Abstract
Hypertension in low-income and middle-income countries (LMICs) is largely undiagnosed and uncontrolled, representing an untapped opportunity for public health improvement. Implementation of hypertension control strategies in low-resource settings depends in large part on cost considerations. However, evidence on the cost-effectiveness of hypertension interventions in LMICs is varied across geographical, clinical and evaluation contexts. We conducted a comprehensive search for published economic evaluations of hypertension treatment programmes in LMICs. The search identified 71 articles assessing a wide range of hypertension intervention designs and cost components, of which 42 studies across 15 countries reported estimates of cost-effectiveness. Although comparability of results was limited due to heterogeneity in the interventions assessed, populations studied, costs and study quality score, most interventions that reported cost per averted disability-adjusted life-year (DALY) were cost-effective, with costs per averted DALY not exceeding national income thresholds. Programme elements that may reduce cost-effectiveness included screening for hypertension at younger ages, addressing prehypertension, or treating patients at lower cardiovascular disease risk. Cost-effectiveness analysis could provide the evidence base to guide the initiation and development of hypertension programmes.
Keywords: health economics, hypertension, review
Key questions.
What is already known?
Implementation of hypertension control strategies in low-resource settings depends in large part on cost considerations, but evidence on the cost-effectiveness of hypertension interventions from low-income and middle-income countries (LMICs) is sparse and varied across geographical, clinical and evaluation contexts.
What are the new findings?
Most interventions that reported cost per averted disability-adjusted life-year were cost-effective using national income thresholds, but gaps in evidence exist on programme elements that can affect cost-effectiveness in LMICs, such as task-sharing, risk-based treatment and standardised treatment protocols.
What do the new findings imply?
Hypertension control is found to be a cost-effective intervention for many LMICs. Gaps in evidence can be filled by economic evaluation of programme elements that include shifting some healthcare tasks to non-physician providers, integrating cardiovascular disease (CVD) risk assessment into treatment decisions and incorporating standardised CVD prevention programmes.
Introduction
Hypertension in low-income and middle-income countries (LMICs) remains largely undiagnosed, untreated and uncontrolled despite being a leading factor in preventable death and disability (Chow et al, 20131; Ibrahim and Damasceno, 20122; Lozano et al, 20183; and WHO, 20134). The suboptimal treatment of hypertension in LMICs represents an untapped opportunity for public health improvement (Frieden and Bloomberg, 2018).5 Recent estimates suggest that nearly 40 million hypertension-related deaths can be avoided over the next 25 years by scaling up hypertension treatment to 70% (Kontis et al, 2019).6
Hypertension management depends on consistent and reliable access to healthcare. Areas with documented shortages of healthcare workers and with limited access to formal healthcare, such as sub-Saharan Africa, have fared the worst in addressing hypertension (Geldsetzer et al, 2019).7 At the population level, weak hypertension control and insufficient cardiovascular disease (CVD) prevention in LMICs can have broad implications that exceed the direct health consequences. For example, clustering of hypertension-related disease in younger adults, which is disproportionately more common in LMICs than high-income countries (Roth et al, 2018),8 has considerable socioeconomic effects, contributing to productivity and income losses at the household level and impeding macroeconomic growth (Bloom et al, 2011).9
While the value of addressing hypertension in LMICs has gained recognition over the past decade, resources in this area remain limited, revealing a gap between health targets and current needs (United Nations (UN), 201110;UN, 201511; and WHO, 2018a12). The transition from goal setting to actual implementation of hypertension control strategies in LMICs depends in large part on cost considerations. Although some economic modelling suggests that both population-level and clinical interventions for hypertension control can be cost-effective (Murray et al, 200313; Jha et al, 201214; Nugent and Brouwer, 201515; Bertram et al, 201816; and WHO, 2018b17), policymakers in individual countries might regard aggregate global estimates to be insufficient evidence for policy formulation in specific country circumstances. To inform policy decisions regarding hypertension approaches in LMICs, we reviewed the current evidence on costs and cost-effectiveness of hypertension interventions across LMICs. The contribution of this study is twofold. First, it provides the first comprehensive review of the evidence on cost-effectiveness of hypertension management programmes in LMICs. This review summarises the available evidence most relevant to policymakers in countries where hypertension management is currently limited or absent, and where decision-makers may be considering additions to health benefit packages without detailed cost or cost-effectiveness information. Second, this review documents the variation among existing studies across study designs and study quality. It produces a standardised quality score and explores contextual differences such as those that may arise between programmes based exclusively on pharmaceutical intervention and programmes that incorporate non-pharmaceutical components; programmes that target hypertension populations with different levels of CVD risk; or programmes applied in countries with different income levels. This too provides informative evidence to decision-makers in LMICs. The results describe a range of clinical programmes and corresponding programme cost and cost-effectiveness estimates from different settings, with varying levels of quality. We found gaps in evidence on programme elements that can affect cost-effectiveness in LMICs, such as shifting of healthcare tasks to non-physician providers, integrating CVD risk assessment into treatment decisions and standardising CVD prevention approaches.
Patient and public involvement
No patients or human subjects were involved in the process of conducting this literature review.
Methods
In March 2019, we searched for articles on economic evaluation of hypertension treatment programmes in LMICs using PubMed, the Cochrane Collaboration Database of Systematic Reviews, the Tufts Cost-Effectiveness Analysis Registry, the UK’s National Institute for Health and Care Excellence (NICE) guidelines, the University of York Centre for Reviews and Dissemination and the Disease Control Priorities (3rd Edition). To guide the search eligibility criteria, we developed a PICOTS table summarising the inclusion and exclusion criteria across the following elements: population, intervention, comparator, outcomes, time frame, settings and study design (Liberati et al, 2009)18 (see online supplementary appendix table A1). The search was performed using Medical Subject Headings (MeSH) and search terms related to hypertension and the pharmacological treatment, diagnosis, screening and management of hypertension. The list of MeSH terms can be found in online supplementary appendix table A2. We also used search terms for world regions; all low-income, lower middle-income and upper middle-income country names; newly classified high-income countries in South America, the Caribbean and the Pacific; and economic terms related to costs and cost-effectiveness. The PubMed search strategy can be found in online supplementary appendix table A3. We performed a supplemental ad hoc literature scan without MeSH terms in May 2020 to account for the lag in indexing and to capture any recent articles. The initial search identified 60 articles for inclusion in the review while the supplemental scan identified an additional 11 relevant publications. Results were not limited by publication date.
bmjgh-2019-002213supp001.pdf (120.3KB, pdf)
An inclusion/exclusion guide was created for reviewing the abstracts and full-text of articles (see online supplementary appendix table A4). Articles were included if they involved an intervention related to clinical screening, treatment and management of hypertension. Articles were excluded if they were designed for other diseases for which hypertension may be a risk factor or common comorbidity, or if they were for surgery patients to address acute events related to hypertension. Articles were excluded if they looked only at the cost of hypertension, with no reference to a specific intervention; only studied the prevalence of hypertension; if they did not involve any clinical setting; or, if they studied knowledge or awareness of hypertension. Studies that were conducted in high-income countries, or in territories or associated states of high-income countries (with the exception of South America, the Caribbean and the Pacific), studies that were published in a foreign language, and any article that was an editorial, review, correspondence or abstract related to study design and protocol were also excluded.
Overall, 595 references were identified: 534 from PubMed and 61 from other databases and sources. Screening abstracts identified 163 articles for full-text review of which 71 were identified as relevant for inclusion in the analysis (see online supplementary appendix table A5). Of these, 42 studies across 15 countries provided estimates of cost-effectiveness, with the rest evaluating costs only. A diagram of the search process is depicted in figure 1. Each of the 42 cost-effectiveness studies underwent a quality assessment based on a 13-question checklist informed by Drummond guidelines for economic evaluation of healthcare programmes (Evers et al, 2005).19 These studies were reviewed and assigned a total score equal to the sum of positive answers to the checklist questions.
Figure 1.
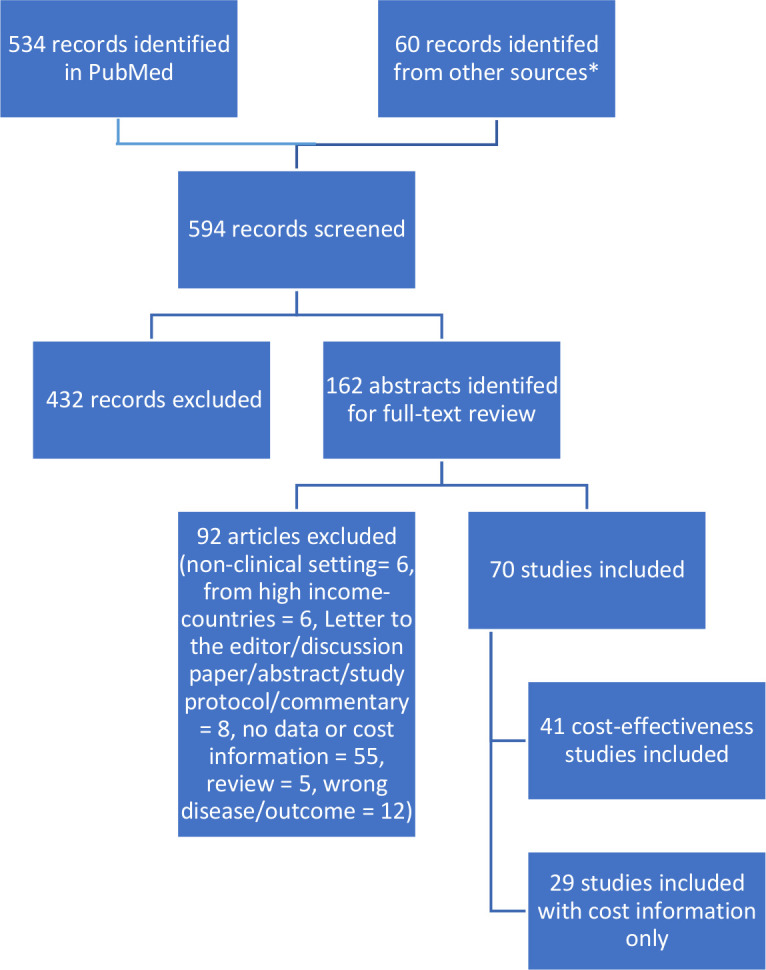
Summary diagram of the costs and cost-effectiveness literature search process. *Other sources searched include the Cochrane Collaboration Database of Systematic Reviews, the Tufts Cost-Effectiveness Analysis Registry, the UK’s National Institute for Health and Care Excellence (NICE) guidelines, the University of York Centre for Reviews and Dissemination and the Disease Control Priorities (3rd Edition). These databases were hand searched using similar terms as the PubMed search strategy found in online supplementary appendix table A3.
Reported indicators included: cost per mm Hg reduction in systolic and/or diastolic blood pressure (table 1), cost per patient with controlled hypertension (table 2), cost per patient with hypertension (table 3), cost per averted disability-adjusted life year (DALY) (table 4) and cost per gained quality-adjusted life year (QALY) (table 5). Estimates were converted to constant 2017 US dollars (US$) and were adjusted to reflect annual amounts where applicable. Two studies reported estimates in purchasing-power-parity (PPP)-adjusted international dollars, which were not converted into US$ because appropriate conversion factors were not available for the blend of countries examined (Ortegon et al, 201220 and Murray et al, 2003). Studies in the above cost-effectiveness categories were further categorised according to intervention type, as follows. ‘Pharm only’ indicates interventions where pharmacotherapy is the only treatment element, encompassing various combinations of drugs and drug classes, different providers and delivery platforms. ‘Pharm plus’ indicates combination programmes that incorporate other forms of treatment for hypertension in addition to medications, such as patient education or lifestyle changes. ‘Other’ indicates interventions that did not evaluate changes in pharmacological treatment. Cost elements included costs of medication, laboratory work, labour, equipment, transportation, provider training and others.
Table 1.
