Abstract
For rare cancers, challenges in establishing standard therapies are greater than those for major cancers, and effective methods are needed. MASTER KEY Project is a multicenter study based in Japan, with two main parts: prospective registry study and multiple clinical trials. Advanced rare cancers, cancers of unknown primary origin, and those with rare tissue subtypes of common cancers are targeted. The registry study accumulates highly reliable consecutive data that can be used for future drug development. The multiple trials are conducted simultaneously, targeting either a specific biomarker or a rare tumor type of interest. The first interim data set from the registry part presented here shows the prevalence of genetic abnormalities, response rates, survival rates, and clinical trial enrollment rates. From May 2017 to April 2019, 560 patients (mean age = 53) were enrolled in the project. Frequent cancer types included soft tissue sarcomas, neuroendocrine tumors, and central nervous system tumors. Among the 528 patients with assessable data, 69% (364/528) had next‐generation sequencing tests, with 48% (176/364) harboring an “actionable” alteration. Seventy‐one (13%) patients have been enrolled in one of the clinical trials, with an accrual rate of 3.94 patients/month. A descriptive analysis of biomarker‐directed or non‐biomarker‐directed treatment survival was performed. This project is expected to accelerate development of treatments for rare cancers and show that comprehensive platform trials are an advantageous strategy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Rare cancers are troublesome for translational science and drug development due to the small sample size of each cancer type. A large number of rare cancers never see the light of day, so better ways of attracting attention to them are needed.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study reveals the first interim data from a platform study that includes a registry and multiple clinical trials, which is intended to enroll more patients into clinical trials and at the same time build a large‐scale database for future drug development.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ As a result of this platform trial that included 528 patients, 66% have been referred and specifically screened for one of the ongoing nonrandomized clinical trials, and 13% of patients have been treated with an investigational drug, with a patient accrual rate of 3.94 patients/month.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Platform trials are an effective means of accelerating development of treatments for patients with rare cancers.
With genomic profiling significantly increasing and becoming routine across multiple cancer types, and new clinical trial designs intended to facilitate drug development, there has been great progress in treatment strategies for many cancer types. In the present age of precision medicine, platforms studies such as the NCI‐MATCH have been conducted. 1 Nonetheless, cancer types with low rates of incidence are frequently overlooked, leading to a lack of evidence for establishing standard therapy.
Critical delays in anticancer drug development, especially for rare cancers (annual incidence of < 6 cases per 100,000 population) have been an issue in the past. 2 , 3 , 4 The main reason for the delay in the approval of drugs for rare cancers compared with common cancers is delays in development, and not the review time taken by the healthcare authorities.
The factors in delays in development for rare cancers can be narrowed down to the following:
Few hospitals carry out precise diagnosis and treatment
Molecular backgrounds are not well investigated
Lack of natural history data
Lack of knowledge of other therapeutic options
Patient accrual takes time, with consequent high costs
Randomized trials are impracticable, making it difficult to set high success rates for approval
Pharmaceutical companies are rarely interested in such a small market, although the approaches of the US Orphan Drug Act (United States) and Orphan Drug/Medical Device Designation System (Japan) have been taken to incentivize drug development for rarer indications to some extent
Recognizing the immediate and urgent need for a new approach for rare cancers, in May 2017, Marker Assisted Selective Therapy in Rare Cancers: Knowledge Database Establishing Registry (MASTER KEY) Project: A Platform (basket/umbrella) Trial with a Registry Study for Rare Cancers 5 was launched. We present here the first integrated solid tumor data set from the MASTER KEY Project.
Methods: MASTER KEY Project Overview
Overall objectives
MASTER KEY Project is a platform trial with a registry study, focused on rare cancers. This study was approved by the National Cancer Center Hospital (NCCH) Institutional Review Board. It consists of two main parts: A prospective registry study and multiple clinical trials (Figure 1 ). The primary objective of the project is to collect consecutive data on biomarkers, patient backgrounds, and prognosis from the registry study, to build a large‐scale highly reliable database that can be used for future drug development. The data will also be shared with the participating pharmaceutical companies to help them in the search for new biomarkers and/or cancer types that could be therapeutic targets. Patients in the registry study can be enrolled in a clinical trial depending on the results of the molecular profiling or cancer type. With some exceptions, the clinical trials will mostly be registration‐directed trials.
Figure 1.
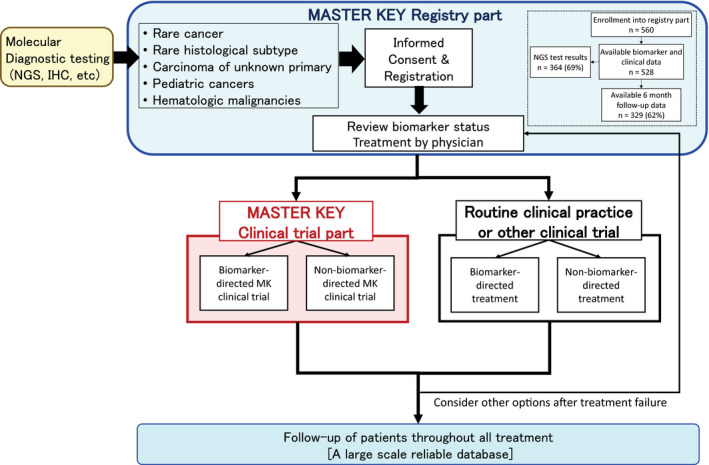
MASTER KEY Project overview. IHC, immunohistochemistry; MK, Master Key; NGS, next‐generation sequencing.
