Abstract
Background
Antibody-dependent cell-mediated cytotoxicity (ADCC) may mediate antitumour activity of IgG1-isotype monoclonal antibody (mAb), suggesting as potential treatment combination of IgG1-mAbs, anti-epidermal growth factor receptor cetuximab and anti-programmed death-ligand-1 avelumab.
Methods
We evaluated ADCC induction in lung cancer cells by lactate dehydrogenase (LDH) release assay. Antitumour activity and safety of cetuximab plus avelumab were explored in a single-arm proof-of-concept study in pre-treated non-small cell lung cancer (NSCLC) patients (pt) (Cetuximab-AVElumab-lung, CAVE-Lung). Search for predictive biomarkers of response was done.
Results
Avelumab plus cetuximab induced ADCC in NSCLC cells in vitro in presence of natural killers (NK) from healthy donors (HD) or NSCLC pt, as effectors. Sixteen relapsed NSCLC pt were treated with avelumab plus cetuximab. Antitumour activity was observed in 6/16 pt, defined by progression free survival (PFS) ≥8 months, with 4 of them still on treatment at data lock time (range, 14–19 months). Of note, 3/6 responders had received as previous line anti-programmed death-1 therapy. In responders, clinical benefit was accompanied by significant increase in LDH release over baseline at the first radiological evaluation (8 weeks) (p=0.01) and by early skin toxicity; while in the 10 non-responders, that had PFS ≤5 months, LDH release tends to reduce. Baseline circulating DNA levels were higher in non-responders compared with responders and HD (p=0.026) and decrease in responders during therapy. Mutations in DNA damage responsive family genes were found in responders.
Conclusion
Cetuximab and avelumab activates NSCLC pt NK cells. Ex vivo evaluation of ADCC, circulating DNA levels and early skin toxicity may predict response to cetuximab plus avelumab in NSCLC.
EUDRACT 2017-004195-58
Keywords: NSCLC, ADCC, cetuximab, avelumab, NK cells
Key questions.
What is already known about this subject?
Antibody-dependent cell-mediated cytotoxicity (ADCC) may be one of the mechanisms of antitumour activity of IgG1-isotype monoclonal antibody (mAb), with consequent activation of both innate and adaptive immune responses and engagement of different types of immune cells. In this respect, the combination of the IgG1-isotype anti-epidermal growth factor receptor (EGFR) mAb cetuximab plus the IgG1-isotype anti-programmed death-ligand-1 (PD-L1) avelumab, with or without chemotherapy, is currently in clinical development in several cancer types, including non-small cell lung cancer (NSCLC), colorectal cancer and head and neck squamous cell carcinoma. Even if neither avelumab or cetuximab are currently approved in NSCLC, they have showed promising clinical activity in PD-L1 positive and in EGFR high NSCLC, respectively.
What does this study add?
To our knowledge, this is the first report on the potential antitumour activity of the combination of the two IgG1-isotype anti-EGFR (cetuximab) and anti-PD-L1 (avelumab) mAbs in a cohort of relapsed NSCLC patients. An extensive preclinical and translational study has also been conducted to elucidate the potential mechanisms and biomarkers of this combination. We found that ex vivo evaluation of ADCC by using natural killers (NK) cells obtained from patient blood samples may represent a predictive biomarker of antitumour response to combined treatment with cetuximab plus avelumab in chemo-refractory metastatic NSCLC.
Key questions.
How might this impact on clinical practice?
The results of the present study provide experimental and clinical evidence for the antitumour and pro-ADCC activity of cetuximab plus avelumab therapy, thus supporting larger clinical studies to confirm these results in various cancer types. The evaluation of drug-induced ADCC by lactate dehydrogenase release assay on patient derived-NK cells represent a novel feasible test for the monitoring of patients response in clinical setting.
Introduction
Antibody-dependent cell-mediated cytotoxicity (ADCC) occurs in patients that are treated with IgG1-based monoclonal antibodies (mAbs), whereas this host immune-mediated response does not occur following treatment with other isotypes (such as IgG2 mAbs).1 2 Several mAbs, including trastuzumab, necitumumab, rituximab or cetuximab, may stimulate ADCC, and this effect is thought to play a relevant role for their antitumour activity.1–3
In this scenario and with the introduction of immune-checkpoint inhibitors (ICIs) in cancer therapy, an effective approach could be the combined treatment with IgG1-isotype mAbs, that are directed against relevant cancer cell molecular targets, such as the anti-epidermal growth factor receptor antibody (EGFR) cetuximab,3 and avelumab, that blocks the programmed death-1/programmed death-ligand-1 (PD-1/PD-L1) axis, in order to potentiate their antitumour efficacy. Treatment with cetuximab may also activate a functional cross-talk between natural killers (NK) cells and dendritic cells (DC), and it may recruit cytotoxic T cells in the tumour microenvironment, thus priming the immune system to be more sensitive to ICI treatment.4–8 Among the currently available ICIs, avelumab is a fully human IgG1-isotype anti-PD-L1 mAb, that, therefore, could have potential ADCC properties.9 In this respect, combined therapy with cetuximab plus avelumab may be able to activate both innate and adaptive immune responses, therefore increasing their antitumour efficacy by the engagement of different types of immune cells. In this respect, the combination of cetuximab plus avelumab with or without chemotherapy is currently in clinical development in several cancers, including non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and head and neck squamous cell carcinoma (HNSCC).10–12
To our knowledge, this is the first report on the potential antitumour activity of the combined treatment with cetuximab plus avelumab in a cohort of NSCLC patients that had progressed after at least one line of therapy for metastatic disease. An extensive preclinical and translational study has also been conducted to elucidate the potential mechanism(s) of antitumour activity of this combination, to define if ADCC could play a role in antitumour activity, and to evaluate if induction of ADCC could be a predictive biomarker of response to therapy.
