Abstract
Background:
The positive association between physical fitness and bone structure has been widely investigated in children and adolescents, yet no studies have evaluated this influence in young children (ie, preschoolers).
Hypothesis:
Fit children will present improved bone variables when compared with unfit children, and no sex-based differences will emerge in the sample.
Study Design:
Cross-sectional study.
Level of Evidence:
Level 3.
Methods:
Handgrip strength, standing long jump (SLJ), speed/agility, balance, and cardiorespiratory fitness (CRF) were assessed using the Assessing FITness levels in PREschoolers (PREFIT) test battery in 92 children (50 boys; age range, 3-5 years). A peripheral quantitative computed tomography scan was performed at 38% of the length of the nondominant tibia. Cluster analysis from handgrip strength, SLJ, speed/agility, and CRF was developed to identify fitness groups. Bone variables were compared between sexes and between cluster groups. The association between individual physical fitness components and different bone variables was also tested.
Results:
Three cluster groups emerged: fit (high values on all included physical fitness variables), strong (high strength values and low speed/agility and CRF), and unfit (low strength, speed/agility, and CRF). The fit group presented higher values than the strong and unfit groups for total and cortical bone mineral content, cortical area, and polar strength strain index (all P < 0.05). The fit group also presented a higher cortical thickness when compared with the unfit group (P < 0.05). Handgrip, SLJ, and speed/agility predicted all bone variables except for total and cortical volumetric bone mineral density. No differences were found for bone variables between sexes.
Conclusion:
The results suggest that global fitness in preschoolers is a key determinant for bone structure and strength but not volumetric bone mineral density.
Clinical Relevance:
Physical fitness is a determinant for tibial bone mineral content, structure, and strength in very young children. Performing physical fitness tests could provide useful information related to bone health in preschoolers.
Keywords: bone mass, preschool, muscular strength, children, fitness
Physical activity is a major determinant for body composition,3 cognition,11 bone mass,22 bone strength,40 and quality of life20 in children. Nonetheless, some studies have suggested that the effect of physical activity on the previously mentioned outcomes is mediated by physical fitness.32 It is clear that both are positively associated, and that while registers of physical activity are usually based on a 7-day window, physical fitness is a robust physiological measure that could reflect the amount of physical activity performed over a longer time span (eg, months).
The effects of physical fitness on health have also been widely studied in children,31 finding positive associations with quality of life,20 metabolic risk factors,38 and bone health,38 among others. Focusing on bone health, 12 of 17 studies included in a meta-analysis by Smith et al38 showed a positive association between muscular fitness and bone mass. Gracia-Marco et al18 evaluated the influence of several components of physical fitness and physical activity on bone mineral content (BMC) in adolescents and found that lower levels of fitness were associated with lower BMC. An interesting finding of the previous study18 was that cardiorespiratory fitness had no influence on BMC in boys, suggesting that the development of different fitness components might act unequally on bone mass.
Both BMC and bone mineral density (BMD), measured using dual energy x-ray absorptiometry (DXA), have been the most researched bone variables. Nevertheless, DXA has several limitations when used in small children,17 and unlike peripheral quantitative computed tomography (pQCT), DXA only provides information about bone quantity without evaluating structure, and therefore bone quality. This might be very important information, as bone strength, which is a fracture risk factor, is determined by both BMD and bone structure.37
Few studies have measured the association between physical fitness and bone structure in children, finding that muscular fitness predicts adolescent bone strength in 17-year-olds23 and that active, fit 7- to 9-year-old boys present greater cortical area and thickness compared with inactive, unfit boys.9 To the best of our knowledge, no studies have evaluated the association between physical fitness and bone structure in very young children (ie, preschoolers). It is important to clarify this effect, as poor bone accrual during growth is associated with increased fracture risk in childhood7,24 and may influence lifelong fracture risk.34
Therefore, the aims of the present study were to (1) determine the association between different physical fitness components and several structural bone parameters in 3- to 5-year-olds, (2) analyze the overall association between physical fitness and bone structure, and (3) determine whether sex-based differences regarding bone structure have already emerged in 3- to 5-year-old children. It was hypothesized that fit children would present improved bone variables when compared with unfit children and that no sex-based differences would be present in the sample.
