Abstract
In December 2019, a novel virus, namely COVID-19 caused by SARS-CoV-2, developed from Wuhan, (Hubei territory of China) used its viral spike glycoprotein receptor-binding domain (RBD) for the entrance into a host cell by binding with ACE-2 receptor and cause acute respiratory distress syndrome (ARDS). Data revealed that the newly emerged SARS-CoV-2 affected more than 24,854,140 people with 838,924 deaths worldwide. Until now, no licensed immunization or drugs are present for the medication of SARS-CoV-2. The present review aims to investigate the latest developments and discuss the candidate antibodies in different vaccine categories to develop a reliable and efficient vaccine against SARS-CoV-2 in a short time duration. Besides, the review focus on the present challenges and future directions, structure, and mechanism of SARS-CoV-2 for a better understanding. Based on data, we revealed that most of the vaccines are focus on targeting the spike protein (S) of COVID-19 to neutralized viral infection and develop long-lasting immunity. Up to phase-1 clinical trials, some vaccines showed the specific antigen-receptor T-cell response, elicit the humoral and immune response, displayed tight binding with human-leukocytes-antigen (HLA), and recognized specific antibodies to provoke long-lasting immunity against SARS-CoV-2.
Keywords: SARS-CoV-2, Spike protein, Protein-based vaccine, Neutralization, Inactivated virus vaccine, Immune response
Graphical abstract
1. Introduction
In late December 2019, another type of deadly zoonotic beta-coronavirus occurred in Wuhan, China, which was initially termed as 2019-nCoV and then COVID-19 by World Health Organization (WHO) [1]. WHO reports revealed that COVID-19 is transmitted by a mean of direct human-to-human contact, respiratory discharges (cough and sneeze) and beads, that could stay up to 8–12 days on the surface [2,3]. Despite the fact that replication duration of COVID-19 was accounted for as between 1 and 14 days [4]. The studies observed that symptoms of COVID-19 are exactly related to the symptoms of patients that were suffering from severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 [5]. However, shortness of breath (respiratory infection), cough, itching, and muscle pain are major coronavirus associated symptoms which caused acute respiratory distress syndrome (ARDS) and ultimately led to death [[6], [7], [8]]. A respiratory viral infection is a worldwide wellbeing concern nowadays that caused hazardous respiratory contamination and extreme pneumonia among people [9,10].
The accumulated data revealed that COVID-19 is a single-stranded positive sense-RNA virus that holds a sizable RNA genome of about 29.88 kb with 12 open reading frames (ORF) and belongs to the β-group of coronavirus [8,11]. The genome-based activity analysis of COVID-19 revealed that it is caused by SARS-CoV-2 which shared over 90% matching structure to bat genome and 89.10% nucleotide resemblance with previously reported beta-coronavirus named as SARS-CoV-1 [12,13]. The data suggested that newly emerged COVID-19 is composed of several significant subunits including membrane protein (M), envelop protein (E), nucleocapsid protein (N), spike glycoproteins (S), several non-structural proteins (nsps), 3′ and 5′-untranslated proteins (UTR) [14,15]. The spike glycoprotein (S) is considered as vital proteins in the composition of coronavirus and helped in the entrance of COVID-19 virus inside the host body by binding to the ACE-2 receptor. The spike protein (see glossary; Supplementary material) is a typical 3D like structure in the receptor-binding domain (RBD) of coronavirus, which helps to sustain van der Waal forces [16]. Due to the loose attachment of RBD on the surface of coronavirus, it (COVID-19) can easily affect multiple hosts like humans and animals (bat, cat, camel, and dog) [17]. The virus entry is mainly mediated by the combining of a viral-spike protein (S) to angiotensin-converting enzyme-2 (ACE-2) (see glossary) of the lung. The reason is that the large surface area of lungs provides higher chances of entry of viruses [14]. Recently, Zhao et al. [18] experimented on eight different ACE-2 receptors of corona-patients and reported that 83% of ACE-2 cells expressed the characteristics of alveolar epithelial type II cells, which act as a reservoir for the entry of the virus. Alveolar epithelial cells type II contains a large amount of different types of viral process genes, including the genes of viral replication [19]. Several types of proteases including human airway trypsin-like protease (HAT), transmembrane protease serine 2 (TMPRSS2), and cathepsins involved in the entry of coronavirus inside the host by unbinding the spike protein and establish greater saturation chances [20].
According to the WHO, the newly emerged COVID-19 affected more than 24,854,140 people with 838,924 deaths globally, with a mortality rate of 3.37% [21]. Based on data, due to high infection rate of the global pandemic, COVID-19, there is a necessity to make a robust and safe vaccine or antiviral drug to control the cases and deaths by a newly emerged virus. Like other viruses such as influenza virus, the antivirals or vaccine are still not present against COVID-19. But some preventive measures and compounds are showing better results to control the effects of coronavirus. Recently, different therapeutic strategies including remdesivir, lopinavir, hydroquinone, and dexamethasone are showing somehow better results but still are not admirable due to dose and time-dependent, increase in AST and ALT levels, decrease in the level of electrolytes, vomiting, and digestive disorders among patients [[22], [23], [24], [25]]. Recently different developments have been done to develop vaccines against COVID-19. Vaccines target glycoproteins and antibodies, which helped in the virus entry into the host cell and produce antibody response [26].
According to emerging evidence, as of April 2020, >115 different vaccine candidates have been started to study, in which 78 candidates have been confirmed as active (at the preclinical stage) and the rest of all confirmed as inactive. The few vaccine candidates, including mRNA-1273 (Moderna), Ad5-nCoV (CanSino Biologicals), pathogen-specific aAPC (Shenzhen Geno-Immune Medical Institute), and INO-4800 (Inovio) presenting the significant results in initial trials and soon will be tested on human [27]. Spike protein (S) of coronavirus are targeted by these vaccines and help in the stimulation of neutralizing antibodies [28]. Different subunits of spike proteins like the S1 and S2 subunits, and the receptor-binding domain (RBD) are the critical elements for the formation of a vaccine against the newly emerged virus (COVID-19) that helped in producing T-cell responses and protective immunity against SARS-CoV-2 [29]. Furthermore, two recombinant proteins that carry a receptor-binding domain (see glossary), and recombinant vectors that code for the receptor-binding domain can also play a useful role in developing a corona-vaccine. Besides, the monoclonal antibodies of recovered corona-patients showing better results in neutralizing the antibodies by targeting the specific domains of SARS-CoV-2 [30,31]. However, with increasing the cases and deaths of COVID-19 day by day, the development of vaccines against SARS-CoV-2 is also required on an urgent basis. The present review investigated the new insight parameters and components to develop a vaccine against newly emerged COVID-19 by targeting the spike protein and epitopes. The present study also aims to share the candidate antibodies and latest development in the formulation of a vaccine against SARS-CoV-2. Further, current challenges and future perspectives, structure, mechanism, immune response, and different components of COVID-19 are studied in the present review for a better understanding.