Methods
Study design, patients eligibility and treatment
The Cetuximab-AVElumab-Lung (CAVE-Lung) trial was an independent, non-profit, single-arm exploratory clinical and translational study sponsored by the Department of Precision Medicine, Università degli Studi della Campania Luigi Vanvitelli, Italy, within the framework of the Gruppo Oncologico dell’ Italia Meridionale (GOIM). Eligibility criteria were: patients with histologically confirmed stage IIIb/IV or recurrent NSCLC, suitable for second or third line treatment after progression to previous therapies. Primary objective of the study was to evaluate the efficacy on overall survival (OS) of cetuximab plus avelumab, as compared with historical data of ICI in same population. Secondary endpoints include the overall response rate and progression free survival (PFS), and the safety profile of the trial drugs. Sixteen patients were enrolled in two Italian academic centres. Two patients were previously treated with ICI. Treatment cycles and doses were as it follows: avelumab, 10 mg/kg intravenous, every 2 weeks and cetuximab, 400 mg/m2by intravenous infusion in 120 min, as first dose, and, subsequently, 250 mg/m2 by intravenous infusion in 60 min, every week. Treatment was administered until disease progression or unacceptable toxicity.
Genomic profiling of tumours
We performed genomic profiling of tumours by next generation sequencing, according Foundation Medicine platform, as previously described.13
Assessment of total circulating plasma cell-free DNA levels
We collected blood serially during treatment from all patients and we analysed cell-free DNA (cfDNA) levels, using the KAPA hgDNA Quantification and QC Kit (Roche), as previously shown.14
Human NSCLC cell lines, peripheral blood mononuclear cells, NK cells and drugs
Human NSCLC cell lines were obtained by the American Type Culture Collection (ATCC, Manassas, Virginia, USA) and were cultured in RPMI-1640 (Sigma-Aldrich) medium which was supplemented with 10% foetal bovine serum (FBS; Life Technologies, Gaithersburg, Maryland, USA) in a humidified atmosphere with 5% CO2. The identity of cell lines was confirmed by Short tandem repeat profiling (Promega). Cetuximab, atezolizumab and panitumumab were purchased from Selleck Chemicals (Munich, Germany). Avelumab, was provided by Merck (Darmstadt, Germany), as part of a Cooperative Research and Development Agreement. Peripheral blood mononuclear cells (PBMCs) from healthy donors (HD) or from NSCLC patients were isolated by Ficoll-Paque Plus (GE Healthcare). NK cells were obtained through magnetic separation by using the NK CELL isolation kit, HUMAN (Miltenyi Biotech 130-092-657) according to the manufacturer’s protocol.
Flow cytometry analysis
For flow cytometry (fluorescence-associated cell sorting; FACS), analysis, cells were washed in staining buffer (SB) (2% FBS, 0,1% sodium azide in phosphate-buffered saline) and after a blocking of 10 min with SB+Ab serum 20%, were stained for 30 min with mouse monoclonal antibodies. The antibodies used were: anti-CD16, anti-CD107a, anti EGFR, anti-major histocompatibility complex type I (MHC-I), anti-PD-L1 (Miltenyi Biotec). Stained cells were washed two times, resuspended in SB and then acquired on a FACS ACCURI C6 (BD Biosciences). Analysis was conducted using ACCURI C6 software (BD Biosciences).
Lactate dehydrogenase cytotoxicity assay
Cytotoxicity was assessed by lactate dehydrogenase (LDH) release from cancer cells into the culture medium. The activity of LDH in the medium was determined using a commercially available kit from Thermo Fisher (Pierce LDH Cytotoxicity Assay Kit). The assay is based on the conversion of lactate to pyruvate in the presence of LDH with parallel reduction of Nicotinamide adenine dinucleotide. The formation of Nicotinamide adenine dinucleotide hydrate from the above reaction results in a change in absorbance at 340 nm. Aliquots of media and warm reagents were mixed in a 96-well plate according to protocol instructions and absorbance was recorded using a microplate spectrophotometer system. Per cent cytotoxicity was calculated according with the formula: (Experimental Value–Effector Cells Spontaneous Control–Target Cells Spontaneous Control)/(Target Cell Maximum Control–Target Cells Spontaneous Control).
Statistical analysis
Statistical analysis was performed using Graphpad Prism software V.6.0 (Graphpad Software, San Diego, California, USA). Data were compared with one-way ANalysis Of VAriance (ANOVA) statistical test followed by Tukey’s test or unpaired t-test; p values less than 0.05 were considered statistically significant. Patient demographics and clinical information were summarised with median and range for continuous variables and frequency and percentage for categorical variables. All statistical analyses were generated using SPSS statistical software (V.18). The Kaplan-Meier method was used to estimate median PFS and median OS.
