tears, and conjunctival secretions [101,[108], [109], [110]].
3.6. Fecal-oral transmission
The role of feces in the transmission of COVID-19 is unclear [111]. There have been suggestions that the gastrointestinal system is a critical route for the spread of this virus [112]. The COVID-19 virus infects cells via the surface angiotensin-converting enzyme 2 (ACE2). Incidentally, it was shown that there are high expressions of ACE2 in gastric glandular, colon and ileum absorptive enterocytes, duodenal and rectal cells suggesting the virus spread via the fecal-oral route [97,107,113]. It seems, that using immunofluorescent staining, the ACE2-positive cells are rarely detected in esophageal mucosa. This is probably of the predominance of esophageal squamous epithelial cells that express less ACE2 than glandular epithelial cells [114].
Specimens collected from the surface of the toilet bowl, in the sink, and the door handles of toilets used COVID-19-positive patients without diarrhea, were all positive for the virus [108]. However, these toilets were negative for the virus after it has been thoroughly sanitized. Therefore, public toilets with poor sanitation may pose infection risks to users through contact transmission. Sharing of toilets between healthy and COVID-19-positive individuals, although still contentious [52], may facilitate the transmission of the infection through the fecal-oral route [115].
It seems that the feces of recovered COVID-19 patients negative for the virus in their respiratory secretions may contain viral RNA that remains viable in feces for long periods. It may be that gastrointestinal infection with this virus and fecal-oral transmission of COVID-19 can still occur even after the respiratory tract of COVID-19 patients had become clear of the virus [116]. Clearance of the COVID-19 virus in the respiratory tract occur within two weeks, whereas the feces can remain positive for viral RNA for longer than 4 weeks, after abatement of fever [117]. Thus, although the respiratory secretion of recovered patients may appear negative for the virus, according to the principles of transmission-based Precautions, the feces should be checked for the presence of the virus, to ensure that they have completely virus-free and completely recovered from the infection [114].
3.7. Transmission through organ transplantation and surgical operations
The COVID-19 virus resides principally in the respiratory tract and its secretions. These pose risks to healthcare providers if these patients require surgery. This is particularly true in organ transplantation procedures. Recipients of organs, especially, because being place in the immunocompromised state, which is necessary for the procedure, are at high risk of being infected with COVID-19 [17,118]. Thus, unless necessary, it would be advisable to delay organ transplantation procedures until the COVID-19 pandemic has gone through its full course and no longer a health threat. However, emergency surgical interventions should be followed in the COVID-19 population in case of severe appendicitis; abortions that need caesarian section, strangulated inguinal hernias, intestinal/respiratory obstructions, severe toothache, aggressive tumor, painful kidney stone and even severe trauma caused by a gunshot or car accident [119].
Although until the beginning of April 2020 there was no published data on cases of transmission of COVID-19 from patients to surgeons, healthcare providers, and other hospitalized patients during the surgical operation, hospitalization, and treatment [120]. But later on, it was confirmed by the Chinese Center for Disease Control and Prevention that 3.8% of reported cases were healthcare personnel from which 15% were classified as severe cases with only 0.2% mortality rate. In COVID-19 patients, there is an evidence of the virus in the sputum (93%), blood (1%), stool (29%), and maybe some organs contain the virus, which may expose surgeons, or operating room staff [121]. Taken together, this suggests that pulmonary and upper respiratory surgeries are likely to be higher risk, but that colorectal and gastrointestinal surgeries may also have a greater risk of transmission [122].
3.7.1. Vertical Transmission
The COVID-19 pandemic puts pregnant women and fetuses at risk of being infected by the virus. This is primarily due to the high expression of ACE2 receptors in the human maternal-fetal interphase. This ACE2 receptor structure of the COVID-19 virus is analogous to that of SARS. Thus, like SARS, COVID-19 can potentially be transmitted vertically [116,123,124]. The first case of possible vertical transmission of COVID-19 was reported in March 2020. The neonate of a COVID-19-positive mother, 2 h after birth, showed elevated blood IgM and cytokine levels [125]. Other cases similarly showed neonates to be positive for the COVID-19 virus, shortly after birth [126], while the amniotic fluid, cord blood, breast milk after the first lactation of their mothers were negative of the virus [127,128]. The mothers acquired the infection in the last trimester of their pregnancy, thus, it is still not clear of the perinatal outcome had the infection occurred early in the pregnancy [129]. Not all neonates of COVID-19-positive mothers acquired the disease [130] because one study showed that none of the neonates born of 31 mothers infected with COVID-19 had the disease [130,130]. Although cases of vertical COVID-19 transmission are few and maybe incidental, the potential of vertical transmission of COVID-19 should not be ruled out.
Learning from the previous SARS outbreak, the Canadian Society of Obstetricians and Gynecologists guidelines suggested immediate clamping of the umbilical cord to avoid potential transmission of the COVID-19 following delivery [131,131]. As an additional precaution, newborns of mothers with suspected or diagnosed COVID-19 infection should be isolated and monitored for clinical manifestations of the infection for 14 days after birth [132]. Since the breast milk of COVID-19-mothers is virus-free, breastfeeding is safe for the newborn [133,134]. However, mothers should be wearing masks during breastfeeding to minimize the risk of transmitting the infection to the newborns through droplets and aerosols [135].
