Abstract
Pressure reactivity index (PRx) and brain tissue oxygen (PbtO2) are associated with outcome in traumatic brain injury (TBI). This study explores the relationship between PRx and PbtO2 in adult moderate/severe TBI. Using the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) high resolution intensive care unit (ICU) sub-study cohort, we evaluated those patients with archived high-frequency digital intraparenchymal intracranial pressure (ICP) and PbtO2 monitoring data of, a minimum of 6 h in duration, and the presence of a 6 month Glasgow Outcome Scale –Extended (GOSE) score. Digital physiological signals were processed for ICP, PbtO2, and PRx, with the % time above/below defined thresholds determined. The duration of ICP, PbtO2, and PRx derangements was characterized. Associations with dichotomized 6-month GOSE (alive/dead, and favorable/unfavorable outcome; ≤ 4 = unfavorable), were assessed. A total of 43 patients were included. Severely impaired cerebrovascular reactivity was seen during elevated ICP and low PbtO2 episodes. However, most of the acute ICU physiological derangements were impaired cerebrovascular reactivity, not ICP elevations or low PbtO2 episodes. Low PbtO2 without PRx impairment was rarely seen. % time spent above PRx threshold was associated with mortality at 6 months for thresholds of 0 (area under the curve [AUC] 0.734, p = 0.003), > +0.25 (AUC 0.747, p = 0.002) and > +0.35 (AUC 0.745, p = 0.002). Similar relationships were not seen for % time with ICP >20 mm Hg, and PbtO2 < 20 mm Hg in this cohort. Extreme impairment in cerebrovascular reactivity is seen during concurrent episodes of elevated ICP and low PbtO2. However, the majority of the deranged cerebral physiology seen during the acute ICU phase is impairment in cerebrovascular reactivity, with most impairment occurring in the presence of normal PbtO2 levels. Measures of cerebrovascular reactivity appear to display the most consistent associations with global outcome in TBI, compared with ICP and PbtO2.
Keywords: autoregulation, brain tissue oxygen, ICP, physiological burden
Introduction
Secondary injury after moderate/severe traumatic brain injury (TBI) is known to drive progressive cellular injury and death, leading to morbidity and mortality. Over the past few decades of TBI care, there have been advancements in guideline-based approaches and neuromonitoring,1 yet the mortality rates for moderate/severe TBI have remained fairly constant.2,3 This is believed to stem from the lack of ability to properly mitigate against secondary insults.
There has been a recent focus on optimizing physiology through the application of advanced continuous cerebral monitoring.1,4 Intracranial pressure (ICP)-based threshold targets have emerged based on both population-wide studies,5 and individualized physiological responses.6,7 Current Brain Trauma Foundation (BTF) guidelines suggest ICP thresholds of 20 or 22 mm Hg.8 Further, brain tissue oxygenation (PbtO2) has emerged as another important physiological target in TBI care, with recent phase II data supporting improved outcomes for those patients receiving ICP- and PbtO2-directed therapy, versus ICP-directed therapy alone,9 prompting a phase III study that is currently under way. These works focus on a PbtO2 threshold of 20 mm Hg. Finally, continuous cerebrovascular reactivity monitoring, through such measures as the pressure reactivity index (PRx), have been derived through the correlation between slow-wave vasogenic fluctuations in ICP and mean arterial pressure (MAP).10 Numerous studies to date have documented the association between PRx and global outcome in adult TBI,3,5,11,12 with recent multi-center data supporting that the strong link with mortality is preserved when adjusting for baseline characteristics and ICP.13 Various thresholds exist for PRx in the adult TBI literature, including: 0, +0.25, and +0.35, based on association with dichotomized 6-month outcomes.5,11
Despite these advances in cerebral physiological monitoring, the behavior of PbtO2 and PRx when assessed concurrently, is poorly characterized. Elevated ICP is a known correlate with impaired cerebrovascular reactivity,14,15 as measured through PRx, and low PbtO2.3,16,17 We also know that dynamically, PbtO2 appears to follow changes in cerebral perfusion pressure (CPP) in TBI.17 Further, we know that vascular reactivity when assessed via PbtO2, does not appear to be related to the more standard ICP-derived PRx.18,19 However, the relationship between derangements in cerebrovascular reactivity and PbtO2 is not well understood. Knowledge of this relationship is crucial prior to adoption of individualized cerebral physiological targets derived from cerebrovascular reactivity, such as optimal cerebral perfusion pressure (CPPopt)20–22 or individualized ICP (iICP) thresholds.6,7 The goal of this study was to provide an exploratory analysis into the relationship between insults in ICP, PbtO2, and PRx, investigating preliminary associations with outcome. This was conducted using the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI)23 high-resolution intensive care unit (HR ICU) sub-study cohort.
Methods
Patient population
All patients from the multi-center CENTER-TBI high resolution ICU monitoring cohort with parenchymal ICP and PbtO2 monitoring, with a 6-month Glasgow Outcome Scale – Extended (GOSE) score, were included in this analysis. Patients with external ventricular drain (EVD) based ICP data were excluded given the interrupted nature of their recordings (i.e., reliable ICP can be recorded only when the drainage is closed). These patients were prospectively recruited between January 2015 and December 2017 from 21 centers in the European Union (EU). All patients were admitted to ICU for their TBI during the course of the study, with high frequency digital signals recorded from their ICU monitors during the course of their ICU stay. All patients predominantly had moderate to severe TBI (moderate = Glasgow Coma Score [GCS] 9–12, and severe = GCS ≤8). A minority of patients (n = 9) were categorized at the time of admission as having less severe TBI, but experienced subsequent early deterioration leading to ICU admission for care and monitoring. All patients in this cohort had invasive ICP monitoring conducted in accordance with the BTF guidelines.8
Ethics
Data used in these analyses were collected as part of the CENTER-TBI study, which had individual national or local regulatory approval; the UK Ethics approval is provided as an exemplar (IRAS No: 150943; REC 14/SC/1370). The CENTER-TBI study (EC grant 602150) has been conducted in accordance with all relevant laws of the EU if directly applicable or of direct effect, and all relevant laws of the country where the recruiting sites were located, including but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force including, but not limited to, the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) (“ICH GCP”) and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects.” Informed consent by the patients and/or the legal representative/next of kin was obtained, accordingly to the local legislations, for all patients recruited in the core data set of CENTER-TBI and documented in the electronic case report form (e-CRF).
