Abstract
In recent times, many scientists have given great attention to nutraceuticals (complementary medicine) as it widely used for promoting health status. In particular for the prevention and treatment of various neurological diseases or disorders without or less adverse effects. The current mini-review was intended to compile all popular (major) nutraceuticals against various neurodegenerative diseases (NDDs) including Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD) with special reference to clinical trials. Preliminary reviews indicated that nutraceuticals like curcumin, resveratrol, Epigallocatechin-3-gallate (EGCG), Coenzyme Q10, ω-3 FA (DHA/EPA/ALA), showed better neuroprotective activity against various NDDs in human setting (clinical trial). Hence this contribution will focus only on those popular nutraceuticals with proposed brief mechanisms (antioxidant, anti-inflammatory, mitochondrial homeostasis, autophagy regulation, promote neurogenesis) and its recommendation. This mini-review would aid common people to choose better nutraceuticals to combat various NDDs along with standard neuroprotective agents and modified lifestyle pattern.
Keywords: Nutraceutical, Neurodegenerative diseases, Clinical trial, Neuroprotective agents
Abbreviations: NDDs, neurodegenerative diseases; PD, Parkinson’s disease; AD, Alzheimer’s disease; HD, Huntington’s disease; ATP, Adenosine triphosphate; BBB, Blood-brain barrier; Nrf2, Nuclear factor-E2-related factor; HO-1, Heme Oxygenase-1; NF-κB, Nuclear factor Kappa B; SIRT1, Sirtuin 1; MAPK, Mitogen-activated protein kinase; JNK, c-Jun N-terminal Kinase; PI3K, Phosphatidylinositol-3-kinase
Graphical abstract
1. Introduction
Neurodegenerative diseases/disorders (NDDs) are a heterogeneous group of various chronic debilitating condition or diseases which, affects the peripheral or central nervous system (PNS and CNS). The major NDDs includes Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), prion disease, motor neuron disease (MND) are mainly caused due to lowered neuronal counts (decreased neural progenitor cells; NPCs) or loss of neuronal integrity (protein tangle/aggregation) as well as lack of communication (decreased neurotransmitters) which eventually results in loss of cognitive (memory impairment), sensory and, motor functions.1,2 NDDs like PD, AD and, HD can result in dementia and depression and thus contribute to elevated mortality and morbidity, (disabilities) which pose an enormous economical burden.3,4 Also, NDDs are highly connected to aging, and hence the prevalence rate is higher in older people than young owing to decline or altered hormone secretion, enhanced oxidative stress, and, neuro-inflammation. Several studies also demonstrated that NDDs have a direct impact on the social-economic status of any community or country as it hampers cognition and physical (unhealthy aging) or motor movement (quality of life) as well as an incurable condition. Also, it brings emotional (psychological) and, physical stress to families of the affected person. It has been estimated that globally about 47 million people might live with dementia (cognitive decline) and, other NDDs by the year 2050.5
The prevalence of NDDs is increasing considerably especially in western countries as compared to Asian countries due to modified lifestyles (food habits and sedentary lifestyles). Pathophysiology denominator of most NDDs includes neuro-oxidative stress, inflammation, altered cellular energetics (mitochondrial dysfunction), calcium overload, autophagy, elevated endoplasmic reticulum (ER) stress and, apoptosis.6 However, the in-depth mechanism of PD, AD and, HD are yet to fully explored (hence its incurable till date). Moreover, the brain is highly prone to oxidative stress owing to high fatty acid content (especially polyunsaturated fatty acids, which contributes to 10% of total dry brain weight), high oxygen consumption and redox signaling (about 20% basal oxygen for ATP production), low antioxidant content with higher neurotransmitter auto-oxidation.7 Currently, various anti-inflammatory (NSAIDs) and antioxidants are recommended to alleviate symptoms and to slow the progression of various NDDs. Therefore, any nutraceuticals which can pass through the blood-brain barrier (BBB) with potent antioxidant, anti-inflammatory, anti-protein aggregation, anti-apoptotic activities as well as suppress abnormal mitochondrial dynamics (multi-faceted along with high bioavailability rate) would be an effective treatment agent against NDDs to slow the progression of neuronal degeneration.6,8
2. Nutraceuticals
Nutraceuticals are the combination of pharmaceutical and nutrition, which was first coined by Stephen L DeFelice. Nutraceuticals refer to any food particles (whole) or a part of the food (purified food product) which renders health or medical benefits including, the prevention and treatment of disease.9,10 Nevertheless, recently nutraceuticals are redefined as a food product or its secondary metabolites that could deliver health benefits (to prevent or treat diseases) in the clinical setting.11,12 Numerous studies have indicated the neuroprotective effect of nutraceuticals (complementary medicine) against various NDDs via regulating energy metabolism, neuro-oxidative stress, neuroinflammation and enhance neurogenesis (improve NPCs proliferation, growth factor, neurotrophins) through various signaling pathways.13,14 However, only a few nutraceuticals are effective in a clinical setting which, complicate the right choice of nutraceuticals for the management of NDDs and its related symptoms. Therefore, this mini-review was intended to compile all popular (major) nutraceuticals against which, might be effective against various NDDs especially PD, AD, HD and, prion disease as well as depression (sequential effect of NDDs) with special reference to clinical trials and its proposed brief mechanism (including in vitro and animal studies). Hence, this contribution would aid common people to choose better nutraceuticals to combat various NDDs along with standard neuroprotective agents and modified lifestyle pattern.