3.8. Viral load and shedding
The pathophysiological characteristics of COVID-19 have not been determined and there is much uncertainty regarding the mechanism of shedding and spread of the virus [136,137]. Recent estimates suggested that COVID-19 has a median incubation period of 3 days (range: 0–24 days) [138] with potential asymptomatic transmission [139,140]. Epidemiological evidence showed the virus can be transmitted during the incubation period [141], especially during the late stages [142]. Interestingly, the viral could not be detected early in diseased patients from the tertiary transmission, thus, it could be speculated that the infectivity of COVID-19 will gradually decrease with the level of transmission [143]. The highest viral load in COVID-19 patients are in the sputum, saliva, and upper airway secretions [144], although the virus may also be found in blood, pharynx, and anus [145].
The most critical characteristics of a COVID-19 virus are their high capacity for shedding, transmission, and spread [146,147]. The median period of COVID-19 viral release from the time patients nasopharyngeal swab tested positive, is 12 days, although some patients shed virus after one week. In these patients, the virus may be present in stool, whole blood, and urine [23,151,152]. In COVID-19-positive patients at Wuhan Pulmonary Hospital, China, there were more positive anal than oral swabs, which suggests a possible fecal-oral route for the transmission of the disease [148].
The COVID-19 virus exhibits gastrointestinal tropism and may account for the frequent occurrence of diarrhea in infected patients. The shedding of the virus in feces facilitates the spread of the disease via fomite transmission and aerosolized toilet plume [149].
The nasopharyngeal viral load of patients with COVID-19 peaks within the first few days of onset of symptoms and viral shedding in nasopharyngeal secretions will persist for at least another 24 days [150]. Most COVID-19 patients show a high prevalence of the virus in saliva. However, saliva viral load decreases with time [151]. Thus, the saliva is a convenient noninvasive sample for the diagnosis of COVID-19, especially, at the stages of the disease.
Patients with symptoms of COVID-19 should be immediately triaged and separated from the community preferably in well-ventilated facilities while keeping safe distances of at least 2 m from other individuals [152]. Contact tracing for exposure to COVID-19-infected people is essential to identify potential hotbeds and to institute disease control and containment measures [[153], [154], [155]]. Healthcare providers are at high risk of acquiring the disease, thus, must be subjected to regular testing, practice self-monitor, report signs of illness, and voluntarily isolate themselves if they exhibit symptoms of COVID-19 [94,156]. Since the COVID-19 virus is known to survive on surfaces for hours or days [157], the healthcare providers must practice strict hygiene, regularly and conscientiously cleaning workspaces and personal items such as stethoscopes, mobile phones, keyboards, dictation devices, landlines, and nametags. The hospital must provide disinfectants for the use of personnel and patients [158].
3.9. Testing strategy to prevent viral transmission
The testing strategies that should be followed to prevent COVID-19 vertical/horizontal modes of transmission during surgery include detection of the viral RNA using Polymerase Chain Reaction (PCR) and detection of the host's response (antibodies) to the virus using serological techniques [159].
The former technique is more accurate and considered as a gold standard for this virus using swabs from the nasopharynx and/or oropharynx. Since COVID-19 can infect anyone and result in transmission before the onset of symptoms or even without individuals ever developing symptoms, testing/screening of asymptomatic patients has been considered [160].
Whereas, the latter includes detection of IgM, IgA, IgG, or total antibodies in the blood although this virus is not typically present in blood in which COVID-19 infection causes white blood cells (WBC) to make antibody proteins that help the immune system identify the viruses and stop them or mark infected cells for destruction. This process is host-dependent and takes 7–11 days after exposure to the virus, although some patients may develop antibodies sooner. Therefore, this technique is not useful in the setting of an acute infection [159].
3.10. Protection strategies
Standard surgical personal protective equipment (PPE) includes a face shield, high-performance filtering mask, waterproof gown, double gloves, and shoe covers that must be implemented properly (Table 1 ) [161]. However, there is some disagreement about the type of respiratory protection N95 respirator, powered air-purifying respirator (PAPR), and standard surgical mask that should be used for surgical procedures on patients with COVID-19. Mask is considered as the main item of PPE as it is easy to obtain, cheap, and available in almost all hospitals including low-income countries. A surgical mask is capable of blocking the gross inhalation of respiratory droplets, while a well-fitted N95 respirator is proficient in filtering aerosols [162,163]. On the other hand, high-performance filtering masks including FFP1, FFP2, and FFP3 masks have a high filtration efficiency of 80%, 94%, and 99% respectively, while N95 mask blocks at least 95% of solid and liquid aerosol test particles. Thus, the FFP3 is likely to be twice as effective as the FFP2 mask, and broadly both are equivalent or superior to an N95 mask.
Table 1.