Academia‐industry collaboration
The project is a collaboration between academic institutions and pharmaceutical companies. As of April 2019, four clinical sites—NCCH, Kyoto University Hospital, Hokkaido University Hospital, and Kyushu University Hospital—were taking part, in partnership with 13 pharmaceutical companies.
Study design
Registry study part
In the registry study, all patient information is collected from registry enrollment until death (or loss to follow‐up), whether the patient receives clinical trial treatment inside the MASTER KEY, clinical trial treatment outside the MASTER KEY, or treatment in routine clinical practice (Figure 1 ). Patients with a certain biomarker or cancer type are offered an ongoing clinical trial. However, this is not the case for all patients since an adequate clinical trial is not necessarily open at the time a patient is identified. For instance, some patients may hold a biomarker of interest but there are no ongoing clinical trials available at the time. Nevertheless, these patients, who would then receive routine clinical practice, will be followed in the registry as part of the historical control data. Analysis in the registry includes cancer type‐specific incidence of genetic abnormalities, relationship between cancer types and prognosis, and effects of individual treatments. The purposes of the registry study part are (i) to establish a large‐scale uniform and longitudinal database to help understand the nature of patients with rare cancers based on reliable biomarkers, tumor response, and survival information; (ii) to establish a reliable historical control data for clinical trials; and (iii) to assign enrolled patients efficiently to the clinical trials.
Main inclusion criteria for the registry study part are:
Age 1 year or older
Histological diagnosis of rare cancer defined as one with annual incidence of < 6/100,000 persons, cancer of unknown primary origin, rare tissue subtype of common cancer, or hematologic malignancy
Incurable progressive disease (metastatic and/or unresectable)
Molecular diagnostic testing has been or will be performed
Written consent
Clinical trial part
The MASTER KEY Project includes multiple clinical trials that are ongoing simultaneously. The treatment regimen for each clinical trial addresses a specific molecular profile (biomarker‐directed trial) or a specific cancer type. A non‐biomarker‐directed clinical trial is also available so that a broader number of patients have the chance to be enrolled in a clinical trial. Patients who are ineligible or who do not provide informed consent for any of the ongoing clinical trials at that time will receive routine clinical care and will be followed up with through the registry to seek future clinical trials.
Patients who meet the criteria for one of the clinical trials will be assigned to one. Each clinical trial will be based on a single‐arm study with the primary end point of response rate. More details of trial design and statistical analysis are shown in the Supplementary Text . Notably, due to the scarce population, all clinical trials under the MASTER KEY Project are, in principle, designed as a single‐arm study with a registration‐directed purpose.
Biomarker analysis
Biomarkers identified in the MASTER KEY Project are defined as those that may be potential predictive markers for treatment. Biomarker testing is done outside of this protocol, which includes immunohistochemical staining, fluorescent in situ hybridization, single gene testing, and next‐generation sequencing (NGS). These tests are conducted at each hospital, research center, or laboratory according to the corresponding protocol. Re‐biopsy is always considered when necessary.
The genes detected in NGS testing are then classified according to their actionable levels. Actionable gene aberrations are those predicted to confer sensitivity/resistance to either an approved targeted agent or an experimental targeted agent currently in clinical trials. Evidence levels are added to each gene aberration according to Clinical Practice Guidance for Next Generation Sequencing in Cancer Diagnosis and Treatment 6 using cancer genome knowledge databases, such as CanDL (https://candl.osu.edu/browse), Cancer Genome Interpreter (https://www.cancergenomeinterpreter.org/biomarkers), CIViC (https://civic.genome.wustl.edu/home), and OncoKB (https://www.oncokb.org/).
Organization and individual committee roles
MASTER KEY is implemented under the control of nine main committees/regular meetings. The responsibility of the committees is to promote smooth execution and ensure a rigorous scientific basis, avoiding subjective or biased evaluations of their performance. The detailed roles of each committee/regular meeting are shown in Figure S1 .
Statistical considerations
Using the data collected in the registry study part, we primarily evaluated the prevalence of any genetic abnormality, prevalence of individual genetic abnormalities and actionability classification, response rate for each treatment, overall survival, and progression‐free survival (PFS) in the enrolled patients. These analyses are performed twice a year in coordination with periodic monitoring activities.
Data standardization
To ensure consistency across centers, a data entry manual describing the core data elements and data definitions is provided. For genomic data, to comply with patient consent agreements at each institution and to ensure patient privacy, raw binary alignment map files are not shared within the MASTER KEY Project, but the clinical sequencing platform, date of sequencing, and specimen collection date are specified, enabling researchers to more carefully compare data sets across cancer types. For clinical data, patient‐level data elements have been identified and defined. This comprises a set of clinical data, which includes sex, date of birth, primary cancer diagnosis, prior treatment, response to treatment, and survival. Primary cancer diagnoses are reported using a modified version of the RareCareNet List of rare cancers established by RareCareNet, 7 which is organized into three tiers. The bottom tier is the World Health Organization (WHO) classification name of individual cancer entities which corresponds to an International Classification of Disease for Oncology, Third Edition morphology and topography code.
The data are followed‐up and updated every 6 months.
Ensuring data quality
We anticipated that the data set in the MASTER KEY Project will be used in applications for drug approval by pharmaceutical companies, as well as for research purposes. Therefore, quality assurance is required for the data within. Data cleaning, central monitoring, site visit monitoring, and auditing are taking place in accordance with the standard operating procedure for each process.
