Abstract
Stromal tumour of uncertain malignant potential of the prostate is a rare tumour with a variable clinical behaviour ranging from incidentally detected indolent tumours that never progress, to aggressive diseases almost identical to sarcomas that may invade surrounding organs or develop metastases. Surgical excision is generally recommended for local diseases; however, owing to its diverse clinical outcomes, optimal management may vary from surgery alone to wide excision combined with chemotherapy and/or radiotherapy. Therefore, preoperative evaluation of the malignant potential of the disease is essential to decide the treatment strategy. Herein, we report a case of stromal tumour of uncertain malignant potential successfully treated with minimally invasive robot-assisted radical prostatectomy alone under the diagnosis of the disease with low malignant potential based on the findings of positron emission tomography with 18F-fluorodeoxyglucose.
Keywords: prostate cancer, pathology, urological surgery
Background
Prostate mesenchymal neoplasms are rare tumours that account for less than 1% of all prostate cancers. According to the WHO classification,1 they are classified into sarcomas and stromal tumour of uncertain malignant potential (STUMP), which has diverse clinical behaviour. Although many cases are incidentally diagnosed with indolent disease, some patients have aggressive diseases which could be histologically accompanied by sarcomatous features and may have high potential for invasion, metastasis and recurrence.2 Considering its diverse biological behaviour, surgical excision is generally recommended.3 Surgery alone with less invasive technique may be appropriate for low malignant potential diseases, while wide excision combined with chemotherapy and/or radiotherapy should be considered in accordance with treatment for prostatic sarcoma.4 However, there is no established treatment strategy for STUMP, owing to the rarity of this tumour and lack of relevant prognostic factors including tumour markers, histological subtypes and imaging findings.5
Positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) is an advanced functional imaging technique which visualises the increased glucose consumption of cancer cells, including prostate cancer.6 Besides being useful in the detection of cancer, FDG-PET is capable of differentiating malignant and benign lesions by means of standardised uptake values (SUV), a measurement of tracer uptake in a region of interest normalised to a distribution volume.7 Here, we present a case of STUMP successfully being treated with minimally invasive robotic prostatectomy under the diagnosis of the disease with low malignant potential based on the FDG-PET findings. To the best of our knowledge, this is the first report describing the usefulness of FDG-PET in the management of STUMP of the prostate.
Case presentation
A 60-year-old man, with a medical history of pancreatic intraductal papillary mucinous neoplasia, duodenal ulcer, pulmonary emphysema and cerebral aneurysm, was incidentally found to have an enlarged prostate suggestive of prostate tumour by abdominal CT scan. Although he did not have any voiding symptoms related to the enlarged prostate, he was referred to the urology clinic for further examination of the prostate tumour.
Investigations
Digital rectal examination revealed an elastic hard tumour in the left lobe of the prostate. His serum prostate-specific antigen (PSA) was within normal limits (2.48 ng/mL, (normal range <4.0 ng/mL)), while other tumour markers showed slight elevation (carcinoembryonic antigen, 18.0 ng/mL (normal range <5.0 ng/mL); cancer antigen 19-9, 71.0 U/mL (normal range <37.0 U/mL)). There were no abnormal findings in the urinalysis.
Prostate volume was measured to be 47 mL by transrectal ultrasound. On MRI, the prostate tumour protruding toward the left seminal vesicle had high signal intensity on T1-weighted images, heterogeneous intensity on T2-weighted images and low apparent diffusion coefficient values on diffusion-weighted images (figure 1). The tumour showed neither early staining in the arterial phase nor washout of the contrast in the venous phase in contrast-enhanced MRI (figure 2). Taken together, these MRI findings indicated the possibility of a prostate mesenchymal tumour.
