Abstract
Background
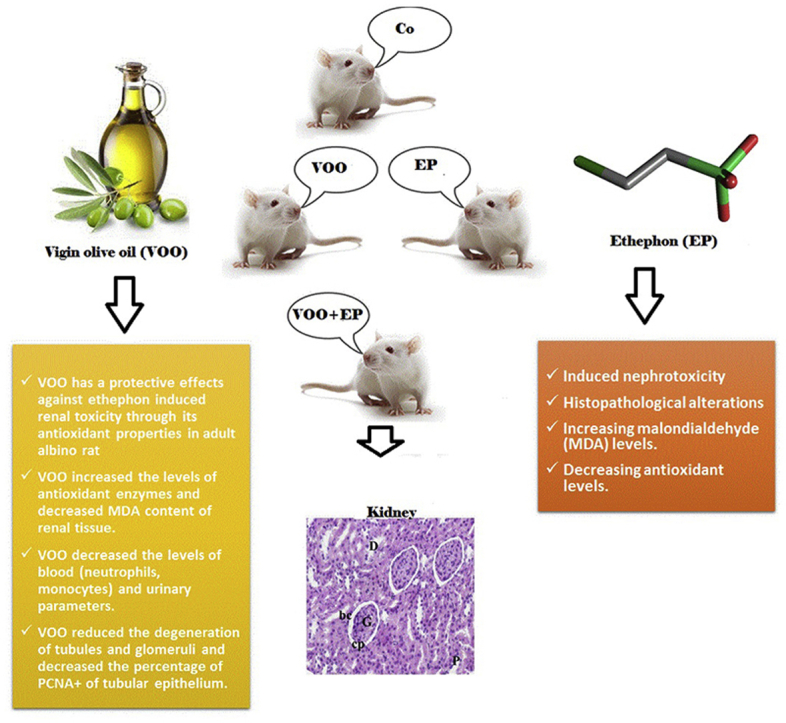
Ethephon (EP) is the most famous plant growth regulator with different adverse effects on kidney function. Virgin Olive Oil (VOO) is considered as a natural source of antioxidant with beneficial effects. Thus, this study was conducted to investigate the effects of VOO on nephrotoxicity induced by EP in rats.
Methods and materials
In this study, 80 male rats (weighing 200–250 g) were divided into four groups including I: control group received normal saline as vehicle, II: received VOO, III: received EP (150 mg/kg/day) for 2 months, IV: received EP (150 mg/kg/day for 2 months, after 2-month pretreatment with VOO. VOO (2 mL/kg/day) and vehicle were administered by gastric gavage for 2 months. At the end, the animals were sacrificed, and their blood and kidneys were used for examinations. Isolated kidneys were used for histopathological and oxidative stress studies.
Results
Significant increases were recorded in blood (neutrophils, monocytes) and urinary parameters as well as malondialdehyde (MDA) content in the group III compared to groups II and I (P˂0.05). Antioxidant enzymes significantly declined and histopathological alterations increased in the group III. In the group IV, significant decreases were recorded in blood and urinary parameters, MDA, and histopathological alterations and a significant increase were found in antioxidant enzymes compared to group III (P˂0.05).
Conclusions
Findings of the present study demonstrated protective effects of VOO in prevention of kidneys against EP -induced toxicity in albino rats.
Keywords: Virgin olive oil, Ethephon, Nephrotoxicity, Kidney, Histopathology, Rat
Graphical abstract
Highlights
-
•
Ethephon as a most famous example of plant growth regulator induced nephrotoxicity and histopathological alterations by increasing malondialdehyde (MDA) content and decreasing antioxidant levels.
-
•
Virgin olive oil (VOO) is considered as a natural source of antioxidant with hypoglycaemic, hypotensive, hepatoprotective cardiovascular effects.
-
•
VOO has a protective effects against ethephon induced renal toxicity through its antioxidant properties in adult albino rat
-
•
VOO increased the levels of antioxidant enzymes and decreased MDA content of renal tissue.
-
•
VOO decreased the levels of blood (neutrophils, monocytes) and urinary parameters.
-
•
VOO reduced the degeneration of tubules and glomeruli and decreased the percentage of PCNA+ of tubular epithelium.
List of abbreviations:
- EP
ethephon
- VOO
Virgin olive oil
- PGRs
plant growth regulators
- MUFA
monounsaturated fatty acids
- UA
Uric acid
- Cr
Creatinine
- BUN
blood urea nitrogen
- RBCs
red blood cells
- WBCs
white blood cells
- Hb
hemoglobin
- HCT
hematocrit
- MDA
malondialdehyde
- CAT
catalase
- GSH
glutathione
- SOD
superoxide dismutase
- GPx
Glutathione peroxidase
- PCNA
proliferating cell nuclear antigen
- H&E
hematoxylin & eosin
- TRI
Masson's trichrome
- PAS
Periodic Acid Schiff
- IHC
immunohistochemical
- DAB
3,3-diaminobenzidine tetrahydrochloride solution
1. Introduction
Extensive use of pesticides leads to renal toxicity in human and experimental animals. Pesticides have been reported as main cause of 27% of acute kidney diseases in the United States.1,2 Pesticides killing insects such as carbonates and organophosphates are known to induce nephrotoxicity.3 In recent decades, using chemicals such as Plant Growth Regulators (PGRs) in agriculture has been associated with many health hazards. Ethephon (EP, 2-chloroethyl phosphonic acid) is the most famous example of plant growth regulators used for artificial ripening through release of ethylene, directly influencing several physiological processes such as ripening and maturation. It is used on some crops including cereals, vegetables, fruits, and crops̓ seeds producing oil.4
Mechanism of action of EP is through release of ethylene followed by being absorbed by the plant and interfering in growth process,and is also used in acceleration of ripening of fruits and vegetables.5 Previous studies showed that, when EP is administered orally, it causes various types of disease including necrotic hepatitis gastroenteritis, and respiratory diseases, in both rats and mice.6,7 Other side effects related to EP administration include a significant increase in the kidney weight, glomeru-losclerosis, nephritis, glomerulonephritis, mineralization of brain, fibrosis of heart, etc.8,9 EP also produces hematological changes and inhibits activity of plasma cholinesterase in intoxicated animals.10 Also, EP has been shown to induce gonadal disorders,and its teratogenic effects have been demonstrated in mice.11,12 EP has been reported to reduce concentrations of DNA and RNA and induce mutagenic effects.13 Additionally, increased oxidative stress and immunotoxicity of EP have been demonstrated in the recent studies.14
The use of medicinal herbs and natural compounds has been proven in treatment and prevention of diseases.15, 16, 17, 18 Antioxidant activities of herbal medicines are effective in reducing toxicities related to toxic agents and different synthetic drugs.19,20 Global consumption of Virgin Olive Oil (VOO) known as a Mediterranean Diet has been significantly increased in the last few decades.21 Consumption of VOO has been suggested to protect individuals against cardiovascular diseases,22 different types of cancer,23 obesity, type II diabetes, and metabolic syndrome.24 Moreover, beneficial effects of VOO have been noticed in healthy individuals.25 Two main compositions of VOO are fatty acid and phenolic antioxidants.25 VOO contains a wide variety of antioxidants such as vitamin E, oleocanthal, carotenoids,and polyphenols like hydroxytyrosol, hydroxytyrosol acetate,and oleuropein, among which oleuropein especially prevents oxidation of Low-Density Lipoprotein (LDL) particles.26,27 Monounsaturated Fatty Acids (MUFA) especially oleic acid with beneficial effects on cardiovascular system constitutes 70–80% of total fatty acids of olive oil.28 Recently, researchers have focused on preventive effects of phenols with natural sources against degenerative diseases mediated by Reactive Oxygen Species (ROS).29,30 Polyphenols of VOO are characterized for their anti-inflammatory features, applied by down-regulation of inflammatory mediators through transcriptional or post-transcriptional mechanisms.31 Hepatoprotective effects of VOO32,33 and its ameliorative effects on nephrotoxicity induced by different agents have been demonstrated so that, nephrotoxicity was ameliorated in animals as a result of administrating VOO or olive leaf extract.34, 35, 36
Therefore, the present study was carried out to investigate the potential protective effects of VOO on some hematological parameters and nephrotoxicity induced by sub-chronic administration of EP in male albino rats.