2. Structure of spike protein (S)
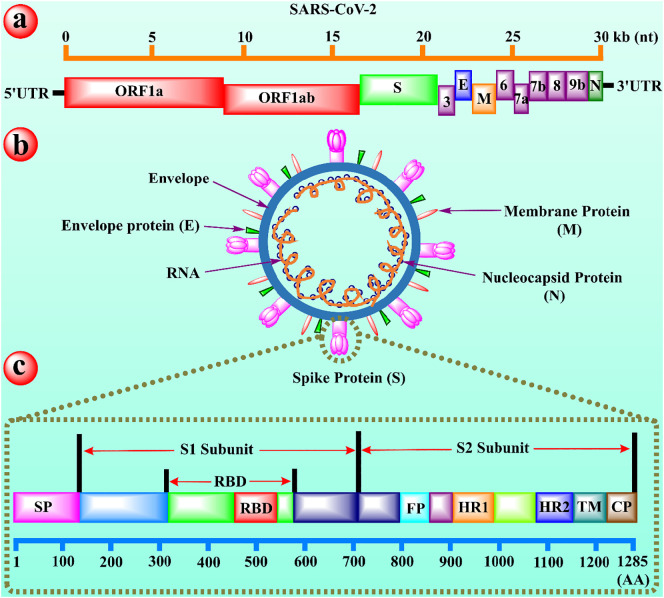
The structure evaluation of coronavirus observed that the structural proteins of COVID-19 are encoded by four main proteins including membrane (M), nucleocapsid (N), envelope (E), and spike protein (S) (see Fig. 1 ) [32]. Among all, the spike protein (S) is the most desired part of viral design that acts as a critical immunogen and also aid in a receptor attachment and infection transmission into host cells. The S protein (1285 amino acids long protein) present in two forms, namely pre-fusion and post-fusion forms. The pre-fusion (on mature virions) possess a homo-trimer shaped structure with three receptor-binding S1 heads on the top of S2 stalk. At the same time, the post-fusion (formed after completion of membrane fusion) contain a coiled like structure with S2 domain [33,34]. According to emerging evidence, the S1 domain of spike protein possesses a C-terminal domain (S1-CTD) and the N-terminal domain (NTD).
Fig. 1.
The schematic illustration of (a) full genome of SARS-CoV-2, (b) representation of viral particles of SARS-CoV-2, and (c) functional domains of spike protein (S) for SARS-C0V-2. (a) The open reading frame 1a and 1ab (ORF 1a and 1ab) encoded by non-structure proteins are represented in red. The green color denotes the spike protein. The purple color reported the accessory proteins, whereas the orange line on the top characterizes the length of genomic viral RNA. (b) The pink, red, and green colors represent spike, membrane, and envelop proteins respectively, while the orange thread inside the envelope shows the viral RNA and small dark blue balls signifies the nucleocapsid protein (N). (c) The S1/S2 subunits are reported on the top with red arrows, while the RBD highlights below the S1/S2 subunits. The pink, red, sky, orange, blue, teal and brown color represents the signal peptide (SP), receptor-binding domain (RBD), fusion peptide (FP), heptad repeat-1 (HR1), heptad repeat-2 (HR2), transmembrane domain (TM), and cytoplasm domain (CP) respectively, whereas the bottom cerulean line highlights the length of amino acids. [60]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In some cases, both of these S1 domains could play a role as an RBD [35,36]. A 394-peptide chain of glutamine deposits in the RBD of spike protein in the S1-CTD domain (present on the tip of S trimer) successfully identified the specific 31-amino residual long lysine chain on the human lung ACE-2 receptor. A single mutation (N501T) in the spike protein of SARS-CoV-2 effectively increased the binding affinity of virus for ACE-2 receptor [37]. A study reported that a critical neutralizing domain is present in RBD of the spike protein of SARS-CoV-2, which could instigate profoundly powerful killing neutralizer reaction and cross-security against unique SARS-CoV-2 strains [38].
3. Mechanism of replication of SARS-CoV-2
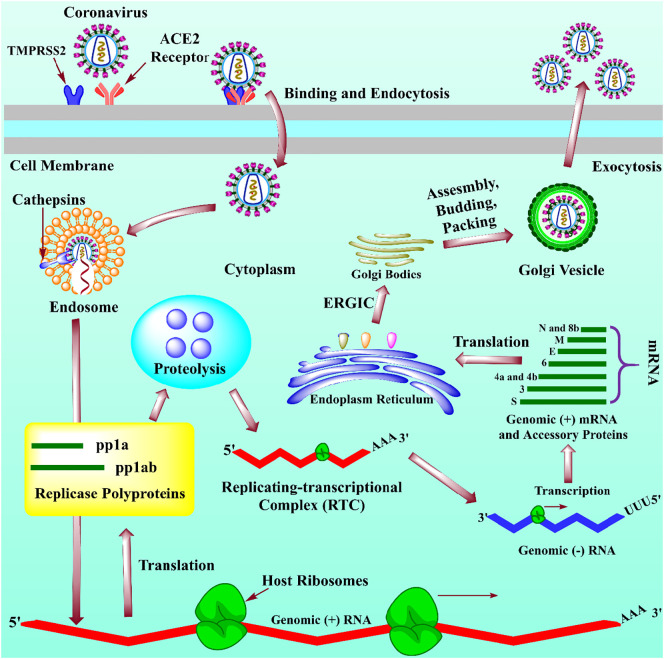
According to emerging evidence, the pathway of replication of newly developed SARS-CoV-2 has a similarity to the previously reported SARS-CoV-1. During the entry of coronavirus, the virus binds to the cell membrane of a host attachment by spike proteins (glycoproteic in nature) to angiotensin-converting enzymes-2 (ACE-2) receptor of human [39]. To facilitate the activation of viral fusion spike protein is sliced at two sites (one cleavage site positioned at the boundary of S1 and S2 domains and other present upstream of the fusion peptide of S2) by the help of cellular proteases like TMPRSS2 which resultingly activated endosomal cathepsins B and L in SARS-CoV-2 [40]. The evidence revealed that the cellular protease, like TMPRSS2 (see glossary) plays an essential role during the entry and circulation of SARS-CoV-2. However, the antiviral or vaccine could be a potential therapeutics against COVID-19 by blocking the regulation of TMPRSS2 enzyme [41]. However, the entry of the virus is cell type and protease specific dependent. Six HBs are framed by the communication of heptad repeats 1 and 2 (HR1 and HR2), and the areas present in a spike protein formed by the embedment of peptides in the layer of endosomes [42]. As a result, an envelope of virus and plasma film is synthesized by the Golgi intermediate compartment. Finally, the newly formed virus RNA genome is discharged from the cell through exocytosis. The detailed pathway is presented in Fig. 2 . To reduce the binding of virus with host receptor known as ACE2, the subunit immunizations for both SARS coronaviruses depend on inspiring a resistant reaction contrary to the spike protein [43].
Fig. 2.
