Abstract
Background:
Anemia is commonly encountered in cancer patients receiving active chemotherapy. Due to adverse events and presumed negative effects on disease-progression and survival, erythropoiesis-stimulating agents are not frequently used. In this study, we assess the efficacy and safety of intravenous ferric carboxymaltose (FCM) to treat cancer-induced anemia (CIA).
Patients and Methods:
We recruited adult cancer patients on active chemotherapy with a hemoglobin (Hb) level ⩽11.0 g/dL. Based on serum ferritin (sFr) and transferrin saturation (TSAT), patients were divided into 3 groups: group I (absolute iron deficiency, n = 26) with sFr < 30 ng/mL and TSAT < 20%; group II (functional iron deficiency, n = 24) with sFr 30–800 ng/mL and TSAT < 20%; and patients with TSAT ⩾ 20% were placed in group III as “others” (n = 34). All patients were treated with intravenous FCM. Serum hepcidin and C-reactive protein were used as biomarkers to predict response.
Results:
A total of 84 patients with a median age (SD) of 53.8 (10.6) were recruited. Baseline median Hb level was 10.2 (range: 8.3–11.0) gm/dL. At week 12, there was a significant increment in Hb level for patients in groups I and II (median increment: 2.35 and 1.5 gm/dL, respectively), with limited response observed in group III, and most of the increment noted as early as week 3 (⩾1.0 g/dL). Responders tended to have lower levels of hepcidin. No clinically significant adverse events were reported; however, asymptomatic hypophosphatemia was observed in 39 (46.4%) patients.
Conclusions:
Intravenous FCM is a safe and effective treatment option for the management of a subgroup of patients with CIA.
The study was registered at ClinicalTrials.gov [Identifier: NCT04246021]
Keywords: anemia, cancer, ferric carboxymaltose, hepcidin
Introduction
Anemia is a commonly encountered problem in cancer patients receiving chemotherapy with or without radiation. Its prevalence in published reports varies and can be as high as 60%.1,2 Anemia can be attributed to a combination of different factors including underlying malignancy-related bleeding due to the anatomic site of the tumor, acquired coagulation defects, poor nutrition, chemotherapy, or radiation therapy.3,4
Common constitutional symptoms of cancer, such as fatigue, weakness, and dyspnea, can be worsened by anemia, and thus negatively affect quality of life (QOL) and performance status.5 In addition, preliminary studies have suggested that anemia, during cancer therapy, can also have a negative effect on survival and loco-regional control after radiation therapy, especially in head and neck and cervical cancers.6 Thus, to improve physical functioning and QOL in patients with cancer, it would be reasonable to take a proactive approach in identifying cancer patients who need treatment for anemia and provide a timely management.
Although blood transfusion is a rapid and effective approach to treat anemia on the short-term, unfortunately its effect is temporary and can be associated with a number of serious adverse events. In addition, treatment outcomes can be negatively affected by transfusion-related immune modulation associated with recurrent red blood cell transfusions.7
Erythropoiesis-stimulating agents (ESA) were proposed as an effective treatment for anemia for several years.8–10 Randomized clinical trials in patients with cancer-related anemia, however, had suggested that although ESAs can significantly increase hemoglobin (Hb) levels, decrease transfusion requirements, and improve QOL,10,11 30–50% of patients do not respond to these agents. This lack of response in cancer patients can partly be attributed to a functional iron deficiency state in a significant subset of patients. In these patients, there is an increase in erythropoiesis, but this exceeds the rate of usable iron delivery, despite adequate iron stores.12 Several randomized trials were conducted to examine the role of intravenous iron in addition to ESAs which would potentially, at least partially, overcome this problem.13,14 Many of these studies have shown significant improvement in a number of parameters in favor of the combination, such as response rates, time to response, reduction in ESA dose, and improvement in QOL.15,16