2. Materials and methods
2.1. Chemicals and drugs
Ethrel, in commercial form containing 48% EP (2-chloroethyl phosphonic acid) was purchased from Chema Industries. Virgin Olive Oil (VOO) was purchased from local market and was analyzed in laboratory in Taif University, Taif, Saudi Arabia (analytical parameters including fatty acids, oxidative stability, and antioxidant composition are demonstrated in Table 1). VOO contained 68.23% of monounsaturates (mainly oleic acid), 16.29% of saturates (palmitic and stearic acids), and 14.21% of polyunsaturated fatty acid. Urea, Uric Acid (UA), Creatinine (Cr) and Blood Urea Nitrogen (BUN) kits were purchased from Biovision Inc., (USA). Other chemicals were purchased from Sigma Company (St. Louis, MO, USA).
Table 1.
Mean values of analytical parameters, fatty acids composition (%) and antioxidant content of VOO.
| Mean | |
|---|---|
| Palmitic acid | 11.21 |
| Palmitoleic acid | 0.69 |
| Stearic acid | 3.69 |
| Oleic acid | 63.38 |
| Linoleic acid | 14.34 |
| Linolenic acid | 0.71 |
| Arachidic acid | 0.69 |
| Gadoleic acid | 0.7 |
| Behenic acid | 2.84 |
| MUFA | 68.33 |
| SFA | 16.3 |
| PUFA | 15.1 |
| Total polyphenols (mg/kg) | 561.3 |
| Chlorophylls (mg/kg) | 11.9 |
| β-Carotene (mg/kg) | 7.31 |
| α- tocopherol (mg/kg) | 480.79 |
Data are expressed as mean values. MUFA: monounsaturated fatty acid; SFA: saturated fatty acids; PUFA: polyunsaturated fatty acid.
2.2. Animals
This study was approved by the Ethics Committee of Animal Experiments at Taif University, Taif, Saudi Arabia,and was performed according to the Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). In this study, 80 male rats (weighing200-250 g) were obtained from Taif laboratory, and were kept under 12h light/12h dark cycle. Rats were housed in plastic cages with free access to food and water, and they were acclimated to laboratory conditions.
2.3. Experimental design
After acclimatization of the rats for 1 week, the rats were randomly divided into four groups, and were placed in separate plastic cages (20 rats for each group) as below:
Group I: Control group received equal volume (2 ml/day) of normal saline (NS, 0.9% NaCl) as vehicle by oral administration (gastric gavage) for 2 months; followed by 2-month administration of NS in equal volume intraperitoneally (IP).
Group II: Received VOO (2 ml/kg/day) for 2 months by oral administration33;, followed by 2- month administration of NS in equal volume IP.
Groups III: Received NS (2 ml/kg/day) for 2 months as a vehicle by oral administration, followed by 2-month administration of EP (150 mg/kg/day).37
Groups IV: Received VOO (2 ml/kg/day) by oral administration as a pretreatment for 2 months; followed by 2-month administration of EP (150 mg/kg/day).
2.4. Samples collection and tissue samples
At end of 4th month, the rats were anesthetized through ether inhalation. Two blood samples were collected from each rat using intracardiac puncture. One sample was left to clot at 37̊C, and was centrifuged at 3000 rpm for 15 min. Then, the serum was separated, and was stored at −20̊C for biochemical analysis. The other one was collected in heparinized tubes to determine hematological parameters. Both kidneys were isolated, and small slices of right kidney were cut, and were cleaned and washed with normal saline, and were fixed for histological examination, and the left kidney in each rat was processed for tissue enzymes.
2.5. Hematological studies
The heparinized blood samples were analyzed regarding number of Red Blood Cells (RBCs), White Blood Cells (WBCs), Hemoglobin concentration (Hb), Hematocrit (HCT), and differential count of polymorphs and lymphocytes according to standard methods using an Animal Blood Counter-ABC vet (Horiba ABX, France)38,39(38, 39)37,38(38, 39).
2.6. Kidney function assay
To assess the kidney function, blood samples were obtained through intra cardiac puncture from each rat at end of 2 nd month, and were left to clot at 37 °C, and were centrifuged at 3000 rpm for 15 min. For biochemical study, levels of urea, uric acid, creatinine, BUN were assessed in serum of control and experimental animals. Levels of serum urea were assessed using the methods proposed by Banday et al.,40 and then levels of serum UA, serum Cr,and BUN were evaluated using the methods introduced by Caraway,41 Natelson et al.,42 and Owen et al., respectively43.
2.7. Oxidative stress assay
Both kidney samples were dissected and put in Petri dishes. After washing with physiological saline, the right kidney was taken for histopathological investigations, and the left one was stored at - 80 °C until using for oxidative stress assay. Collected tissues were grinded with liquid nitrogen in a mortar. Then, grinded tissues (0.5 g) were homogenized in 2 mL of 50 mM phosphate buffer (pH 7.8) containing 1 mM EDTA and 1% PVP. The homogenate was centrifuged under cooling at 15,000×g for 20 min, and the supernatant was stored at −80̊C until using for determination of Catalase (CAT),44 Glutathione (GSH),45 and Superoxide Dismutase (SOD)46 activities as well as content of Malondialdehyde (MDA).47
2.7. Histopathological studies
Samples obtained from each kidney were fixed in 10% buffered formalin solution for 72 h. Then, tissue processing was performed, and tissues were embedded in paraffin blocks. Serial sections (3–5 μm) were prepared using microtome. The sections were stained with Hematoxylin & Eosin (H&E) to study general histological features, Masson's Trichrome (TRI) stain was used for detecting collagen fibers (grade of interstitial fibrosis), Periodic Acid Schiff (PAS) stain was applied to demonstrate mucopolysaccharides.48 Images were taken at 400 × magnifications using a light microscope (ZEISS, Axiolab) for each type of staining, and all histopathological studies were analyzed using a blind method by the same histologist.