Cost per mm Hg reduction in systolic and/or diastolic blood pressure (2017 US$)
| Country income group | Country | Author | Study type | Sample size | Study design | Provider | Intervention details | Time period | Cost elements | Intervention subgroup | Cost – systolic (2017 US$) | Cost – diastolic (2017 US$) |
| Lower middle | India | Anchala | Pharm plus | 1638 | Cluster randomised control study | Doctors | Primary healthcare physicians received training to use decision support system (DSS) software for management of HTN or received chart-based support with HTN guidelines on a poster. | 1 year | Drugs, laboratories, labour, travel/transportation/per diem, building overhead costs, depreciation, equipment costs and office supplies, training costs, intervention development costs, translation charges. | Decision support system | 37.82 | |
| Chart-based support | 99.29 | |||||||||||
| Upper middle | South Africa | Anderson A | Pharm only | 1473 | Meta-analysis | Not specified | Comparison of the angiotensin receptor blockers (ARBs) currently available in South Africa: candesartan, losartan, irbesartan and valsartan. | 1 year | Drugs | Candesartan | 4.6 | |
| Losartan | 5.47 | |||||||||||
| Irbesartan | 6.11 | |||||||||||
| Valsartan | 6.77 | |||||||||||
| Upper middle | Argentina | Augustovski | Pharm plus | 1432 | Cluster randomised control study | Community health workers, doctors | Multicomponent strategy that included community health worker home-based intervention, physician education and a text-messaging intervention. | 1.5 years | Drugs, laboratories, labour, costs of medical visit or screening - not further disaggregated, equipment costs and office supplies, intervention development costs, training costs, health education/promotion/ media costs. | Control group | 15.37 | 29.57 |
| Intervention group | 19.51 | 32.72 | ||||||||||
| Upper middle | China | Bai | Pharm plus | 818 | Observational study | Doctors, nurses, pharmacists, other | Community health centres that are part of a chronic disease control government programme. Components of intervention include classifying patients into four groups based on BP and risk; conduct diet, exercise, smoking and drinking interventions consisting of educational sessions, supervision and face-to-face consultation as necessary; standardise drug therapies according to 2005 Chinese national guidelines for hypertension prevention and control; conduct follow-up visits on a regular basis; provide other services, such as physician recommendations, if necessary. | 1 year | Labour, building overhead costs, depreciation, equipment costs and office supplies, health education/promotion costs. | Best case scenario - based on the lowest per capita cost and greatest blood pressure reduction of the community health centres | 0.35 | 0.75 |
| Community health centre in Beijing | 0.61 | 1.05 | ||||||||||
| Overall - all three community health centres | 0.67 | 1.33 | ||||||||||
| Community health centre in Hangzhou | 0.75 | 1.61 | ||||||||||
| Community health centre in Chengdu | 0.83 | 1.62 | ||||||||||
| Worst case scenario - based on the highest per capita cost and smallest blood pressure reduction of the community health centres | 1.76 | 3.43 | ||||||||||
| Blend | Blend | Basu | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | A ‘treat-to-target’ (TTT) strategy in which BP therapy is titrated until blood pressures fall below a threshold, a ‘benefit-based, tailored’ (BBT) strategy in which BP therapy is initiated for patients with high estimated CVD risk, and a hybrid strategy that combines TTT and BBT. | Simulation period: 10 years | Drugs, costs of medical services - including patient-borne costs | BBT - China | 0.12 | |
| Hybrid - China | 0.13 | |||||||||||
| TTT - China | 0.14 | |||||||||||
| BBT - India | 0.17 | |||||||||||
| TTT - India | 0.2 | |||||||||||
| Hybrid - India | 0.28 | |||||||||||
| Upper middle | Argentina | He | Pharm plus | 1357 | Cluster randomised control study | Community health workers, doctors | Intervention clinics implemented a community health worker-led home-based programme including health coaching, and BP monitoring. Physicians at the clinics received online education course on HTN management, and patients received individualised text messages. Control clinics maintained usual care: monthly visits after initiation of antihypertensive treatment and every 3 to 6 months for patients with controlled BP. | 18 months | Drugs, laboratories, labour, costs of medical visits or screening not further disaggregated, equipment costs, intervention development costs, training costs, media costs | Usual care | 5.59 | 10.56 |
| Intervention | 9.25 | 14.06 | ||||||||||
| Lower middle | Pakistan | Jafar | Other | 1044 | Cluster randomised control study | Community health workers, doctors | Family-based home health education by community health workers and special training of general practitioners on treatment and management of HTN. | 2 years | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, building overhead costs, training costs, health education/promotion/ absenteeism or lost productivity and fruits and vegetables. | Home health education and general practitioner training | 54.72 | |
| Home health education only | 83.01 | |||||||||||
| General practitioner training only | 113.53 | |||||||||||
| Low | Nepal | Krishnan | Pharm plus – modelled |
Not applicable | Hypothetical population-level model | Community health workers | Community health workers provide blood pressure screening, lifestyle counselling, referrals and follow-up on adherence to antihypertensive medication via home visits | 1 year | Drugs, labour, travel, training costs, administrative costs | Adults aged 25 to 65 with hypertension | 1.64 | |
| All adults aged 25 to 65 | 0.51 | |||||||||||
| Upper middle | Brazil | Obreli-Neto | Pharm plus | 200 | Randomised controlled clinical trial | Doctors, nurses, pharmacists | The control group received the usual care offered by the primary healthcare unit (medical and nurse consultations). The intervention group received the usual care plus a pharmaceutical care intervention. | 3 years | Drugs, labour and cost of medical visit or screening - not further disaggregated. | Intervention group (cost per patient divided by average change during study period) | 12.67 | 19.69 |
| Lower middle | India | Patel | Pharm only | 60 | Observational study | Not specified | Comparing two beta blockers - nebivolol and metoprolol. | 2 months | Drugs | Nebivolol 2.5 mg | 0.57 | 0.81 |
| Nebivolol 5 mg | 0.64 | 1.02 | ||||||||||
| Metoprolol 25 mg | 0.89 | 1.07 | ||||||||||
| Metoprolol 50 mg | 1.07 | 1.31 | ||||||||||
| Nebivolol 10 mg | 1.09 | 1.3 | ||||||||||
| Metoprolol 100 mg | 1.13 | 1.29 | ||||||||||
| Upper middle | Brazil | Tsuji | Pharm only | 418 | Observational study | Not specified | Traditional treatment (hydrochlorothiazide and atenolol) and current treatment (losartan and amlodipine) were evaluated in patients with grade 1 or 2 hypertension. For patients with grade 3 hypertension, a third drug was added to the treatment combinations: enalapril was added to the traditional treatment, and hydrochlorothiazide was added to the current treatment. | 1 year | Drugs | Traditional: Grade 1 or 2 HTN | 44.68 | 66.47 |
| Traditional: Grade 3 HTN | 81.73 | 107.88 | ||||||||||
| Current: Grade 3 HTN | 82.82 | 103.52 | ||||||||||
| Current: Grade 1 or 2 HTN | 90.45 | 130.77 | ||||||||||
| Upper middle | China | Wang X | Pharm plus | 436 | Randomised controlled trial | Doctors | Provider training in guideline-oriented primary healthcare HTN management programme covering detection, evaluation, non-pharmaceutical and pharmaceutical treatment, follow-up and management, two-way referral, prevention and health education for hypertension. | 1 year | Drugs, labour, travel/transportation/per diem and training costs. | PP analysis rural intervention | 3.73 | 5.99 |
| ITT analysis rural intervention | 3.85 | 6.22 | ||||||||||
| ITT analysis rural control | 4.8 | 9.1 | ||||||||||
| ITT analysis urban intervention | 5.32 | 15.22 | ||||||||||
| PP analysis urban intervention | 5.37 | 15.76 | ||||||||||
| PP analysis rural control | 5.55 | 11.09 | ||||||||||
| ITT analysis urban control | 7.94 | 34.8 | ||||||||||
| PP analysis urban control | 9.06 | 51.96 | ||||||||||
| Upper middle | China | Wang Z | Pharm only | 623 | Observational study | Not specified | Treatment with nitrendipine with hydrochlorothiazide, or treatment with nitrendipine with metoprolol. | 6 months | Drugs, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem | Nitrendipine + hydrochlorothiazide. Women. | 1.47 | 3.05 |
| Nitrendipine + hydrochlorothiazide. Men. | 1.47 | 2.95 | ||||||||||
| Nitrendipine + hydrochlorothiazide. 65 years and older. | 1.47 | 2.95 | ||||||||||
| Nitrendipine + hydrochlorothiazide. All patients. | 1.47 | 2.95 | ||||||||||
| Nitrendipine + hydrochlorothiazide. Under 65 years old. | 1.58 | 3.37 | ||||||||||
| Nitrendipine + metoprolol. Women. | 1.89 | 3.89 | ||||||||||
| Nitrendipine + metoprolol. 65 years and older. |
2 | 3.89 | ||||||||||
| Nitrendipine + metoprolol. All patients. |
2 | 4 | ||||||||||
| Nitrendipine + metoprolol. Men. |
2.1 | 4.1 | ||||||||||
| Nitrendipine + metoprolol. Under 65 years old. |
2.31 | 4.52 |
‘Pharm only’ indicates interventions or studies in which pharmacotherapy is the only form of treatment for hypertension. This includes testing various combinations of drugs and drug classes, different providers and delivery platforms. ‘Pharm plus’ indicates combination programmes that incorporated other forms of treatment for hypertension, such as patient education or lifestyle changes. ‘Other’ indicates a programme in which there was no pharmacological treatment.
BP, blood pressure; CVD, cardiovascular disease; HTN, hypertension; ITT, intention-to-treat; PP, per protocol; US$, US dollars.
Table 2.
Annual cost per patient with controlled hypertension (blood pressure brought below defined threshold) (2017 US$)
| Country income group | Country | Author | Intervention type | Sample size | Study design | Provider | Intervention details | Cost elements | Intervention subgroup | Cost (2017 US$) |
| Upper middle | Malaysia | Alefan | Pharm only | 600 | Observational | Doctors, nurses, pharmacists | Comparing different antihypertensive drug classes and combinations: Diuretics, BB, ACEIs, CCBs, prazosin, diuretics and ACEIs and other combinations | Drugs, laboratories, labour, and travel/transportation/per diem. | Diuretics | 626.78 |
| Beta blockers | 840.89 | |||||||||
| ACE Inhibitors | 977.54 | |||||||||
| Prazosin | 1004.07 | |||||||||
| Diuretics and beta blockers | 1172.96 | |||||||||
| Calcium channel blockers | 1446.8 | |||||||||
| Other combinations | 2509.7 | |||||||||
| Upper middle | Thailand | Pannarunothaai | Pharm only | 81 | Cross-sectional | Not specified | All cases of diabetes and hypertension that registered and made use of the urban health centre from 1994 to 1996 were included in group 1. All diabetic and hypertension patients who resided in the catchment area of the hospital, and visited the regional hospital from 1994 to 1996, were included in group 2. Group 3 included patients identified by the accidental sampling of diabetic and hypertension patients attending the regional hospital in 1997. | Drugs, cost of medical visit or screening - not further disaggregated, and travel/transportation/per diem. | Group 1 | 183.97 |
| Group 2 | 229.18 | |||||||||
| Group 3 | 231.05 |
ACEIs, ACE inhibitors; BB, beta blockers; CCBs, calcium channel blockers; US$, US dollars.
Table 3.