Results
Landscape of the first interim integrated MASTER KEY Project registry cohort
From May 2017 to April 2019, 560 patients consented. Among them, 528 could be evaluated for biomarkers, background characteristics, and treatment. Basic patient characteristics are shown in Table 1 . The biomarker and clinical data for the 528 patients are only for NCCH patients, as the first data cutoff was set before data were submitted by the other member institutions. As the MASTER KEY evolves, data from other member institutions as well as biomarker data will accumulate because gene panels will keep evolving with new custom panels being introduced.
Table 1.
Patient characteristics
| N = 560 | |
|---|---|
| Age | |
| Mean (range) | 53 (2–84) |
| Sex | |
| Male | 253 |
| Female | 307 |
| PS | |
| 0 | 313 |
| 1 | 194 |
| 2 | 14 |
| 3 | 2 |
| 4 | 1 |
| Unknown | 36 |
| Relapse or unresectable | |
| Relapse after curative treatment | 285 |
| Unresectable at diagnosis | 241 |
| Other | 4 |
| Unknown | 30 |
| Previous chemotherapy lines | |
| Median (range) | 2 (0–10) |
PS, performance status.
Cancer type overview
The spectrum of cancer types across the registry study and the primary lesions are shown in Figure 2a and Table S1 . The most common cancer type was soft tissue sarcoma (n = 174). Soft tissue sarcoma consists of more than 30 pathological subtypes, making it difficult to conduct trials specifically for a certain subtype. Of note, glial tumors of the central nervous system ranked third. This indicates the need for promoting drug development in this area as surgery, not pharmacotherapy, is the main treatment for glial tumors, which has limited survival benefit for many patients. Rare pathological subtypes of common cancers, such as those of breast, stomach, colon, pancreas, and lung cancers, were also included since such rare subtypes behave differently from common pathological types, and therefore tend to respond differently to drugs.
Figure 2.
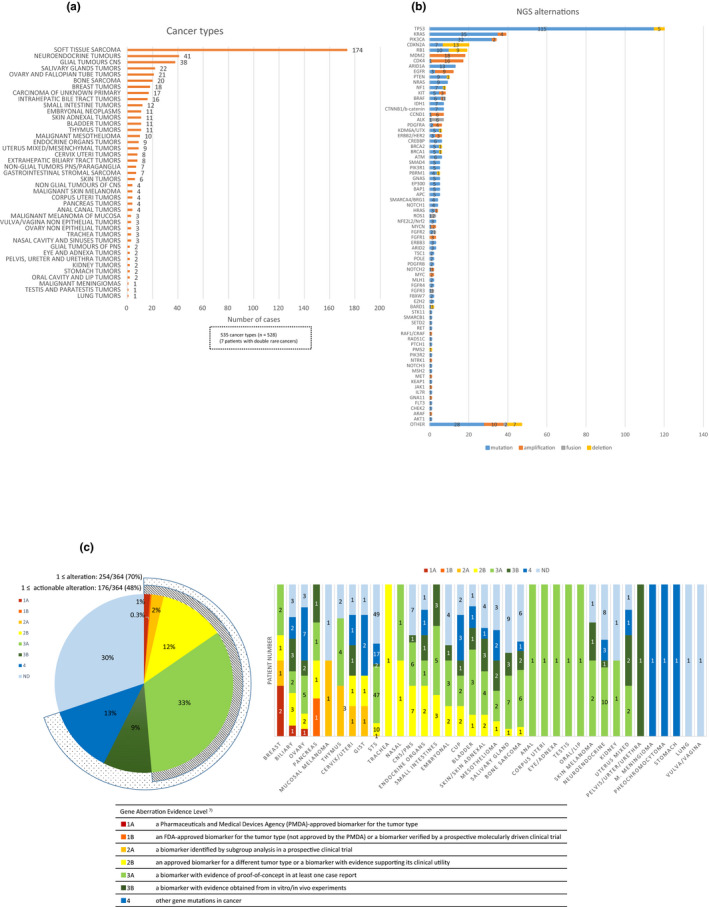
Cancer types and gene alterations in NGS testing. (a) Number of enrolled patients by cancer type (n = 528). (b) Overall alterations in the 364 patients in NGS testing. Mutations, amplifications, fusions, and deletions are distinguished by color. (c) Genes classified according to potential clinical “actionability” by level of evidence. CNS, central nervous system; NGS, next‐generation sequencing; PNS, peripheral nervous system.
Seven patients had double rare cancers. Their clinical features are shown in Table S2 . Three patients had a concurrent neuroendocrine tumor. Two patients had double cancers, arising from the ovary and uteri, respectively, which had different histologies. All except one patient had no relapse or only local relapse for the second cancer. Therefore, NGS testing was conducted only for the more aggressive cancer types needing systemic therapy. One patient with the double ovary and uterine cancer showed PMS2 "protein" loss in immunohistochemistry (IHC), suggesting a relationship with Lynch Syndrome.
