Abstract
Background:
In April 2015, in collaboration with the US Centers for Disease Control and Prevention and Gilead Sciences, the country of Georgia embarked on the world’s first hepatitis C elimination program. We aimed to assess progress toward elimination targets 3 years after the start of the elimination program.
Methods:
We constructed a hepatitis C virus (HCV) care cascade for adults in Georgia, based on the estimated 150 000 persons aged ≥18 years with active HCV infection. All patients who were screened or entered the treatment program during April 2015–March 2018 were included in the analysis. Data on the number of persons screened for HCV were extracted from the national HCV screening database. For the treatment component, we utilized data from the Georgia National HCV treatment program database. Available treatment options included sofosbuvir and ledipasvir/sofosbuvir–based regimens.
Results:
Since April 2015, a cumulative 974 817 adults were screened for HCV antibodies; 86 624 persons tested positive, of whom 61 925 underwent HCV confirmatory testing. Among the estimated 150 000 adults living with chronic hepatitis C in Georgia, 52 856 (35.1%) were diagnosed, 45 334 (30.2%) initiated treatment with direct-acting antivirals, and 29 090 (19.4%) achieved a sustained virologic response (SVR). Overall, 37 256 persons were eligible for SVR assessment; of these, only 29 620 (79.5%) returned for evaluation. The SVR rate was 98.2% (29 090/29 620) in the per-protocol analysis and 78.1% (29 090/37 256) in the intent-to-treat analysis.
Conclusions:
Georgia has made substantial progress in the path toward eliminating hepatitis C. Scaling up of testing and diagnosis, along with effective linkage to treatment services, is needed to achieve the goal of elimination.
Keywords: HCV, elimination, cascade, Georgia
Introduction of highly effective direct-acting antivirals (DAAs) for hepatitis C virus (HCV) with cure rates exceeding 90% have resulted in a paradigm shift in the response to the HCV epidemic [1]. In 2016 the World Health Assembly endorsed the Global Health Sector Strategy on Viral Hepatitis 2016–2021, which calls for the elimination of viral hepatitis as a public health threat by 2030 [2]. The World Health Organization (WHO) defines elimination as 90% reduction in the incidence of HCV infection and 65% reduction in HCV-related mortality, to be achieved through diagnosing 90% of people living with HCV infection and treating 80% of those diagnosed. As of February 2019, 124 countries reported national hepatitis plans to be in place, yet only 12 countries (Australia, Egypt, France, Georgia, Iceland, Italy, Japan, Mongolia, the Netherlands, Spain, Switzerland, and the United Kingdom) were on track to meet the WHO targets [3, 4]. A major barrier on the road to elimination is limited domestic and international investments in hepatitis C programs, particularly in resource-limited countries [5]. Lack of finances affects access to testing, diagnostics, and treatment, resulting in significant gaps in the HCV care cascade. Global progress analysis shows that only 14 million people with chronic hepatitis C were diagnosed and a cumulative 5 million people out of estimated 71 million persons living with chronic hepatitis C globally received DAA treatment [3, 6].
Georgia is a small Eastern European country (population of 3.7 million people), with a lower-middle–income economy and healthcare expenditures accounting for 8.44% of gross domestic product [7]. The country’s high burden of hepatitis C has been known since 2002, when the first population-based survey found anti-HCV prevalence of 6.7% among the adult general population of the capital city, Tbilisi [8]. However, because of economic reasons, access to treatment was limited to only those who could afford to pay, with an estimated 400 persons getting HCV treatment with pegylated interferon (IFN) and ribavirin (RBV) annually at a cost of $10 000 is cost per patient per treatment course (unpublished data).
In 2011 the government of Georgia substantially stepped up its efforts by first implementing a national program to provide free access to HCV treatment for patients with human immunodeficiency virus (HIV)/HCV coinfection (implemented in collaboration with the Global Fund to Fight AIDS, TB, and Malaria since 2011). In 2013, the government provided free treatment in the penitentiary system and negotiated a 60% price reduction for the general population (since 2013). A total of 1685 patients were treated through these initiatives between 2011 and 2014 with the dual IFN/RBV combination [9].