Recommended personal protective equipment (PPE) to be used against COVID-19 infection, according to the setting, personnel and type of activity. Reprinted from World Health Organization, 2020.
| Setting | Target personnel or patients | Activity | Type of PPE or procedure |
|---|---|---|---|
| Healthcare facilities | |||
| Inpatient facilities | |||
| Patient room | Healthcare workers | Providing direct care to COVID-19 patients. | Medical mask Gown Gloves Eye protection (goggles or face shield). |
| Aerosol-generating procedures performed on COVID-19 patients. | Respirator N95 or FFP2 standard, or equivalent. Gown Gloves Eye protection Apron |
||
| Cleaners | Entering the room of COVID-19 patients. | Medical mask Gown Heavy duty gloves Eye protection (if risk of splash from organic material or chemicals). Boots or closed work shoes |
|
| Visitors | Entering the room of a COVID-19 patient | Medical mask Gown Gloves |
|
| Other areas of patient transit (e.g., wards, corridors). | All staff, including healthcare workers. | Any activity that does not involve contact with COVID-19 patients. | No PPE required |
| Triage | Healthcare workers | Preliminary screening not involving direct contact | Maintain spatial distance of at least 1 m. No PPE required |
| Patients with respiratory symptoms. | Any | Maintain spatial distance of at least 1 m. Provide medical mask if tolerated by patient. |
|
| Patients without respiratory symptoms. | Any | No PPE required | |
| Laboratory | Lab technician | Manipulation of respiratory samples. | Medical mask Gown Gloves Eye protection (if risk of splash) |
| Administrative areas | All staff, including healthcare workers. | Administrative tasks that do not involve contact with COVID-19 patients. | No PPE required |
| Outpatient facilities | |||
| Consultation room | Healthcare workers | Physical examination of patient with respiratory symptoms. | Medical mask Gown Gloves Eye protection |
| Healthcare workers | Physical examination of patients without respiratory symptoms. | PPE according to standard precautions and risk assessment. | |
| Patients with respiratory symptoms. | Any | Provide medical mask if tolerated. | |
| Patients without respiratory symptoms. | Any | No PPE required | |
| Cleaners | After and between consultations with patients with respiratory symptoms. | Medical mask Gown Heavy duty gloves Eye protection (if risk of splash from organic material or chemicals). Boots or closed work shoes |
|
| Waiting room | Patients with respiratory symptoms. | Any | Provide medical mask if tolerated. Immediately move the patient to an isolation room or separate area away from others; if this is not feasible, ensure spatial distance of at least 1 m from other patients. |
| Patients without respiratory symptoms. | Any | No PPE required | |
| Administrative areas | All staff, including healthcare workers. | Administrative tasks | No PPE required |
| Triage | Healthcare workers | Preliminary screening not involving direct contact | Maintain spatial distance of at least 1 m. No PPE required |
| Patients with respiratory symptoms. | Any | Maintain spatial distance of at least 1 m. Provide medical mask if tolerated. |
|
| Patients without respiratory symptoms. | Any | No PPE required | |
| Community | |||
| Home | Patients with respiratory symptoms. | Any | Maintain spatial distance of at least 1 m. Provide medical mask if tolerated, except when sleeping. |
| Caregiver | Entering the patient's room, but not providing direct care or assistance. | Medical mask | |
| Caregiver | Providing direct care or when handling stool, urine or waste from COVID-19 patient being cared for at home. | Gloves Medical mask Apron (if risk of splash) |
|
| Healthcare workers | Providing direct care or assistance to a COVID-19 patient at home | Medical mask Gown Gloves Eye protection |
|
| Public areas (e.g., schools, shopping malls, train stations). | Individuals without respiratory symptoms | Any | No PPE required |
| Points of entry | |||
| Administrative areas | All staff | Any | No PPE required |
| Screening area | Staff | First screening (temperature measurement) not involving direct contact | Maintain spatial distance of at least 1 m. No PPE required |
| Staff | Second screening (i.e., interviewing passengers with fever for clinical symptoms suggestive of COVID-19 disease and travel history). | Medical mask Gloves | |
| Cleaners | Cleaning the area where passengers with fever are being screened. | Medical mask Gown Heavy duty gloves Eye protection (if risk of splash from organic material or chemicals). Boots or closed work shoes |
|
| Temporary isolation area | Staff | Entering the isolation area, but not providing direct assistance. | Maintain spatial distance of at least 1 m. Medical mask Gloves |
| Staff, healthcare workers | Assisting passenger being transported to a healthcare facility. | Medical mask Gown Gloves Eye protection |
|
| Cleaners | Cleaning isolation area | Medical mask Gown Heavy duty gloves Eye protection (if risk of splash from organic material or chemicals). Boots or closed work shoes |
|
| Ambulance or transfer vehicle | Healthcare workers | Transporting suspected COVID-19 patients to the referral healthcare facility. | Medical mask Gowns Gloves Eye protection |
| Driver | Involved only in driving the patient with suspected COVID-19 disease and the driver's compartment is separated from the COVID-19 patient. |
Maintain spatial distance of at least 1 m. No PPE required |
|
| Assisting with loading or unloading patient with suspected COVID-19 disease. | Medical mask Gowns Gloves Eye protection |
||