The mechanism of replication of newly emerged SARS-CoV-2. The spike protein (S) of COVID-19 binds to the ACE-2 membrane receptor, which is facilitated by the protease, named TMPRSS2, and enters the cell. After the entry of viral RNA into the cell, the translocation of open reading frame-1a and 2b (ORF-1a and ORF-2b) produced two major types of polyproteins, namely pp1a and pp1ab (yellow box) to yield 16 different types of nonstructural proteins (nsps) and help in proteolysis (skybox) and form the replicating-transcriptional complex (RTC; red), which yield in a negative-sense RNAs (-RNA) (blue line). These newly formed (−) RNAs act as template strands for the formation of positive-sense RNA of the viral genome (green lines). The transcription in (−) RNAs further leads to the synthesis of accessory and structural proteins (green lines). Finally, the genomic RNA and structural proteins are assembled into a viral nucleocapsid by ER (blue) and move to Golgi intermediate compartment (brown) for envelope formation and released outside the cell by exocytosis [41,112,113]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Innate immune response to COVID-19 infection
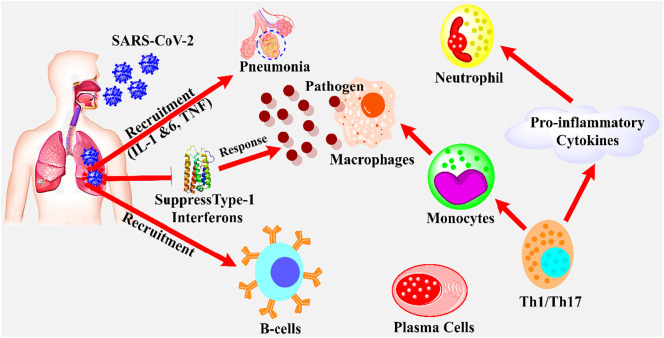
To prevent the viral infections, there is a need for the natural killer cells to perceive the entrance of virus into a host cell. According to accumulated data, only limited researches are presented yet on the innate immune response to SARS-CoV-2 infection. Recently, an ongoing project (consist of 35 cases) in Buali Hospital, Iran revealed a significant reduction of 45% (1100 μ/L) in lymphocytes, improvement in total neutrophil (48%), C-reactive protein (99%), and serum IL-6 (58%). The results also reported that the amount of lymphocytopenia and neutrophilia are correlated with infection mortality and disease severity [13,44]. Similarly, another report based on 99 cases in Wuhan, China, claimed 35% reduction in total lymphocytes, 38, 84, and 52% enhancement in neutrophils, C-reactive proteins, and serum IL-6, respectively [45]. The immune responses associated with the diseases of lung may have resulted in a large amount of cytokines (see glossary) production, which provides the 1st line defense against viral infection [46]. In addition, the humoral cells of the body are responsible for antibodies production against viral, which could play a significant role in preventing viral infection by killing them [47]. The innate immune system could effectively identify the situation during the incursion of virus, usually called pathogen-associated molecular patterns (PAMPs). However, the endosomal RNA receptors, RIG, cytosolic RNA sensor, TLR7, and TLR8 quickly recognized these PAMPs (present in the form of ssRNA on the viral genome) during the replication of virus and cause stimulation of numerous signaling pathways and transcription factors including NF-κB, IRF-3, AP-1 and IRF-7 [48,49]. Ultimately, these nuclear factors, especially AP-1 and NF-κB, activated the expression of genes that encoded the chemokines (CXCL8 and CCL2), and cytokines (IL-1 and TNF) which lead to the inflammatory responses. Similarly, other nuclear factors, namely IRF3 and IRF7, increased the production of INF-type 1 (IFNα and INFβ), which significantly helped in the reduction of viral dissemination and replication (see Fig. 3 ) [50].
Fig. 3.
The representation of the immune response of host during the infection of SARS-CoV-2. The SARS-CoV-2 binds to the human ACE-2 receptor and inter lung and infected the cells, which arrest the type-1 interferon (INF) to replicate viral RNA genome. The suppression in INF-1 leads to the production of specific first-line defense against pathogens. The B-cells and plasma cells could effectively neutralize the SARS-CoV-2 by producing specific antibodies against virus. The specific cells, namely Th1/Th17 could lead to the activation of pro-inflammatory cytokines such as INF-γ, and monocytes/macrophages which may result in the recruitment of neutrophil production and pathogens ingestion respectively [44].
5. Strategies to develop a vaccine against SARS-CoV-2
Based on the increasing incidences of newly emerged COVID-19, there is necessary to make a successful and safe vaccine against SARS-CoV-2 to control its present pandemic and future recurrence. The present lethal coronavirus (COVID-19) shared the structure homology with previously reported beta-coronaviruses, including MERS-CoV (50%) and especially SARS-CoV (79.6%) [27]. Based on their structure homology, the genome accession number (GenBank), MN975262.1, MN994468.1, MN908947.3, MN985325.1, MN938384.1, NC_045512.2, MN997409.1, and MN988713.1 could facilitate the formulation of a vaccine against SARS-CoV-2 [51]. Accumulated data reported that the formation of a vaccine falls into one of the following types; viral vectors (25 projects and one is under clinical trial), DNA and mRNA-based (20 projects and one from each type is under clinical trial), protein-based (28 projects and mostly on S protein), virus-like particle (VLP) (5 projects), inactivated or live-attenuated virus (7 projects and two are under clinical trial), and epitope vaccine [52,53]. Antibodies are the best and conservative means to anticipate and control desirable infections. Till more than 40 pharmaceutical industries and scholarly foundations all over the world have propelled their plans on immunization improvement against SARS-CoV-2 [54]. Herein, we discussed the new insights and latest developments in each class of vaccines to formulate a strong and effective vaccine against SARS-CoV-2.
5.1. Protein-based vaccine
The vaccine development gained special attention during the last few decades, including the development of RNA, DNA, and protein-based vaccines against several viruses like the influenza virus, and Ervebo virus. The proteins are the important constituents in the structural activities of coronavirus, which are involved in the transmission, entry, and replication of viruses. The data suggested that proteins could be ideal targets for the development of vaccines [13,55]. The emerging evidence revealed that the viral spike (S) protein vaccine showed the higher neutralizing titers (see glossary) against SARS-CoV than other vaccine candidates. Based on accumulated data, the S protein is the preferred site of vaccine formation against previously emerged coronaviruses (MERS and SARS-CoVs) because S protein could easily be encountered and allow the body to make an immune response more efficiently than other proteins [55,56]. Based on these points, the potential parts of S protein, which are used as antigens in immunization improvement, integrate the entire extent of this protein and vaccine development [57]. Pallesen et al. [58] reported that the S ectodomain of coronavirus with G4 showed a glycosylated loop variation in a structure of the virus, and observed the four major conformational sites in a trimer for the binding of each RBD either relatedly or tightly to a receptor accessible confirmation. However, the structure-based design of coronavirus could provide a prospective way for the formulation of a vaccine.
Many pharmaceutical industries and universities start working for the development of vaccines against COVID-19. Recently, the AstraZeneca and the University of Oxford began to combined effort to develop a spike protein (S) vaccine, namely AZD1222 against newly emerged SARS-CoV-2 in chimpanzee to generate the DNA for the spike antigen and to grow the vigorous T cell and B cell responses for better prophylaxis with less dose [59]. Besides, another company who made a vaccine against Ebola virus, namely CanSino Biologics (China) started a project to create a safe and efficient S protein antigen-based vaccine (Ad5-nCoV) against SARS-CoV-2 that is undergoing the phase-1 clinical trial on individuals aged between 18 and 60 years under ClinicalTrial.gov: NCT04313127 [60]. Similarly, the two top vaccine producer companies, including GlaxoSmithKline and Sanofi start working on the formation of spike protein-based vaccine against COVID-19 to trigger the immune response, and they reported that they would begin phase-1 clinical trials later this year [59].
Besides, the subunits of S protein, including receptor-binding domain (RBD), N-terminal domain (NTD), and C-terminal domain (CTD) also consider as a vital target for the progress of a vaccine. The study reported that antibodies that are attached to the N-terminal domain of the S1 subunit of coronavirus demonstrated an active killing movement, which indicates that NTD is applicable in balance for vaccine development. Jiaming and his research group [61] experimented on BALB/c immunized mice at two different doses (5 and 10 μg) to investigate the effect of recombinant NTD (rNTD) for vaccine development and immunogenicity. They revealed that high administration of 10 μg significantly reduced the lung infection caused by a coronavirus in a recombinant vaccinated mouse model. They also observed a robust T-cell immune retort in the serum of vaccinated mice (rNTD) with CpG and aluminum adjuvant. Geo et al. [62] experimented on BALB/c mice (n = 9) to check the RBD, S, and N-protein antibody response after 1–6 weeks of first immunization at 6 μg dose (accessed by ELIZA). They observed that SARS-CoV-2 linked RBD and S-protein immunoglobulin G (IgG) (see glossary) instantly start to develop inside the immunized mice at 6th week with the peak titer of 409,600 (100 μg/mL) and 819,200 (200 μg/mL) respectively.
Interestingly, the peak titer of N-specific IgG in antibody response was about 30 times fewer than RBD in vaccinated mice. But recently, another study stated that the N-specific IgG is reported as one of the most effective proteins in COVID-19 recovered patients as a diagnostic marker and vaccine development [63]. The study revealed that the RBD of specific IgG showed about the half of the value of antibody response than S protein, and suggested that the RBD is a meticulously associated immunogen to the recovered patients of COVID-19 [62]. However, the S protein and RBD of SARS-CoV-2 might be an innovative possible target for the formulation of the vaccine.