Biomarker overview: Assessing clinical actionability
Biomarker data within the MASTER KEY Project include mutation, copy number, rearrangement, protein expression, and chromosome defect data. Although NGS approaches are currently the mainstream, we must not forget that IHC staining is still the most cost effective, simple means of identifying the features of a cancer. IHC staining to identify biomarkers that might lead to treatment options was carried out for half of the patients (n = 269), and the results of NGS tests were available for 364 patients (69%) (Table S3 ). The results of the gene analysis are shown in Figure 2b . NGS platforms included NCC Oncopanel (National Cancer Center, Tokyo, Japan), 8 FoundationOne CDx (Foundation Medicine, Cambridge, MA), Guardant360 (Guardant Health, Redwood City, CA), and Oncomine Cancer Research Panel (Thermo Fisher Scientific, Waltham, MA). Tumor mutation burden (TMB) results were obtained when possible, and are shown in Figure S2 . The results are limited because for some cancers, TMB was only reported for one patient. However, we found that central/peripheral nervous system tumors had a relatively high TMB. A total of 48% of patients with NGS results had a Level 1 to 3A alteration indicative of treatment with either a US Food and Drug Administration (FDA) or Pharmaceuticals and Medical Devices Agency (PMDA)‐approved drug or a drug with supporting evidence showing clinical utility towards the alteration. 6 These “druggable” alterations correspond to evidence levels A‐C in the guidelines published by the Association for Molecular Pathology, American College of Medical Genetics (ACMG), American Society of Clinical Oncology, and College of American Pathologists 9 as shown in Figure 2c . The frequencies of druggable alterations varied widely across cancer type, from those in rare subtypes of breast cancer, which showed high frequency, followed by those in biliary tract tumors and ovarian cancer, to tumor types with few actionable alterations, such as neuroendocrine tumors, bone sarcomas, and soft tissue sarcomas.
Treatment decisions: Clinical trials within MASTER KEY
The treatment actions that were taken after enrollment into MASTER KEY are summarized in Table 2 . The majority of patients had some kind of pharmacotherapy treatment, while others had radiotherapy alone, surgery, or transplantation. Cancers of the CNS were more likely to be treated with radiotherapy and surgery, as opposed to pharmacotherapy due to the lack of pharmacotherapy options.
Table 2.
Treatments after registry study enrollment among patients with 6‐month follow‐up data
| N = 329 | |
|---|---|
| Clinical trial (all pharmacotherapy) | 64 a |
| Routine clinical practice | |
| Pharmacotherapy | 148 a |
| Radiotherapy | 51 a |
| Surgery | 17 a |
| Chemoradiotherapy | 6 |
| Bone marrow (auto) transplantation | 1 |
Includes more than one treatment per patient.
Recent commentaries have questioned the clinical utility of matching patients to drugs based on tumor molecular profiling, 10 , 11 , 12 largely based on the low frequency of patients matched to current targeted therapy trials and a lack of data from clinical trials assessing the added benefit of molecular profiling. This statement could be emphasized for rare cancers, as patients in this population have less access to clinical trials or appropriate treatment. Nonetheless, patients in this study were able to receive a targeted therapy according to their biomarker as shown in Table 3 . Ten sarcoma patients received a biomarker‐directed treatment.
Table 3.
Details of pharmacotherapy regimens in patients with 6‐month follow‐up data
| Target biomarker | Cancer type | Treatment | n = 212 |
|---|---|---|---|
| Biomarker‐directed regimens | 32 | ||
| ALK | |||
|
CLTC/ALK Fusion CTNNA1/ALK Fusion ITSN2/ALK Fusion EML4/ALK Fusion |
STS Salivary gland carcinoma Bone sarcoma Cholangiocarcinoma |
ALK TKI ALK TKI ALK TKI ALK TKI |
2 1 1 1 |
| BRAF | |||
| BRAF V600E | Nonglial tumor of CNS, STS | BRAF inhibitor + MEK inhibitor | 2 |
| Cell cycle gene | |||
|
CDK4 amplification CCND1 amplification CDKN2A homozygous deletion |
STS Breast carcinoma STS |
CDK4/6 inhibitor CDK4/6 inhibitor CDK4/6 inhibitor + hormone therapy |
1 1 1 |
| FGFR | |||
|
FGFR2/CRIP1 Fusion FGFR2 rearrangement (FISH) |
Skin carcinoma Cholangiocarcinoma |
FGFR inhibitor FGFR inhibitor |
1 1 |
| HER2 | |||
| HER2 amplification | Breast carcinoma | HER2 inhibitor + Capecitabine | 1 |
| HER2 amplification | Breast carcinoma | T‐DM1 | 1 |
| HER2 amplification | Breast carcinoma | HER2 inhibitor | 1 |
| HER3 | |||
| HER3 protein overexpression | Breast carcinoma | HER3 inhibitor | 1 |
| Homologous recombination deficiency (HRD) | |||
|
BRCA1 Q1721X BRCA2 E1299X |
Breast carcinoma Breast carcinoma |
PARP inhibitor PARP inhibitor |
1 1 |
| IDH | |||