1.7.1. H&E staining
In H&E staining, tubular lesion was assessed using a grading scale ranged from 0 to 5,which is expressed as follows: 0:no damage, 1:unicellular patchy isolated degeneration, 2: less than 25%, 3 = 25–50%, 4: 50–75%,and 5: more than 75% tubular degeneration.49,50 In this way, 20 fields from each section were randomly selected to evaluate (10 sections for each group).
2.7.2. TRI staining
To investigate deposition of tubulointerstitial collagen, 20 fields were randomly selected in each TRI-stained section (10 sections for each group). Grade of interstitial fibrosis as a semiquantitative score was defined as follows: 0: no fibrosis, 1: 1–25% fibrosis (mild), 2: 26–50% fibrosis (moderate), and 3: >50% fibrosis (severe).51
2.7.3. PAS staining
PAS staining was used to show tubular injury using a semiquantitative score. This score evaluates percentage of tubular epithelial cells atrophy, tubular dilatation, and thickening of tubular basement membrane in cortex and corticomedullary junction as follows: 0: none; 1: 10%; 2: 10–25%; 3: 26–45%; 4: 46–75%; and 5: >75% of tubules. 20 fields were randomly selected on each slide section (10 sections for each group).52
2.7.3. Immunohistochemical staining
For immunohistochemical (IHC) localization of Proliferating Cell Nuclear Antigen (PCNA), paraffin sections of kidney were deparaffinized and hydrated. Hydrogen peroxide (3% for 5 min) was used for blocking endogenous peroxidase activity. Renal sections were incubated with PCNA monoclonal antibody (Dako Corporation, Carpentaria, CA) over night, and were washed with Phosphate- Buffered Saline (PBS) for 5 min. Then, monoclonal antibody was linked with biotinylated goat anti-mouse IgG antibody (Daco, LASB Universal Kit) for 30 min. After being washed with PBS for 5 min, sections were incubated with peroxidase -conjugated streptavidin for 30 min. A brown colored reaction was developed by exposing sections to 3,3-diaminobenzidine tetrahydrochloride solution (DAB) for 5 min,and then they were washed in distilled water. Sections were counter stained with hematoxylin53. To calculate percentage of PCNA + cells, number of stained cells, and total number of cells were counted. For this purpose, 10 fields were randomly selected from each sections (10 kidney sections from each animal group) to be studied.
2.9. Statistical analyses
Statistical analysis was conducted using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).Results were expressed as mean ± SEM. Comparison of means was performed using one -way ANOVA and post-hoc Tukey. P-Values of <0.05 were considered statistically significant.
3. Results
3.1. Effects of VOO on hematological parameters in rats with sub-chronic EP
As shown in Table 2, there was a significant decrease in the RBC and total WBC count, hemoglobin levels and lymphocyte percentage and a significant increase in the percentage of neutrophils and monocytes in rats treated with EP (group III) compared to groups II and I (P˂0.05, Table 2). RBC and total WBC count, hemoglobin levels and lymphocyte percentage significantly increased and lymphocyte percentage and significantly decreased in the group IV (treated with EP and VOO) compared to group III (P˂0.05, Table 2). No significant changes were recorded in hematological parameters between groups II and I.
Table 2.
Effects of VOO on hematological parameters in rats with sub-chronic EP.
| Parameters | I | II | III | IV |
|---|---|---|---|---|
| RBCs ( × 106/μL) | 5.44 ± 0.55 | 5.21 ± 0.22 | 3.54 ± 0.82 * | 5.78 ± 0.01# |
| Hb (g/dl) | 12.01 ± 0.23 | 12.05 ± 0.44 | 9.99 ± 0.82* | 11.97 ± 0.56# |
| HCT(%) | 50.99 ± 0.96 | 50.01 ± 0.17 | 40.97 ± 0.19* | 49.95 ± 0.67# |
| WBCs( × 103/μl) | 5.21 ± 0.14 | 5.13 ± 0.17 | 9.72 ± 0.11* | 5.20 ± 0.87# |
| Lymphocytes (%) | 35.85 ± 0.57 | 35.92 ± 0.01 | 26.41 ± 0.66* | 36.14 ± 0.40# |
| Eosinophils (%) | 0.01 ± 0.02 | 0.02 ± 0.03 | 0.03 ± 0.03 | 0.01 ± 0.01 |
| Basophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Neutrophils (%) | 53.85 ± 0.32 | 53.89 ± 0.54 | 62.42 ± 0.52* | 54.40 ± 0.43# |
| Monocytes (%) | 6.11 ± 0.21 | 6.05 ± 0.44 | 8.98 ± 0.01* | 6.99 ± 0.01# |
RBC: red blood cells/Hb: Hemoglobin/HCT:/WBCs: white blood cells/HCT: hematocrit.
N = 6 in each group/Results were expressed as mean ± SEM/*P < 0.05 (significant difference compared to groups I &II)/#P < 0.05 (significant difference in comparison with group III)/I: control and received normal distilled water orally, II: received VOO (2 mL/kg). III: received EP (150 mg/kg) for 2 months, IV: Received EP, after successive administration of VOO.
3.2. Effects of VOO on kidney function parameters in rats with sub-chronic EP
As depicted in Table 3, a significant increase was recorded in the levels of serum urea, UA, Cr, and BUN in animals received EP compared to groups II and I (P˂0.05, Table 3). Animals in the group IV showed a significant decrease in the levels of serum urea, UA, Cr, and BUN compared to group III (P˂0.05, Table 3). No significant alteration was recorded in values of serum urea, uric acid, Cr, and BUN between animals treated with VOO (II) and the control group (I).
Table 3.
Effects of VOO on kidney function parameters in rats with sub-chronic.
| Parameters | I | II | III | IV |
|---|---|---|---|---|
| Urea (mg/dl) | 32.16 ± 3.23 | 32.67 ± 2.96 | 75.88 ± 3.1 * | 35.27 ± 1.12# |
| UA (mg/dl) | 5.02 ± 0.12 | 5.89 ± 0.22 | 9.12 ± 0.32* | 5.96 ± 0.42# |
| Cr (mg/dl) | 1.31 ± 0.02 | 1.15 ± 0.01 | 3.95 ± 0.12* | 1.12 ± 0.07# |
| BUN (mg/dl) | 18.01 ± 3.03 | 18.32 ± 2.92 | 29.91 ± 1.24* | 19.01 ± 2.44# |
UA: Uric acid/Cr: Creatinine/BUN: Blood urea nitrogen.
N = 6 in each group/Results were expressed as mean ± SEM/*P < 0.05 (significant difference compared to groups I &II)/#P < 0.05 (significant difference in comparison with group III)/I: control and received normal distilled water orally, II: received VOO (2 mL/kg). III: received EP (150 mg/kg) for 2 months, IV: Received EP, after successive administration of VOO.
3.3. Effects of VOO on oxidative stress parameters in rats with sub-chronic EP
As illustrated in Table 4, there was significant decrease in the SOD, CAT,and GSH levels and a significant increase in the MDA level in rats treated with EP (group III) compared to groups II and I (P˂0.05, Table 4). Animals treated with EP and VOO (group IV) exhibited a significant increase in the SOD, CAT, and GSH levels and a significant reduction in the MDA level compared to group III (P˂0.05, Table 4). No significant changes were recorded in values of SOD, CAT, GSH, and MDA level between groups II and I.