In April 2015 Georgia launched the world’s first national hepatitis C elimination program in a partnership with the US Centers for Disease Control and Prevention (CDC) and a commitment from Gilead Sciences to provide its DAAs to treat all Georgians living with HCV infection free of charge [9]. Georgia was selected as the first model country for elimination because of the small size of the country and high burden of hepatitis C, strong political will, existence of human and technical capacities, and best-practice experience in ensuring universal access to HIV and TB treatments.
The program aims to decrease HCV prevalence by 90% primarily through a test-and-treat approach strengthened by effective prevention measures. The national elimination strategy set ambitious targets to diagnose 90% of people with HCV, to treat 95% of those diagnosed, and to cure 95% of those treated by 2020.
We assessed the progress toward achieving national targets by quantifying the national-level HCV care cascade after 3 years of the elimination program.
Methods
The National Hepatitis C Elimination Program
The national strategy to eliminate hepatitis C builds on delivering a comprehensive response to HCV including advocacy and awareness, surveillance, and primary prevention, with a test-and-treat approach being the cornerstone of this strategy.
Active case-finding activities are implemented through healthcare and community-based programs. Current screening efforts include targeted testing of high-risk populations (people who inject drugs [PWID], men who have sex with men, prisoners, HIV-positive persons, persons with tuberculosis, patients on hemodialysis, and people with hemophilia); universal screening of all hospitalized patients, pregnant women and military recruits; and mandatory screening of donated blood. For self-referred persons, testing services are available at HCV care provider sites. Outreach screening campaigns are conducted for general and high-risk populations.
During the initial phase of the program, antibody-positive persons were referred to HCV care provider sites for confirmatory testing. In 2017 for healthcare-based screening, an HCV reflex testing approach was implemented, with antibody-positive blood specimens immediately sent for confirmation to designated sites. HCV RNA and HCV core antigen tests are used for confirming the diagnosis. Persons with confirmed infection are notified about the diagnosis and provided with information about HCV care sites. Referral support services are available for PWID.
At the beginning of the program, in April 2015, only 4 specialty clinics were authorized to provide care and treatment services, all located in the capital city of Tbilisi. Over the following 3 years, the number of authorized clinics expanded to 31, all specialty clinics, with locations throughout the country.
After confirming chronic infection, patients undergo pretreatment evaluation including genotype testing and liver fibrosis assessment using Fibrosis-4 (FIB-4) score or transient elastography. Advanced fibrosis was defined as FIB-4 score of >3.25 or liver stiffness >9.5 kPa on transient elastography [10, 11]. Patients are monitored for treatment efficacy and safety throughout the treatment duration. Sustained virologic response (SVR) is assessed 12–24 weeks after completing the treatment course using HCV RNA quantitative assay (HCV core antigen is only used for confirming active HCV infection and not for determining SVR).
During the first year, sofosbuvir (SOF) was the only DAA available within the program. SOF was used in combination with RBV, with or without IFN, depending on the HCV genotype and level of fibrosis. In March 2016, a fixed-dose combination of ledipasvir (LDV)/SOF became available, and the drug has been recommended in all genotypes with or without RBV depending on the HCV genotype and level of fibrosis. All persons failing initial SOF-based regimens were re-treated with LDV/SOF with extended duration, or in combination with RBV and/or IFN. An expert group of Georgian clinicians in consultation with international experts developed national treatment protocols that provide an easy guide for selecting appropriate treatment regimen by genotype, level of fibrosis, and previous treatment experience.
During the initial phase of the program (April 2015–May 2016), treatment was offered only to patients with advanced liver fibrosis [12]. Beginning in June 2016, treatment was expanded to all people with HCV regardless of degree of liver damage.
All drugs within the elimination program are provided free of charge to patients. Screening, confirmation, and SVR testing are also free. Pretreatment evaluation and treatment monitoring are free for registered socially vulnerable persons, whereas others are required to co-pay on a sliding scale based on income status. During the 3 years of program implementation, costs of co-payment have been reduced for patients, and some testing has been eliminated. As a result, the maximum co-payment amount for a 3-month course of treatment has been reduced from $306 to $128.
