Abstract
Background
Daratumumab, a monoclonal antibody used to treat relapsed or refractory multiple myeloma, can interfere with protein electrophoresis and immunofixation assays. False-positive immunofixation results due to daratumumab can cause uncertainty regarding the status of a patient's disease and lead to potential misclassification of their response to therapy. The Hydrashift 2/4 Daratumumab assay (Sebia) was recently cleared by the Food and Drug Administration for resolving daratumumab interference on immunofixation. Here, we evaluate the performance of the Hydrashift assay in multiple myeloma patients receiving treatment with daratumumab-based regimens.
Methods
Waste serum samples from multiple myeloma patients (n = 40) receiving daratumumab were analyzed by standard immunofixation and the Hydrashift assay. Results from these tests were compared and were evaluated along with pretreatment serum protein electrophoresis and immunofixation results, if available.
Results
The Hydrashift assay shifted the migration of daratumumab in patient samples. In 27 cases, the patient's M protein was distinguishable from daratumumab by standard immunofixation. In these cases, the Hydrashift assay confirmed that the IgGκ band was daratumumab and helped identify the presence of treatment-related oligoclonal bands. There were 11 instances in which the patient's IgGκ M protein comigrated with daratumumab. In all 11 cases, the Hydrashift assay confirmed the presence of residual M protein. Finally, in 2 patients whose pretreatment immunofixation results were not available, the Hydrashift assay confirmed that the IgGκ band visible on immunofixation was due to daratumumab alone.
Conclusions
The Hydrashift 2/4 Daratumumab assay is a useful tool to clarify the source of an IgGκ band on immunofixation and allow a patient's M protein to be viewed without interference.
Impact Statement
This manuscript presents results from the Hydrashift 2/4 Daratumumab assay, a commercially available, Food and Drug Administration-cleared kit that distinguishes daratumumab from disease-related M proteins on immunofixation. Application of the Hydrashift assay in patients receiving this monoclonal antibody therapy allows the source of an IgGκ band to be confirmed and a patient's M protein to be viewed without interference. This will benefit myeloma patients who are receiving daratumumab therapy through more accurate monitoring of their disease status.
Daratumumab, a fully human IgG1κ monoclonal antibody (mAb) that binds CD38, gained Food and Drug Administration (FDA)4 approval in 2015 for the treatment of relapsed or refractory multiple myeloma. Because of its potent antimyeloma activity, daratumumab is being considered for inclusion as part of frontline therapy in several clinical trials. However, daratumumab and other mAb therapeutics can cause a false-positive interference on serum protein electrophoresis (SPEP) and immunofixation (IF) assays (1–4), which are routinely used to monitor myeloma patients. If daratumumab is misinterpreted as the patient's disease biomarker on SPEP/IF studies, clinical decisions may be affected and the patient's response to therapy based on International Myeloma Working Group uniform response criteria (5) may be misclassified. Therefore, laboratory tools that can discriminate between the false-positive interference and disease-related paraprotein (M protein) are greatly needed.
The Hydrashift 2/4 Daratumumab assay (Sebia) was recently cleared by the FDA to distinguish daratumumab from disease-related M proteins on IF. The Hydrashift assay is a commercial version of the Daratumumab Interference Reflex Assay (6, 7) and can be performed with existing instruments and standard supplies. A monoclonal murine antiidiotypic antibody against daratumumab (antidaratumumab antiserum) is used to shift the migration of daratumumab on the gel, which allows the presence of the drug to be confirmed and the patient's M protein to be viewed without interference. In this study, we evaluated the performance of the Hydrashift assay in 40 multiple myeloma patients who were receiving daratumumab-based therapy.
METHODS
Patient samples
The study was conducted with the approval of the Institutional Review Board at Memorial Sloan Kettering Cancer Center under protocol number 17-376. A clinical database search was performed to identify patients who had routine SPEP and IF studies when receiving a daratumumab-based treatment regimen. Waste clinical samples from 40 patients were collected on the basis of sample availability and sample volume. Samples were obtained from the Clinical Chemistry Laboratory at Memorial Sloan Kettering Cancer Center according to institutional guidelines and were stored at −20 °C until analysis. Clinical patient characteristics, including the isotype of the patients' endogenous M protein, date of last daratumumab infusion, daratumumab cycle number, doses administered, and concurrent medication use, were collected through chart review (Table 1). If available, results from pretreatment SPEP and IF were collected to evaluate the migration pattern of the patient's original disease-related M-protein band.
Table 1.
