Abstract
Purpose:
The purpose of this study was to determine the impact of food on gastric pH and the ability of over the counter betaine hydrochloride (BHCl) acid to reacidify gastric pH after food-induced elevations in gastric pH.
Methods:
This open-label cross over clinical study (NCT02758015) included 9 subjects who were randomly assigned to one of 16 possible, 4-period cross-over sequences to determine the impact and relationship of food and gastric pH with acid supplementation. Subjects were administered various doses (1500mg, 3000mg and 4500mg) of betaine hydrochloride (BHCl) to determine the ability of acid supplementation to reacidify gastric pH after the elevation of gastric pH caused by the ingestion of food.
Results:
Following the administration of food and the resulting elevation in gastric pH, time to return to baseline gastric pH levels without acid supplementation was 49.7 ± 14.0 minutes. Administering 4500mg of BHCl acid in capsules was able to reacidify gastric pH levels back to baseline following the administration of food in approximately 17.3 ± 5.9 minutes. AUCpH of each treatment were similar and not statistically different. Mean max pH following the administration of food was 3.20 ± 0.55.
Conclusion:
The ability of food to elevate and maintain gastric pH levels in the presence of acid supplementation was made evident throughout the study. A 4500mg dose of BHCl was required to reacidify gastric pH after the administration of food. This study details the difficulty faced by clinicians in dosing a poorly soluble, weakly basic drug to patients receiving acid reducing agents where administration with food is recommended to avoid gastric side effects.
Trial Registration:
Keywords: betaine hydrochloride, meal effects, gastric pH, gastric reacidification
INTRODUCTION
The impact of drug solubility and dissolution is important when considering the absorption of orally administered drugs. Absorption is influenced by both a combination of physicochemical properties of the drugs in question [distribution-coefficient or partition coefficient (log P), native ionic state, and acid dissociation constant (pKa)] and also physiological factors of the gastrointestinal tract.1–3 Gastric pH, a physiological factor, plays an essential role in determining the appropriate physiological parameters facilitating solubility and absorption.3 The ability of varying levels of gastric pH to have an effect on drug absorption and systemic bioavailability has been well investigated previously.1,3–8 Weakly basic drugs that have a pH-dependent solubility profile and are orally administered are most vulnerable to varying gastric pH levels. When the gastric pH levels rise above the pKa of weakly basic drugs then pH-based interactions, particularly regarding drug-solubility, are emphasized. These pH-based interactions can have clinically meaningful implications resulting in toxicity or reduced therapeutic efficacy. Gastric pH levels are often elevated by commonly prescribed medications, acid-reducing agents (ARAs), which are generally prescribed for gastrointestinal diseases. According to a report, The Burden of Digestive Diseases in the United States, released by the National Institute of Health (NIH) in 2008,9 there were over 60-million annual prescriptions filled at pharmacies in the United States in 2004 for the treatment of gastroesophageal reflux disease (GERD). Proton pump inhibitors (PPIs), such as omeprazole and H2-receptor antagonists (H2-RAs), such as ranitidine, are the most common classes of ARAs used for GERD. Furthermore, it has been estimated that in the United States PPI’s have been the preferred treatment for greater than 50% of digestive disorders, which amounts to more than $11 billion dollars annually10 and results in ARAs being the most widely prescribed medication in North America.11 The risk of polypharmacy is abundant and it is important for the medical community to address and mitigate these risks.
Prior data in 2013 by Yago et al. successfully demonstrated the ability of betaine hydrochloride (BHCl) to transiently and rapidly re-acidify gastric pH in healthy volunteers pretreated with rabeprazole to induce hypochlorhydria.1 BHCl is an over the counter (OTC) nutraceutical that is used as a digestive aid, and has clinical efficacy in homocystinuria.1 With a pKa of 1.83,12 BHCl dissociates to a large extent to give zwitterionic betaine, protons and chloride ions, with virtually complete dissociation being observed up to millimolar BHCl concentration levels in water12, thus acidifying gastric fluid. BHCl was observed to rapidly and temporarily increase gastric acidity in subjects with drug-induced hypochlorhydria (pH >4).1 The mean onset of reacidification (pH <3) was approximately 6.3 minutes and the decrease in pH was transient, with pH < 3 lasting for only about 73 minutes. These findings led to a successful follow-up study demonstrating the ability of a single dose of 1500mg of BHCl to fully restore the lost Cmax and AUC0-∞ of dasatinib in healthy fasting volunteers after dosing with rabeprazole.5
Based on findings from that study, the utilization of BHCl was explored, to regain lost bioavailability of atazanavir (ATV) in healthy volunteers pretreated with rabeprazole under fed conditions.6 In that study, Faber et al. demonstrated statistically significant reductions in Cmax and AUC of ATV when healthy subjects were pretreated with rabeprazole, as was expected. However, the administration of 1500mg BHCl under fed conditions in healthy volunteers only restored 13% and 12% of ATV Cmax and AUC, respectively. These increases were not statistically significant. We noted that the inability to recover lost bioavailability due to rabeprazole-induced achlorhydria was multi-factorial. One reason being that ATV was dosed with ritonavir (RTV), a cytochrome P450 enzyme inhibitor that serves to boost or increase serum concentrations of ATV by preventing its metabolism. RTV is also affected by gastric pH changes and we postulated that ATV was not appropriately boosted due to the decrease in RTV bioavailability. Another factor further complicating this study was the administration of food prior to ATV and RTV dosing. This was done to replicate clinical settings in which food is recommended in the label to be administered with ATV and RTV to decrease GI toxicity. Based on a review of previous data from the Benet Lab1,5,6, increases in gastric pH when fasting subjects were not taking concomitant ARAs and are fed were observed. Gastric pH levels rise from baseline values between 0.5 and 1 and increase to a pH plateau between 2 and 3. Conversely, when fasting subjects are fed a standardized meal in the presence of ARA induced achlorhydria (baseline pH > 4), their pH decreases and plateaus at a pH between 2 and 3. These observations demonstrate the complexity of the gastric pH effect of food. The dose of BHCl used in Faber et al.’s study (1500mg) was unable to overcome the gastric effects of a meal and reacidify gastric pH in the setting of ARA induced achlorhydria. The appropriate dose of BHCl to overcome this gastric pH-food effect is of interest and could be clinically significant information for the medical community in dosing basic drugs in GERD patients.
