Abstract
Urine from pro-œstrus female rodents evokes increased levels of sexually-motivated behaviors in males, including sniffing and scent marking of the urine spot as well as activation of brain reward regions. Stressors such as social defeat can adversely impact urine scent marking behavior in male rodents, an effect that can be mitigated with anti-depressant drugs. Persistent pain is also known to be a potent stressor, producing elevated levels of plasma corticosterone as well as reduced sucrose preference and reduced social interaction. However, the effect of persistent pain on sexually-motivated behavior is unknown. Here, we compared urine scent marking behavior in male rats for up to 3 weeks following intra-articular injection of Complete Freund’s Adjuvant (CFA) or sham injection. CFA-injected rats exhibited profound and ongoing deficits in static weight bearing capacity. CFA-induced persistent inflammatory pain increased plasma corticosterone levels and reduced urine scent marking behavior in male rats. Moreover, while the vast majority of injured rats showed decreased urine scent marking preference for the pro-œstrus female urine spot, male rats with higher baseline scent marking preference also exhibited higher post-injury scent marking preference, more sniffing behavior and lower levels of plasma corticosterone, compared to those with lower baseline scent marking preference. Overall, scent marking behavior may be an ethologically relevant behavioral predictor of persistent pain-induced stress in rats, representing a novel translational approach to understanding chronic pain comorbidities.
Keywords: Rat, Inflammation, Pain, Sexual, Stress
1. Introduction
Sexual/reproductive behaviors reflect one of the most potent natural drives, where loss of sexual drive in humans may be a consequence of severe stress and/or mental illness reviewed in [29,79]. Male rodents are potently attracted to urine from pro-estrous females [21,47], resulting in activation of brain reward regions in these males [47]. In rats and mice, the social defeat paradigm is considered to be a model of chronic stress and/or depression [12,23,34], and has been shown to reduce sexually-motivated urine scent marking behavior in male mice in a fluoxetine-dependent manner [44]. In humans, chronic pain is also considered to be a potent stressor [74,76] that can lead to comorbid depression [1,2] and as well as reduced sexual drive [53]. Indeed, among the most severely impacted portion of the chronic pain population are those experiencing comorbid depression [75]. In rodents, persistent pain following nerve injury or adjuvant-induced inflammation is a known stressor that can increase plasma corticosterone [43,60], depression-like behavior [78], anxiety-like behavior [55,59,67,78] and anhedonia (i.e. reduced sucrose preference [30]). However, very little is known about the effects of persistent pain on rodent sexual behavior.
We hypothesized that scent marking behavior will be reduced in male rats experiencing persistent adjuvant-induced inflammation. Considering that depression is seen in only a subset of the chronic pain population (20–35%) [19,51,73], we further hypothesized that injured rats will also exhibit inter-individual variability in the degree of scent marking behavior post-injury. In humans, pre-surgical affective states have been linked with persistent post-surgical pain [15,26,58,62,72],reviewed in [33] and mood disturbance [58]. While no link between pre-injury affective states and post-injury pain or affect has been reported in rodent models of chronic pain, there is some evidence for such a relationship in models of chronic stress [13,42,44,65,70]. Therefore, using a model of persistent hind limb inflammatory pain, we assessed whether or not post-injury outcomes such as pain, stress and urine scent marking were associated with baseline (pre-injury) scent marking behavior. Taken together, we sought to assess the effects of persistent pain on urine scent marking, and to determine if basal scent marking behavior was predictive of post-injury outcomes.
2. Methods
2.1. Animals
A total of 26 male Long Evans rats (Charles River) between 250–275 g were used for all experiments (n = 12–14/group). Rats were kept on an inverted 12 h/12 h light/dark cycle (lights out at 9am) and were only tested during the early portion of their waking cycle. All animals were pair-housed with a cage mate of the same experimental group and given free access to food and water. Each cage was randomly assigned to either the sham or injured experimental conditions. We opted to randomly assign each cage to either the sham or injured condition in order to avoid the potential disruption of dominant-submissive hierarchies that could occur by mixing experimental conditions within the same cage. While experimenters were blinded to the experimental condition, hind limb swelling in the injured group was evident during assessment of weight bearing capacity. Experimenters were blinded to the experimental condition for analysis of plasma corticosterone, scent marking and all video analysis. Each behavioral procedure was always performed by the same male experimenter. Animals were habituated to each experimental apparatus for 15–30 min on three consecutive days prior to baseline testing. All protocols were reviewed and approved by National Institute of Neurological Disorders and Stroke/National Institute on Deafness and other Communication Disorders Animal Care and Use Committee (NINDS/NIDCD ACUC). Experiments were in accordance with the NINDS/NIDCD ACUC and the International Association for the Study of Pain (IASP) guidelines for the care and use of experimental animals.
2.2. Peripheral inflammation
A total of 25ul of Complete Freund’s Adjuvant (CFA; 1 mg/ml Mycobacterium tuberculosis, Sigma Alderich) was injected into the tibial–tarsal joint of the left hind paw in isoflurane-anesthetized (5% in O2) rats according to the method described in [60]. Control (sham) animals underwent the same procedure including needle insertion but without irritant injection in order to prevent potential volume-related damage to the ankle joint. Animals were immediately returned to their home cage for observation.
2.3. Weight bearing
Static weight bearing capacity was measured with an Incapacitance Meter (IITC Life Science, model 600). Similar to humans with a painful injury to the ankle or knee, the static weight bearing assay reflects the rodent’s unwillingness or incapacity to bear weight on the injured limb. As such, it can be considered an ethologically relevant measure of mechanical hypersensitivity within the injured joint rather than on the skin. To measure static weight bearing, we used a commercially available device (Incapacitance Meter, IITC Life Science, model 600) and employed a commonly used approach [8,60]. Briefly, the rat was placed on an inclined plane within a small transparent Perspex enclosure where its tail was gently held to minimize excessive movement. When the animal had finished exploring the enclosure and hind paws were stably resting on the two strain gauges, the measurement was taken, expressed as the proportion of total weight placed on the ipsilateral and contralateral hind paws. Three measurements of 5 s each were recorded for each animal and a mean was calculated. The entire procedure lasted no more than approximately two minutes for each animal. For analysis, weight bearing was expressed as the proportion of ipsilateral to contralateral scores. A proportion of 1 reflects equal weight bearing capacity on both hind paws.
2.4. Urine scent marking
We compared urine scent marking behavior in male rats (n = 12 sham; n = 14 CFA) at baseline and weekly for the three weeks following CFA injection (Fig. 1A). Briefly, 0.1 ml of urine pooled from 6 female rats in the pro-estrous stage was blotted onto an absorbent paper uniformly covering the floor of a large (45 × 60 cm), grey acrylic arena (Fig. 1B). The female urine spot was marked with pencil to differentiate it from urine deposited by the male rat during testing. Male rats were placed individually into the arena and allowed to scent mark for 10 min and then returned to their home cage. The paper was immediately removed, air-dried overnight and stained with ninhydrin (Tritech Forensics, Southport, NC), which stains the amines found in urine. Dried sheets were imaged using a large format scanner and analyzed for urine-marking area in a circular region (25 cm diameter) around the female urine spot as well as in the remaining area. A representative example of scent marking from an uninjured male rat can be seen in Fig. 1C. The primary outcome was marking preference, based on the area of marking in the circular zone around the female urine spot compared to the remaining arena (Fig. 1C). Specifically, because the circular zone around the female urine spot is 4.5 times smaller than the remaining arena, the preference score is defined as scent marking area (or time spent in the circular zone) multiplied by 4.5, all divided by scent marking area (or time spent) in the remaining arena; Fig. 1C). A marking preference greater than 1 corresponds to a preference for the female urine zone, while a score of 1 reflects chance or random behavior. A preference score of less than 1 represents active avoidance of scent marking in the female urine zone. To assess the predictability of baseline marking preference on post-injury outcomes, the mean baseline marking preference score was calculated from all rats and used to define the low and high baseline marking preference groups. To measure time preference for the female urine zone, sniffing behavior of the female urine spot and locomotion (total distance covered in the arena), movement of each rat within the arena was recorded with a video camera and analyzed using TopScan software (CleverSys, Inc).
Fig. 1.