Methods
Study Design and Participants
This study is part of the PREFIT project (Assessing FITness levels in PREschoolers; http://profith.ugr.es/prefit), a multicentric study developed in 10 Spanish cities aiming to include 3000 preschool children to develop physical fitness reference values with the PREFIT physical assessment battery and to determine the association of physical fitness and several health outcomes. The present study focused on the sample from Zaragoza, Spain, the only center in which bone structure was measured, and is not representative of the entire Spanish population of preschoolers. The study protocol was approved by the Review Committee for Research Involving Human Subjects from the University of Granada (No. 845) and by the ethics committee of the University of Zaragoza (CEICA CP18/2014) and it adheres to the Helsinki Declaration of 1961 (revision of Fortaleza 2013). Signed informed consent was retrieved from the parents or legal guardians of all participants.
In order to be included, participants had to be (1) in preschool years 1, 2, or 3 (starting ages 3, 4, or 5 years, respectively) and (2) healthy and not taking medications affecting bones. Those participants who did not present complete fitness and bone data were excluded from final analyses. Bone scans showing any sign of movement were omitted from all analyses. From the initial 139 participants (77 boys, 62 girls), 47 scans (27 boys, 20 girls) were excluded from the analysis due to movement artifact (37%). Therefore, to guarantee high-quality data, all analyses for the present study were conducted with 92 participants (50 boys, 42 girls).
Physical Fitness Assessment
Physical fitness was assessed with the PREFIT physical assessment battery,30 which is known to be feasible and reliable in preschoolers.6 The PREFIT battery was performed to measure physical fitness. To obtain the highest level of performance, participants were verbally encouraged throughout the duration of all tests. The performed tests were the following:
Handgrip strength tests (handgrip): This was measured using a handgrip dynamometer (TKK 5001, grip A; Takei) (range, 0-100 kg; accuracy, 0.5 kg). Children were in a standing position maintaining the arm of the tested side straight down with the shoulder slightly abducted (~10° not touching the rest of the body), the elbow in 0° of flexion, the forearm in neutral position, and the wrist in 0° of flexion. The best value of 4 attempts (2 trials with each hand) was chosen.
Lower limb explosive strength (SLJ): This test consisted of jumping horizontally, with both feet at the same time, the maximum distance over a nonslippery and hard surface with the feet immediately behind the starting line and separate from each other, approximately at shoulder width. Children performed 3 jumps with 1 to 2 minutes of rest between attempts. The best value of 3 attempts (in centimeters) was used for analysis.
Balance (standing on 1 leg; balance): the single-leg stance test was used to evaluate static balance. Each leg was scored and calculated as the length of time balance was maintained. Children were allowed to use their arms if necessary to maintain the balance position as long as possible. The timer was activated when the free leg left the ground, and the test ended when the children were not able to maintain the required position (eg, moved the supporting foot, heel, or toe from the original position) or when the free leg touched the ground. The mean of 2 attempts was registered.
Speed/agility (4 × 10-m shuttle run; agility): Participants ran back and forth 4 times along a 10-m track at the fastest speed possible. At the end of each track section, the participants had to touch the hand of a researcher, crossing the finish line with both feet. Children performed the test twice with 1 to 2 minutes of rest between attempts. The best result (fastest time) was used for analysis.
Cardiorespiratory fitness (PREFIT 20-m shuttle run; laps): This test required participants to run back and forth between 2 lines set 20 m apart. Running pace was determined by audio signals emitted from a prerecorded compact disc; the initial velocity was 6.5 km/h, which was increased by 0.5 km/h/min. The test also required that 2 researchers run with the preschool children, one in front and the other behind, forming an imaginary band in motion that helped maintain the correct speed. The test was finished when the participant failed to reach the end lines concurrent with the audio signals on 2 consecutive occasions or the child stopped because of fatigue.
Relative handgrip strength (Rel_Handgrip): This was calculated as the absolute handgrip strength divided by body weight.
The PREFIT battery also includes anthropometric measurements, with weight measured to the nearest 0.1 kg using a body composition analyzer (TANITA BC420 SMA) and height assessed to the nearest 0.1 cm using a stadiometer (SECA 213).