The aim of the study was to characterize the effect of a standardized meal on fasting stomach pH as well as the time course and potency of increasing doses (1500mg, 3000mg, 4500mg) of betaine hydrochloride on gastric acidity in fed healthy volunteers. The hypotheses were that the higher doses of BHCl would result in a faster time to baseline pH after the administration of food and that the AUCpH would decrease proportionally as the dose of BHCl was increased. The primary outcome variable is time to baseline gastric pH after the administration of BHCl. Secondary outcomes included AUCpH, max pH, and the effect of food on gastric pH.
MATERIALS AND METHODS
We aimed to recruit non-smoking, healthy volunteers between the ages of 18-64 (per protocol), who were not taking concomitant medications (National Clinical Trials ID: #NCT02758015). The Committee on Human Research of the University of California, San Francisco (UCSF), approved this study on 08/29/2016 and the research was conducted at the UCSF Clinical Research Center. Recruitment was conducted using fliers around the campus, and telephone pre-screen interviews were conducted for interested subjects. Once deemed eligible via telephone screening and after providing written informed consent, patients’ eligibility for the study was determined during an in-person screening visit. The 2-hour screening visit consisted of routine laboratory value measurements (complete blood count, complete metabolic panel), medical history review, physical examination and baseline gastric pH measurement using the Heidelberg pH Diagnostic System (Heidelberg Medical, Inc., Mineral Bluff, GA) to confirm normochlorhydria (fasting gastric pH <4).
As previously described by Yago et al.,1 this FDA approved radiotelemetry system utilizes a small Heidelberg capsule that is indigestible. The Heidelberg pH Diagnostic system is a micro-electric transmitter device designed specifically for inter-abdominal pH monitoring and consists of a miniature pH electrode and battery-operated transmitter encapsulated within a sealed polyacrylate (plastic) capsule, 7mm in diameter and 15 mm long. This capsule is tethered to a thin, surgical string that has been pre-measured to allow the capsule to sit in the stomach when swallowed. Upon swallowing and proper placement, the string was taped to the patient’s cheek to ensure the capsule did not continue through the gastrointestinal tract. The pH information from the capsule was transmitted to a transceiver that interfaces with a PC computer to capture pH data at 1-second intervals. The pH capsule and Heidelberg System are not radioactive and are specifically designed to safely monitor intragastric and intraesophageal pH. Subjects who were able to tolerate the screening procedure and did not have achlorhydria (pH > 2) were eligible for the study. Subjects were not eligible if they had any past medical history of or current gastrointestinal diseases, concomitant medication use, ingestion of grapefruit juice within 7 days of the study days, pertinent allergies, or gastrointestinal intolerances (e.g., lactose intolerance). No subjects experienced adverse effects using the Heidelberg System or the BHCl capsules during any phase of the clinical study. The BHCl capsules are made from Hypromellose resulting in solubility that is independent of pH.13
This study was a randomized prospective, open-label, four period crossover repeated measures clinical study in which subjects were randomized into one of four periods using Latin square randomization. The four periods were as follows: 1) standardized meal only, 2) standardized meal + 1500mg BHCl, 3) standardized meal + 3000mg BHCl and 4) standardized meal + 4500mg BHCl. The minimum washout period was 48 hours between subject visits based on previous literature.1,5 The BHCl was administered with 250ml of water. Subjects entered each period fasting for at least 8 hours. On each study day, regardless of the period, baseline gastric pH levels were measured for 15 minutes. After baseline measurements, each subject was given a standard breakfast meal, containing a total of 310 calories. The meal consisted of one 160-calorie standard peach flavored yogurt (1.5g of fat, 32g carbohydrates, and 6g of protein) and a 150-calorie breakfast egg and cheese sandwich (8g of fat, 12g carbohydrates, and 8g of protein). These foods were chosen to standardize a typical light breakfast meal that consisted of about 45%-65% of calories from carbohydrates, 20%-35% from fat and 10%-35% from protein.14 This food composition was similar to the meals used in the prior study by Faber et al. Each subject had 15 minutes to finish the food, and at the 15-minute mark, the subjects were given the BHCl.