Baseline characteristics.
| Patient characteristics, n = 40 | |
|---|---|
| Age: median (range), years | 72 (48–85) |
| Sex: | Men: n = 26 (65%), Women: n = 14 (35%) |
| Diagnosis: | Multiple myeloma: n = 35 |
| Smoldering multiple myeloma: n = 1 | |
| Amyloid light-chain amyloidosis: 1 | |
| Amyloid light-chain amyloidosis and multiple myeloma: 1 | |
| Multiple myeloma & POEMS syndrome and CLL/SLLa: 1 | |
| Amyloid light-chain amyloidosis and Waldenstrom macroglobulinemia: 1 | |
| Isotype of endogenous paraprotein: | IgGκ: 17 |
| IgGλ: 9 | |
| IgAκ: 2 | |
| IgAλ: 4 | |
| IgMκ: 0 | |
| IgMλ: 1 | |
| Light chain κ: 3 | |
| Light chain λ: 3 | |
| IgAλ/IgGλ biclonal: 1 | |
| Prior lines of therapy: median (range) | 2 (0–10) |
| Regimen at time of evaluationb: | Daratumumab monotherapyc: n = 9 |
| Daratumumab + bortezomib ± steroid: n = 7 | |
| Daratumumab + lenalidomide ± steroid: n = 7 | |
| Daratumumab + pomalidomide ± steroid: n = 14 | |
| Daratumumab + carfilzomib ± steroid: n = 1 | |
| Daratumumab + immunomodulatory imide drug + investigational mAb clinical trial: n = 2 | |
| Days from last daratumumab infusion to laboratory assessment: median (range) | 12 (2–57) |
| Prior doses of daratumumab: median (range) | 10 (2–25) |
POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma.
All daratumumab doses are 16 mg/kg administered intravenously.
One patient received daratumumab as part of a clinical trial.
IF and Hydrashift 2/4 Daratumumab assays
Patient samples were run on both the Hydrashift assay and a standard IF gel for comparison. Both assays were performed on the Hydrasys 2 system (Sebia) using the Hydragel 4 IF kit (Sebia). The Hydrashift procedure is similar to that of a normal IF, except that the antidaratumumab antiserum is applied to the gel with an additional applicator. A positive control sample was run daily. Both assays were performed according to the manufacturer's instructions.
RESULTS
We evaluated the performance of the Hydrashift assay with 40 samples from multiple myeloma patients who were receiving daratumumab and who had a variety of M-protein isotypes (Table 1). The Hydrashift assay reliably shifted the migration of daratumumab in patient samples. Shift of the daratumumab-related band was most evident in samples in which daratumumab was present at sufficient concentration and was distinct from the patient's M-protein band on IF. There were 27 instances in which the patient's M protein and an additional IgGκ band were distinguishable by IF alone. Bands could be distinguished by IF alone if the M protein was an isotype other than IgGκ (n = 20) or if the M protein was an IgGκ (n = 5) or free κ (n = 2) with a different migration pattern than daratumumab. In 26 of these 27 cases, the Hydrashift assay result confirmed that the additional band was daratumumab. A representative example is shown in Fig. 1A. In 1 case, after application of the antidaratumumab antisera, the additional IgGκ band remained and the daratumumab–antidaratumumab complex was visible on the Hydrashift gel (Fig. 1B). This indicates that the patient had an oligoclonal IgGκ protein that comigrated with daratumumab.
Fig. 1. Examples in which the patient's M protein and an additional IgGκ band (marked with an arrow) are visible by IF.
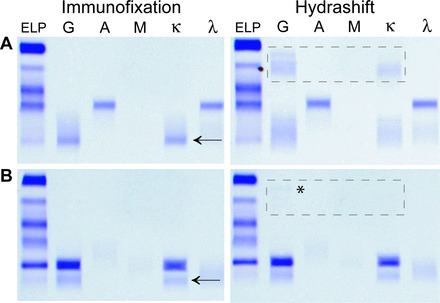
The IF result (left) for a patient with an IgAλ M-protein. When run on the Hydrashift assay (right), the IgGκ band disappears, and the daratumumab–antidaratumumab complex appears as a broad smear in the α region (highlighted with box) (A). The result indicates that the IgGκ band visible on IF was daratumumab. The IF result (left) for a patient with an IgGκ M protein with a different migration pattern than daratumumab (B). The Hydrashift result (right) shows the presence of 2 IgGκ bands and a faint daratumumab–antidaratumumab complex (highlighted with box and *). This result indicates the presence of an oligoclonal IgGκ that comigrates with daratumumab.