Annual cost per hypertension patient (2017 US$)
| Country income group | Country | Author | Study type | Sample size | Study design | Provider | Intervention details | Cost elements | Intervention subgroup | Cost (2017 US$) |
| Upper middle | Mexico | Arredondo | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | Analysis of healthcare costs of changes in epidemiological profile in Mexico, using hypertension as one of four tracer diseases. | Drugs, laboratories, labour, equipment costs and office supplies | Total hospital and ambulatory costs per case of hypertension | 904.73 |
| Upper middle | Malaysia | Alefan | Pharm only | 600 | Observational | Doctors, nurses, pharmacists | Comparing different antihypertensive drug classes and combinations: Diuretics, BB, ACEIs, CCBs, prazosin, diuretics and ACEIs and other combinations | Drugs, laboratories, labour, and travel/transportation/per diem. | Diuretics | 522.32 |
| Diuretics + beta blockers | 614.41 | |||||||||
| Beta blockers | 626.32 | |||||||||
| ACE inhibitors | 651.69 | |||||||||
| Calcium channel blockers | 723.4 | |||||||||
| Prazosin | 753.06 | |||||||||
| Other combinations | 826.64 | |||||||||
| Lower middle | India | Anchala | Pharm plus | 1638 | Cluster randomised control trial | Doctor | Primary healthcare physicians received training to use decision support system (DSS) software for management of HTN or received chart-based support with HTN guidelines on a poster. | Drugs, laboratories, labour, travel/transportation/per diem, building overhead costs, depreciation, equipment costs and office supplies, training costs, intervention development costs, translation charges. | Chart-based support | 356.47 |
| Decision support system | 383.15 | |||||||||
| Upper middle | Argentina | Augustovski | Pharm plus | 1432 | Cluster randomised control trial | Community health workers, doctors | Multicomponent strategy that included community health worker home-based intervention, physician education and a text-messaging intervention. | Drugs, laboratories, labour, costs of medical visit or screening - not further disaggregated, equipment costs and office supplies, intervention development costs, training costs, health education/promotion/ media costs. | Intervention group | 202.85 |
| Control group | 102.49 | |||||||||
| Upper middle | China | Bai | Other | 818 | Observational study | Doctors, nurses, pharmacists, other | Community health centres that are part of a chronic disease control government programme. Components of intervention include classifying patients into four groups based on BP and risk; conduct lifestyle education sessions, supervision, and one-on-one sessions; standardise drug therapies according to 2005 Chinese national guidelines; conduct follow-up visits on a regular basis; provide other services, such as physician recommendations, if necessary. | Labour, building overhead costs, depreciation, equipment costs and office supplies, and health education/promotion costs. | Community health centre in Beijing | 6.19 |
| Community health centre in Chengdu | 6.35 | |||||||||
| Overall - all three community health centres | 8.19 | |||||||||
| Community health centre in Hangzhou | 13.38 | |||||||||
| Blend | Blend | Basu | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | A ‘treat-to-target’ (TTT) strategy in which BP therapy is titrated until blood pressures fall below a threshold, a ‘benefit-based, tailored’ (BBT) strategy in which BP therapy is initiated for patients with high estimated CVD risk, and a hybrid strategy that combines TTT and BBT. | Drugs, costs of medical services - including patient-borne costs | TTT - India | 48.88 |
| TTT - China | 57.41 | |||||||||
| BBT - India | 76.57 | |||||||||
| Hybrid - China | 87.69 | |||||||||
| Hybrid - India | 90.72 | |||||||||
| BBT - China | 99.14 | |||||||||
| Upper middle | Brazil | Bueno | Pharm only | 377 | Cross-sectional study | Not specified | Analysis of the association between physical activity level and healthcare costs among hypertensive non-institutionalised older people. | Drugs, cost of medical visit or screening - not further disaggregated | Activity level: active | 36.08 |
| Activity level: insufficiently active | 144.51 | |||||||||
| Activity level: sedentary | 158.81 | |||||||||
| Upper middle | Mexico | Calvo-Vargas | Pharm only | Not reported | Longitudinal study | Not specified | Analysis of the annual cost of antihypertensive medications with the cost of medical consultations and laboratory tests. | Drugs, laboratories, cost of medical visit or screening - not further disaggregated | Annual cost of treatment with diuretics | 90.3 |
| Annual cost of treatment with beta blockers | 176.54 | |||||||||
| Annual cost of treatment with calcium channel blockers | 451.65 | |||||||||
| Annual cost of treatment with ACE inhibitors | 701.3 | |||||||||
| Upper middle | Brazil | Cazarim | Pharm plus | 51 | Quasi-Experimental study | Doctors, pharmacists | Prior to intervention, the public health service did not offer pharmaceutical care for hypertension. Intervention involved blood pressure measurements and CV risk measures, analysis of medications and test results, education in health matters with guidelines on patient behaviours, adherence to treatment and, when necessary, interventions in pharmacotherapy | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, building overhead costs, equipment costs and office supplies, and absenteeism or lost productivity. | Intervention period | 203.85 |
| Pre-intervention period | 205.15 | |||||||||
| Post-intervention period | 222.31 | |||||||||
| Upper middle | South Africa | Gaziano | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Intervention included screening for HTN and six different eligibility criteria for initiating pharmacological treatment (two BP-based criteria and four risk-based criteria) and a no treatment scenario in which individuals are screened but not treated. | Drugs and cost of medical visit or screening - not further disaggregated. | Screened - no treatment | 80.55 |
| Eligibility: absolute risk >40% | 80.66 | |||||||||
| Eligibility: absolute risk >30% | 81.3 | |||||||||
| Eligibility: absolute risk >20% | 84.57 | |||||||||
| Eligibility: absolute risk >15% | 87.9 | |||||||||
| Eligibility: 1995 South African guidelines - target level 160/95 | 88.83 | |||||||||
| Eligibility: Current guidelines - target level 140/90 | 93.22 | |||||||||
| Upper middle | Argentina | He | Pharm plus | 1357 | Cluster randomised control study | Community health workers, doctors | Intervention clinics implemented a community health worker-led home-based programme including health coaching, and BP monitoring. Physicians at the clinics received online education course on HTN management, and patients received individualised text messages. Control clinics maintained usual care: monthly visits after initiation of antihypertensive treatment and every 3 to 6 months for patients with controlled BP. | Drugs, laboratories, labour, costs of medical visits or screening not further disaggregated, equipment costs, intervention development costs, training costs, media costs | Intervention | 119.07 |
| Usual care | 45.07 | |||||||||
| Lower middle | Pakistan | Jafar | Other | 1044 | Cluster randomised control study | Community health workers, doctors | Family-based home health education by community health workers and special training of general practitioners on treatment and management of HTN. | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, building overhead costs, training costs, health education/promotion/ absenteeism or lost productivity and fruits and vegetables. | Home health education only | 232.42 |
| Home health education and general practitioner training | 295.49 | |||||||||
| General practitioner training only | 317.89 | |||||||||
| Upper middle | China | Le | Pharm only | 9396 | Cross-sectional study | Not specified | Estimation of the economic burden of hypertension using cross-sectional health examination and questionnaire survey. Care includes outpatient visits, hospitalisation and medication. | Drugs, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, absenteeism or lost productivity, other unspecified | Men | 609.38 |
| Women | 511.14 | |||||||||
| Age 19 to 44 years old | 326.33 | |||||||||
| Age 45 to 59 years old | 427.73 | |||||||||
| Age 60 years and older | 654.35 | |||||||||
| Overall | 547.78 | |||||||||
| Upper middle | South Africa | Makkink | Pharm only | 28 165 | Observational study | Not specified | ACE inhibitors compared with angiotensin receptor blockers (ARBs) in management of hypertension. Data analysed for 2 years, 2010 and 2011. | Drugs and other unspecified costs. | ACE inhibitor (year 2010) | 574.06 |
| ACE inhibitor (year 2011) | 625.06 | |||||||||
| ARB (year 2010) | 727.3 | |||||||||
| ARB (year 2011) | 866.27 | |||||||||
| Combined (year 2010) | 2019.93 | |||||||||
| Combined (year 2011) | 2417.85 | |||||||||
| Upper middle | Brazil | Obreli-Neto | Pharm plus | 200 | Randomised controlled clinical trial | Doctors, nurses, pharmacists | The control group received the usual care offered by the primary healthcare unit (medical and nurse consultations). The intervention group received the usual care plus a pharmaceutical care intervention. | Drugs, labour and cost of medical visit or screening - not further disaggregated. | Control group | 73.15 |
| Intervention group | 97.14 | |||||||||
| Lower middle | Kenya | Oyando | Pharm only | 212 | Cross-sectional study | Not specified | Examination of patient costs associated with obtaining care for HTN in public healthcare facilities. | Drugs, laboratories, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem | Overall median annual hypertension care cost at a public facility | 282.7 |
| Overall mean annual hypertension care cost at a public facility | 476.5 | |||||||||
| Upper middle | Argentina | Perman | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Doctors, medical students, health workers | Usual hypertension care (primary care physicians) compared with a new hypertension programme that added personal and telephone contact with patients by medical students; support with diet and activity; educational material; workshops; and, electronic health records. Programme was for middle-class patients 65 years or older. | Drugs, laboratories, labour, building overhead costs, equipment costs and office supplies, health education/promotion | Hypertension programme | 240.43 |
| Usual care | 196.50 | |||||||||
| Lower middle | India | Praveen | Pharm only | 62 194 | Cross-sectional study | Not specified | Comparing the BP lowering treatment eligibility standards compared with an untreated population. The different treatment standards were: (1) current practice (not further defined); (2) treating people with HTN using the 140/90 mm Hg threshold; (3) treatment according to the new Indian NPCDCS guidelines (drug therapy recommended in patients with CVD risk 20% to 30% and BP levels ≥140/90 mm Hg or CVD risk of ≥30% and BP levels ≥130/80 mm Hg; (4) treating everyone in the intermediate and high risk categories (regardless of BP level); and (5) treating only those in the high risk category (regardless of BP level). | Drugs, costs of medical visit or screening - not further disaggregated | Treatment of all above 55 years of age | 34.92 |
| Treatment of all at high risk | 35.07 | |||||||||
| Treatment of all above 45 years of age | 35.08 | |||||||||
| Treatment according to NPCDCS guidelines | 35.13 | |||||||||
| Treatment of all at intermediate and high risk | 35.18 | |||||||||
| Current practice (undefined) | 35.23 | |||||||||
| Treatment of patients with BP greater than 140/90 mm Hg | 54.56 | |||||||||
| Lower middle | Kenya | Subramanian | Pharm only | Not reported | Observational study | Doctors and others | Analysis of payment data on CVD, diabetes, breast and cervical cancer and respiratory diseases from Kenyatta National Hospital, the main tertiary public hospital and the Kibera South Health Centre - a public outpatient facility, and private sector practitioners and hospitals. A treatment framework was developed using an itemisation cost approach to estimate payments. | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated | Public facility - monotherapy - costs to patient | 25.64 |
| Public facility - two drug combination therapy - costs to patient | 67.25 | |||||||||
| Public facility - three drug combination treatment - costs to patient | 81.2 | |||||||||
| Public facility - four drug combination therapy - costs to patient | 110.33 | |||||||||
| Public facility - patients with resistant hypertension (high BP despite use of combination medications) - costs to patient | 159.36 | |||||||||
| Private facility - monotherapy - costs to patient | 418.2 | |||||||||
| Private facility - two drug combination therapy - costs per patient | 596.44 | |||||||||
| Private facility - three drug combination therapy - costs per patient | 948.06 | |||||||||
| Private facility - resistant hypertension (high BP despite the use of combination medications) - costs to patient | 987.17 | |||||||||
| Upper middle | China | Wang X | Pharm plus | 436 | Randomised controlled trial | Doctors | Provider training in guideline-oriented HTN management programme covering detection, evaluation, non-pharmaceutical and pharmaceutical treatment, follow-up and management, two-way referral, prevention and health education for hypertension. | Drugs, labour, travel/transportation/per diem, and training costs. | Rural intervention group - intention-to-treat analysis | 70.58 |
| Rural intervention group - per protocol analysis | 73.03 | |||||||||
| Rural control group - intention-to-treat analysis | 80.12 | |||||||||
| Rural control group - per protocol analysis | 86.52 | |||||||||
| Urban intervention group - intention-to-treat analysis | 108.05 | |||||||||
| Urban intervention group - per protocol analysis | 116.63 | |||||||||
| Urban control group - intention-to-treat analysis | 135.71 | |||||||||
| Urban control group - per protocol analysis | 155.87 | |||||||||
| Upper middle | China | Xie | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | A computer simulation model to project the consequences and cost-effectiveness of intensive hypertension control (reducing systolic/diastolic BP to 133/76 mm Hg) compared with standard hypertension control (based on the Chinese guidelines for the management of hypertension in 2011, involves the reduction of systolic/diastolic BP to 140/90 mm Hg). | Drugs, cost of medical visit or screening - not further disaggregated, monitoring costs | Standard - all men | 58.92 |
| Standard - all women | 63.27 | |||||||||
| Intensive - all men | 69.21 | |||||||||
| Standard - all men and all women | 70.96 | |||||||||
| Intensive - all men and all women | 70.96 | |||||||||
| Intensive - all women | 72.99 |
ACEIs, ACE inhibitors; BB, beta blockers; BP, blood pressure; CCBs, calcium channel blockers; CVD, cardiovascular disease; HTN, hypertension; NPCDCS, National Program on Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke; US$, US dollars.