Several later studies raised serious concerns about the safety of ESAs in cancer patients, such as shortened overall survival or time to tumor progression, especially among patients whose Hb levels increased to above 12.0 g/dL.17–19 These concerns, in addition to a higher observed incidence of thromboembolic events,20,21 resulted in a significant decline in the utilization of these agents, especially among patients treated with a curative intent.22 The aforementioned concerns and issues with both blood transfusions and ESAs resulted in state of confusion among clinicians and, in turn, in undertreatment of anemia in cancer patients.23,24
Iron plays an important role in Hb synthesis and inadequate supply is a major element in the pathogenesis of anemia. Intravenous iron therapy is routinely used in the non-cancer setting to treat iron-deficiency anemia, especially in patients with severe anemia and in those who failed or could not tolerate oral formulations.25,26 Absolute iron deficiency anemia (AIDA) occurs when iron delivery is impaired because iron stores are depleted. Functional iron deficiency anemia (FIDA), on the other hand, occurs when access to iron stores is restricted or too slow to keep up with the process of erythropoiesis.27 In cancer, tumor cells interact with the immune system leading to a chronic state of inflammation. Pro-inflammatory cytokines are also released leading in turn to increased release of hepcidin; a small (25 amino acids) peptide produced by the liver.28 Hepcidin is a regulator of iron homeostasis; 29,30 it binds to ferroportin-expressing cells inhibiting iron transport across cell membranes and decreasing accessibility to the iron stores.31 At high enough circulating levels, it also impairs gastrointestinal absorption of dietary iron.32,33 This state of inhibited absorption and blocked release from stores leads to an increased frequency of iron-restricted erythropoiesis. Hence, it was proposed that patients identified with FIDA may benefit from extra doses of iron to overcome the inhibitory effect of hepcidin.
In a previous pilot study conducted by our group, intravenous iron without ESAs was investigated in a group of non-iron deficient anemic cancer patients on active treatment with chemotherapy. Patients were given 12 short weekly infusions of ferric sucrose while receiving their planned chemotherapy regimens. For the 15 patients who completed at least 9 weekly iron treatment doses, the mean increment in Hb level was 1.7 gm/dL (median, 1.1 gm/dL; range, –1.9 gm/dL to 3.2 gm/dL). No treatment-related adverse events were reported.34
Ferric carboxymaltose (FCM), is a widely used intravenous iron formulation, used to treat iron deficiency anemia in different clinical settings including pregnancy-related anemia and anemia in patients with chronic renal failure.26,35
This study aims to investigate the effect of FCM, without ESAs, on anemic cancer patients undergoing chemotherapy; stratified by status of iron deficiency, that is, AIDA versus FIDA.
Methods
Patients
All enrolled patients were adults, at least 18 years of age, and were about to start a cycle of chemotherapy with or without radiation therapy within 1 week of inclusion. Inclusion criteria included an established diagnosis of a non-myeloid malignancy, anemia with a Hb levels of ⩽11.0 g/dL, a life expectancy of more than 24 weeks, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients should not have received ESAs or iron therapy within the 30 days preceding enrollment.
Exclusion criteria included any evidence of hemolysis, gastrointestinal bleeding, leukoerythroblastic features on blood film, or concomitant folate or vitamin B12 deficiencies. Patients were also excluded if they had elevated serum ferritin (⩾800 ng/mL) or transferrin saturation (TSAT) ⩾50%, or a personal or family history of hemochromatosis. Other exclusion criteria were pregnancy or lactation, liver dysfunction (grade 2 or higher based on National Cancer Institute Common Toxicity Criteria: Bilirubin >1.5 normal, Transaminases >2.5 normal), renal dysfunction (serum creatinine levels ⩾2.0 mg/dL), active infection requiring systemic antibiotics, comorbidities precluding study participation, known hypersensitivity to intravenous iron, red blood cell transfusion within the preceding 2 weeks, or any investigational agent within 30 days before enrollment. Patients with prior gastric surgery and those planned to receive definitive radiotherapy alone without chemotherapy were excluded as well.
During the study period, patients were not allowed to take any iron-containing, vitamins, minerals, or herbal supplements. Blood transfusions were permitted at the primary physician’s discretion, and this was documented and recorded. Changes to the chemotherapy plan and duration, as suggested by the treating physicians, were permitted, and did not trigger study withdrawal.