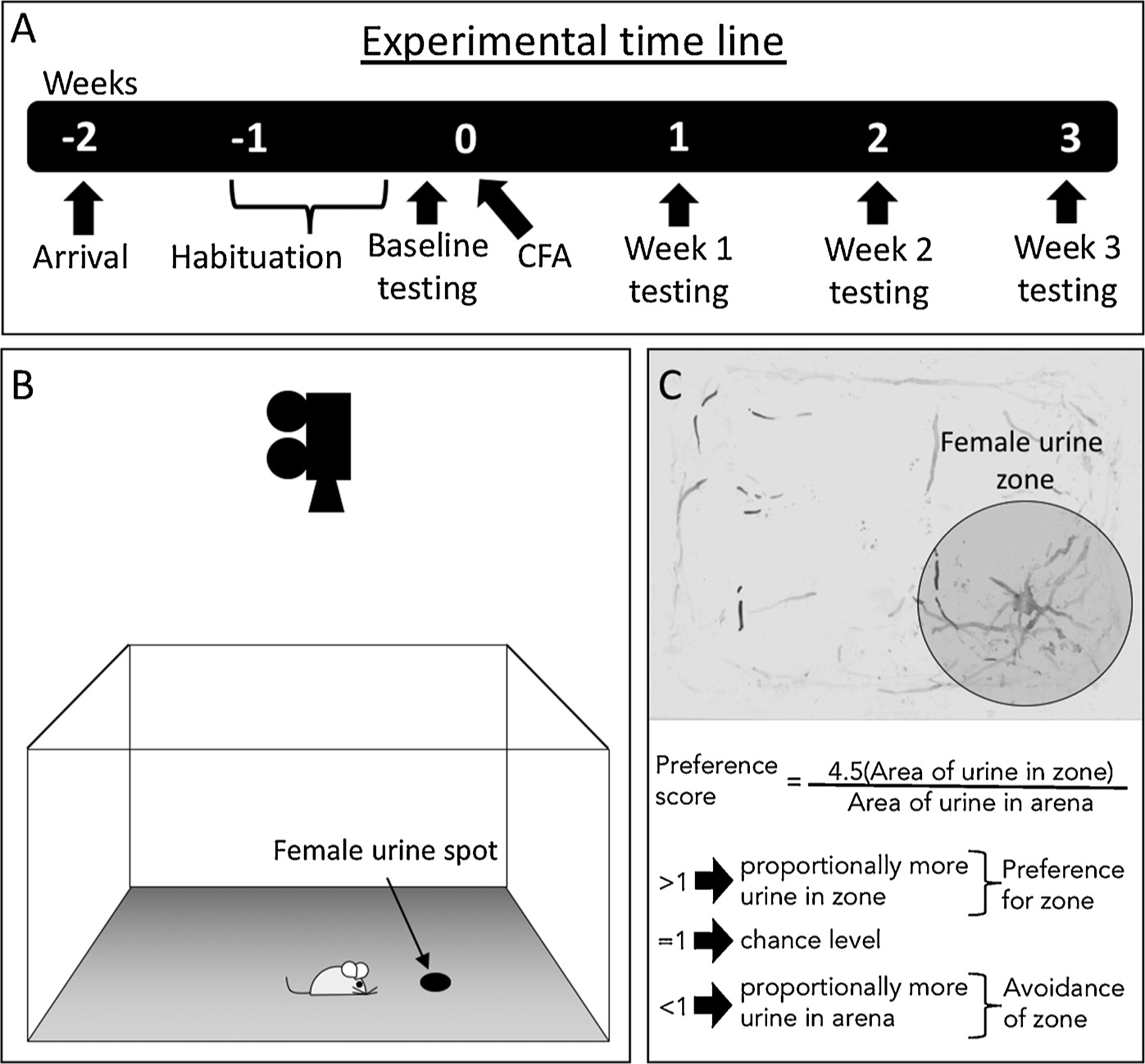
Experimental time line and methodology. (A) Experimental time line. (B) Rats spent 10 min in an arena with an absorbent paper floor and a spot of urine harvested from pro-œstrus female rats. Behavior was recorded from above. (C) Representative image of urine scent marking, with equation to calculate preference scores for the female urine zone (i.e. marking and time preference).
2.5. Plasma corticosterone
Plasma corticosterone was assessed twice; once at baseline and again at week 3 post-CFA, using an enzyme-linked immunosorbent assay (ELISA; Enzo Life Sciences kit #ADI-900–097). As described previously [60], rats were placed in a soft folded towel (new towel for each rat) where they were gently restrained. A small 0.5 mm incision to the tip of the tail was made, from which 20–30 µl of blood was harvested using heparin-treated capillary tubes. The entire procedure lasted approximately 2 min and was performed between 9–11 a.m. Blood samples were kept at 4 °C until centrifugation (2 min at 16,000RPM; Iris CritSpin) to separate plasma from serum. Plasma was pipetted into individually labeled sample tubes and stored at −80 °C until ELISA processing. Plasma levels of corticosterone were assessed according to the small sample volume instructions from Enzo Life Sciences kit #ADI-900–097. Briefly, samples were brought to room temperature and 10 µl of each sample was diluted (40:1) with assay buffer. 100 µl of samples and standards were pipetted into the appropriate wells of the ELISA plate. Conjugate (50 µl) and antibody (50 µl) were added to each well and incubated in darkness for 2 h on a shaker (500 RPM) at room temperature. The plate was then washed 3 times with washing buffer and gently tapped on an absorbent paper to remove remaining moisture. A total of 200 µl of p-nitrophenyl phosphate substrate was added to each well and incubated in darkness for 1 h at room temperature without shaking. Then, 50 µl of ‘stop’ solution was added to each well and the absorbance was immediately measured with a Wallac 1420 model plate reader with a 405 nm filter. All samples, controls and standards were measured in duplicate. Plasma levels of corticosterone were expressed in picograms/milliliter (pg/ml). One plasma sample for a sham rat was lost.
2.6. Statistical analysis
Statistical analysis was performed with GraphPad Prism. Two-way mixed design repeated measures ANOVA (time as a within-subjects factor and experimental group as between-subjects factor), Pearson correlation or Chi Square (χ2) analyses were used where indicated. Normality of group distributions was assessed with the D’Agostino-Pearson test. In some cases, data sets did not pass normality testing. Therefore, the Mann-Whitney U, a non-parametric test that does not require the assumption of normal distributions, was used to assess group differences following two-way mixed design repeated measures ANOVA. Results are reported as mean ± standard error. For χ2, an increased marking preference was defined as post-injury marking preferences greater than 100% of baseline marking preference. All post-injury marking preference scores less than 100% of baseline were considered as decreased. In no cases were post-injury marking preference scores equal to 100%. Descriptions of statistics and outcomes for these analyses can be found in Table 1. In all cases, p < 0.05 was considered significant.
Table 1.
Description of statistical analysis and results.
| Figure | Panel | Title | Statistic | Result |
|---|---|---|---|---|
| 2 | A | Weight bearing (Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 33.14, p < 0.0001****; Time – F(3,72) = 36.07, p < 0.0001****; Group–F(1,24) = 97.95, p = 0.0001*** |
| B | Plasma corticosterone (Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(1,23) = 5.537, p = 0.028*; Time – F(1,23) = 0.110, p = 0.744; Group– F(1,23) = 1.872, p = 0.184 | |
| C | Change in plasma corticosterone | χ2 | χ2(1) = 11.4, p = 0.0007*** | |
| 3 | A | Marking preference (Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 2.764, p = 0.048*; Time – F(3,72) = 1.657, p = 0.184; Group– F(1,24) = 0.144, p = 0.708 |
| B | Change in marking preference | χ2 | χ2(1) = 3.869, p = 0.025* | |
| C | CFA– marking preference (inter-individual variability) | No analysis | No analysis | |
| D | Sham – marking preference (inter-individual variability) | No analysis | No analysis | |
| E | Marking area in zone and arena (Sham and CFA) | No analysis | No analysis | |
| 4 | A | Ankle width (mm; Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 36.58, p < 0.0001****; Time – F(3,72) = 21.56, p < 0.0001****; Group–F(1,24) = 33.32, p < 0.0001**** |
| B | Mass (g; Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 22.85, p < 0.0001****; Time – F(3,72) = 295.5, p < 0.0001****; Group–F(1,24) = 7.36, p < 0.012* | |
| C | Marking area (cm2; Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 0.061, p = 0.980; Time – F(3,72) = 2.777, p = 0.047*; Group– F(1,24) = 0.109, p = 0.744 | |
| D | Sniffing urine spot (s; Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 0.159, p = 0.924; Time – F(3,72) = 0.373, p = 0.773; Group– F(1,24) = 0.329, p = 0.572 | |
| E | Time preference (Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 0.148, p = 0.930; Time – F(3,72) = 1.804, p = 0.154; Group– F(1,24) = 0.000, p = 0.985 | |
| F | Total distance (cm; Sham vs. CFA) | Two-way mixed design ANOVA | Interaction – F(3,72) = 0.1.883, p = 0.140; Time – F(3,72) = 15.11, p < 0.0001****; Group– F(1,24) = 0.641, p = 0.431 | |
| 5 | A | Week 3 weight bearing vs. baseline marking preference | Pearson correlation | CFA: r=−0.235; p = 0.418; Sham: r = 0.107: p = 0.742 |
| B | Week 3 corticosterone vs. week 3 ankle width | Pearson correlation | CFA: r=−0.468; p = 0.091; Sham: r = 0.177: p = 0.603 | |
| C | Week 3 corticosterone vs. week 3 marking preference | Pearson correlation | CFA: r=−0.315; p = 0.273; Sham: r=−0.029: p = 0.933 | |