| IDH1 R132H | Glial tumor of CNS | IDH inhibitor | 1 |
| KIT | |||
| KIT V559A | Thymus carcinoma | Multi‐TKI | 1 |
| MDM2 | |||
| MDM2 amplification | STS | MDM2 inhibitor | 3 |
| Mismatch repair deficiency | |||
| Loss in either of following four proteins: MLH1/MSH2/MSH6/PMS2 |
Skin carcinoma STS Glial tumor of CNS Uterine cancer |
PD‐1 inhibitor PD‐1 inhibitor PD‐1 inhibitor PD‐1 inhibitor |
1 1 1 1 |
| ROS1 | |||
| GOPC/ROS1 Fusions | Small bowel carcinoma | Selective TKI | 1 |
| ER | |||
| ER protein overexpression | Breast carcinoma | Hormone therapy | 1 |
| PDGFR | |||
| PDGFB rearrangement (FISH) | STS | Multi‐TKI | 2 |
| Other | |||
| (not identified) | Paraganglioma | Pan‐ERBB inhibitor | 1 |
| Non‐biomarker‐directed regimens | 180 | ||
|
1. STS 2. Salivary gland carcinoma, etc. |
PD‐1 inhibitor | 30 | |
|
1. STS 2. Glial tumor of CNS |
Eribulin Mesilate | 21 | |
|
1. Salivary gland carcinoma 2. Nonglial tumors, etc. |
Platinum containing | 18 | |
|
1. STS 2. Breast carcinoma, etc. |
Doxorubicin Hydrochloride containing | 12 | |
| 1. STS | Pazopanib Hydrochloride | 13 | |
| 1. STS | Trabectedin | 10 | |
|
1. Glial tumor of CNS 2. STS |
Temozolomide | 9 | |
|
1. STS 2. Bladder carcinoma, etc. |
Taxane containing | 7 | |
|
1. Salivary gland carcinoma 2. Thymus carcinoma, etc. |
TS‐1 | 6 | |
| 1. Glial tumor of CNS | Bevacizumab | 5 | |
|
1. STS 2. Melanoma of mucosa |
Dacarbazine | 5 | |
| 1. STS | Ifosfamide containing | 5 | |
|
1. STS 2. CUP |
Irinotecan Hydrochloride Hydrate containing | 5 | |
|
1. Bladder carcinoma 2. STS, etc. |
Cell cycle gene inhibitor | 3 | |
|
1. Breast carcinoma 2. Vulva carcinoma, etc. |
Vinca alkaloid containing | 4 | |
| 1. Breast carcinoma | Capecitabine | 3 | |
|
1. Breast carcinoma 2. Bladder carcinoma, etc. |
Gemcitabine Hydrochloride | 3 | |
|
1. Cholangiocarcinoma 2. CUP, etc. |
PD‐L1 inhibitor | 3 | |
|
1. Small bowel carcinoma 2. CUP |
Fluorouracil containing | 2 | |
| 1. STS | Cyclophosphamide Hydrate containing | 2 | |
|
1. GIST 2. CUP |
Multi TKI | 2 | |
| 1. Neuroendocrine tumor | Amrubicin Hydrochloride | 1 | |
| 1. Bladder carcinoma | ATR inihibitor | 1 | |
| 1. Bone sarcoma | Everolimus + Multi TKI | 1 | |
| 1. Nonglial tumor | FGFR inhibitor | 1 | |
| 1. Bladder carcinoma | MDM2 inhibitor | 1 | |
| 1. STS | MET inhibitor | 1 | |
| 1. CUP | mTOR inhibitor | 1 | |
| 1. Neuroendocrine tumor | Octreotide Acetate | 1 | |
| 1. Bladder carcinoma | PARP inhibitor | 1 | |
| 1. Embryonal neoplasm | Regorafenib Hydrate | 1 | |
| 1. STS | Tissue factor inhibitor | 1 | |
| 1. CUP | Trifluridine/Tipiracil Hydrochloride | 1 | |
CNS, central nervous system; CUP, carcinoma of unknown primary; FGFR, fibroblast growth factor receptor; FISH, fluorescent in situ hybridization; GIST, gastrointestinal stromal sarcoma; STS, soft tissue sarcoma; T‐DM1, trastuzumab emtansine; TKI, tyrosine kinase inhibitor.
MASTER KEY had eight ongoing clinical trials, of which seven are registration‐directed as of April 2019 (Table 4 ), with a few more in process to start in late 2019. The trials not only include biomarker‐directed therapy trials, but also those for a certain histological cancer type, and those for patients with no specific biomarker. Thus, some trials provide a treatment option for those for whom no standard treatment is available or those with no significant biomarker, who would otherwise have no treatment option.
Table 4.
Clinical trials inside MASTER KEY Project, number of patients enrolled in each trial from the project, and accrual time
| Sponsor | Target population | Study drug | Progress | Open date | Trial ID | Accrual from MK (n) a | Accrual time (mo) b |
|---|---|---|---|---|---|---|---|
| Pharma | BRAF V6 00E | Dabrafenib + Trametinib | Active, not recruiting | 2017/11 |
JapicCTI‐173743 |
5 | 8 |
| NCCH | dMMR/MSI‐high | Nivolumab | Ongoing | 2018/4 | JMA‐IIA00344 | 4 | 13 |
| NCCH | All rare cancers | Nivolumab | Ongoing | 2018/4 | JMA‐IIA00345 | 48 | 13 |
| NCCH | HER2 Carcinosarcoma | DS‐8201a | Ongoing | 2018/1 | UMIN000029506 | 5 | 16 |
| NCCH | ALK | Alectinib | Ongoing | 2018/7 | JMA‐IIA00364 | 3 | 10 |
| Pharma | Malignant mesothelioma | Ad‐SGE‐REIC | Ongoing | 2018/8 | JapicCTI‐184040 | Not open to public | |
| Pharma | Adenoid cystic carcinoma | Liposomal Eribulin | Active, not recruiting | 2018/8 | JapicCTI‐173649 | 3 | 5 |
| NCCH | MDM2 intimal sarcoma | DS‐3032b | Ongoing | 2018/12 | JMA‐IIA00402 | 3 | 5 |
| Biomarker A | Drug A | In preparation | TBA | ||||
| Disease B | Drug B | In preparation | TBA | ||||
| Disease C | Drug C | In preparation | TBA | ||||
| Biomarker D | Drug D | In preparation | TBA | ||||
| Total number of patients enrolled in a MK clinical trial | 71/528 (13%) | ||||||
MK, Master Key; NCCH, National Cancer Center Hospital; Pharma, pharmaceutical industry; TBA, to be announced.