Based on prior studies, we noted a large effect size with a mean change in gastric pH of 4.2 with a standard deviation of 0.3 after the administration of betaine hydrochloride in healthy volunteers (N=6) pre-treated with multiple doses of rabeprazole.1 We calculated that a sample size of 8 would have a power of 0.8 to demonstrate a significant effect. We assumed we might have up to a 20% drop out rate; therefore, we aimed to recruit 10 healthy volunteers for the study.
The Heidelberg machine occasionally would send an erroneous data point to the computer system. These were determined by visually observing one-data point that would jump up several pH points in a given second and back to baseline the next second. These erroneous pH values were cleaned up using R Version 3.6.1. During data-clean up, individual’s data were sectioned off into different segments: baseline, food administration, and BHCl administration. In the baseline portion, any data values that jumped from patient’s baseline by more than several pH points in a given second were eliminated. For example, if pH readings are consistently measured between 0.5-1.5 in the baseline phase, any reading that jumped to greater than pH 3 in one second were determined to be erroneous. If the pH level jumped in either direction, but was maintained there, then these values were not eliminated in the data-clean up phase. This type of cleaning was applied to each section of an individual’s data. As previously discussed, median pH intervals for every minute were chosen for the analysis based on previous literature and thus 1-second erroneous data did not significantly impact the analyses.
Gastric pH data were collected at 1-second intervals by the Heidelberg system for the duration of each study visit, which was 3-4 hours. After the administration of BHCl, subjects were monitored for a total of 3 hours. However, based on an interim analysis, the protocol was later amended to 2 hours of monitoring after the administration of BHCl providing ample time for gastric pH to return to baseline. Given the vast amount of gastric pH data attained every second for each subject from each visit, the median pH data at 1-minute intervals were used for analyses. It has been previously shown that 1-minute intervals can sufficiently depict the pH-time profile1,15 The calculation of time to baseline was determined from the time of administration of BHCl until the gastric-pH had reached the baseline levels; in the food only arm the time began at the administration of food (comparison 1). A separate time to baseline calculation (comparison 2) was performed with the starting time point being 15-minutes after the administration of food, specifically in the food-only arm. This allowed for a consistent measure of time to baseline across all treatment arms since in the BHCl arms, the BHCl was administered 15-minutes after the administration of food. Baseline gastric pH levels were determined from gastric-pH measurements collected by the Heidelberg machine during the beginning of each study visit for each volunteer. Areas under the pH versus time curves (AUCpH) were calculated using the linear Trapezoidal Rule, beginning with the administration of food until baseline gastric pH levels were reached. The administration of food was chosen as the starting point for AUCpH to keep consistency among treatment arms.
A repeated measures analysis of variance (ANOVA) with Tukey’s test for multiple comparisons was used to determine statistical significance across all treatments. Geometric mean ratios and 90% confidence intervals were calculated for between-group treatment comparisons. Each subject was randomized to receive a standardized meal and 3 varying doses of BHCl. We measured the time to baseline gastric pH prior to and after the administration of a BHCl dose in the presence of a standardized meal, which resulted in elevated pH levels. The null hypothesis is that there is no difference in the time to baseline gastric pH with increasing doses of betaine hydrochloride compared to the control (standardized meal only and no betaine hydrochloride). The level of significance was set at 0.05 (alpha). Statistical calculations and descriptive analyses were done using Sigmaplot 12.5 (Systat Software, San Jose CA) and GraphPad Prism 8.2.0 (La Jolla, Ca). We also calculated the AUCpH using the linear trapezoidal method as a secondary outcome to determine if there is a significant difference between groups. The null hypothesis for this outcome is that there is no difference in the AUCpH between the groups.
RESULTS
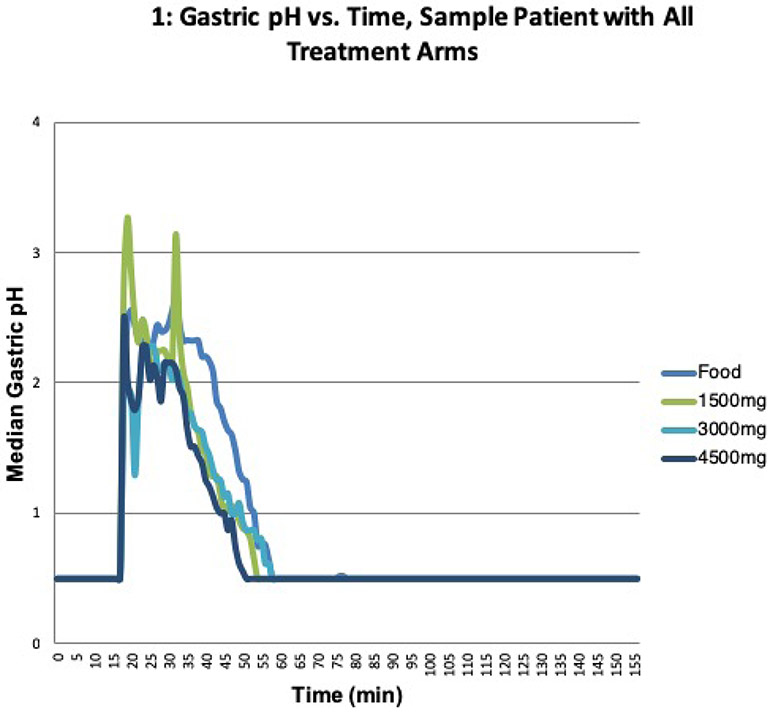
We enrolled nine healthy, non-smoking volunteers between the ages of 18 and 64. The protocol was approved by the University of California San Francisco Institutional Review Board, and all subjects signed the approved consent. Subject demographics are summarized in Table I. The breakdown of males and females was uniform, and the age range for subjects was 21-59 years old. The mean baseline pH across all nine volunteers was 0.75. Ethnicity, established by self-reporting, not obtained by genetic analysis, was collected as a means of gathering baseline demographics. Figure 1 illustrates a representative pH study profile along with the markings demonstrating the administration of food, BHCl and baseline levels.