The nutraceuticals chosen for this mini-review were obtained from several databases (search engine) including, Google Scholar, Web of Science, Scopus, PubMed, Science Direct. Using a combination of various keywords such as “neuroprotection, anti-neurotoxicity, nutraceuticals, complementary medicine” in a clinical setting (clinical trials) as well as a cell line or in-vitro and animal studies (pre-clinical studies) were also included but only for explaining the proposed brief neuroprotective mechanism. Thus, the author’s clear intention is to include only effective neuroprotective nutraceuticals (positive impact) against various neurodegenerative conditions in a clinical setting for better understating (mechanism) and recommendation (treatment/prevention).
2.1. Curcumin
Curcumin is an active ingredient of turmeric (Curcuma longa L). which belongs to the ginger family Zingiberaceae and commonly grown in tropical and sub-tropical countries. Turmeric has been used in various cuisine (flavoring/coloring agent) and also folklore remedy for treating various diseases/ailments (therapeutic function). Curcumin (major curcuminoid), is the main contributor for various biological functions of turmeric and hence used as a nutraceutical (most extensively used nutraceuticals).15 Curcumin displays an array of therapeutic functions like anti-inflammation, antioxidant, anti-obesity, anti-cancer, anti-diabetic, anti-microbial as well as neuroprotective and cardioprotective properties.16,17 Furthermore, curcumin is economical and well-tolerated (safe) and can effectively pass through the Blood-brain barrier (BBB) and thus exhibit strong neuroprotection. However, the major disadvantage of curcumin is the limited bioavailability (poor absorption and rapid metabolism), to overcome this bioavailability challenge, curcumin is combined with piperine to form curcumin-piperine complex or complexed with liposomes or nanoparticles (enhance bio-availability).18
2.1.1. Proposed neuroprotective mechanism
Copious studies have confirmed the neuroprotective activity of curcumin through improving antioxidant status (Nrf2 signaling pathway) and exhibit anti-inflammatory properties by inhibiting neuronal damage via suppressing microglial, astrocytes activation by inhibiting NF-κB, TLR4/RAGE, JNK, ERK, and MAPK (p38) signaling pathway.19,20 Anti-amyloidogenic property or anti-protein aggregation/misfolding (Aβ amyloid/β secretase, α-synuclein, tau, and prions protein) of curcumin was reported through modulating PPAR-γ, restoring innate immune system (via type I macrophages) as well as restore autophagy machinery and the ubiquitin-proteasome system (regulating SIRT-1/mTOR) and thus alleviate AD and neuro-inflammatory symptoms.18,21 Anti-depression (alter HPA axis) activity was reported by lowering cortisol and inhibit monoamine oxidase A and B and also improve neurotrophic factors.22,23 Moreover, curcumin can enhance neuroplasticity (improve neuron production) and inhibit acetyl/butyryl choline esterase (AChE/BuChE; anti-AD) and monoamine oxidases (anti-PD) activities and improve mitochondrial dynamics or membrane potential (mitigating mitochondrial dysfunction by alleviating calcium overload) as well as ameliorate neurotropic growth factors (BDNF, GDNF.18,23 Moreover, curcumin is also employed for detecting amyloid plaque (imaging system-MRI) in AD model and might help in early diagnosis and effective treatment.24
2.1.2. Clinical trial evidence
Baum and his colleagues25 conducted a placebo-controlled pilot clinical trial in Chinese AD patients (n = 36) by administrating 1 or 4 g of curcumin for six months showed a slight improvement in cognitive function (due to slower decline in neural loss) as compared to placebo (but no significant changes). Another clinical trial carried out by treating with curcumin for 4–8 weeks displayed modest anti-depressant effect in major depression disorder (MDD) patients.26 Sanmukhani and others,27 concluded that treatment with curcumin (1000 mg/day) would effectively and safely lower the Hamilton Depression Rating Scale (HDRS-17) in MDD patients. Most of the clinical trials were conducted with curcumin at dosage 0.1 g–1.5 g and in few cases, 4 or 5 g were also used. Overall, curcumin showed some positive effect against various NDDs especially against AD, and depression in humans, which were confirmed by a systemic review conducted by Voulgaropoulou et al.28 Nevertheless, further trials would be needed to confirm the effect neuroprotective functions against various NDDs in clinical settings.