Table 4.
Cost per averted disability-adjusted life year (2017 US$D, unless indicated otherwise)
| Country income group | Country | Author | Study type | Sample size | Study design | Provider | Intervention details | Cost elements | Intervention subgroup | Cost (2017 US$) | 2017 country GDP per capita |
| Blend | Blend | Basu | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | A ‘treat-to-target’ (TTT) strategy in which BP therapy is titrated until blood pressures fall below a threshold, a ‘benefit-based, tailored’ (BBT) strategy in which BP therapy is initiated for patients with high estimated CVD risk, and a hybrid strategy that combines TTT and BBT. | Drugs, costs of medical services - including patient-borne costs | BBT - China | 220.90 | 8826 |
| BBT - India | 290.61 | 1939 | |||||||||
| Hybrid - India | 371.58 | 1939 | |||||||||
| TTT - India | 412.85 | 1939 | |||||||||
| Hybrid - China | 449.25 | 8826 | |||||||||
| TTT - China | 450.80 | 8826 | |||||||||
| Lower middle | Ghana | Gad | Pharm only – modelled | Not applicable | Hypothetical population-level model | Not specified | A core treatment model was used to estimate the long-term costs and health effects of the five main classes of antihypertensive drugs and a ‘no intervention” comparator: ACE inhibitors (ACEI), angiotensin receptor blockers (ARB), beta blockers (BB), calcium channel blockers (CCB), thiazide-like diuretics | Drugs, cost medical visits not further disaggregated | Diuretics | 61.24 | 2025 |
| CCB | 799.35 | 2025 | |||||||||
| ACEI | 1555.47 | 2025 | |||||||||
| ARB | 1808.72 | 2025 | |||||||||
| BB | 1462.90 | 2025 | |||||||||
| Lower middle | Vietnam | Ha | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Doctors, nurses | Comparison of a set of personal and non-personal prevention strategies to reduce CVD in Vietnam, including mass media campaigns for reducing consumption of salt and tobacco, drugs for lowering blood pressure or cholesterol, and combined pharmacotherapy for people at varying levels of absolute risk of a cardiovascular event. | Drugs, laboratories, labour, travel/transportation/per diem and media costs. | Education and individual treatment (beta-blocker and diuretic) for treatment of SBP >160. | 94.24 | 2343 |
| Education and individual treatment (beta-blocker and diuretic) for treatment of SBP >140. | 268.83 | 2343 | |||||||||
| Upper middle | Thailand | Khonputsa | Pharm only – modelled | Not applicable | Hypothetical population-level model | Doctors | Analysis of monotherapy and combination therapy of thiazide diuretics (D), CCB, BB, ACEI and ARB. Cost-effectiveness analysis includes cost-offsets, that is, the cost of disease treatments that are avoided by prevention. The study calculated cost-effectiveness figures using the lowest cost generic and the median cost medication shown in the Ministry of Health website. The figures reported in this table are based on the median cost. | 10-year CVD risk 5% to 9.9%, D+CCB+ACEI | 2077.34 | 6578 | |
| 10-year CVD risk 5% to 9.9%, D | 692.45 | 6578 | |||||||||
| 10-year CVD risk 5% to 9.9%, CCB | 1483.41 | 6578 | |||||||||
| 10-year CVD risk 5% to 9.9%, ACEI | 2934.65 | 6578 | |||||||||
| 10-year CVD risk 5% to 9.9%, BB | 6594.72 | 6578 | |||||||||
| 10-year CVD risk 5% to 9.9%, ARB | 10 221.82 | 6578 | |||||||||
| 10-year CVD risk 10% to 19.9%, D | 286.87 | 6578 | |||||||||
| 10-year CVD risk 10% to 19.9%, CCB | 890.29 | 6578 | |||||||||
| 10-year CVD risk 10% to 19.9%, ACEI | 1912.47 | 6578 | |||||||||
| 10-year CVD risk 10% to 19.9%, BB | 5935.25 | 6578 | |||||||||
| 10-year CVD risk 10% to 19.9%, ARB | 7583.93 | 6578 | |||||||||
| 10-year CVD risk 20% and up, CCB | 309.95 | 6578 | |||||||||
| 10-year CVD risk 20% and up, ACEI | 956.24 | 6578 | |||||||||
| 10-year CVD risk 20% and up, BB | 3627.10 | 6578 | |||||||||
| 10-year CVD risk 20% and up, ARB | 4616.31 | 6578 | |||||||||
| Low | Nepal | Krishnan | Pharm plus – modelled | Not applicable | Hypothetical population-level model | Community health workers provide blood pressure screening, lifestyle counselling, referrals and follow-up on adherence to antihypertensive medication via home visits | Drugs, labour, travel, training costs, administrative costs | Adults aged 25 to 65 with hypertension | 568.16 | 911 | |
| All adults aged 25 to 65 | 401.23 | 911 | |||||||||
| Upper middle | Sri Lanka | Lung | Pharm only – modelled | Not applicable | Hypothetical population-level model | Doctors | The intervention group received the triple pill consisting of amlodipine, telmisartan and chlorthalidone (with discontinuation of current monotherapy, if applicable) as part of their usual hypertension clinic visits. There were scheduled clinic visits at 6, 12 and 24 weeks (end of study), which included blood pressure measurement, potential changes in medications in line with local guidelines at the discretion of the treating physician, and assessment of adverse events. | Drugs, cost of outpatient and inpatient visits not further disaggregated | Usual care | 1323.46 | 4105 |
| Intervention group | 1693.92 | 4105 | |||||||||
| Blend | Blend | Murray* | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Seventeen non-personal and personal health-service interventions or combinations, including salt reduction through voluntary agreements with industry and salt intake legislation, health education campaigns and treatment and education for hypertension. Hypertension treatment for people with BP above two thresholds (140 and 160) was a standard regimen of beta blockers and diuretics. Treatment for people with absolute risk of cardiovascular event over next 10 years based on four thresholds (35%, 25%, 15% and 5%) with a statin, diuretic, beta blocker and aspirin. | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, building overhead costs, training costs and media costs. | Eligibility: SBP above 160 (SE Asia) | 51.24* PPP dollars |
n/a |
| Eligibility: SBP above 160 (Latin America) | 115.30* PPP dollars |
n/a | |||||||||
| Eligibility: SBP above 140 (SE Asia) | 128.11* PPP dollars |
n/a | |||||||||
| Eligibility: SBP above 140 (Latin America) | 264.76* PPP dollars |
n/a | |||||||||
| Eligibility: SBP above 160 (Europe) | 288.96* PPP dollars |
n/a | |||||||||
| Eligibility: SBP above 140 (Europe) | 646.25* PPP dollars |
n/a | |||||||||
| Treatment of risk above 35% (Latin America) | 37.26* PPP dollars |
n/a | |||||||||
| Treatment of risk above 25% (Latin America) | 52.67* PPP dollars |
n/a | |||||||||
| Treatment of risk above 15% (Latin America) | 76.87* PPP dollars |
n/a | |||||||||
| Treatment of risk above 5% (Latin America) | 132.38* PPP dollars |
n/a | |||||||||
| Treatment of risk above 25% (Europe) | 239.14* PPP dollars |
n/a | |||||||||
| Treatment of risk above 15% (Europe) | 306.04* PPP dollars |
n/a | |||||||||
| Treatment of risk above 5% (Europe) | 446.97* PPP dollars |
n/a | |||||||||
| Treatment of risk above 25% (SE Asia) | 46.97* PPP dollars |
n/a | |||||||||
| Treatment of risk above 15% (SE Asia) | 68.33* PPP dollars |
n/a | |||||||||
| Treatment of risk above 5% (SE Asia) | 109.61* PPP dollars |
n/a | |||||||||
| Low | Tanzania | Ngalesoni | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | Pharmaceutical treatment with ACE inhibitors and diuretics modelled for four different risk levels. Very high risk is categorised as having SBP of 160 to 179 and being a smoker; high risk is having SBP of 160 to 179 and not being a smoker; moderate risk is having SBP of 140 to 159; and low risk is having SBP of 120 to 139. | Drugs, costs of medical visit or screening - not further disaggregated | Moderate risk | 2616.98 | 936 |
| High risk | 1761.58 | 936 | |||||||||
| Very high risk | 1533.00 | 936 | |||||||||
| Low risk | 1419.41 | 936 | |||||||||
| Blend | Sub-Saharan Africa region and South East Asia region | Ortegon | Pharm plus – modelled | Not applicable | Hypothetical population-level model | Not specified | Cost-effectiveness analysis of 123 single or combined prevention and treatment strategies for cardiovascular disease, diabetes and smoking. Relevant interventions were treatment with beta blockers and diuretics and along with patient education for two eligibility criteria (those with SBP above 140 and those above 160). | Drugs, laboratories, cost of medical visit or screening not further disaggregated, intervention development cost, training cost, media cost, monitoring and evaluation cost, other unspecified costs | Sub-Saharan Africa, eligibility: SBP >160 | 180.95* PPP dollars |
n/a |
| Sub-Saharan Africa, eligibility: SBP >140 | 504.36* PPP dollars | n/a | |||||||||
| South East Asia, eligibility: SBP >160 | 182.24* PPP dollars | n/a | |||||||||
| South East Asia, eligibility: SBP <140 | 621.14* PPP dollars | n/a | |||||||||
| Lower middle | India | Praveen | Pharm only | 62 194 | Cross-sectional study | Not specified | Comparing the BP lowering effect of treatment eligibility standards compared with an untreated population. The different treatment standards were: (1) current practice (not further defined); (2) treating people with HTN using the 140/90 mm Hg threshold; (3) treatment according to the new Indian NPCDCS guidelines (drug therapy recommended in patients with CVD risk 20% to 30% and BP levels ≥140/90 mm Hg or CVD risk of ≥30% and BP level’s ≥130/80 mm Hg; (4) treating everyone in the intermediate and high risk categories (regardless of BP level); and (5) treating only those in the high risk category (regardless of BP level). | Drugs, costs of medical visit or screening - not further disaggregated | Treatment of all at high risk | 213.72 | 1939 |
| Treatment of all at intermediate and high risk | 241.03 | 1939 | |||||||||
| Treatment according to NPCDCS guidelines | 365.43 | 1939 | |||||||||
| Current practice (undefined) | 380.27 | 1939 | |||||||||
| Treatment of patients with BP greater than 140/90 mm Hg | 459.66 | 1939 | |||||||||
| Treatment of all above 55 years of age | 472.51 | 1939 | |||||||||
| Treatment of all above 45 years of age | 601.69 | 1939 | |||||||||
| Low | Tanzania | Robberstad | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | Fourteen pharmaceutical interventions of primary prevention of cardiovascular disease, four of which specifically target hypertension exclusively. | Drugs, cost of medical visit or screening - not further disaggregated, travel/transportation/per diem, building overhead costs (utilities, maintenance, and so on), equipment costs and office supplies | Diuretics | 106.68 | 936 |
| Beta blockers | 412.93 | 936 | |||||||||
| Calcium channel blockers | 1374.33 | 936 | |||||||||
| Diuretics and beta blockers | 155.63 | 936 | |||||||||
| Lower middle | Nigeria | Rosendaal | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Population-level hypertension screening and subsequent antihypertensive treatment for high CVD risk individuals in the context of the KSHI programme. Two eligibility strategies: first was CVD risk and BP level, in which all individuals with HTN stage 1 combined with a 10-year CVD risk greater than 20% as well as all individuals with stage 2 HTN regardless of risk were treated. The second was CVD based only, in which all individuals with 10-year CVD risk greater than 20% were eligible. Three estimates of relative risk reduction, based on (1) Lawes, (2) Rapsomaniki and (3) Framingham. | Labs, labour, cost of medical visit or screening - not further disaggregated, building overhead costs, and training costs. | Treatment eligibility: Risk based. Risk reduction: Lawes et al | 3649.84 | 1968 |
| Treatment eligibility: Risk + HTN. Risk reduction based: Lawes et al | 3998.39 | 1968 | |||||||||
| Treatment eligibility: Risk based. Risk reduction: Rapsomaniki et al | 11 553.36 | 1968 | |||||||||
| Treatment eligibility: Risk based. Risk reduction: Framingham score. | 13 616.78 | 1968 | |||||||||
| Treatment eligibility: Risk + HTN. Risk reduction: Rapsomaniki et al | 17 138.03 | 1968 | |||||||||
| Treatment eligibility: Risk + HTN. Risk reduction: Framingham score. | 21 268.82 | 1968 | |||||||||
| Upper middle | Argentina | Rubinstein* | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Population and clinical interventions, including mass media campaigns to promote tobacco cessation, reduction of salt in bread, bupropion for tobacco cessation, high blood pressure treatment, high cholesterol treatment and polypill strategy for people with CVD risk greater than 20%. | Drugs, laboratories, labour, cost of medical visit or screening - not further disaggregated, trainings costs and media costs. | Lifestyle change promotion and pharmacological therapy to achieve BP control. | 2596.97 | 14 401 |
| Low | Ethiopia | Tolla | Pharm plus – modelled | Not applicable | Hypothetical population-level model | Not specified | Analysis included cost-effectiveness analysis of 15 interventions; relevant interventions were antihypertensive treatment with 25 mg hydrochlorothiazide and 50 mg atenolol per day. Patients assumed to have four visits to a health centre for the first year followed by three visits per year for the remaining 9 years. Additionally, 20% will have 1.5 visit per year at primary hospital. | Drugs, laboratories, cost of medical visit or screening not further disaggregated, intervention development cost, training cost, media cost, monitoring and evaluation cost, other unspecified costs | Eligibility: SBP >160 | 80.18 | 768 |
| Eligibility: SBP >140 | 166.86 | 768 |
BP, blood pressure; CVD, cardiovascular disease; HTN, hypertension; KSHI, Kwara State Health Insurance; n/a, not available; NPCDCS, National Program on Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke; PPP, purchasing-power-parity; SBP, systolic blood pressure; SE Asia, South East Asia; US$, US dollars.
