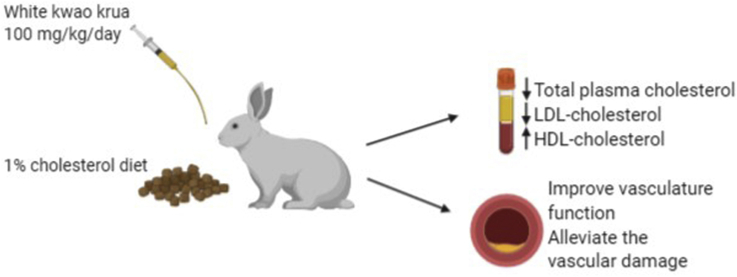
Abstract
Background and aim
White kwao krua is an edible plant that grows in Southeast Asia. It is very rich in natural phytoestrogens. Previous clinical studies revealed that the use of White kwao krua as a hormone replacement therapy has beneficial effects on the lipid profile of menopause women. In this present study, we utilized the hypercholesterolemia rabbit model to demonstrate the effect of White kwao krua on the daily intake of high-fat diet.
Experimental procedure
We induced hypercholesterolemia in rabbits by feeding with high-fat diet (1% cholesterol-containing diet). The animals were maintained 12 weeks for the experimentation. The White kwao krua supplement was administered 100 mg/kg/day, and the effects were monitored comparing with Statins and turmeric. Blood was collected periodically to monitor the plasma cholesterol level and the oxidative susceptibility of isolated LDL-cholesterol. At the end of the experiment, the aorta was collected from the animal and performed endothelial-dependent relaxation and endothelial-independent relaxation assays. The relative ratio of intima to media layer was microscopically evaluated from hematoxylin/eosin-stained tissues.
Results and conclusion
We showed that the White kwao krua supplement reduced LDL-cholesterol about 40% compared with high-fat diet consumption alone. Administration of White kwao krua had significantly prolonged the susceptibility of LDL-cholesterol to oxidation. Besides, it led to the improvement of vascular function by recovering endothelium-dependent relaxation and alleviating vascular structure impairment induced by high-fat dietary intake. Together, we suggest that White kwao krua should be used as a dietary supplement to reduce the atherogenesis in high-fat dietary consumption.
Section
Dietary therapy/nutrients supplements.
Taxonomy
Inflammation, Disease.
Keywords: Cholesterol, Vasorelaxation, Antioxidant, Anti-atherosclerotic effect, Pueraria mirifica
Graphical abstract
Highlights
-
•
Daily administration of White kwao krua reduced LDL-cholesterol following high-fat diet consumption.
-
•
White kwao krua had an antioxidant effect, that led to the prolonged susceptibility of LDL to oxidation.
-
•
The cholesterol-induced vascular damage was alleviated by White kwao krua supplement.
-
•
The impairment of vasorelaxation following cholesterol intake was rescued by White kwao krua supplement.
List of abbreviations:
- LDL
low-density lipoprotein
- oxLDL
oxidized-LDL
- HDL
high-density lipoprotein
- Ach
Acetylcholine
- SNP
Sodium nitroprusside
1. Introduction
Atherosclerosis is the major cause of the cardiovascular disease (CVD), the predominant cause of death worldwide. Several previous studies demonstrated that dyslipidemia, oxidation, and inflammation related to the pathogenesis of atherosclerosis.1 The increase of plasma low-density lipoprotein (LDL) from the diet passes through the endothelial barrier and deposits on the arterial wall, plays a crucial role in atherosclerosis development. The accumulation of LDL transforms into oxidized-LDL (oxLDL) and constitutes the hallmark of the disease. The presence of oxLDL not only produces self-reactive oxygen species (ROS) but also activates endothelial cells to release more ROS and pro-inflammatory cytokines.2 This increases the leukocyte migration, subendothelial space retention,3 leukocyte adhesion to the vascular endothelial cells,4 and even synergizes the effect of air-pollutant-induced vascular inflammation.5
Turmeric has been widely used as herbal remedies in China, India, and Southeast Asia to cure various diseases. Several biological effects such as antioxidant, anti-inflammatory, and anti-atherogenic effects have been observed in this plant.6 Curcuminoids, the yellow pigmented-fraction of turmeric, consist of three major chemical substances namely curcumin, demethoxycurcumin, and bis-demethoxycurcumin. Previous studies have shown that turmeric-derived curcuminoids have a hypocholesterolemic effect. It can reduce oxidative stress and it decreases fatty streak lesions in the thoracic and abdominal aorta of the animal model.7, 8, 9
White kwao krua (Pueraria mirifica) is a native plant in the Indochina region that is widely used in cosmetics. Previous chemical analysis revealed the presence of phytoestrogens, including isoflavonoids, coumestrans, and chromenes in this plant.10, 11, 12, 13, 14, 15, 16, 17 The phytoestrogens are safe to use for therapy and do not cause mutagenesis. In addition, White kwao krua has shown the beneficial potency of anti-carcinogenic effect by inhibiting hepatic cytochrome P450 in a rat model.18 The previous clinical trial reported that estrogen could reduce total blood cholesterol and LDL-cholesterol levels.19 In addition, several clinical studies revealed that the supplementation of White kwao krua, as a hormone replacement therapy has shown a beneficial effect on plasma lipid profile.20,21 In this study, we demonstrate the anti-atherogenic properties of White kwao krua crude powder in the hypercholesterolemia animal model comparing with turmeric crude powder and Statins, which previously described as anti-atherogenic agents.