2.2. Coenzyme Q10 (CoQ10)
Coenzyme Q10 (CoQ10) is an electron acceptor (ubiquitous molecules) and plays a crucial role in energy metabolism (ATP production via ETC) as well as acts as a potent antioxidant (lipophilic). Its major food sources are organ meat, fatty fish, broccoli and, cauliflower. It is safe and well-tolerated in all types of subjects with minimal adverse effects.29 CoQ10 possesses a wide range of therapeutic effects including anti-inflammatory, antioxidant, anti-hyperlipidemic, anti-hyperglycemic, and, cardioprotective activities.30,31 Moreover, it can pass through BBB and reported to be effective against various NDDs.10,32 CoQ10 has poor water-soluble property (lipophilic) and hence show modest bioavailability in human setting and hence it can be complexed or delivered using nanoparticles or any drug-emulsifying delivering system.33
2.2.1. Proposed neuroprotective mechanism
CoQ10 shows potent neuroprotective property through improving endogenous antioxidant system via improving the Nrf2/HO-1 (ARE) signaling pathway as well as to attenuate inflammatory markers (cytokines and microglial activation) by suppressing NF-κB signaling pathway and thus protect the nigrostriatal dopaminergic system.30,34 Many reports have indicated that the administration of CoQ10 can considerably improve the mitochondrial function (mitochondrial dysfunction via altered calcium flow) as well as neuronal function and locomotory function through modulating PPAR-γ and Akt signaling pathway.31,32 Also, CoQ10 could effectively inhibit AChE activity (anti-AD property) as well as inhibit α-syn aggregation and subsequent Lewy bodies formation (anti-PD activity).35
2.2.2. Clinical trial evidence
The first trial conducted by Shults and his co-workers36 demonstrated that supplementation of CoQ10 would considerably enhance the activity of mitochondria (Complexes-I/II/III) and thus suggest that CoQ10 treatment might be useful for treating PD and its related symptoms. The reduced form of CoQ10 (ubiquinol-10) was shown to be safe and well-tolerated at the dosage of 300 mg and improve the symptoms of PD by lowering total Unified Parkinson’s Disease Rating Scale (UPDRS) scores through reversing mitochondrial abnormalities.37 However, a meta-analysis conducted by Zhu and others38 including, eight clinical trials concluded that CoQ10 is well tolerable (safe) but not improved the UPDRS scores and motor function than placebo. A double-blind clinical trial conducted with 69 bipolar depression showed that supplementation of CoQ10 for 8 weeks at a dose of 200 mg/day could considerably improve the symptoms of depression owing to its antioxidant and anti-inflammatory property.35 Altogether, CoQ10 supplementation markedly lowered PD, depression and its related symptoms, which were evidenced from the various clinical trial and can be recommended as a complementary therapy.
2.3. Resveratrol
Resveratrol is a stilbene (polyphenol) that belongs to the phytoalexin family and usually found in red grapes, red cherries, peanut, pomegranate, and, berries. Resveratrol, is one of the popular nutraceuticals as it shows various health-promoting properties due to its anti-inflammatory, anti-diabetic, anti-cancer, antioxidant, anti-hyperlipidemic activity as well as cardioprotective and neuroprotective properties (due to better BBB crossing property).6,39 It is also safe and well tolerable by all types of people. One of the biggest disadvantages of resveratrol is the poor bioavailability due to high metabolizing rate (instability), poor lipophilic property and hence it’s esterified (food matrix) with piperine or quercetin as well as by liposomal-encapsulation.40
2.3.1. Proposed neuroprotective mechanism
Resveratrol notably improves BBB integrity via enhancing antioxidant system through upregulating Nrf2/HO-1 and PI3K/Akt signaling pathway as well as effectively attenuate the inflammatory response via regulating NF-κB and JNK/MAPK signaling pathway.41,42 Reports have confirmed that resveratrol could positively modulate SIRT1 (restore autophagy by lowering ER stress), PPAR, ERK and AMPK (PGC-1α) and thus regulate mitochondrial function (energy metabolism) and improve neuronal survival rate as well as stimulate neurogenesis (increase BDNF/GDNF and enhance NPCs proliferation and renewal).9,43,44 The major neuroprotective property of resveratrol is by acting as anti-protein aggregation/misfolding or anti-amyloidogenic property (anti-amyloidogenesis) by abolishing the neurofibrillary tau protein tangles or Aβ protein formation and deposition (hyperphosphorylation) and thereby improve cognition function.43,45 In addition, it restores the levels of ATG4 and thus facilitate the degradation of polyQ Huntingtin (PolyQ-Htt) protein aggregation (restore autophagy activity) and thereby protect neurons from dopamine toxicity.46
2.3.2. Clinical trial evidences
As compared to the placebo group, the resveratrol supplemented (200 mg) for 26 weeks significantly improved the cognitive function (word retention) as well as cerebral blood flow in the healthy adult.47 Plasma and cerebrospinal fluid (CSF) beta-amyloid (Aβ) 42 and Aβ40 levels were considerably higher in resveratrol treated group (for 52 weeks) than placebo group as well as considerably lowered the MMP-9 and thus strengthen the CNS and improved BBB.48 A randomized, placebo-controlled trial conducted by Evans and et al.49 concluded that regular consumption of 150 mg of trans-resveratrol for 14 weeks elicited 17% elevation in cerebrovascular responsiveness (CVR) and verbal memory as well as mood (cognition) as compared to placebo in postmenopausal women. However, the recent systematic review and meta-analysis performed with ten studies have revealed that the administration of resveratrol did not provide significant improvement in overall cognitive function but slightly improved some cognitive parameters.50 Taking together, resveratrol can significantly improve cognitive function in healthy and various NDDs patients. Further large-scale trials are needed to confirm the neuroprotective effect of resveratrol in humans.