Results
Combination of cetuximab plus avelumab mediates ADCC by NK cells in vitro
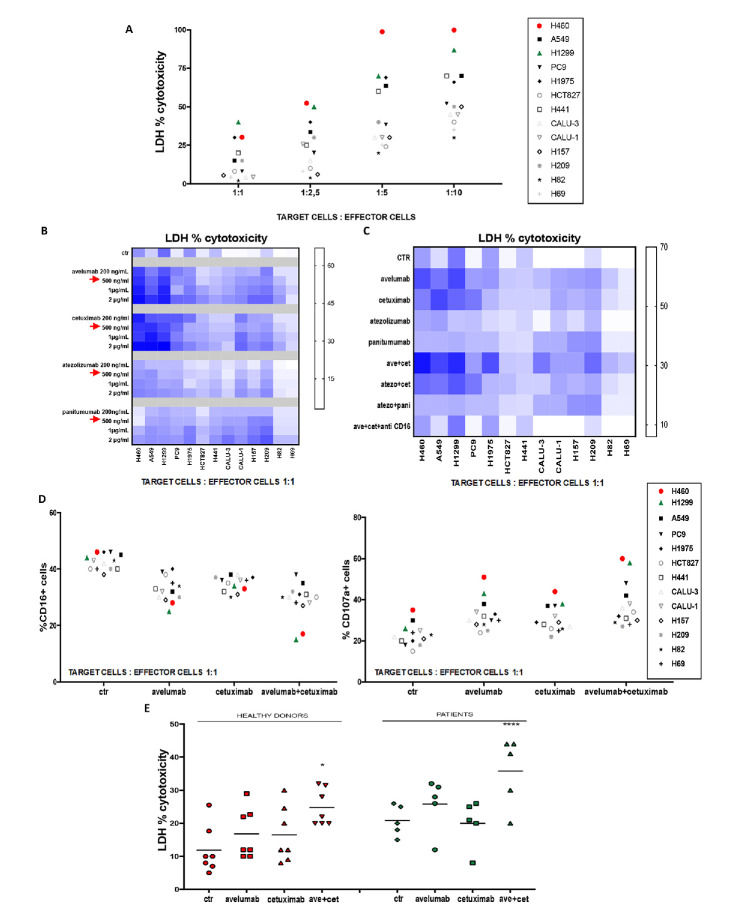
To evaluate the potential induction of ADCC by IgG1-isotype mAb treatment, we developed an in vitro LDH cytotoxicity assay by using NK cells, that were derived from PBMCs isolated from HD (as effector cells), in a panel of 13 different human lung cancer cell lines (as target cells). Histological as well as key gene mutation profiles of these cell lines are representative of human lung cancer (online supplementary table 1). Flow cytometric analysis detected on these human cancer cell lines a wide range of expression of EGFR, MHC-I and PD-L1 (online supplementary table 1). H460 and H1299 were the most sensitive lung cancer cell lines to NK-induced ADCC following treatment with either avelumab or cetuximab (figure 1A, B), probably due to their high expression levels of PD-L1 and EGFR, along with very low MHC-I surface expression, since it is known that high levels of MHC-I on tumour cells can inhibit NK-mediated lysis. Thus, we selected H1299 cells as a good model for next in vitro experiments. In contrast, treatment with two IgG2 isotype mAbs, that are directed against EGFR (panitumumab) or PD-L1 (atezolizumab), was unable to activate ADCC in this panel of 13 human lung cancer cell lines. The combined treatment with cetuximab plus avelumab synergistically enhanced cytotoxicity on lung cancer cells, as measured by the Chou and Talalay method (figure 1C, online supplementary table 2). As an example, in H460 lung adenocarcinoma cells, single agent treatment with cetuximab or avelumab determined 23.5% or 30% LDH release, respectively, whereas their combination led to 85% LDH release. NK cell-mediated ADCC occurs when CD16 (FcγRIII) on NK effector cells interacts with the fragment crystallisable (Fc) portion of IgG1-isotype antibodies that opsonise target cells.15 To determine whether CD16 engagement is a critical component of NK-induced ADCC mediated by avelumab or by cetuximab treatment, the LDH cytotoxicity assay was performed in the presence of an anti-CD16 neutralising antibody. Anti-C16 treatment significantly inhibited LDH release, suggesting that CD16 plays a major role for NK-induced ADCC, which is mediated by cetuximab and avelumab (figure 1C). Treatment with cetuximab or avelumab determined a decrease in CD16 expression in NK cells with release of CD107a, another marker of induction of ADCC,16 thus further supporting the evidence of innate immunity activation following avelumab or cetuximab treatment (figure 1D). Next, we analysed LDH release by using, as effector cells, NK cells, that were isolated from PBMCs of five metastatic NSCLC patients before first line therapy, in order to compare their efficiency to NK cells that were isolated from PBMCs of HD with H1299 as target cells. We detected avelumab plus cetuximab-mediated ADCC by both HD’s and patients’ NK cells, but the effect in NSCLC patients was amplified and of statistical significance (figure 1E).
Figure 1.
In vitro evidence of natural killers (NK) activation and cytotoxic lytic activity following cetuximab and/or avelumab treatment. (A) Lactate dehydrogenase (LDH) cytotoxicity assay was performed on 13 lung cell lines, which were used as target cells, and were cocultured with NK cells from healthy donors (HD), which were used as effector cells. Different target: effector cell ratios were used to test cancer cell cytotoxicity. (B) Heat map graph representation of LDH cytotoxicity assay, which was performed on 13 lung cancer cell lines. Cancer cell lines were treated with different doses of avelumab, cetuximab, atezolizumab or panitumumab, as indicated. Intensity of the blue colour indicates the degree of LDH release, as reported on the quantitative scale on the right. (C) Heat map graph representation of LDH cytotoxicity assay, which was performed on 13 lung cell lines that were cocultured with NK cells isolated from HD in a 1:1 ratio and with: avelumab, cetuximab, atezolizumab or panitumumab alone or in combination. Treatments with avelumab and/or cetuximab were also performed in the presence of an anti-CD16 blocking antibody. Intensity of the blue colour indicates the degree of LDH release, as reported on the quantitative scale on the right. (D) Flow cytometric analysis of CD16 (left panel) and CD107A (right panel) expression on NK cells from HD (effector cells), which were cocultured with different human cancer cell lines (target cells), in a 1:1 ratio. (E) LDH cytotoxicity assay was performed on supernatants obtained from cocultures of the H1299 lung cancer cell line (as target cells) and of NK cells which were obtained from seven HD or from five NSCLC patients (as effector cells), in a 1:1 ratio. H1299 cells were treated for 24 hours with avelumab, cetuximab or the combination of both drugs. After 24 hours, NK cells were added and cocultured for 4 hours. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001 by one-way ANalysis Of VAriance (ANOVA) test.