For the remaining 13 cases, daratumumab could not be distinguished from the patient's disease by IF alone because only a single IgGκ band was visible, and these patients had a history of IgGκ or free κ myeloma. Pretreatment SPEP and IF results depicting the migration of the original clone were available for 11 of these patients; these results showed that their IgGκ M protein comigrated with daratumumab. The Hydrashift assay demonstrated that the disease band was present in all 11 cases with baseline SPEP and IF results. Compared to the standard IF, the IgGκ band did not shift after application of antidaratumumab antisera, but the daratumumab complex was observed (example shown in Fig. 2A). Pretreatment IF results were not available for 2 patients, one with IgGκ myeloma and the other with κ light chain myeloma. The Hydrashift assay demonstrated that the IgGκ band visible on IF was due to daratumumab only and that the patients' disease was not detectable (example shown in Fig. 2B).
Fig. 2. Examples in which a single IgGκ band is visible by IF.
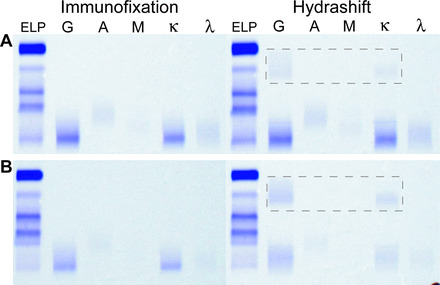
The IF result (left) for a patient with an IgGκ M protein that comigrates with daratumumab (A). The Hydrashift result (right) shows the IgGκ band and the daratumumab–antidaratumumab complex in the α region (highlighted with box), indicating that the patient's M protein is still present after daratumumab has been shifted. The IF result (left) from a patient with a history of IgGκ myeloma but whose original serum studies were not available for comparison (B). The Hydrashift result (right) shows the disappearance of the IgGκ band and appearance of the daratumumab–antidaratumumab complex (highlighted with box). This indicates that the band visible on standard IF was due entirely to daratumumab and that the patient's disease is not detectable.
DISCUSSION
Several strategies have been suggested to help properly interpret immunofixation results for patients who are receiving daratumumab or other mAb drugs (1, 3, 8). Medication history and original serum studies can aid laboratorians in properly interpreting the SPEP and IF results. However, if baseline serum studies showing the migration pattern of the patient's original clone are not available or if the patient's IgGκ M protein comigrates with daratumumab, one cannot infer whether the IgGκ band visible on immunofixation represents daratumumab, the patient's M protein, or concurrent presence of both monoclonal antibodies.
Ambiguous IF results related to the above scenarios are fairly common. Among 17 IgGκ patients in our cohort, 65% (11/17) had M proteins that comigrated with daratumumab. Comigration with daratumumab was determined by a review of all SPEP/IF results in conjunction with a record of the patient's original disease clone. Review of current SPEP/IF results alongside historical results is standard practice at our center, and we maintain a digital library of all SPEP/IFs that is searchable by medical record number. However, this approach has its limitations and may not be practical at other institutions. Use of the Hydrashift assay at reference laboratories, for example, may be especially important given their limited access to medical history and prior laboratory results.
The Hydrashift 2/4 Daratumumab assay is a useful tool to confirm the source of the IgGκ band on IF. In cases in which the patient's M protein is already distinguishable from daratumumab on IF, the Hydrashift assay is helpful in identifying the presence of treatment-related oligoclonal band(s). And, in cases in which the M protein comigrates with daratumumab, the Hydrashift removes the interference, allowing scientists and physicians to properly interpret if the patient's disease is present. This latter situation is when the Hydrashift assay is most consequential because there is potential to mistake daratumumab as the patient's disease. We found that the Hydrashift assay was easily performed using existing laboratory instruments and only involves a simple modification of the standard IF procedure.
There are some limitations to the assay, however. The Hydrashift assay is only specific for daratumumab and will not work for other mAb therapeutics. This limitation will become increasingly problematic as several new therapeutic mAbs are under investigation for use in multiple myeloma. For example, 2 patients in this study were receiving an investigational regimen including daratumumab and another therapeutic mAb drug. Although a monoclonal protein band representing this additional drug was not visible by IF for either patient, other mAb drugs could cause a false-positive interference on IF (1, 4, 8). Interference by other mAb drugs would not be resolved by use of the Hydrashift assay without the development of antisera specific for each drug. Additional complementary diagnostics with shift methods accompanying new mAb drugs or a more universal approach, perhaps such as mass spectrometry (4, 9), will be needed in the future to mitigate the interference caused by other mAbs. In addition, a solution to the interference with SPEP is needed to provide accurate quantification of the M protein. Currently, the Hydrashift assay only addresses the false-positive interference on IF.