Data collection
As part of recruitment to the multi-center high resolution ICU cohort of CENTER-TBI, all patients had demographic, injury, and imaging data prospectively recorded. Similarly, all patients had high frequency digital signals from ICU monitoring recorded throughout their ICU stay, with the goal of initiating recording within 24 h of ICU admission. All digital ICU signals were further processed (see Signal Acquisition and Signal Processing sections). For the purpose of this study, basic admission demographics and centrally reported computed tomography (CT) variables for the first available CT of each patient were extracted.24 They included: age, admission best GCS motor score and pupillary reactivity (bilaterally reactive, unilateral reactive, bilateral unreactive), Marshall CT Classification,25 Rotterdam CT score,26 and presence or absence of traumatic subarachnoid hemorrhage (tSAH), extradural hematoma (EDH), pre-hospital hypotension, and pre-hospital hypoxia. CENTER-TBI data version 2.1 was accessed for the purpose of this study, via Opal database software.27
Signal acquisition
Arterial blood pressure (ABP) was obtained through arterial lines connected to pressure transducers. ICP was acquired from an intra-parenchymal strain gauge probe (Codman ICP MicroSensor; Codman & Shurtleff Inc., Raynham, MA), parenchymal fiberoptic pressure sensor (Camino ICP Monitor, Integra Life Sciences, Plainsboro, NJ, USA; https://www.integralife.com/). PbtO2 monitoring occurred via invasive parenchymal monitoring (Licox probe; Integra, Licox Brain Oxygen Monitoring System, Plainboro, NJ), typically placed in the frontal lobe. All signals were recorded using digital data transfer or digitized via an A/D converter (DT9803; Data Translation, Marlboro, MA), where appropriate; sampled at frequency of 100 Hertz (Hz) or higher, using the ICM+ software (Cambridge Enterprise Ltd, Cambridge, UK, http://icmplus.neurosurg.cam.ac.uk) or Moberg CNS Monitor (Moberg Research Inc, Ambler, PA, USA, https://www.moberg.com) or a combination of both. Signal artefacts were removed using both manual and automated methods prior to further processing or analysis.
Signal processing
Post-acquisition processing of the above-described signals was conducted using ICM+ (Cambridge Enterprise Ltd, Cambridge, UK, http://icmplus.neurosurg.cam.ac.uk). CPP was determined as MAP – ICP. Ten second moving averages (updated every 10 sec to avoid data overlap) were calculated for all recorded signals: ICP, ABP (which produced MAP), CPP, and PbtO2. PRx was calculated as the moving correlation coefficient between 30 consecutive 10 sec mean windows of ICP and MAP, updated every minute.
Data were time averaged and down sampled to minute-by-minute resolution for the entire duration of recording for each patient. Grand mean values of all physiological variables were calculated per patient. In addition, the following post-processing of this physiological data occurred in R (R Core Team [2018]. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. https://www.R-project.org/).
-
1.
% time spent with ICP >20 mm Hg8 - Was determined across the entire recording period.
-
2.
% time spent with PbtO2 < 20 mm Hg9,28 – Was determined across the entire recording period.
-
3.
% time spent with PRx above threshold: For each patient the % of time spent above the following clinically defined thresholds were calculated across the entire recording period: 0, +0.25, +0.35.5,11 All of these thresholds for PRx have been defined in previous published literature as statistically significant for association with 6-month global outcome in adult TBI patients.
-
4.
% time with normal/abnormal PRx and normal/abnormal PbtO2 values - For each patient, across the entire recording period, we determined
a. % time with PRx above threshold and PbtO2 < 20 mm Hg
b. % time with PRx above threshold and PbtO2 > 20 mm Hg
c. % time with PRx below threshold and PbtO2 < 20 mm Hg
Statistical analysis
All statistical analysis was conducted using R and XLSTAT (Addinsoft, New York, NY; https://www.xlstat.com/en/) add-on package to Microsoft Excel (Microsoft Office 15, Version 16.0.7369.1323). Normality of continuous variables was assessed via the Shapiro–Wilks test, in which all variables displayed non-parametric characteristics, and are hence displayed as median (range) or median (interquartile range [IQR]) in the summary of characteristics in Table S1. Various box and contour plots were produced to describe the mean and % time physiological variables for the entire cohort.
Mean % time physiological metrics were compared between dichotomized 6-month GOSE, using Mann–Whitney U testing. GOSE was dichotomized into: Alive/Dead, and Favorable/Unfavorable (with ≤4 denoting unfavorable outcome). For all testing described, α was set at 0.05 for significance. No correction for multiple comparisons was made.
Univariate logistical regression (ULR) was conducted, comparing each % time physiological variable to both dichotomized GOSE defined outcomes. Area under the receiver operating curve (AUC), 95% confidence intervals (CIs), and p values for the univariate models are reported. All AUCs and 95% CIs for ULR were determined using bootstrapping techniques with 2000 iterations, with only the statistically significant results reported in Table S2. Comparison of ULR model AUCs was conducted using Delong's test.
For those physiological variables reaching significance in ULR analysis, multi-variable logistical regression (MLR) models were created, adjusting for baseline admission characteristics and % time with ICP >20 mm Hg, assessing the relationship with dichotomized 6-month GOSE-defined outcomes. These models adjusted for the following baseline admission characteristics (in addition to % time with ICP >20 mm Hg): age, admission GCS motor score, pupillary response, and Marshall CT grade.