esmoopen-2020-000753supp001.pdf (53.3KB, pdf)
esmoopen-2020-000753supp002.pdf (42.6KB, pdf)
Proof of concept of antitumour activity and safety of avelumab plus cetuximab in the treatment of metastatic NSCLC patients: the CAVE-Lung trial
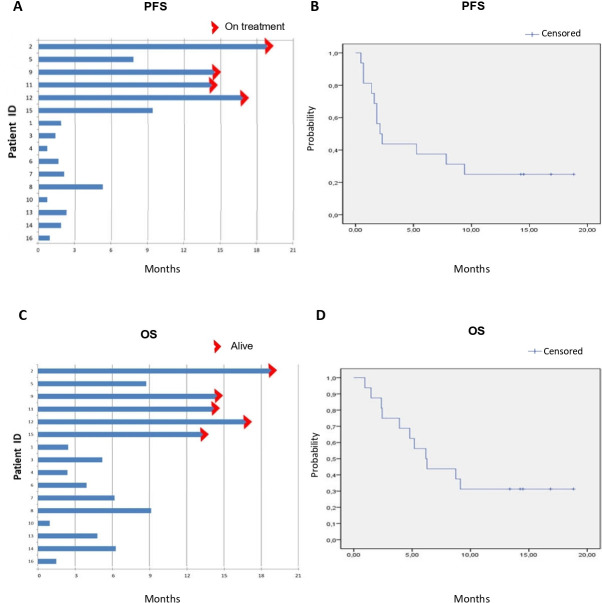
The therapeutic combination of the anti-PDL1 mAb avelumab with the anti-EGFR mAb cetuximab was tested in a small single-arm, proof of concept clinical study. From September 2018 to February 2019, 16 patients with advanced or metastatic NSCLC, including 7 squamous and 9 adenocarcinomas, that were previously treated with at least one line of therapy, received a combination of cetuximab plus avelumab. Patient characteristics are summarised in table 1. Median age was 70 years with one patient aged more than 75 years. Eleven patients received treatment as second line of therapy; four patients were treated in third line, whereas one patient had the experimental treatment as fourth line. The latter patient had a tumour harbouring EGFR exon 19 deletion, whereas all the other patients had an EGFR wild-type tumour. In particular, the patient harbouring EGFR mutation was treated in first line with EGFR-tyrosine kinase inhibitor and then with chemotherapy agents; EGFR mutation was re-evaluated after chemotherapy and it was found negative. Another patient with EGFR wild-type tumour received treatment with EGFR-inhibitor as maintenance after platinum therapy. All patients received at least one dose of the experimental treatment. Overall, 216 cycles of therapy were delivered. Moreover, four patients were still on treatment at the time of data lock (15 April 2020), having received therapy with cetuximab plus avelumab for 14–19 months, respectively. The observed safety profile was consistent with what has been previously reported for single agents cetuximab or for avelumab (table 2). Overall, cetuximab plus avelumab treatment was well tolerated. The most frequent adverse events were skin toxicity with maculo-papular or acneiform rash and serum lipase increase, that was, however, not associated with significant changes in pancreatic function. In one patient treatment was discontinued for grade 3 serum amylase increase. Only one patient experienced a delay in cetuximab administration with subsequent dose reduction for grade 3 skin toxicity, that was managed with local and systemic anti-inflammatory and antibiotic treatment. Of interest, this patient is currently on treatment with cetuximab plus avelumab for 19 months with a total of 41 cycles received so far with stable disease (SD), as best antitumour response. Radiological assessment for response evaluation was performed every 8 weeks. Fourteen out of 16 patients were evaluable for response. Eight patients presented SD as best response, disease progression (PD) was observed in the other six patients, including two patients with KRAS G12V mutation and one patient with STK11 D194N mutation in their tumours. No major responses were observed. Five patients were alive and four of them were still on treatment at the time of data lock (15 April 2020). Median PFS and median OS in the intention to treat (ITT) population of 16 patients were 2.1 months (95% CI 1.2 to 3.0 months) and 6.2 months (95% CI 4.0 to 8.3 months), respectively (figure 2). However, according to clinical outcome, two different patient groups could be identified following treatment with cetuximab plus avelumab. In fact, six patients had a PFS of 8 months or longer (responders) with four of them still on treatment at the time of data lock (range, 14–19 months of treatment); whereas nine patients had a PD and one patient a PFS shorter than 5 months as best response (non-responders) (figure 2). The clinical benefit in all responders was accompanied by early onset of any grade skin toxicity (within 6 weeks from starting treatment)Interestingly, G3 skin toxicity was reported in the two patients with longer PFS (17 and 19 months, respectively), that were still on treatment at the time of data analysis.
Table 1.
Baseline characteristics of patients enrolled in phase II Cetuximab-AVElumab-Lung trial
| Characteristics | n (%) |
| Age, years | |
| Median | 70 |
| Range | 43–85 |
| Age ≥75 years | 1 (6.2) |
| Male sex | 13 (81.2) |
| Race | |
| White | 16 (100) |
| Asian | 0 |
| Black | 0 |
| Other | 0 |
| ECOG performance-status score | |
| 0 | 10 (62.5) |
| 1 | 6 (37.5) |
| Smoking status | |
| Current | 1 (6.2) |
| Former smoker | 12 (75) |
| Never smoker | 3 (18.7) |
| EGFR mutation | 1 (6.2) |
| Histology | |
| Squamous carcinoma | 7 (43.8) |
| Adenocarcinoma | 9 (56.2) |
| ALK translocation | 0 |
| PD-L1 expression | |
| <1% | 7 (43.7) |
| 1%–49% | 2 (12.5) |
| ≥50% | 1 (6.2) |
| Unknown | 6 (37.5) |
| KRAS mutation * | 2 |
| No. of prior systemic regimens | |
| 1 | 11 (68.7) |
| 2 | 4 (25) |
| 3 | 1 (6.2) |
| Type of last systemic therapy | |
| Platinum-based therapy | 10 (62.5) |
| Single agent chemotherapy (no platinum) | 2 (18.7) |
| EGFR-TKI | 1 (12.5) |
| ICI treatment | 3 (18.75) |
| Best response to previous systemic treatment | |
| Complete or partial response | 6 (37.5) |
| Stable disease | 6 (37.5) |
| Progressive disease | 4 (25%) |
*For three patients, KRAS mutation could not be tested due to insufficient pathological material.