Number of patients enrolled in the clinical trial from MASTER KEY registry study.
The accrual period from the start of each clinical trial until the cutoff of date of April 2019.
Enrolling patients into clinical trials in a timely manner
Within MASTER KEY Project, 350 patients (66%) have been referred and specifically screened for one of the ongoing clinical trials in a period of 18 months from the launch of the first MASTER KEY clinical trial. Among the screened patients, 71 (13%) have been treated with an investigational drug, which is attractive for patients that lack other treatment options (Table 4 ). The accrual time for each trial is shown in the far right column of Table 4 , from which a patient accrual rate of 3.94 patients/month has been estimated.
Clinical benefit
Among the patients who had a follow‐up of at least 6 months, the median PFS in the overall population, defined as the time from the start of a pharmacotherapy to disease progression or death from any cause, was estimated at 4.34 months (Figure 3a ). We displayed the two Kaplan‐Meier curves in patients who were treated within a clinical trial and those who were treated as routine clinical practice (Figure 3b ). The PFS in patients who were treated with a biomarker‐directed therapy (“matched” therapy) and those treated with a non‐biomarker‐directed therapy are also shown in Figure 3c . This population consists of the 32/212 (15%) patients who were treated with a “matched” drug, which included ALK inhibitors, BRAF inhibitors, immune‐checkpoint inhibitors, HER2 inhibitors, and fibroblast growth factor receptor inhibitors (Table 3 ).
Figure 3.
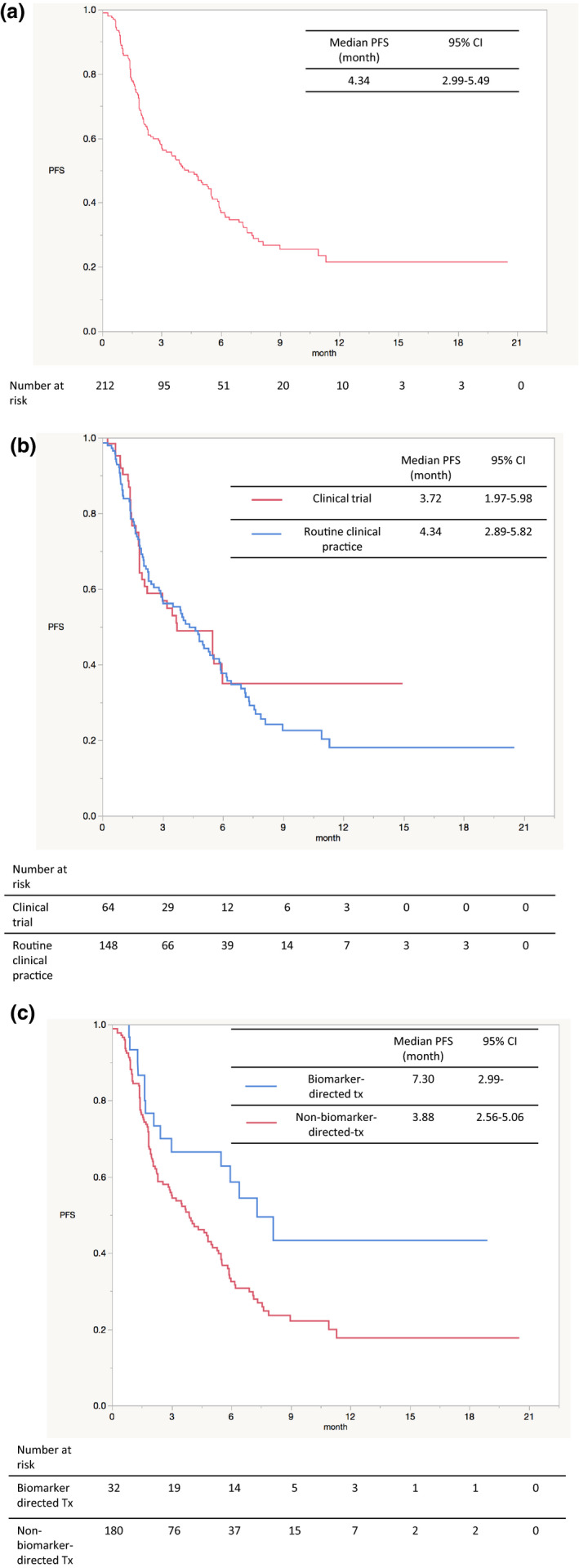
Kaplan‐Meier survival curves for progression‐free survival are plotted for (a) overall population with at least 6 months of follow‐up, (b) those receiving clinical trial therapy and routine clinical practice therapy, (c) those receiving biomarker‐directed therapy and non‐biomarker‐directed therapy. CI, confidence interval; CNS, central nervous system; CUP, carcinoma of unknown primary; FDA, US Food and Drug Administration; M. MENINGIOMA, malignant meningioma; PFS, progression‐free survival; PNS, peripheral nervous system.
The overall response rate was reported for patients who had undergone diagnostic imaging testing and tumor assessment at the time of data cutoff. The results are shown in Table S4 . The patients receiving biomarker‐directed therapy showed a response rate of 40.0% and a median duration of response of 164.5 days, while the patients receiving non‐biomarker‐directed therapy showed a response rate of 9.8% and a median duration of response of 84 days.
Discussion
Comparison with other platform studies
MASTER KEY Project is the first platform trial focused only on rare cancers with a quality controlled registry study that includes extensive biomarker and clinical data. An Australian group has initiated a similar platform for identifying molecular changes of therapeutic relevance using a master protocol, 13 but the trials based on it focus more on multiple treatment arms rather than on quality controlled registry data.