Results
Patient demographics
A total of 43 patients were identified with parenchymal ICP and PbtO2 monitoring, with a documented 6-month GOSE. Table S1 summarizes the baseline patient characteristics and physiology variables. The median age was 46 (IQR: 31–65), with 35 being male. The median admission GCS motor score was 3 (IQR: 1–5), with 8 having had a hypoxic episode, 3 having had a hypotensive episode, and 32 demonstrating bilaterally reactive pupils. The median Marshall CT grade was 3 (IQR: 2–6), and duration of high-frequency physiological recording was 137.4 h (IQR: 89.2–174.5).
% time spent above physiological thresholds
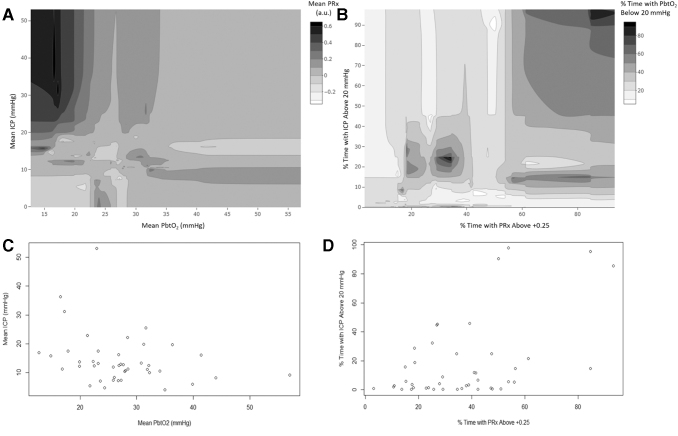
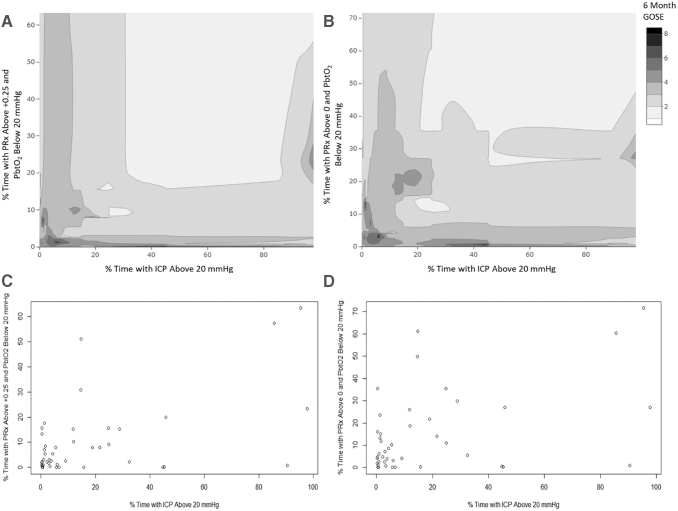
Table S1 provides a summary for the % time spent above/below threshold for ICP, PbtO2 and PRx, with the median values of: 5.8% (IQR: 1.2–23.2), 19.0% (IQR: 3.9–34.6), 60.9% (IQR: 42.9–70.2) for % time above ICP of 20 mm Hg, % time with PbtO2 < 20 mm Hg, and % time with PRx >0, respectively. Figure 1 provides multi-variable contour plots demonstrating the general relationship among ICP, PbtO2 and PRx, displaying that extreme impairments in cerebrovascular reactivity are seen with high ICP and low PbtO2 values. Figure 2 displays descriptive contour plots of % time with ICP >20 mm Hg, % time with PRx >0/+0.25 and PbtO2 < 20 mm Hg, and raw 6-month GOSE score. This highlights the association between improved outcomes with overall optimized ICP, PbtO2, and PRx.
FIG. 1.
Multi-variable contour plots: ICP, PbtO2, and PRx. (A) Contour plot of mean ICP, PbtO2, and PRx, demonstrating grossly impaired PRx values, during high ICP and low PbtO2. (B) Contour plot of % time with ICP >20 mm Hg, % time with PbtO2 < 20 mm Hg, and % time with PRx > +0.25, again highlighting that extreme impairment in cerebrovascular reactivity and ICP lead to reduction in PbtO2 values. (C) Data density plot for A. (D) Data density plot for B. Mean and % time values were derived across the patient's entire recording period. a.u., arbitrary units; ICP, intracranial pressure; MAP, mean arterial pressure; PbtO2, brain tissue oxygen, PRx, pressure reactivity index (correlation between slow-waves of ICP and MAP).
FIG. 2.
Cohort contour plots: % time ICP >20, % time PRx >0/+0.25 and PbtO2 < 20 mm Hg, and 6-month Glasgow Outcome Scale –Extended (GOSE). (A) Contour plot of % time with ICP >20 mm Hg, % time with PRx > +0.25/ PbtO2 < 20 mm Hg, and 6-month GOSE. (B) Contour plot of % time with ICP >20 mm Hg, % time with PRx >0/ PbtO2 < 20 mm Hg, and 6-month GOSE. (C) Data density plot for A. (D) Data density plot for B. Note the superior outcomes (i.e., higher GOSE scores) for more time spent with lower ICP and PRx and higher PbtO2. Mean and % time values were derived across the patient's entire recording period. ICP, intracranial pressure; MAP, mean arterial pressure; PbtO2, brain tissue oxygen; PRx, pressure reactivity index (correlation between slow-waves of ICP and MAP).