A national HCV treatment database was established at the launch of the program. The database undergoes periodic modifications and improvements. The database collects case-based information, including demographic, laboratory, and clinical data, on each person enrolled in the treatment program using a standardized protocol. In 2017, a national electronic HCV screening database was launched to collect data from all sites providing HCV screening services in the country.
HCV Care Cascade
We constructed a 7-stage HCV care cascade for adult persons (aged ≥18 years): (1) estimated number of persons with chronic HCV infection; (2) number of persons diagnosed with chronic HCV infection; (3) number of persons who initiated HCV treatment; (4) number of persons who completed HCV treatment; (5) number of persons eligible for assessment of SVR; (6) number of persons assessed for SVR; and (7) number of persons who achieved SVR.
The estimate of the number of persons living with HCV infection is based on a 2015 countrywide population-based survey, which showed that 5.4% of the adult general population (150 000 persons) has chronic HCV infection [13]. All persons who were screened or entered the treatment program during April 2015–March 2018 were included in the analysis. Data on the number of persons screened for HCV were extracted from the national HCV screening database. For the treatment component, we utilized data from the Georgia National HCV treatment program database including all data on persons tested for chronic HCV infection through SVR. Both HCV RNA testing and HCV core antigen testing are available for confirming active HCV infection and are both included in the analysis. Patients were eligible for SVR assessment at least 12 weeks and no later than 24 weeks after completing treatment. SVR was defined as an undetectable plasma level of HCV RNA 12–24 weeks after completing treatment.
SVR rates were calculated using per-protocol and intent-to-treat analyses. The per-protocol approach included only those with complete SVR data, whereas the intent-to-treat analysis included all persons eligible for SVR assessment. All analyses were performed with SAS version 9.3 software (SAS Institute, Cary, North Carolina).
Results
After the start of the elimination program, a cumulative 974 817 adults (35% of the adult general population of Georgia) were screened for HCV infection. Overall, 86 624 persons tested positive for HCV antibodies, of whom 61 925 (71.5%) underwent HCV confirmatory testing with either HCV RNA or HCV core antigen. Chronic HCV infection was confirmed in 52 856 of 61 925 (85.4%) persons; overall, approximately 35% of the 150 000 estimated number of people with chronic HCV infection were identified by the elimination program (Figure 1). Among 52 856 persons with a confirmed diagnosis, 50.6% were in the age category 18–45 years, 77.9% were men, and 55.0% were from the capital city of Tbilisi.
Figure 1.
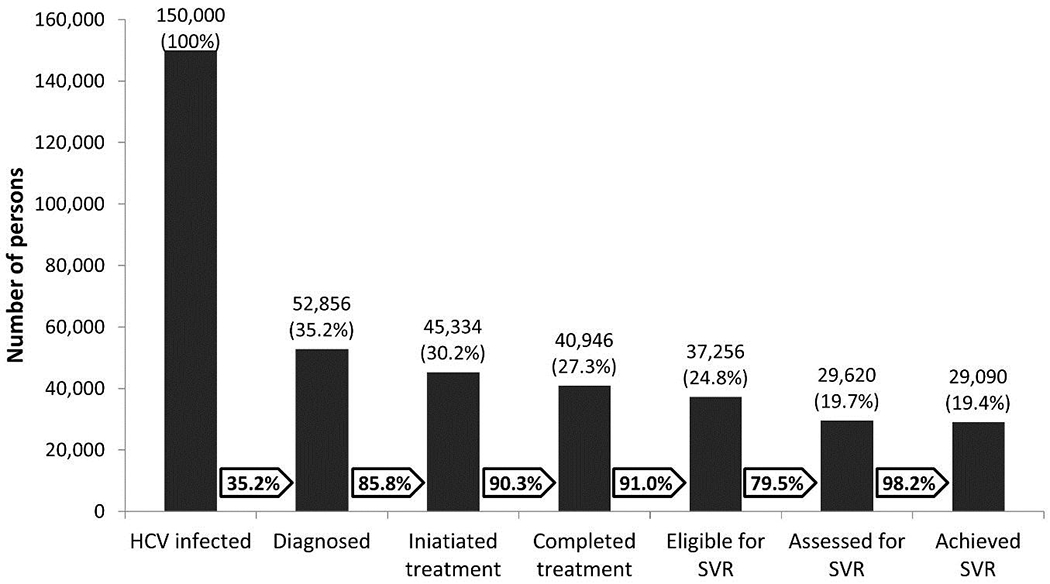
HCV care cascade in the country of Georgia, March 2018.