| D | Week 3 corticosterone vs. baseline marking preference | Pearson correlation | CFA: r=−0.580; p = 0.030*; Sham: r=−0.234: p = 0.750 | |
| E | Low/high baseline marking preference | |||
| F | Low/high baseline marking preference (ankle width) | Two-way mixed design ANOVA | Interaction – F(1,12) = 3.143, p = 0.102; Time – F(1,12) = 39.1, p < 0.0001****; Group– F(1,12) = 1.086, p = 0.318 | |
| G | Low/high baseline marking preference (mass) | Two-way mixed design ANOVA | Interaction – F(1,12) = 0.158, p = 0.698; Time – F(1,12) = 195.2, p < 0.0001****; Group– F(1,12) = 2.0, p = 0.183 | |
| H | Low/high baseline marking preference (weight bearing) | Two-way mixed design ANOVA | Interaction – F(1,12) = 0.759, p = 0.401; Time – F(1,12) = 37.97, p < 0.0001****; Group– F(1,12) = 273, p = 0.495 | |
| I | Low/high baseline marking preference (total distance) | Two-way mixed design ANOVA | Interaction – F(1,12) = 030, p = 0.865; Time – F(1,12) = 7.139, p = 0.020*; Group– F(1,12) = 0.022, p = 0.886 | |
| J | Low/high baseline marking preference (total marking area)) | Two-way mixed design ANOVA | Interaction – F(1,12) = 0.175, p = 0.683; Time – F(1,12) = 1.2, p = 0.295; Group– F(1,12) = 0.759, p = 0.401 | |
| K | Low/high baseline marking preference (time preference) | Two-way mixed design ANOVA | Interaction – F(1,12) = 0.440, p = 0.520; Time – F(1,12) = 1.285, p = 0.279; Group– F(1,12) = 1.8839, p = 0.195 | |
| L | Low/high baseline marking preference (marking preference) | Two-way mixed design ANOVA | Interaction – F(1,12) = 0.523, p = 0.483; Time – F(1,12) = 14.76, p = 0.0023**; Group– F(1,12) = 17.78, p = 0.0012** | |
| M | Low/high baseline marking preference (sniffing) | Two-way mixed design ANOVA | Interaction – F(1,12) = 1.884, p = 0.195; Time – F(1,12) = 0.004, p = 0.953; Group– F(1,12) = 10.13, p = 0.0079** | |
| N | Low/high baseline marking preference (corticosterone) | Two-way mixed design ANOVA | Interaction – F(1,12) = 1.58, p = 0.233; Time – F(1,12) = 4.257, p = 0.061; Group– F(1,12) = 1.977, p = 0.185 | |
| Results section; correlations (no figure) | Baseline marking preference vs. week 3 total marking area | Pearson correlation | CFA: r = 0.231; p = 0.428; Sham: r = 0.125: p = 0.698 | |
| Baseline marking preference vs. week 3 distance | Pearson correlation | CFA: r = 0.220; p = 0.451; Sham: r=−0.076: p = 0.815 | ||
| Baseline marking preference vs. week 3 marking preference | Pearson correlation | CFA: r = 0.338; p = 0.237; Sham: r = 0.100: p = 0.756 | ||
| Baseline marking preference vs. week 3 sniffing | Pearson correlation | CFA: r = 0.272; p = 0.348; Sham: r=−0.284: p = 0.371 | ||
| Baseline marking preference vs. week 3 time preference | Pearson correlation | CFA: r = 0.210; p = 0.471; Sham: r = 0.021: p = 0.949 | ||
| Week 3 weight bearing vs. week 3 marking preference | Pearson correlation | CFA: r=−0.044; p = 0.882; Sham: r = 0.031: p = 0.924 | ||
| Week 3 weight bearing vs. week 3 corticosterone | Pearson correlation | CFA: r=−0.075; p = 0.799; Sham: r = 0.208: p = 0.540 | ||
3. Results
While weight bearing capacity of the sham rats remained at baseline levels for the duration of the study, two-way mixed design analysis of variance (ANOVA) shows that the CFA group exhibited persistent weight bearing deficits at all post-injection time points (Fig. 2A; Table 1). Considering that CFA-induced inflammation can, over time, lead to deficits in affective behavior [55,59,67,78] and elevated stress [43,60], we assessed the effects of CFA-induced inflammatory pain on plasma corticosterone (Fig. 2B). While comparable at baseline, week 3 plasma corticosterone level for the CFA rats (1362.0 ± 156.2 pg/ml) was significantly higher than that of the sham group (676.7 ± 88.7 pg/ ml; Fig. 2B, Table 1). However, week 3 post-CFA corticosterone levels in both the CFA and sham groups were not significantly different from their respective baseline levels. Chi square analysis indicated that while plasma corticosterone levels tended to decrease between baseline and week 3 in shams, they increased in the CFA group (Fig. 2C; Table 1).
Fig. 2.
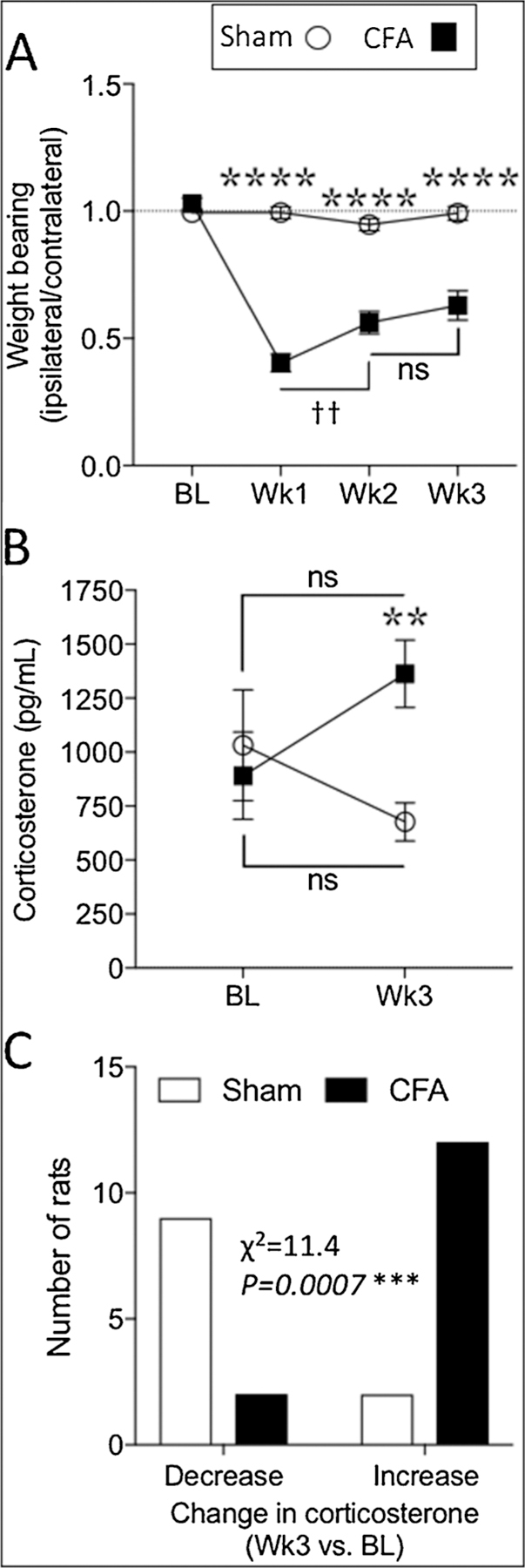
CFA-induced persistent inflammation produced ongoing hypersensitivity and stress. Compared to rats with sham injections, rats with CFA exhibited prolonged static weight bearing deficits (A) as well as increased plasma corticosterone levels (B). Chi square analysis indicated that corticosterone levels tended to decrease in shams and increase in CFA-injected rats (C). Two-way mixed design ANOVA (A, B) or Chi square analysis (C); p < 0.05 considered significant in all cases (Sham n = 12; CFA n = 14). Stars (*) represent a significant comparison between CFA and sham groups. ** p < 0.01; *** p < 0.001; **** p < 0.0001. Daggers (†) represent a significant comparison between the time points indicated. †† p < 0.01; ns = not significant.
In terms of scent marking behavior, the sham group exhibited robust marking preference for the female urine zone at baseline and for all three post-injection weeks (Fig. 3A; Table 1), demonstrating a preference for marking in the female urine zone compared to the remainder of the arena. However, while the CFA group exhibited a similar preference for the female urine zone at baseline and for two weeks after injection, a significant reduction was observed at three weeks (Fig. 3A; Table 1). Chi square (χ2) analysis indicated that compared to the sham group, significantly more CFA-injected rats exhibited a reduced marking preference at the 3-week time point (Fig. 3B; Table 1). Indeed, while 12 of the 14 CFA-injected rats exhibited a decrease in scent marking behavior at 3 weeks post-injection (Fig. 3C), only 6 of 12 rats in the sham group showed a decrease at 3 weeks (Fig. 3D). Interestingly, while none of the rats from the sham group exhibited active avoidance of the female urine zone at the 3-week time point (i.e. marking preference < 1; Fig. 3D), 4 out of 14 CFA-injected rats actively avoided the female urine zone at the 3 weeks (28.6%; Fig. 3C).