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ICI, immune-checkpoint inhibitor; KRAS, Kirsten rat sarcoma gene; PD-L1, programmed death-ligand-1; TKI, tyrosine kinase inhibitor.
Table 2.
Treatment-related adverse event reported in patients treated with avelumab plus cetuximab in phase II Cetuximab-AVElumab-Lung trial
| Event | Any grade n (%) |
Grade 3 or 4 n (%) |
| Any event | 16 (100) | 4 (25)* |
| Constipation | 2 (13) | 0 |
| Diarrhoea | 2 (13) | 0 |
| Mucositis | 1 (6.7) | 0 |
| Anorexia | 2 (13) | 0 |
| Fatigue | 5 (31.3) | 0 |
| Pain | 2 (13) | 0 |
| ALT increase | 4 (25) | 0 |
| AST increase | 2 (13) | 0 |
| Lipase increase | 4 (25) | 2 (13) |
| Amylase increase | 3 (20) | 1 (6.7) |
| Hyperglycaemic | 1 (6.7) | 0 |
| Thyroid dysfunction | 0 (0) | 0 |
| Arthralgia | 3 (20) | 0 |
| Haemoptysis | 1 (6.7) | 0 |
| Cough | 2 (13) | 0 |
| Dyspnoea | 8 (53) | 0 |
| Skin toxicity | 10 (62.5) | 2 (12.5) |
| Early skin toxicity | 6 (37.5) | 2 (12.5) |
| Any, leading to treatment discontinuation | 0 | 1 (6.7) |
| Any, leading to dose reduction | 0 | 1 (6.7) |
*One patient experienced both lipase and amylase G3 increase.
ALT, Alanine transaminase; AST, Aspartate transaminase.
Figure 2.
Progression free survival (PFS) and overall survival (OS) for the 16 patients that were treated with cetuximab and avelumab in the Cetuximab-AVElumab-Lung trial. (A) Individual patient PFS. (B) Kaplan-Meier estimate for PFS. Median PFS, 2.1 months (95% CI 1.2 to 3.0 months). (C) Individual patient OS. (D) Kaplan-Meier estimate for PFS. Median OS, 6.2 months (95% CI 4.0 to 8.3 months).
Analysis of cfDNA levels showed significant higher baseline levels in non-responders as compared with responders and HD (p=0.026). In addition, cfDNA levels downregulated during treatment, at week 8 and 18 in responders, while they tend to increase in non-responders. Of interest, three of these six patients had received ICI as previous line of therapy and two of them are still on treatment at time of data lock thus suggesting an activity of the experimental combination beyond progression to second line immunotherapy. In particular, these three patients experienced pulmonary progression disease after 6, 7 and 7 months of second line therapy with an anti-PD-1 drug and benefited from avelumab plus cetuximab for 8, 14.5 and 15 months, respectively. Tumour PD-L1 expression was 60%, 5% and unknown for the three patients. Both anti-PD-1 drug and combination of avelumab plus cetuximab produced SD as radiological best response, although a decrease in cfDNA was observed. Noteworthy, we detected in those three pts mutations in DNA damage responsive (DDR) family, that have been associated with innate immune activation; specifically, the first patient presented STK11/TP53 co-mutation, the second CHK2/ATM/BRCA2 co-mutation and the third MDM2 mutation. According to our previous publication, presence of STK11/TP53 co-mutation and DDR alterations defines a subgroup of NSCLC with features of immune responsiveness.17
Ex vivo evidence of NK activation in NSCLC patients treated with avelumab plus cetuximab in the CAVE-Lung trial and its correlation with antitumour activity
In order to study the potential induction of ADCC following treatment with avelumab plus cetuximab, NK cells were isolated from PBMCs from patients enrolled in the CAVE-Lung study. LDH release assay was performed on human H1299 lung cancer cells as target cells, which were cocultured with NK cells, that were isolated from PBMCs of five responding patients. Blood was collected at baseline and, sequentially, after each of five cycles of treatment with avelumab plus cetuximab. Baseline levels of LDH release from cocultures of target cells with patients’ NK cells were significantly higher than HD’s NK cells and a progressive increase in LDH release was observed during treatment as compared with baseline values for patients (figure 3A). These results suggest that a sustained activation of NK cells occurs in these patients following treatment with anti-PD-L1 and anti-EGFR IgG1-isotype mAbs. We next evaluated LDH release at baseline and at the time of the first radiological evaluation at 8 weeks of treatment with cetuximab plus avelumab for the ITT population of 16 patients. As shown in figure 3B, in six patients, that had a PFS of 8 months or longer (responders), a significant increase in ADCC over baseline levels was observed (p=0.01 by unpaired t-test). In contrast, in ten patients, that were not responding to the experimental treatment, a decrease in ADCC was detected as compared with baseline values. Difference between baseline levels of responders and non-responders was also significant (p=0.05 by unpaired t-test).
Figure 3.