Table 5.
Cost per gained quality-adjusted life year (2017 US$)
| Upper middle | Argentina | Augustovski | Pharm plus | 1432 | Cluster Randomised control trial | Community health workers, doctors | Multicomponent strategy that included community health worker home-based intervention, physician education and a text-messaging intervention. | Drugs, laboratories, labour, costs of medical visit or screening - not further disaggregated, equipment costs and office supplies, intervention development costs, training costs, health education/promotion/ media costs. | Intervention group | 235.88 | 14 401 |
| Control group | 124.99 | 14 401 | |||||||||
| Upper middle | China | Chen | Pharm only - modelled | Not applicable | Hypothetical cohort model | Not specified | Analysis of costs of pharmaceutical treatment for high-range prehypertensive patients (130 to 139/85 to 89 mm Hg) without CVD. | Drugs, labour and cost of medical visit or screening - not further disaggregated. | Treatment with ramipril or candesartan for prehypertension. | 13 454.18 | 8826 |
| Lower middle | Nigeria | Ekwunife | Pharm only - modelled | Not applicable | Hypothetical cohort model | Doctors | Clinical outcomes and costs during a life cycle of 30 years for 1000 people under alternative intervention scenarios for thiazide diuretics (D), beta blockers (BB), ACE inhibitor (ACEI) and calcium channel blocker (CCB). Three different treatment eligibility criteria were analysed: low risk (10-year CVD risk <15%), medium risk (10-year CVD risk 15% to 20%) and high risk (>20%). | Drugs, labs, cost of medical visits not further disaggregated | Low risk, D | 2978.55 | 1968 |
| Medium risk, D | 1489.28 | 1968 | |||||||||
| High risk, D | 1489.28 | 1968 | |||||||||
| High risk, CCB | 14 319.97 | 1968 | |||||||||
| Upper middle | South Africa | Gaziano | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Intervention included screening for HTN and six different eligibility criteria for initiating pharmacological treatment (two BP-based criteria and four risk-based criteria) and a no treatment scenario in which individuals are screened but not treated. | Drugs and cost of medical visit or screening - not further disaggregated. | No treatment | 103.04 | 6160 |
| Absolute risk >40% | 103.17 | 6160 | |||||||||
| Absolute risk >30% | 103.95 | 6160 | |||||||||
| Absolute risk >20% | 108.06 | 6160 | |||||||||
| Absolute risk >15% | 112.29 | 6160 | |||||||||
| 1995 South African guidelines - target level 160/95 | 113.51 | 6160 | |||||||||
| Current guidelines - target level 140/90 | 119.08 | 6160 | |||||||||
| Upper middle | China | Gu* | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Not specified | Hypertension screening, essential medicines programme implementation, and hypertension control programme administration, using different treatment eligibility criteria. | Drugs, labs, cost of medical visit or screening - not further disaggregated, and side effect costs. | Control BP in all persons living with CHD or stroke | 65.55 | 8826 |
| Status quo case | 65.73 | 8826 | |||||||||
| Treat all stage 2 HTN patients to goal of 140/90 if 35 to 64 and 150/90 if 65 or older | 72.27 | 8826 | |||||||||
| Treat all stage 2 and stage 1 to goal of 140/90 if 35 to 64 and 150/90 if 65 or older | 75.16 | 8826 | |||||||||
| Lower middle | Vietnam | Nguyen* | Pharm plus - modelled | Not applicable | Hypothetical population-level model | Doctors | Different intervals for screening (one-off, annual, biannually, biannually until 55 or 60 years old and then annually until death) and varying ages to start screening (35, 45 or 55 years old). Diagnosed patients in both the screening and non-screening scenarios were assumed to be receiving treatment for hypertension at the community health centre and antihypertensive drugs would be prescribed according to the Ministry of Health guidelines. | Drugs, cost of medical visit or screening - not further disaggregated, and travel/transportation/per diem. | Start screening at 55, man, biannual plus increase coverage by 20% | 127.3 | 2343 |
| Start screening at 55, woman, one-off | 331.98 | 2343 | |||||||||
| Start screening at 55, man, biannual | 791.27 | 2343 | |||||||||
| Start screening at 45, man, one-off | 1594.35 | 2343 | |||||||||
| Start screening at 55, man, annual plus increase coverage by 20% | 1624.46 | 2343 | |||||||||
| Start screening at 55, woman, biannual plus increase coverage by 20% | 2830.04 | 2343 | |||||||||
| Start screening at 55, man, annual | 2911.22 | 2343 | |||||||||
| Start screening at 45, man, biannual plus increase coverage by 20% | 3900.30 | 2343 | |||||||||
| Start screening at 55, woman, biannual | 4264.68 | 2343 | |||||||||
| Start screening at 45, woman, one-off | 4600.48 | 2343 | |||||||||
| Start screening at 45, man, biannual | 6111.74 | 2343 | |||||||||
| Start screening at 55, woman, annual plus increase coverage by 20% | 6946.83 | 2343 | |||||||||
| Start screening at 45, man, annual plus increase coverage by 20% | 9701.40 | 2343 | |||||||||
| Start screening at 55, woman, annual | 9708.26 | 2343 | |||||||||
| Start screening at 35, man, one-off | 11 218.38 | 2343 | |||||||||
| Start screening at 45, man, annual | 14 323.60 | 2343 | |||||||||
| Start screening at 45, woman, biannual plus increase coverage by 20% | 14 409.74 | 2343 | |||||||||
| Start screening at 35, man, biannual plus increase coverage by 20% | 19 288.84 | 2343 | |||||||||
| Start screening at 45, woman, biannual | 19 566.32 | 2343 | |||||||||
| Start screening at 35, man, biannual | 27 910.45 | 2343 | |||||||||
| Start screening at 45, woman, annual plus increase coverage by 20% | 30 029.26 | 2343 | |||||||||
| Start screening at 45, woman, annual | 40 220.82 | 2343 | |||||||||
| Start screening at 35, man, annual plus increase coverage by 20% | 42 155.92 | 2343 | |||||||||
| Start screening at 35, woman, one-off | 48 678.53 | 2343 | |||||||||
| Start screening at 35, man, annual | 60 277.68 | 2343 | |||||||||
| Start screening at 35, woman, biannual plus increase coverage by 20% | 111 095.98 | 2343 | |||||||||
| Start screening at 35, woman, biannual | 147 448.13 | 2343 | |||||||||
| Start screening at 35, woman, annual plus increase coverage by 20% | 218 276.41 | 2343 | |||||||||
| Start screening at 35, woman, annual | 289 176.35 | 2343 | |||||||||
| Upper middle | Brazil | Obreli-Neto | Pharm plus | 200 | Randomised controlled clinical trial | Doctors, nurses, pharmacists | The control group received the usual care offered by the primary healthcare unit (medical and nurse consultations). The intervention group received the usual care plus a pharmaceutical care intervention. | Drugs, labour and cost of medical visit or screening - not further disaggregated. | Intervention group | 206.69 | 9821 |
| Control group | 2031.99 | 9821 | |||||||||
| Upper middle | China | Xie | Pharm only - modelled | Not applicable | Hypothetical population-level model | Not specified | A computer simulation model to project the consequences and cost-effectiveness of intensive hypertension control (reducing systolic/diastolic BP to 133/76 mm Hg) compared with standard hypertension control (based on the Chinese guidelines for the management of hypertension in 2011, involves the reduction of systolic/diastolic BP to140/90 mm Hg). | Drugs, cost of medical visit or screening - not further disaggregated, monitoring costs | Standard - all men and all women | 73.95 | 8826 |
| Standard - all men | 71.94 | 8826 | |||||||||
| Standard - all women | 76.14 | 8826 | |||||||||
| Intensive - all men and all women | 85.19 | 8826 | |||||||||
| Intensive - all men | 83.58 | 8826 | |||||||||
| Intensive - all women | 87.00 | 8826 |
BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; HTN, hypertension; US$, US dollars.
Results
Study characteristics
Thirty-six of the identified studies were conducted in upper-middle-income countries (UMICs), 30 studies were from low-income and lower-middle-income countries (LLMICs) and five studies included countries of different income levels. Studies reported costs of hypertension treatment, cost-effectiveness of hypertension treatment or both. Twenty-five of the studies included only medication costs, while the remaining studies included health system costs and other services such as laboratory tests, health provider time and other screening costs. Study designs included longitudinal (seven studies), cross-sectional (four studies), modelled or simulated (22 studies), randomised control trials (seven studies) and retrospective cohort studies (two studies).
After conducting the quality assessment based on the 13-question checklist informed by Drummond guidelines for economic evaluation of healthcare programmes (Evers et al, 2005),19 the average quality score of the studies was 7.8. Modelled studies and randomised control trials tended to be higher quality, with average scores of 9.6 and 8.4, respectively. Longitudinal, cross-sectional and retrospective cohort studies were lower quality, with average scores of 5.0, 4.3 and 3.0, respectively (table 6).