Abbreviations: HCV, hepatitis C virus; SVR, sustained virologic response.
A total of 45 334 persons initiated treatment, 85.8% of identified persons with chronic HCV infection (Figure 1). Among them, 44.1% had genotype 1, 19.9% had genotype 2, and 34.1% had genotype 3. With regard to treatment regimens, 14.0% received SOF and 83.8% received LDV/SOF-based regimens as initial treatment; 2.2% were re-treated with LDV/SOF after failure of initial SOF-based treatment.
During the initial phase of the program (April 2015–May 2016), when treatment was prioritized for persons with severe liver disease, on average 661 patients were started on treatment monthly; this increased to 2619 patients per month during June–December 2016, decreased to 1232 patients per month during January–December 2017, and further to 985 patients per month during January–March 2018 (Figure 2).
Figure 2.
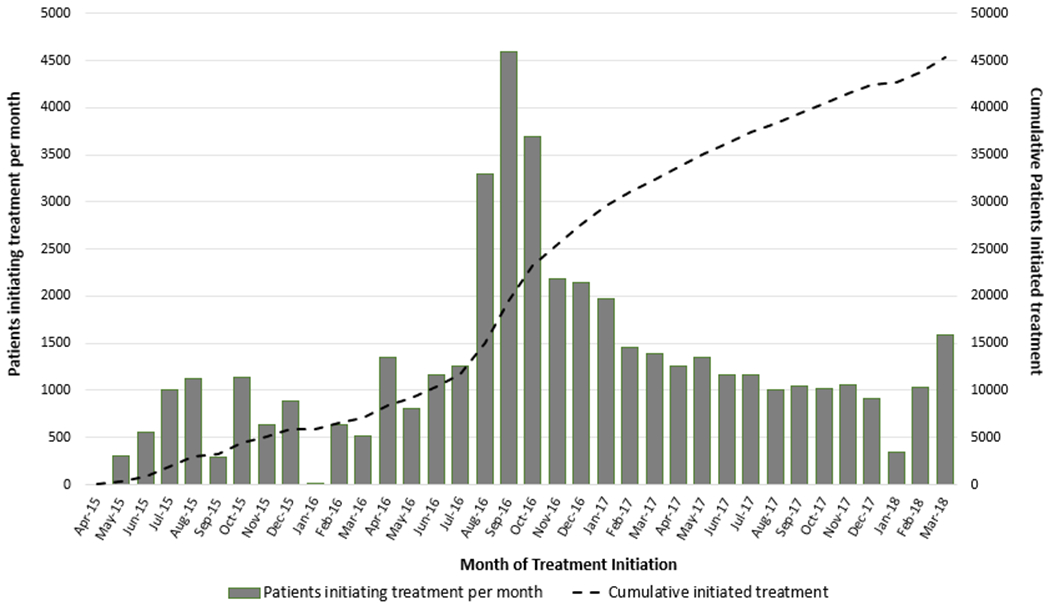
Hepatitis C virus treatment uptake in the country of Georgia, April 2015–March 2018.
Of 45 334 persons initiating treatment, 40 946 (90.3%) completed it and 3052 (6.7%) were still on therapy by the end of March 2018 (Figure 1). Of those who began treatment, 421 (0.9%) died during their treatment, 221 (0.5%) discontinued treatment because of adverse events, and 694 (1.5%) self-discontinued or were lost to follow-up.