Table 4.
Effects of VOO on stress oxidative parameters in rats with sub-chronic EP.
| Parameters | I | II | III | IV |
|---|---|---|---|---|
| SOD (μgr/gr protein) | 19.98 ± 0.65 | 9.33 ± 0.02 | 14.14 ± 0.23* | 20.08 ± 0.03# |
| CAT(U/mg protein) | 90.21 ± 4.20 | 89.20 ± 4.98 | 49.90 ± 7.18* | 91.10 ± 1.05# |
| GSH (μgr/mg protein) | 170.68 ± 13.93 | 169.87 ± 13.13 | 110.96 ± 34.12* | 169.45 ± 23.67# |
| MDA (noml/g protein) | 38 ± 0.34 | 37 ± 0.45 | 61 ± 0.18* | 37 ± 0.71# |
SOD: Superoxide Dismutase/CAT: Catalase/GSH: Glutasthione/MDA: Malondialdehyde.
N = 6 in each group/Results were expressed as mean ± SEM/*P < 0.05 (significant difference compared to groups I &II)/#P < 0.05 (significant difference in comparison with group III)/I: control and received normal distilled water orally, II: received VOO (2 mL/kg). III: received EP (150 mg/kg) for 2 months, IV: Received EP, after successive administration of VOO.
3.4. Effects of VOO on histopathological alterations in rats with sub-chronic EP
3.4.1. H&E staining
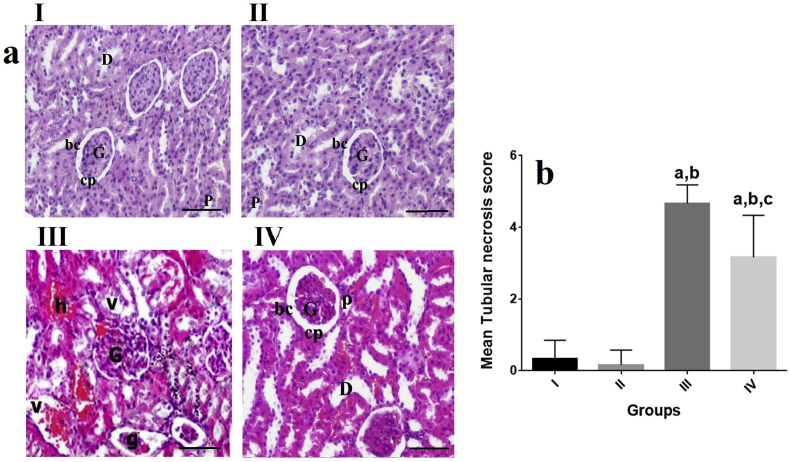
H&E staining and light microscopic examination showed that, the kidney tissue of control group (group I) and VOO group (group II) has a normal architecture for glomerulus and tubules. Typical thick cubic epithelium in proximal convoluted tubules and lower cubic epithelium in distal convoluted tubules were observed. The tubules were found to have a relatively regular distinct lumen, glomerular capsule were lined with a flat epithelium (Fig. 1 (a)). In the rats treated only with EP (group III), most areas of tubular damages of kidney were seen to range from mild to severe. These tubular damages appeared as degeneration of epithelia of renal tubules characterized by mononuclear cells infiltration and vacuolation of cytoplasm. Also, some vascular glomeruli were prominently enlarged and tightly filled the Bowman's capsule resulting in absence of capsular spaces. Moreover, hyperaemia of the kidney vessels was observed (Fig. 1 (a)). In the group IV, a marked reduction was recorded in the toxic effect on the kidney as mild hyperaemia in the kidney vessels, as well as a decrease in the degenerative changes in tubular epithelium (Fig. 1 (a)).
Fig. 1.
Effects of VOO on tubular changes in rats with sub-chronic EP a) Renal tissue of groups II and I with normal architecture Renal tissue of group III with severe tubular damages and degeneration of renal tubules with distinct mononuclear cells infiltration Renal tissue of group IV with mild hyperaemia in the kidney vessels and decreased degenerative changes H&E staining ( × 100) b) Effects of VOO on tubular necrosis score in rats with sub-chronic EP aP˂0.05 compared to group I, bP˂0.05 compared to group II, cP˂0.05 compared to group III. I: control group, II: VOO group, III: EP treated group, IV: VOO pretreatment and EP treated group (G) Renal glomeruli, (bc) Glomerular capsule, (cp) capsular space, (P) proximal convoluted tubules, (D) distal convoluted tubules, (g) Atrophied glomeruli, (h) Intertubular hemorrhage, (v) vaculles, (h) hemorrhage.
As shown in Fig. 1 (a), (b), there was a significant enhancement in the mean tubular necrosis score of groups III and IV compared to groups II and I (P˂0.05). Mean tubular necrosis score reduced in the group IV (treated with EP and VOO) compared to group III (P˂0.05, Fig. 1(b)). No significant changes were recorded in mean tubular necrosis score between groups II and I.
3.4.2. TIR staining
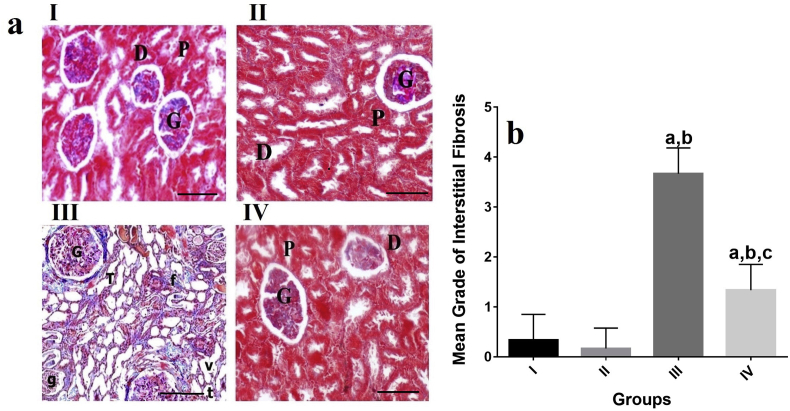
Light microscopic examination of the kidney of the control group (group I) and VOO group (group II) revealed normal distribution of collagen in these groups based on TIR staining. A significant increase was also found in the distribution of collagen (grade of interstitial fibrosis) in the kidney of rats treated with EP (group III) (P˂0.05, Fig. 2(a), (b)). In the kidney tissue of rats treated with EP in the group IV, a prominent reduction was observed in the toxic effect of EP on the kidney with nearly normal distribution of collagen (P˂0.05, Fig. 2(a) and (b)).
Fig. 2.
Effects of VOO on collagen distribution in rats with sub-chronic EP a) Renal tissue of groups II and I with normal distribution of collagen Renal tissue of group III with increased distribution of collagen Renal tissue of group IV with nearly normal distribution of collagen Masson's trichrome staining ( × 100). b) Effects of VOO on grade of interstitial fibrosis in rats with sub-chronic aP˂0.05 compared to group I, bP˂0.05 compared to group II, cP˂0.05 compared to group III I: control group, II:VOO group, III: EP treated group, IV: VOO pretreatment and EP treated group (G) Renal glomeruli, (bc) Glomerular capsule, (cp) capsular space, (P) proximal convoluted tubules, (D) distal convoluted tubules, (g) Atrophied glomeruli, (h) Intertubular hemorrhage, (v) vaculles, (h) hemorrhage, (t) Tubular necrosis, (f) Fibrosis.