Table I:
Subject Demographics
| Total N | 9 |
| Sex | |
| Male | 4 |
| Female | 5 |
| Race/Ethnicity | |
| White | 5 |
| Asian | 3 |
| Black | 1 |
| Baseline pH of All Subjects | |
| Mean | 0.75 |
| Range | 0.5 – 2.1 |
| Age (years) | |
| Mean +/− SD | 40 +/− 13 |
| Range | 21 – 59 |
Fig 1.
The gastric pH vs time plot for a sample patient from each treatment arm: food only, 1500mg of BHCl, 3000mg of BHCl and 4500mg of BHCl.
Time to baseline:
There were two comparisons made for the time to baseline outcome, comparison 1 (beginning when food was administered) and comparison 2 (beginning 15 min following food administration equivalent to when BHCl was administered). The primary outcome of mean time to baseline after food administration is shown for comparison 1 in Figure 2a (Mean Time to Baseline) and for comparison 2 in Figure 3a (Mean Time to Baseline).
Fig 2.
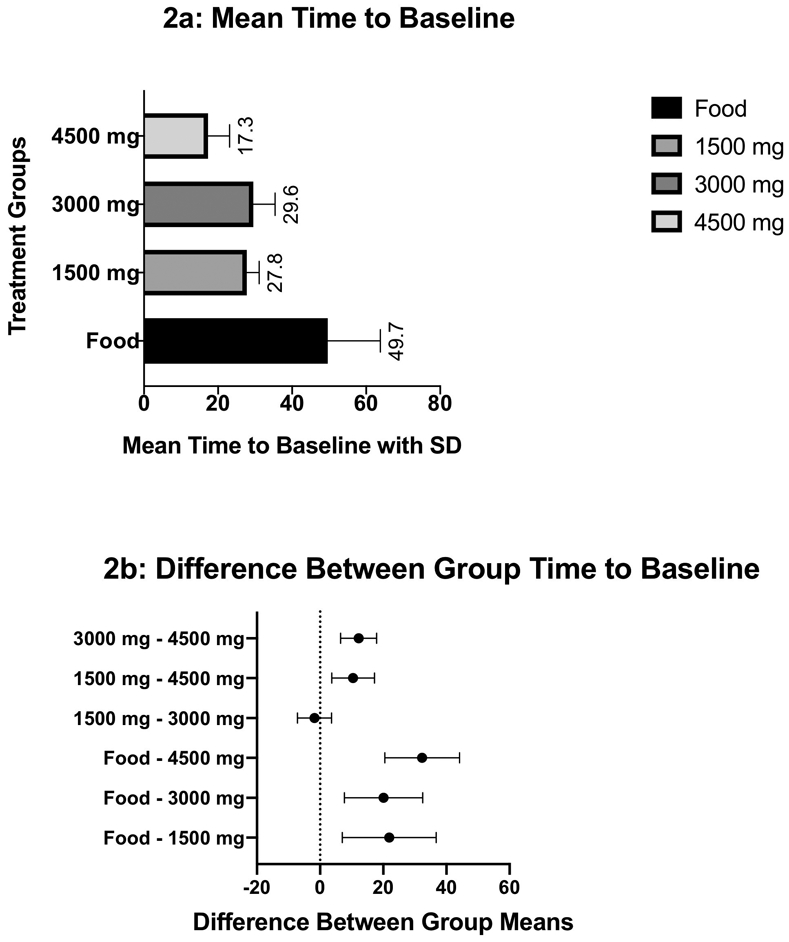
Figure 2a depicts the mean time to baseline for comparison 1 with the standard deviations shown as error bars. The calculation of time to baseline was determined from the time of administration of BHCl until the gastric-pH had reached the baseline levels. In the food only arm of comparison 1, the time began at the administration of food. Figure 2b illustrates the difference between group means with 90% confidence interval error bars.
Fig 3.

Figure 3a depicts the mean time to baseline for comparison 2 with the standard deviations shown as error bars. The calculation of time to baseline was determined from the time of administration of BHCl until the gastric-pH had reached the baseline levels. In the food only arm of comparison 2, the starting time point was 15-minutes after the administration of food. Figure 3b illustrates the difference between group means with 90% confidence interval error bars.
When individually comparing pairwise differences in mean time to baseline between treatment groups and the control group (Table II), all BHCl treatment arms compared to the food-only control arm showed a statistically significant decreases in the time to baseline pH. When comparing the BHCl treatment groups to one another, the higher doses of BHCl showed statistically significant decrease in time to baseline to lower doses of BHCl except for the 1500mg arm compared to the 3000mg arm where the difference was insignificant (P=0.810, CI [−7.20 to 3.64]). The geometric mean ratios are consistent with these results as well. The difference in means of the time to baseline, the p-values, the 90% CI of the difference in means, and the geometric mean ratios are given in Table II.