2. Material and methods
2.1. Plant materials
White kwao krua crude powder was kindly given by Assoc. Prof. Dr. Amphawan Apisariyakul (Chiangmai University, Thailand). The plant was collected from Baan Tak, Lampang province, the north region of Thailand during March and April 2015 (Voucher herbarium specimens number 23028, identified and deposited at Royal Forest Department, Ministry of Agriculture and Cooperatives, Thailand). In brief, the tuberous roots of Pueraria mirifica were dried and pulverized to get a fine powder. The powder was kept at 4 °C and protected from light. The aliquot of White kwao krua powder was freshly suspended in distilled water at the time of administration and given at the final dose of 100 mg/kg/day as previously described.10,22 The presence of phytoestrogens in White kwao krua crude powder had been confirmed in previous studies and was not shown here.10,18
Dried turmeric crude powder was kindly given by Prof. Dr. Apichart Suksamrarn (Ramkhamhaeng University, Thailand). The plant crude powder was prepared as previously described.23 In brief, the rhizomes of Curcuma longa were dried and pulverized to a fine powder. The powder was suspended in distilled water at the time of administration and given at the final dose of 100 mg/kg/day.
2.2. Animals
Thirty male New Zealand White rabbits weighing 2.00 kg were used in this study following the CCAC Guide to the Care and Use of Experimental Animal (1993; Vol.1) and the guidelines of the national laboratory animal center, Thailand. After two weeks period of adaptation, the rabbits were randomized and divided into five groups and exposed to dietary treatments. The first group was fed with normal rabbit chow served as a baseline (n = 6). The second group, high-fat feeding group, was fed with 1% cholesterol-containing diet for twelve weeks. The other groups were fed with fed 1% cholesterol-containing diet supplement with 5 mg/day Statins, 100 mg/kg/day of turmeric or 100 mg/kg/day of White kwao krua, respectively. The blood sample was drawn from a central ear vein at the beginning of the experiments and every four weeks intervals thereafter. The collected blood samples were centrifuged at 3500 rpm for 15 min. Plasma was separated and kept at −20 °C until performing further experiments. At the end of experiments, the animals were premedicated with ketamine (25 mg/kg body weight, intramuscular injection) followed by general anesthesia with thiopental via the marginal ear vein (initial dose 40 mg plus maintenance dose as required). Blood samples were taken, and the animals were euthanized with overdose thiopental. The aorta was immediately isolated and placed in fresh ice-cold Krebs buffer before the measurement of vascular endothelium-dependent and endothelium-independent relaxation assay.
2.3. Lipid profile measurement
Plasma lipid profiles were determined by enzymatic colorimetric method (VITROS Chemistry, UK). This experiment was performed by Professional Laboratory Management Corp (Bangkok, Thailand).
2.4. Oxidized-LDL formation measurement
Low-density lipoprotein (LDL) was isolated from the plasma sample as described by Redgrave et al.24 EDTA (1 mg/mL) was used throughout all the steps of LDL isolation and was eliminated by dialysis with 10 mM phosphate-buffered saline (PBS) pH 7.4, at 4 °C for 24 h. The EDTA free-LDL was then solubilized in PBS buffer. Protein concentrations were determined by Lowry method and adjusted to 75 μg of LDL protein/mL before performing the experiment. 2.5 μM of CuSO4 was used to stimulate LDL-oxidation. The formation of conjugated diene compound was monitored at 234 nm every 10 min interval. The lag-phase was determined to indicate the susceptibility of LDL to oxidation.
2.5. Measurement of vascular functions
After the animal was euthanized, the aortic rings were immediately placed into organ baths containing Krebs bicarbonate buffer solution pH 7.4, 37 °C, and continuously aerated with 95% O2:5% CO2. The aortic rings were connected to force transducers to measure isometric tension. Each aortic ring was pre-constricted to baseline tension of 2 g for at least 60 min. The aortic ring was induced contraction with 1 μM noradrenaline, then induced relaxation with 1 μM acetylcholine to determine endothelial integrity. Thereafter, the aortic ring was repeatedly washed with Krebs buffer until the tension had returned to the baseline value. After pre-contraction with 1 μM norepinephrine, vascular endothelium-dependent relaxations was determined by using acetylcholine (1 nM–100 μM), whereas endothelium-independent relaxations was determined by using sodium nitroprusside (1 nM–100 μM). The contraction was reported as a percentage of the initial contraction to 1 μM of norepinephrine.
2.6. Atherosclerotic plaque investigation
The proximal aorta was dissected freely of adventitial tissue and cleared of remaining blood by 0.9% NaCl infusion. The specimens were fixed in 10% formalin buffer for 24 h, before embedding in paraffin. The embedded tissue was cut cross-section of approximately 5 μm and stained with hematoxylin/eosin. Thereafter, the images of intima and media layers were analyzed by using Adobe Photoshop Program. The results were reported as the ratio of plaque relative to the media layer.
2.7. Statistical analysis
All data were present as mean ± standard error of the mean (SEM). The statistical analysis of conjugated diene formation and the plaque formation in vasculature were analyzed using one-way analysis of variance (one-way ANOVA) and two-way analysis of variance (two-way ANOVA) followed by Dunnett’s post hoc analysis. The significant difference between mean was indicated at the level of P-value < 0.05.
3. Results
3.1. The administration of White kwao krua alters plasma lipid profile by reducing LDL-cholesterol and slightly increasing HDL-cholesterol
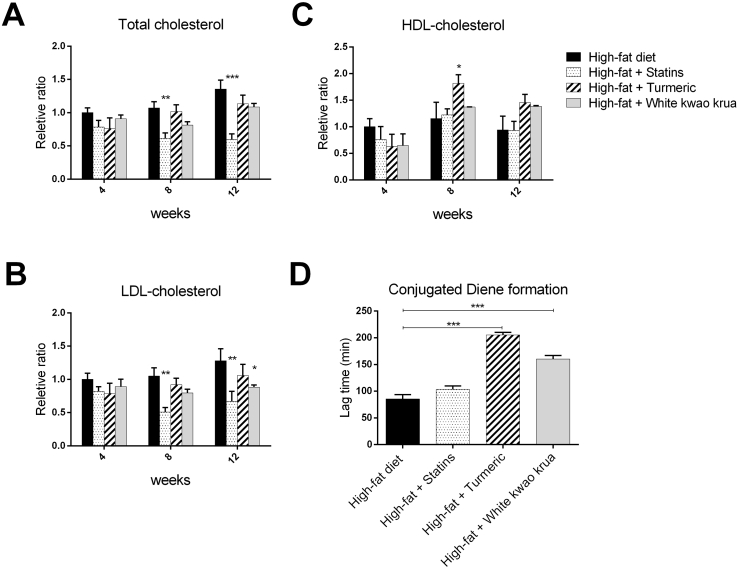
High-fat diet consumption is one of the major causes of Atherosclerosis. The consumption of high lipid content food increases LDL-cholesterol level, the hallmark of the disease. In this study, we fed the rabbit animal model with high-fat diet (1% cholesterol-containing diet) for twelve weeks and monitored the alteration of plasma lipid profile following the treatment. Upon the high-fat diet feeding, total plasma cholesterol elevated and maintained at approximately 30–40 folds compared to a normal diet (4th-12th week). We next further investigated the effects of White kwao krua on blood lipid profiles comparing with Statins, a cholesterol-lowering drug, and turmeric crude powder, an anti-atherogenic herb, when supplemented with the high-fat diet consumption. The plasma cholesterol profiles are shown in Table .1. Its relative ratio is shown in Fig. 1A-C.