3.4.3. PAS staining
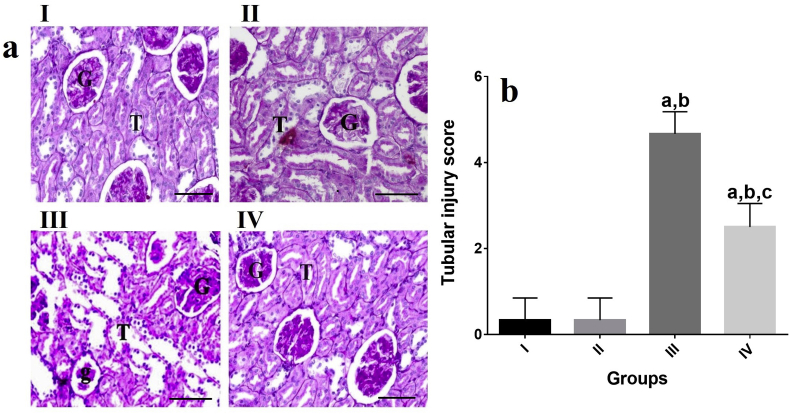
Light microscopic observations of the kidney tissue of rats in groups II and I revealed strong positive PAS reaction in glomeruli, proximal, and distal convoluted tubules of rats (Fig 3(b)). The kidney tissues of the rats exposed only to EP (group III) showed a marked reduction in the PAS reaction in glomeruli, proximal, and distal convoluted tubules of rats (P˂0.05, Fig 3(b)). PAS reaction of the kidney tissues of the rats exposed to EP and VOO (group IV) showed a moderate increased intensity of PAS positive reaction in glomeruli, proximal ,and distal convoluted tubules of the kidney compared to group III (P˂0.05, Fig 3(b)).
Fig. 3.
Effects of VOO on collagen distribution and PAS reaction in rats with sub-chronic EP a) Renal tissue of groups II and I with normal distribution of collagen Renal tissue of group III with increased distribution of collagen Renal tissue of group IV with nearly normal distribution of collagen Masson's trichrome staining ( × 100). b) I: Renal tissue of groups II and I with strong positive PAS reaction in glomeruli, proximal, and distal convoluted tubules Renal tissue of group III with marked reduction in PAS reaction Renal tissue of group IV with moderate increased intensity of PAS positive reaction PAS staining ( × 100) I: control group, II: VOO group, III: EP treated group, IV: VOO pretreatment and EP treated group (G) Renal glomeruli, (bc) Glomerular capsule, (cp) capsular space, (P) proximal convoluted tubules, (D) distal convoluted tubules, (g) Atrophied glomeruli, (h) Intertubular hemorrhage, (v) vaculles, (h) hemorrhage, (t) Tubular necrosis, (f) Fibrosis.
3.4.4. IHC staining
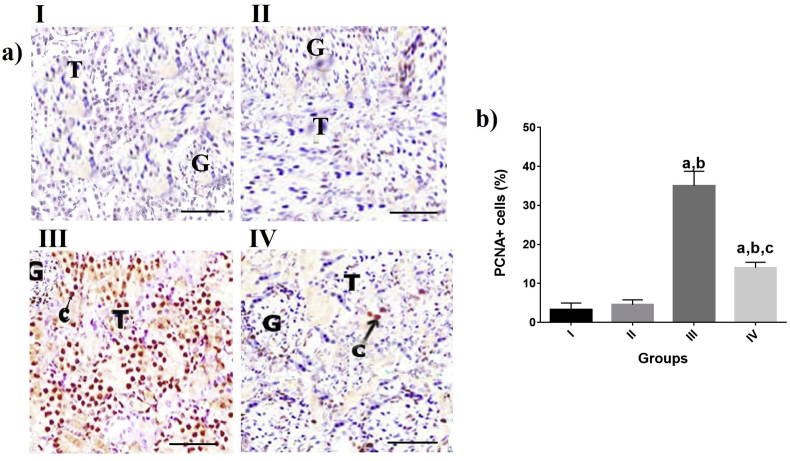
IHC staining revealed a significant enhancement in the percentage of PCNA+ cells in groups III and IV compared to groups II and I (P˂0.05, Fig 4 (a),(b)). Percentage of PCNA+ cells reduced in the group IV (treated with EP and VOO) compared to group III (P˂0.05 Fig 4 (a), (b)). No significant changes were recorded in percentage of PCNA+ cells between groups II and I.
Fig. 4.
Effects of VOO on percentage of PCNA positive cells in rats with sub-chronic a) Renal tissue of different groups with anti-PCNA staining ( × 100) aP˂0.05 compared to group I, bP˂0.05 compared to group II, cP˂0.05 compared to group III I: control group, II: VOO group, III: EP treated group, IV: VOO pretreatment and EP treated group (G) Renal glomeruli, (T) Renal Tubules, (c) PCNA expression.
4. Discussion
Plant growth regulators such as EP have been used to improve quality and yield of crops54, and oral administration of EP has been found to have toxic effects on different organs such as the kidney7. So, the present study was designed to investigate the effects of EP on hematological alterations and also biochemical and histopathological changes of renal tissues of albino rats. In addition, the protective effects of VOO against EP-induced nephrotoxicity were studied.
EP induces its toxic effects through different mechanisms. El Raouf and Girgis (2011) used different doses of EP ((50, 100,and 150 mg/kg bw/day) for 3 weeks in mice and observed that EP reduced DNA and RNA concentrations of different tissues such as the brain, liver,and kidney, and also decreased cholinesterase enzyme activity, protein content, hemoglobin,and body weight of dams and fetuses.12 Also, Wang et al. (2017)55 and Abou-Zeid et al. (2018)4 observed that EP significantly decreased levels of Hb and Hct in mice. Also, Abou-Zeid et al. (2018) reported that EP decreased number of peripheral blood lymphocytes due to its toxic effect on lymphoid organs.4 In parallel with these studies, the present study revealed that administrated EP influenced hematopoietic parameters and decreased levels of RBC, Hb, Hct, and also total WBC count and lymphocyte. Additionally, levels of serum urea, uric acid, Cr, and BUN significantly elevated in EP-treated rats. El-Okazy et al. (2008) stated that EP elevated levels of serum urea and Cr dose-dependently in mice.56 Moreover, El Raouf and Girgis (2011) demonstrated that EP increased levels of serum urea and Cr in pregnant mice.12
Additionally, EP reduced levels of antioxidant enzymes such as SOD, CAT, and GSH, while elevated levels of MDA (as lipid peroxidation landmark) in the renal tissue. In agreement with these results, Wang et al. (2010) evaluated the effects of EP on oxidative stress in mice. Their results demonstrated that SOD and GSH activities significantly reduced and contents of MDA increased in blood and spleen.So, EP induced oxidative stress in mice.57 In a similar study, Abou-Zeid et al. (2018) evaluated the protective effects of green tea on EP-induced oxidative stress and immunotoxicity in mice, and indicated that EP enhanced level of MDA and declined SOD, CAT, Glutathione peroxidase (GPx),and GSH activities in spleen and thymus of mice.58 The elevation in the MDA level as a marker of lipid peroxidation increases ROS production and subsequently disturbs cell membrane function and integrity.