Fig. 3.
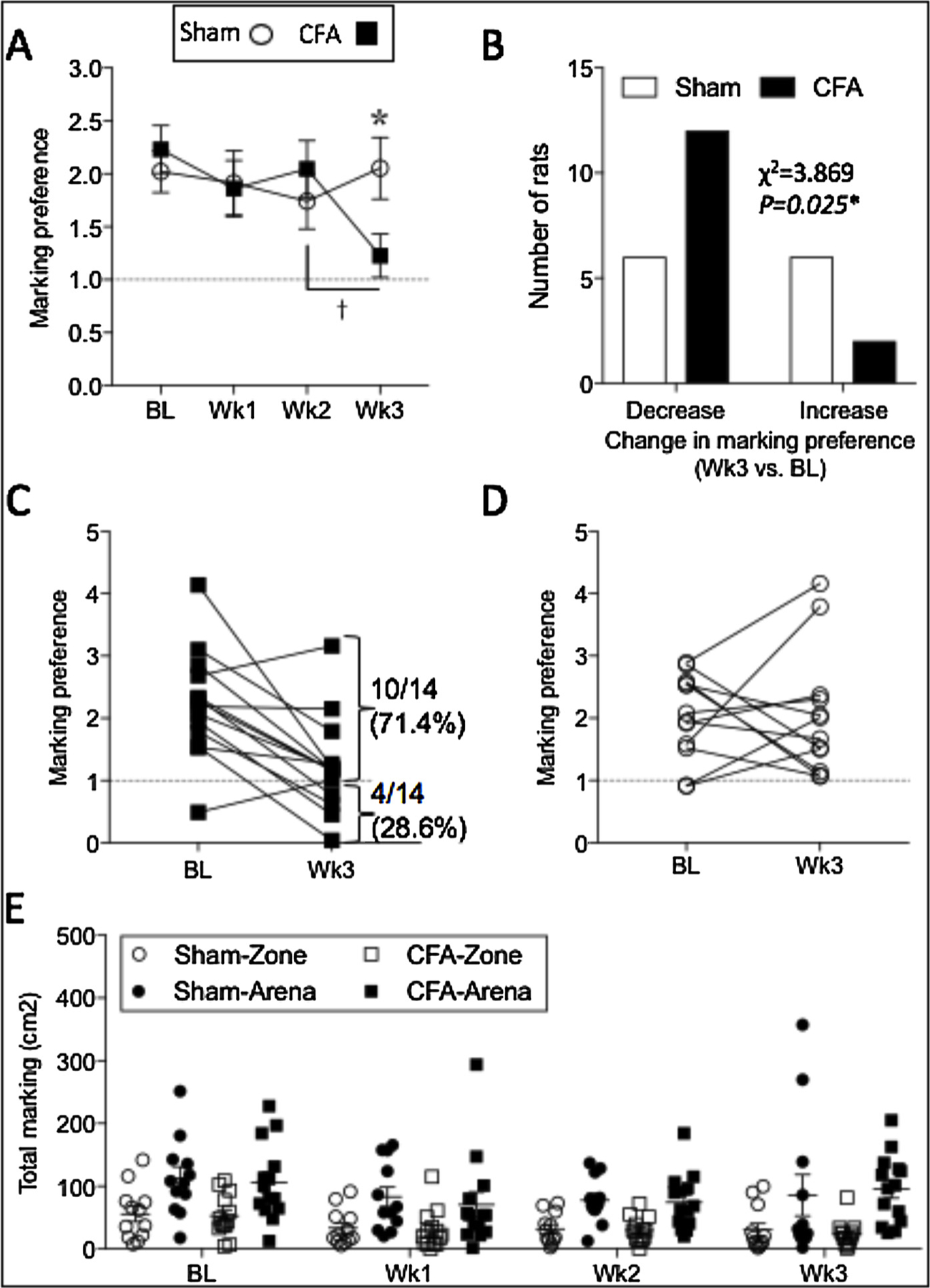
CFA-induced persistent inflammation reduced scent marking preference for the female urine spot. Urine scent marking preference for the female urine spot was reduced at 3 weeks post-CFA (A). Chi square analysis indicated that a greater proportion of the CFA group exhibited decreased marking preference compared to shams (B). Individual differences in baseline and post-injury marking preference in the CFA group (C) and the sham group (D). Note that approximately 29% of the CFA group exhibited active avoidance for scent marking in the female urine zone (C). Raw data for urine scent marking in the female urine zone and the remaining arena (E). Two-way mixed design ANOVA (A) or Chi square analysis (B); p < 0.05 considered significant in all cases (Sham n = 12; CFA n = 14). Stars (*) represent a significant comparison between CFA and sham groups. * p < 0.05. Daggers (†) represent a significant comparison between the time points indicated. † p < 0.05.
Fig. 4.
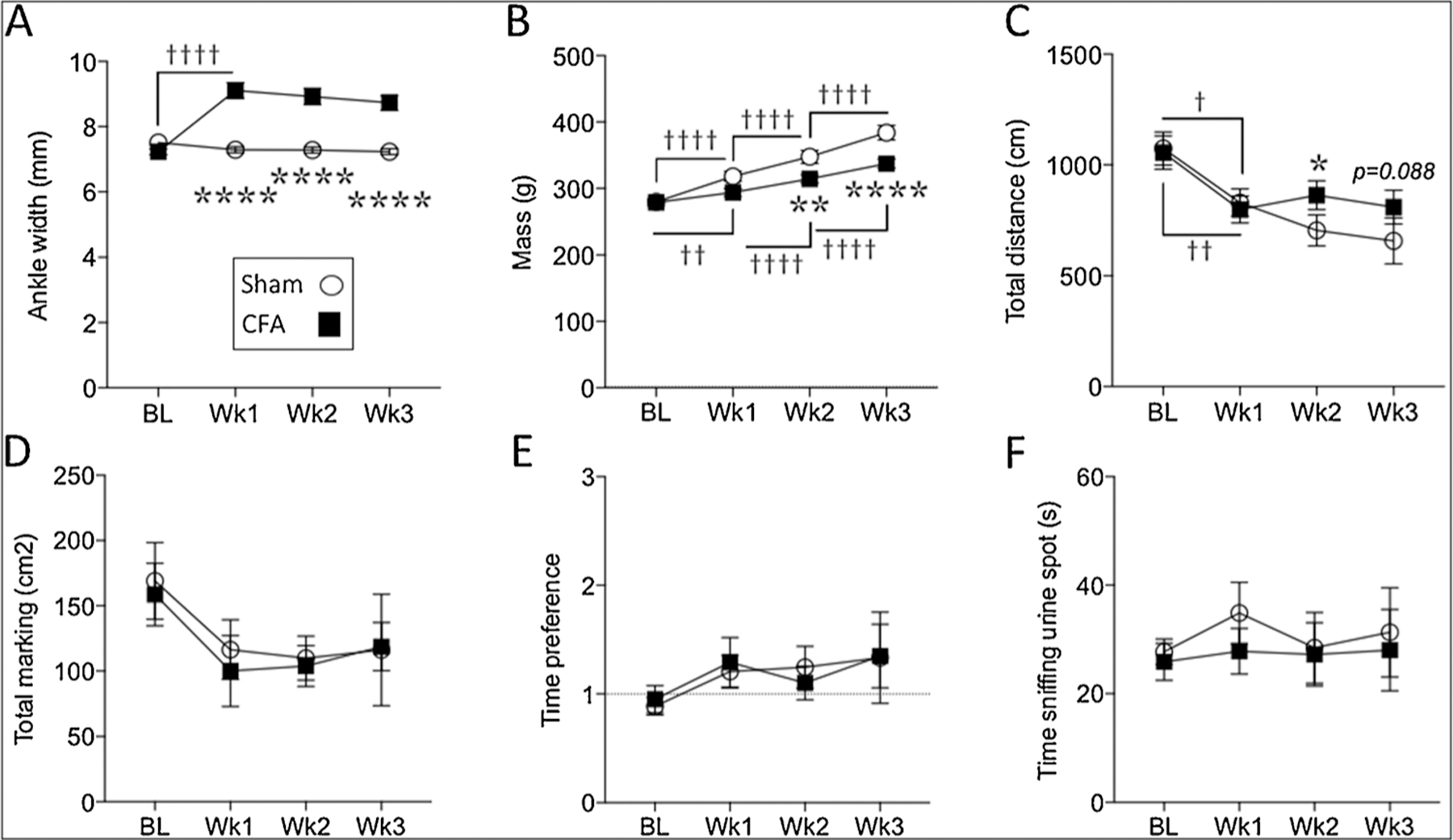
At the group level, CFA-induced persistent inflammation increased ipsilateral ankle width (A), slower weight gain (B) and differences in distance covered (C), but did not alter overall marking area (D), time preference for the female urine zone (E) or sniffing of the female urine spot (F). Two-way mixed design ANOVA. Stars (*) represent a significant comparison between CFA and sham groups. * p < 0.05; ** p < 0.01; **** p < 0.0001. Daggers (†) represent a significant comparison between the time points indicated. † p < 0.05; †† p < 0.01; ††††p < 0.0001.