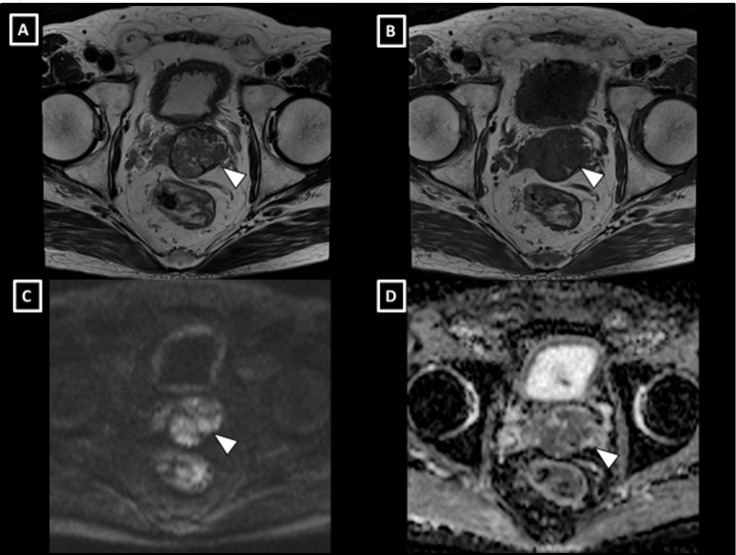
Figure 1.
MRI findings of the prostate tumour. T2-weighted (A) and T1-weighted (B) images revealed the tumour, which showed expansive progression with a clear capsule. The tumour presented with high signal intensity on diffusion-weighted images (C) and had low apparent diffusion coefficient values (D).
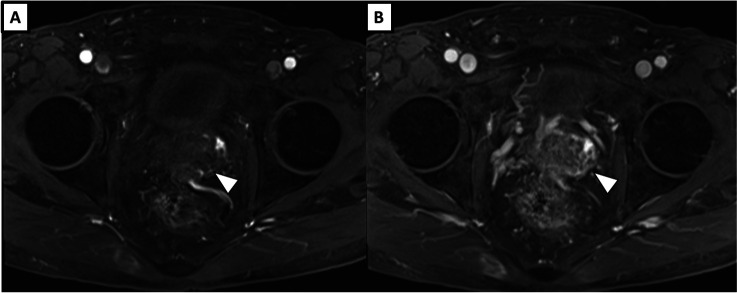
Figure 2.
Dynamic contrast-enhanced MRI in arterial phase (A) and in venous phase (B) revealed hypovascularity of the tumour.
FDG-PET showed no abnormal accumulation of FDG in the prostate (SUVmax 3.4), nor in the bone, lymphatic system or other organs (figure 3), indicating not only the absence of distant metastases but also the low malignant potential in the primary prostate tumour.
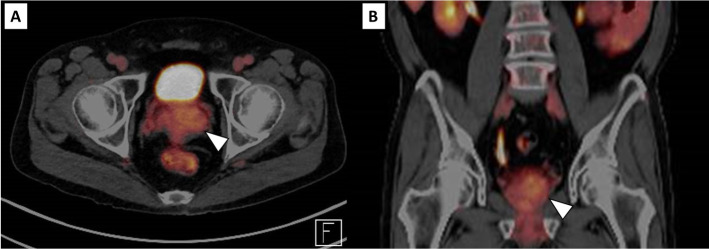
Figure 3.
FDG-PET imaging in axial (A) and in coronal (B) section showing low FDG uptake (SUVmax 3.4) in the primary prostate tumour, suggestive of low malignant disease. FDG-PET, positron emission tomography with 18F-fluorodeoxyglucose.
Systematic needle biopsy of the prostate suggested STUMP as the most probable diagnosis, although the possibility of prostatic sarcoma could not be completely excluded due to the small amount of biopsied samples.
Differential diagnosis
Although prostate adenocarcinoma and benign prostatic hyperplasia were suspected at his first presentation, low serum PSA levels compared with the large tumour size indicated a low possibility of these diseases. The large tumour showing an expansive progression with clear tumour capsule revealed on MRI suggested the possibility of prostate mesenchymal neoplasms. Moreover, as the tumour did not show a cystic appearance, cystic prostate neoplasms such as mucinous adenocarcinoma and cystic epithelial tumour were not suspected.
Histological examination of tumour tissue acquired by needle biopsy suggested STUMP of the prostate as the most probable diagnosis, although prostatic sarcoma could not be entirely excluded from the differential diagnosis. Based on the findings of FDG-PET showing that the primary prostate tumour had low FDG uptake, this tumour was assumed to have low malignant potential. Concordantly, we diagnosed this tumour as STUMP of the prostate with low malignant potential.