| No direct contact with patient with suspected COVID-19, but no separation between driver's and patient's compartments. | Medical mask | ||
| Patient with suspected COVID-19 disease. | Transport to the referral healthcare facility. | Medical mask if tolerated | |
| Cleaners | Cleaning after and between transport of patients with suspected COVID-19 disease to the referral healthcare facility. | Medical mask Gown Heavy duty gloves Eye protection (if risk of splash from organic material or chemicals). Boots or closed work shoes |
|
| Special considerations for rapid response teams assisting with public health investigations | |||
| Community | |||
| Anywhere | Rapid response team investigators. | Interview suspected or confirmed COVID-19 patients or their contacts. | No PPE if done remotely (e.g., by telephone or video conference). Remote interview is the preferred method. |
| In-person interview of suspected or confirmed COVID-19 patients without direct contact. | Medical mask Maintain spatial distance of at least 1 m. The interview should be conducted outside the house or outdoors, and confirmed or suspected COVID-19 patients should wear a medical mask if tolerated. |
||
| In-person interview with asymptomatic contacts of COVID-19 patients. | Maintain spatial distance of at least 1 m. No PPE required The interview should be performed outside the house or outdoors. If it is necessary to enter the household environment, use a thermal imaging camera to confirm that the individual does not have a fever, maintain spatial distance of at least 1 m and do not touch anything in the household environment. |
||
In this regard, a study in 4 hospitals at Wuhan, China on 420 fully protected (implemented appropriate PPE) healthcare professionals (116 doctors and 304 nurses) with an average age of 35.8 years and an average work of 16.2 h each week in intensive care units that had direct contact with COVID-19 patients and performed at least one aerosol-generating procedure for 6–8 weeks has been conducted. The results revealed that during the deployment period, none of the participants reported COVID-19 related symptoms and when the participants returned home, they all tested negative for COVID-19 specific nucleic acids and IgM or IgG antibodies [164].
3.11. Recently developed vaccines
Very recently chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19) expressing the SARS-CoV-2 spike protein has been developed in the University of Oxford, UK in collaboration with AstraZeneca Company in a ½ phase, single, blind randomized controlled trial on healthy adults aged 18–55 years with no history of COVID-19 infection or COVID-19-like symptoms. But, this vaccine produced side effects in vaccinated individuals such as pain, slight fever, chills, muscle ache, headache, and malaise which were reduced by the use of prophylactic paracetamol. However, it presented an acceptable safety profile, and homologous boosting improved antibody responses [165].
Moreover, a recombinant adenovirus type-5-vectored COVID-19 (Ad5-vectored COVID-19) vaccine in healthy adults aged 18 years or older with no history of HIV and COVID-19 infections were developed in Beijing Institute of Biotechnology, Wuhan, China in a randomized, double-blind, placebo-controlled, phase 2 trial. This vaccine seemed to be safe and induced significant immune responses in the majority of recipients after a single immunization [166].
Furthermore, a COVID-19 RNA Vaccine Candidate (BNT162b1) has been developed by both Pfizer and BioNTech Companies, Germany in the placebo-controlled, observer-blinded dose-escalation study among healthy adults, 18–55 years of age, randomized to receive 2 doses, separated by 3 weeks, of various amounts of a lipid nanoparticle-formulated, nucleoside-modified, mRNA vaccine that encodes trimerized SARS-CoV-2 spike glycoprotein RBD. Local reactions and systemic events were seemed to be dose-dependent, generally mild to moderate, and transient [167].
Finally, the mRNA-1273 vaccine is developed by Moderna Company, the USA in phase 1, dose-escalation, open-label trial including 45 healthy adults, ages 18–55 years who received 3 different doses of 2 vaccinations at 4 weeks interval. Besides, this vaccine produces immune response but more than half of the participants showed adverse effects such as fatigue, chills, headache, myalgia, and pain at the injection site especially after 2nd vaccination with high doses of mRNA-1273 [168].
4. Conclusion
COVID-19 infection may have begun from animal-to-human infection, however, the virus has evolved into a form that causes rapid human-to-human transmission through aerosols, droplets, and direct contact. Nosocomial transmission of COVID-19 patients to healthcare works can be controlled by instituting adequate safety measures. However, control of COVID-19 transmission in the community may not be as straightforward because this depends on strict societal compliance to the standard operating procedure for control of the COVID-19 pandemic, which includes social distancing, wearing masks, personal hygiene, and avoiding crowds. These precautions are even more critical for the elderly and those with comorbidities such as cardiovascular and kidney diseases, diabetes, and other chronic diseases. Measures were taken by the government to lock down the country are probably the most effective means of controlling the pandemic. However, the country mustn't be too hasty in the lifting of lockdown because of the pressure to restart the economy. Lifting lockdown prematurely, when the disease is not adequately contained, poses the risk of a flare-up in second and third wave pandemic.