Fig. 3E illustrates the raw scores for scent marking area in the female urine zone and the remaining arena.
In addition to marking preference, we also assessed other outcomes (Fig. 4A–F). CFA-injected rats exhibited a significant increase in ankle width at one-week post-CFA (Fig. 4A; Table 1). In addition, rats in the CFA group gained weight at a significantly slower rate than sham rats (Fig. 4B; Table 1). In terms of total distance covered, a significant main effect for time indicates that, overall, rats covered less distance over time. However, rats in the CFA group covered significantly more distance than shams at week-2 and to some extent at week-3 post-CFA (Fig. 4C; Table 1). In terms of total marking area, a significant main effect for time indicated a decrease in the total area of urine deposited in the arena (Fig. 4D; Table 1). However, no significant differences were observed between sham and CFA groups at any time point, suggesting that the inflammation-induced reduction in marking preference is a function of where the rats choose to mark (i.e. zone versus arena) rather than how much they mark overall. Baseline time preference for the female urine zone was close to chance level in both shams and CFA groups (Fig. 4E), indicating that rats spent proportionately equal amounts of time in the female urine zone as in the rest of the arena. While the time preference seemed to increase slightly over time, no main effect for time or group was found, and there was no significant difference between sham and CFA groups at any time point (Fig. 4E; Table 1). In terms of time spent sniffing the female urine spot, both sham and CFA groups exhibited similar amounts of time sniffing the urine spot at all time points (Fig. 4F; Table 1).
While pre-surgical affective state is predictive of chronic post-surgical pain in humans [26,62,72]; reviewed in [33], we did not find a correlation between baseline scent marking preference and week 3 wt bearing capacity for the CFA group (Fig. 5A; Table 1). To determine if severity of CFA-induced weight bearing deficits were related to week 3 marking preference or corticosterone level, we assessed the relationship between week-3 wt bearing and week-3 scent marking or corticosterone: Neither scent marking nor corticosterone were correlated with weight bearing at week-3 time points (Table 1). We also assessed the relationship between baseline scent marking and week-3 measures of total marking area, distance, marking preference, sniffing and time preference, but no other significant correlations with baseline marking preference were observed (Table 1). There was a non-significant trend toward a relationship between week-3 corticosterone and week-3 ankle width in the CFA group, where higher corticosterone appeared to be related to smaller ankle width (Fig. 5B; Table 1). While no correlation was found between week-3 corticosterone and week-3 marking preference (Fig. 5C; Table 1), CFA-injected rats exhibiting active avoidance of the female urine zone (i.e. marking preference less than 1) seemed to have relatively high levels of plasma corticosterone. We did observe a significant correlation between baseline marking preference and week-3 corticosterone (Fig. 5D; Table 1). Therefore, we grouped CFA-injected animals into either low or high baseline marking preference (i.e. either less than or more than the mean basal marking preference of 2.13; Fig. 5E) and assessed post-injury outcomes. High versus low baseline marking preference groups did not identify differences in ankle width (Fig. 5F; Table 1), body mass (Fig. 5G; Table 1), weight bearing (Fig. 5H; Table 1), total distance (Fig. 5I; Table 1), total marking area (Fig. 5J; Table 1) or time preference (Fig. 5K; Table 1). However, in terms of scent marking preference, high versus low baseline marking preference groups remained significantly segregated at 3 weeks post-CFA, where high baseline markers remained high at week-3 post-CFA (Fig. 5L; Table 1). In addition, they also identified significant differences in week-3 sniffing behavior (Fig. 5M; Table 1) and plasma corticosterone level (Fig. 5N; Table 1). Therefore, while basal scent marking does not appear to be predictive of post-injury hypersensitivity, it is predictive of post-injury stress-related outcomes and sexually-motivated behavior, whereby CFA-injected rats in the higher baseline marking preference group exhibited lower plasma corticosterone at three weeks post-injury, along with more sniffing behavior and greater marking preference for the female urine zone.
Fig. 5.
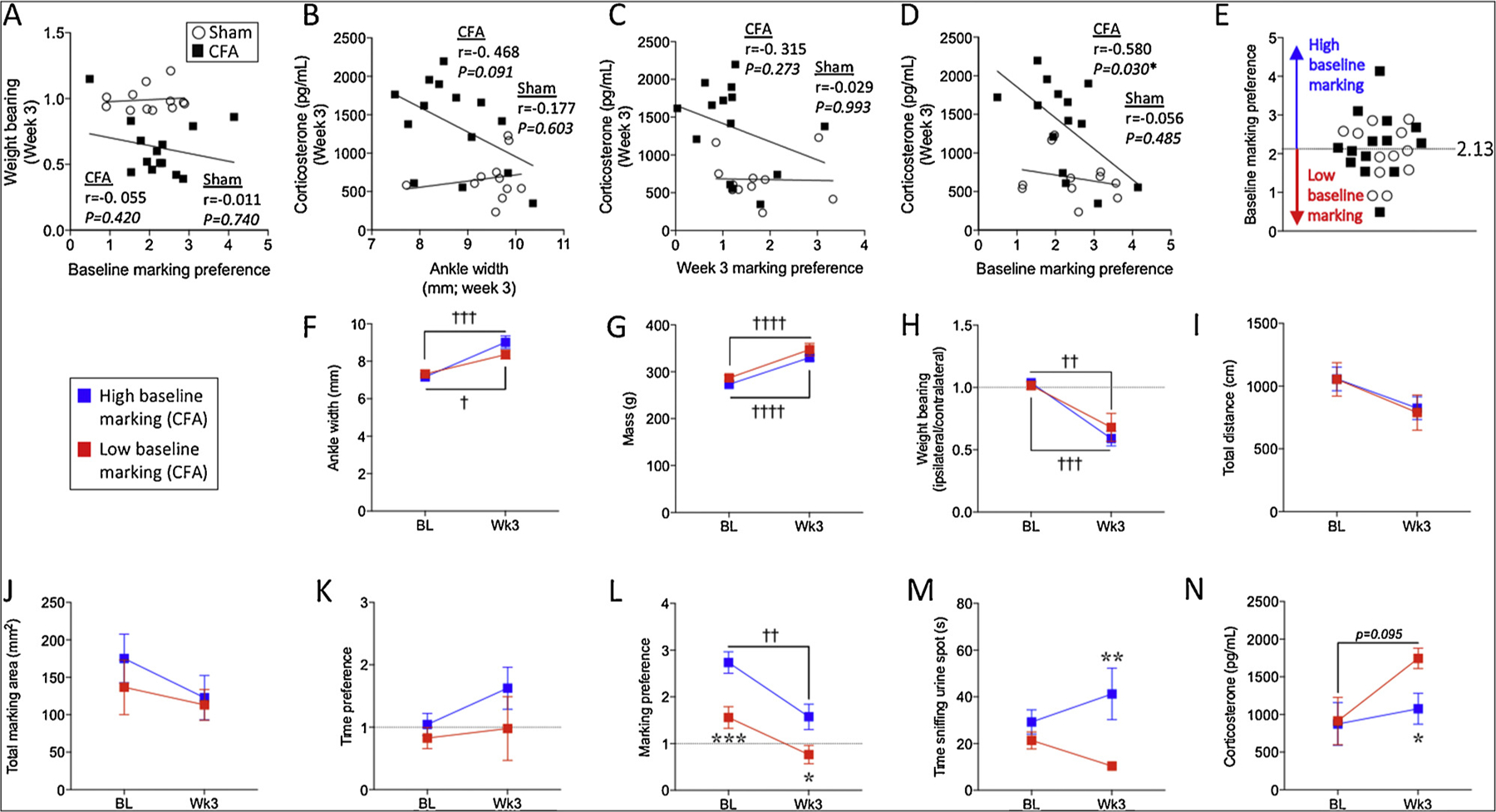
Baseline marking preference predicts post-injury stress, marking preference and sniffing behavior. Baseline marking preference scores are not correlated with week 3 wt bearing capacity in the CFA group (A). Week-3 corticosterone levels are not significantly correlated with ankle width at the three week time point (B). While week 3 corticosterone levels are not correlated with week 3 marking preference (C), they are significantly correlated with baseline marking preference (D). The mean baseline marking preference defined low versus high baseline marking groups (E). Based on this approach, n = 6 CFA-injected rats had low baseline marking (red squares) whereas n = 8 had high baseline marking (blue squares). CFA-injected rats with high baseline urine scent marking preference were no different from those with low baseline urine scent marking preference in terms of ankle width (F), body mass (G), weight bearing capacity (H), total distance (I), total marking area (J) and time preference (K). However, CFA-injected rats with high urine scent marking preference exhibited greater marking preference for the female urine zone (L), greater sniffing behavior of the female urine spot (M) and lower levels of plasma corticosterone (N) at 3 weeks post-injury. Pearson correlations were used in panel A–D. Two-way mixed design ANOVA’s were used in panels F–N. In panels F–N, stars (*) represent a significant comparison between high and low baseline marking groups at the time point indicated. * p < 0.05; ** p < 0.01; *** p < 0.001. Daggers (†) represent a significant comparison between the time points indicated. † p < 0.05; †† p < 0.01; †††p < 0.001; ††††p < 0.0001 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
Considering that the number of pain studies using awake, behaving rodents has been steadily increasing over the last 40 years [52], there is an urgent need for improved translational validity of pre-clinical research [4,32,61]. While some common mental health comorbidities of the human chronic pain condition are mirrored in rodent models of persistent pain [11], a better understanding of the impacts of persistent pain on ethologically relevant rodent behaviors such as sexual/reproductive behaviors may improve pre-clinical research outcomes. In most mammals, males use urine scent marking to demarcate territorial boundaries as well as to signal dominance, health and reproductive status reviewed in [10,28,36]. Indeed, male mice will preferentially scent mark over and around urine specifically from pro-œstrus females [9], an effect that is diminished in a fluoxetine-dependent manner following social defeat stress [44]. Moreover, scent marking preference in mice is closely aligned with social interaction [44] (an assay often used to measure stress resilience [41]) and sucrose preference [44] (a well-known rodent assay of anhedonia [77]). Here, we assessed the impact of persistent inflammatory pain on urine scent marking behavior in rats. We found that male scent marking preference for the female urine zone was reduced three weeks after induction of CFA-induced inflammation, in line with studies showing reduced sexual behavior in rodent models of chronic stress [14,20,27,31,35,44,63,64]. On the other hand, our findings do not correspond with Farmer et al., who show that female mounting behavior in zymosan or carrageenan-injected male mice was equivalent to vehicle-injected males [25], suggesting that acute/sub-chronic pain does not influence sexual behavior in male mice. Numerous methodological differences between our study and Farmer et al. [25] may explain these apparently divergent results (i.e. species, pain model and behavioral testing approaches). However, the most crucial differences involve pain duration and the nature of the different outcome measures. With regard to pain duration, considering that we did not observe CFA-induced effects until three weeks post-injection, it seems reasonable that Farmer et al. did not observe any changes in sexual behavior at the 3–4 hours post-injection time point used in their study [25]. Indeed, our results clearly show that scent marking preference was unaffected until week 3 post-CFA, indicating that a substantial amount of time with pain is required to alter this form of sexually motivated behavior. In terms of mounting behavior versus scent marking behavior, while both are related to sexual reproduction, they likely differ in many other respects and may not be similarly impacted by persistent pain. Just as persistent CFA-induced pain may have differing effects on measures or sub-measures of anxiety-like behavior (i.e. open field, elevated plus, light/dark box) [59], persistent pain may not affect mounting behavior in the same way as it affects scent marking behavior.