Based on serum iron, ferritin, total iron-binding capacity and TSAT levels, patients were categorized into 3 groups as illustrated in Table 1. In total, 26 (31.0%) patients were in group I with AIDA, 24 (28.6%) were in group II with FIDA, while 34 (40.5%) were in group III (others). Written informed consents were obtained by one of the investigators from all patients before enrollment. Study protocol and supporting documents were approved by our local institutional review board (King Hussein Cancer Center, KHCC IRB) under the number: 15-KHCC-107. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as contained in the US Code of Federal Regulations that governs the protection of human subjects on clinical trials.
Table 1.
Patient grouping according to serum ferritin and TSAT levels.
| Group | Ferritin (ng/mL) |
TSAT | |
|---|---|---|---|
| I | Absolute iron deficiency | <30 | <20% |
| II | Functional iron deficiency | 30–800 | <20% |
| III | Others | any | ⩾20% |
TSAT, Transferrin saturation.
Intravenous iron therapy
Based on body weight and Hb level, patients received one or two doses of FCM and were then followed up for a total of 12 weeks. FCM (Ferrinject®, Vifor Pharma, Bern, Switzerland) dose was calculated based on both body weight and baseline Hb level as illustrated in Table 2.
Table 2.
Ferric carboxymaltose dose.
| Baseline hemoglobin (gm/dL) | Body weight |
|
|---|---|---|
| <70 Kg | >70 Kg | |
| <10.0 | 1500 mg | 2000 mg |
| ⩾10.0 | 1000 mg | 1500 mg |
FCM was administered as a single short intravenous infusion if the needed dose was 1000 mg, diluted in 100 mL of 0.9% saline over 15–30 min. If the needed FCM dose was over 1000 mg, an initial dose of 1000 mg was given as mentioned previously, and the remaining dose was given the following week in a similar fashion. During the infusion, patients were monitored for all potential adverse events.
Assessment
Hb level was checked every 3 weeks, mostly in-line with the scheduled chemotherapy protocol visits, and then at week 12. Patients were considered responders if they showed a minimum of 1.0 gm/dL increment in Hb level. Laboratory monitoring of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, alkaline-phosphatase, gamma-glutamyl transferase and phosphorous were done at baseline and then repeated at week 3, while ferritin level was done at baseline and then repeated at week 12. A total of 4 follow-up visits were required for the study at weeks 3, 6, 9, and 12.
In addition, tests for C-reactive protein (CRP) and serum hepcidin level [Hepcidin 25 (bioactive) HS ELISA, DRG Diagnostics, Marburg, Germany], were performed on the blood samples drawn at baseline.
Statistical analysis
Hb levels were recorded as absolute levels per patient and as mean, median, and range for the whole group of patients and for each subgroup throughout the 12 weeks of the study. The same was done to show the changes of Hb levels per each 3-week period. Comparisons of means of Hb levels were made between baseline Hb and Hb levels on weeks 3, 6, 9, and 12, using Wilcoxon rank test. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), and a significance criterion of p < 0.05 was used. The datasets generated and analyzed during the study are available from the corresponding author on reasonable request
Results
A total of 84 patients were consented and recruited to the study. The majority (70 patients; 83.3%) were females and the median age [standard deviation, (SD)] was 53.8 years (10.6 years). Breast cancer was the most common primary tumor, encountered in 48 (57.1%) patients, followed by colorectal cancer in 19 (22.6%) patients and gynecological tumors in 8 (9.5%) patients. The majority of the patients had advanced-stage disease; 44 (52.4%) patients with stage IV disease and 21 (25.0%) with stage III disease. All patients were receiving active chemotherapy which varied according to the primary cancer site, and these included platinum, taxanes, anthracyclines, cyclophosphamide, high dose ifosfamide, vincristine, vinblastine, bleomycin, and others. Many of the included patients had their chemotherapy treatment as second-line or beyond. Patients’ characteristics across treatment groups including age, primary tumor, and active anticancer treatment are summarized in Table 3.