5.2. Inactivated and live attenuated virus vaccines
The inactivated vaccine (known as Whole Killed Virus: WKV) is an important vaccine that possesses the ability to cease the replication cycle of the virus, and it is generally prepared by neutralizing the virus through radiation or heat, and chemicals like formaldehyde. According to emerging evidence, the inactivated virus vaccine is one of the safe and effective vaccines, which can easily be prepared with much fewer cost as compared to nucleic acid vaccines [64]. The WKV vaccine could significantly target the subunits of viruses, including S and E proteins, ORF, matrix (M), and boost the immune response against viruses [65]. The WKV vaccine has attained special attention over the decades due to its several advantages against viruses, particularly SARS-CoV, that could easily neutralize the virus antibodies [64]. Most recently, a study experimented on non-human primates (rhesus macaques), rat, and mice (BALB/c) at two different doses (3 and 6 μg) for the investigation of protective potential and immunogenicity of purified inactivated virus vaccine candidate (PiCoVacc). They revealed that PiCoVacc significantly neutralized the effect of ten SARS-CoV-2 strains and demonstrated the partial or complete protective potential against SARS-CoV-2 in macaques deprived of any development of the antibody-dependent disease. The data suggested that the inactivated virus vaccine candidate (PiCoVacc) could be proved as a novel option for a vaccine development against COVID-19 [62]. Another study demonstrated that inactivated virus vaccine also showed a protective behavior against previously emerged coronavirus, namely SARS-CoV-1, but phase-1 clinical trials were dried due to several reasons including a low number of cases and lack of funds, but few neutralizing monoclonal antibodies (see glossary) development by inactivated virus vaccine like CR3022 that could be a potent cross-protection against COVID-19 [55,66].
Based on the accumulated data, the live attenuated and inactivated virus vaccines are reported as one of the safest and effective vaccines against previously emerged viruses, namely influenza virus [67]. The live attenuated virus vaccine could be developed by diagnosing the newly emerged transmitted SARS-CoV-2 through a possible fewer risk of pathogenesis like enhanced anti-inflammatory cytokines, minimum lung infection, and fewer neutrophil influx as compared to wild type virus [51]. The inactivated and live attenuated virus vaccines consider the whole virus as a vaccine. A study evaluated the performance of live attenuated vaccine, by initiating alteration (Y6398H) into Orf1a/b polyprotein (nsp14) of mice, which showed the significant replication reduction behaviors of coronavirus in mice after day five intracerebral inoculation [52]. Similarly, a recent study suggested that the development of oral live attenuated virus vaccine could be a potential target to diminish the lung infections triggered by SARS-CoV-2, due to its initial infection in guts which resultingly boosted up the mucosa that associated with immune system during the early immune response against COVID-19 [68]. Another study also reported that the live attenuated virus vaccine could easily be administrated and serve as a community spread to rapidly develop herd immunity against the pathogens of COVID-19 [69].
Based on the multiple target's abilities to live attenuated vaccines, it could be the earliest licensed vaccine against newly emerged SARS-CoV-2. Recently, many institutes, including Zhejiang University, the Chinese Centers for Disease Control and Prevention, and the Chinese Academy of Sciences, successfully isolated the viral strains of SARS-CoV-2 and start working on the formulation of live attenuated vaccines against COVID-19. In addition, Codagenix, Inc. developed the live attenuated vaccine with the collaboration of Serum Institute of India, Ltd. against SARS-CoV-2 that is under phase-1 clinical trial [70]. However, few limitations could also be manifested during the expansion of inactivated or live attenuated vaccines against SARS-CoV-2. First, the live attenuated vaccines could regain its virulent effect in an in vivo or cell culture. Second, the coronavirus could prevent seepage from the immunity developed by these vaccines through quick progression. Thus, the manufacturer should be aware and careful during the development of these (inactivated and live attenuated) vaccines against SARS-CoV-2 [71].
5.3. mRNA-based vaccine
According to emerging evidence, the development of vaccines is one of the most important ways to mitigate viral infections. Currently, several vaccines, including mRNA-based vaccines, are under clinical trials. mRNA was the first vaccine that goes under clinical trial due to the fast development process towards manufacturing the vaccine against SARS-CoV-2 [72]. Generally, the mRNA immunization development depends on several factors, including the determination of foreign particle, sequence arrangements, the protection of newly developed nucleotides, the development of transmission frameworks, assessment of invulnerable reaction, and safety assessment. According to accumulated data, two well-planned procedures were used to build up an mRNA antibody of the virus. The first procedure involves the utilization of mRNA to communicate with the area of RBD and S protein of coronavirus. Currently, the potential of this antibody is under assessment. The second procedure involves the utilization of this mRNA to communicate with infection [29,73]. Currently, the undergoing projects are following one of the above-reported procedures for the formulation of an mRNA-based vaccine.
Recently, one of the most reputed companies, namely Moderna Therapeutics, start work for the formulation of an mRNA-based vaccine (mRNA-1273) against SARS-CoV-2 and enroll 45 healthy participants (aged 18–55) for clinical trials. They reported that mRNA-1273 has the ability to mimic the natural infection to robust the immune response more efficiently. The Moderna Therapeutics also stated that this vaccine (mRNA-1273) gained the advantage over other traditional vaccines to target the spike protein of coronavirus, but still is under phase-1 clinical trial and will be available at the start of 2021 [74]. Corbett et al. [75] experimented on mice to investigate the immunogenicity and potential ability of spike trimer (see glossary) of an mRNA-1273 vaccine against newly emerged SARS-CoV-2 and revealed that mRNA-1273 successfully protected the nose and lung of mice in a phase-1 clinical trial and neutralized the antibody and induce CD8 T-cell responses without any immune-dependent infection. Its phase-2 clinical trials will be started soon. In addition, Zeng and his research group conducted a systematic de novo study to evaluate the untranslated regions (UTRs) of mRNA to facilitate the development of vaccine and protein production. They observed that optimal combination of 5′ and 3′ (NASAR) showed a better efficiency of about 5–10 times greater than the tested endogenous UTRs, and the lipid-based nanoparticle delivery of mRNA NASAR into SARS-CoV-2 infected body significantly expressed the antigens both in vivo and in vitro studies. They suggested the development of novel mRNA NASAR could be an important clue for the discovery of mRNA based vaccine against COVID-19 [76].
5.4. DNA-based vaccine
Antibodies that are made up of DNA contained circular DNA molecules that encoding at least one foreign particle, and these antibodies are considered as better, as compared to other antibodies [77,78]. The DNA-based vaccine could effectively target several variants of coronavirus including the S1 domain, and prefusion stabilized ectodomain with furring cleavage, spike protein, receptor-binding domain (RBD), cytoplasmic tail, and transmembrane domain [79,80]. The DNA-based vaccine composition is an expensive and sophisticated method, which consists of double-stranded plasmids that are usually designed with the help of a computer/smart device to generate an immune retort against the virus. A specific device, named CELLECTRA is used to generate the electric pulse under the skin to usher the DNA-based vaccine [81,82]. Several institutes have started the work to create a DNA-based vaccine against SARS-CoV-2. Recently, Inovio Pharmaceutical (Plamouth, PA) starts working for the formulation of a DNA-based vaccine candidate (INO-4800), which conducted an initial U.S human clinical trial (30 participants) in April and planned to conduct more trials in China and South Korea in June in more than 3000 participants. In his initial clinical trial, the INO-4800 showed a better immune response against SARS-CoV-2 with no serious adverse effect. If all will go according to the plan, the Inovio Pharmaceutical will produce about 1 million vaccines by the start of 2021 [74].