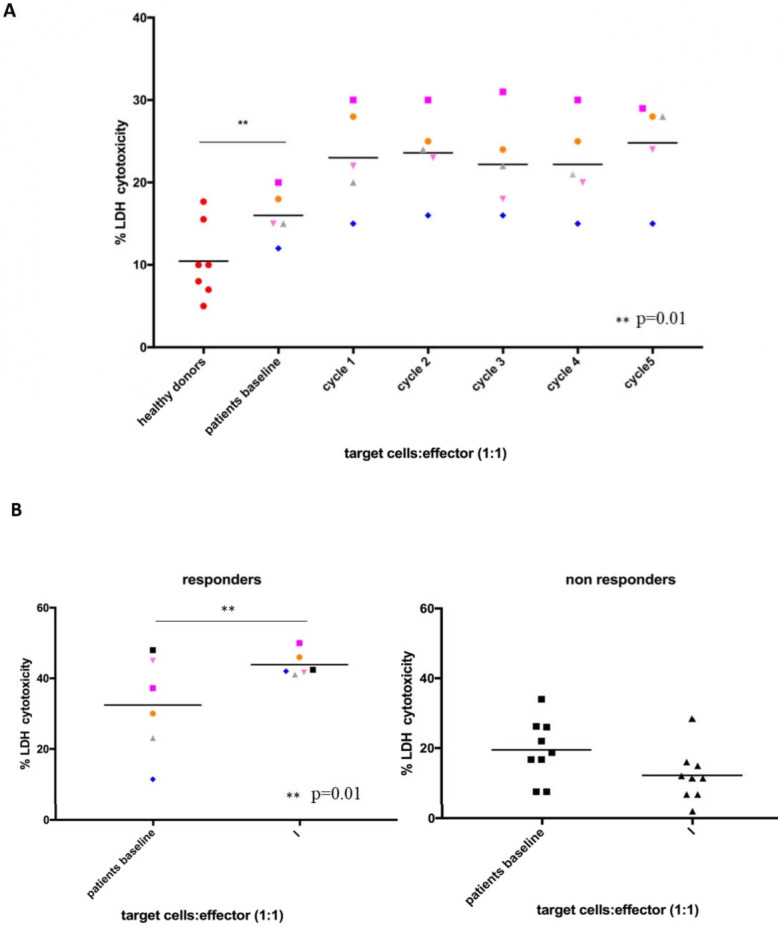
Ex vivo evidence of natural killers (NK) activation and cytotoxic lytic activity for the 16 patients that were treated with cetuximab plus avelumab in the Cetuximab-AVElumab-Lung (CAVE-Lung) trial. (A) Lactate dehydrogenase (LDH) cytotoxicity assay at baseline (before the first cycle of therapy) and during treatment. LDH cytotoxicity assay was performed on the supernatants which were obtained from cocultures of the H1299 lung cancer cell line (as target cells) and of NK cells obtained from seven healthy donors or from five NSCLC patients (as effector cells), in a 1:1 ratio. The five patients were treated according to the CAVE-Lung study. Blood samples were collected at each cycle of therapy with cetuximab plus avelumab for five cycles. *p≤0.05; **p≤0.01; ***p≤0.001 by one way ANalysis Of VAriance (ANOVA) test. (B) LDH cytotoxicity assay was performed on supernatants from cocultures of the H1299 lung cancer cell line (as target cells) and by using NK cells (as effector cells) obtained from the blood of 6 responder (left panel) and from 10 non-responder (right panel) patients, as defined in the results. For each patient, blood samples were collected at baseline and at the time of the first radiological evaluation (after 8 weeks of treatment). *p≤0.05; **p≤0.01; ***p≤0.001 by unpaired t-test.
Discussion
The introduction of ICIs, that target the PD-1/PD-L1 axis, has completely reshaped the therapeutic scenario for locally advanced and metastatic NSCLC patients.18 19 Four ICI mAbs, of which two anti-PD-1 and two anti-PD-L1 molecules (pembrolizumab, nivolumab, atezolizumab and durvalumab), are currently available with different treatment indications in the management of NSCLC patients.19 20 IgG antibodies regulate the immune system by interaction of their Fc region with specific receptors (FcγR) on the surface of immune cells. Any IgG subtype can lead to different immune responses: nivolumab and pembrolizumab are IgG4 isotype anti-PD-1 antibodies, with high affinity to FcγR but with low ADCC properties; durvalumab and atezolizumab are anti-PD-L1 antibodies of IgG1-isotype, without any FcγR-binding and effector functions. Avelumab is a fully human IgG1 anti-PD-L1 mAb, and it is the only currently available ICI potentially able to activate ADCC. Although it is approved for the treatment of locally advanced or metastatic urothelial cancer, for metastatic Merkel cell carcinoma and for metastatic renal clear cell carcinoma, avelumab is not currently registered for the treatment of NSCLC. In fact, a randomised phase III study, that assessed the efficacy and safety of avelumab compared with docetaxel in metastatic NSCLC patients after first line platinum-based therapy, failed to demonstrate a survival advantage.21 However, in the patient subgroup with high PD-L1 expression median PFS and median OS were significantly longer for avelumab as compared with docetaxel treatment. In this respect, a randomised phase III trial is currently ongoing to evaluate the role of avelumab in the first line treatment of metastatic NSCLC with high PD-L1 expression.22
Several studies have evaluated EGFR blockade in NSCLC patients. Two phase III trials, the First-Line ErbituX in lung cancer (FLEX) and the BMS-099, that were investigating the addition of cetuximab to standard chemotherapy in the first line setting, had contradictory results.23 24 Although the difference in OS as compared with chemotherapy was similar in both studies (approximately 1.3-month increase in median OS, with 11%–13% reduction in death risk), this difference was statistically significant only in the FLEX study.23 Of note, a meta-analysis of the cetuximab trials has shown that early onset skin toxicity of any grade was associated with better outcome (median OS of 15.0 vs 8.8 months, HR, 0.63; p≤0.0001).25 Further, in the FLEX study, cetuximab plus chemotherapy was significantly better in those patients with squamous cell histology and with higher EGFR protein expression.26
In light of the potential ADCC that can be activated by IgG1-isotype mAbs, such as cetuximab or avelumab, in the present study we have evaluated if their combination may be of therapeutic value in NSCLC. This hypothesis has been studied first in vitro in a panel of human lung cancer cell lines, which have been exposed to cetuximab plus avelumab in the presence of NK cells that have been isolated from the peripheral blood of HD or of NSCLC patients. A synergistic effect in terms of ADCC was observed by cetuximab plus avelumab treatment of human lung cancer cells, being this effect stronger for cancer cell lines with higher expression of PD-L1 and EGFR. In addition, similar levels of ADCC were observed by using, as effector cells, NK cells derived from either HD or NSCLC patients, suggesting that NSCLC patients may retain functional NK cells, which can be activated by cetuximab plus avelumab.