Table II:
Time From Food Administration to Baseline pH
| Comparison 1 | Difference of Means (time to baseline in mins) |
GMR | 90% CI of Difference in Means |
t | P Value | Significant |
|---|---|---|---|---|---|---|
| Control vs. 4500mg | 32.3 | 0.34 | 20.5 to 44.2 | 8.15 | <0.001 | Yes |
| Control vs. 1500mg | 21.9 | 0.58 | 7.04 to 36.7 | 5.52 | <0.001 | Yes |
| Control vs. 3000mg | 20.1 | 0.61 | 7.72 to 32.5 | 5.07 | <0.001 | Yes |
| 3000mg vs. 4500mg | 12.2 | 0.56 | 6.53 to 17.9 | 3.08 | 0.001 | Yes |
| 1500mg vs. 4500mg | 10.4 | 0.59 | 3.68 to 17.2 | 2.63 | 0.013 | Yes |
| 3000mg vs. 1500mg | 1.80 | 1.05 | -7.20 to 3.64 | 0.45 | 0.810 | No |
For the second comparison (comparison 2), the starting time point in the food-only control arm began 15-minutes after the administration of food (Figure 3). When individually comparing pairwise differences in time to baseline between treatment periods and the control group (Table III), the 4500mg BHCl treatment arm was statistically significant when compared to the control arm, the 1500mg BHCl arm and the 3000mg BHCl arm. All other comparisons between in the difference in mean time to baseline between the treatment groups were statistically insignificant (Table III).
Table III:
Time From BHCL to Baseline pH
| Comparison 2 | Difference of Means (time to baseline in mins) |
GMR | 90% CI of Difference in Means |
t | P Value | Significant |
|---|---|---|---|---|---|---|
| Control vs. 4500mg | 17.3 | 0.51 | 5.50 to 29.2 | 4.34 | 0.017 | Yes |
| Control vs. 1500mg | 6.90 | 0.87 | −7.96 to 21.7 | 1.74 | 0.611 | No |
| Control vs. 3000mg | 5.11 | 0.91 | −7.28 to 17.5 | 1.29 | 0.689 | No |
| 3000mg vs. 4500mg | 12.2 | 0.57 | 6.53 to 17.9 | 3.08 | 0.001 | Yes |
| 1500mg vs. 4500mg | 10.4 | 0.59 | 3.68 to 17.2 | 2.63 | 0.013 | Yes |
| 3000mg vs. 1500mg | 1.80 | 1.05 | −7.20 to 3.64 | 0.45 | 0.810 | No |
Mean Max pH
The mean max pH was measured to determine if there was a significant difference in post-prandial pH among the treatment arms (Table IV). The results demonstrated no difference between the groups, ensuring that each arm had similar gastric pH effects resulting from the administration of a standardized meal.
Table IV:
Mean Max pH
| N= 9 | Mean Max pH | STDV | Mean Max pH (all arms) | p-value |
|---|---|---|---|---|
| Food only | 3.02 | 0.62 | 3.2 | p = 0.670 |
| 1500mg | 3.30 | 0.49 | ||
| 3000mg | 3.17 | 0.52 | ||
| 4500mg | 3.30 | 0.45 |
Area Under the Curve, pH
The area under the curve was assessed to determine the impact on the pH vs. time curve of varying doses of betaine hydrochloride. The AUCpH was relatively stable regardless of the dose of BHCl administered. The differences in the mean values among the treatment groups are not great enough to exclude the possibility that the differences are due to random sampling variability; there is not a statistically significant difference (P = 0.990). The data are presented in Table V.
Table V:
AUCpH (From Food administration)
| N= 9 | Mean AUCpH | STDV | p-value |
|---|---|---|---|
| Food only | 68.1 | 34.4 | p = 0.990 |
| 1500mg | 67.0 | 17.8 | |
| 3000mg | 70.1 | 17.0 | |
| 4500mg | 69.7 | 27.0 |
DISCUSSION
These study results demonstrate the powerful effect of food on elevating and maintaining increased gastric pH and the impact of BHCl in overcoming this pH effect. The ability of food to elevate gastric pH and maintain elevated pH levels in the presence of BHCl is much more powerful than what was previously seen in drug-induced achlorhydria using ARAs. In the latter study, administration of BHCl was able to quickly and transiently decrease pH. In this study, there were two major comparisons. In the first comparison, there was a statistical difference between each of the BHCl arms compared to the food-only control arm in decreasing gastric pH. All but one (1500mg vs. 3000mg) of the treatment arms showed a statistically significant difference when compared to one another in their difference in mean time to baseline. These lower doses were likely not high enough to counter the strong gastric buffering effect of food. The null hypothesis was that there would be no difference in doses of BHCl dosing in time to baseline. We rejected the null hypothesis as the 4500mg BHCl dosing reached baseline significantly faster than the other doses, reaching baseline at 17.3 minutes. There was no correlation or significance between the AUCpH and the dose of BHCl. After administration of BHCl, the data showed fluctuations in pH as the pH moved towards baseline versus a linear decrease in gastric pH. These fluctuations could further explain the powerful buffering effect of food at attempting to maintain elevated gastric pH and the insignificant differences in AUCpH.