2.4. Epigallocatechin-3-gallate (EGCG)
Epigallocatechin-3-gallate (EGCG) is one of the major catechins of green tea (polyphenol), which contributes to several beneficial functions especially cardioprotective, renoprotective, hepatoprotective and neuroprotective properties due to its biological activities like antioxidant, anti-inflammatory, anti-microbial, anti-platelet aggregation, pro-autophagy and anti-proliferative properties.51,52 EGCG is one of the well-known nutraceuticals owing to the above indicated therapeutic functions. EGCG can effectively pass through BBB and significantly lower neurological disorders. Nevertheless, owing to high water-soluble property, it can absorb properly (poor bio-availability) and hence fused with piperine/DHA/EPA (esterified) or complexed with liposomes or nanoparticles.53,54
2.4.1. Proposed neuroprotective mechanism
The major neuroprotective function of EGCG is exerted by its anti-amyloidogenic property which, includes the inhibition of Aβ1-42 amyloid fibril aggregation or production as well as suppress alpha-secretase/Synuclein protein/peptide misfolding via modulating apoptosis and autophagy (upregulating transcription factor EB; TFEB) pathway.14,55,56 Moreover, it enhances the neuronal growth factor (BDGF/GDNF) by inactivating microglial cells and improve antioxidant status via modulating various signaling pathways Nrf2/HO-1 (antioxidant) and NF-κB/JNK/MAPK signaling pathway (anti-inflammatory).1,57 ECGC is reported to lower the loss of nigrostriatal dopamine as well as reduce the photoreceptor degeneration and huntingtin protein (PolyQ-Htt) misfolding and thus improve motor function in the various animal model.58 In addition, it downregulates the downstream protein involved in ERK1/2 and PKC signaling pathway molecules and thus inhibiting Aβ amyloid/synuclein aggregation/accumulation as well as improve neurogenesis (increasing NPCs proliferation).8,59
2.4.2. Clinical trial evidence
A cohort study conducted by Tomata and his co-workers60 concluded that consumption of green tea catechin (rich in EGCG) would concomitantly improve cognition performance and memory in older Japanese subjects. Another trial carried out by supplementing a single dose of EGCG (300 mg) in healthy subjects significantly improved cognitive function, calmness as compared to placebo group.61 Noguchi-Shinohara and others,62 demonstrated that higher consumption of green tea (rich in EGCG) was associated with improved brain function (MCI) in the older Japanese population. However, a systematic review performed by including twenty one studies has evidenced that no single component (EGCG or theanine or caffeine) of green or black tea showed a beneficial effect on cognition, but when it combined together it showed a potential cognitive or neuroprotective effect and thus showcasing the holistic effect.63 To support the above statement, another review conducted by Kakutani and his colleagues64 by included three cohort and five cross-sectional studies, of which two cohort and four cross-sectional studies displayed positive neuroprotective effect of green tea polyphenol (catechin) by reducing the risk of dementia, Alzheimer’s disease, and, cognitive impairment. Hence, the consumption of green tea polyphenol or catechin could effetely improve brain/cognition activity than single components like EGCG or caffeine or theanine.
2.5. Polyunsaturated fatty acids (ω-3 FA)
Polyunsaturated fatty acids or omega-3- fatty acids (ω-3 FA) are classified as essential fatty acids as it cannot be synthesized by a human. The three major ω-3 FA includes α-linolenic acid (ALA; Short-chain FA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA; Long chained FA) play an array of beneficial function in the human system and commonly found in fatty fishes (salmon, sardines), flaxseed, walnuts.65 Especially, DHA and EPA are reported to showcase a wide spectrum of pharmaceutical activities such as anti-inflammatory, immunomodulatory, antioxidant anti-obesity, anti-hyperlipidemic (hypertriglyceridemia) and anti-diabetic as well as cardioprotective (improve endothelial function), neuroprotective activities.66,67 Recently studies have indicated that ω-3 FA can be beneficial for short term intervention, however, long-term intervention ends up in several adverse effects.68
2.5.1. Proposed neuroprotective mechanism
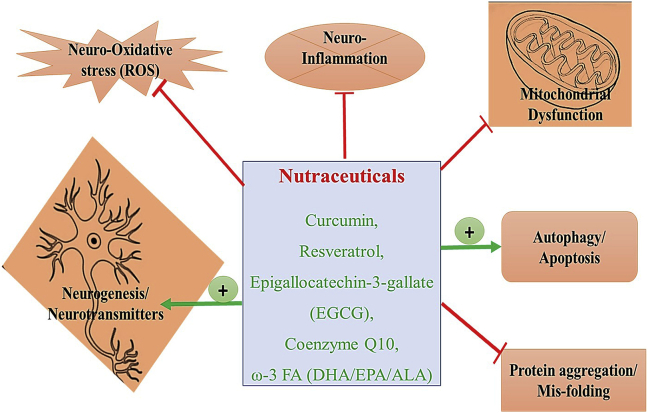
Polyunsaturated fatty acids or omega-3- fatty acids (ω-3 FA) can improve brain activity owing to anti or pro-oxidant (especially due to double bond) as well as display anti-inflammatory properties by inactivating microglia/astrocytes via JNK and PPAR-γ signaling pathway.69,70 It also mitigates amyloid β plaque (senile plaque) as well as aggregation of tau protein (hyperphosphorylation) via enhancing α-β 42 phagocytosis. Moreover, it effectively inhibits β/γ secretase enzyme (anti-amyloidogenic) as well as enhance neurotransmitter production and improve neurogenesis via producing neurotrophic growth factors and thus demonstrates its neuroprotective function.71,72 The compiled neuroprotective function (mechanism of action) of all popular nutraceuticals is shown in Fig. 1.