With the support of preclinical experimental evidence of cetuximab plus avelumab-induced ADCC, which could translate in a better antitumour efficacy, we conducted a small size, single-arm, proof of concept, exploratory clinical study (the CAVE-Lung trial) in 16 metastatic NSCLC patients, that had failed one or more lines of therapy. Overall, treatment with cetuximab plus avelumab was well tolerated. Cetuximab-related skin toxicity was the most frequent adverse event. Median PFS and median OS were 2.1 months and 6.2 months, respectively, for the ITT patient population. However, a clear evidence of potentially relevant antitumour activity of cetuximab plus avelumab therapy was observed in a subgroup of patients. In fact, 6/16 patients had, as best antitumour response, a PFS of 8 months or longer. At the time of data analysis for this report, 5/16 patients are alive, with four of them still on treatment with PFS ranging from 14 to 19 months. Clinical activity in the six responders was accompanied by reduction of cfDNA levels, a non-invasive marker of survival prediction in lung cancers14 and emerging early known marker of response to immunotherapy.27 Moreover, all these six patients experienced cetuximab-related skin toxicity within 6 weeks from start of treatment. In particular, we identified among responders a subgroup of three patients that obtained clinical benefit from the experimental combination, although a previous failure of anti-PD-1 therapy, thus suggesting that targeting PD-L1 and enhancing ADCC with combination of avelumab plus cetuximab might overcome resistance to anti-PD-1. Furthermore, the genomic profiling of these three tumours underline the hypothesis that alterations in DDR genes could promote the interplay between innate and adaptive immunity involved in response to ADCC inducing immunotherapy.17
Furthermore, in this report we also provide evidence that the combination of cetuximab plus avelumab induces NK cell-driven ADCC in patients that were enrolled in the CAVE-Lung trial. Induction in ADCC may be monitored during therapy and it could represent a potential biomarker of response. In fact, the evaluation of this activation by LDH release assay can be performed ex vivo by using, as targets, a human cancer cell line and, as effectors, patient-derived NK cells, which are isolated from circulating PBMCs, thus allowing to monitor this innate immune response during treatment. In this respect, in responding patients a significant and sustained increase in ADCC, as assessed by LDH release assay, was observed during treatment as compared with baseline values for at least five cycles of cetuximab plus avelumab. Moreover, at the first radiological assessment at 8 weeks of treatment, a significant increase in ADCC was detected only in the six responding patients, whereas a reduction in ADCC was found in the ten non-responding patients. Also, baseline levels in responders patients are higher than HD and non-responders patients, thus encouraging future studies on a larger number of patients to explore the predictive value of baseline levels. Our results suggest that an increase in cancer cell cytotoxicity as assessed by LDH release assay in presence of patient derived-NK cells after 8 weeks of treatment as compared with baseline may represent a predictive biomarker for clinical activity of avelumab plus cetuximab.
Further preclinical and translational studies should investigate additional effect of avelumab to the antitumour microenvironment, including a potential depletion of PD-L1 positive immunosuppressive antigen-presenting cells and effect on human leucocyte antigen expression. Moreover, considering the negative prognostic role of high EGFR expression on tumour associated macrophages and myeloid cells demonstrated in other cancer types,28 29 cetuximab may directly inhibit immune suppressive signals of tumour microenvironment. Also, we speculate that CD16 genotyping may influence the induction of ADCC30 and that other mechanisms could be implicated in the activity of combination of cetuximab plus avelumab, such as antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity and inhibition of other immune checkpoint pathway. A limit of the present study is represented by the low number of samples available, from the patients enrolled in a proof of concept clinical trial.
Collectively, the results of the present study provide the first experimental and clinical evidence for the antitumour and pro-ADCC activity of cetuximab plus avelumab therapy in the clinical context of a proof of concept exploratory trial in NSCLC patients, relapsed after chemotherapy or immunotherapy. Larger clinical studies are needed to confirm these results and to validate the predictive value of analysis of ADCC on patients’ NK and of DDR related genomic alterations as novel biomarker for this combination. In this respect, a multicentre, single arm, phase II study is currently ongoing to evaluate the potential clinical activity of cetuximab plus avelumab treatment in chemo-refractory rat sarcoma gene (RAS) wild-type metastatic CRC patients.31
Footnotes
Twitter: @Carminia Della Corte
Contributors: MF, CMDC, FM and FC designed the study and wrote the paper. RDL, FS, GV, MLI, FP, VS, AM, EM contributed to clinical trial, collection and analysis of clinical data. GB, RDL and CMDC performed translational experiments. MF, GB, VC, CMDC and FM made figures. VF performed statistical analysis. MF, FM and FC supervised the trial. FM and FC supervised the translational studies.
Funding: The CAVE-Lung clinical trial was in part supported by a research grant from Merck, Darmstadt, Germany to FC.
Competing interests: MF: family relation with Merck; AM: Consultancy and Advisory Boards: Roche, AstraZeneca, Boehringer, Pfizer, Takeda, BMS, MSD; EM: Consultancy and Advisory Boards: Eli Lilly, Sanofi, Cellgene, Servier; FC: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Ipsen; FM: Advisory Boards MSD, Lilly; Institutional Research Grants: AstraZeneca.
Patient consent for publication: Not required.