Table 3.
Baseline patients’ characteristics (n = 84).
| Variables | All patients (n = 84) |
Group I absolute iron deficiency (n = 26) |
Group II functional iron deficiency (n = 24) |
Group
III others (n = 34) |
|
|---|---|---|---|---|---|
| Age (years) | Mean (SD) | 53.8 (10.6) | 52.6 (7.8) | 54.5 (12.8) | 51.9 (11.0) |
| Gender | Female | 70 (83.3%) | 20 (76.9%) | 19 (79.2%) | 31 (91.2%) |
| Male | 14 (16.7%) | 6 (23.1%) | 5 (20.8%) | 3 (8.8%) | |
| Primary tumor | Breast cancer | 48 (57.1%) | 15 (57.7%) | 11 (45.8%) | 22 (64.7%) |
| Colorectal cancer | 19 (22.6%) | 7 (26.9%) | 8 (33.3%) | 4 (11.8%) | |
| Gynecological cancer | 8 (9.5%) | 3 (11.5%) | 2 (8.3%) | 3 (8.8%) | |
| Others | 9 (10.7%) | 1 (3.8%) | 3 (12.5%) | 5 (14.7%) | |
| Stage | I | 4 (4.8%) | 3 (11.5%) | – | 1 (2.9%) |
| II | 15 (17.9%) | 4 (15.4%) | 3 (12.5%) | 8 (23.5%) | |
| III | 21 (25.0%) | 10 (38.5%) | 3 (12.5%) | 8 (23.5%) | |
| IV | 44 (52.4%) | 9 (34.6%) | 18 (75.0%) | 17 (50.0%) | |
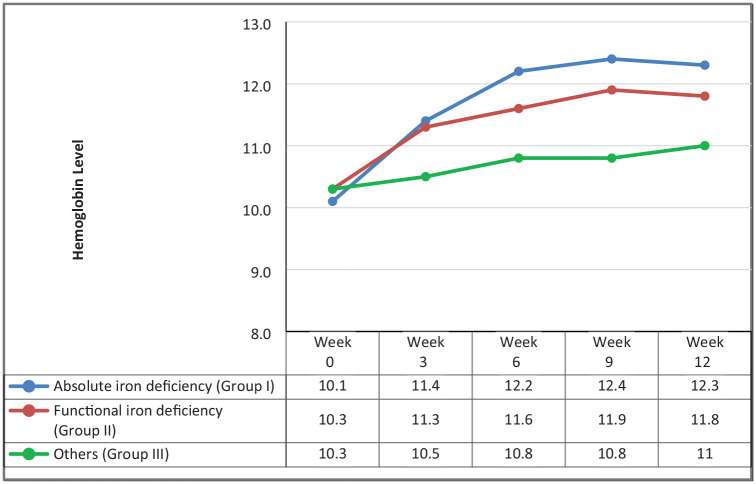
The mean Hb level of the 84 patients at baseline was 10.1 gm/dL (median, 10.2 gm/dL; range, 8.3–11.0 gm/dL). Table 4 details baseline values for Hb, serum ferritin, iron and TSAT for the three study groups. Patients in group I had a median baseline Hb level of 10.1 gm/dL (range, 8.4 gm/dL–10.9 gm/dL). Following the FCM therapy, median Hb increased to 11.4 gm/dL on week 3, 12.2 gm/dL on week 6 and 12.3 gm/dL on week 12. The median change in Hb at week 12 compared with baseline was 2.35 (–1.0, 4.6) gm/dL. Baseline Hb for group II was 10.3 gm/dL, increased to 11.3 gm/dL on week 3 and 11.8 gm/dL on week 6 and then reached 11.8 gm/dL on week 12. The median change in Hb at week 12 for this group was 1.5 (–1.0, 3.7) gm/dL. Patients in group III had a baseline Hb of 10.3 gm/dL that increased to 10.8 gm/dL on week 6 and 11.0 gm/dL on week 12. Their median change in Hb at week 12 was 0.5 (–1.6, 3.3) gm/dL. Figure 1 illustrates the course of Hb changes among the 3 groups during the 12 weeks of follow up.