When looking at comparison 2, there was a statistically significant difference between the 4500mg BHCl arm and both the control, the 1500mg arm and the 3000mg arm.. The 1500mg and the 3000mg doses did not provide a faster time to baseline when compared to the control arm (Table III), and the time to baseline for these two arms were similar to one another. As mentioned previously, these doses were not high enough to counteract the strong gastric pH effect of food.
The potential for drug interactions resulting from PPI’s prescribed for digestive disorders is clear, however, the concomitant use of ARAs is also abundant in the setting of other clinical diseases. Two clinical areas very well studied include HIV and oncology, where studies have shown reduced systemic exposure to the therapeutic drug with concomitant ARA dosing.11,16–19 Approximately 33% of patients receiving an anticancer agent are also simultaneously prescribed an ARA,11 and many of these cancer agents have product labels (e.g. erlotinib) stating that ARAs should not be taken concomitantly.19 These pH interactions depend on the type of ARA used, the dose of ARA and the time of ARA administration relative to the pH dependent drug. Of the ARAs, the PPIs are the most potent as they are responsible for reducing acid in the stomach by irreversibly binding to the gastric proton pump (H+/K+ ATPase) at the secretory surface of parietal cells.20 PPIs drastically elevate gastric pH and thus cause the greatest concern for drug-drug interactions. A primary example of a compound that highlights these interactions is atazanavir (ATV), a weakly basic protease inhibitor with a pKa= 4.25.21 In a study by Zhu et al.22, low dose omeprazole (20mg) was co-administered to 56 healthy volunteers given ATV/RTV once daily. The resulting ATV area under the curve (AUC0-24) and trough concentrations (Cmin), in the presence of low dose omeprazole (20mg), were reduced by 42% (90% CI of geometric mean ratio: 0.44–0.75) and 46% (90% CI of geometric mean ratio: 0.41–0.71), respectively.22 The decrease in AUC0-24 and Cmin are significant. This pharmacokinetic interaction was also found to be dose-dependent as a previous study looking at the impact of omeprazole 40mg on ATV bioavailability demonstrated a decrease of about 75%.22
Another important factor to consider when discussing drug absorption is the effect of food on the physiology of the gastrointestinal tract, including gastric pH and its ability to affect the bioavailability of drugs. The ability of food to raise gastric pH levels has been well studied and documented.23–25 Furthermore, food impacts bioavailability through a number of variables, such as delayed gastric emptying, direct dissolution and absorption effects, physically or chemically interacting with drug molecules, biotransformation, stimulation of bile flow, and increased splanchnic blood flow. Furthermore the food content, the timing of food versus drug administration, and the biopharmaceutics classification system (BCS) class of the drug are also vital considerations.3,24,26 Despite the ample amount of research regarding the effect of food on drug bioavailability, a mechanistic prediction of the impact of food on specific chemical entities or a class of drugs has not been fully established.24
Food serves as a buffer and elevates gastric pH like ARA's do albeit in a different way, thus having similarly profound effects on pharmacokinetic parameters for weakly basic drugs that exhibit pH-dependent solubility.3,6 Molecules that have pH dependent solubility must be ionized to be soluble, however, absorption via diffusion through the lipid bilayer will be enhanced for unionized compounds. Weakly basic drugs, especially poorly water soluble and highly permeable drugs3 (BCS class II) will dissolve more readily in the acidic environment of the stomach as they will be ionized. However, as gastric pH levels rise after a meal or the administration of ARAs, weakly basic drugs become unionized and precipitate due to a reduction in their solubility. The percent of ionization for weakly basic drugs is therefore vulnerable to the pH in its environment. The Henderson-Hasselbalch equation demonstrates the importance of having a molecule in a pH range that will allow it to be ionized for solubility and unionized for absorption. For example, a weakly basic drug (BCS class II), such as ATV with a pKa of 4.2, will be 50% ionized and 50% unionized when the pH = pKa. In order for a weakly basic drug to be ionized and solubilized, we want the pH < pKa to ensure a high proportion of ionized to unionized drug. In contrast, absorption from the small intestine will be facilitated by the unionized state. This occurs when the pH is greater than the pKa of the drug. A majority of drug absorption occurs in the upper portion of the small intestine (jejunum and duodenum).3
Given the complexity of drug solubility and the number of variables that contribute to drug bioavailability, it is imperative to develop and discuss methods to alleviate these pH-based interactions. In 1994, Chin et al. demonstrated the ability of an acidic beverage such as Coca-Cola (pH of 2.5) to improve the absorption of ketoconazole in the presence of omeprazole induced achlorhydria.4 The area under the curve (AUC0-∞) and the maximum plasma concentration (Cmax) were increased by more than 10-fold with treatment of Coca-Cola in comparison to ketoconazole alone.4 Another example utilizing Coca-Cola as a reacidfying agent involved posaconazole. In this study, Coca-Cola was successful in regaining lost bioavailability due to drug-induced achlorhydria.27 Another study utilizing Coca-Cola and ATV in healthy subjects pretreated with omeprazole was not able to demonstrate any considerable recovery of bioavailability.28 Of these three studies, the ketoconazole and posaconazole examples demonstrate the potential viability of reacidification strategies and the necessity for research.