Table 1.
Plasma total cholesterol level (mg/dL), Plasma LDL-cholesterol levels (mg/dL), Plasma HDL-cholesterol levels (mg/dL), Plasma triglyceride levels (mg/dL) of the rabbits.
| Groups | Time period (weeks) |
|||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| Plasma total cholesterol level (mg/dL) | ||||
| Normal diet (baseline)a | 41.2 ± 4.2 | 35.9 ± 3.8 | 36.7 ± 3.2 | 37.1 ± 5.1 |
| High-fat diet | 40.6 ± 3.1 | 1102.3 ± 89.7 | 1179.9 ± 115.2 | 1483.8 ± 156.3 |
| High-fat + Statins | 43.7 ± 1.2 | 870 ± 124.05 | 690.7 ± 102.2∗∗ | 672.3 ± 106.5∗∗∗ |
| High-fat + Turmeric | 43.4 ± 4.4 | 848.7 ± 184.6 | 1121.3 ± 124.7 | 1246.5 ± 152.1 |
| High-fat + White kwao krua | 45.5 ± 2.9 | 1004 ± 76.1 | 902.7 ± 69.3 | 1195.7 ± 71.9 |
| Plasma LDL-cholesterol levels (mg/dL) | ||||
| Normal diet (baseline)a | 10.7 ± 2.2 | 8.3 ± 2.1 | 9 ± 1.9 | 9.5 ± 2.9 |
| High-fat diet | 12.3 ± .1 | 1009.0 ± 92.7 | 1058.3 ± 127.2 | 1291.8 ± 179.3 |
| High-fat + Statins | 11.7 ± 1.4 | 827.0 ± 72.7 | 517.0 ± 69.4∗∗ | 677.7 ± 153.5∗∗∗ |
| High-fat + Turmeric | 9.3 ± 3.1 | 799.8 ± 150.3 | 929.6 ± 97.8 | 1069.4 ± 167.4 |
| High-fat + White kwao krua | 9.0 ± 2.8 | 901.4 ± 112.1 | 805.7 ± 55.3 | 892 ± 34.5∗ |
| Plasma HDL-cholesterol levels (mg/dL) | ||||
| Normal diet (baseline)a | 28.3 ± 1.5 | 28.0 ± 2.3 | 23.0 ± 3.9 | 26.7 ± 2.8 |
| High-fat diet | 27.7 ± 3.8 | 169 ± 22.1 | 185.7 ± 44.6 | 159.3 ± 37.7 |
| High-fat + Statins | 26.3 ± 3.9 | 134.7 ± 36.1 | 195.7 ± 17.3 | 158 ± 25.1 |
| High-fat + Turmeric | 29.3 ± 2.9 | 117.2 ± 32.5 | 279.3 ± 24.2∗ | 232 ± 22.6 |
| High-fat + White kwao krua | 27.4 ± 3.8 | 119.1 ± 31.9 | 216.1 ± 1.3 | 221.2 ± 3.5 |
All values are means ± SEM, Two-way ANOVA, Dunnett post-hoc test.
a The level of plasma cholesterol in normal diet group was significantly different from high-fat dietfeeding group throughout the study (week 4–12).
∗, P-value < 0.05, ∗∗, P-value < 0.01, ∗∗∗, P-value < 0.001 compared to high-fat diet feeding group, respectively.
Fig. 1.
The graphs represent the ratio of (A) Plasma total cholesterol level (mg/dL), (B) LDL-cholesterol, and (C) HDL-cholesterol of supplement groups relatively to high-fat diet feeding group. (D) This graph represents the lag time of conjugated diene formation in high-fat diet, high-fat diet supplemented with 5 mg/day of Statins, 100 mg/kg/day of turmeric, or 100 mg/kg/day of White kwao krua for twelve weeks.
Data show as Mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
The supplementation of Statins decreased total-blood cholesterol 46–76% and LDL-cholesterol 54%–61% starting from week 8th (P-value<0.01), while it did not affect HDL-cholesterol. Similar to Statins, White kwao krua also had an LDL-cholesterol-lowering effect at week 12th (40% decrease, P-value<0.05), while increased HDL-cholesterol 44% (although the HDL increasing level was not statistically significant). Turmeric crude powder, in this study, had no effect on LDL-cholesterol (throughout week 4th-12th), but significantly increased HDL-cholesterol (52–66% increasing at week 8th). Together, we show that White kwao krua has altered plasma lipid profile by significantly reducing LDL-cholesterol and slightly increasing HDL-cholesterol. These results suggest that it may consequently reduce the plaque formation and progression of Atherosclerosis.