Of 40 946 patients who completed treatment, 37 256 (91.0%) persons were eligible for SVR assessment (Figure 1). Of these, 29 620 (79.5%) returned for SVR assessment within 24 weeks following completion of treatment.
Among 29 620 persons assessed for SVR, 29 090, including 512 re-treated persons, achieved a cure, representing 19.4% of the total estimated population with chronic HCV infection. The SVR rate was 98.2% (29 090/29 620) in the per-protocol analysis, and 78.1% (29 090/37 256) in the intent-to-treat analysis. Per-protocol SVR rates were 98.5% (12 781/12 979) for genotype 1, 98.3% (6162/6266) for genotype 2, and 97.7% (9606/9830) for genotype 3. Patients with advanced fibrosis had an SVR of 97.3% (10 209/10 491), compared with 98.7% (18 672/18 915) among those without advanced fibrosis. Initial SOF-based treatment yielded an SVR of 82.1% (4170/5080), whereas initial LDV/SOF treatment resulted in an SVR of 98.4% (24424/24812). Re-treatment with LDV/SOF-based regimens also proved to be effective, with an overall SVR rate of 93.1% (512/550).
Discussion
Georgia has made substantial progress in the path toward eliminating hepatitis C as a major public health threat. Over the first 3 years of the elimination program, >52 000 patients were diagnosed, >45 000 initiated treatment, and 98.2% of those assessed for SVR achieved a cure. Mathematical modeling showed that these efforts already had an impact in terms of reducing prevalence of chronic infection by 21% and total incidence by 19% [14].
Georgia has emerged as a leader in the global fight against HCV infection [15]. To the best of our knowledge, Iceland is the only country that might have already reached the WHO’s service coverage targets for diagnosis and treatment, but the country has a very low burden of hepatitis C, with only 1100 persons estimated to live with the disease [16].
Along with successes, Georgia faces serious challenges on the road to elimination. The modeling showed that achieving elimination by 2020 in Georgia will require treating 4000 persons a month, whereas retaining the current rate of treating around 1000 persons per month will achieve elimination in 2025 [14]. Our analysis shows that treatment uptake has been declining in the country, resulting from the significant gap at the stage of diagnosis. This decline can be attributed to the phenomenon coined as diagnostic burnout, when fewer newly diagnosed persons are available for treatment.
A similar situation has been observed in other countries implementing elimination programs. Australia experienced a 63% decline in treatment uptake, from a peak of 13 109 prescriptions per quarter to 5320 prescriptions per quarter [17]. Egypt treated >2 million people with DAAs, while there are >4 million people with undiagnosed HCV infection [18].
Georgia’s overall success in eliminating hepatitis will depend on the ability to identify 90% of people living with HCV infection. The country is ramping up widescale HCV screening, including door-to-door screening in selected areas of the country, and has already tested close to 1 million persons. However, many at-risk Georgians remain unscreened, underlining the need for targeted services for those at highest risk of HCV infection [19]. Scaling up screening efforts alone is not sufficient if people with positive HCV antibodies are not engaged in care. As shown in our study, among almost 87 000 persons with positive anti-HCV antibodies in Georgia, 24 699 (28.5%) did not get confirmatory testing for chronic HCV infection, which is an unacceptably high dropout rate. Linkage to care has been a significant challenge elsewhere; for example, in Australia, only 47% of antibody-positive persons received HCV RNA testing [20].
Improvements are needed in the subsequent stages of the cascade as well. Overall, 85.8% of diagnosed persons initiated treatment. This compares favorably to recent international reports [21–23], but it also falls short of the national target of treating 95% of diagnosed persons. The treatment completion rate was very high, with a loss to follow-up rate of just 1.5%. A significant loss of patients occurred after completion of treatment when 20.5% of persons eligible for SVR assessment did not return for the final test, despite the fact that SVR testing is free to all. For the time being, only 19% of the estimated number of HCV patients have had documented cure. However, if we assume that SVR rates are same in lost and returning persons, the proportion of cured persons increases to 24%, but this needs to be validated as patients who do not return for final testing may have lower cure rates.