4.1. Strength and limitation of the studies
The review applied a systematic and rigorous search strategy to retrieve relevant articles based on the objectives of this review. The information in this review is current at the point of publication. New information on the COVID-19 pandemic is being published every day, however, much is still not known about the COVID-19 pandemic. For example, there are gaps in knowledge on the mode, source, and mechanism of transmission of COVID-19 and the rate of mutation of the virus in the community. This information must be acquired to ensure containment of the pandemic through the use of vaccines or institutions of new normal physical and psychological behaviors, which with luck will eradicate the virus from the community.
Ethical approval
No patient samples were used.
Funding
No fund is obtained for this review article.
Author contribution
Heshu S. Rahman: Conceptualization, Writing-Original draft preparation, Reviewing and Editing.
Masrur S. Aziz: Data Analysis, Resources and Visualization.
Ridha H. Hussein: Writing-Original draft preparation.
Hemn H. Othman: Writing-Original draft preparation.
Shirwan H. Omer: Writing-Original draft preparation.
Eman S. Khalid: Resources and Visualization.
Nusayba A. Abdulrahman: Resources and Visualization.
Kawa Amin: Writing-Original draft preparation.
Rasedee Abdullah: Writing-Original draft preparation, Reviewing and Editing.
Conflict of interest statement
Authors declared that there is no conflict of interest to this manuscript.
Guarantor
Prof. Dr. Heshu Sulaiman Rahman.
Research registration number
There is no human participants and this is a review article.
Consent
No studies on patients has been done and this is a review article.
Acknowledgments
Our sincere thanks go to all the experts who contributed to the writing of this review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijso.2020.08.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Centers for Disease Control (CDC)-2019 . 2020. Novel Coronavirus (2019-nCoV)2020 Feb 1. [Google Scholar]
- 2.Olivencia G.R., Membrillo de Novales F.J., Rosado M.V.G., Figueras A.I.L., Alonso P.C., Estébanez M. 2020. Quarantine for SARS-CoV-2 in Spain: how did we do it? [Google Scholar]
- 3.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2):575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Novel coronavirus (2019-nCoV) situation report. 2020;9(29) [Google Scholar]
- 6.Fang Y., Nie Y., Penny M. Transmission dynamics of the COVID-19 outbreak and effectiveness of government interventions: a data-driven analysis. J Med Virol. 2020;92(6):645–659. doi: 10.1002/jmv.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly. 2020;150(1112) doi: 10.4414/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- 8.Qian G.Q., Yang N.B., Ding F., Ma A.H.Y., Wang Z.Y., Shen Y.F., et al. Epidemiologic and clinical characteristics of 91 Hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM: Int J Med. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haider N., Yavlinsky A., Simons D., Osman A.Y., Ntoumi F., Zumla A., et al. Passengers' destinations from China: low risk of novel coronavirus (2019-nCoV) transmission into Africa and South America. Epidemiol Infect. 2020;148:e41. doi: 10.1017/S0950268820000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J., Ouyang W., Chua M.L., Xie C. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. medRxiv. 2020 doi: 10.1101/2020.02.22.20025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.China N.H.C.o.P.s.R.o Prevent guideline of 2019-nCoV. http://wwwnhcgovcn/xcs/yqfkdt/202001/2020
- 12.Woo P.C., Lau S.K., Li K.S., Poon R.W., Wong B.H., Tsoi H.W., et al. Molecular diversity of coronaviruses in bats. Virology. 2006;351(1):180–187. doi: 10.1016/j.virol.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmood Z.H., Sleman R.R., Uthman A.U. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet Microbiol. 2011;150(1–2):21–27. doi: 10.1016/j.vetmic.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T., Hu J., Xiao J., He G., Kang M., Rong Z., et al. Time-varying transmission dynamics of Novel coronavirus pneumonia in China. bioRxiv. 2020 doi: 10.1101/2020.01.25.919787. [DOI] [Google Scholar]
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z., et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Instructions for use: LightMix modular SARS and Wuhan CoV E-gene. TIB MolBiol; Berlin: 2020. Cat. No. 53-0776-96 V. Instructions for Use: LightMix Modular SARS and Wuhan CoV E-gene. Instructions for Use: LightMix Modular SARS and Wuhan CoV E-gene Berlin: TIB MolBiol (2020) Cat No 53-0776-96, V2001122020. [Google Scholar]
- 20.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020:1–10. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralph R., Lew J., Zeng T., Francis M., Xue B., Roux M., et al. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J Infect Dev Countries. 2020;14(1):3–17. doi: 10.3855/jidc.12425. [DOI] [PubMed] [Google Scholar]
- 23.Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., et al. Isolation of Infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microb Infect. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group R.C. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Commission N.H. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) Chin Med J (Engl) 2020;133(9):1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guardian T. Vol. 20.35. BST; 2020. (First trial for potential Covid-19 drug shows it has no effect). [Google Scholar]
- 27.Liu X., Wu Y., Ali S.T. Close contacts and household transmission of SARS-CoV-2 in China: a content analysis based on local Heath Commissions' public disclosures. medRxiv. 2020 doi: 10.1101/2020.03.02.20029868. [DOI] [Google Scholar]
- 28.Centers for Disease Control (CDC) 2020. Healthcare personnel preparedness checklist for 2019-nCoV. [Google Scholar]
- 29.World Health Organization (WHO) 2020. Novel Coronavirus (2019-nCoV) advice for the public. [Google Scholar]