3.2. White kwao krua decreases the susceptibility of LDL to oxidation
After the discovery of LDL incorporated with an atherosclerotic plaque by Goldstein and Brown in 1977,25 several scientists have urged to understand the role of LDL in atherogenesis. In 1978, Henricksen et al. found the cytotoxicity of LDL when incubated with endothelial cells.26 Later, they found that the structure of treated LDL after incubated with endothelial cells was greater in negative charge (oxidized).27 This observation led to the extensive works of oxLDL as an early marker of atherosclerosis. In this part, we measured the susceptibility of LDL to oxidation by monitoring the lag time of conjugated diene formation. The longer lag time indicates the low susceptibility of LDL to become oxidized. The results showed that the high-fat diet-feeding group had the shortest lag time (about 85 min), indicating the highest susceptibility of LDL to oxidation. The daily administration of White kwao krua prolonged the LDL to oxidation to about 160 min (Fig. 1D). The lag time of White kwao krua was shorter than turmeric but both of them decreased the susceptibility of LDL to oxidation.
3.3. White kwao krua improves vascular endothelial function and rescues vascular structure impairment
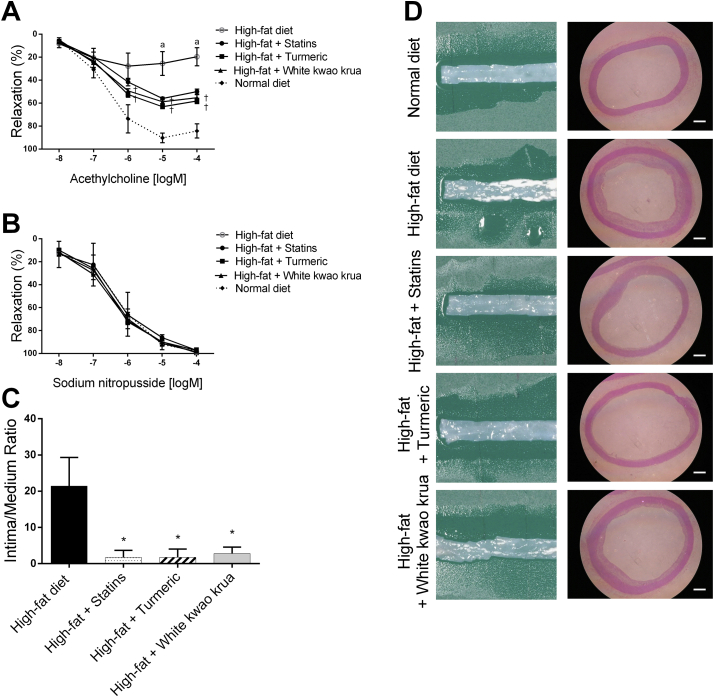
The accumulation of LDL-cholesterol from high-fat diet consumption resulted in the impairment of vascular function. We found that the consumption of high-fat diet for twelve weeks significantly abolished the endothelial-dependent relaxation (Ach-meditated relaxation) to approximately 27.8 ± 11.4% of maximal contract comparing with the normal diet group of 90.2 ± 1.7%, whereas endothelial-independent relaxation (SNP-meditated relaxation) was not affected (Fig. 2b). In this present study, we showed that the administration of 100 mg/kg/day White kwao krua significantly recovered the Ach-meditated relaxation to the level of 63.1 ± 1.7% of maximal contract at 10 μM acetylcholine. This observation was similar to turmeric and Statins supplement groups. The maximum relaxant percentage was observed at 1 μM acetylcholine and no longer induced relaxation in higher concentration, while in the normal diet, the maximum relaxant percentage was observed at 10 μM acetylcholine. This observation suggested the changes in acetylcholine receptor availability and its downstream pathways following high-fat diet consumption. Interestingly, the supplementation of Statins, turmeric, and White kwao krua not only increased the maximum relaxation percentage but also recovered the acetylcholine dose-response to the level similar to a normal diet group. This suggested the restoration of acetylcholine receptor availability and vasodilation-mediated by acetylcholine. Yet, it has not been further tested in this study.
Fig. 2.
The graphs show (A) concentration-response curve of the endothelium-dependent relaxations induced by Acetylcholine, and (B) endothelium-independent relaxations induced by sodium nitropusside of isolated aortic rings at week 12th. (C) the ratio of plaque in intima to media layer. (D) shows the representative images of aortic sections unstained (left) and hematoxilin/eosin stained cross section under 40X total magnification (right). Scale bar indicates 500 μm.
Data show as Mean ± SEM, a = all supplement groups were significantly different from normal diet. † = the extract administration group was NOT significantly different from Statins administration. ∗P < 0.05.
We further investigated the preventive effect on atherosclerosis plaque formation following the administration of White kwao krua. The sectioned specimens were stained with hematoxylin/eosin and determined the intimal/medial cross-section ratio. The data were normalized with the thickness of blood vessels in the normal diet group. Upon the consumption of high-fat diets for twelve weeks, we observed the thickening of the intima layer of the artery, which caused by the proliferation and migration of vascular smooth muscle cells from the media layer. This resulted in the narrowing of the vascular lumen and consequently reduced blood flow. Following the administration of White kwao krua daily, it significantly reduced the thickening of the intima layer about 7.65 folds less than high-fat diet feeding alone. Taken together, we found that the consumption of high-fat diets increased vascular smooth muscle cell proliferation and migration to the intima layer. This resulted in the narrowing of the vascular lumen. The smooth muscle-mediated vasorelaxation was not affected, while the endothelial-mediated vasorelaxation was impaired by high-fat diet consumption. The daily supplementation of White kwao krua along with high-fat diet consumption reduced the thickening of the arterial wall, as well as improved the endothelial-mediated vasorelaxation. This observation was similar to the Statins and turmeric, which were previously known as anti-atherogenic agents. Suggesting that White kwao krua can be used as a dietary supplement to prevent atherogenesis.