Bone Measurements
A Stratec XCT-2000 L scanner (Stratec Medizintechnik) that has been proven to be a valid device to measure bone in 3- to 4-year-old children4 was used to evaluate the nondominant tibia. This device is a translate-rotate, small computed tomography scanner that acquires a transaxial image and allows for measurement of the tibia and radius. For the obtained image to be valid for posterior analyses, participants must stay completely still for the entire duration of the scans. We have seen that the radius is more sensitive to movement than the tibia14,26; therefore, only the tibia was analyzed. Participants were distracted with a cartoon that was played for the duration of the scan with the aim of reducing movement.
The coefficient of variation between measurements for the phantom is <1%. In vivo coefficients of variation for several measures of pQCT in our laboratory have been described elsewhere.13 The nondominant tibia was selected for measurements, as recommended by the International Society for Clinical Densitometry.1 The diaphyseal tibia results (located at 38% of the total tibial length) were used, maintaining the reference line at the tibial endplate. Participants were seated in a stationary chair, adjusted to the appropriate height. The tibial length from the distal end of the medial malleolus to the medial knee joint cleft was measured. A tibial adjustable fastening belt was used to hold the limb and to limit motion during the scan. Every limb was centered in the imaging field. The scanner was positioned on the distal tibia, and a coronal computed radiograph (scout view) was performed to manually locate a reference line on the distal end of the tibia. The measurement sites were located proximal to this reference line by a distance corresponding to 38% (diaphyseal tibia) of the tibial length.
Bone Outcomes
Data for total and cortical bone at 38% of the tibial length were registered. Volumetric BMD (vBMD), BMC, and area were derived for both cortical and total bone. Additionally, cortical thickness was measured. Bone strength was established with respect to torsion (polar strength strain index [SSIPOL]) and bending with regard to the x-axis (Frac_X).
Statistical Analysis
Power calculation and sample size estimations were computed based on the primary outcome of the multicentric PREFIT project.5 The present study was based on a secondary analysis using data from a single research center from the multicentric project, as only 1 center collected bone data. Nonetheless, the sample size was similar to a previous study that also measured bone structure in preschool children, which presented a sample of 101 children (53 boys).4
Sample characteristics are presented as means and standard deviations or frequencies. Sex-based differences for bone variables were evaluated using analyses of covariance (ANCOVAs), adjusting for age and tibial length. The association between different fitness variables and bone was evaluated using linear regression models. Two models were created for each physical fitness variable, with model 1 only including age, sex, and tibial length (the same model for all physical fitness variables) and model 2 including all the variables from model 1 plus the physical fitness variable.
Cluster analysis was performed to identify groups of physical fitness and compare bone variables among groups. As no sex-based differences for bone variables were found, cluster analyses were performed for the entire sample.
First, z-scores were calculated for Rel_handgrip, SLJ, speed/agility, and laps. As Rel_handgrip and SLJ both express strength, they were grouped into a single variable ((SLJ z-score + Rel_handgrip z-score)/2), called “strength.” Balance was not included, as the linear regression coefficients were all nonsignificant, suggesting that it was not associated with bone structure or strength. Although the linear regression coefficients for cardiorespiratory fitness were also nonsignificant, we decided to include this variable in the cluster analysis, as previous studies suggest that it is an important fitness variable regarding bone mass in children and adolescents.42
Second, hierarchical cluster analysis was performed with the 3 fitness z-score values (strength, speed/agility, and laps), finding that the final cluster solutions were always highly influenced by age, with those classified as fit being mostly 5-year-old participants (preschool grade 3; 5- to 6-year-olds) and those classified as unfit being mostly 3- to 4-year-olds (preschool grade 1). These age differences also emerged when stratifying by grade (into 3 groups), as those classified as fit were always significantly older than those classified as unfit. Therefore, to control for the important effect of age and the possible effect of sex, linear regressions were performed with physical fitness components as the dependent variable and age and sex as independent variables. Standardized regression residuals were saved, and the cluster analysis was developed from the standardized regression residuals of the 3 previously described variables: strength, speed/agility, and laps.
To be consistent with clustering methods reported in previous studies,33,35 2 types of cluster analyses were used: hierarchical clustering and k-means clustering. To reduce the sensitivity of the Ward method to outliers, individual outliers and multivariate outliers (those with high Mahalanobis values distance) were investigated. First, a hierarchical cluster analysis was initially used, as the numbers of clusters in the data were unknown beforehand. The number of clusters was determined by examining dendrograms, which suggested a solution of 3 cluster groups.