We have demonstrated the importance of increasing doses of BHCl, specifically that the highest dose of BHCl (4500mg) is the most potent in terms of time to re-acidify gastric pH to baseline levels in the presence of food. The probable explanation for the inability for Faber et al. to replicate previous studies from Yago et al. was due to the powerful food effect on increasing and maintaining elevated gastric pH even in the presence of 1500mg BHCl. Previous studies did not include food, and as a result, managing the impact of food on gastric pH is complex. The effect of food is often regarded as a minor factor that can be manipulated by adjusting dose administration of various drugs in relation to the food. However, as we have demonstrated, the gastric food effect on pH is much more powerful than anticipated. This study has served as a means of elucidating the gastric pH effect and suggests that the elevation in gastric pH seen with food is much different than ARA-induced hypochlorhydria.
This study reflects the conundrum faced by clinicians in administering weakly basic drugs in patients receiving ARAs. Clinically, it is recommended to dose atazanavir with food to minimize GI irritation from the drug formulation. But the concomitant meal will markedly increase gastric pH, resulting in poor bioavailability due to solubility issues that can lead to a higher chance of treatment failure and drug resistance. The clinician can recommend that patients wait about 50 minutes when food pH effects are diminished. But we do not know if this would still provide the positive effects of the meal on reducing gastric irritation. Alternatively, we show here that a large BHCl dose (4500 mg) will yield a rapid (~17 min) decrease in stomach pH that will allow solubilization, as we demonstrated earlier in fasted subjects receiving ARAs with a 1500 mg BHCl dose.
Although the most potent dose of BHCl, in terms of fastest time to baseline was the 4500mg dose, the AUCpH values did not inversely decrease as the dose of BHCl was increased. We expected that as the dose of BHCl was increased, we would see a faster time to baseline pH and as a result, the area under the time vs. pH curve would be the smallest. The data showed no significant differences among the different groups. One postulate as to why the AUCpH did not decrease with the increasing doses of the BHCl acid is because of the fluctuations in pH after the administration of the acid, further demonstrating the gastric pH effect of the food and its ability to maintain elevated pH levels. The decline of the slope on the pH vs. time curve was non-linear, with fluctuations in the pH that contributed to the AUCpH.
Limitations
The standardized meal we chose to administer for was based on the Institute of Medicine’s recommendation for caloric breakdown of fat, protein and carbohydrates. This is not necessarily a standard diet for many individuals in the USA. Since the impact of food on gastric pH is quite complex, different types of food could potentially alter the gastric pH response and thereby alter drug availability.
Another limitation was the Heidelberg device. We dealt with a range of technical issues ranging from interference in the transmission of pH data from the capsule to the computer to devices sending erroneously inaccurate values to the pH laptop. This resulted in a lot of data clean up that was required in order to ensure consistency. We worked with Heidelberg to help overcome the technical problems.
The sample size of N=9 was thought to be adequately powered based on previous studies, however, a larger sample size may have helped to tease out the between-treatment effects of the various BHCl doses. Moreover, the FDA does not regulate the standards of nutraceutical medicines as strictly as pharmaceutical drugs. The BHCl used was from a facility compliant with the FDA’s Good Manufacturing Process (GMP) requirements. Quantitative mass spectrometry was not performed to ensure the dosing of the BHCl nutraceutical capsules to ensure the accuracy of the dosing.
In addition to the above-mentioned limitations, we also dealt with some patient difficulty in swallowing the BHCl capsules. These capsules came in only 750mg doses. When subjects were taking 4500mg, they were required to swallow six of these capsules, which are larger than standard vitamin pills. This could have been one source of the fluctuation we observed in the pH vs. time graphs due to the various capsules opening and releasing its acidic content at various times in the gastric environment. Additionally, these fluctuations may have been enhanced by food-induced increase in gastric fluid viscosity, resulting in less BHCl disintegration and dissolution.
Lastly, the variability of water administered in the different periods could potentially lead to inconsistency in the gastric pH readings. Subjects who were unable to swallow all six BHCl capsules were given up to an additional 250 ml if desired. Additionally, in the food only arm subjects were able to drink up to 250 ml of water during the administration of food. The water administered during food was unlikely to affect the accuracy of BHCl to re-acidify gastric pH given both water and food serve as a buffer. It is unlikely that such a dosing paradigm could be used in patients.
CONCLUSION
Overall, this study has provided valuable data and insight concerning the environment of gastric reacidification strategies in the presence of food. Previous data by Yago et al. has suggested the impactful and translational role that orally administered BHCl could have on solubility and absorption of weakly basic drugs in the presence of drug-induced hypochlorhydria. The process is complicated with multiple factors, including BHCl disintegration and dissolution relative to gastric emptying, and a potential increase in gastric viscosity and BHCl. We sought to determine the effect of food on gastric pH as well as the impact of increasing doses of BHCl on gastric pH reacidification in the presence of food. The clinical relevance of these data suggest that when giving patients weakly basic medications that require low gastric pH for solubility and proper absorption, the medication must be given about an hour after the administration of food or approximately 20 minutes if using 4500mg of BHCl. The gastric pH effect of food is best overcome with the 4500mg dose of BHCl, but this is likely an unacceptably large dose for continuous administration using the given the available dosage forms discussed. More concentrated capsules or powder formulations of BHCl would be a more feasible option in a clinical setting.
ACKNOWLEDGEMENTS
This study was funded by the Benet Fund for Excellence through contributions and through Dr. Benet’s consultation and expert witness fees, as well as Board of Directors remunerations, all of which are made payable to the Regents of the University of California to support the research studies of the Benet Laboratory. The clinical portion of the study was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004.