4. Discussions
Cardiovascular disease increases with age-dependent mechanistic components, especially, the increase of oxidative stress leads to the misconduct of gene expression and signaling pathways.28 Diet plays a role in modulating the level of oxidative stress, which associates with the intake of food-containing oxidants and antioxidants.29,30 Hypercholesterolemia, in particular total plasma cholesterol exceeds 160 mg/dL, is primarily due to the high-fat diet consumption. It has been instituted as a risk factor for the augmentation of atherosclerotic cardiovascular disease.31,32 The earliest appearance lesion in experimental atherosclerosis is the presence of foam cells, which consists of monocyte-derived macrophages containing LDL. The incubation of LDL on macrophage does not generate foam cells but the incubation with oxLDL does. This suggests the oxidative modification of LDL before initiating atherosclerotic plaque.33 Lipid peroxidation chain reaction generates oxLDL that leads to the initial step of atherosclerotic plaque formation.31,33,34 The uptake of oxLDL is mediated by scavenger receptor that recognizes oxLDL but not native LDL. However, the oxLDL does not activate the scavenging downstream pathway. It subsequently accumulates in macrophage and ultimately becomes foam cells. The consumption of moderate dietary cholesterol additional to a normal diet not only increases plasma cholesterol but also increases the susceptibility of LDL to oxidation in regardless of the lipid content of a normal diet.35 HDL, on the other hand, is known as a protective factor against atherosclerosis.36 It prevents atherosclerosis by inhibiting the oxLDL-stimulating monocyte infiltration.37
The elevated plasma cholesterol infiltrates and accumulates on vascular endothelial cells. It results in the impairment of endothelial-dependent vasorelaxation in both human and animal models.38, 39, 40 In general, the endothelial vascular function is regulated by vasodilators such as nitric oxide (NO) and prostacyclin, and vasoconstrictor such as thromboxane A2, free radical and endothelin. The previous study showed that the inhibition of NO by long term administration of competitive NOS inhibitor, l-NAME sustained hypertension in rats.41 In addition to the vasodilator effect, NO has anti-atherogenic properties by inhibiting monocyte adhesion and smooth muscle cell proliferation. The mechanisms underlying cholesterol-induced endothelial dysfunction may due to the reduction of endothelial NO production, decrease of NO activity, or increase of NO degradation.42,43 The lack of functional NO attenuates vascular smooth muscle response and alters the secondary messenger systems. Intracellular ROS are key players who enhance the vascular oxidative stress. They act as signaling molecules to promote cell adhesion molecules expression, which facilitates the adherence of leukocyte and initiates atherosclerotic plaques.44 Thus, the improvement of endothelial function by the treatment of cholesterol-lowering drug or antioxidant therapy can alleviate atherosclerosis.45,46
The atherosclerosis model has been well generated in rabbit species due to its tendency to develop severe hypercholesterolemia after feeding with high cholesterol diet.47 In addition, the degree of an aortic lesion showed markedly thickening of the intima wall, which strongly proportionate to the level of plasma cholesterol.48 In this current study, plasma total cholesterol markedly increased by 40-folds in rabbits receiving 1% cholesterol diet (named as high-fat diet feeding group). The plasma cholesterol concentration change in this study was similar to previous studies as Chumark et al., in 2008 and Srisawat et al., in 2003.49,50 To evaluate the anti-atherogenic properties of White kwao krua, we administered White kwao krua powder suspension together with high-fat diet consumption and compared to Statins and turmeric powder, both were characterized for having anti-atherogenic effect in previous studies.7, 8, 9,51 We found that the administration of White kwao krua to high-fat diet alleviated the vascular function impairment and reduced the vascular lumen thickening.
White kwao krua supplement delayed the LDL to oxidation and decreased the plasma LDL-cholesterol at week 12th. Besides, there seemed to be a decreasing trend of total cholesterol, while showed an increasing trend of HDL-cholesterol at all time points (although the results did not reach statistical significance). In comparison with Statins and turmeric powder, White kwao krua exhibited the improvement of vascular function and structure similarly. However, the mechanism of action of Statins and turmeric was different. Statins alleviate the atherosclerosis progression through the inhibition of HMG-Co A reductase enzyme, which is the rate-limiting step in the biosynthesis of cholesterol. It increases LDL-receptor and leads to the decrease of plasma LDL-Cholesterol.51, 52, 53 In this study, Statins decreased plasma total cholesterol and LDL-cholesterol at week 8th of administration, while turmeric had non-significance result on LDL level in the entire study. On the other hand, the administration of turmeric resulted in the inhibition of the LDL to oxidation, while Statins had non-significance result of antioxidant property. The antioxidant effect of turmeric administration was believed to be related to curcuminoids component as it was previously described for having antioxidant properties.7,54,55 Together suggested that either reduction of plasma LDL or inhibition of LDL to oxidation could ease the vascular impairment induced by high-fat diet consumption. In this present study, we found that White kwao krua has a plasma LDL lowering effect as well as delays LDL to oxidation. Nonetheless, the mechanisms of action underlying these two properties are undetermined, and has not been identified in this present study.