Yu et al. [83] experimented on 35 different rhesus macaques to assess the performance of DNA-based vaccine against SARS-CoV-2. They demonstrated that the vaccinated macaques showed excellent results in the reduction of viral infection in the nasal mucosa and bronchoalveolar of macaques with >3.7 and > 3.1 log10 titers as compared to control and showed remarkable development of immune and humoral response in vaccinated macaques against SARS-CoV-2. Smith and co-workers constricted a synthetic DNA-based vaccine (INO-4800) to target the spike protein of SARS-CoV-2 and showed that INO-4800 significantly neutralized antibody response of newly emerged COVID-19 in guinea pig and mice, and boosted up the expression to block the S protein to bind with ACE2 receptor in vitro, and suggested that synthetic DNA vaccine candidate, namely INO-4800 might be a strong choice against COVID-19 [84]. Similarly, Modjarrad et al. [85] experiment on the DNA-based vaccine candidate, namely DLS-5300 against MERS-CoV in a phase-1 clinical trial (ClinicalTrial.gov. NCT 02670187) at three different concentration of doses as 0.67, 2, and 6 mg in 75 healthy candidates (aged 18–50 years). The results revealed no serious adverse effect and immune response was dose-dependent, at 6 mg the candidates showed the maximum immune response against MERS-CoV. However, future study is required to check the efficiency of DNA-based vaccine (DLS-5300) against newly emerged SARS-CoV-2, that could be a novel target.
5.5. Peptide or epitope-based vaccine
The previously reported studies revealed that the computation strategy is one of the effective techniques for the development of vaccines against many lethal infections, including malaria, cancer, and dengue. This technique worked by recognition of novel T cell epitopes, and MHC 1 and 2 molecules (see glossary) for the development of specific vaccines associated with the Transporter of Antigen Presentation (TAP) agents [86,87]. This vaccine is made up of antibodies related to the segments of immaculate foreign particles and is generally arranged by substance amalgamation procedures. These antibodies are simpler in readiness and also have efficient control [88]. Based on its previous tremendous application in other diseases like dengue, the epitope-based virus could be an essential option in the formulation of vaccines against SARS-CoV-2. Currently, many projects are undergoing vaccine formation. Khan and co-workers successfully recognized the 15 distinct antigenic peptides (including B and T cells) on the surface of the glycoprotein of newly emerged SARS-CoV-2. They reported that only 9 peptides including WTAGAAAYY, LTDEMIAQY, VKQLSSNFG, QTQTNSPRRARS, VITPGTNTS, IRASANLAA, FGAGAALQ, FGAISSVLN, and FAMQMAYRF, have more than 90% resemblance to the peptides of closely related SARS-CoV, and these peptides are tightly bonded to the HLA (human-leukocytes-antigen) which showed high T cell response against the virus. They suggested that these peptides could prove an innovative antigen target for the formulation of peptide or epitope-based vaccine against SARS-CoV-2 [89]. Sharp et al. [90] performed an in-silico experiment on a multi-epitope vaccine and stated that this vaccine efficiently provoked the cell-mediated immune and humoral response. They also observed that the obtained sequence is about 86.3% in the appropriate region of Ramachandran plot, which docked with toll-like receptor-8 (see glossary) and provided new hope for the formulation of an epitope-based vaccine against SARS-CoV-2. Ahmad et al. [30] experimented for the documentation of epitopes of SARS-CoV-2 for vaccine development, they observed the novel T-cell and B-cell epitopes (total 27: 16 N and 11 S) in the structure of spike (S) and nucleocapsid (N) proteins of SARS-CoV-2 without any mutation, that could be suggested as a probable target against COVID-19 and epitope-based vaccine development by enhancing the immune response. Similarly, Feng et al. [91] detected a total of 61 B-cell and 499 T-cell epitopes, out of which 19 B-cell and 34 T-cell epitopes showed the highest potential for a vaccine development and tight connection to the human-leukocytes-antigen (HLA) alleles that are responsible for T cell response. However, more in vivo and in vitro experiments are mandatory for the further clarification of epitopes for vaccine formulation against SARS-CoV-2.
5.6. Subunit-based vaccine
Subunit immunizations include at least one antigen with solid immunogenicity to prepared to efficiently vaccine that activates the host immune system. This kind of antibody is more secure and simpler to create because it did not contain any live virus for the development of a vaccine. However, it frequently requires the development of adjuvants (see glossary) to induce a solid defensive safe reaction. But like other vaccines like inactivated or live attenuated vaccine, the subunit vaccine is less immunogenic that could be improved by adding the suitable adjuvants [92,93]. Up until few foundations have started working on the formulation of a subunit-based vaccine to target the virus. The University of Queensland has begun work to build up this type of subunit body, which depends upon the molecular lamp methodology [94].
Similarly, Clover Biopharmaceuticals Inc. started the initial validation in building up an antibody competitor against SARS-CoV-2 by utilizing the “Trimer-Tag” innovation [95]. Liu and his research group designed a novel S1-subunit based nano-vaccine against SARS-CoV-2 and stated that nano-vaccine showed the strong immune and humoral response and effectively elicited the T-cell response by triggering CD4+ and CD8+ cells, which to minimize the viral load in affected persons. The results also revealed that the nano-vaccine provoke the IgA antibody, which provides the mucosal protection against viral load [96]. Kalita et al. [97] composed a 33 highly antigenic subunit-based epitopes vaccine, which comprises helper T-lymphocytes (HTC), cytotoxic T-lymphocytes (CTC; see glossary), adjuvant, and B-cell against SARS-CoV-2, and reported that vaccine is thermostable, non-toxic and effectively produce an immune and humoral response, and give new hope for the formulation of subunit-based epitope vaccine against newly emerged SARS-CoV-2.
5.7. Recombinant vaccine
The recombinant protein is known as one of the emerging fields for the development of a vaccine against viruses due to several properties including tight binding to specific ACE-2 receptor, provoke immune protection against viral infections, increase antibody-dependent viral entry, and promote antigenicity against virus like SARS-CoV [52]. The accumulated data stated that the recombinant ACE-2 (rACE-2) receptor exerted the potential targets against the protection of many diseases, including acute Ang 2-induced hypertension, and severe lung injury [98,99]. Besides, rACE-2 showed the rapid cure rate with a half-life of about 60 min in mice as well as humans [100]. Lei et al. [101] designed the recombinant protein by composing the Fc region of IgG1 and ACE-2 receptor of human and reported that the recombinant fusion protein significantly neutralized the COVID-19 in vitro and could be a potential cue for the formulation of recombinant protein vaccine against SARS-CoV-2. Similarly, Chen and co-workers developed a recombinant adenovirus vaccine against SARS-CoV. They observed that this vaccine showed a remarkable antigen-specific cellular and humoral response against SARS-CoV in macaques, and suggested that this vaccine could be a new hope against newly emerged SARS-CoV-2 due to structure homology with SARS-CoV-1 [102].