Table 4.
Baseline hemoglobin and iron study profiles.
| Variables | All patients (n = 84) |
Group I absolute iron deficiency (n = 26) |
Group II functional iron deficiency (n = 24) |
Group
III others (n = 34) |
p-value | |
|---|---|---|---|---|---|---|
| Baseline Hb | Mean (SD)Median (Range) | 10.1 (0.6)10.2 (8.3, 11.1) | 10.1 (0.7)10.1 (8.4, 10.9) | 10.3 (0.7)10.3 (8.3, 11.0) | 10.3 (0.5)10.3 (8.8, 11.1) | 0.4283 |
| Serum iron | Mean (SD)Median (Range) | 48.2 (22.2)43 (15–113) | 34.5 (10.2)35.5 (19.0, 52.0) | 34.9 (12.5)33.5 (15.0, 65.0) | 68.1 (19.1)66.5 (20.0, 113) | <0.0001 |
| Ferritin | Mean (SD) Median (Range) |
120 (132) 67.1 (5.2–537) |
16.2 (6.7) 18.1 (5.2, 26.7) |
164 (156) 82.7 (31.8, 573) |
168 (120) 139 (7.8, 457) |
<0.0001 |
| TSAT | Mean (SD) Median (Range) |
17.7 (8.6) 16.8 (5, 37) |
10.7 (3.5) 10.0 (5.0, 17.0) |
12.9 (4.5) 13.5 (6.0, 19.0) |
26.5 (5.1) 25.0 (20.0, 37.0) |
<0.0001 |
| CRP | Mean (SD) Median (Range) |
18.5 (36.6) 6.5 (0.016, 221) |
8.0 (7.8) 4.1 (0.3, 30.4) |
45.5 (60.2) 20.9 (0.4, 221) |
7.6 (7.5) 5.6 (0.016, 34.8) |
0.0004 |
CRP, C-reactive protein; Hb, hemoglobin; TSAT, transferrin saturation.
Figure 1.
Hemoglobin changes following intravenous iron infusion.
Among the whole study population, a total of 50 (59.5%) patients had an increment in Hb ⩾ 1.0 gm/dL (responders) at week-12. Response rate was the highest among the patients with AIDA (80.8%). However, patients with FIDA had also a high repose rate (70.8%) and both were significantly higher than group III, with a response rate of 35.3% (p = 0.00027; Table 5). A total of 6 (7.1%) patients received blood transfusions.
Table 5.
Response rates per patient subgroup*.
| Groups | Number of patients | Response n (%) |
p-value |
|---|---|---|---|
|
Group I
Absolute iron deficiency |
26 | 21 (80.8%) | 0.00027** |
|
Group II
Functional iron deficiency |
24 | 17 (70.8%) | |
|
Group III
Others |
34 | 12 (35.5%) | |
| Total | 84 | 50 (59.5%) |
Response defined as a Hb increment ⩾1.0 gm/dL at week 12.
Group I and II versus group III.
CRP level, a marker of inflammation, was elevated in patients with FIDA with a mean level (SD) of 45.5 (60.2) mg/L. This was significantly higher than in those with AIDA [8.0 (7.8) mg/L] and in those in group III [7.6 (7.5) mg/L, p = 0.0004].
Serum hepcidin level was performed on all patients at baseline. The mean (SD) hepcidin level was 15.1 (22.5) ng/mL. Levels were lowest in group I (AIDA) [mean (SD) level was 1.7 (1.1) ng/mL], significantly lower than those with FIDA (group II) with a mean level of 25.7 [(33.1) ng/mL] and those in group III [17.7 (16.8) ng/mL, p < 0.0001; Table 6]. In addition, among the 50 patients who showed significant response, 39 (78.0%) had low hepcidin levels, this included all of the 21 (100%) responders in group I and 11 (64.7%) of the 17 responders in group II (p = 0.0018).