Fig. 1.
The compiled neuroprotective function (mechanism of action) of all popular nutraceuticals.
2.5.2. Clinical trial evidence
Morris and his colleague73 conducted a clinical trial by including 131 subjects developed Alzheimer’s disease and found that consumption of fish oil rich in DHA could considerably lower (60%) the risk of incident Alzheimer’s disease as compared to non-fish oil consumed AD subjects. A meta-analysis (6 RCT) also showed that higher consumption of fish oil was associated with a 36% reduction in the risk of AD. Moreover, increment (by 100 g) in fish oil consumption was associate with further 11% reduction in the risk of AD.74 A randomized, clinical trial indicate that supplementation of ω-3 FA from fish oil (4 capsules/day for 3 months) in parkinsonian patients with depression showed a significant decline in the levels of Montgomery Asberg rating scale (MADRS) and improved other mood scales as compared to control group (Vit E).75 A small open-label study performed in ten mild to moderated bipolar disorder subjects by treating with 1.5–2 g of ω-3 FA (DHA/EPA) for 6 months achieved a 50% reduction in HRS score and thus considerably reduce the depression rate.76 Lee and others77 conducted a randomized, double-blind, placebo-control trial by supplementation of DHA (fish oil) could considerably improve the working and verbal memory in mild cognitive impairment (MCI) patients. Most of the clinical trials were conducted with small-sized trial and hence further clinical trials with large size subjects are needed to confirm the beneficial effect of ω-3 FA against various neurological diseases or disorders.
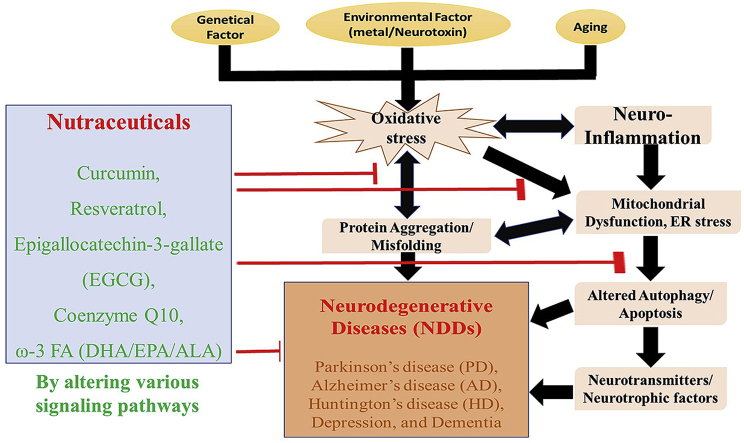
The overall schematic representation of the mini-review was represented in Fig. 2. The major limitation of nutraceuticals is its low bioavailability and rapid metabolism to secondary metabolites (crossing BBB and its effect on gut-brain axis) as well as lack of strong pharmacokinetics/dynamic data (dose, stability, toxicity, effective administration route) and its specific mode of action (multi-faceted) in the human setting.72 The major advantage of this contribution is by including only popular nutraceuticals with potent neuroprotective properties against various NDDs only in human settings and hence it will be useful for the common people to get the real picture of those popular nutraceuticals and be recommended along with standard neuro-therapeutic agents.
Fig. 2.
The overall schematic representation of the mini-review.
3. Conclusion and future perspectives
The current mini-review focusses only on popular (major) nutraceuticals like curcumin, resveratrol, EGCG, Coenzyme Q10, ω-3 FA (DHA/EPA/ALA) and revealed that supplementation of those nutraceuticals showed promising neuroprotective activity (well-tolerable) by improving cognitive function and neurotropic signaling pathway (improve neurogenesis) owing to antioxidant, anti-inflammatory, anti-protein aggregation, autophagy modulatory properties as well as regulate mitochondrial homeostasis in clinical settings. However, most of the clinical trial included in this review recruit only small size samples (a few healthy subjects) with short treatment regimen along with conventional neuroprotective drugs. Hence, in future a large-scale well-controlled clinical trial should be conducted in various NDDs patients (PD/AD/HD) with those popular nutraceuticals alone (need to confirm the bio-availability and nutrigenomic data) and along with neuroprotective therapeutic agents (inter-comparison). Overall, this mini-review would aid common people to choose better nutraceuticals to combat various NDDs along with standard neuroprotective agents and modified lifestyle pattern.