White kwao krua is a phytoestrogens enriched plant.10, 11, 12, 13, 14, 15, 16, 17 Previous studies revealed that at least 17 chemical components containing estrogenic activity were found in its tuberous roots, leave and stem, but predominantly found in tuberous roots.56 Among these components, they could be categorized into 3 major groups including isoflavonoids, coumestrans, and chromenes. Ten isoflavonoids included daidzin, daidzein genistin, genistein, kwakhurin, kwakhurin hydrate, tuberosin, puerarin, mirificin and puemiricarpene; four coumestrans included coumestrol, mirificoumestan, mirificoumestan glycol and mirificoumestan hydrate; and three chromenes included deoxymiroestrol, miroestrol, and isomiroestrol.56, 57, 58, 59 All of these are structurally similar to 17β-estradiol. Among these, miroestrol (may be deoxymiroestrol derivative) were firstly characterized for having the highest estrogenic activity over other subsequent isolated phytoestrogens.16,17,20 According to the study done by Chansakaow et al., 100 g of White kwao krua dried powder collected from Thailand in 1996 contained approximately 46 mg of daidzein and approximately 2–3 mg of miroestrol and deoxymiroestrol.16 Whereas, another previous study done by Malaivijitnond in 2004 revealed 51 mg of daidzin, 8.1 mg of daidzein, 12 mg of genistin, 20 mg of genistein, and 96 mg of puerarin were presented in 100g of dried root White kwao krua powder.60 Indicating that, the amount of each component are varied among cultivar and collected time. The estrogenic bioassay of chromenes group showed that purified miroestrol from White kwao krua had 4 times less estrogenic activity compared to 17β-estradiol.61 Although miroestrol was the first phytoestrogen mentioned in this plant, it later showed that miroestrol could be the derivative of deoxymiroestrol following the oxidation.16,62 In comparison, deoxymiroestrol has higher estrogenic activity than miroestrol about ten times more.7 Due to the containing of phytoestrogens in White kwao krua, it was widely used as a hormone replacement in Asian menopause women.20,63 Previous clinical studies showed that White kwao krua supplement not only increased the estrogen level, it also increased plasma HDL and Apo1A, while decreased plasma LDL and ApoB.20 As a result, this suggested that White kwao krua supplementation may decrease the risk factor toward cardiovascular diseases such as Atherosclerosis in menopause women. Besides, the previous study in the ovariectomy mouse model showed that the supplement of White kwao krua powder or purified miroestrol derived from White kwao krua increased the level of antioxidants enzymes such as glutathione peroxidase, superoxide dismutase, and catalase (65). This, at lease, suggested that the antioxidant effect of White kwao krua is partly due to miroestrol components (or deoxymiroestrol derivative). The meta-analysis of the effect of soy-derived protein on the plasma lipid profile revealed that the consumption of higher containing isoflavonoids derived-soy protein had a high correlation with the decrease of plasma total plasma cholesterol and LDL-cholesterol independently to the soy protein level.64 Suggesting that the plant-derived isoflavonoids may have an LDL lowering effect. However, the mechanisms of action are still underdetermined. The purification of each phytoestrogen, as well as other components, is required to identify the mode of action of each component on atherosclerosis. Rather, the anti-atherogenic properties of White kwao krua may derive from the synergistic effects of multiple components in this plant, instead of one single compound. In conclusion, the supplementation of White kwao krua crude powder could lower plasma LDL level, as well as delayed the LDL to oxidation, and ultimately mitigated the vascular impairment induced by high-fat diet consumption.
5. Conclusion
This study indicates that White kwao krua has an anti-atherogenic effects. It reduces LDL-cholesterol level, prolongs LDL to oxidation, improves vascular function, and decreases atherosclerotic plaque formation in the animal model. Suggesting that the supplementation of daily White kwao krua along with diet could moderate the atherogenesis and should be used as the dietary supplement.
Funding source
This work was supported by National Research Council of Thailand.
Declaration of competing interest
This statement is to certify that all authors have no conflict of interest to declare.
Acknowledgements
The authors would like to thank Assoc. Prof. Dr. Yupin Sanvarinda for her helpful advice and encouragement of this paper. We also would like to thanks Assoc. Prof. Dr. Amphawan Apisariyakul for kindly provide White kwao krua, and Prof. Dr. Apichart Suksamrarn for kindly provide Turmeric powder for using in this study. The graphical abstract was prepared under BioRender Web application.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Spagnoli L.G., Bonanno E., Sangiorgi G., Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007;48(11):1800–1815. doi: 10.2967/jnumed.107.038661. [DOI] [PubMed] [Google Scholar]
- 2.Honjo T., Otsui K., Shiraki R. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium. 2008;15(3):137–141. doi: 10.1080/10623320802125433. 2008/01/01. [DOI] [PubMed] [Google Scholar]
- 3.Gu L., Okada Y., Clinton S.K. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2(2):275–281. doi: 10.1016/s1097-2765(00)80139-2. 1998/08/01/ [DOI] [PubMed] [Google Scholar]
- 4.Quehenberger O. Thematic review series: the immune system and atherogenesis. Molecular mechanisms regulating monocyte recruitment in atherosclerosis. JLR (J Lipid Res) 2005;46(8):1582–1590. doi: 10.1194/jlr.R500008-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Gong K.W., Zhao W., Li N. Air-pollutant chemicals and oxidized lipids exhibit genome-wide synergistic effects on endothelial cells. Genome Biol. 2007;8(7):R149. doi: 10.1186/gb-2007-8-7-r149. 2007/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohman A., Sudjadi Devi, Ramadhani D., Nugroho A. Analysis of curcumin in curcuma longa and Curcuma xanthorriza using FTIR spectroscopy and chemometrics. Res J Med Plant. 2015 08/04;9:179–186. [Google Scholar]