A better understanding of the reasons behind these gaps in the cascade will be essential for achieving the goal. One of the potential barriers is geographic accessibility of HCV services, with almost all service delivery points located in cities and large towns. Acknowledging this issue, the elimination program is bringing services to communities through integrating screening and treatment services within primary healthcare and within harm reduction services for PWID. Decentralization has been shown to be beneficial in international settings and it is expected to improve engagement in the entire HCV care continuum from testing through cure, particularly for vulnerable populations [24–27]. The decentralization process was initiated in May 2018 and after 1 year, integrated models were operational in 10 primary care centers and 4 harm reduction sites with the intention of countrywide expansion so that integrated models are available at least in each district of Georgia.
Another potential barrier is the need for co-payment for diagnostics. In line with a public health approach, diagnostic and treatment monitoring algorithms have been simplified over time to remove viral load monitoring during treatment and to minimize the number of other tests. High cure rates were retained with the simplified approach, while the maximum co-payment significantly decreased by 139%, to $128 per 3-month treatment course. A further decrease in costs can be achieved by removing genotyping testing after the introduction of pan-genotypic treatment with velpatasvir/SOF.
High cure rates achieved within the program are worth mentioning as well. As would be expected, LDV/SOF was highly effective in genotype 1, similar to data from other real-world cohorts [28, 29]. Interestingly, LDV/SOF in combination with RBV was also highly effective in genotype 2 and most challenging in genotype 3, with SVR rates comparable to those shown with newer-generation DAAs [30, 31]. High cure rates observed in the Georgian program underscores the effectiveness of the service delivery model, which relies on simplified modalities that can be successfully replicated in nonspecialty settings, which is important in light of the ongoing decentralization process.
Our analysis has strengths and limitations. The national treatment database, which contains information on all diagnosed persons enrolled in the elimination program, provides accurate treatment-related information on a national level. On the other hand, data available in the national system have limited ability to answer questions as to why people are lost to follow-up along the continuum of care, and thus we were able to conduct only a quantitative analysis to report the country’s progress toward elimination targets. In addition, the national HCV screening and treatment databases are 2 separate systems, which did not allow us to accurately track transition from screening through diagnosis and care. Additional studies are needed to better understand the reasons behind the gaps in the cascade.
In summary, Georgia has an unprecedented opportunity to eliminate hepatitis C as a major public health threat. Closing the gaps in the HCV care continuum, along with implementing prevention interventions, will be critical for achieving this goal. Strong governmental commitment coupled with effective local and international partnerships provide a basis for turning this ambitious goal into reality. If the goal is reached, this will be the first case in the history of medicine when chronic infection is eliminated with medicines and without a vaccine.
Acknowledgments:
The authors gratefully acknowledge Gilead Sciences for donating direct-acting antivirals to the Georgian national hepatitis C elimination program at no cost.
Footnotes
Potential conflicts of interest S. Z. has received honoraria for consultancy and lectures from AbbVie, Gilead, Janssen, Merck Sharp & Dohme, and Intercept. N. A. has received honoraria for consultancies from Merck, Gilead, Echosens, Ligand, and Shionogi. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and not necessarily the official position of the US Centers for Disease Control and Prevention.
References
- 1.Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol 2015; 62:S87–99. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 3.Bulterys M Global progress towards hepatitis elimination—an update In: The Intentional Liver Congress 2019. International Liver Congress, Vienna, Austria, 10–14 April 2019. [Google Scholar]
- 4.CDA Foundation. Polaris observatory. Available at: http://cdafound.org/polaris/. Accessed 20 April 2019.
- 5.Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Lancet Gastroenterology and Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology and Hepatology Commission. Lancet Gastroenterol Hepatol 2019; 4:135–84. [DOI] [PubMed] [Google Scholar]
- 6.Hutin YJ, Bulterys M, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc 2018; 21(Suppl 2):e25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Bank. Georgia country profile. Available at: https://data.worldbank.org/country/georgia. Accessed 29 April 2019.