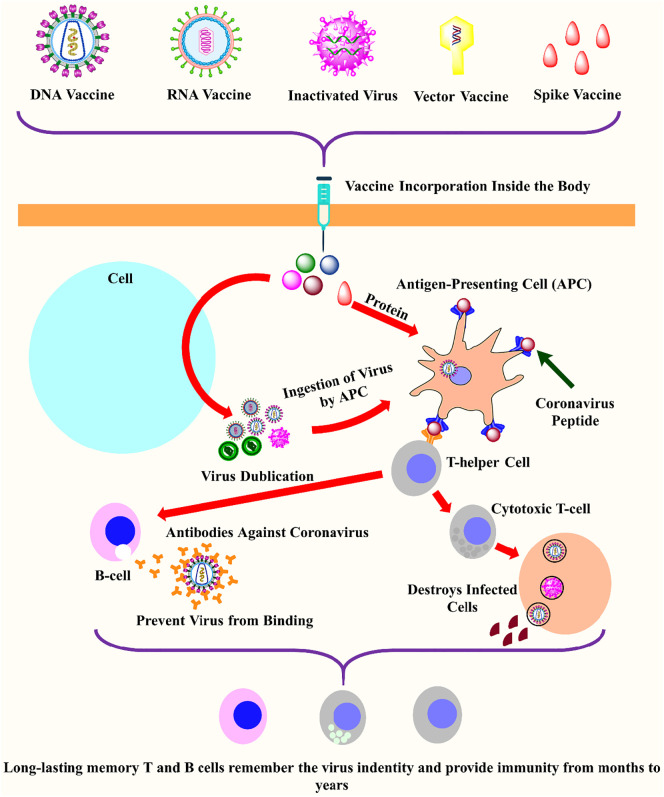
Table 1 illustrates some essential vaccine candidates that are presently under clinical trials for the formulation of an active vaccine against the pandemic of SARS-CoV-2. In contrast, Fig. 4 explained the general mechanism of action of different vaccines to provoke the humoral and immune retort against SARS-CoV-2.
Table 1.
The list of some vaccine candidates which are currently under clinical trials for the development of a safe and effective vaccine against SARS-CoV-2.
| Vaccine | Phase | Clinical Trial number | Target | Enroll participant | Institute | Sponsor |
|---|---|---|---|---|---|---|
| mRNA-1273 | Phase-1 | NCT04283461 | S | 155 | Emory Vaccine Center-The Hope Clinic, Decatur, Georgia, United State | National Institute of Allergy and Infectious Disease (NIAID) |
| Gam-COVID-Vac | Phase-1/2 | NCT04436471 | Undisclosed | 38 | Main military clinical hospital named after academician N. N. Burdenko Moscow, Russian Federation | Gamaleya Research Institute of Epidemiology and Microbiology, Russia |
| Pathogen-specific aAPC | Phase-1 | NCT04299724 | S | 100 | Shenzhen Geno-immune Medical Institute Shenzhen, Guangdong, China | Shenzhen Geno-Immune Medical Institute |
| Antigen-specific CTLs, injection and infusion of LV-SMENP-DC vaccine | Phase-1/2 | NCT04276896 | S trimer | 100 | Shenzhen Second People's Hospital Shenzhen, Guangdong, China | Shenzhen Geno-Immune Medical Institute |
| BNT162a1 | Phase-1 | NCT04368728 | NSP5, 3CLpro, Mpro | 7600 | NYU Langone Health New York, New York, United States | Pfizer/Biotech SE |
| Recombinant novel coronavirus vaccine | Phase-2 | NCT04341389 | Incorporated antigens in SARS-CoV-2- | 508 | Hubei Provincial Center for Disease Control and Prevention Wuhan, Hubei, China | CanSino Biologics Inc. |
| BCG | Phase-4 | NCT04369794 | S | 1000 | Hospital das Clínicas Unicamp Campinas, SP, Brazil | University of Campinas, Brazil |
| SCB-2019 | Phase-1 | NCT04405908 | S | 150 | Linear Clinical Research Ltd. Nedlands, Western Australia, Australia | Clover Biopharmaceuticals, AUS Pty Ltd. |
| bac-TRL-Spike | Phase-1 | NCT04334980 | S trimer | 84 | Canadian center for Vaccinology Dalhousie University, IWK Health Centre Halifax, Nova Scotia, Canada | Symvivo Corporation |
| Inactivated SARS-CoV-2 vaccine | Phase-1/2 | NCT04412538 | Undisclosed | 942 | West China Second University Hospital, Sichuan University, China | Chines Academy of Medical Sciences |
| ChAdOx1 nCoV-19 | Phase-1/2 | NCT04324606 | S | 1090 | University Hospital Southampton NHS Foundation Trust Southampton, Hampshire, United Kingdom | University of Oxford |
| IMM-101 | Phase-3 | NCT04442048 | S | 1500 | Canadian Cancer Trials Group | Canadian Cancer Trials Group, BioCan Rx |
| INO-4800 | Phase-1 | NCT04336410 | S | 120 | University of Pennsylvania Philadelphia, Pennsylvania, United States |
Inovio Pharmaceuticals |
S: Spike protein. This information is taken from [111].
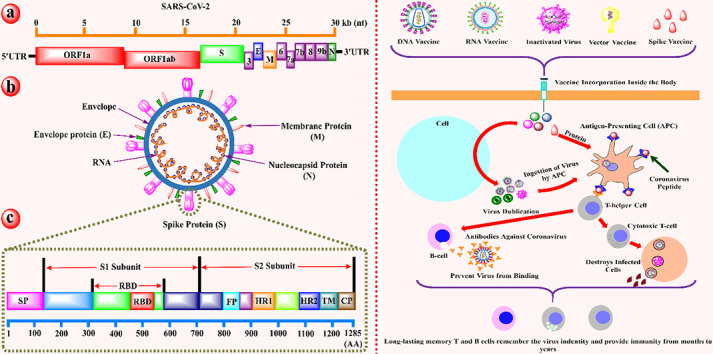
Fig. 4.
The schematic mechanism of action of vaccine candidates to elicit an immune response against SARS-CoV-2. Different types of vaccines including DNA, RNA, protein, inactivated virus, live-attenuated virus, and vector-based vaccines inter inside the body to elicit the humoral and immune response against viruses. After the entry of the vaccine inside the human body, they enter into the human cell (sky color), where the translation of viral RNA occurs to produce more viral genome copies. In the next step, the specialized cells called antigen-presenting cells (APCs; orange) ingested the virus and activates the T-helper cells (grey) by binding through the virus peptides (imperial purple). The T-helper cells activate the cytotoxic T-cell (grey) and B-cell (light pink). The B-cells produced the antibodies (orange) which blocked the virus and marked the virus for destruction, similarly, cytotoxic T-cell destroys the virus-infected cells. Finally, the T-cells and B-cells remembred the invading pathogens for long-terms to provide a strong immune response [53]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
6. Current challenges and future perspectives
Based on the present pandemic situation caused by newly emerged SARS-CoV-2, there is a need to make a vaccine on urgent bases. After investigation, it is revealed that the calculated Ro value of SARS-CoV-2 is 2.2. This indicates that one contaminated person could contaminate more 2.2 persons. However, by using different precautionary measures, isolation, and quarantine activities, the Ro value could be decreased [103]. No doubt, the vaccine formulation process is not only expensive and time taking but also very complicated that sometimes gives a very low success rate. Currently, more than 40 pharmaceutical industries and various international institutes are working for the development of several types of vaccines. Still, all are under phase-1 clinical trials, and no vaccine has been licensed yet [104]. Vaccines elicit the immune response against viruses, but currently, one of the major concerns is that no one knows what kind of immune response is required against SARS-CoV. One of the basic alarms is the disadvantages of several vaccines, including the unstable under physiological condition, low immunogenicity, organ failure, required high dose, and more adjuvants. However, these disadvantages need to be addressed and improved on urgent bases in the next version of vaccine development [105,106]. However, the delivery of vaccine both in immunization and modality modes are also significant issues which also needs to be revised to minimize the off-target effects. Based on data, it is suggested that aerosol route or oral administration could be a better way for vaccine delivery that might significantly induce a mucosal response.
Similarly, DNA plasmid or vector could also be used for the delivery of a vaccine to its target site [51,107,108]. Previously due to negligence and improper attention of government towards this task, no proper improvement in vaccine development is seen especially against MERS and SARS-CoVs. The government and health departments do not have a proper budget to manage it. In 20 years the SARS-CoV is considered to be 3rd epidemic war [7].