K-means cluster analysis was therefore performed with 3 possible solutions. This approach minimizes the within-cluster variance and maximizes the between-cluster distance so that resulting clusters are as homogeneous as possible. K-means cluster analysis is considered superior to hierarchical methods because it is less sensitive to outliers and has been found to result in greater within-cluster homogeneity and between-cluster heterogeneity.10
Analyses of variance (ANOVAs) with the z-scores of the fitness variables and the raw fitness variables were performed to classify and name the 3 cluster groups. ANOVAs were performed to evaluate anthropometric differences among groups, and chi-square tests were developed to compare the sex and school year distribution among groups. Finally, age-, sex-, and tibial length–adjusted ANCOVAs were performed to compare bone variables among the 3 defined cluster groups.
Results
Participant Characteristics and Physical Fitness
A total of 92 children were included in the study. Regarding physical fitness, data for handgrip, Rel_handgrip, SLJ, speed/agility, balance, and cardiorespiratory fitness are presented for the entire sample and stratified by sex in Table 1.
Table 1.
Descriptive characteristics of preschool children (mean ± SD)
| Entire Sample (N = 92) | Boys (n = 50) | Girls (n = 42) | |
|---|---|---|---|
| Age, y | 4.81 ± 0.76 | 4.85 ± 0.69 | 4.76 ± 0.84 |
| School year, a 1/2/3 | 23/40/29 | 10/24/16 | 13/16/13 |
| Weight, kg | 18.6 ± 2.8 | 18.7 ± 2.3 | 18.5 ± 3.3 |
| Height, cm | 107.1 ± 6.7 | 107.3 ± 5.8 | 106.8 ± 7.7 |
| BMI, kg/m2 | 16.2 ± 1.2 | 16.2 ± 1.2 | 16.1 ± 1.3 |
| Tibial length, mm | 227.8 ± 19.3 | 226.3 ± 16.8 | 229.4 ± 22.0 |
| Physical fitness | |||
| Mean handgrip, kg | 6.87 ± 2.47 | 6.89 ± 2.44 | 6.84 ± 2.53 |
| Rel_handgrip, kg/weight | 0.36 ± 0.10 | 0.36 ± 0.11 | 0.36 ± 0.09 |
| Standing long jump, cm | 81.70 ± 20.99 | 85.06 ± 20.47 | 77.69 ± 21.12 |
| Speed run time 4 × 10 m, s | 16.49 ± 2.26 | 16.22 ± 2.06 | 16.80 ± 2.46 |
| Standing 1 leg, s | 14.70 ± 12.17 | 13.76 ± 11.83 | 15.81 ± 12.61 |
| PREFIT 20-m SRT, number of laps | 20.43 ± 11.69 | 22.44 ± 13.03 | 18.05 ± 9.51 |
BMI, body mass index; Rel, relative; SRT, shuttle run test.
School year 1/2/3 indicates the number of participants in each group from preschool year 1, year 2, and year 3.
pQCT Variables
From the initial 139 participants, 47 scans presented movement (37%). No differences were found between sexes for any of the bone variables (all P > 0.05) (Table 2).
Table 2.
Sex differences in bone mass (adjusted by age and tibial length) a
| Tibia 38% Variables | Boys (n = 50) | Girls (n = 42) |
|---|---|---|
| Total BMC, g | 1.188 ± 0.099 | 1.161 ± 0.097 |
| Total area, mm2 | 160.366 ± 14.404 | 156.421 ± 14.433 |
| Total vBMD, mg/cm3 | 741.026 ± 41.571 | 742.837 ± 41.652 |
| Cortical thickness, mm | 2.803 ± 0.212 | 2.718 ± 0.214 |
| Cortical BMC, g | 1.042 ± 0.092 | 1.015 ± 0.091 |
| Cortical area, mm2 | 101.081 ± 9.242 | 97.511 ± 9.261 |
| Cortical vBMD, mg/cm3 | 1031.010 ± 35.560 | 1042.091 ± 35.631 |
| SSIPOL, mm3 | 360.825 ± 45.714 | 350.453 ± 45.806 |
| Frac_X, N | 743.204 ± 92.645 | 745.426 ± 92.830 |
BMC, bone mineral content; Frac_X, bone strength with regard to the x-axis; SSIPOL, polar strength strain index; vBMD, volumetric bone mineral density.