In addition to urine scent marking behavior, we also measured the males’ sniffing behavior of the female urine spot. We found that while scent marking behavior decreased by three weeks post-CFA, no change in sniffing behavior was observed at any time point. This is in contrast Malkesman et al. [47] who showed that sniffing behavior was reduced 24 h after a learned helplessness paradigm involving electric shock. Our study is different from Malkesman et al. in a number of ways. Firstly, we monitored sniffing behavior during a much later time frame (1–3 weeks post-CFA) where potential early changes in sniffing may have resolved. It is also possible that CFA-induced persistent inflammatory pain and electric shock-induced learned helplessness have differential effects on sniffing behavior. As such, the findings described in Malkesman et al. cannot be directly compared to our findings. Another potential difference between sniffing behavior and urine scent marking behavior is important to note: While sniffing the female urine spot may reflect a purely passive, appetitive behavior, urine scent marking is an active communicative behavior that may identify the male rat as willing to compete for access to the female. We speculate that while the CFA-injected male rat may certainly continue to be interested in the female urine spot during persistent pain states (i.e. continued sniffing), it’s willingness to compete for access to the female may be attenuated (i.e. reduced scent marking) even in the restricted environment of our testing arena. However, this hypothesis requires experimental validation.
In the context of stress, elevated corticosterone is generally interpreted as a marker of stress [22,37]. Persistent pain can alter corticosterone levels in rodents [43,60] but see [3,78] and cortisol in humans [76]. Moreover, sustained stress reviewed in [22,49,57,66] or sustained elevation of circulating corticosterone [46,68,81] can eventually produce anxiety- and depression-like behavior in rodents. In rodent models of persistent pain, delayed onset of anxiety- and depression-like behaviors after injury has been repeatedly shown [45,59,67,78,80]. Considering that depression has been described as “an end product of failed adaptation to chronic emotional stress” [57], the delayed effects of CFA-induced pain on scent marking preference may reflect a diminished capacity to cope adaptively to an ongoing stressor, possibly leading to the onset of depression- or anxiety-like behavior.
In addition to changes in scent marking, we also showed that plasma levels of the stress-related hormone corticosterone were significantly greater than shams three weeks after CFA. While comparisons with baseline corticosterone levels were not statistically significant for either the sham or CFA groups, corticosterone levels in the sham group did appear to decrease between baseline and week 3, and the corticosterone levels in the CFA group appeared to increase. Given that the corticosterone response is well known to decrease with repeated exposure to a stressor [5,18,39,56], it is possible that the apparent reduction observed in our sham group represents ongoing acclimation to the blood sampling procedures and environment. As such, the statistically significant difference in corticosterone levels between the sham and CFA groups at week 3 to some extent represent a failure to habituate to the experimental procedures/environment.
In another vein, given that corticosterone may evoke either anti-inflammatory or pro-inflammatory effects in some contexts reviewed in [69], another potential explanation could be that CFA-induced increase of corticosterone could alter the degree of peripheral inflammation. Indeed, there was a non-significant trend toward decreased ankle width in rats with higher week-3 corticosterone, suggesting that under these experimental conditions corticosterone may exert anti-inflammatory effects. Nonetheless, other studies have noted that the level of stress-induced corticosterone had no effect of CFA-induced peripheral inflammation, as measured by paw volume [16,17].
Given that 20–35% of the human chronic pain population reports comorbid depression [19,51,73], it seems reasonable to expect that some level of inter-individual variability in scent marking behavior may occur in rodent models of persistent pain. Indeed, despite the overall decrease in marking preference three weeks after CFA, approximately 29% of the CFA group exhibited active avoidance of scent marking in the female urine zone (i.e. marking preference less than 1), whereas 71% retained a positive marking preference for the female urine zone. Interestingly, those CFA-injected rats that actively avoided marking within the female urine zone also exhibited relatively high levels of plasma corticosterone. These results seem to indicate that the degree of impact in rodents may be as variable as in human chronic pain patients, where some individuals are more severely impacted than others. In this context of inter-individual variability in post-CFA outcomes, we further show that pre-injury marking preference is associated with post-injury scent marking and stress, but not pain. Specifically, rodents with high baseline scent marking preference also exhibited high post-injury marking preference, more time sniffing the female urine spot as well as lower levels of the stress hormone corticosterone. However, no difference in post-CFA weight bearing capacity was found. In other words, rodents with higher basal scent marking preference seem to show greater stress resilience in spite of ongoing pain.
Pre-surgical affective states such as anxiety are related to post-surgical pain ratings in humans [15,26,58,62,72],reviewed in [33]. Nonetheless, evidence from a rodent model of neuropathic pain indicates that pre-injury affective behaviors do not predict the level of post-injury pain [40]. While CFA-induced inflammation is not considered a model of post-surgical pain, we, too, found no relationship between pre-CFA marking preference and post-CFA hypersensitivity. This apparent disagreement between human and rodent findings may be related to time course and/or species differences, as well as to a potential lack of homology between rodent pain assays and the measures used in humans (i.e. weight bearing capacity in rodents may not necessarily translate into self-reported pain ratings in humans). On the other hand, the association between baseline marking preference and post-injury stress is consistent with a number of rodent studies showing that baseline anxiety, pessimism-like behavior, social status, sucrose preference and scent marking preference can predict resilience/vulnerability to subsequent social defeat stress [13,39,42,44,65,70]. Overall, our findings suggest that basal scent marking may be a predictor of resilience or vulnerability to CFA-induced persistent inflammatory pain, akin to human studies demonstrating that the level of pre-surgical affective disturbance is the best predictor of post-surgical affective disturbance [50,58].
In terms of limitations, of primary concern is that the scent marking preference approach described in this study was examined in males only. Given that persistent pain is more prevalent in females [38,54], follow-up studies should address female scent marking preference. Indeed, research from the 1970’s and 1980’s shows that female rodents prefer urine from males high in testosterone [9,71], but see [7] and will preferentially scent mark urine from male rats, especially during the pro-œstrus part of their cycle [6,9,24,48]. However, to date, female scent marking behavior has received little attention in the context of its potential relevance to mental health and stress resilience.