Based on accumulated data, we suggested that the development of a ideal vaccine should possess some major properties, including 1) the perfect vaccine should be well-coordinated and strongly elicit the T-lymphocyte immunity that could lead to the stimulation of cytotoxic T-lymphocytes and T-helper cells to control or block the replication of SARS-CoV-2. 2) The ideal vaccine should boost the immune responses and develops long-lasting neutralizing antibodies against COVID-19 antigens that could provoke both adaptive and innate immunities during the case of entry of any foreign particles to induce antigen-presenting cells (APCs; see glossary) attack. 3) The ideal vaccine should possess limited or no serious adverse effects. 4) The ideal vaccine should be the simplest and reactive. 5) The ideal vaccine should easily be administrated in a single dose of orally or intravascularly. 6) The vaccine should possess the long-lasting storage ability at room temperature, and 7) the ideal vaccine should be target-specific and high accurate towards SARS-CoV-2 [109,110].
7. Concluding remarks
Based on the increasing incidences of COVID-19, there is urgently needed to formulate a vaccine against SARS-CoV-2. Based on emerging evidence, we recommended several vaccine categories including subunits, epitopes, mRNA, protein, especially S protein, recombinant adenovirus, and fusion proteins, inactivated and live attenuated virus for the formulation of a timely required vaccine against SARS-CoV-2. More than 40 pharmaceutical industries and institutes are started works on more than 100 projects for the development of different vaccines against COVID-19. These vaccines significantly neutralized the SARS-CoV-2 and developed a strong immune and humoral response against the virus. We suggested that the receptor-binding domain (RBD), a component of S protein, might show a noteworthy role in neutralizing the antibodies because it inhibits the entrance of the virus into the host by blocking the attachment of the virus to the host membrane and also block the virus membrane fusion which could help in vaccine development. But the site-specific glycan defense creates difficulties in targeting the specific antigen. However, the vaccine development will be a challenging task, and it did not guarantee the success against SARS-CoV-2, because no efficient and reliable vaccine is developed yet against the AIDS/HIV even after the 30 years of occurrence. It is recommended that further study is needed to discover the more target sites instead of only S protein for better inside into understanding and also suggested that Cryptic epitopes based studies should be conducted on urgent bases to identify more antibody targets to generate a better long-lasting and stable immunity against SARS-CoV-2.
8. Outstanding questions
-
1.
Would the development of vaccines be active without any severe damage?
-
2.
Are plasma transplant and dexamethasone (alone or in combination with other drugs) would showed the better results against COVID-19 than vaccine development?
-
3.
Would the formulation of live-attenuated and inactivated virus vaccines be useful? What would be happened if coronavirus escape from the immunity developed by inactivated virus vaccine by rapid growth evaluation or live-attenuated virus regains its virulent effect inside the body?
-
4.
Could the use of CRISPR/Cas9 genome editing technology help in the identification of novel sequence or subunit to aid the development of a subunit-based or epitope-based vaccine against SARS-CoV-2.
-
5.
Does the temporary blockage of the ACE-2 receptor of the lung during viral infection will be helpful during the medication and reduce the further viral infection?
-
6.
Does the successful development of vaccines will also be useful against HIV? Because coronavirus and HIV shared the replication cycle.
-
7.
If the vaccine failed to exert the admirable results, what will be the next option?
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Hereby, we extend our gratitude to A.Q Research Group, Pakistan, for reviewing the article and providing helpful comments.
CRediT authorship contribution statement
A. Hazafa, and S. N. Chaudhry: Conceived the presented data, Writing - original draft, Software, & Supervision. A. Siddique, S. Bilal, and U. Kalsoom: Developed the theory, Formal analysis, & Investigation. A. Kainaat and S. Abbas: Software. A. Zafar, N. Zafar, and M. Mumtaz: Revision.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2020.118421.
Appendix A. Supplementary data
Supplementary material
References
- 1.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020;80(5):554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., Fang C., Huang D., Huang L.-Q., Huang Q. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Medical Research. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park M., Cook A.R., Lim J.T., Sun Y., Dickens B.L. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 2020;9(4):967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazafa A., Ur-Rahman K., Haq I.-U.-., Jahan N., Mumtaz M., Farman M., Naeem H., Abbas F., Naeem M., Sadiqa S. The broad-spectrum antiviral recommendations for drug discovery against COVID-19. Drug Metab. Rev. 2020;52(3):408–424. doi: 10.1080/03602532.2020.1770782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong J.E., Leo Y.S., Tan C.C. COVID-19 in Singapore—current experience: critical global issues that require attention and action. Jama. 2020;323(13):1243–1244. doi: 10.1001/jama.2020.2467. [DOI] [PubMed] [Google Scholar]
- 13.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singhal T. A review of coronavirus disease-2019 (COVID-19) The Indian Journal of Pediatrics. 2020:1–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. 2020. Single-cell RNA Expression Profiling of ACE2, the Putative Receptor of Wuhan 2019-nCov, BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(1):1–9. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Coronavirus disease (COVID-19). Situation report. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Available at:
- 22.Nyarko R.O., Boateng E., Kahwa I., Boateng P.O. 2020. A Comparison Analysis on REMDESIVIR, FAVIPIRAVIR, Hydroxychloroquine, ChloroquinE and Azithromycin in the Treatment of Corona Virus Disease 2019 (COVID-19)-A Review. (Preprint) [Google Scholar]
- 23.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., Yu W., Zhang J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jubeen F., Liaqat A., Sultan M., Iqbal S.Z., Sajid I., Sher F. Green synthesis and biological evaluation of novel 5-fluorouracil derivatives as potent anticancer agents. Saudi Pharmaceutical Journal. 2019;27(8):1164–1173. doi: 10.1016/j.jsps.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nisa Z.U., Zafar A., Sher F. Assessment of knowledge, attitude and practice of adverse drug reaction reporting among healthcare professionals in secondary and tertiary hospitals in the capital of Pakistan. Saudi Pharmaceutical Journal. 2018;26(4):453–461. doi: 10.1016/j.jsps.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyon M.P., Du L., Tseng C.-T.K., Seid C.A., Pollet J., Naceanceno K.S., Agrawal A., Algaissi A., Peng B.-H., Tai W. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36(14):1853–1862. doi: 10.1016/j.vaccine.2018.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaimes J.A., André N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically-sensitive activation loop. J. Mol. Biol. 2020;432(10):3309–3325. doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsaadi E.A., Neuman B.W., Jones I.M. A fusion peptide in the spike protein of MERS coronavirus. Viruses. 2019;11(9):825. doi: 10.3390/v11090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert review of vaccines. 2017;16(1):55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 32.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8):e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walls A.C., Tortorici M.A., Bosch B.-J., Frenz B., Rottier P.J., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531(7592):114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & molecular immunology. 2020:1–8. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang J., Wan Y., Liu C., Yount B., Gully K., Yang Y., Auerbach A., Peng G., Baric R., Li F. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020;16(3):e1008392. doi: 10.1371/journal.ppat.1008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriarty L.F. Public health responses to COVID-19 outbreaks on cruise ships—worldwide, February–March 2020. MMWR Morb. Mortal. Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmann C.C., Silverman R.H. COVID-19: Coronavirus replication, pathogenesis, and therapeutic strategies. Cleve. Clin. J. Med. 2020;87(6):321–327. doi: 10.3949/ccjm.87a.20047. [DOI] [PubMed] [Google Scholar]
- 42.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Innes E.A. Vaccination against toxoplasma gondii: an increasing priority for collaborative research? Expert review of vaccines. 2010;9(10):1117–1119. doi: 10.1586/erv.10.113. [DOI] [PubMed] [Google Scholar]