Declaration of competing interest
All authors declared that no conflict of interest regarding this review paper.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.03.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Farkhondeh T., Yazdi H.S., Samarghandian S. The protective effects of green tea catechins in the management of neurodegenerative diseases: a review. Curr Drug Discov Technol. 2019;16:57–65. doi: 10.2174/1570163815666180219115453. [DOI] [PubMed] [Google Scholar]
- 2.Adefegha S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J Diet Suppl. 2018;15:977–1009. doi: 10.1080/19390211.2017.1401573. [DOI] [PubMed] [Google Scholar]
- 3.Agnihotri A., Aruoma O.I. Alzheimer’s disease and Parkinson’s disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics, and environmental chemicals. J Am Coll Nutr. 2020;39:16–27. doi: 10.1080/07315724.2019.1683379. [DOI] [PubMed] [Google Scholar]
- 4.Wimo A., Jönsson L., Bond J., Prince M., Winblad B., International A.D. The worldwide economic impact of dementia 2010. Alzheimer Dement. 2013;9:1–10. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Qiu C., De Ronchi D., Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatr. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 6.Pourhanifeh M.H., Shafabakhsh R., Reiter R.J., Asemi Z. The effect of resveratrol on neurodegenerative disorders: possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr Pharmaceut Des. 2019;25:2178–2191. doi: 10.2174/1381612825666190717110932. [DOI] [PubMed] [Google Scholar]
- 7.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing L., Zhang H., Qi R., Tsao R., Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. 2019;67:1029–1043. doi: 10.1021/acs.jafc.8b06146. [DOI] [PubMed] [Google Scholar]
- 9.Venkatakrishnan K., Chiu H.F., Wang C.K. Popular functional foods and herbs for the management of type-2-diabetes mellitus: a comprehensive review with special reference to clinical trials and its proposed mechanism. J Funct Foods. 2019;57:425–438. [Google Scholar]
- 10.Chiu H.F., Shen Y.C., Venkatakrishnan K., Wang C.K. Popular functional foods and nutraceuticals with lipid lowering activity and in relation to cardiovascular disease, dyslipidemia, and related complications: an overview. J Food Bioactives. 2018;2:16–27. [Google Scholar]
- 11.Daliu P., Santini A., Novellino E. A decade of nutraceutical patents: where are we now in 2018? Expert Opin Ther Pat. 2018;28:875–882. doi: 10.1080/13543776.2018.1552260. [DOI] [PubMed] [Google Scholar]
- 12.Santini A., Novellino E. Nutraceuticals: shedding light on the grey area between pharmaceuticals and food. Expet Rev Clin Pharmacol. 2018;11:545–547. doi: 10.1080/17512433.2018.1464911. [DOI] [PubMed] [Google Scholar]
- 13.Pandareesh M.D., Kandikattu H.K., Razack S. Nutrition and nutraceuticals in neuroinflammatory and brain metabolic stress: implications for neurodegenerative disorders. CNS Neurol Disord - Drug Targets. 2018;17:680–688. doi: 10.2174/1871527317666180625104753. [DOI] [PubMed] [Google Scholar]
- 14.Dadhania P.V., Trivedi P.P., Vikram A., Nand Tripathi D. Nutraceuticals against neurodegeneration: a mechanistic insight. Curr Neuropharmacol. 2016;14:627–640. doi: 10.2174/1570159X14666160104142223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatakrishnan K., Chiu H.F., Wang C.K. Extensive review of popular functional foods and nutraceuticals against obesity and its related complications with a special focus on randomized clinical trials. Food Funct. 2019;10:2313–2329. doi: 10.1039/c9fo00293f. [DOI] [PubMed] [Google Scholar]
- 16.Mantzorou M., Pavlidou E., Vasios G., Tsagalioti E., Giaginis C. Effects of curcumin consumption on human chronic diseases: a narrative review of the most recent clinical data. Phytother Res. 2018;32:957–975. doi: 10.1002/ptr.6037. [DOI] [PubMed] [Google Scholar]
- 17.Perrone L., Squillaro T., Napolitano F., Terracciano C., Sampaolo S., Melone M.A. The autophagy signaling pathway: a potential multifunctional therapeutic target of curcumin in neurological and neuromuscular diseases. Nutrition. 2019;11:1881. doi: 10.3390/nu11081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari S.K., Agarwal S., Seth B. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 19.Limanaqi F., Biagioni F., Busceti C.L. Phytochemicals bridging autophagy induction and alpha-synuclein degradation in parkinsonism. Int J Mol Sci. 2019;20:3274. doi: 10.3390/ijms20133274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teter B., Morihara T., Lim G.P. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer’s pathogenesis. Neurobiol Dis. 2019;127:432–448. doi: 10.1016/j.nbd.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatami M., Abdolahi M., Soveyd N., Djalali M., Togha M., Honarvar N.M. Molecular mechanisms of curcumin in neuroinflammatory disorders: a mini review of current evidences. Endocr Metab Immune Disord - Drug Targets. 2019;19:247–258. doi: 10.2174/1871530319666181129103056. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni S.K., Bhutani M.K., Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacol. 2008;201(3):435. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 23.Maes M., Kallaur A.P., Lopes J., Oliveira S.R., Ramon D., Reiche E.M. Immune-inflammatory and oxidative and nitrosative stress (IO & NS) pathways in depression and multiple sclerosis (MS): shared IO & NS pathways but less hyper-acute neuro-inflammation explain the increased incidence of depression in MS. Neurol Psychiatr Brain Res. 2016;1:16–17. [Google Scholar]