Values are given as mean ± SD. No differences were found between boys and girls (all P > 0.05).
Influence of Physical Fitness on Bone Content, Structure, and Strength
Linear regression analyses showed similar results for Rel_handgrip, SLJ, and speed/agility, as they all increased r2 of model 1 for total and cortical BMC, total and cortical area, SSIPOL, and Frac_X (from 2% to 5%; all P < 0.05) (Table 3). SLJ and agility also increased the cortical thickness r2 of model 1 (both P < 0.05) (Table 3).
Table 3.
Linear regression coefficients for the influence of physical fitness on tibial values a
| Tot.BMC | Tot.Area | Tot.vBMD | Crt.Thick | Crt.BMC | Crt.Area | Crt.vBMD | SSIPOL | Frac_X | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | r 2 b | 0.550* | 0.477* | 0.067* | 0.434* | 0.539* | 0.536* | 0.031 | 0.502* | 0.542* |
| Rel_handgrip (B) | 0.414* | 57.20* | −20.58 | 0.565* | 0.366* | 37.65* | −35.96 | 186.30* | 371.91* | |
| Change r2 | 0.037* | 0.044* | 0.002 | 0.022* | 0.033* | 0.038* | 0.007 | 0.042* | 0.037* | |
| Total r2 | 0.587 | 0.522 | 0.068 | 0.455 | 0.573 | 0.574 | 0.038 | 0.544 | 0.570 | |
| Standing long jump (B) | 0.003* | 0.366* | −0.054 | 0.005* | 0.002* | 0.271* | −0.373 | 1.016* | 2.427* | |
| Change r2 | 0.043* | 0.048* | <0.001 | 0.039* | 0.040* | 0.052* | 0.019 | 0.033* | 0.042* | |
| Total r2 | 0.592 | 0.525 | 0.067 | 0.473 | 0.579 | 0.588 | 0.050 | 0.534 | 0.583 | |
| 4 × 10-m, s (B) | −0.035* | −3.388* | −6.803 | −0.071* | −0.033* | −3.189* | 0.702 | −11.15* | −25.05* | |
| Model 2 | Change r2 | 0.058* | 0.033* | 0.036 | 0.072* | 0.057* | 0.058* | 0.001 | 0.032* | 0.036* |
| Total r2 | 0.608 | 0.511 | 0.102 | 0.506 | 0.597 | 0.594 | 0.032 | 0.534 | 0.578 | |
| Balance, s (B) | <0.001 | 0.063 | −0.270 | −0.001 | −0.001 | <0.001 | −0.498 | 0.208 | 0.157 | |
| Change r2 | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 | <0.001 | 0.016 | 0.001 | <0.001 | |
| Total r2 | 0.551 | 0.478 | 0.070 | 0.435 | 0.540 | 0.536 | 0.047 | 0.709 | 0.542 | |
| Laps, s (B) | 0.001 | 0.085 | −0.067 | <0.001 | 0.001 | 0.041 | 0.211 | 0.279 | 0.441 | |
| Change r2 | 0.001 | 0.002 | <0.001 | <0.001 | 0.002 | 0.001 | 0.004 | 0.001 | 0.001 | |
| Total r2 | 0.551 | 0.479 | 0.067 | 0.434 | 0.542 | 0.537 | 0.035 | 0.503 | 0.542 |
Crt.Area, cortical area; Crt.BMC, cortical bone mineral content; Crt.Thick, cortical thickness; Crt.vBMD, cortical volumetric bone mineral density; Frac_X, fracture load in the x-axis; SSIPOL, polar strength strain index; Tot.Area, total area; Tot.BMC, total bone mineral content; Tot.vBMD, total volumetric bone mineral density.
Unstandarized Beta (B) coefficients and r2 for each of the fitness predictors adjusted by age and sex.
From the age and sex prediction model.
P < 0.05 for the included fitness variable.
Both total and cortical vBMD were unaffected by all fitness components (all P > 0.05) (Table 3). Balance and laps did not modify model 1 predictions (all P > 0.05) (Table 3).