- 44.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev. Med. Virol. 2020;30(3):e2107. doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Channappanavar R., Perlman S. Springer; 2017. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology, Seminars in Immunopathology; pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 48.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng X., Van Geelen A., Buckley A.C., O’Brien A., Pillatzki A., Lager K.M., Faaberg K.S., Baker S.C. Coronavirus endoribonuclease activity in porcine epidemic diarrhea virus suppresses type I and type III interferon responses. J. Virol. 2019;93(8) doi: 10.1128/JVI.02000-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang W., Yang Y., Rao Y., Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. npj Vaccines. 2020;5(1):1–3. doi: 10.1038/s41541-020-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Central Science. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 54.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 57.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020:1–6. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 58.Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. 2017;114(35):E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395(10239):1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uddin M., Mustafa F., Rizvi T.A., Loney T., Suwaidi H.A., Al-Marzouqi A.H.H., Eldin A.K., Alsabeeha N., Adrian T.E., Stefanini C. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12(5):526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiaming L., Yanfeng Y., Yao D., Yawei H., Linlin B., Baoying H., Jinghua Y., Gao G.F., Chuan Q., Wenjie T. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine. 2017;35(1):10–18. doi: 10.1016/j.vaccine.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L., Liu W., Zheng Y., Jiang X., Kou G., Ding J., Wang Q., Huang Q., Ding Y., Ni W. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020;22:206–211. doi: 10.1016/j.micinf.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pandey S.C., Pande V., Sati D., Upreti S., Samant M. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci. 2020;117956 doi: 10.1016/j.lfs.2020.117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaecher S.R., Mackenzie J.M., Pekosz A. The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus-infected cells and incorporated into SARS-CoV particles. J. Virol. 2007;81(2):718–731. doi: 10.1128/JVI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging microbes infections. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grohskopf L.A., Alyanak E., Broder K.R., Walter E.B., Fry A.M., Jernigan D.B. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2019–20 influenza season. MMWR Recommendations reports. 2019;68(3):1. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanal M.G., Duby R.C. An oral live attenuated vaccine strategy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2/2019-nCoV) Research Ideas Outcomes. 2020;6:e53767. [Google Scholar]
- 69.Nuismer S.L., Basinski A., Bull J.J. Evolution and containment of transmissible recombinant vector vaccines. Evol. Appl. 2019;12(8):1595–1609. doi: 10.1111/eva.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen J.W., Chen J.M. Potential of live pathogen vaccines for defeating the COVID-19 pandemic: history and mechanism. J. Med. Virol. 2020;92:1–6. doi: 10.1002/jmv.25920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27(4):757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Molecular Therapy-Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricks D. Race for a coronavirus vaccine: thanks in part to institutional support, CanSino biologics, Moderna therapeutics, and other developers are exploring diverse approaches against SARS-CoV-2. Genetic Engineering Biotechnology News. 2020;40(5):16–18, 20. [Google Scholar]
- 75.Corbett K.S., Edwards D., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schafer A., Ziwawo C.T., DiPiazza A.T. 2020. SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng C., Hou X., Yan J., Zhang C., Li W., Zhao W., Du S., Dong Y. bioRxiv; 2020. Leveraging mRNAs Sequences to Express SARS-CoV-2 Antigens In Vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlos W.G., Dela C.C., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201(4):P7. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 78.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. Jama. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 79.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Z.-y., Kong W.-p., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrushina I., Hovakimyan A., Harahap I., Davtyan H., Antonyan T., Chailyan G., Kazarian K., Antonenko M., Jullienne A., Hamer M.M. Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol. Dis. 2020;104823 doi: 10.1016/j.nbd.2020.104823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J., Kumar S.A., Jhan Y.Y., Bishop C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018;80:31–47. doi: 10.1016/j.actbio.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith T.R., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., Reuschel E.L., Robb M.L., Racine T., Oh M.-d. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19(9):1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shahsavandi S., Ebrahimi M.M., Sadeghi K., Mahravani H. Design of a heterosubtypic epitope-based peptide vaccine fused with hemokinin-1 against influenza viruses. Virol. Sin. 2015;30(3):200–207. doi: 10.1007/s12250-014-3504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielsen M., Lundegaard C., Lund O., Keşmir C. The role of the proteasome in generating cytotoxic T-cell epitopes: insights obtained from improved predictions of proteasomal cleavage. Immunogenetics. 2005;57(1–2):33–41. doi: 10.1007/s00251-005-0781-7. [DOI] [PubMed] [Google Scholar]
- 88.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan A., Alam A., Imam N., Siddiqui M.F., Ishrat R. 2020. Design of an Epitope-Based Peptide Vaccine against the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A Vaccine Informatics Approach, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharp K., Dange D. Application of in-silico reverse vaccinology for designing multi-epitope vaccine against coronavirus. Chemrxiv. 2020 doi: 10.26434/chemrxiv.12345653.v1. [DOI] [Google Scholar]
- 91.Feng Y., Qiu M., Zou S., Li Y., Luo K., Chen R., Sun Y., Wang K., Zhuang X., Zhang S. 2020. Multi-Epitope Vaccine Design Using an Immunoinformatics Approach for 2019 Novel Coronavirus in China (SARS-CoV-2), bioRxiv. [Google Scholar]
- 92.Du L., Zhou Y., Jiang S. Taylor & Francis; 2017. The latest advancements in Zika virus vaccine development. [DOI] [PubMed] [Google Scholar]
- 93.George P.J., Tai W., Du L., Lustigman S. The potency of an anti-MERS coronavirus subunit vaccine depends on a unique combinatorial adjuvant formulation. Vaccines. 2020;8(2):251. doi: 10.3390/vaccines8020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Current tropical medicine reports. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biopharmaceuticals C. 2020. Clover Initiates Development of Recombinant Subunit-trimer Vaccine for Wuhan Coronavirus (2019-Ncov) [Google Scholar]
- 96.Liu L., Liu Z., Chen H., Liu H., Gao Q., Cong F., Gao G., Chen Y. 2020. Subunit Nanovaccine with Potent Cellular and Mucosal Immunity for COVID-19. [DOI] [PubMed] [Google Scholar]
- 97.Kalita P., Padhi A., Zhang K.Y., Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 2020;104236 doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y. Angiotensin-converting enzyme 2 protects from lethal avian influenza a H5N1 infections. Nat. Commun. 2014;5(1):1–7. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu H., Xie Z., Li T., Zhang S., Lai C., Zhu P., Wang K., Han L., Duan Y., Zhao Z. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52(9):783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 101.Lei C., Fu W., Qian K., Li T., Zhang S., Ding M., Hu S. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. BioRxiv. 2020 doi: 10.1101/2020.02.01.929976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y., Qin C., Wei Q., Li R., Gao H., Zhu H., Deng W., Bao L., Wei T. Protection of rhesus macaque from SARS-coronavirus challenge by recombinant adenovirus vaccine. BioRxiv. 2020 doi: 10.1101/2020.02.17.951939. [DOI] [Google Scholar]
- 103.Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pang J., Wang M.X., Ang I.Y.H., Tan S.H.X., Lewis R.F., Chen J.I.-P., Gutierrez R.A., Gwee S.X.W., Chua P.E.Y., Yang Q. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020;9(3):623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan J., Yang J., Hu Z., Yang Y., Shang W., Hu Q., Zheng Y., Peng H., Zhang X., Cai X. Safe staphylococcal platform for the development of multivalent nanoscale vesicles against viral infections. Nano Lett. 2018;18(2):725–733. doi: 10.1021/acs.nanolett.7b03893. [DOI] [PubMed] [Google Scholar]
- 109.Funk C.D., Laferrière C., Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 111.NIH U.S national library of medicine. ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/results?cond=Covid+vaccine Available at:
- 112.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim Y.-I., Kim S.-G., Kim S.-M., Kim E.-H., Park S.-J., Yu K.-M., Chang J.-H., Kim E.J., Lee S., Casel M.A.B. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material