- 7.Ramírez-Tortosa M.C., Mesa M.D., Aguilera M.C. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147(2):371–378. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 8.Quiles José L., Mesa M.D., Ramírez-Tortosa César L. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler Thromb Vasc Biol. 2002 2002/07/01;22(7):1225–1231. doi: 10.1161/01.atv.0000020676.11586.f2. [DOI] [PubMed] [Google Scholar]
- 9.Olszanecki R., Jawien J., Gajda M. Effect of curcumin on atherosclerosis in apoE/LDLR - double knockout mice. J Physiol Pharmacol : An Off. J. Pol. Physiol. Soc. 2005 12/01;56:627–635. [PubMed] [Google Scholar]
- 10.Sanvarinda Y., Phivthong-ngam L., Wattanapitayakul S. 2003. Antioxidative Effect of Pueraria Mirifica. Conference Proceedings-Chemistry; p. 87. 2003. [Google Scholar]
- 11.Cherdshewasart W., Subtang S., Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharmaceut Biomed Anal. 2007;43(2):428–434. doi: 10.1016/j.jpba.2006.07.013. 2007/01/17/ [DOI] [PubMed] [Google Scholar]
- 12.Lee J.H., Kim J.Y., Cho S.-H. Determination of miroestrol and isomiroestrol from pueraria mirifica (white kwao krua) in dietary supplements by LC–MS-MS and LC–Q-orbitrap/MS. J Chromatogr Sci. 2017;55(3):214–221. doi: 10.1093/chromsci/bmw171. [DOI] [PubMed] [Google Scholar]
- 13.Cho J.-G., Park H.-J., Huh G.-W. Flavonoids from Pueraria mirifica roots and quantitative analysis using HPLC. Food Science and Biotechnology. 2014;23(6):1815–1820. 2014/12/01. [Google Scholar]
- 14.Udomsin O., Juengwatanatrakul T., Yusakul G., Putalun W. Chromene stability: the most potent estrogenic compounds in White Kwao Krua (Pueraria candollei var mirifica) crude extract. Journal of Functional Foods. 2015;19:269–277. 2015/12/01/ [Google Scholar]
- 15.Shimokawa S., Kumamoto T., Ishikawa T. Quantitative analysis of miroestrol and kwakhurin for standardisation of Thai miracle herb ’Kwao Keur’ (Pueraria mirifica) and establishment of simple isolation procedure for highly estrogenic miroestrol and deoxymiroestrol. Nat Prod Res. 2013 2013/03;27(4-5):371–378. doi: 10.1080/14786419.2012.695370. [DOI] [PubMed] [Google Scholar]
- 16.Chansakaow S., Ishikawa T., Seki H., Sekine K., Okada M., Chaichantipyuth C. Identification of deoxymiroestrol as the actual rejuvenating principle of “kwao keur”, pueraria mirifica. The known miroestrol may Be an artifact. J Nat Prod. 2000;63(2):173–175. doi: 10.1021/np990547v. 2000/02/01. [DOI] [PubMed] [Google Scholar]
- 17.Chansakaow S., Ishikawa T., Sekine K. Isoflavonoids from pueraria mirifica and their estrogenic activity. Planta Med. 2000 09/01;66:572–575. doi: 10.1055/s-2000-8603. [DOI] [PubMed] [Google Scholar]
- 18.Lawanprasert S., Phivthong-ngam L., Srichairat S., Niwattisaiwong N., Charoenkul K., Chaichantipayuth C. Effects of Pueraria mirifica subchronic exposure on hepatic cytochrome P450 in rats fed with normal and high -cholesterol diets. Thai Journal of Pharmacology. 2006 01/01;28:21–32. [Google Scholar]
- 19.Miller V.T., LaRosa J., Barnabei V. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the postmenopausal estrogen/progestin interventions (PEPI) trial. J Am Med Assoc. 1995;273(3):199–208. [PubMed] [Google Scholar]
- 20.Okamura S., Sawada Y., Satoh T. Pueraria mirifica phytoestrogens improve dyslipidemia in postmenopausal women probably by activating estrogen receptor subtypes. Tohoku J Exp Med. 2009 01/01;216:341–351. doi: 10.1620/tjem.216.341. [DOI] [PubMed] [Google Scholar]
- 21.Manonai J., Chittacharoen A., Udomsubpayakul U., Theppisai H., Theppisai U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause. 2008;15(3) doi: 10.1097/gme.0b013e31815c5fd8. [DOI] [PubMed] [Google Scholar]
- 22.Wattanapitayakul S.K., Chularojmontri L., Srichirat S. Effects of Pueraria mirifica on vascular function of ovariectomized rabbits. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2005 Jun;88(Suppl 1):S21–S29. [PubMed] [Google Scholar]
- 23.Piyachaturawat P., Gansar R., Suksamrarn A. Choleretic effect of curcuma comosa rhizome extracts in rats. Int J Pharmacogn. 1996;34(3):174–178. 1996/01/01. [Google Scholar]
- 24.Redgrave T.G., Roberts D.C.K., West C.E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65(1):42–49. doi: 10.1016/0003-2697(75)90488-1. 1975/05/12/ [DOI] [PubMed] [Google Scholar]
- 25.Goldstein a L.J., Brown S.M. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46(1):897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 26.Henriksen T., Evensen S.A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Investig. 1979;39(4):361–368. doi: 10.3109/00365517909106120. 1979/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen T., Mahoney E.M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe J-i, Berk B.C. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc Med. 1998;8(2):59–64. doi: 10.1016/S1050-1738(97)00133-3. 1998/02/01/ [DOI] [PubMed] [Google Scholar]
- 29.Sies H., Stahl W., Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135(5):969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 30.Murphy R.C., Johnson K.M. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J Biol Chem. 2008;283(23):15521–15525. doi: 10.1074/jbc.R700049200. 2008 June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witztum J.L. Susceptibility of low-density lipoprotein to oxidative modification. Am J Med. 1993 1993/04/01;94(4):347–349. doi: 10.1016/0002-9343(93)90143-d. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y., Blumenthal R.S., Flaws J.A. Non–high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. JAMA Internal Medicine. 2001;161(11):1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 33.Witztum J.L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 12/01;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parthasarathy S., Steinberg D., Witztum J.L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43(1):219–225. doi: 10.1146/annurev.me.43.020192.001251. 1992/02/01. [DOI] [PubMed] [Google Scholar]