- 30.Critical preparedness, readiness and response actions for COVID-19. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/critical-preparedness-readiness-and-response-actions-for-covid-19 [Google Scholar]
- 31.Kucharski A.J., Russell T.W., Diamond C., Funk S., Eggo R.M., group C.n.w. Early dynamics of transmission and control of 2019-nCoV: a mathematical modelling study. medRxiv. 2020 doi: 10.1101/2020.01.31.20019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Wang W., Zhao X., Zai J., Zhao Q., Li Y., et al. Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020;92(5):501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan C., Liu L., Guo W., Yang A., Ye C., Jilili M., et al. Prediction of epidemic spread of the 2019 novel coronavirus driven by spring festival transportation in China: a population-based study. Int J Environ Res Publ Health. 2020;17(5):1679. doi: 10.3390/ijerph17051679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraemer M., Cummings D., Funk S., Reiner R., Faria N., Pybus O., et al. Reconstruction and prediction of viral disease epidemics. Epidemiol Infect. 2019:147. doi: 10.1017/S0950268818002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L., et al. Genomic diversity of SARS-CoV-2 in coronavirus disease 2019 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanmugaraj B., Malla A., Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2):148. doi: 10.3390/pathogens9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele E.J., Lindley R.A. Analysis of APOBEC and ADAR deaminase-driven Riboswitch Haplotypes in COVID-19 RNA strain variants and the implications for mono-strain vaccine design. Res Rep.1-35.
- 38.Gostic K., Gomez A.C., Mummah R.O., Kucharski A.J., Lloyd-Smith J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;9 doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahim S.H., Memish Z.A. COVID-19–the role of mass gatherings. Trav Med Infect Dis. 2020;34:101617. doi: 10.1016/j.tmaid.2020.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R., Liu H., Li F., Zhang B., Liu Q., Li X., et al. Transmission and epidemiological characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infected Pneumonia (COVID-19): preliminary evidence obtained in comparison with 2003-SARS. medRxiv. 2020 doi: 10.1101/2020.01.30.20019836. [DOI] [Google Scholar]
- 42.Chinese Center for Disease Control and Prevention . 2020. 585 environmental samples from the South China Seafood Market in Wuhan, Hubei Province, China.http://www.chinacdc.cn/yw_9324/202001/t20200127_211469 [Google Scholar]
- 43.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilocca B., Soggiu A., Musella V., Britti D., Sanguinetti M., Urbani A., et al. Molecular basis of COVID-19 relationships in different species: a one health perspective. Microb Infect. 2020;22(4–5):218–220. doi: 10.1016/j.micinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T.M., Rui J., Wang Q.P., Zhao Z.Y., Cui J.A., Yin L. A mathematical model for simulating the phase-based transmissibility of a novel Coronavirus. Infect Dis Poverty. 2020;9(1):1–8. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L., Yang J.R., Zhang Z., Lin Z. Genomic variations of SARS-CoV-2 suggest multiple outbreak sources of transmission. medRxiv. 2020 doi: 10.1101/2020.02.25.20027953. [DOI] [Google Scholar]
- 47.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Review. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q., Zhao S., Gao D., Lou Y., Yang S., Musa S.S., et al. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int J Infect Dis. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microb Infect. 2020;(2292):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020;92960:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lau S.K., Li K.S., Huang Y., Shek C.-T., Tse H., Wang M., et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6):2808–2819. doi: 10.1128/JVI.02219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020 doi: 10.1101/2020.01.22.914952. [DOI] [Google Scholar]
- 54.Ji W., Wang W., Zhao X., Zai J., Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. 2020;29(4):433–439. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong M.C., Cregeen S.J.J., Ajami N.J., Petrosino J.F. Evidence of recombination in coronaviruses implicating pangolin origins of nCoV-2019. Biorxiv. 2020 doi: 10.1101/2020.02.07.939207. [DOI] [Google Scholar]
- 56.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.J., et al. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan pangolins. BioRxiv. 2020 doi: 10.1101/2020.02.17.951335. [DOI] [Google Scholar]
- 57.Lam T.T.Y., Shum M.H.H., Zhu H.C., Tong Y.G., Ni X.B., Liao Y.S., et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv. 2020 doi: 10.1101/2020.02.13.945485. [DOI] [PubMed] [Google Scholar]
- 58.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T., Hu J., Kang M., Lin L., Zhong H., Xiao J., et al. 2020. Transmission dynamics of 2019 novel Coronavirus (2019-nCoV) [DOI] [Google Scholar]
- 60.Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M., et al. Epidemiological, clinical and virological characteristics of 74 cases of Coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jalava K. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 2020;226:113490. doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson R.N. Novel Coronavirus outbreak in Wuhan, China, 2020: intense surveillance Is vital for preventing sustained transmission in new locations. J Clin Med. 2020;9(2):498. doi: 10.3390/jcm9020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imai N., Dorigatti I., Cori A., Riley S., Ferguson N. 2020. Estimating the potential total number of novel Coronavirus cases in Wuhan City, China. [DOI] [Google Scholar]