Table 6.
Hepcidin levels according to group.
| Group | Number of patients | Mean (SD) ng/mL |
Median (Range) ng/mL |
|---|---|---|---|
|
Group I
Absolute iron deficiency |
26 | 1.7 (1.1) | 1.2 (0.3, 4.8)* |
|
Group II
Functional iron deficiency |
24 | 25.7 (33.1) | 13.7 (1.0, 123)* |
|
Group III
Others |
34 | 17.7 (16.8) | 10.5 (0.7,59.4) |
| Total | 84 | 15.1 (22.5) | 4.6 (0.3, 123) |
p-value between group I and group II <0.0001.
No immediate infusion-related adverse events were reported. Specifically, no skin rash, itching, or anaphylaxis. The highest ferritin level among patients who completed the planned iron treatments was 4184 ng/mL in one patient, with 5 other patients developing levels in the 2000 ng/mL range. Hypophosphatemia (serum phosphate concentration <2.0 mg/dL) was observed in 39 (46.4%) patients; more in patients with AIDA (17: 65.4%; Table 7). All patients with hypophosphatemia were asymptomatic, were treated with oral phosphate supplements, and this subsided with the next blood testing.
Table 7.
Treatment and complications according to patient group.
| Variables | Absolute
iron deficiency (n = 26) |
Functional
iron deficiency (n = 24) |
Others (n = 34) |
p-value | |
|---|---|---|---|---|---|
|
Dose of FCM
(mg) |
Mean (SD) Median (Range) |
1577 (306) 1500 (1000, 2000) |
1500 (330) 1500 (1000, 2000) |
1397 (320) 1500 (1000, 2000) |
0.0974 |
|
Ferritin level (wk 12)
(mg/mL) |
Mean (SD) Median (Range) |
589 (509) 442 (177, 2794) |
838 (875) 577 (168, 4184) |
956 (667) 802 (166, 2687) |
0.0247 |
|
Phosphorus level (wk 3)
(mg/dL) |
Mean (SD) Median (Range) |
1.8 (0.7) 1.5 (1.1, 3.4) |
2.5 (1.0) 3.3 (1.0, 5.4) |
2.2 (0.9) (1.0, 4.1) |
0.0124 |
|
Hypophosphatemia
n (%) |
17 (65.4%) | 6 (25.0%) | 16 (47.1%) | 0.0421 | |
FCM, ferric carboxymaltose.
Discussion
Our study clearly demonstrates the efficacy of FCM in anemic cancer patients with either AIDA or FIDA. Response to FCM infusion was evident as early as 3 weeks after starting the treatment. In both AIDA and FIDA groups, a 1.3 and 1.25 gm/dL median increment, respectively, was noted at week 3. We have not checked the response earlier than week 3, but even a response as early as this should prevent treatment escalation with blood transfusions or ESAs. The lack of response among patients in group III is clinically expected; patients with a TSAT ⩾ 20% and/or serum of ferritin >800 ng/mL are unlikely to respond. In such situations, other treatment options can be tried early on.
While concerns may be raised about the rapidity of intravenous infusion of FCM, we have not encountered any serious adverse events including anaphylaxis. Hypophosphatemia was commonly encountered; however, it was clinically insignificant, transient, and did not mandate any intervention other than oral phosphate supplementation. The high incidence of hypophosphatemia encountered in our patients is consistent with previously published data. In a recent prospective study, the incidence of hypophosphatemia 2 weeks after treatment, similarly defined as a serum phosphate concentration of <2.0 mg/dL, was 38.7%.36 In another retrospective study, a similar decrease in phosphate at week 2 following FCM infusion was seen in 64 of 164 (39%) patients.37
Despite the small number of patients enrolled in our study, a correlation between hepcidin level and the type of anemia was evident; patients with FIDA had significantly higher levels of hepcidin. In addition, there might be some correlation between low hepcidin level and response to iron therapy. This was most pronounced in patients with AIDA, where all responders had low levels and in patients with FIDA where almost two-thirds of responders had low levels as well. CRP level should also be explored as a potential marker for response to intravenous iron therapy.