Treatment
Irrespective of whether this tumour was STUMP or sarcoma, surgical resection would be recommended; however, surgical management would depend on the aggressiveness of the disease. Based on the clinical diagnosis of STUMP with low malignant potential, we performed minimally invasive robot-assisted radical prostatectomy without wide excision of adjacent organs. The tumour had no strong adhesion or direct invasion to surrounding tissues. Although dissection between the prostate and the rectum was challenging owing to its large size and the shape protruding dorsally, the tumour could be safely dissected with adequate surgical margin (figure 4).
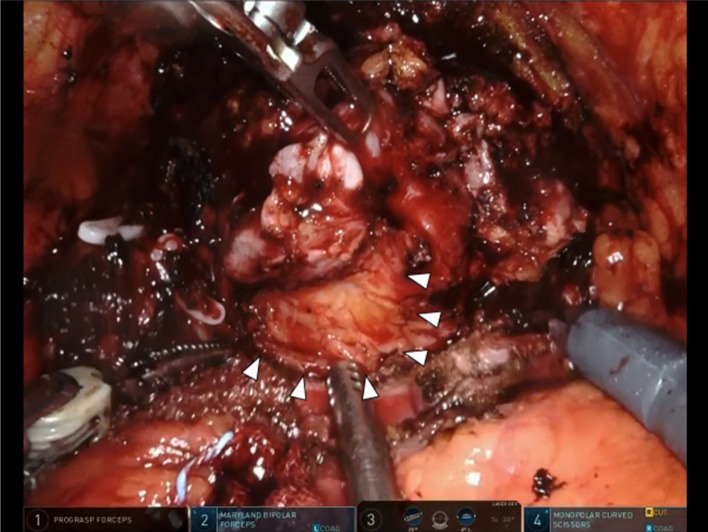
Figure 4.
Intraoperative image of robot-assisted radical prostatectomy showing the yellowish tumour with expansive protrusion.
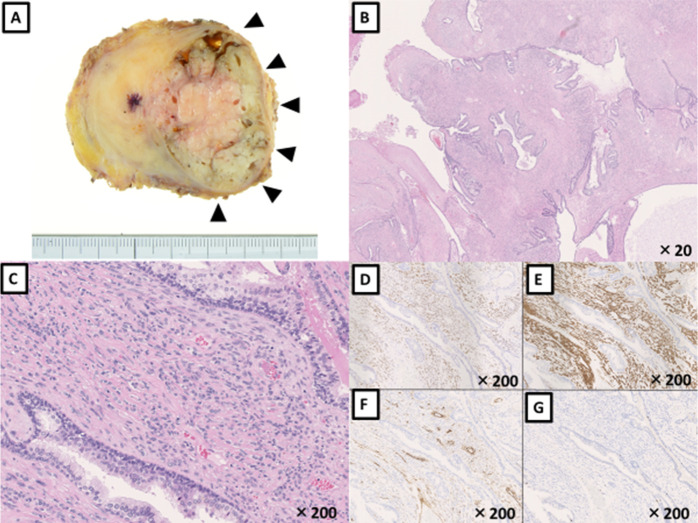
Macroscopically, the tumour presented as a multiloculated greyish-white mass with a clear capsule (figure 5A). Histologically, the tumour demonstrated hypercellular stroma that consisted of fusiform cells with eosinophilic cytoplasm. No mitotic figures or necrosis were observed and the associated epithelial elements were non-neoplastic (figure 5B, C). Immunohistochemical analysis revealed that these tumour cells were positive for progesterone receptor, smooth muscle actin and CD34, but negative for Ki-67, consistent with a final diagnosis of STUMP with low malignant potential (figure 5D–G).
Figure 5.
Tissue analyses of radical prostatectomy specimen. (A) Macroscopic finding, (B and C) H&E staining and immunohistochemistry for (D) progesterone receptor, (E) smooth-muscle actin, (F) CD34 and (G) Ki-67.