With the need to distinguish drug from disease on serum immunofixation and the recent FDA clearance of the Hydrashift assay, it is likely that this test will be rapidly adopted by clinical laboratories. The assay may be most useful when M-protein levels are approximately equal to or less than the concentration of daratumumab (approximately 1 g/L) (2, 6, 7). Distinguishing drug from low levels of disease is important for determining if patients have achieved a complete response by International Myeloma Working Group criteria (5) and whether to perform minimal residual disease testing via bone marrow aspirate sampling. Considering emerging data from ongoing clinical trials (NCT02874742, NCT03290950, NCT02195479, NCT01998971) (10, 11), regimens containing daratumumab may gain FDA approval for use in newly diagnosed multiple myeloma patients in the very near future. The Hydrashift assay will likely become critically important and commonly used for patients receiving potent daratumumab-based regimens as upfront therapy, as a significant proportion of those treated will be expected to achieve a complete response with modern combination therapies (12).
Guidelines for appropriate use are necessary to properly implement the Hydrashift assay into laboratory workflows and routine clinical practice. Use of the assay will likely vary based on the setting (i.e., reference vs hospital-based laboratory) and whether medication history and pretreatment SPEP/IF results are available. Future work should focus on determining the optimal timing for best use of this assay to help clinicians accurately assess patients' disease status.
Acknowledgments
The authors would like to acknowledge Sebia for providing reagents and funding for this study, and Dana Terranova, Technical Product Specialist at Sebia Inc., for performing the Hydrashift assay.
4 Nonstandard abbreviations
- FDA
Food and Drug Administration
- mAb
monoclonal antibody
- SPEP
serum protein electrophoresis
- IF
immunofixation.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Employment or Leadership: L. Ramanathan, Memorial Sloan Kettering Cancer Center.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: K. Thoren, Y. Maakaroun, Sebia Inc; M.J. Pianko, Memorial Sloan Kettering Cancer Center Mortimer J. Lacher Fellowship, NIH/National Center for Advancing Translational Sciences (UL1TR00457); Memorial Sloan Kettering Cancer Center Core Grant: P30 CA008748.
Expert Testimony: None declared.
Patents: None declared.
Role of Sponsor: The sponsor, Memorial Sloan Kettering Cancer Center, had a role in the design of the study, choice of enrolled patients and final approval of the manuscript.
References
- 1. Murata K, McCash SI, Carroll B, Lesokhin AM, Hassoun H, Lendvai N, et al. Treatment of multiple myeloma with monoclonal antibodies and the dilemma of false positive M-spikes in peripheral blood. Clin Biochem 2018;51:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCudden CR, Jacobs JFM, Keren D, Caillon H, Dejoie T, Andersen K. Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin Biochem 2018;51:72–9. [DOI] [PubMed] [Google Scholar]
- 3. Cenaj O, Dahlin JL, Buencamino DM, Laubach JP, Jarolim P. 74-Year-old female with new monoclonal protein on serum immunofixation electrophoresis. Clin Biochem 2018;51:97–100. [DOI] [PubMed] [Google Scholar]
- 4. Willrich MAV, Ladwig PM, Andreguetto BD, Barnidge DR, Murray DL, Katzmann JA, et al. Monoclonal antibody therapeutics as potential interferences on protein electrophoresis and immunofixation. Clin Chem Lab Med 2016;54:1085–93. [DOI] [PubMed] [Google Scholar]
- 5. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328–46. [DOI] [PubMed] [Google Scholar]
- 6. McCudden C, Axel AE, Slaets D, Dejoie T, Clemens PL, Frans S, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med 2016;54:1095–104. [DOI] [PubMed] [Google Scholar]
- 7. van de Donk NW, Otten HG, El Haddad O, Axel A, Sasser AK, Croockewit S, et al. Interference of daratumumab in monitoring multiple myeloma patients using serum immunofixation electrophoresis can be abrogated using the daratumumab IFE reflex assay (DIRA). Clin Chem Lab Med 2016;54:1105–9. [DOI] [PubMed] [Google Scholar]
- 8. McCudden CR, Voorhees PM, Hainsworth SA, Whinna HC, Chapman JF, Hammett-Stabler CA, et al. Interference of monoclonal antibody therapies with serum protein electrophoresis tests. Clin Chem 2010;56:1897–9. [DOI] [PubMed] [Google Scholar]
- 9. Mills JR, Kohlhagen MC, Dasari S, Vanderboom PM, Kyle RA, Katzmann JA, et al. Comprehensive assessment of M-proteins using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin Chem 2016;62:1334–44. [DOI] [PubMed] [Google Scholar]
- 10. Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018;378:518–28. [DOI] [PubMed] [Google Scholar]
- 11. Jakubowiak AJ, Chari A, Lonial S, Weiss BM, Comenzo RL, Wu K. Daratumumab (DARA) in combination with carfilzomib, lenalidomide, and dexamethasone (KRd) in patients (pts) with newly diagnosed multiple myeloma (MMY1001): an open-label, phase 1b study [Abstract]. J Clin Oncol 2017;35. [Google Scholar]
- 12. Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med 2017;281:365–82. [DOI] [PubMed] [Google Scholar]