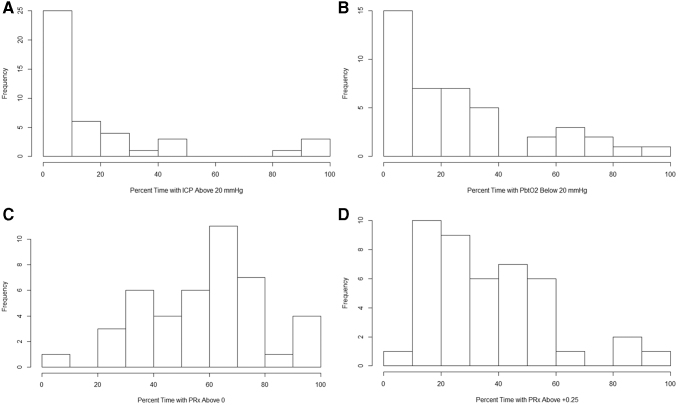
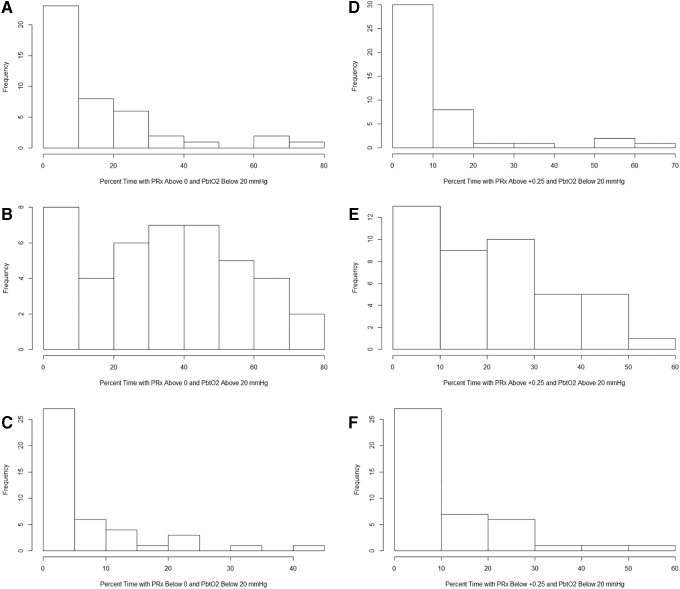
With that said, Figure 3 provides the population histograms of % time above/below threshold for ICP, PbtO2, and PRx of 0/+0.25, and demonstrates that among these three physiological metrics, impaired cerebrovascular reactivity, not elevated ICP or low PbtO2, dominates the landscape of physiological derangement during the acute ICU phase. This is also highlighted by the histograms in Figure 4, which demonstrate that the % time with impaired cerebrovascular reactivity and low PbtO2, is much less than the % time spent with impaired cerebrovascular reactivity and normal PbtO2 values. Further, Figure 4 also displays that the % time with normal cerebrovascular reactivity and low PbtO2 values is overall low across the cohort.
FIG. 3.
Cohort histogram plots - % time with ICP, PbtO2, and PRx beyond threshold. (A) Histogram of % time with ICP >20 mm Hg. (B) Histogram of % time with PbtO2 < 20 mm Hg. (C) Histogram % time with PRx above 0. (D) Histogram % time with PRx > +0.25. Mean and % time values were derived across the patient's entire recording period. Frequency, number of patients; ICP, intracranial pressure; MAP, mean arterial pressure; PbtO2, brain tissue oxygen; PRx, pressure reactivity index (correlation between slow waves of ICP and MAP).
FIG. 4.
Cohort histograms: % time with combined PRx and PbtO2 derangements. (A) Histogram of % time with PRx >0 and PbtO2 < 20 mm Hg. (B) Histogram of % time with PRx >0 and PbtO2 > 20 mm Hg. (C) Histogram of % time with PRx < below 0 and PbtO2 < 20 mm Hg. (D) Histogram of % time with PRx > +0.25 and PbtO2 < 20 mm Hg. (E) Histogram of % time with PRx > +0.25 and PbtO2 > 20 mm Hg. (F) Histogram of % time with PRx < +0.35 and PbtO2 < 20 mm Hg. Mean and % time values were derived across the patient's entire recording period. Frequency, number of patients; ICP, intracranial pressure; MAP, mean arterial pressure; PbtO2, brain tissue oxygen; PRx, pressure reactivity index (correlation between slow waves of ICP and MAP).
Association with dichotomized 6-month outcomes
Comparing % time physiological variables between the two dichotomized 6-month outcomes, only a few demonstrated statistically significant difference in mean values between groups. Table 1 highlights the statistically significant results only. Percent time with PRx >0, +0.25, and +0.35 was higher in the mortality group (p = 0.0007, p = 0.005, p = 0.005, respectively), whereas PRx >0 and +0.25 were not statistically different between those in the favorable and unfavorable outcome categories. Percent time with ICP >20 mm Hg, or PbtO2 < 20 mm Hg, or the combined PRx and PbtO2 % time metrics, were not statistically different between either of the group dichotomizations (p > 0.05 for all).
Table 1.
Summary of Physiological Measurements on Alive/Dead or Favorable/Unfavorable Outcome Groups – Mann–Whitney U Testing – Significant Results Only
| Variable | Mean (± SD) |
p value | Mean (± SD) |
p value | ||
|---|---|---|---|---|---|---|
| Alive | Dead | Favorable | Unfavorable | |||
| Number of patients | 34 | 9 | 19 | 24 | ||
| % time with PRx >0 | 52.8 (20.8) | 71.0 (15.0) | 0.0007 | 50.9 (19.2) | 62.1 (21.0) | 0.08 |
| % time with PRx > +0.25 | 31.6 (17.7) | 49.2 (21.9) | 0.005 | 28.7 (14.7) | 41.2 (22.0) | 0.05 |
| % time with PRx > +0.35 | 23.9 (15.1) | 40.5 (23.8) | 0.005 | 20.8 (11.3) | 33.1 (21.5) | 0.04 |
All bolded p values are those <0.05 when comparing the variables between alive/dead and favorable/unfavorable outcome groups. favorable, Glasgow Outcome Scale of 5–8, unfavorable, Glasgow Outcome Scale of 1–4.
ICP, intracranial pressure; MAP, mean arterial blood pressure; PRx, pressure reactivity index (correlation between ICP and MAP), SD, standard deviation.