Also, in MASTER KEY, most of the clinical trials are conducted as registration directed. Although the intention of many other basket or umbrella trials, among them the NCI‐MATCH 1 and MoST trial, 13 has been signal finding, trials conducted under the MASTER KEY are all for small populations, making it difficult to conduct subsequent later‐phase trials. Therefore, clinical trials under the MASTER KEY are directly aimed at obtaining PMDA approval and national health reimbursement under Japan’s “Conditional Early Approval System.” 14
In addition, quality‐assured clinical data from the ongoing registry study is being expeditiously accumulated, which includes data from both clinical trials and routine clinical practice. This makes the MASTER KEY registry unique compared with other basket/umbrella trials, and its utilization for future drug development, including new drug applications, is anticipated.
It should be noted that the limitations on the comparison of outcomes between treatment groups shown in Figure 3b , c , include confounding between prognostic and predictive biomarkers, patient selection bias, and concerns regarding nonconcurrent historical controls, such as stage migration and improved standard of care, which lead to progressive improvements in the control arms over time. To address these issues, it is possibly useful to use informational design, that is, a study design in which a subpopulation of the overall patients are randomly enrolled into an informational cohort to provide randomized controlled information to evaluate the treatment effect at the end of the trial. 15
Lessons learned and future challenges
Patients with rare cancers from all over Japan are now seeking new treatment options. According to an earlier study, 16 when patients were offered the chance to participate in a cancer clinical trial, 75% assented. However, even in the United States, only 3–5% of adult cancer patients are currently enrolled in clinical trials, suggesting that both medical staff and patients lack the information needed for enrollment into a clinical trial. This is similar in Japan, and a low patient participation rate is more evident for patients with rare cancers. MASTER KEY has the advantage for both clinicians and patients of allowing them to be constantly aware of ongoing clinical trials. The activities of the committees within the project, especially those of the Case Review Committee, have raised the awareness of clinicians, enabling the clinical trial accrual rate in MASTER KEY to be as high as 13%.
Collaboration with “Rare Cancer Japan,” 17 a patient advocacy group, has enhanced our awareness of the critical need for the development of treatments for rare cancers. Patient advocacy is not only important to patients, it is also of great importance during the entire clinical trial process as well as to all involved in it. This can help patients, patient advocates, and other stakeholders gain insights into the best ways of informing patients of clinical trials, and what steps to take in finding the "right one."
Although 176/364 (48%) patients had an “actionable” biomarker based on the results of an NGS test, this did not lead to the majority of patients being given a “matched” drug. There are several possible reasons for this: (i) The NGS platform in current widespread use may not be suitable for patients with rare cancers, (ii) investigational drugs for rare cancers are limited in number, or (iii) patients had progressive disease at the time of testing and were not eligible for further treatment. In the NGS results in our study, the actionable alteration rate was slightly lower than in recent studies including common cancers, in which 59.4% (111/187) of patients harbored actionable alterations. 8 At NCCH, an NGS platform specially indicated for sarcomas and pediatric patients is being developed. Also, for rare cancers, we realized that biomarker modalities other than NGS, such as IHC, can play a crucial role in determining drug options.
We anticipate that other benefits of MASTER KEY will be its increased ability to delineate the clinical significance of somatic mutations (particularly regarding new indications for approved drugs) and to perform the data‐driven selection of high‐yield tumors likely to contain actionable mutations for clinical trials. Therefore, since the data collected in MASTER KEY should be of great value to stakeholders worldwide, we are seeking collaborations with other organizations in Asia and groups participating in the International Rare Cancers Initiative.
Regarding short‐term goals, enrolling patients and finishing the ongoing clinical trials to enable new drugs to be approved with the support of high‐quality registry data will provide patients with the personalized care they need. The inclusion of Kyoto University Hospital, Kyushu University Hospital, Hokkaido University Hospital, and other institutions in 2020 will further accelerate the patient accrual rate. An academic‐industry‐government partnership will help ensure the success of such studies as MASTER KEY.
Conclusions
The results of this study suggest that patients with rare cancers could be involved in clinical trials more effectively through platform studies such as MASTER KEY Project, which would lead to accelerated development of treatments for this patient population. They also show that biomarker testing is an essential aspect of the treatment of rare cancers. We will continue this project with the aim of bringing new drugs to patients as early as possible.
Funding
This project was funded by the following collaborative companies (in alphabetical order): Astellas Pharma Inc., Bayer Yakuhin, Ltd, Bristol‐Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd., Eisai Co. Ltd., Kyorin Pharmaceutical Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. The project was also partly funded by the Japan Agency for Medical Research and Development "Project of Translational and Clinical Research Core Centers” (17lk1503003j0001, 18lk1503003j0002, 19lk1503003j0003).