Table 6.
Quality assessment of 34 reviewed cost-effectiveness studies
| Author | (1) Was a well-defined question posed in answerable form? | (2) Did the study examine both costs and effects of the service or programme? | (3) Did the study involve a comparison of alternatives? | (4) Was a viewpoint for the analysis stated? | (5) Was a do-nothing alternative considered? | (6) Were the capital costs, as well as operating costs, included? | (7) Were the cost and consequences valued credibly? | (8) Were currencies updated and converted clearly and appropriately? | (9) Were costs and consequences that occur in the future discounted to their present value? | (10) Was there any justification given for the discount rate used? | (11) Were the incremental costs generated by one alternative over another compared with the additional effects generated? | (12) Was a sensitivity analysis performed? | (13) Did the study discuss the generalisability of the results to other setting and patient/client groups? | Total score (out of 13 equally weighted) | Type of study design |
| Alefan | No | Yes | Yes | Yes | No | No | Yes | Yes | No | No | No | Yes | Yes | 7 | Longitudinal |
| Amira | No | Yes | Yes | No | No | No | Yes | No | No | No | No | No | No | 3 | Cross-sectional |
| Anchala et al47 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 10 | RCT |
| Anderson, AN | No | Yes | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | 7 | Cross-sectional |
| Augustovski et al28 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 11 | RCT |
| Bai et al21 | No | Yes | No | Yes | No | Yes | Yes | Yes | No | No | No | No | Yes | 5 | Longitudinal |
| Basu et al33 | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 9 | Modelled |
| Cazarim24 | No | Yes | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | No | 7 | Longitudinal |
| Chen et al46 | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 9 | Modelled |
| Das | No | Yes | Yes | No | No | No | No | No | No | No | No | No | No | 2 | Longitudinal |
| Edwards | No | Yes | Yes | No | No | No | Yes | Yes | No | No | No | No | No | 4 | Longitudinal |
| Ekwunife et al42 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 | Modelled |
| Gad et al38 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Modelled |
| Gaziano et al34 | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 | Modelled |
| Gu et al31 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 | Modelled |
| Ha36 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Modelled |
| He | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 10 | RCT |
| Ilesanmi | No | Yes | Yes | No | No | No | Yes | Yes | No | No | No | No | No | 4 | Cross-sectional |
| Jafar et al22 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12 | RCT |
| Jiang | No | Yes | Yes | No | No | No | Yes | No | No | No | No | No | No | 3 | Cross-sectional |
| Khonputsa et al39 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | 9 | Modelled |
| Krishnan et al43 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 | Modelled |
| Lung et al40 | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | 8 | Modelled |
| Makkink | No | Yes | Yes | Yes | No | No | No | No | No | No | No | No | No | 3 | Retrospective cohort study |
| Murray et al13 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Modelled |
| Ngalesoni et al45 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 | Modelled |
| Nguyen et al37 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | 9 | Modelled |
| Ortegon | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 11 | Modelled |
| Pandey | No | Yes | Yes | No | No | No | Yes | Yes | No | No | No | Yes | No | 5 | Modelled |
| Pannarunothaai | No | Yes | No | No | No | Yes | Yes | No | No | No | No | No | No | 3 | Retrospective cohort study |
| Patel | No | Yes | Yes | No | No | No | No | No | No | No | No | No | No | 2 | RCT |
| Perman | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 13 | Modelled |
| Praveen et al49 | No | Yes | Yes | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | 7 | RCT |
| Robberstad et al35 | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 | Modelled |
| Rosendaal et al44 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 11 | Modelled |
| Rubinstein et al29 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 11 | Modelled |
| Tolla et al41 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Modelled |
| Tsuji | No | Yes | Yes | No | No | No | Yes | No | No | No | Yes | No | Yes | 5 | Longitudinal |
| Verguet | No | Yes | Yes | No | Yes | No | Yes | Yes | No | No | No | Yes | No | 6 | Modelled |
| Wang, Xin et al48 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | 7 | RCT |
| Wang, Zengwu | No | Yes | Yes | No | No | No | Yes | Yes | No | No | Yes | No | Yes | 5 | Longitudinal |
| Xie et al32 | No | Yes | No | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | 7 | Modelled |
RCT, randomised controlled trial.
Fifty-four studies described pharmaceutical-only interventions using various combinations of antihypertensive drugs and drug classes. Fifteen studies assessed pharmaceutical treatment plus at least one other component, such as providing physician training, implementing treatment guidelines or offering lifestyle advice. A small number of studies did not include pharmaceutical treatment and instead assessed cost-effectiveness of activities such as physician training, lifestyle education (Bai et al, 201321 and Jafar et al, 2011),22 or loaning out blood pressure self-measurement devices (Calvo-Vargas et al, 2001).23 Four different delivery platforms were represented across studies: community-based services; health centres providing basic medical care and staffed by a physician, nurse or mid-level healthcare provider; first-level hospitals that have the capacity to perform surgery and provide inpatient care; and referral or speciality hospitals that include general specialists and provide secondary and tertiary services. As such, care was provided by a range of providers that included physicians, nurses, pharmacists and community health workers.
Cost and cost-effectiveness evidence
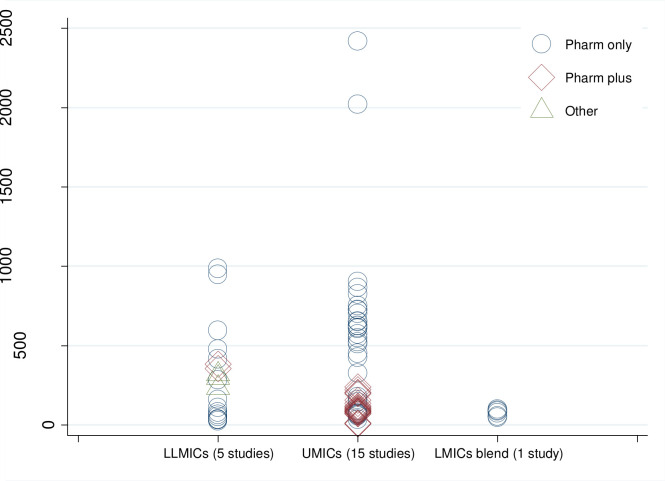
Study results were reported across five outcome types: Cost per mm Hg reduction in systolic and/or diastolic blood pressure (13 studies; table 1); annual cost per patient with controlled hypertension (2 studies; table 2); annual cost per patient with hypertension (21 studies, 7 of which did not include a cost-effectiveness analysis; table 3); cost per averted DALY (14 studies; table 4); and cost per gained QALY (8 studies; table 5). Significant variability was present across studies due to cost differences even across studies with like interventions. For example, two interventions in UMICs both providing patient risk assessment, education, pharmacotherapy and adherence monitoring reported substantially different per patient costs for the intervention—US$6.19 to US$13.38 per patient in China (Bai et al, 2013) compared with US$203.85 in Brazil (Cazarim and Pereira, 2018).24 In this example, the analysis in China did not include the cost of drugs whereas the analysis in Brazil included indirect costs such as the cost of absenteeism resulting from missing work for doctor’s appointments. Across all the types of interventions, the range of estimates of the annual intervention cost per hypertension patient was wider in UMICs (ranging from US$6.2 for a non-drug intervention programme in China to US$2418 for a Pharm only programme in South Africa) than in LLMICs (ranging from US$25.6 for a Pharm only programme in Kenya public facilities to US$987 for a Pharm only programme in Kenya private facilities). Nonetheless, almost all studies in all countries yielded results below US$1000 per patient for any intervention (figure 2).
Figure 2.
Annual cost per treated hypertension patient in hypertension management programmes (2017 US$). Notes: Estimates from 21 studies. LLMICs: India, Kenya and Pakistan; UMICs: Argentina, Brazil, China, Malaysia, Mexico and South Africa. ‘Pharm only’ indicates interventions where pharmacotherapy is the only treatment element. ‘Pharm plus’ indicates combination programmes that incorporate other forms of treatment for hypertension in addition to medications. ‘Other’ indicates interventions that did not evaluate changes in pharmacological treatment. LMICs, low-income and middle-income countries; LLMICs, low-income and lower-middle-income countries; UMICs, upper-middle-income countries; US$, US dollars.
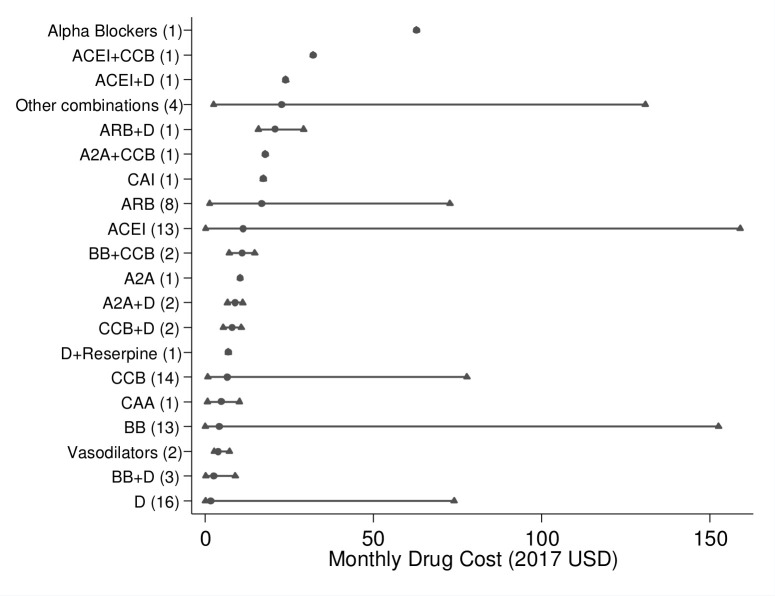
Median monthly drug costs were less than US$50 for the 23 studies with medication-specific costs of treatment by drug or drug combination group (figure 3); however, the lowest and highest monthly costs illustrate a wide range across contexts. The widest cost range was observed for monotherapy with angiotensin-converting enzyme inhibitors (ACEI) (US$0.18 to US$159 with a median monthly cost of US$11) and beta blockers (BB) (US$0.11 to US$153 with a median monthly cost of US$4.25), obtained from 13 studies for each medication type. Other commonly evaluated monotherapy plans focussed on diuretics (16 studies, with estimates ranging from US$0.12 to US$74 with a median of US$1.77), calcium channel blockers (CCB) (14 studies, with estimates ranging from US$0.79 to US$78 with a median of US$6.56) and angiotensin-II receptor blockers (ARB) (8 studies, with estimates ranging from US$1.37 to US$73 with a median of US$17). Other less common treatment plans, such as multiple-drug therapies and monotherapies involving alpha blockers, alpha-2 agonists, central acting antiadrenergics and central adrenergic inhibitors, had very limited representation with one to two studies each. Monotherapies with diuretics, BB and CCB were less costly while ACEI or ARB monotherapy incurred a higher median cost(figure 3). However, drug price variability across studies, reflecting cross-country differences in price, procurement and delivery context, prevents robust comparison of costs across treatment plans.
Figure 3.
Range of monthly drug cost (2017 US$) by treatment type (minimum, median, and maximum values). Notes: Estimates from 23 studies reporting costs of medication treatment only. A2A, alpha-2 agonists; ACEI, ACE inhibitors; ARB, angiotensin-2 receptor blockers; BB, beta blockers; CAA, central acting antiadrenergics; CAI, central adrenergic inhibitors; CCB, calcium channel blockers; D, diuretics; US$, US dollars.