- 65.Ung C.O.L. Community pharmacist in public health emergencies: quick to action against the coronavirus 2019-nCoV outbreak. Res Soc Adm Pharm. 2020;16(4):583–586. doi: 10.1016/j.sapharm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020;9(1):1–7. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boldog P., Tekeli T., Vizi Z., Dénes A., Bartha F.A., Röst G. Risk assessment of novel coronavirus COVID-19 outbreaks outside China. J Clin Med. 2020;9(2):571. doi: 10.3390/jcm9020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou C. Evaluating new evidence in the early dynamics of the novel coronavirus COVID-19 outbreak in Wuhan, China with real time domestic traffic and potential asymptomatic transmissions. medRxiv. 2020 doi: 10.1101/2020.02.15.20023440. [DOI] [Google Scholar]
- 70.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4):2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Respiratory Therapy Group, Chinese Medical Association Respiratory Branch Expert consensus on respiratory therapy related to new Coronavirus infection in severe and critical patients. Chin J Tubercul Respir Med. 2020;11(12):4909–4917. [Google Scholar]
- 74.Wua Y.C.W., Chena C.S., Chan Y.J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng R., Xu Y., Wang W., Ning G., Bi Y. Spatial transmission of COVID-19 via public and private transportation in China. Trav Med Infect Dis. 2020;34:101662. doi: 10.1016/j.tmaid.2020.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu T., Chen C., Zhu Z., Cui M., Chen C., Dai H., et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoecklin S.B., Rolland P., Silue Y., Mailles A., Campese C., Simondon A., et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25(6):2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X., Zai J., Wang X., Li Y. Potential of large “first generation” human-to-human transmission of 2019-nCoV. J Med Virol. 2020;92(4):448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Y., Chen H., Chen L., Cheng B., Diao P., Dong L., et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33(4) doi: 10.1111/dth.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu Q., Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologist need to know. J Med Virol. 2020;92(6):564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aziz P.Y., Hadi J.M., Aram M.S., Aziz S.B., Rahman H.S., Ahmed H.A., et al. The strategy for controlling COVID-19 in Kurdistan Regional Government (KRG)/Iraq: identification, epidemiology, transmission, treatment, and recovery. Int J Surg Open. 2020;25:41–46. doi: 10.1016/j.ijso.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belser J.A., Rota P.A., Tumpey T.M. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77(1):144–156. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koenig K.L., Bey C.K., McDonald E.C. 2019-nCoV: the identify-isolate-inform (3I) tool applied to a novel emerging coronavirus. West J Emerg Med. 2020;21(2):184. doi: 10.5811/westjem.2020.1.46760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong J., Goh Q.Y., Tan Z., Lie S.A., Tay Y.C., Ng S.Y., et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anesth/Journal canadien d'anesthésie./Journal canadien d'anesthésie. 2020;67:732–745. doi: 10.1007/s12630-020-01620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(9):1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su L., Ma X., Yu H., Zhang Z., Bian P., Han Y., et al. The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emerg Microb Infect. 2020;9(1):707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouadma L., Lescure F.X., Lucet J.C., Yazdanpanah Y., Timsit J.F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Basile C., Combe C., Pizzarelli F., Covic A., Davenport A., Kanbay M., et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35(5):737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cook T., El-Boghdadly K., McGuire B., McNarry A., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020;75(6):785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Pan Z., Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. medRxiv. 2020 doi: 10.1101/2020.02.18.20021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Publ Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin Med. 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dodd R.Y., Stramer S.L. COVID-19 and blood safety: help with a dilemma. Transfus Med Rev. 2020 doi: 10.1016/j.tmrv.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang A., Tong Z., Wang H., Dai Y., Li K., Liu J., et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong X., Cao Y.Y., Lu X.X., Zhang J.J., Du H., Yan Y.Q., et al. Eleven faces of Coronavirus disease 2019. Allergy. 2020;75:1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv. 2020 doi: 10.1101/2020.01.30.927806. [DOI] [Google Scholar]
- 114.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Danchin A., Ng T.W.P., Turinici G. A new transmission route for the propagation of the SARS-CoV-2 Coronavirus. medRxiv. 2020 doi: 10.1101/2020.02.14.20022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xing Y., Ni W., Wu Q., Li W., Li G., Tong J., et al. Prolonged presence of SARS-CoV-2 in feces of pediatric patients during the convalescent phase. medRxiv. 2020 doi: 10.1101/2020.03.11.20033159. [DOI] [Google Scholar]
- 118.Kumar D., Manuel O., Natori Y., Egawa H., Grossi P., Han S.H., et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patriti A., Baiocchi G.L., Catena F., Marini P., Catarci M. Emergency general surgery in Italy during the COVID-19 outbreak: first survey from the real life. World J Emerg Surg. 2020;15(36):1–7. doi: 10.1186/s13017-020-00314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 122.Ortega R., Gonzalez M., Nozari A., Canelli R. Personal protective equipment and Covid-19. N Engl J Med. 2020;382(26):1. doi: 10.1056/NEJMvcm2014809. [DOI] [PubMed] [Google Scholar]
- 123.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 124.Li M., Chen L., Xiong C., Li X. The ACE2 expression of maternal-fetal interface and fetal organs indicates potential risk of vertical transmission of SARS-COV-2. bioRxiv. 2020 doi: 10.1101/2020.02.27.967760. [DOI] [Google Scholar]