5. Conclusions
Overall, persistent pain represents a form of chronic stress that can place a heavy burden on an individuals’ capacity to cope. Nonetheless, only 20–35% of the chronic pain population exhibits comorbid depression [19,51,73]. Cross-sectional studies of chronic pain in humans are often faced with a great deal of variability in pain etiology, intensity, duration and treatment parameters that can impair the identification of bio-psychosocial predictors of mental health outcomes. Rodent models of chronic pain, however, permit greater control of physiological, genetic and environmental factors, as well as consistent pain-induction procedures followed by reproducible measures of pain intensity (i.e. mechanical or thermal hypersensitivity). Despite this relative consistency, a high level of inter-individual variability in affective/motivational outcomes is often reported. Here, using a well-characterized rat model of persistent inflammatory pain, we have found that while injured rats generally showed a reduced scent marking preference, some animals were impacted more severely than others. Importantly, it was the animals with lower baseline marking preference that ended up more severely impacted, where the severity of post-injury stress and scent marking were related to pre-injury marking preference, despite similar levels of CFA-induced hypersensitivity. While these findings certainly require further validation using other models and measures, they support the targeting of basal affective/motivational states as predictors of stress reactivity and/or resilience. Overall, our study highlights the impact of chronic pain on affective/motivational functioning and suggests that basal scent marking behavior is predictive of post-injury stress outcomes, possibly reflecting a novel behavioral marker of stress resilience. Finally, the urine scent marking assay we describe provides a straightforward methodology that can be easily and rapidly employed by other investigators to study stress resilience, allowing for improved identification of biomarkers that could be studied in humans.
Acknowledgements
This research was supported by the Division of Intramural Research of the National Center for Complementary and Integrative Health, National Institutes of Health.
Footnotes
Disclosures
This research was supported by the Division of Intramural Research of the National Center for Complementary and Integrative Health, National Institutes of Health.
The authors have no competing or conflicting interests to declare.
References
- [1].Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C, Comorbid depression, chronic pain, and disability in primary care, Psychosom. Med 68 (2006) 262–268. [DOI] [PubMed] [Google Scholar]
- [2].Bair MJ, Wu JW, Damush TM, Sutherland JM, Kroenke K, Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients, Psychosom. Med 70 (2008) 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Benedetti M, Merino R, Kusuda R, Ravanelli MI, Cadetti F, dos Santos P, Zanon S, Lucas G, Plasma corticosterone levels in mouse models of pain, Eur. J. Pain 16 (2012) 803–815. [DOI] [PubMed] [Google Scholar]
- [4].Berge OG, Predictive validity of behavioural animal models for chronic pain, Br. J. Pharmacol 164 (2011) 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhatnagar S, Huber R, Nowak N, Trotter P, Lesions of the posterior para-ventricular thalamus block habituation of hypothalamic-pituitary-Adrenal responses to repeated restraint, J. Neuroendocrinol 14 (2002). [DOI] [PubMed] [Google Scholar]
- [6].Birke LIA, Effects of estradiol and progesterone on scent-marking behavior of female rats, Horm. Behav 18 (1984) 95–98. [DOI] [PubMed] [Google Scholar]
- [7].Birke LIA, Sadler D, The rate of scent marking by male-rats and consequent olfactory preferences of female rats, Behav. Neural Biol 39 (1983) 116–122. [DOI] [PubMed] [Google Scholar]
- [8].Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS, Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis, Osteoarthr. Cartil 11 (2003) 821–830. [DOI] [PubMed] [Google Scholar]
- [9].Brown RE, Odor preference and urine-marking scales in male and female rats - effects of Gonadectomy and sexual experience on responses to conspecific odors, J. Comp. Physiol. Psych 91 (1977) 1190–1206. [Google Scholar]
- [10].Brown RE, Mammalian social odors: a critical review, Adv. Study Behav 10 (1979) 103–162. [Google Scholar]
- [11].Bushnell MC, Case LK, Ceko M, Cotton VA, Gracely JL, Low LA, Pitcher MH, Villemure C, Effect of environment on the long-term consequences of chronic pain, Pain 156 (Suppl. 1) (2015) S42–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS, Animal models of anxiety disorders and stress, Rev. Bras. Psiquiatr 35 (Suppl 2) (2013) S101–S111. [DOI] [PubMed] [Google Scholar]
- [13].Castro JE, Diessler S, Varea E, Marquez C, Larsen MH, Cordero MI, Sandi C, Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity, Psychoneuroendocrinology 37 (2012) 1209–1223. [DOI] [PubMed] [Google Scholar]
- [14].Chen G, Yang B, Chen J, Zhu L, Jiang H, Yu W, Zang F, Chen Y, Dai Y, Changes in male rat sexual behavior and brain activity revealed by functional magnetic resonance imaging in response to chronic mild stress, J. Sex. Med 15 (2018) 136–147. [DOI] [PubMed] [Google Scholar]
- [15].Choiniere M, Watt-Watson J, Victor JC, Baskett RJ, Bussieres JS, Carrier M, Cogan J, Costello J, Feindel C, Guertin MC, Racine M, Taillefer MC, Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: a 2-year prospective multicentre study, CMAJ 186 (2014) E213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chover-Gonzalez AJ, Harbuz MS, Tejedor-Real P, Gibert-Rahola J, Larsen PJ, Jessop DS, Effects of stress on susceptibility and severity of inflammation in adjuvant-induced arthritis, Ann. N.Y. Acad. Sci 876 (1999) 276–286. [DOI] [PubMed] [Google Scholar]
- [17].Chover-Gonzalez AJ, Tejedor-Real P, Harbuz MS, Gibert-Rahola J, Larsen PJ, Jessop DS, A differential response to stress is not a prediction of susceptibility or severity in adjuvant-induced arthritis, Stress 2 (1998) 221–226. [DOI] [PubMed] [Google Scholar]
- [18].Cole MA, Kalman BA, Pace TWW, Topczewski F, Lowrey MJ, Spencer RL, Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation, J. Neuroendocrinol 12 (2000) 1034–1042. [DOI] [PubMed] [Google Scholar]
- [19].Currie SR, Wang JL, Chronic back pain and major depression in the general Canadian population, Pain 107 (2004) 54–60. [DOI] [PubMed] [Google Scholar]
- [20].D’Aquila PS, Brain P, Willner P, Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression, Physiol. Behav 56 (1994) 861–867. [DOI] [PubMed] [Google Scholar]
- [21].Davies VJ, Bellamy D, Olfactory response of mice to urine and effects of gonadectomy, J. Endocrinol 55 (1972) 11–&. [DOI] [PubMed] [Google Scholar]
- [22].de Kloet ER, Joels M, Holsboer F , Stress and the brain: from adaptation to disease, Nat. Rev. Neurosci 6 (2005) 463–475. [DOI] [PubMed] [Google Scholar]
- [23].Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A, Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response, Biol. Psychiatry 76 (2014) 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eliasson M, Meyerson BJ, Sexual preference in female rats during estrous-cycle, pregnancy and lactation, Physiol. Behav 14 (1975) 705–710. [DOI] [PubMed] [Google Scholar]
- [25].Farmer MA, Leja A, Foxen-Craft E, Chan L, MacIntyre LC, Niaki T, Chen M, Mapplebeck JC, Tabry V, Topham L, Sukosd M, Binik YM, Pfaus JG, Mogil JS, Pain reduces sexual motivation in female but not male mice, J. Neurosci 34 (2014) 5747–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Geller EJ, Babb E, Nackley AG, Zolnoun D, Incidence and risk factors for pelvic pain after mesh implant surgery for the treatment of pelvic floor disorders, J. Minim. Invasive Gynecol 24 (2017) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gorzalka BB, Hanson LA, Brotto LA, Chronic stress effects on sexual behavior in male and female rats: mediation by 5-HT2A receptors, Pharmacol. Biochem. Behav 61 (1998) 405–412. [DOI] [PubMed] [Google Scholar]
- [28].Gosling LM, Roberts SC, Scent-marking by male mammals: cheat-proof signals to competitors and mates, Adv. Stud. Behav 30 (2001) 169–217. [Google Scholar]
- [29].Graziottin A, Libido: the biologic scenario, Maturitas 34 (2000) S9–S16. [DOI] [PubMed] [Google Scholar]
- [30].Gregoire S, Wattiez AS, Etienne M, Marchand F, Ardid D, Monoarthritis-induced emotional and cognitive impairments in rats are sensitive to low systemic doses or intra-amygdala injections of morphine, Eur. J. Pharmacol 735 (2014) 1–9. [DOI] [PubMed] [Google Scholar]
- [31].Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R, Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions, Physiol. Behav 84 (2005) 571–577. [DOI] [PubMed] [Google Scholar]
- [32].Hariri AR, Holmes A, Finding translation in stress research, Nat. Neurosci 18 (2015) 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA, Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review, Eur. J. Pain 13 (2009) 719–730. [DOI] [PubMed] [Google Scholar]
- [34].Hollis F, Kabbaj M, Social defeat as an animal model for depression, ILAR J 55 (2014) 221–232. [DOI] [PubMed] [Google Scholar]