Conflict of Interest
All authors report grants for the institute from multiple companies participating in this project during the conduct of the study. Outside of the present study, the following authors report grants and/or other payments: A.H. reports grants and personal fees from Ono Pharmaceutical; personal fees from Astellas Pharma, Abbvie, Nippon Boehringer Ingelheim, Kissei Pharmaceutical, Pfizer, and Nippon Shinyaku. A.K. reports personal fees from Eli Lilly, Taiho Pharmaceutical, Novartis, Takara, and Eisai. K.Y. reports personal fees from Eisai, Novartis, Taiho Pharmaceutical, Pfizer, and AstraZeneca; grants and personal fees from Ono Pharmaceutical; and grants from Daiichi Sankyo. K.N. reports personal fees from Chugai Pharmaceutical, Taiho Pharmaceutical, and Bayer. T.N. reports grants from Pfizer, other payments from Novartis, Bayer, Eisai, and Taiho Pharmaceutical. T.Shim. reports grants from Novartis, Eli Lilly, Daiichi Sankyo, Bristol‐Myers Squibb, Eisai, AbbVie, AstraZeneca, Takeda Oncology, Incyte, Chordia Therapeutics, 3D‐Medicine, from Symbio Pharmaceuticals, PharmaMar, Five Prime, and Astellas Pharma; and personal fees from Taiho Pharmaceutical, Boehringer Ingelheim, Chugai Pharmaceuticals, and Ono Pharmaceutical. N.Y. reports grants from Chugai Pharmaceutical, Taiho Pharmaceutical, Eisai, Eli Lilly, Quintiles, Astellas Pharma, Bristol‐Myers Squibb, Novartis, Daiichi Sankyo, Pfizer, Boehringer Ingelheim, Kyowa‐Hakko Kirin, Bayer, Ono Pharmaceutical, Janssen Pharma, MSD, Merck, and Takeda; and personal fees from Ono Pharmaceutical, Chugai Pharmaceutical, AstraZeneca, Pfizer, Eli Lilly, Bristol‐Myers Squibb, Eisai, Otsuka, Takeda, Boehringer Ingelheim, Cimic, and Sysmex. Y.F. reports grants and other payments from Japan Agency for Medical Research and Development, grants and other payments from The Ministry of Health Labor and Welfare of Japan, during the conduct of the study; and other payments from AstraZeneca, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical, Novartis Pharma, SRL Inc, and Bristol‐Myers Squibb and contributed to the study until March 2019. All other authors declared no competing interest for this work.
Author Contributions
H.S.O., Y.F., K.Y., K.N., A.H., and T.Shib. wrote the manuscript. H.S.O., Y.F., K.Y., K.N., A.H., and T.Shib. designed the research. H.S.O., Y.F., K.Y., T.Shim., A.K., T.N., T.Su., N.Y., and K.N. performed the research. H.S.O., S.N.N., A.H., and T.Shib. analyzed the data.
Supporting information
Acknowledgments
We thank all the patients who agreed to take part in the project, the members of the Rare Cancers Japan (Patient advocacy), the members of the Rare Cancer Center of National Cancer Center Hospital, Tokyo, Japan, and all of the investigators and study teams from the participating institutions: NCCH, Kyoto University Hospital, Hokkaido University Hospital, and Kyushu University Hospital. We also thank the participating companies who funded the project.
References
- 1. National Cancer Institute: Molecular Analysis for Therapy Choice (NCIMATCH <https://www.cancer.gov/about‐cancer/treatment/clinical‐trials/nci‐supported/nci‐match>. Accessed July 31, 2019.
- 2. Fujiwara, Y. & Ono, S. Regulatory review of new therapeutic agents. N. Engl. J. Med. 376, 2598 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Yonemori, K. et al The notorious "drug lag" for oncology drugs in Japan. Investig. New Drug 29, 706–712 (2011). [DOI] [PubMed] [Google Scholar]
- 4. Yamashita, K. , Kaneko, M. & Narukawa, M. A significant anticancer drug approval lag between Japan and the United States still exists for minor cancers. Clin. Pharmacol. Ther. 105, 153–160 (2019). [DOI] [PubMed] [Google Scholar]
- 5. Okuma, H.S. & Fujiwara, Y. Have we found the key to unravel treatment development lags for rare cancers? MASTER KEY Project. Clin. Pharmacol. Ther. 106, 491–492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sunami, K. et al Clinical practice guidance for next‐generation sequencing in cancer diagnosis and treatment (Edition 1.0). Cancer Sci. 109, 2980–2985 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. RARECARENet <http://www.rarecarenet.eu/>. Accessed July 31, 2019. [Google Scholar]
- 8. Sunami, K. et al Feasibility and utility of a panel testing for 114 cancer‐associated genes in a clinical setting: a hospital‐based study. Cancer Sci. 110, 1480–1490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li, M.M. et al Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 19, 4–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tannock, I.F. & Hickman, J.A. Limits to personalized cancer medicine. N. Engl. J. Med. 375, 1289–1294 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Prasad, V. Perspective: the precision‐oncology illusion. Nature 537, S63 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Joyner, M.J. , Paneth, N. & Ioannidis, J.P.A. What happens when underperforming big ideas in research become entrenched? JAMA 316, 1355–1356 (2016). [DOI] [PubMed] [Google Scholar]
- 13. Thavaneswaran, S. et al Cancer molecular screening and therapeutics (MoST): a framework for multiple, parallel signal‐seeking studies of targeted therapies for rare and neglected cancers. Med. J. Aust. 209, 354–355 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Pharmaceuticals and Medical Devices Agency <https://www.pmda.go.jp/english/>. Accessed July 24, 2019
- 15. Chen, C. , Li, N. , Shentu, Y. , Pang, L. & Beckman, R.A. Adaptive informational design of confirmatory phase III trials with an uncertain biomarker effect to improve the probability of success. Stat. Biopharm. Res. 8, 237–247 (2016). [Google Scholar]
- 16. Albrecht, T.L. et al Influence of clinical communication on patients' decision making on participation in clinical trials. J. Clin. Oncol. 26, 2666–2673 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rare Cancers Japan <https://www.rarecancersjapan.org/en/>. Accessed July 31, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