- 125.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. Jama. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woodward A. 2020. A pregnant mother infected with the Coronavirus gave birth, and her baby tested positive 30 hours later.https://www.businessinsider.com/wuhan-coronavirus-in-infant-bornfrom-infected-mother-2020-2 [Google Scholar]
- 128.Murphy S. 2020. Newborn baby tests positive for coronavirus in London.https://www.theguardian.com/world/2020/mar/14/newborn-baby-tests-positive-for-coronavirus-in-london [Google Scholar]
- 129.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A., et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):1–5. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maxwell C., McGeer A., Tai K.F.Y., Sermer M., Farine D., Basso M., et al. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS) 2009;107(1):82–86. doi: 10.1016/j.ijgo.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen D., Yang H., Cao Y., Cheng W., Duan T., Fan C., et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynecol Obstet. 2020;149(2):130–136. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poon L.C., Yang H., Kapur A., Melamed N., Dao B., Divakar H., et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynecol Obstet. 2020;149(3):273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang H., Wang C., Poon L.C. Novel Coronavirus infection and pregnancy. 2020;55(4):435–437. doi: 10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woo P.C., Lau S.K., Wong B.H., Chan K.-H., Chu C.-M., Tsoi H.-W., et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brüssow H. The novel coronavirus–a snapshot of current knowledge. Microb Biotechnol. 2020;13(3):607–612. doi: 10.1111/1751-7915.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020 doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 140.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ki M. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li P., Fu J.B., Li K.F., Chen Y., Wang H.L., Liu L.J., et al. Transmission of COVID-19 in the terminal stage of incubation period: a familial cluster. Int J Infect Dis. 2020;96:452–453. doi: 10.1016/j.ijid.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kam K.Q., Yung C.F., Cui L., Tzer Pin Lin R., Mak T.M., Maiwald M., et al. A well infant with Coronavirus disease 2019 with high viral load. Clin Infect Dis. 2020;71(15):847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microb Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B., et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 150.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.To K.K.W., Tsang O.T.Y., Yip C.C.Y., Chan K.H., Wu T.C., Chan J.M.C., et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen P. 2020. Study on the virus transmission based on data analysis of confirmed cases of COVID-19 coronavirus in China (III)https://osf.io/7yzwv [Google Scholar]
- 153.Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I., Clapham H.E., et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Global Health. 2020;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Bonsall D.G., et al. Quantifying dynamics of SARS-CoV-2 transmission suggests that epidemic control and avoidance is feasible through instantaneous digital contact tracing. medRxiv. 2020 doi: 10.1101/2020.03.08.20032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Biscayart C., Angeleri P., Lloveras S., Chaves T.D.S.S., Schlagenhauf P., Rodríguez-Morales A.J. The next big threat to global health? 2019 novel coronavirus (2019-nCoV): what advice can we give to travellers?–Interim recommendations January 2020, from the Latin-American society for Travel Medicine (SLAMVI) Trav Med Infect Dis. 2020;33:101567. doi: 10.1016/j.tmaid.2020.101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Interim infection prevention and control recommendations for patients with confirmed coronavirus disease 2019 (COVID-19) or persons under investigation for COVID-19 in healthcare settings. 2020. https://www.cdc.gov/coronavirus/2019- ncov/infection-control/control-recommendations Available from: [Google Scholar]
- 159.Al-Muharraqi M. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery-continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg. 2020;58(5):503–505. doi: 10.1016/j.bjoms.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patel R., Babady E., Theel E.S., Storch G.A., Pinsky B.A., George K.S., et al. Report from the American Society for Microbiology COVID-19 international summit, 23 march 2020: value of diagnostic testing for SARS–CoV-2/COVID-19. Am Soc Microbiol. 2020 doi: 10.1128/mBio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.World Health Organization . World Health Organization; Geneva . 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 February 2020.https://extranet.who.int/iris/restricted/handle/10665/331215 [Google Scholar]
- 162.Brat G.A., Hersey S., Chhabra K., Gupta A., Scott J. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferioli M., Cisternino C., Leo V., Pisani L., Palange P., Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29(155):200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu M., Cheng S.Z., Xu K.W., Yang Y., Zhu Q.T., Zhang H., et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369:1–6. doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jonathan T.M. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults. Lancet. 2020 doi: 10.1016/S0140-6736(20)31605-6. https://www.practiceupdate.com/content/immunogenicity-and-safety-of-a-recombinant-adenovirus-type-5-vectored-covid-19-vaccine-in-healthy-adults/104129/55/20/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S.P., et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. medRxiv. 2020 doi: 10.1101/2020.06.30.20142570. [DOI] [Google Scholar]
- 168.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.