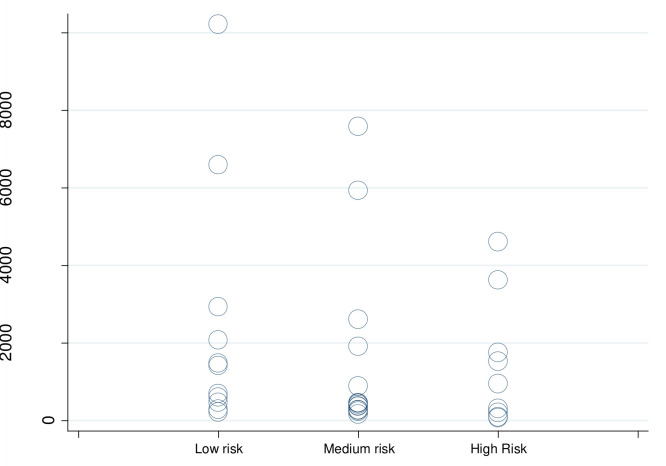
Of the 42 cost-effectiveness evaluations, 6 studies reported cost per averted DALY while also reporting differences across at least two CVD risk levels. Figure 4 describes the range of estimates across risk groups, in 2017 US$. Despite the wide range of cost-effectiveness estimates, most occurred below US$1000 per averted DALY. There was some indication that higher cost-effectiveness is associated with focussing on higher-risk patients (figure 4).
Figure 4.
Cost per DALY averted, by CVD risk (in '000s 2017 US$). Notes: Estimates from six studies reporting risk-specific estimates across multiple CVD risk levels (Basu, Ha, Khonputsa, Ngalesoni, Praveen, Tolla). CVD, cardiovascular disease; DALY, disability-adjusted life year; US$, US dollars.
A common threshold for cost-effectiveness determination in LMICs is based on per capita gross domestic product (GDP), where an intervention is considered cost-effective if the cost per DALY averted or QALY gained is less than three times the annual per capita country GDP, and very cost-effective if the cost per DALY averted or QALY gained does not exceed the annual per capita GDP. Despite some limitations of the GDP threshold approach (Marseille et al, 201425 and Bertram et al, 2016),26 we used it as a guideline to compare cost-effectiveness across studies reporting DALY-based and QALY-based cost-effectiveness indicators. Hypertension interventions were found to be cost-effective in the majority of evaluations using the GDP threshold (tables 4 and 5). As figure 4 illustrates, most cost-effectiveness estimates in our review were clustered below US$1000 per averted DALY—well below the average 2017 GDP per capita for lower-middle income countries of $2188 (FRED,27 suggesting they could be very cost-effective for lower-middle income countries. Favourable cost-effectiveness levels using the GDP threshold were found for programmes in Argentina (Augustovski et al, 201828 and Rubinstein et al, 201029), Brazil (Obreli-Neto et al, 201530), China (Gu et al, 201531; Xie et al, 201832; Basu et al, 201633), South Africa (Gaziano et al, 200534), Tanzania (Robberstad et al, 200735), Vietnam (Ha and Chisholm, 201136 and Nguyen et al, 201637), India (Basu et al, 201633), Ghana (Gad et al, 202038), Thailand (Khonputsa et al, 201239), Sri Lanka (Lung et al, 201940), Ethiopia (Tolla et al, 201641), Nigeria (Ekwunife et al, 201342) and Nepal (Krishnan et al, 2019.43 A small number of studies indicated that cost-effectiveness thresholds were more difficult to meet in lower-income countries; for example, cost-effectiveness was not established for select intervention scenarios reported in Nigeria (Rosendaal et al, 201644 and Ekwunife et al, 2013) and Tanzania (Ngalesoni et al, 201645 and Robberstad et al, 2007) (table 4). Factors that were associated with not meeting the cost-effectiveness thresholds for their respective countries included treatment of patients at lower risk for CVD (Ekwunife et al, 2013 and Khonputsa et al, 2012), screening for hypertension at younger ages (for example, at age 35 vs 55, Nguyen et al, 2016), and addressing prehypertension (Chen et al, 201746 (table 5).
Several studies evaluated non-pharmaceutical interventions in addition to medication treatment. One study found a complex strategy that included community health worker home-based visits, physician education and text messaging promoting lifestyle change and medication adherence was less cost-effective than usual care (Augustovski et al, 2018). By contrast, three other studies estimated that interventions for hypertension management such as physician training were more cost-effective than usual care (Anchala et al, 201547; Jafar et al, 2011; and Wang et al, 201348).
Discussion
The range of estimated costs and cost-effectiveness of hypertension programmes is wide, both across and within countries, reflecting heterogeneity in intervention design, cost components and country context. We broadly distinguished between intervention designs that involved pharmaceutical treatment only and those that included non-pharmaceutical components, such as provider or patient training, and between countries with different income levels. We did not observe clear distinctions in programme cost-effectiveness based on country group or inclusion of non-pharmaceutical programme elements; however, the large majority of interventions that reported cost per averted DALY were found to be cost-effective using national income thresholds, with costs per averted DALY not exceeding the average GDP per capita of lower-middle income nations. Some exceptions were observed in lower-income countries, where the cost-effectiveness cut-off, as defined by national GDP, is lower. This might suggest that hypertension management programmes in lower-income countries may warrant special consideration in terms of minimising costs relative to outcomes. However, the potential need to accommodate programmes in LMICs to lower cost-effectiveness thresholds is not necessarily generalisable. For example, a recent study from Nepal, a low-income country, detailed very high cost-effectiveness of a community-based hypertension management programme relative to its income threshold (Krishnan et al, 2019). Relatively higher costs per averted DALY were observed in scenarios that expanded treatment to younger age groups or to prehypertension, suggesting that more targeted treatment may improve cost-effectiveness. Median drug costs for monotherapies involving diuretics, beta blockers and calcium channel blockers appeared to be lower than those involving ACE inhibitors or combinations.
While this review did not establish a clear pattern in cost-effectiveness when comparing estimates of cost per averted DALY by patient CVD risk across studies, individual studies indicated that hypertension treatment tends to be more cost-effective when applied to populations at higher CVD risk (Ngalesoni et al, 2016; Praveen et al, 201849; Ha and Chisholm, 2011; Khonputsa et al, 2012; and Tolla et al, 2016), pointing to an important area for future research on the role of risk-tailored treatment. Hypertension treatment guidelines in LLMICs can be strengthened by further evidence translating the use of simple risk assessments based on age, smoking status and obesity into population-level efficiencies in CVD prevention (Kaptoge et al, 2019).50
In addition to the low comparability across intervention programmes in LMICs, this review is subject to a number of limitations. We did not review the economic literature for the potential of behavioural modifications such as low-sodium diet, healthy weight, physical activity and eliminating tobacco use (WHO, 2011)51 to control blood pressure. Such modifications have been promoted at the population level through national policies on taxation and/or regulation of products containing trans-fatty acids, excess sodium, tobacco and added sugar and the WHO has summarised those results in online supplementary appendix 3 of the Global Action Plan for Non-Communicable Diseases (WHO, 201752; Task Force on Fiscal Policy for Health, 201953; and WHO, 201354). Studies in this review did not specifically aim to evaluate improved access to medications; rather, they described the relative cost-effectiveness of different treatment approaches, or, less frequently, the relative effectiveness of the same treatment approach across different study groups. Three studies that compared the cost per hypertension patient with treatment relative to no treatment found, as expected, that costs increased with the initiation of treatment (Cazarim and Pereira, 2018; Gaziano et al, 2005; and Obreli-Neto et al, 2015). This review does not assess the cost-effectiveness of population-level approaches that can improve hypertension and is mostly limited to studies with health-systems perspective rather than societal perspective. Programme evaluation from the health system perspective rather than the social perspective presents a narrower view of hypertension interventions. Another limiting aspect is that many studies did not specify the type of provider involved in the intervention, precluding inferences about costs associated with different provider type or delivery platform. Comparisons of drug class combinations were limited by lack of information on underlying drivers of drug price such as generic or brand status or type of drug within a drug class.
To reduce the knowledge gap about factors that can influence the cost-effectiveness of hypertension programmes, future research can focus on programme elements that may be particularly relevant to low-resource settings, such as the uptake of healthcare tasks by non-physician providers and the assessment of patient CVD risk in treatment determination. Using community health workers (CHW) in the provision of chronic disease care has been associated with increased cost-effectiveness in the USA (Kim et al, 201655), and has been similarly regarded in LMICs (Jeet et al, 201756 and Krishnan et al, 2019), but evidence specific to hypertension care costs is mostly lacking. Additional studies focussing on the role of CHW in improving the cost-effectiveness of hypertension interventions can help inform health strategies in areas where access to care is otherwise limited. Standardisation of cost evaluation platforms can streamline economic assessment across countries. An example of a mechanism for evaluating the costs of standardised CVD prevention approaches is provided by the costing mechanism for the HEARTS package of clinical guidelines for CVD prevention in primary care (WHO, 201657 and WHO, 201758). A list of standard cost elements to track during implementation of hypertension management programmes is included in table 7, which summarises the leading cost indicators of HEARTS programme components, including establishment of treatment protocols, training of healthcare providers in lifestyle counselling and risk-based management, ensuring access to essential medicines and promoting task sharing and systems for patient monitoring. Additional evidence on the cost-effectiveness of introducing non-physician health workers in healthcare delivery can inform future approaches to address physician scarcity (Seidman and Atun, 201759; Jafar et al, 2011; and Chen et al, 200460).
Table 7.
Key cost elements of standardised programme implementation: WHO Global Hearts Initiative, HEARTS technical package for CVD prevention in primary care.
| HEARTS element |
Description | Cost elements |
| H: Healthy lifestyles | Counselling on lifestyle changes, including tobacco cessation, dietary modification, avoiding harmful use of alcohol and increasing physical activity | Training of healthcare providers |
| Provider time for patient screening and counselling | ||
| Health promotion materials | ||
| E: Evidence-based treatment protocols | Adopting simple, standard treatment protocols for use in primary care for the management of CVD, including secondary prevention and management of high blood pressure and diabetes | Provider time for patient screening |
| Provider time for physical exam | ||
| Provider time for laboratory tests | ||
| A: Access to essential medicines and technologies | Continuous availability of essential medicines and basic technology in primary healthcare | Inventory of core medicines |
| Inventory of diagnostic supplies (eg, blood pressure measurement devices, laboratory supplies) | ||
| R: Risk-based Management | Incorporating CVD risk assessment for treatment and referral | Training of healthcare providers in risk assessment |
| Provider time for establishing patient risk profile using risk charts | ||
| T: Team care and task sharing | Incorporating team-based care and non-physician healthcare providers in primary care | Training of healthcare providers |
| Training of supervisors | ||
| Change in provider time across types of healthcare providers (physicians, nurses, community health workers) | ||
| S: Systems for monitoring | Establishing patient records for follow-up, tracking and reporting health outcomes | Technology (software, hardware) |
| Supplies (if using paper materials) | ||
| Administrative staff | ||
| Training of administrative staff |
CVD, cardiovascular disease.
Although CVD death rates have decreased worldwide since 1990, improvements have not been evenly distributed across countries, and have showed signs of slowing down (GBD, 2018). Both domestic and external financing for non-communicable diseases across LMICs remains low (IHME, 2019). The results of this review suggest that hypertension control approaches can be a cost-effective way to prevent premature CVD in LMICs across a variety of population, clinical and health system contexts.
Footnotes
Handling editor: Lei Si
Contributors: GS conducted a comprehensive literature search. All authors contributed to the analysis, drafting and editing of the manuscript.
Funding: GS and RN received support from the CDC Foundation with funds provided by Resolve to Save Lives, a division of Vital Strategies. Resolve to Save Lives is funded by grants from Bloomberg Philanthropies; the Bill and Melinda Gates Foundation; and Gates Philanthropy Partners, which is funded with support from the Chan Zuckerberg Foundation. The funders had no role in the design of this study and did not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request. As a review article, this article reports data from previously published studies.