Outcome and follow-up
The patient recovered well after surgery without any surgical complications. He was followed-up every 3 months and underwent serum PSA testing and abdominal CT scan at each visit; at the time of publication, he had been followed-up for 6 months and remained disease-free without any adjuvant therapy.
Discussion
STUMPs are rare mesenchymal neoplasms with divergent clinical outcomes ranging from indolent tumours which never progress to aggressive tumours almost identical to prostatic sarcomas.2 Due to the diversity and the unpredictability of their clinical outcomes and prognosis, patients with STUMPs are generally assumed to require surgical excision and close follow-up.3 8 As optimal surgical management may vary from surgery alone to wide excision combined with chemotherapy and/or radiotherapy4 depending on their aggressiveness, preoperative evaluation of the potential for malignancy of the disease is essential to decide treatment strategy. In this case report, we described a case of STUMP of the prostate that was successfully treated with minimally invasive robot-assisted radical prostatectomy alone. A diagnosis of disease with low malignant potential was made based on the FDG-PET findings, suggesting the clinical utility of FDG-PET in surgical management of STUMP of the prostate.
MRI is the preferred imaging study for the evaluation of prostate tumours as it has excellent resolution of signals in soft tissue masses. Typical MRI features of prostate STUMPs include a large, expansive, well-defined mass, a cystic mass interspaced by septa and solid component showing highly heterogeneous signals on T2-weighted images, and discrete enhancement on septa and solid areas.9 Although these features are quite different from those of prostate adenocarcinoma in that they show low signal intensity in T2-weighted images, other prostate lesions such as mucinous adenocarcinoma10 and cystic epithelial tumours11 may have a similar appearance. The prostate sarcomas usually present as heterogeneous solid lesions; however, large tumours may show cystic areas of variable sizes, which represent areas of necrosis instead of true cystic components. Thus, the differential diagnosis of STUMP based on MRI findings is sometimes difficult. Furthermore, no radiological features have been proven to be associated with aggressiveness and prognosis of STUMPs of the prostate.
FDG-PET, a functional imaging technique that visualises the enhanced glucose metabolism by cancer cells, is widely used in the management of various cancers. FDG-PET can be used for guided biopsy, diagnosis, initial staging, therapy response assessment, radiation treatment planning, restaging and prognostication. As for prostate adenocarcinoma, FDG-PET is useful in the detection of the primary tumour,12 initial staging13 and prognosis assessment.14 FDG-PET is also useful in clinical management of sarcomas,15 including prostate sarcoma.16 17 FDG-PET can evaluate tumour aggressiveness by means of SUV, as aggressive tumours tend to be FDG avid. The metabolic activity determined by SUV can distinguish between aggressive and indolent subtypes and has been reported to be useful in the surgical planning of uterine sarcoma,7 retroperitoneal liposarcoma18 and musculoskeletal lesions.19 Recently, Yamazaki et al reported that the absence of FDG uptake was useful to discriminate STUMP from malignant cystic epithelial tumour of the prostate.20 Concordant with these reports, the current case suggests the clinical usefulness of FDG-PET in surgical planning of STUMP of the prostate.
Recently, imaging methods targeting prostate-specific membrane antigen (PSMA) have been developed, such as PSMA-PET that provides higher detection rates compared with FDG-PET and is replacing the latter method in management of prostate adenocarcinoma.21 Furthermore, PSMA is expressed in various organs other than the prostate and a subset of soft tissue tumours,22 indicating that PSMA-PET could be useful for detection and evaluation of aggressiveness of soft tissue tumours including STUMP and prostate sarcoma. However, the potential role of PSMA-PET in clinical decision-making for soft tissue tumours still needs to be evaluated in future studies.