We compared each % time physiological metric in association with the dichotomized outcomes using ULR. Table S2 summarized the statistically significant associations. For alive/dead outcome, only % time with ICP >20 mm Hg (AUC 0.648, p = 0.006), % time with PRx >0 (AUC 0.734, p = 0.003), > +0.25 (AUC 0.747, p = 0.002) and > +0.35 (AUC 0.745, p = 0.002), were found to be statistically significant, but not different from one another (p > 0.05 using Delong's test). Whereas for favorable/unfavorable outcome, % time with PRx > +0.25 (AUC 0.679, p = 0.034) and +0.35 (AUC 0.690, p = 0.030), % time with PbtO2 < 20 mm Hg (AUC 0.682, p = 0.030), and % time with PRx >0 and PbtO2 < 20 mm Hg (AUC 0.661, p = 0.041), were found to be statistically significant, but not different from one another (using Delong's test). During MLR modelling, none of the PRx/PbtO2 variables retained significance when adjusting for baseline admission characteristics. ICP also fell out of significance when adjusting for baseline admission characteristics in this cohort. However, lack of significance in MLR may just reflect low power in this small cohort.
Discussion
Through this preliminary multi-center analysis using the CENTER-TBI HR ICU Sub-Study cohort, we have been able to display some interesting and important relationships among ICP, PbtO2, PRx and 6-month outcome. A few deserve highlighting.
First, across the entire recording period for all patients, it appears that impaired cerebrovascular reactivity dominated the impaired cerebral physiology seen in this adult TBI cohort. This can be seen both in the cohort summary of mean % times beyond threshold, and population histograms for the various physiological metrics. It is of note that very little time was spent with ICP >20 mm Hg and PbtO2 < 20 mm Hg (Fig. 3). This suggests that in the current environment of BTF-based therapeutic strategies in adult TBI care, cerebrovascular reactivity remains independent to treatment effect, whereas ICP and PbtO2 remain responsive. This parallels past larger population analysis regarding therapeutic intensity and cerebrovascular reactivity in the wider CENTER-TBI HR cohort.3,29 Further, this lack of treatment effect and persistent cerebrovascular dysfunction seen in this cohort, appears to also display stronger associations with dichotomized 6-month outcome, compared with % time above ICP of 20 mm Hg, and % time with PbtO2 < 20 mm Hg. Again, this strong association between cerebrovascular reactivity and global outcome has been previously described.12,13 Therefore, the question is raised regarding the determinants of impaired cerebrovascular reactivity in TBI. In general, if ICP-directed therapies appear to have limited impact on cerebrovascular reactivity, then future investigation is warranted into other potential driving factors involved. Such work may allow directed pharmacological manipulation of cerebrovascular reactivity status. These types of investigations are the ongoing work of various laboratories.
Second, the time spent with derangements in PRx and PbtO2 were highlighted. It is known that elevations in ICP can lead to impaired cerebrovascular reactivity,14,15 as measured through PRx. However, the relationship between PRx and PbtO2 is less clear. We were able to display (Fig. 1) that during extreme impairments in cerebrovascular reactivity, both ICP and PbtO2 tend to be abnormal. Similarly, Figure 2 supports that optimization of ICP, PbtO2, and PRx leads to overall better GOSE at 6 months. However, the amount of time spent with impaired cerebrovascular reactivity and low PbtO2 is relatively limited in this population, whereas isolated impairment in cerebrovascular reactivity, with normal PbtO2 levels, tends to dominate the overall physiology when looking at variations in derangements of PRx and PbtO2 (Figure 4). Therefore, one does not always display low PbtO2 values during impaired cerebrovascular reactivity, with impaired cerebrovascular reactivity alone appearing to be the more common event in moderate/severe TBI.
Third, cerebrovascular reactivity is generally impaired in the presence of isolated low PbtO2 in this cohort. This suggests that many of the low PbtO2 episodes are occurring in the presence of impaired cerebrovascular reactivity. This may simply suggest that the pathological state required to cause a reduction in brain tissue oxygenation is so severe that autoregulation is impaired. Alternatively, and more tantalizingly, it may be that abnormal cerebrovascular reactivity is an important factor in the development of reduced brain tissue oxygen tension, perhaps by limiting the brain's ability to mount compensatory changes in cerebral blood flow in response to oxygen supply–demand mismatch. We know from previous works that PbtO2 tends to follow changes in CPP in TBI.17,30,31 Figure S1 provides a contour plot of mean ICP, CPP, and PbtO2, demonstrating that in general, PbtO2 values increase with increasing CPP, corroborating these previously described results. Such relationships among cerebrovascular reactivity, CPP, and PbtO2 required further validation and exploration in larger cohorts with this type of high frequency physiological recordings.
Finally, the association with global dichotomized outcome at 6 months favors more consistent and robust associations with impaired cerebrovascular reactivity, over elevated ICP and low PbtO2. It must be acknowledged that these results are not preserved in multi-variable logistical regression analysis, which may reflect a tenuous relationship or small sample size. However, recent work from the larger CENTER HR cohort supports independent association between cerebrovascular reactivity measures and global outcome, above and beyond ICP monitoring, during multi-variable adjustment for baseline characteristics.13 Therefore, future larger studies with high-frequency ICP and PbtO2 physiological data may perhaps reveal similar outcome relationships between PRx and PbtO2.
Limitations
Despite the interesting results described, they remain preliminary in nature and have some limitations. The overall cohort size is small at 43 patients. Therefore, it remains difficult to extrapolate the findings from this group regarding ICP, PRx, and PbtO2 to other moderate/severe TBI populations. Consequently, any statistically significant/non-significant findings described in this article must be taken as purely preliminary and exploratory in nature. However, the fact at we did obtain highly significant results for some of the associations studied even in this extremely small sample is a testimony to the importance of those effects. In addition, all patients underwent active treatment for their TBI in accordance with BTF guidelines and local protocols. Therefore, all physiology data recorded represents treated physiological data, not the natural history of untreated moderate/severe TBI. As such, the limited time spent with abnormal ICP and PbtO2 values in this cohort may reflect effective management. However, lack of extremes seen for ICP and PbtO2 may also just reflect the small cohort size, or the fact that in this particular cohort the rate of extreme physiology values just happened to be low, regardless of intervention. Finally, we specifically did not correct for multiple comparisons, as this was designed to be an exploratory pilot analysis. Future validation of the findings here will require extensive large multi-center populations of TBI patients with ICP and PbtO2 monitoring, with prospective archiving of high-frequency digital physiology. Such work would also benefit from investigation into the association between PbtO2 and individualized physiological targets, such as CPPopt and iICP thresholds. Similarly, future investigations will require investigation into the higher frequency time-series relationships between both slow-waves of ICP and PbtO2 signals, and their derived metrics such as PRx and oxygen reactivity metrics.