- 35.Schwab U.S., Ausman L.M., Vogel S. Dietary cholesterol increases the susceptibility of low density lipoprotein to oxidative modification. Atherosclerosis. 2000;149(1):83–90. doi: 10.1016/s0021-9150(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 36.Lin K.-Y., Chen Y.-L., Shih C.-C., Pan J.-P., Chan W.-E., Chiang A.-N. Contribution of HDL-apolipoproteins to the inhibition of low density lipoprotein oxidation and lipid accumulation in macrophages. J Cell Biochem. 2002;86(2):258–267. doi: 10.1002/jcb.10210. 2002/01/01. [DOI] [PubMed] [Google Scholar]
- 37.Mertens A.N.N., Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. Faseb J. 2001;15(12):2073–2084. doi: 10.1096/fj.01-0273rev. 2001/10/01. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs M., Plane F., Bruckdorfer K.R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol. 1990;100(1):21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. 1990/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeiher A.M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83(2):391–401. doi: 10.1161/01.cir.83.2.391. 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 40.Kolodgie F.D., Virmani R., Rice H.E., Mergner W.J. Vascular reactivity during the progression of atherosclerotic plaque. A study in Watanabe heritable hyperlipidemic rabbits. Circ Res. 1990;66(4):1112–1126. doi: 10.1161/01.res.66.4.1112. 1990/04/01. [DOI] [PubMed] [Google Scholar]
- 41.Böger R.H., Bode-Böger S.M., Gerecke U., Frölich J.C. Long term administration of L-arginine, L-NAME, and the exogenous NO donor molsidomine modulates urinary nitrate and cGMP excretion in rats. Cardiovasc Res. 1994;28(4):494–499. doi: 10.1093/cvr/28.4.494. [DOI] [PubMed] [Google Scholar]
- 42.Steinbrecher U.P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta Lipids Lipid Metabol. 1988;959(1):20–30. doi: 10.1016/0005-2760(88)90145-2. 1988/03/04/ [DOI] [PubMed] [Google Scholar]
- 43.Davignon J., Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23_suppl l_1) doi: 10.1161/01.CIR.0000131515.03336.f8. 2004/06/15. III-27-III-32. [DOI] [PubMed] [Google Scholar]
- 44.Stoll G., Bendszus M. Inflammation and atherosclerosis. Stroke. 2006;37(7):1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. 2006/07/01. [DOI] [PubMed] [Google Scholar]
- 45.Egashira K., Hirooka Y., Kai H. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89(6):2519–2524. doi: 10.1161/01.cir.89.6.2519. 1994/06/01. [DOI] [PubMed] [Google Scholar]
- 46.Levine Glenn N., Frei B., Koulouris Spyridon N., Gerhard Marie D., Keaney John F., Vita Joseph A. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93(6):1107–1113. doi: 10.1161/01.cir.93.6.1107. 1996/03/15. [DOI] [PubMed] [Google Scholar]
- 47.Shore B., Shore V. Rabbits as a model for the study of hyperlipoproteinemia and atherosclerosis. In: Day C.E., editor. Atherosclerosis Drug Discovery. Springer US; Boston, MA: 1976. pp. 123–141. [DOI] [PubMed] [Google Scholar]
- 48.Bocan T.M.A., Bak Mueller S., Mazur M.J., Uhlendorf P.D., Quenby Brown E., Kieft K.A. The relationship between the degree of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis. 1993;102(1):9–22. doi: 10.1016/0021-9150(93)90080-e. 1993/08/01/ [DOI] [PubMed] [Google Scholar]
- 49.Srisawat S., Phivthong-ngam L., Unchern S., Chantharaksri U., Govitrapong P., Sanvarinda Y. Improvement of vascular function by chronic administration of a cyclo-oxygenase inhibitor in cholesterol-fed rabbits. Clin Exp Pharmacol Physiol. 2003;30(5-6):405–412. doi: 10.1046/j.1440-1681.2003.03850.x. 2003/05/01. [DOI] [PubMed] [Google Scholar]
- 50.Chumark P., Khunawat P., Sanvarinda Y. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008;116(3):439–446. doi: 10.1016/j.jep.2007.12.010. 2008/03/28/ [DOI] [PubMed] [Google Scholar]
- 51.Bittencourt M.S., Cerci R.J. Statin effects on atherosclerotic plaques: regression or healing? BMC Med. 2015;13:260. doi: 10.1186/s12916-015-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conde K., Pineda G., Newton R.S., Fernandez M.L. Hypocholesterolemic effects of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors in the Guinea pig: atorvastatin versus simvastatin. Biochem Pharmacol. 1999;58(7):1209–1219. doi: 10.1016/s0006-2952(99)00203-8. 1999/10/01/ [DOI] [PubMed] [Google Scholar]
- 53.Oranje W.A., Sels J.-P.J.E., Rondas-Colbers G.J.W.M., Lemmens P.J.M.R., Wolffenbuttel B.H.R. Effect of atorvastatin on LDL oxidation and antioxidants in normocholesterolemic type 2 diabetic patients. Clin Chim Acta. 2001;311(2):91–94. doi: 10.1016/s0009-8981(01)00549-6. 2001/09/25/ [DOI] [PubMed] [Google Scholar]
- 54.Alwi I., Santoso T., Suyono S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta medica Indonesiana. 2008 11/01;40:201–210. [PubMed] [Google Scholar]
- 55.Qin S., Huang L., Gong J. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J. 2017;16(1):68. doi: 10.1186/s12937-017-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front Med. 2012;6(1):8–21. doi: 10.1007/s11684-012-0184-8. 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 57.John L.I., Satoshi T., Stanley Z.D. A chemical investigation of pueraria mirifica roots. Z Naturforsch C Biosci. 1986;41(4):403–408. [Google Scholar]
- 58.John L.I., Satoshi T., Stanley Z.D. Coumestans from the roots of pueraria mirifica. Z Naturforsch C Biosci. 1988;43(1-2):5–10. [Google Scholar]
- 59.John L.I., Satoshi T., Stanley Z.D. Minor isoflavones from the roots of pueraria mirifica. Z Naturforsch C Biosci. 1989;44(9-10):724–726. [Google Scholar]
- 60.Malaivijitnond S., Kiatthaipipat P., Cherdshewasart W., Watanabe G., Taya K. Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J Pharmacol Sci. 2004 2004/12;96(4):428–435. doi: 10.1254/jphs.fpj04029x. [DOI] [PubMed] [Google Scholar]
- 61.J H.E.H., W H.B., P G.S. The effect OF miroestrol ON vaginal cornification, pituitary function and pregnancy IN the rat. J Endocrinol. 1961;22(3):293–302. doi: 10.1677/joe.0.0220293. [DOI] [PubMed] [Google Scholar]
- 62.Cain J.C. Mirœstrol: an œstrogen from the plant pueraria mirifica. Nature. 1960;188(4753):774–777. doi: 10.1038/188774a0. 1960/12/01. [DOI] [PubMed] [Google Scholar]
- 63.Manonai J., Chittacharoen A., Udomsubpayakul U., Theppisai H., Theppisai U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause. 2008;15(3):530–535. doi: 10.1097/gme.0b013e31815c5fd8. [DOI] [PubMed] [Google Scholar]
- 64.Zhan S., Ho S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81(2):397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]