Most patients with prostate STUMP or sarcoma present with urinary obstruction because of large tumour size. Transurethral resection of the prostate performed for symptom palliation may reveal the presence of these prostate mesenchymal tumours; however, most cases are diagnosed with a transrectal ultrasound-guided prostate needle biopsy.8 As expected, most patients do not have elevated PSA levels in contrast to those with large prostate size. STUMP is sometimes difficult to distinguish from low-grade prostatic sarcoma by morphology, especially in cases with predominant, large, bizarre and degenerative nucleolus.3 Furthermore, some cases of STUMP have progressed to prostatic sarcoma on subsequent biopsy and some sarcomas are associated with concomitant STUMP, leading to the hypothesis that STUMP may have the ability to undergo malignant transformation.3
Treatment of STUMP may range from active surveillance to surgical excision with chemoradiation and outcomes appear to be related to the degree of sarcomatous differentiation of the tumour. Herawi reported a series of 50 cases involving patients with pure STUMP not associated with sarcoma (n=36), STUMP associated with sarcoma (n=7) and pure sarcoma (n=7). Of the 36 patients with pure STUMP, 14 underwent active surveillance after biopsy or transurethral resection without evidence of disease progression, 5 showed local tumour progression and required repeated transurethral resections and 14 underwent immediate radical prostatectomy without evidence of recurrence (three were lost to follow-up). In contrast, all patients with a sarcoma component underwent radical prostatectomy. Of those, four had disease recurrence and eight had no evidence of disease recurrence (two were lost to follow-up). No correlation between the histological features of STUMP and progression to sarcoma was found.3
While active surveillance could be selected for patients with pure STUMP, particularly those receiving maximal transurethral resection, it is not generally acceptable for patients with biopsy-proven STUMP due to the lack of correlation between prostate biopsy and final surgical pathology, risk of sarcomatous differentiation and lack of long-term surveillance data.23 Instead, we believe that patients with biopsy-proven STUMP warrant active treatment with surgical excision,3 8 chemoradiation or combination of these therapies which is the preferred treatment for prostatic sarcoma.4 In the current case, the lack of accumulation of FDG in the tumour suggested the low malignant potential of STUMP, which was further supported by the final pathology results that revealed no sarcoma component; thus, close follow-up without any adjuvant therapy was considered to be safe. The successful treatment of prostate STUMP by minimally invasive robot-assisted radical prostatectomy without any adjuvant therapy has also been reported by Leong et al.24
Learning points.
STUMP (sarcomas and stromal tumour of uncertain malignant potential) is a rare mesenchymal tumour of the prostate, which is associated with variable clinical outcomes and warrants surgical resection.
Histological findings and imaging studies including MRI are insufficient in discriminating STUMPs with low malignant potential from those with high malignant potential or sarcomas.
FDG-PET (positron emission tomography with 18F-fluorodeoxyglucose) may have the capability to evaluate the malignant potential of STUMP and may be useful for surgical planning.
Acknowledgments
The authors would like to thank Professor Masahiro Yashi for technical instruction and supervision of robot-assisted radical prostatectomy.
Footnotes
Contributors: Patient was under the care of IS. Acquisition and interpretation of clinical information were done by IS and TKijima. Pathological analysis including immunohistochemistry was performed by AO. Report was written by IS and TKijima. Supervised by TKamai.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Humphrey PA, Moch H, Cubilla AL, et al. . The 2016 who classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol 2016;70:106–19. 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 2.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol 1998;22:148–62. 10.1097/00000478-199802000-00002 [DOI] [PubMed] [Google Scholar]
- 3.Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol 2006;30:694–704. 10.1097/00000478-200606000-00004 [DOI] [PubMed] [Google Scholar]