Although larger than many published studies in the field, our study population is still small, and some caution is required before generalizing our results. However, several conclusions can be made. First, FCM is safe and effective in treating anemia in select cancer patients undergoing chemotherapy. Second, patients with AIDA can be easily identified based on ferritin and TSAT levels and do respond to FCM. Third, patients with FIDA, especially those with low hepcidin levels, have high response rates, justifying a treatment trial. Fourth, patients with TSAT ⩾ 20% and/or serum ferritin >800 ng/mL are unlikely to respond, and FCM should not be used. Larger studies evaluating and confirming these conclusions are highly needed to improve the management of this commonly encountered clinical problem.
Footnotes
Author contributions: Conception and design: Hikmat Abel-Razeq
Provision of study materials or patients: Salwa S. Saadeh, Razan Malhis, Sameer Yasser, Hazem Abdulelah, Rana Eljaber, Amer Kleib
Collection and assembly of data: Salwa S. Saadeh, Razan Malhis, Sameer Yasser, Hazem Abdulelah, Rana Eljaber, Amer Kleib, Rouba Ismael
Data analysis and interpretation: Hikmat Abel-Razeq, Salwa S. Saadeh,
Manuscript Writing: Hikmat Abel-Razeq, Salwa S. Saadeh
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
Authors’ note: Prior Presentation: Presented at the annual European Society of Medical Oncology (ESMO) Congress, Barcelona, Spain, 27 September-1 October 2019.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee (KHCC IRB) and with the 1964 Helsinki declaration and its later amendments
Written informed consent was obtained by one of the investigators from all individual participants included in the study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by unrestricted grant from Hikma Pharmaceuticals, Amman-Jordan Company had no access to data or manuscript prior to its publication.
ORCID iDs: Hikmat Abdel-Razeq  https://orcid.org/0000-0003-2833-6051
https://orcid.org/0000-0003-2833-6051
Salwa S. Saadeh  https://orcid.org/0000-0002-1052-9093
https://orcid.org/0000-0002-1052-9093
Contributor Information
Hikmat Abdel-Razeq, Department of Medical oncology, King Hussein Cancer Center, 202 Queen Rania Al-Abdullah, Amman, 11941, Jordan; School of Medicine, University of Jordan, Amman, Jordan.
Salwa S. Saadeh, Department of Medical oncology, King Hussein Cancer Center, Amman, Jordan
Razan Malhis, Department of Medical oncology, King Hussein Cancer Center, Amman, Jordan.
Sameer Yasser, Department of Medical oncology, King Hussein Cancer Center, Amman, Jordan.
Hazem Abdulelah, Department of Medical oncology, King Hussein Cancer Center, Amman, Jordan.
Rana Eljaber, Department of Pharmacy, King Hussein Cancer Center, Amman, Jordan.
Amer Kleib, Department of Nursing, King Hussein Cancer Center, Amman, Jordan.
Rouba Ismael, Office of Scientific Affairs and Research, King Hussein Cancer Center, Amman, Jordan.