- 24.Cheng K.K., Chan P.S., Fan S. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI) Biomaterials. 2015;44:155–172. doi: 10.1016/j.biomaterials.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Baum L., Lam C.W., Cheung S.K. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer’s disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 26.Lopresti A.L., Maes M., Maker G.L., Hood S.D., Drummond P.D. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. 2014;167:368–375. doi: 10.1016/j.jad.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Sanmukhani J., Satodia V., Trivedi J. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res. 2014;28:579–585. doi: 10.1002/ptr.5025. [DOI] [PubMed] [Google Scholar]
- 28.Voulgaropoulou S.D., van Amelsvoort T.A., Prickaerts J., Vingerhoets C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: a systematic review of pre-clinical and clinical studies. Brain Res. 2019;1725 doi: 10.1016/j.brainres.2019.146476. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S., Dubey K.K. CoQ10 a super-vitamin: review on application and biosynthesis. 3 Biotech. 2018;8:249. doi: 10.1007/s13205-018-1271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q10: from bench to clinic in aging diseases, a translational review. Crit Rev Food Sci Nutr. 2019;59:2240–2257. doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 31.Spindler M., Beal M.F., Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatric Dis Treat. 2009;5:597. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Zhang Y., Xu H. Neuroprotection of coenzyme Q10 in neurodegenerative diseases. Curr Top Med Chem. 2016;16:858–866. doi: 10.2174/1568026615666150827095252. [DOI] [PubMed] [Google Scholar]
- 33.Balakrishnan P., Lee B.J., Oh D.H. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72. doi: 10.1016/j.ijpharm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Jing L., He M.T., Chang Y. Coenzyme Q10 protects astrocytes from ROS-induced damage through inhibition of mitochondria-mediated cell death pathway. Int J Biol Sci. 2015;11:59. doi: 10.7150/ijbs.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrpooya M., Yasrebifar F., Haghighi M., Mohammadi Y., Jahangard L. Evaluating the effect of coenzyme Q10 augmentation on treatment of bipolar depression: a double-blind controlled clinical trial. J Clin Psychopharmacol. 2018;38:460–466. doi: 10.1097/JCP.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 36.Shults C.W., Haas R.H., Passov D., Beal M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol: Off J Am Neurol Assoc Child Neurol Soc. 1997;42:261–264. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- 37.Yoritaka A., Kawajiri S., Yamamoto Y. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Park Relat Disord. 2015;21:911–916. doi: 10.1016/j.parkreldis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Z.G., Sun M.X., Zhang W.L., Wang W.W., Jin Y.M., Xie C.L. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol Sci. 2017;38:215–224. doi: 10.1007/s10072-016-2757-9. [DOI] [PubMed] [Google Scholar]
- 39.Berman A.Y., Motechin R.A., Wiesenfeld M.Y., Holz M.K. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precision Oncol. 2017;1:1–9. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coimbra M., Isacchi B., van Bloois L. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm. 2011 Sep 20;416(2):433–442. doi: 10.1016/j.ijpharm.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 41.Moussa C., Hebron M., Huang X. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J Neuroinflammation. 2017;14:1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui Y., Chengyong T., Cheng L., Haixia H., Yuanda Z., Weihua Y. Resveratrol attenuates the cytotoxicity induced by amyloid-β 1–42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res. 2018;43:297–305. doi: 10.1007/s11064-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 43.Gomes B.A., Silva J.P., Romeiro C.F. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y., Li X., Zhu J.X. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasinetti G.M., Wang J., Ho L., Zhao W., Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2015;1852:1202–1208. doi: 10.1016/j.bbadis.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidoni C., Secomandi E., Castiglioni A., Melone M.A., Isidoro C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem Int. 2018;117:174–187. doi: 10.1016/j.neuint.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Witte A.V., Kerti L., Margulies D.S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner R.S., Thomas R.G., Craft S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer’s disease. Neurol. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans H., Howe P., Wong R. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrition. 2017;9:27. doi: 10.3390/nu9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marx W., Kelly J.T., Marshall S. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials. Nutr Rev. 2018;76:432–443. doi: 10.1093/nutrit/nuy010. [DOI] [PubMed] [Google Scholar]
- 51.Venkatakrishnan K., Chiu H.F., Cheng J.C. Comparative studies on the hypolipidemic, antioxidant and hepatoprotective activities of catechin-enriched green and oolong tea in a double-blind clinical trial. Food Funct. 2018;9:1205–1213. doi: 10.1039/c7fo01449j. [DOI] [PubMed] [Google Scholar]