- 8.Stvilia K, Tsertsvadze T, Sharvadze L, et al. Prevalence of hepatitis C, HIV, and risk behaviors for blood-borne infections: a population-based survey of the adult population of T’bilisi, Republic of Georgia. J Urban Health 2006; 83:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program—Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015; 64:753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and Fibrotest. Hepatology 2007; 46:32–6. [DOI] [PubMed] [Google Scholar]
- 11.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54. [DOI] [PubMed] [Google Scholar]
- 12.Gvinjilia L, Nasrullah M, Sergeenko D, et al. National progress toward hepatitis C elimination—Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:1132–5. [DOI] [PubMed] [Google Scholar]
- 13.Hagan LM, Kasradze A, Salyer SJ, et al. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 2019; 19(Suppl 3):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker J, Gvinjilia L, Nasrullah M, Gamkrelidze A, Morgan J, Vickerman P. Interim evaluation and projected impact of the hepatitis C virus elimination program in Georgia. J Hepatol 2018; 68(Suppl 1):S142. [Google Scholar]
- 15.Pedrana A, Howell J, Schröder S, et al. Eliminating viral hepatitis: the investment case. Doha, Qatar: World Innovation Summit for Health, 2018. [Google Scholar]
- 16.Olafsson S, Fridriksdottir RH, Tyrfingsson T, et al. Iceland may already have reached the WHO 2030 targets for diagnosis and treatment of hepatitis C virus infection: results from the treatment as prevention for hepatitis C (Trap HepC) program. J Hepatol 2019; 70(Suppl 1):e337–8. [Google Scholar]
- 17.Doyle JS, Scott N, Sacks-Davis R, Pedrana AE, Thompson AJ, Hellard ME; Eliminate Hepatitis C Partnership. Treatment access is only the first step to hepatitis C elimination: experience of universal anti-viral treatment access in Australia. Aliment Pharmacol Ther 2019; 49:1223–9. [DOI] [PubMed] [Google Scholar]
- 18.Esmat G, El-Sayed MH, Hassany M, Doss W, Waked I; National Committee for the Control of Viral Hepatitis. One step closer to elimination of hepatitis C in Egypt. Lancet Gastroenterol Hepatol 2018; 3:665. [DOI] [PubMed] [Google Scholar]
- 19.Nasrullah M, Sergeenko D, Gvinjilia L, et al. The role of screening and treatment in national progress toward hepatitis C elimination—Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2017; 66:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. Sydney: Kirby Institute, University of New South Wales, 2018. [Google Scholar]
- 21.Janjua NZ, Kuo M, Yu A, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine 2016; 12:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier MM, Ross DB, Chartier M, Belperio PS, Backus LI. Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. Am J Public Health 2016; 106:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby Institute. Monitoring hepatitis C treatment uptake in Australia (issue 9). Sydney: Kirby Institute, University of New South Wales, 2018. [Google Scholar]
- 24.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364:2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cachay ER, Hill L, Ballard C, et al. Increasing hepatitis C treatment uptake among HIV-infected patients using an HIV primary care model. AIDS Res Ther 2013; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyle C, Kwakwa H, Viner K. Integrating routine HCV testing in primary care: lessons learned from five federally qualified health centers in Philadelphia, Pennsylvania, 2012–2014. Public Health Rep 2016; 131(Suppl 2): 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kattakuzhy S, Gross C, Emmanuel B, et al. ASCEND Providers. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med 2017; 167:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapper EB, Bacon BR, Curry MP, et al. Real-world effectiveness for 12 weeks of ledipasvir-sofosbuvir for genotype 1 hepatitis C: the Trio Health study. J Viral Hepat 2017; 24:22–7. [DOI] [PubMed] [Google Scholar]
- 29.Buggisch P, Wursthorn K, Stoehr A, et al. Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany. PLoS One 2019; 14:e0214795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster GR, Afdhal N, Roberts SK, et al. ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 31.Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018; 378: 354–69. [DOI] [PubMed] [Google Scholar]