ABREVIATIONS
- BHCl
Betaine Hydrochloride
- ARA
Acid Reducing Agent
- GERD
Gastroesophageal Reflux Disease
- PPI
Proton Pump Inhibitor
Footnotes
Conflict of Interests: None
REFERENCES
- 1.Yago MR, Frymoyer AR, Smelick GS, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10(11):4032–4037. doi: 10.1021/mp4003738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DA, Waterbeemd H van de, Walker DK. Pharmacokinetics and Metabolism in Drug Design. Wiley-VCH Verlag GmbH; 2001. [Google Scholar]
- 3.Abuhelwa AY, Williams DB, Upton RN, Foster DJR. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm. 2017;112:234–248. doi: 10.1016/j.ejpb.2016.11.034 [DOI] [PubMed] [Google Scholar]
- 4.Chin TWF, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39(8): 1671–1675. doi: 10.1128/AAC.39.8.1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yago MR, Frymoyer A, Benet LZ, et al. The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. AAPS J. 2014; 16(6): 1358–1365. doi: 10.1208/s12248-014-9673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faber KP, Wu H-F, Yago MR, et al. Meal effects confound attempts to counteract rabeprazole-induced hypochlorhydria decreases in atazanavir absorption. Pharm Res. 2017;34(3):619–628. doi: 10.1007/s11095-016-2090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eley T, Luo FR, Agrawal S, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol. 2009;49:700–709. doi: 10.1177/0091270009333854 [DOI] [PubMed] [Google Scholar]
- 8.Kletzl H, Giraudon M, Abt M, Hamilton M, Lum BL. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs. 2015:565–572. doi: 10.1097/CAD.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 9.Everhart J Burden of Digestive Diseases in the United States Report. 2008. [Google Scholar]
- 10.Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930–937. doi: 10.1007/s11606-013-2345-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smelick GS, Heffron TP, Chu L, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. MolPharm. 2013;10(11):4055–4062. doi: 10.1021/mp400403s [DOI] [PubMed] [Google Scholar]
- 12.Dawson RM et al. Data for Biochemical Research. 3rd ed. New York: Oxford University Press; 1986. [Google Scholar]
- 13.Sykora MA, Mahaguna V. Method to Recover a Lipophilic Drug From Hydroxypropyl Methylcellulose Matr i x Tablets Cl. 2001;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids -.; 2005. doi: 10.17226/10490 [DOI] [PubMed] [Google Scholar]
- 15.Knapp MJ, Berardi RR, Dressman JB, Rider JM, Carver PL. Modification of gastric pH with oral glutamic acid hydrochloride. Clin Pharm. 1991; 10(11):866–869. [PubMed] [Google Scholar]
- 16.Falcon RW, Kakuda TN. Drug interactions between HIV protease inhibitors and acid-reducing agents. Clin Pharmacokinet. 2008;47(2):75–89. doi: 10.2165/00003088-200847020-00001 [DOI] [PubMed] [Google Scholar]
- 17.Budha NR, Frymoyer A, Smelick GS, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–213. doi: 10.1038/clpt.2012.73 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wu F, Lee SC, Zhao H. pH-Dependent drug-drug interactions for weak base drugs: Potential implications for new drug development. Clin Pharmacol Ther. 2014;96(2):266–277. doi: 10.1038/clpt.2014.87 [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen RWF, Peric R, Hussaarts KG, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non – small-cell lung cancer. J Clin Oncol. 2016;34(12): 1309–1315. doi: 10.1200/JCO.2015.65.2560 [DOI] [PubMed] [Google Scholar]
- 20.Prilosec® [Prescribing Information], AstraZeneca Pharmaceuticals LP; Wilmington, DE: June 2018. [Google Scholar]
- 21.Kis O, Zastre J a, Ramaswamy M, Bendayan R. pH Dependence of organic aniontransporting polypeptide 2B1 in Caco-2 cells : Potential role in antiretroviral drug oral bioavailability and drug – drug interactions. J Pharmacol Exp Ther. 2010;334(3):1009–1022. doi: 10.1124/jpet.110.166314 [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Persson A, Mahnke L, et al. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51(3):368–377. doi: 10.1177/0091270010367651 [DOI] [PubMed] [Google Scholar]
- 23.Dressman JB, Berardi RR, Dermentzoglou LC, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–761. doi: 10.1023/A:1015827908309 [DOI] [PubMed] [Google Scholar]
- 24.Singh B Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–255. [DOI] [PubMed] [Google Scholar]
- 25.Netzer P, Eschenmoser K, Halter F, Inauen W. Impact of food intake on the antisecretory effect of low-dose ranitidine and famotidine. Aliment Pharmacol Ther. 1999;13(3):407–412. [DOI] [PubMed] [Google Scholar]
- 26.FDA. Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies. 2002. [Google Scholar]
- 27.Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole. Clin Pharmacokinet. 2011;50(11):725–734. doi: 10.2165/11592630-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 28.Agarwala SG, Eley T, Wang Y, Hughes E, Grasela D. Pharmacokinetic interaction between Atazanavir and Omeprazole in Healthy Subjects. Presented at the 3rd IAS Conference on HIV Pathogenesis and Treatment http://www.medadvocates.org/resources/conferences/3rd_ias/05-156b_agarwala_109.pdf. Published 2005. [Google Scholar]