Histopathological results indicated that, the kidney of the rats treated with EP showed degeneration of epithelia of renal tubules characterized by mononuclear cells infiltration and tubular cytoplasmic vacuolation. Increased tubular necrosis score and enlargement of vascular glomeruli with increased distribution of collagen were recorded in EP group. Moreover, PAS positive reaction decreased in glomeruli, proximal, and distal convoluted tubules in EP group. In similar studies, EP caused vacuolation and degeneration of renal tubular epithelium, interstitial hemorrhage,and multifocal mononuclear cells infiltration between the renal tubules.4 Percentage of PCNA positive epithelial cells increased in EP group. This increase in the expression of PCNA in sub-chronic condition may be associated with tissue regeneration as a repair response. Nakopoulou et al. (1997) demonstrated that PCNA + cells were observed in abnormal condition more frequent than normal kidneys. Furthermore, in glomerulonephritis, enhanced PCNA expression in tubular epithelial cells may be correlated with their proposed function and role in the renal injury progression.59 So, these findings suggest that, EP -induced kidney dysfunction (elevation of serum urea, uric acid, Cr, and BUN) is associated with enhanced oxidative stress biomarkers and subsequent histopathological alterations in the renal tissue.
Nowadays, there is considerable interest in administration of natural dietary antioxidants as a tool to suppress oxidative stress and potential protective and therapeutic agents against free radicals.60, 61, 62
Different studies suggested that herbal medicine and their derivatives can be used in clinic or as preventive treatment to reduce tissue damages induced by toxic agents through their antioxidant and anti-inflammatory characteristics.63,64 Olive oil has been shown to be rich in antioxidant polyphenols with free radical -scavenging features.65 In this study, VOO was used in a pretreatment program to ameliorate toxic effects of EP on the kidney. VOO could be effective against EP -induced toxicity by improving hematological values and renal function. Also, it was found that oxidative stress and histopathological alterations reduced in the group treated with VOO and EP. Histopathological studies revealed a mild hyperaemia in the kidney vessels, reduced degenerative alterations in tubular epithelium and cystic dilatation and decreased distribution of collagen in the group IV. Abdelgayoum et al. (2015) evaluated the effects of VOO on amikacin-induced nephrotoxicity in rats and demonstrated that VOO could decline amikacin-induced nephrotoxicity by improving the kidney function and tubular necrosis.34 In a parallel investigation, Wani et al. (2018) stated that administration of extra-VOO reduced cadmium -induced nephrotoxicity in mice. These therapeutic characteristics of VOO were applied by improving levels of the kidney function parameters (blood urea and serum Cr) and antioxidant (SOD, GPx,and CAT) markers and also histopathological alterations.36 Capasso et al. (2008) proved that hydroxytyrosol (DOPET), a natural olive oil antioxidant diminished cyclosporine -induced nephrotoxicity in rats.66 Necib et al. (2013) reported the reduction of oxidative stress and improvement of the kidney function in mercuric chloride -induced nephrotoxicity in rats.67
Furthermore, antioxidant properties of olive oil have been presented in in -vitro studies neutralizing toxic species and even preventing early stages of their formation.68 In an experimental study, Khalatbary et al. (2017) showed that VOO consumption attenuated deltamethrin-induced hepatotoxicity in mice. They stated that these beneficial effects of VOO might be related to its anti-oxidative, anti-apoptotic,and anti-inflammatory characteristics.69 Additionally, Ghorbel et al. (2017) stated that VOO abrogated acrylamide-induced nephropathy through modulation of biochemical factors (MDA, hydrogen peroxide (H2O2), Protein Carbonyls (PCOs), glutathione, Non-Protein Thiols (NPSHs), vitamin C CAT, SOD, GPx, Lactate Dehydrogenase (LDH), Cr, urea, and uric acid(urinary volume and creatinine clearance levels) and histological alterations (tubular dilatation, glomeruli fragmentation, glomeruli Bowman's space enlargement, hemorrhage,and infiltration of leucocytes) in rats.70
Expression of PCNA increased in the EP group pretreated with VOO (group IV). These findings suggest that VOO could accelerate tissue regeneration against EP-induced nephrotoxicity. Administration of VOO in a pretreatment program process resulted in a significant lower number of proliferating cells compared to the EP group. As VOO accelerated renal regeneration after EP treatment, the decrease in the PCNA expression may prove the fact that, VOO promotes cell proliferation until renal histology and morphology is replaced. PCNA is an auxiliary protein of DNA polymerase δ present in S (synthesis)-phase of cell cycle and plays a critical role in initiation of cell proliferation.71 PCNA expression is directly correlated with rates of cellular proliferation and DNA synthesis.72,73 Bledsoe et al. (2008) demonstrated that PCNA positive cells increased in the renal cortex 1 day (acute phase) after gentamicin treatment as a toxic agent, which reduced 2 weeks later (chronic phase).74
Conclusion
Results of the present study revealed that administration of EP could induce nephrotoxicity and kidney dysfunction by increased oxidative stress and renal tissue necrosis. However, pretreatment with VOO provided a renoprotective condition against EP through its antioxidant and free radical scavenging activities. Thus, it can be concluded that VOO might be considered as a beneficial dietary supplement for a person who is exposed to EP.