References
- 1. Ludwig H, Van Belle S, Barrett-Lee P, et al. The European cancer anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004; 40: 2293–2306. [DOI] [PubMed] [Google Scholar]
- 2. Ludwig H, Müldür E, Endler G, et al. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 2013; 24: 1886–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grotto H. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol 2007; 25: 12–21. [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Razeq HN. Cancer-related anemia. Saudi Med J 2004; 25: 15–20. [PubMed] [Google Scholar]
- 5. Crawford J, Cella D, Cleeland C, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002; 95: 888–895. [DOI] [PubMed] [Google Scholar]
- 6. Thomas G. The effect of hemoglobin level on radiotherapy outcomes: the Canadian experience. Semin Oncol 2001; 28: 60–65. [DOI] [PubMed] [Google Scholar]
- 7. Connor J, O’Shea A, McCool K, et al. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian cancer; potential impact of patient blood management on cancer outcomes. Gynecol Oncol 2018; 151: 294–298. [DOI] [PubMed] [Google Scholar]
- 8. Gordon D, Nichols G, Ben-Jacob A, et al. Treating anemia of cancer with every-4-week darbepoetin alfa: final efficacy and safety results from a phase II, randomized, double-blind, placebo-controlled study. Oncologist 2008; 13: 715–724. [DOI] [PubMed] [Google Scholar]
- 9. Ferrario E, Ferrari L, Bidoli P, et al. Treatment of cancer-related anemia with epoetin alfa: a review. Cancer Treat Rev 2004; 30: 563–575. [DOI] [PubMed] [Google Scholar]
- 10. Littlewood T, Bajetta E, Nortier J, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001; 19: 2865–2874. [DOI] [PubMed] [Google Scholar]
- 11. Gabrilove J, Cleeland C, Livingston R, et al. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol 2001; 19: 2875–2882. [DOI] [PubMed] [Google Scholar]
- 12. Aapro M, Österborg A, Gascón P, et al. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol 2012; 23: 1954–1962. [DOI] [PubMed] [Google Scholar]
- 13. Auerbach M, Ballard H, Trout J, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 2004; 22: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 14. Auerbach M, Silberstein P, Webb R, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol 2010; 85: 655–663. [DOI] [PubMed] [Google Scholar]
- 15. Henry D, Dahl N, Auerbach M, et al. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007; 12: 231–242. [DOI] [PubMed] [Google Scholar]
- 16. Bastit L, Vandebroek A, Altintas S, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alfa administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 2008; 26: 1611–1618. [DOI] [PubMed] [Google Scholar]
- 17. Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003; 362: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 18. Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005; 23: 5960–5972. [DOI] [PubMed] [Google Scholar]
- 19. Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non–small-cell lung cancer with disease-related anemia. J Clin Oncol 2007; 25: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 20. Bennett CL. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008; 299: 914. [DOI] [PubMed] [Google Scholar]
- 21. Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med 2008; 168: 2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizzo JD, Brouwers M, Hurley P, et al. American society of clinical oncology/American society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Oncol Pract 2010; 6: 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdel-Razeq H, Hijjawi S, Abdulelah H, et al. The impact of recently published negative erythropoiesis-stimulating agent studies on the clinical management of cancer-related anemia at a single center. Hematol Oncol Stem Cell Res 2010; 3: 78–83. [DOI] [PubMed] [Google Scholar]
- 24. Ludwig H, Aapro M, Bokemeyer C, et al. A European patient record study on diagnosis and treatment of chemotherapy-induced anaemia. Support Care Cancer 2014; 22: 2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. Epub ahead of print 17 June 2013. DOI: 10.1111/trf.12289. [DOI] [PubMed] [Google Scholar]
- 26. Qunibi WY, Martinez C, Smith M, et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant 2010; 26: 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 2002; 48: 1066–1076. [PubMed] [Google Scholar]
- 28. Sheetz M, Barrington P, Callies S, et al. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol 2019; 85: 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoffel NU, Lazrak M, Bellitir S, et al. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica 2019; 104: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arezes J, Nemeth E. Hepcidin and iron disorders: new biology and clinical approaches. Int J Lab Hematol 2015; 37: 92–98. [DOI] [PubMed] [Google Scholar]
- 31. Ruchala P, Nemeth E. The pathophysiology and pharmacology of hepcidin. Trends Pharmacol Sci 2014; 35: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology 2013; 2013: 1–8. [DOI] [PubMed] [Google Scholar]
- 33. Sangkhae V, Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv Nutr 2017; 8: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdel-Razeq H, Abbasi S, Saadi I, et al. Intravenous iron monotherapy for the treatment of non-iron-deficiency anemia in cancer patients undergoing chemotherapy: a pilot study. Drug Des Devel Ther 2013; 7: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wyck DBV, Mangione A, Morrison J, et al. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009; 49: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 36. Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol 2018; 93: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bager P, Hvas CL, Dahlerup JF. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol 2017; 83: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]