Finally, raw physiological signal variability, such as ABP variability, may impact the strength of correlation metrics derived from ABP or CPP, such as cerebrovascular reactivity metrics. This is an important aspect to consider when interpreting the strength of the relationships displayed in both this work, and others on cerebrovascular reactivity measures. At this time, the CENTER-TBI HR Sub-Study has various approved projects underway, evaluating raw and derived signal variability/complexity, and their association with both patient outcome, and various other cerebral physiological metrics. As such, it was not explored here. At this time, the link between ABP and ICP variability and the strength of derived correlation metrics is unclear, and is an important aspect for future study. Therefore, we must further re-emphasize the exploratory nature of the results highlighted in this article, which require future validation and investigation.
Conclusion
Impaired cerebrovascular reactivity is seen during concurrent episodes of elevated ICP and low PbtO2. However, the majority of the deranged cerebral physiology seen during the acute ICU phase is impairment in cerebrovascular reactivity, with most impairment occurring in the presence of normal PbtO2 levels. Measures of cerebrovascular reactivity appear to display the most consistent associations with global outcome in TBI, compared with ICP and PbtO2.
CENTER-TBI High Resolution Sub-Study Participants and Investigators
Audny Anke, Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway; Ronny Beer, Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria; Bo-Michael Bellander, Department of Neurosurgery & Anesthesia & Intensive Care Medicine, Karolinska University Hospital, Stockholm, Sweden; Erta Beqiri, NeuroIntensive Care, Niguarda Hospital, Milan, Italy; Andras Buki, Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Hungary; Manuel Cabeleira, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom; Marco Carbonara, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy ; Arturo Chieregato, NeuroIntensive Care, Niguarda Hospital, Milan, Italy; Giuseppe Citerio, NeuroIntensive Care Unit, Department of Anesthesia & Intensive Care, ASST di Monza, Monza, Italy/ School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy; Hans Clusmann, Department of Neurosurgery, Medical Faculty RWTH Aachen University, Aachen, Germany; Endre Czeiter, Department of Neurosurgery, University of Pecs and MTA-PTE Clinical Neuroscience MR Research Group and Janos Szentagothai Research Centre, University of Pecs, Hungarian Brain Research Program (Grant No. KTIA 13 NAP-A-II/8), Pecs, Hungary; Marek Czosnyka, Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke's Hospital, Cambridge, UK; Bart Depreitere, Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium; Ari Ercole, Division of Anaesthesia, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom; Shirin Frisvold, Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway; Raimund Helbok, Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria; Stefan Jankowski, Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; Danile Kondziella, Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark; Lars-Owe Koskinen, Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden; Ana Kowark, Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany; David K. Menon, Division of Anaesthesia, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom; Geert Meyfroidt, Intensive Care Medicine, University Hospitals Leuven, Leuven, Belgium; Kirsten Moeller, Department Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark; David Nelson, Department of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden; Anna Piippo-Karjalainen, Helsinki University Central Hospital, Helsinki, Finland; Andreea Radoi, Department of Neurosurgery, Vall d'Hebron University Hospital, Barcelona, Spain; Arminas Ragauskas, Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania; Rahul Raj, Helsinki University Central Hospital, Helsinki, Finland; Jonathan Rhodes, Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, United Kingdom; Saulius Rocka, Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania; Rolf Rossaint, Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany; Juan Sahuquillo, Department of Neurosurgery, Vall d'Hebron University Hospital, Barcelona, Spain; Oliver Sakowitz, Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany/ Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany; Peter Smielewski, Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom; Nino Stocchetti, Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy; Nina Sundström, Department of Radiation Sciences, Biomedical Engineering, Umea University, Umea, Sweden; Riikka Takala, Perioperative Services, Intensive Care Medicine, and Pain Management , Turku University Central Hospital and University of Turku, Turku, Finland; Tomas Tamosuitis, Neuro-intensive Care Unit, Kaunas University of Health Sciences, Kaunas, Lithuania; Olli Tenovuo, Rehabilitation and Brain Trauma, Turku University Central Hospital and University of Turku, Turku, Finland; Peter Vajkoczy, Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany; Alessia Vargiolu, NeuroIntensive Care Unit, Department of Anesthesia & Intensive Care, ASST di Monza, Monza, Italy; Rimantas Vilcinis, Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania; Stefan Wolf, Department of Neurosurgery, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Alexander Younsi, Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany; Frederick A. Zeiler, Division of Anaesthesia, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom/ Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
Supplementary Material
Contributor Information
Collaborators: the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) High-Resolution ICU (HR ICU) Sub-Study Participants and Investigators, Audny Anke, Ronny Beer, Bo-Michael Bellander, Erta Beqiri, Andras Buki, Manuel Cabeleira, Marco Carbonara, Arturo Chieregato, Giuseppe Citerio, Hans Clusmann, Endre Czeiter, Marek Czosnyka, Bart Depreitere, Ari Ercole, Shirin Frisvold, Raimund Helbok, Stefan Jankowski, Danile Kondziella, Lars-Owe Koskinen, Ana Kowark, David K. Menon, Geert Meyfroidt, Kirsten Moeller, David Nelson, Anna Piippo-Karjalainen, Andreea Radoi, Arminas Ragauskas, Rahul Raj, Jonathan Rhodes, Saulius Rocka, Rolf Rossaint, Juan Sahuquillo, Oliver Sakowitz, Peter Smielewski, Nino Stocchetti, Nina Sundström, Riikka Takala, Tomas Tamosuitis, Olli Tenovuo, Peter Vajkoczy, Alessia Vargiolu, Rimantas Vilcinis, Stefan Wolf, Alexander Younsi, and Frederick A. Zeiler
Funding Information
Data used in preparation of this manuscript were obtained in the context of CENTER-TBI, a large collaborative project with the support of the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA) and from Integra LifeSciences Corporation (USA). D.K.M. was also supported by funding from the National Institute for Health Research (NIHR, UK) through a Senior Investigator award and the Cambridge Biomedical Research Centre (BRC) at the Cambridge University Hospitals NHS Foundation Trust. The study also received additional support from the NIHR Clinical Research network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care, UK. F.A.Z. receives research support from the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), the Canadian Institutes for Health Research (CIHR), the University of Manitoba Vice President Research and International (VPRI) Research Investment Fund (RIF), the University of Manitoba Centre on Aging, the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg. M.C.is supported by NIHR Cambridge BRC. P.J.H. is supported by the NIHR (Research Professorship, Cambridge BRC, Global Health Research Group on Neurotrauma) and the Royal College of Surgeons of England.