Conflict of interest
Authors declared no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.08.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Taber S.S., Pasko D.A. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf. 2008;7(6):679–690. doi: 10.1517/14740330802410462. [DOI] [PubMed] [Google Scholar]
- 2.Hedaiaty M. An update on lead-related nephrotoxicity. J Ren. Endocrinol. 2018;4(1):3. [Google Scholar]
- 3.Li S., Cao C., Shi H. Effect of quercetin against mixture of four organophosphate pesticides induced nephrotoxicity in rats. Xenobiotica. 2016;46(3):225–233. doi: 10.3109/00498254.2015.1070443. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Zeid S.M. Ameliorating effects of green tea on ethephon-induced immunotoxicity and oxidative stress in mice. Int J Pharm Sci Sci Res. 2018;4:1. [Google Scholar]
- 5.Kidd H., James D.R. 1991. The Agrochemicals Handbook. [Google Scholar]
- 6.Yazar S., Baydan E. The subchronic toxic effects of plant growth promoters in mice. Ankara Univ Vet Fak Derg. 2008;55:17–21. [Google Scholar]
- 7.Bhadoria P., Nagar M., Bahrioke V., Bhadoria A. Effect of ethephon on the liver in albino rats: a histomorphometric study. Biomed J. 2015;38(5) doi: 10.4103/2319-4170.155589. [DOI] [PubMed] [Google Scholar]
- 8.Bhadoria P., Nagar M., Bharihoke V., Bhadoria A.S. Ethephon, an organophosphorous, a fruit and vegetable ripener: has potential hepatotoxic effects? J Fam Med Prim Care. 2018;7(1):179–183. doi: 10.4103/jfmpc.jfmpc_422_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhadoria P., Nagar M., Bahrioke V., Bhadoria A.S. Effect of ethephon on the liver in albino rats: a histomorphometric study. Biomed J. 2015;38(5):421–427. doi: 10.4103/2319-4170.155589. [DOI] [PubMed] [Google Scholar]
- 10.Deka M., Dutta U. Study of subchronic exposure of ethephon induced cytomorphological and numerical alterations in the blood picture of Albino Rat. Clarion. 2015;4(2):13–24. [Google Scholar]
- 11.Dutta U. Evaluation of ethephon induced oxidative stress to gonadal disorder and its amelioration by ethanolic extract of shoot of Bambusa balcooa Roxb. in Albino rat. Toxicol Lett. 2015;2(238):S269–S270. [Google Scholar]
- 12.El Raouf A., Girgis S. Mutagenic, teratogenic and biochemical effects of ethephon on pregnant mice and their fetuses. Glob Vet. 2011;6:251–257. [Google Scholar]
- 13.Hodjat M., Baeeri M., Rezvanfar M.A., Rahimifard M., Gholami M., Abdollahi M. On the mechanism of genotoxicity of ethephon on embryonic fibroblast cells. Toxicol Mech Methods. 2017;27(3):173–180. doi: 10.1080/15376516.2016.1273425. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Zeid S.M., Allam T., El-Bahrawy A., Mohamed M. Ameliorating effects of green tea on ethephon-induced immunotoxicity and oxidative stress in mice. Int J Pharm Sci Sci Res. 2018;4:1. [Google Scholar]
- 15.Ghorbani R., Mokhtari T., Khazaei M., Salahshoor M., Jalili C., Bakhtiari M. The effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. Int J Morphol. 2014;32(3) [Google Scholar]
- 16.Jalili C., Salahshoor M., Yousefi D. Morphometric and hormonal study of the effect of utrica diocia extract on mammary glands in rats. Int J Morphol. 2015;33(3):983–987. [Google Scholar]
- 17.Golalipour M.J., Gharravi A.M., Ghafari S., Afshar M. Effect of Urtica dioica on morphometric indices of kidney in streptozotocin diabetic rats--a stereological study. Pak J Biol Sci: PJBS. 2007;10(21):3875–3879. doi: 10.3923/pjbs.2007.3875.3879. [DOI] [PubMed] [Google Scholar]
- 18.Golalipour M.J., Ghafari S., Afshar M. RELATED ARTICLES Editorial Protective role of Urtica dioica L.(Urticaceae) extract on hepatocytes morphometric changes in STZ diabetic Wistar rats. Turk J Gastroenterol. 2010;21:262–269. doi: 10.4318/tjg.2010.0098. [DOI] [PubMed] [Google Scholar]
- 19.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghan Shahreza F. Oxidative stress, free radicals, kidney disease and plant antioxidants. Immunopathol Persat. 2017;3(2):e11. [Google Scholar]
- 21.Serreli G., Deiana M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants (Basel, Switzerland) 2018;7(12) doi: 10.3390/antiox7120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covas M.I., de la Torre R., Fito M. Virgin olive oil: a key food for cardiovascular risk protection. Br J Nutr. 2015;113(Suppl 2):S19–S28. doi: 10.1017/S0007114515000136. [DOI] [PubMed] [Google Scholar]
- 23.Psaltopoulou T., Kosti R.I., Haidopoulos D., Dimopoulos M., Panagiotakos D.B. Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011;10:127. doi: 10.1186/1476-511X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckland G., Gonzalez C.A. The role of olive oil in disease prevention: a focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br J Nutr. 2015;113(Suppl 2):S94–S101. doi: 10.1017/S0007114514003936. [DOI] [PubMed] [Google Scholar]
- 25.Piroddi M., Albini A., Fabiani R. Nutrigenomics of extra-virgin olive oil: a review. Biofactors. 2017;43(1):17–41. doi: 10.1002/biof.1318. [DOI] [PubMed] [Google Scholar]
- 26.Masella R., Giovannini C., Varì R. Effects of dietary virgin olive oil phenols on low density lipoprotein oxidation in hyperlipidemic patients. Lipids. 2001;36(11):1195–1202. doi: 10.1007/s11745-001-0832-3. [DOI] [PubMed] [Google Scholar]
- 27.Coni E., Di Benedetto R., Di Pasquale M. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids. 2000;35(1):45–54. doi: 10.1007/s11745-000-0493-2. [DOI] [PubMed] [Google Scholar]
- 28.López-Miranda J., Pérez-Jiménez F., Ros E. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20(4):284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Crespo M.C., Tomé-Carneiro J., Dávalos A., Visioli F. Pharma-nutritional properties of olive oil phenols. Transfer of new findings to human nutrition. Foods. 2018;7(6):90. doi: 10.3390/foods7060090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manna C., D'Angelo S., Migliardi V. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J Agric Food Chem. 2002;50(22):6521–6526. doi: 10.1021/jf020565+. [DOI] [PubMed] [Google Scholar]
- 31.Cicerale S., Lucas L.J., Keast R.S. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23(2):129–135. doi: 10.1016/j.copbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Amamou F., Nemmiche S., kaouthar Meziane R., Didi A., Yazit S.M., Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem Toxicol. 2015;78:177–184. doi: 10.1016/j.fct.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim M.A.B., Wani F.A., Rahiman S. Hepatoprotective effect of olive oil and camel milk on acetaminophen-induced liver toxicity in mice. Int J Med Sci Public Health. 2017;6(1):186–194. [Google Scholar]
- 34.Abdel-Gayoum A.A., Al-Hassan A.A., Ginawi I.A., Alshankyty I.M. The ameliorative effects of virgin olive oil and olive leaf extract on amikacin-induced nephrotoxicity in the rat. Toxicol Rep. 2015;2:1327–1333. doi: 10.1016/j.toxrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalatbary A.R., Ghabaee D.N.Z., Ahmadvand H., Amiri F.T., Lehi S.T. Deltamethrin-induced hepatotoxicity and virgin olive oil consumption: an experimental study. Iran J Med Sci. 2017;42(6):586. [PMC free article] [PubMed] [Google Scholar]
- 36.Wani F.A., Ibrahim M.A., Moneim M.M.A., Almaeen A.R.H.A. Cytoprotectant and anti-oxidant effects of olive oil on cadmium induced nephrotoxicity in mice. Open J Pathol. 2017;8(01):31. [Google Scholar]
- 37.Wolterink G., Inoue K., Zarn J. JMPR/World Health Organization; 2015. ETHEPHON; pp. 227–273. [Google Scholar]