- 4.Janet NL, May A-W, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol 2009;32:27–9. 10.1097/COC.0b013e31817b6061 [DOI] [PubMed] [Google Scholar]
- 5.Murer LM, Talmon GA. Stromal tumor of uncertain malignant potential of the prostate. Arch Pathol Lab Med 2014;138:1542–5. 10.5858/arpa.2013-0212-RS [DOI] [PubMed] [Google Scholar]
- 6.Sanz G, Robles JE, Giménez M, et al. . Positron emission tomography with 18fluorine-labelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int 1999;84:1028–31. 10.1046/j.1464-410x.1999.00349.x [DOI] [PubMed] [Google Scholar]
- 7.Lee EYP, Khong P-L, Tse KY, et al. . Differentiation of aggressive and indolent subtypes of uterine sarcoma using maximum standardized uptake value. Nucl Med Commun 2013;34:1–1189. 10.1097/MNM.0000000000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansel DE, Herawi M, Montgomery E, et al. . Spindle cell lesions of the adult prostate. Mod Pathol 2007;20:148–58. 10.1038/modpathol.3800676 [DOI] [PubMed] [Google Scholar]
- 9.Muglia VF, Saber G, Maggioni G, et al. . Mri findings of prostate stromal tumour of uncertain malignant potential: a case report. Br J Radiol 2011;84:e194–6. 10.1259/bjr/67699443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JM, Lee HJ, Lee SE, et al. . Pictorial review: unusual tumours involving the prostate: radiological-pathological findings. Br J Radiol 2008;81:907–15. 10.1259/bjr/68294775 [DOI] [PubMed] [Google Scholar]
- 11.Paner GP, Lopez-Beltran A, So JS, et al. . Spectrum of cystic epithelial tumors of the prostate: most cystadenocarcinomas are ductal type with intracystic papillary pattern. Am J Surg Pathol 2016;40:886–95. 10.1097/PAS.0000000000000618 [DOI] [PubMed] [Google Scholar]
- 12.Minamimoto R, Uemura H, Sano F, et al. . The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med 2011;25:21–7. 10.1007/s12149-010-0424-4 [DOI] [PubMed] [Google Scholar]
- 13.Oyama N, Akino H, Suzuki Y, et al. . The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol 1999;29:623–9. 10.1093/jjco/29.12.623 [DOI] [PubMed] [Google Scholar]
- 14.Jadvar H, Groshen SG, Quinn DI. Association of overall survival with glycolytic activity of castrate-resistant prostate cancer metastases. Radiology 2015;274:624–5. 10.1148/radiol.14141593 [DOI] [PubMed] [Google Scholar]
- 15.Becher S, Oskouei S. Pet imaging in sarcoma. Orthop Clin North Am 2015;46:409–15 10.1016/j.ocl.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 16.Oldan JD, Chin BB. Fdg PET/CT imaging of prostate carcinosarcoma. Clin Nucl Med 2016;41:629–31. 10.1097/RLU.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 17.Lai R, Ding C. 18F-Fdg PET/CT imaging in an adolescent patient with primary prostatic stromal sarcoma. Clin Nucl Med 2019;44:45–7. 10.1097/RLU.0000000000002372 [DOI] [PubMed] [Google Scholar]
- 18.Rhu J, Hyun SH, Lee K-H, et al. . Maximum standardized uptake value on 18F-fluorodeoxyglucose positron emission tomography/computed tomography improves outcome prediction in retroperitoneal liposarcoma. Sci Rep 2019;9:6605. 10.1038/s41598-019-43215-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parghane RV, Basu S. Dual-time point 18F-FDG-PET and PET/CT for Differentiating Benign From Malignant Musculoskeletal Lesions: Opportunities and Limitations. Semin Nucl Med 2017;47:373–91. 10.1053/j.semnuclmed.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki M, Yoshida K, Terayama N, et al. . Ct and MRI findings of a stromal tumour of uncertain malignant potential of the prostate. Eur J Radiol Open 2020;7:100233 10.1016/j.ejro.2020.100233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterzing F, Kratochwil C, Fiedler H, et al. . (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging 2016;43:34–41. 10.1007/s00259-015-3188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitkötter B, Trautmann M, Grünewald I, et al. . Expression of PSMA in tumor neovasculature of high grade sarcomas including synovial sarcoma, rhabdomyosarcoma, undifferentiated sarcoma and MPNST. Oncotarget 2017;8:4268–76. 10.18632/oncotarget.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidikov L, Maroni P, Kim S, et al. . 67-Year-Old male with prostatic stromal tumor of uncertain malignant potential. Oncology 2019;33:685007. [PubMed] [Google Scholar]
- 24.Leong JY, Chandrasekar T, Sebastiano C, et al. . Prostatic stromal tumors of uncertain malignant potential. Urology 2019;132:e3–4. 10.1016/j.urology.2019.06.023 [DOI] [PubMed] [Google Scholar]