- 52.Chiu H.F., Lin T.Y., Shen Y.C., Venkatakrishnan K., Wang C.K. Improvement of green tea polyphenol with milk on skin with respect to antioxidation in healthy adults: a double-blind placebo-controlled randomized crossover clinical trial. Food Funct. 2016;7:893–901. doi: 10.1039/c5fo01271f. [DOI] [PubMed] [Google Scholar]
- 53.Mereles D., Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int J Mol Sci. 2011;12:5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Rio D., Calani L., Cordero C., Salvatore S., Pellegrini N., Brighenti F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition. 2010;26:1110–1116. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Prasanth M.I., Sivamaruthi B.S., Chaiyasut C., Tencomnao T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients. 2019;11:474. doi: 10.3390/nu11020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezai-Zadeh K., Shytle D., Sun N. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer’s transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pervin M., Unno K., Ohishi T., Tanabe H., Miyoshi N., Nakamura Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules. 2018;23:1297. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelsey N.A., Wilkins H.M., Linseman D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakrawarti L., Agrawal R., Dang S., Gupta S., Gabrani R. Therapeutic effects of EGCG: a patent review. Expert Opin Ther Pat. 2016;26:907–916. doi: 10.1080/13543776.2016.1203419. [DOI] [PubMed] [Google Scholar]
- 60.Tomata Y., Kakizaki M., Nakaya N. Green tea consumption and the risk of incident functional disability in elderly Japanese: the Ohsaki Cohort 2006 Study. Am J Clin Nutr. 2012;95:732–739. doi: 10.3945/ajcn.111.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scholey A., Downey L.A., Ciorciari J. Acute neurocognitive effects of epigallocatechin gallate (EGCG) Appetite. 2012;58:767–770. doi: 10.1016/j.appet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Noguchi-Shinohara M., Yuki S., Dohmoto C. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PloS One. 2014;9 doi: 10.1371/journal.pone.0096013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancini E., Beglinger C., Drewe J., Zanchi D., Lang U.E., Borgwardt S. Green tea effects on cognition, mood and human brain function: a systematic review. Phytomedicine. 2017;34:26–37. doi: 10.1016/j.phymed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Kakutani S., Watanabe H., Murayama N. Green tea intake and risks for dementia, Alzheimer’s disease, mild cognitive impairment, and cognitive impairment: a systematic review. Nutrition. 2019;11:1165. doi: 10.3390/nu11051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kondo K., Morino K., Nishio Y. A fish-based diet intervention improves endothelial function in postmenopausal women with type 2 diabetes mellitus: a randomized crossover trial. Metabolism. 2014;63:930–940. doi: 10.1016/j.metabol.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Belchior T., Paschoal V.A., Magdalon J. Omega-3 fatty acids protect from diet-induced obesity, glucose intolerance, and adipose tissue inflammation through PPARγ-dependent and PPARγ-independent actions. Mol Nutr Food Res. 2015;59:957–967. doi: 10.1002/mnfr.201400914. [DOI] [PubMed] [Google Scholar]
- 67.Saini R.K., Keum Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance—a review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 68.Lange K.W., Nakamura Y., Gosslau A.M., Li S. Are there serious adverse effects of omega-3 polyunsaturated fatty acid supplements? J Food Bioactives. 2019;7:1–7. [Google Scholar]
- 69.Eckert G.P., Lipka U., Muller W.E. Omega-3 fatty acids in neurodegenerative diseases: focus on mitochondria. Prostaglandins Leukot Essent Fatty Acids. 2013;88:105–114. doi: 10.1016/j.plefa.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 70.Dong Y., Xu M., Kalueff A.V., Song C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur J Nutr. 2018;57:1781–1791. doi: 10.1007/s00394-017-1462-7. [DOI] [PubMed] [Google Scholar]
- 71.Hopperton K.E., Trépanier M.O., Giuliano V., Bazinet R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-β 1-40 in mice. J Neuroinflammation. 2016;13:257. doi: 10.1186/s12974-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avallone R., Vitale G., Bertolotti M. Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int J Mol Sci. 2019;20:4256. doi: 10.3390/ijms20174256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris M.C., Evans D.A., Bienias J.L. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer’s disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 74.Wu S., Ding Y., Wu F., Li R., Hou J., Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 75.da Silva T.M., Munhoz R.P., Alvarez C. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111:351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Osher Y., Bersudsky Y., Belmaker R.H. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatr. 2005;66:726–729. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 77.Lee L.K., Shahar S., Chin A.V., Yusoff N.A. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacol. 2013;225:605–612. doi: 10.1007/s00213-012-2848-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.