References
- 1.Chow CK, Teo KK, Rangarajan S, et al. . Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013;310:959–68. 10.1001/jama.2013.184182 [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim MM, Damasceno A. Hypertension in developing countries. The Lancet 2012;380:611–9. 10.1016/S0140-6736(12)60861-7 [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Fullman N, Abate D, et al. . Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related sustainable development goals for 195 countries and territories: a systematic analysis for the global burden of disease study 2017. The Lancet 2018;392:2091–138. 10.1016/S0140-6736(18)32281-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization A global brief on hypertension, 2013. Available: https://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/
- 5.Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet 2018;391:709–12. 10.1016/S0140-6736(17)32443-1 [DOI] [PubMed] [Google Scholar]
- 6.Kontis V, Cobb LK, Mathers CD, et al. . Three public health interventions could save 94 million lives in 25 years. Circulation 2019;140:715–25. 10.1161/CIRCULATIONAHA.118.038160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geldsetzer P, Manne-Goehler J, Marcus M-E, et al. . The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. The Lancet 2019;394:652–62. 10.1016/S0140-6736(19)30955-9 [DOI] [PubMed] [Google Scholar]
- 8.Roth GA, Abate D, Abate KH, et al. . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom DE, Cafiero ET, Jané-Llopis E, et al. . The global economic burden of noncommunicable diseases. Geneva: World Economic Forum, 2011. [Google Scholar]
- 10.United Nations General Assembly Political Declaration of the high-level meeting of the general assembly on the prevention and control of noncommunicable diseases, 2011. Available: https://digitallibrary.un.org/record/720106
- 11.United nations sustainable development goals. Available: https://sustainabledevelopment.un.org/sdgs
- 12.World Health Organization Noncommunicable diseases and their risk factors. third un high-level meeting on NCDS, 2018. a. Available: http://www.who.int/ncds/governance/third-un-meeting/about/en/
- 13.Murray CJL, Lauer JA, Hutubessy RCW, et al. . Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. The Lancet 2003;361:717–25. 10.1016/S0140-6736(03)12655-4 [DOI] [PubMed] [Google Scholar]
- 14.Jha P, Nugent R, Verguet S, et al. . Chronic disease control. Copenhagen consensus center challenge paper, 2012. Available: https://www.copenhagenconsensus.com/sites/default/files/chronicdisease.pdf
- 15.Nugent R, Brouwer E. Economic Benefit-Cost analysis of select secondary prevention interventions in LMIC. Glob Heart 2015;10:319–21. 10.1016/j.gheart.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Bertram MY, Sweeny K, Lauer JA, et al. . Investing in non-communicable diseases: an estimation of the return on investment for prevention and treatment services. The Lancet 2018;391:2071–8. 10.1016/S0140-6736(18)30665-2 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Saving lives, spending less: a strategic response to noncommunicable diseases, 2018. b. Available: https://apps.who.int/iris/bitstream/handle/10665/272534/WHO-NMH-NVI-18.8-eng.pdf?ua=1
- 18.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evers S, Goossens M, de Vet H, et al. . Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240–5. 10.1017/S0266462305050324 [DOI] [PubMed] [Google Scholar]
- 20.Ortegón M, Lim S, Chisholm D, et al. . Cost effectiveness of strategies to combat cardiovascular disease, diabetes, and tobacco use in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ 2012;344:e607 10.1136/bmj.e607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai Y, Zhao Y, Wang G, et al. . Cost-Effectiveness of a hypertension control intervention in three community health centers in China. J Prim Care Community Health 2013;4:195–201. 10.1177/2150131912470459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafar TH, Islam M, Bux R, et al. . Cost-Effectiveness of community-based strategies for blood pressure control in a low-income developing country. Circulation 2011;124:1615–25. 10.1161/CIRCULATIONAHA.111.039990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. . Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model’. Blood Press Monit 2001;6:225–32. 10.1097/00126097-200110000-00001 [DOI] [PubMed] [Google Scholar]
- 24.Cazarim MdeS, Pereira LRL. Cost-Effectiveness analysis of pharmaceutical care for hypertensive patients from the perspective of the public health system in Brazil. PLoS One 2018;13:e0193567 10.1371/journal.pone.0193567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marseille E, Larson B, Kazi DS, et al. . Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ 2015;93:118–24. 10.2471/BLT.14.138206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertram MY, Lauer JA, De Joncheere K, et al. . Cost-Effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94:925–30. 10.2471/BLT.15.164418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Federal reserve bank economic data. Available: https://fred.stlouisfed.org/series/NYGDPPCAPCDLMC
- 28.Augustovski F, Chaparro M, Palacios A, et al. . Cost-Effectiveness of a comprehensive approach for hypertension control in low-income settings in Argentina: trial-based analysis of the hypertension control program in Argentina. Value Health 2018;21:1357–64. 10.1016/j.jval.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein A, Colantonio L, Bardach A, et al. . Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC Public Health 2010;10 10.1186/1471-2458-10-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obreli-Neto PR, Marusic S, Guidoni CM, et al. . Economic evaluation of a pharmaceutical care program for elderly diabetic and hypertensive patients in primary health care: a 36-month randomized controlled clinical trial. J Manag Care Spec Pharm 2015;21:66–75. 10.18553/jmcp.2015.21.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu D, He J, Coxson PG, et al. . The cost-effectiveness of low-cost essential antihypertensive medicines for hypertension control in China: a modelling study. PLoS Med 2015;12:e1001860 10.1371/journal.pmed.1001860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, He T, Kang J, et al. . Cost-Effectiveness analysis of intensive hypertension control in China. Prev Med 2018;111:110–4. 10.1016/j.ypmed.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Yudkin JS, Sussman JB, et al. . Alternative strategies to achieve cardiovascular mortality goals in China and India: a microsimulation of target- versus risk-based blood pressure treatment. Circulation 2016;133:840–8. 10.1161/CIRCULATIONAHA.115.019985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaziano TA, Steyn K, Cohen DJ, et al. . Cost-Effectiveness analysis of hypertension guidelines in South Africa: absolute risk versus blood pressure level. Circulation 2005;112:3569–76. 10.1161/CIRCULATIONAHA.105.535922 [DOI] [PubMed] [Google Scholar]
- 35.Robberstad B, Hemed Y, Norheim OF. Cost-effectiveness of medical interventions to prevent cardiovascular disease in a sub-Saharan African country--the case of Tanzania. Cost Eff Resour Alloc 2007;5:3. 10.1186/1478-7547-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ha DA, Chisholm D. Cost-Effectiveness analysis of interventions to prevent cardiovascular disease in Vietnam. Health Policy Plan 2011;26:210–22. 10.1093/heapol/czq045 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen T-P-L, Wright EP, Nguyen T-T, et al. . Cost-Effectiveness analysis of screening for and managing identified hypertension for cardiovascular disease prevention in Vietnam. PLoS One 2016;11:e0155699. 10.1371/journal.pone.0155699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gad M, Lord J, Chalkidou K, et al. . Supporting the development of evidence-informed policy options: an economic evaluation of hypertension management in Ghana. Value Health 2020;23:171–9. 10.1016/j.jval.2019.09.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khonputsa P, Veerman LJ, Bertram M, et al. . Generalized cost-effectiveness analysis of pharmaceutical interventions for primary prevention of cardiovascular disease in Thailand. Value Health Reg Issues 2012;1:15–22. 10.1016/j.vhri.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 40.Lung T, Jan S, de Silva HA, et al. . Fixed-Combination, low-dose, triple-pill antihypertensive medication versus usual care in patients with mild-to-moderate hypertension in Sri Lanka: a within-trial and modelled economic evaluation of the triumph trial. Lancet Glob Health 2019;7:e1359–66. 10.1016/S2214-109X(19)30343-2 [DOI] [PubMed] [Google Scholar]
- 41.Tolla MT, Norheim OF, Memirie ST, et al. . Prevention and treatment of cardiovascular disease in Ethiopia: a cost-effectiveness analysis. Cost Eff Resour Alloc 2016;14:10. 10.1186/s12962-016-0059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekwunife OI, Okafor CE, Ezenduka CC, et al. . Cost-Utility analysis of antihypertensive medications in Nigeria: a decision analysis. Cost Eff Resour Alloc 2013;11:2. 10.1186/1478-7547-11-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan A, Finkelstein EA, Kallestrup P, et al. . Cost-Effectiveness and budget impact of the community-based management of hypertension in Nepal study (COBIN): a retrospective analysis. Lancet Glob Health 2019;7:e1367–74. 10.1016/S2214-109X(19)30338-9 [DOI] [PubMed] [Google Scholar]
- 44.Rosendaal NTA, Hendriks ME, Verhagen MD, et al. . Costs and cost-effectiveness of hypertension screening and treatment in adults with hypertension in rural Nigeria in the context of a health insurance program. PLoS One 2016;11:e0157925 10.1371/journal.pone.0157925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngalesoni FN, Ruhago GM, Mori AT, et al. . Cost-Effectiveness of medical primary prevention strategies to reduce absolute risk of cardiovascular disease in Tanzania: a Markov modelling study. BMC Health Serv Res 2016;16:185 10.1186/s12913-016-1409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T, Yu D, Cornelius V, et al. . Potential health impact and cost-effectiveness of drug therapy for prehypertension. Int J Cardiol 2017;240:403–8. 10.1016/j.ijcard.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 47.Anchala R, Kaptoge S, Pant H, et al. . Evaluation of effectiveness and Cost‐Effectiveness of a clinical decision support system in managing hypertension in resource constrained primary health care settings: results from a cluster randomized trial. J Am Heart Assoc 2015;4:e001213 10.1161/JAHA.114.001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Li W, Li X, et al. . Effects and cost-effectiveness of a guideline-oriented primary healthcare hypertension management program in Beijing, China: results from a 1-year controlled trial. Hypertens Res 2013;36:313–21. 10.1038/hr.2012.173 [DOI] [PubMed] [Google Scholar]
- 49.Praveen D, Peiris D, MacMahon S, et al. . Cardiovascular disease risk and comparison of different strategies for blood pressure management in rural India. BMC Public Health 2018;18:1264. 10.1186/s12889-018-6142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaptoge S, Pennells L, De Bacquer D, et al. . World Health organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–45. 10.1016/S2214-109X(19)30318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Economic Forum, WHO From burden to “best buys”: reducing the economic impact of non-communicable diseases in low- and middle-income countries. Geneva: World Economic Forum, 2011. http://www.who.int/nmh/publications/best_buys_summary.pdf [Google Scholar]
- 52.World Health Organization Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases: Appendix 3 of the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020. Geneva: WHO, 2017. [Google Scholar]
- 53.Task Force on Fiscal Policy for Health Health taxes to save lives: employing effective excise taxes on tobacco, alcohol, and Sugary beverages. chairs: Michael R. Bloomberg and Lawrence H. summers. New York: Bloomberg Philanthropies, 2019. https://www.bloomberg.org/program/public-health/task-force-fiscal-policy-health/ [Google Scholar]
- 54.World Health Organization Global action plan for the prevention and control of NCDS 2013–20. Geneva: World Health Organization, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K, Choi JS, Choi E, et al. . Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health 2016;106:e3–28. 10.2105/AJPH.2015.302987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeet G, Thakur JS, Prinja S, et al. . Community health workers for non-communicable diseases prevention and control in developing countries: evidence and implications. PLoS One 2017;12:e0180640 10.1371/journal.pone.0180640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization Hearts: technical package for cardiovascular disease management in primary health care. Geneva, Switzerland: World Health Organization, 2016. http://www.who.int/cardiovascular_diseases/hearts/Hearts_package.pdf [Google Scholar]
- 58.World Health Organization Who model list of essential medicines, 2017. Available: https://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1 [DOI] [PubMed]
- 59.Seidman G, Atun R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Hum Resour Health 2017;15:29 10.1186/s12960-017-0200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L, Evans T, Anand S, et al. . Human resources for health: overcoming the crisis. The Lancet 2004;364:1984–90. 10.1016/S0140-6736(04)17482-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-002213supp001.pdf (120.3KB, pdf)