- 38.Wintrob M.M. fourth ed. Lea and Febiger Company; Philadelphia: 1965. Clinical Hematology; p. 411. [Google Scholar]
- 39.Walencik J., Witeska M. The effects of anticoagulants on hematological indices and blood cell morphology of common carp (Cyprinus carpio L.) Comp. Biochem. Physiol. Toxicol. Pharmacol. : CBP. 2007;146(3):331–335. doi: 10.1016/j.cbpc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Banday A.A., Farooq N., Priyamvada S., Yusufi A.N., Khan F. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008;82(9-10):450–459. doi: 10.1016/j.lfs.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Jing-hong L., Mi-mi G. Comparison between two methods of preventing Amiodaroneinduced phlebitis. 岭南心血管病杂志: 英文版. 2014;15(3):198–202. [Google Scholar]
- 42.Nateson S., Scott M., Beffa C. A repid method for the estimation of urea in biological fluid by means of the reaction between diacetely and urea. Am J Clin Pathol. 1951;21:275–281. doi: 10.1093/ajcp/21.3_ts.275. [DOI] [PubMed] [Google Scholar]
- 43.Owen J., Iggo B., Scandrett F., Stewart C. The determination of creatinine in plasma or serum, and in urine; a critical examination. Biochem J. 1954;58(3):426. doi: 10.1042/bj0580426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aebi H. Catalase. In: Bergmeyer H.U., editor. Methods of Enzymatic Analysis. second ed. Academic Press; 1974. pp. 673–684. [Google Scholar]
- 45.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A., Dutt S., Bagler G., Ahuja P.S., Kumar S. Engineering a thermo-stable superoxide dismutase functional at sub-zero to >50 degrees C, which also tolerates autoclaving. Sci Rep. 2012;2:387. doi: 10.1038/srep00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 48.Bancroft J.D., Gamble M. Elsevier Health Sciences; 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- 49.Mousleh R., Al Laham S., Al-Manadili A. The preventive role of pioglitazone in glycerol-induced acute kidney injury in rats during two different treatment periods. Iran J Med Sci. 2018;43(2):184–194. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J., Brown R.P., Shaw M. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicol Pathol. 2008;36(3):397–409. doi: 10.1177/0192623308315832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirata M., Tashiro Y., Aizawa K., Kawasaki R., Shimonaka Y., Endo K. Epoetin beta pegol alleviates oxidative stress and exacerbation of renal damage from iron deposition, thereby delaying CKD progression in progressive glomerulonephritis rats. Physiol. Rep. 2015;3(12) doi: 10.14814/phy2.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi H.Y., Moon S.J., Ratliff B.B. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087853. e87853-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu S.M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem : Off. J. Histochem. Soc. 1981;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 54.Recent developments in the chemical control of tree growth. Quinlan J., editor. Sys Symp Res Dev Orchard Plant. 1980;114 [Google Scholar]
- 55.Wang S., Jin H., Tang Q. The effect of ethephon on immune system in male offspring of mice. Environ Toxicol Pharmacol. 2017;49:119–123. doi: 10.1016/j.etap.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 56.El-Okazy A.M. The effects of combination of gibberellic acid-3 (GA3) and ethephon (2-chloroethyl phosphonic acid)(plant growth regulators) on some physiological parameters in mice. J Egypt Public Health Assoc. 2008;83(1-2):67–86. [PubMed] [Google Scholar]
- 57.Wang B., Zhao W., Liang S-z, Li H-l, Wang G-j. Effect of ethephon on oxidative stress in Kunming mice. Chin J Pesticide Sci. 2010;1 [Google Scholar]
- 58.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 59.Nakopoulou L., Stefanaki K., Salpigidis K. The value of proliferating cell nuclear antigen (PCNA)/cyclin in the assessment of cell proliferation in glomerulonephritis. Histol Histopathol. 1997;12(3):655–662. [PubMed] [Google Scholar]
- 60.Mokhtari T., Faghir Ghanesefat H., Hassanzadeh G. Effects of Flaxseed oil supplementation on renal dysfunction due to ischemia/reperfusion in rat. J. Basic Res. Med. Sci. 2017;4(1) [Google Scholar]
- 61.Bagheri A., Talei S., Hassanzadeh N. The neuroprotective effects of flaxseed oil supplementation on functional motor recovery in a model of ischemic brain stroke: upregulation of BDNF and GDNF. Acta Med Iran. 2018;55(12):785–792. [PubMed] [Google Scholar]
- 62.Ashaari Z., Hassanzadeh G., Alizamir T., Yousefi B., Keshavarzi Z., Mokhtari T. The flavone luteolin improves central nervous system disorders by different mechanisms: a review. J Mol Neurosci. 2018;65(4):491–506. doi: 10.1007/s12031-018-1094-2. [DOI] [PubMed] [Google Scholar]
- 63.Nasri H. Herbal drugs and new concepts on its use. J. Prev. Epidemiol. 2016;1(1) [Google Scholar]
- 64.Rafieian-Kopaei M., Baradaran A., Rafieian M. Plants antioxidants: from laboratory to clinic. J. Nephropathol. 2013;2(2) doi: 10.12860/JNP.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassanzadeh K., Akhtari K., Hassanzadeh H., Zarei S.A., Fakhraei N., Hassanzadeh K. The role of structural C--H compared with phenolic OH sites on the antioxidant activity of oleuropein and its derivatives as a great non-flavonoid family of the olive components: a DFT study. Food Chem. 2014;164:251–258. doi: 10.1016/j.foodchem.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 66.Capasso G., Di Gennaro C.I., Della Ragione F. In vivo effect of the natural antioxidant hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol Dial Transplant : Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2008;23(4):1186–1195. doi: 10.1093/ndt/gfm784. [DOI] [PubMed] [Google Scholar]
- 67.Necib Y., Bahi A., Zerizer S., Abdennour C., Boulakoud M. Effect of virgin olive oil (Olea Europea. L) on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Biochem. Biotechnol. 2013;9(4):415–422. [Google Scholar]
- 68.Petroni A., Blasevich M., Salami M., Papini N., Montedoro G.F., Galli C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb Res. 1995;78(2):151–160. doi: 10.1016/0049-3848(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 69.Khalatbary A.R., Ghabaee D.N.Z., Ahmadvand H., Amiri F.T., Lehi S.T. Deltamethrin-induced hepatotoxicity and virgin olive oil consumption: an experimental study. Iran J Med Sci. 2017;42(6):586–592. [PMC free article] [PubMed] [Google Scholar]
- 70.Ghorbel I., Elwej A., Fendri N. Olive oil abrogates acrylamide induced nephrotoxicity by modulating biochemical and histological changes in rats. Ren Fail. 2017;39(1):236–245. doi: 10.1080/0886022X.2016.1256320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J., Pan X., Fu H. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep. 2017;7(1):10114. doi: 10.1038/s41598-017-10693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y., Xiong W., Yang J. Attenuation of inflammation by emodin in lipopolysaccharide-induced acute kidney injury via inhibition of toll-like receptor 2 signal pathway. Iran. J. Kidney Dis. 2015;9(3) [PubMed] [Google Scholar]
- 73.Schönenberger F., Deutzmann A., Ferrando-May E., Merhof D. Discrimination of cell cycle phases in PCNA-immunolabeled cells. BMC Bioinf. 2015;16:180. doi: 10.1186/s12859-015-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bledsoe G., Shen B., Yao Y.-Y. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol Sci. 2008;102(2):433–443. doi: 10.1093/toxsci/kfn008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.