Author Disclosure Statement
P.S. and M.C. receive part of licensing fees for the software ICM+ (Cambridge Enterprise Ltd, UK) used for data collection and analysis in this study. M.C. has a consultancy agreement with Integra, and P.S. has consultancy agreements with Integra Life Sciences and Pressura Neuro Ltd. D.K.M. has consultancy agreements and/or research collaborations with GlaxoSmithKline Ltd., Ornim Medical, Shire Medical Ltd, Calico Inc., Pfizer Ltd., Pressura Ltd., Glide Pharma Ltd., and NeuroTraumaSciences LLC. The other authors have nothing to disclose.
Supplementary Material
References
- 1. Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G., Diringer M.N., Stocchetti N., Videtta W., Armonda R., Badjatia N., Bösel J., Chesnut R., Chou S., Claassen J., Czosnyka M., De Georgia M., Figaji A., Fugate J., Helbok R., Horowitz D., Hutchinson P., Kumar M., McNett M., Miller C., Naidech A., Oddo M., Olson D., O'Phelan K., Provencio J.J., Puppo C., Riker R., Roberson C., Schmidt M., and Taccone F. (2014). The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: evidentiary tables: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 21, Suppl. 2, S297–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Büki A., Chesnut R.M., Citerio G., Coburn M., Cooper D.J., Crowder A.T., Czeiter E., Czosnyka M., Diaz-Arrastia R., Dreier J.P., Duhaime A.-C., Ercole A., van Essen T.A., Feigin V.L., Gao G., Giacino J., Gonzalez-Lara L.E., Gruen R.L., Gupta D., Hartings J.A., Hill S., Jiang J.-Y., Ketharanathan N., Kompanje E.J.O., Lanyon L., Laureys S., Lecky F., Levin H., Lingsma H.F., Maegele M., Majdan M., Manley G., Marsteller J., Mascia L., McFadyen C., Mondello S., Newcombe V., Palotie A., Parizel P.M., Peul W., Piercy J., Polinder S., Puybasset L., Rasmussen T.E., Rossaint R., Smielewski P., Söderberg J., Stanworth S.J., Stein M.B., von Steinbüchel N., Stewart W., Steyerberg E.W., Stocchetti N., Synnot A., Te Ao B., Tenovuo O., Theadom A., Tibboel D., Videtta W., Wang K.K.W., Williams W.H., Wilson L., Yaffe K., and InTBIR Participants and Investigators. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 [DOI] [PubMed] [Google Scholar]
- 3. Donnelly J., Czosnyka M., Adams H., Cardim D., Kolias A.G., Zeiler F.A., Lavinio A., Aries M., Robba C., Smielewski P., Hutchinson P.J.A., Menon D.K., Pickard J.D., and Budohoski K.P. (2019). Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85, E75–E82 [DOI] [PubMed] [Google Scholar]
- 4. Khellaf A., Khan D.Z., and Helmy A. (2019). Recent advances in traumatic brain injury. J. Neurol. 266, 2878–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorrentino E., Diedler J., Kasprowicz M., Budohoski K.P., Haubrich C., Smielewski P., Outtrim J.G., Manktelow A., Hutchinson P.J., Pickard J.D., Menon D.K., and Czosnyka M. (2012). Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 16, 258–266 [DOI] [PubMed] [Google Scholar]
- 6. Lazaridis C., DeSantis S.M., Smielewski P., Menon D.K., Hutchinson P., Pickard J.D., and Czosnyka M. (2014). Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J. Neurosurg. 120, 893–900 [DOI] [PubMed] [Google Scholar]
- 7. Zeiler F.A., Ercole A., Cabeleira M., Beqiri E., Zoerle T., Carbonara M., Stocchetti N., Menon D.K., Lazaridis C., Smielewski P., Czosnyka M., and CENTER-TBI High Resolution ICU Sub-Study Participants and Investigators (2019). Patient-specific ICP epidemiologic thresholds in adult traumatic brain injury: A CENTER-TBI validation study. J. Neurosurg. Anesthesiol. [Epub ahead of print] [DOI] [PubMed]
- 8. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W.J., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 9. Okonkwo D.O., Shutter L.A., Moore C., Temkin N.R., Puccio A.M., Madden C.J., Andaluz N., Chesnut R.M., Bullock M.R., Grant G.A., McGregor J., Weaver M., Jallo J., LeRoux P.D., Moberg D., Barber J., Lazaridis C., and Diaz-Arrastia R.R. (2017). Brain oxygen optimization in severe traumatic brain injury phase-II: A phase II randomized trial. Crit. Care Med. 45, 1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czosnyka M., Smielewski P., Kirkpatrick P., Laing R.J., Menon D., and Pickard J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–19 [DOI] [PubMed] [Google Scholar]
- 11. Zeiler F.A., Donnelly J., Smieleweski P., Menon D., Hutchinson P.J., and Czosnyka M. (2018). Critical thresholds of ICP derived continuous cerebrovascular reactivity indices for outcome prediction in non-craniectomized TBI patients: PRx, PAx and RAC. J. Neurotrauma 35, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 12. Zeiler F.A., Ercole A., Cabeleira M., Zoerle T., Stocchetti N., Menon D.K., Smielewski P., Czosnyka M., and CENTER-TBI High Resolution Sub-Study Participants and Investigators. (2019). Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir. (Wien) 161, 1217–1227 [DOI] [PMC free article] [PubMed]