Ethics approval: The trial was conducted according to the ethical principles for medical research involving human subjects and protocol was approved by Ethical Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Lo Nigro C, Ricci V, Vivenza D, et al. . Evaluation of antibody-dependent cell-mediated cytotoxicity activity and cetuximab response in KRAS wild-type metastatic colorectal cancer patients. World J Gastrointest Oncol 2016;8:222–30. 10.4251/wjgo.v8.i2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin P, Grillo-López AJ, Link BK, et al. . Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998;16:2825–33. 10.1200/JCO.1998.16.8.2825 [DOI] [PubMed] [Google Scholar]
- 3.Gotwals P, Cameron S, Cipolletta D, et al. . Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017;17:286–301. 10.1038/nrc.2017.17 [DOI] [PubMed] [Google Scholar]
- 4.Trivedi S, Srivastava RM, Concha-Benavente F, et al. . Anti-Egfr targeted monoclonal antibody isotype influences antitumor cellular immunity in head and neck cancer patients. Clin Cancer Res 2016;22:5229–37. 10.1158/1078-0432.CCR-15-2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SC, Srivastava RM, López-Albaitero A, et al. . Natural killer (NK): dendritic cell (dC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res 2011;50:248–54. 10.1007/s12026-011-8231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava RM, Lee SC, Andrade Filho PA, et al. . Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013;19:1858–72. 10.1158/1078-0432.CCR-12-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellucci R, Martin A, Bommarito D, et al. . Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 2015;4:e1008824. 10.1080/2162402X.2015.1008824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue Y, Hazama S, Suzuki N, et al. . Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci 2017;108:455–60. 10.1111/cas.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins JM, Gulley JL. Product review: avelumab, an anti-PD-L1 antibody. Hum Vaccin Immunother 2019;15:891–908. 10.1080/21645515.2018.1551671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan A, Gulley JL, Spigel DR, et al. . Avelumab (anti–PD-L1) in patients with platinum-treated advanced NSCLC: 2.5-year follow-up from the javelin solid tumor trial. JCO 2018;36:9090 10.1200/JCO.2018.36.15_suppl.9090 [DOI] [Google Scholar]
- 11.Stein A, Binder M, Al-Batran S-E, et al. . Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (mCRC): results of the safety run-in phase of the phase II AVETUX trial (AIO-KRK-0216). JCO 2018;36:3561 10.1200/JCO.2018.36.15_suppl.3561 [DOI] [Google Scholar]
- 12.Elbers JBW, Zuur CL, Tesselaar ME, et al. . Radiotherapy with concurrent Avelumab and cetuximab as primary treatment in patients with locally advanced squamous cell carcinoma of the head and neck: a phase-IB feasibility trial in patients unfit for cisplatin (NCT02938273). JCO 2018;36:e18019 10.1200/JCO.2018.36.15_suppl.e18019 [DOI] [Google Scholar]
- 13.De Falco V, Poliero L, Vitello PP, et al. . Feasibility of next-generation sequencing in clinical practice: results of a pilot study in the Department of Precision Medicine at the University of Campania 'Luigi Vanvitelli'. ESMO Open 2020;5:e000675. 10.1136/esmoopen-2020-000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cargnin S, Canonico PL, Genazzani AA, et al. . Quantitative analysis of circulating cell-free DNA for correlation with lung cancer survival: a systematic review and meta-analysis. J Thorac Oncol 2017;12:43–53. 10.1016/j.jtho.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Ferris RL, Lenz H-J, Trotta AM, et al. . Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018;63:48–60. 10.1016/j.ctrv.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aktas E, Kucuksezer UC, Bilgic S, et al. . Relationship between CD107a expression and cytotoxic activity. Cell Immunol 2009;254:149–54. 10.1016/j.cellimm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 17.Della Corte CM, Sen T, Gay CM, et al. . Sting pathway expression identifies NSCLC with an Immune-Responsive phenotype. J Thorac Oncol 2020;15:777–91. 10.1016/j.jtho.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planchard D, Popat S, Kerr K, et al. . Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29:iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, Villegas A, Daniel D, et al. . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 20.Planchard D, Popat S, Kerr K, et al. . Correction to: Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:863–70. 10.1093/annonc/mdy474 [DOI] [PubMed] [Google Scholar]
- 21.Barlesi F, Vansteenkiste J, Spigel D, et al. . Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (javelin lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468–79. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 22.Reck M, DeGreen HP, Rose AL, et al. . Avelumab (MSB0010718C; anti-PD-L1) vs platinum-baseddoubletas first-line treatment for metastatic or recurrent PD-L1-positive non-small-celllungcancer: the phase 3 javelin lung 100 trial. J Clin Oncol 2016. [Google Scholar]
- 23.Pirker R, Pereira JR, Szczesna A, et al. . Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (flex): an open-label randomised phase III trial. Lancet 2009;373:1525–31. 10.1016/S0140-6736(09)60569-9 [DOI] [PubMed] [Google Scholar]
- 24.Lynch TJ, Patel T, Dreisbach L, et al. . Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911–7. 10.1200/JCO.2009.21.9618 [DOI] [PubMed] [Google Scholar]
- 25.Gatzemeier U, von Pawel J, Vynnychenko I, et al. . First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the flex phase 3 study. Lancet Oncol 2011;12:30–7. 10.1016/S1470-2045(10)70278-3 [DOI] [PubMed] [Google Scholar]
- 26.O'Byrne KJ, Gatzemeier U, Bondarenko I, et al. . Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 flex study. Lancet Oncol 2011;12:795–805. 10.1016/S1470-2045(11)70189-9 [DOI] [PubMed] [Google Scholar]
- 27.Kitahara M, Hazama S, Tsunedomi R, et al. . Prediction of the efficacy of immunotherapy by measuring the integrity of cell-free DNA in plasma in colorectal cancer. Cancer Sci 2016;107:1825–9. 10.1111/cas.13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanaya H, Natarajan A, Komposch K, et al. . EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol 2014;16:972–81. 10.1038/ncb3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivatsa S, Paul MC, Cardone C, et al. . EGFR in Tumor-Associated Myeloid Cells Promotes Development of Colorectal Cancer in Mice and Associates With Outcomes of Patients. Gastroenterology 2017;153:e10:178–90. 10.1053/j.gastro.2017.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatjiharissi E, Xu L, Santos DD, et al. . Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood 2007;110:2561–4. 10.1182/blood-2007-01-070656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinelli E, Ciardiello D, Martini G, et al. . Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann Oncol 2020;31:30–40. 10.1016/j.annonc.2019.10.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000753supp001.pdf (53.3KB, pdf)
esmoopen-2020-000753supp002.pdf (42.6KB, pdf)