- [35].Hou G, Xiong W, Wang M, Chen X, Yuan TF, Chronic stress influences sexual motivation and causes damage to testicular cells in male rats, J. Sex. Med 11 (2014) 653–663. [DOI] [PubMed] [Google Scholar]
- [36].Hurst JL, Beynon RJ, Scent wars: the chemobiology of competitive signalling in mice, Bioessays 26 (2004) 1288–1298. [DOI] [PubMed] [Google Scholar]
- [37].Joels M, The stressed brain of rodents and humans, Acta Physiol 221 (2017) 8–8. [Google Scholar]
- [38].Johannes CB, Zhou XL TK Le, Johnston JA, Dworkin RH, The prevalence of chronic pain in United States adults results of an internet-based survey, J. Pain 11 (2010) 1230–1239. [DOI] [PubMed] [Google Scholar]
- [39].Kim JG, Jung HS, Kim KJ, Min SS, Yoon BJ, Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice, Neurosci. Lett 555 (2013) 137–142. [DOI] [PubMed] [Google Scholar]
- [40].Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E, Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy, Pain 80 (1999) 341–346. [DOI] [PubMed] [Google Scholar]
- [41].Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions, Cell 131 (2007) 391–404. [DOI] [PubMed] [Google Scholar]
- [42].Larrieu T, Cherix A, Duque A, Rodrigues J, Lei H, Gruetter R, Sandi C, Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress, Curr. Biol 27 (2017) 2202–2210 e2204. [DOI] [PubMed] [Google Scholar]
- [43].Le Coz GM, Anton F, Hanesch U, Glucocorticoid-mediated enhancement of glutamatergic transmission may outweigh anti-inflammatory effects under conditions of neuropathic pain, PloS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lehmann ML, Geddes CE, Lee JL, Herkenham M, Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice, Plos One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu YT, Shao YW, Yen CT, Shaw FZ, Acid-induced hyperalgesia and anxio-depressive comorbidity in rats, Physiol. Behav 131 (2014) 105–110. [DOI] [PubMed] [Google Scholar]
- [46].Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T, Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations, Behav. Genet 39 (2009) 192–201. [DOI] [PubMed] [Google Scholar]
- [47].Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK, The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents, Biol Psychiat 67 (2010) 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Matochik JA, White NR, Barfield RJ, Variations in scent marking and ultrasonic vocalizations by long-evans rats across the estrous-cycle, Physiol. Behav 51 (1992) 783–786. [DOI] [PubMed] [Google Scholar]
- [49].McEwen BS, Wingfield JC, The concept of allostasis in biology and biomedicine, Horm. Behav 43 (2003) 2–15. [DOI] [PubMed] [Google Scholar]
- [50].McKenzie LH, Simpson J, Stewart M, A systematic review of pre-operative predictors of post-operative depression and anxiety in individuals who have undergone coronary artery bypass graft surgery, Psychol. Health Med 15 (2010) 74–93. [DOI] [PubMed] [Google Scholar]
- [51].Miller LR, Cano A, Comorbid chronic pain and depression: who is at risk? J. Pain 10 (2009) 619–627. [DOI] [PubMed] [Google Scholar]
- [52].Mogil JS, Simmonds K, Simmonds MJ, Pain research from 1975 to 2007: a categorical and bibliometric meta-trend analysis of every Research Paper published in the journal, Pain, Pain 142 (2009) 48–58. [DOI] [PubMed] [Google Scholar]
- [53].Monga TN, Tan G, Ostermann HJ, Monga U, Grabois M, Sexuality and sexual adjustment of patients with chronic pain, Disabil. Rehabil 20 (1998) 317–329. [DOI] [PubMed] [Google Scholar]
- [54].Nahin RL, Estimates of pain prevalence and severity in adults: United States, 2012, J. Pain 16 (2015) 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T, Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala, Neuropsychopharmacology 31 (2006) 739–750. [DOI] [PubMed] [Google Scholar]
- [56].Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN, Effect of stressor intensity on habituation of the adrenocortical stress response, Physiol. Behav 43 (1988). [DOI] [PubMed] [Google Scholar]
- [57].Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB, Posttraumatic stress disorder: a state-of-the-science review, J. Psychiatr. Res 40 (2006) 1–21. [DOI] [PubMed] [Google Scholar]
- [58].Page MG, Watt-Watson J, Choiniere M, Do depression and anxiety profiles over time predict persistent post-surgical pain? A study in cardiac surgery patients, Eur. J. Pain 21 (2017) 965–976. [DOI] [PubMed] [Google Scholar]
- [59].Parent AJ, Beaudet N, Beaudry H, Bergeron J, Berube P, Drolet G, Sarret P, Gendron L, Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain, Behav. Brain Res 229 (2012) 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell MC, Modest amounts of voluntary exercise reduce pain- and stress-related outcomes in a rat model of persistent hind limb inflammation (vol 18, pg 687, 2017), J. Pain 18 (2017) 1016–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Prabhakar S, Translational research challenges: finding the right animal models, J. Investig. Med 60 (2012) 1141–1146. [DOI] [PubMed] [Google Scholar]
- [62].Reddi D, Curran N, Chronic pain after surgery: pathophysiology, risk factors and prevention, Postgrad. Med. J 90 (2014) 222–227. [DOI] [PubMed] [Google Scholar]
- [63].Retana-Marquez S, Salazar ED, Velazquez-Moctezuma J, Effect of acute and chronic stress on masculine sexual behavior in the rat, Psychoneuroendocrinology 21 (1996) 39–50. [DOI] [PubMed] [Google Scholar]
- [64].Retana-Marquez S, Vigueras-Villasenor RM, Juarez-Rojas L, Aragon-Martinez A, Torres GR, Sexual behavior attenuates the effects of chronic stress in body weight, testes, sexual accessory glands, and plasma testosterone in male rats, Horm. Behav 66 (2014) 766–778. [DOI] [PubMed] [Google Scholar]
- [65].Rygula R, Papciak J, Popik P, Trait pessimism predicts vulnerability to stress-induced anhedonia in rats, Neuropsychopharmacolgy 38 (2013) 2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sandi C, Pinelo-Nava MT, Stress and memory: behavioral effects and neurobiological mechanisms, Neural Plast 2007 (2007) 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Seminowicz DA, Laferriere AL, Millecamps M, Yu JSC, Coderre TJ, Bushnell MC, MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain, Neuroimage 47 (2009) 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Skorzewska A, Lehner M, Wislowska-Stanek A, Krzascik P, Ziemba A, Plaznik A, The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats, Horm. Behav 65 (2014) 6–13. [DOI] [PubMed] [Google Scholar]
- [69].Sorrells SF, Sapolsky RM, An inflammatory review of glucocorticoid actions in the CNS, Brain Behav. Immun 21 (2007) 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Spierling SR, Mattock M, Zorrilla EP, Modeling hypohedonia following repeated social defeat: individual vulnerability and dopaminergic involvement, Physiol. Behav 177 (2017) 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Taylor GT, Haller J, Regan D, Female rats prefer an area vacated by a high testosterone male, Physiol. Behav 28 (1982) 953–958. [DOI] [PubMed] [Google Scholar]
- [72].Theunissen M, Peters ML, Schepers J, Maas JW, Tournois F, van Suijlekom HA, Gramke HF, Marcus MA, Recovery 3 and 12 months after hysterectomy: epidemiology and predictors of chronic pain, physical functioning, and global surgical recovery, Medicine (Baltimore) 95 (2016) e3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Browne MAO, Posada-Villa J, Seedat S, Watanabe M, Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders, J. Pain 9 (2008) 883–891. [DOI] [PubMed] [Google Scholar]
- [74].Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen JN, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P, The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans, Brain 136 (2013) 815–827. [DOI] [PubMed] [Google Scholar]
- [75].Valkanoff TA, Kline-Simon AH, Sterling S, Campbell C, Von Korff M, Functional disability among chronic pain patients receiving long-term opioid treatment, J. Soc. Work Disabil. Rehabil 11 (2012) 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Van Uum SHM, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Koren G, Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress, Stress 11 (2008) 483–488. [DOI] [PubMed] [Google Scholar]
- [77].Willner P, Towell A, Sampson D, Sophokleous S, Muscat R, Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant, Psychopharmacology 93 (1987) 358–364. [DOI] [PubMed] [Google Scholar]
- [78].Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M, A time-dependent history of mood disorders in a murine model of neuropathic pain, Biol. Psychiatr 70 (2011) 946–953. [DOI] [PubMed] [Google Scholar]
- [79].Zemishlany Z, Weizman A, The impact of mental illness on sexual dysfunction, Adv. Psychosom. Med 29 (2008) 89–106. [DOI] [PubMed] [Google Scholar]
- [80].Zhang GF, Wang J, Han JF, Guo J, Xie ZM, Pan W, Yang JJ, Sun KJ, Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model, Neurosci. Lett 631 (2016) 7–12. [DOI] [PubMed] [Google Scholar]
- [81].Zhao Y, Ma R, Shen J, Su H, Xing DM, Du LJ, A mouse model of depression induced by repeated corticosterone injections, Eur. J. Pharmacol 581 (2008) 113–120. [DOI] [PubMed] [Google Scholar]