- 13. Zeiler F.A., Ercole A., Beqiri E., Cabeleira M., Thelin E.P., Stocchetti N., Steyerberg E.W., Maas A., Menon D., Czosnyka M., and Smieleweski P. (2019). Association between Cerebrovascular Reactivity Monitoring and Mortality is preserved when adjusting for baseline admission characteristics in Adult TBI: a CENTER-TBI Study. J. Neurotrauma [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 14. Donnelly J., Smielewski P., Adams H., Zeiler F.A., Cardim D., Liu X., Fedriga M., Hutchinson P., Menon D.K., and Czosnyka M. (2020). Observations on the cerebral effects of refractory intracranial hypertension after severe traumatic brain injury. Neurocrit. Care. 32, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Czosnyka M., Aries M., Weersink C., Wolf S., Budohoski K., Dias C., Lewis P., Smielewski P., and Kordasti S. (2016). “Solid red line”: an observational study on death from refractory intracranial hypertension. Acta Neurochir. Suppl. 122, 113–116 [DOI] [PubMed] [Google Scholar]
- 16. Xie Q., Wu H.-B., Yan Y.-F., Liu M., and Wang E.-S. (2017). Mortality and outcome comparison between brain tissue oxygen combined with intracranial pressure/cerebral perfusion pressure-guided therapy and intracranial pressure/cerebral perfusion pressure-guided therapy in traumatic brain injury: a meta-analysis. World Neurosurg. 100, 118–127 [DOI] [PubMed] [Google Scholar]
- 17. Budohoski K.P., Zweifel C., Kasprowicz M., Sorrentino E., Diedler J., Brady K.M., Smielewski P., Menon D.K., Pickard J.D., Kirkpatrick P.J., and Czosnyka M. (2012). What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br. J. Anaesth. 108, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeiler F.A., Donnelly J., Menon D.K., Smielewski P., Zweifel C., Brady K., and Czosnyka M. (2017). Continuous autoregulatory indices derived from multi-modal monitoring: each one is not like the other. J. Neurotrauma 34, 3070–3080 [DOI] [PubMed] [Google Scholar]
- 19. Andresen M., Donnelly J., Aries M., Juhler M., Menon D., Hutchinson P., and Smielewski P. (2018). Further controversies about brain tissue oxygenation pressure-reactivity after traumatic brain injury. Neurocrit. Care 28, 162–168 [DOI] [PubMed] [Google Scholar]
- 20. Steiner L.A., Czosnyka M., Piechnik S.K., Smielewski P., Chatfield D., Menon D.K., and Pickard J.D. (2002). Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 30, 733–738 [DOI] [PubMed] [Google Scholar]
- 21. Aries M.J.H., Czosnyka M., Budohoski K.P., Steiner L.A., Lavinio A., Kolias A.G., Hutchinson P.J., Brady K.M., Menon D.K., Pickard J.D., and Smielewski P. (2012). Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 40, 2456–2463 [DOI] [PubMed] [Google Scholar]
- 22. Beqiri E., Smielewski P., Robba C., Czosnyka M., Cabeleira M.T., Tas J., Donnelly J., Outtrim J.G., Hutchinson P., Menon D., Meyfroidt G., Depreitere B., Aries M.J., and Ercole A. (2019). Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9, e030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maas A.I.R., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., Hill S., Legrand V., Sorgner A., and CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 24. Vande Vyvere T., Wilms G., Claes L., Martin Leon F., Nieboer D., Verheyden J., van den Hauwe L., Pullens P., Maas A.I.R., Parizel P.M., and Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Investigators and Participants. (2019). Central versus,. J. Neurotrauma 36, 1080–1092 [DOI] [PubMed] [Google Scholar]
- 25. Marshall L.F., Marshall S.B., Klauber M.R., Van Berkum Clark M., Eisenberg H., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9, Suppl.1, S287–292 [PubMed] [Google Scholar]
- 26. Maas A.I.R., Hukkelhoven C.W.P.M., Marshall L.F., and Steyerberg E.W. (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 27. Doiron D., Marcon Y., Fortier I., Burton P., and Ferretti V. (2017). Software Application Profile: Opal and Mica: open-source software solutions for epidemiological data management, harmonization and dissemination. Int. J. Epidemiol. 46, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G., Diringer M.N., Stocchetti N., Videtta W., Armonda R., Badjatia N., Bösel J., Chesnut R., Chou S., Claassen J., Czosnyka M., De Georgia M., Figaji A., Fugate J., Helbok R., Horowitz D., Hutchinson P., Kumar M., McNett M., Miller C., Naidech A., Oddo M., Olson D., O'Phelan K., Provencio J.J., Puppo C., Riker R., Roberson C., Schmidt M., and Taccone F. (2014). The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 21, Suppl. 2, S282–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeiler F.A., Ercole A., Beqiri E., Cabeleira M., Aries M., Zoerle T., Carbonara M., Stocchetti N., Smieleweski P., Czosnyka M., and Menon D.K. (2019). Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir. (Wien) [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 30. Bragin D.E., Statom G.L., Yonas H., Dai X., and Nemoto E.M. (2014). Critical cerebral perfusion pressure at high intracranial pressure measured by induced cerebrovascular and intracranial pressure reactivity. Crit. Care Med. 42, 2582–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawryluk G.W.J., Phan N., Ferguson A.R., Morabito D., Derugin N., Stewart C.L., Knudson M.M., Manley G., and Rosenthal G. (2016). Brain tissue oxygen tension and its response to physiological manipulations: influence of distance from injury site in a swine model of traumatic brain injury. J. Neurosurg. 125, 1217–1228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.