Cluster Analysis
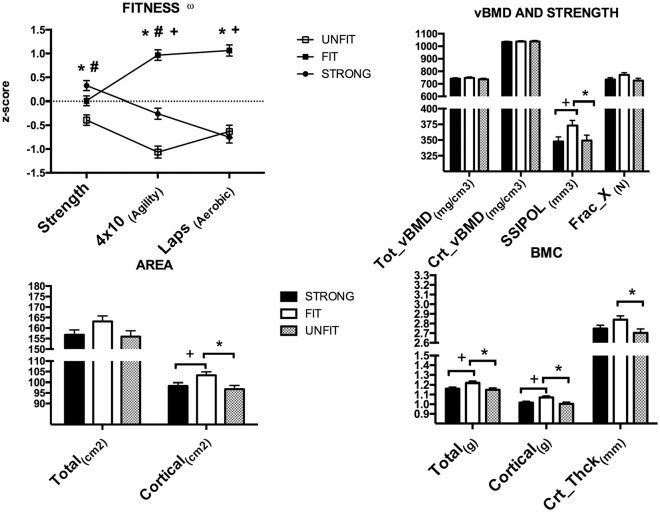
The 3 physical fitness clusters are presented in Figure 1. Cluster 1 was labeled as “strong,” as it was characterized by high levels of strength, average levels of speed/agility, and low levels of cardiorespiratory fitness. Cluster 2 was labeled as “fit,” as it presented similar values to the strong group for the strength variables and also presented high values for both speed/agility and laps. Finally, cluster 3 was labeled “unfit,” as it showed the lowest values for strength and speed/agility and similar values for laps to the strong group. Statistical differences among the groups are described in Figure 1. Descriptive characteristics and group composition are presented in Table 4.
Figure 1.
Physical fitness z-scores and adjusted by tibial length and age bone content, structure, and strength according to cluster group. BMC, bone mineral content; Crt_Thck, cortical thickness; Frac_X, bone strength with regard to the x-axis; SSIPOL, polar strength strain index; vBMD, volumetric bone mineral density. ωAgility z-scores were inverted, with lower z-scores representing low agility values. *Significant difference between fit and unfit groups. #Significant difference between strong and unfit groups. +Significant difference between fit and strong groups.
Table 4.
Anthropometric and fitness differences among cluster groups
| Strong (n = 37) | Fit (n = 29) | Unfit (n = 26) | |
|---|---|---|---|
| Age, y | 4.88 ± 0.72 | 4.80 ± 0.71 | 4.71 ± 0.89 |
| Weight, kg | 18.51 ± 2.85 | 18.59 ± 2.63 | 18.73 ± 2.91 |
| Height, cm | 107.27 ± 6.63 | 107.69 ± 5.25 | 106.04 ± 8.19 |
| BMI, kg/m2 | 16.01 ± 1.24 | 15.97 ± 1.27 | 16.55 ± 0.99 |
| Tibial length, mm | 228.32 ± 18.60 | 228.52 ± 17.33 | 226.08 ± 22.78 |
| Sex, males (%)/females (%) | 19 (51)/18 (49) | 16 (55)/13(45) | 15 (58)/11 (42) |
| School year, a 1/2/3 | 6/19/12 | 8/11/10 | 9/10/7 |
| Physical fitness | |||
| Mean handgrip, kg | 7.46 ± 2.329 b | 7.19 ± 2.42 b | 5.68 ± 2.39 |
| Rel_handgrip, kg/weight | 0.40 ± 0.10 b | 0.38 ± 0.09 b | 0.30 ± 0.10 |
| Standing long jump, cm | 87.19 ± 20.02 b | 85.48 ± 19.99 b | 69.65 ± 19.14 |
| Speed run time 4 × 10 m, s | 16.33 ± 1.92 b | 15.36 ± 1.76 b , c | 17.98 ± 2.46 |
| Standing 1 leg, s | 16.00 ± 12.42 | 15.03 ± 10.40 | 12.48 ± 13.73 |
| Laps, number of laps | 16.84 ± 8.09 | 31.31 ± 11.21 b , d | 13.42 ± 7.40 |
BMI, body mass index; Rel_handgrip, relative handgrip.
School year 1/2/3 indicates the number of participants in each group from preschool year 1, year 2, and year 3.
Significant difference with the unfit group (P < 0.05).
Tendency toward a difference between the strong and fit groups (P = 0.06).
Significant difference between the strong and fit groups (P < 0.05).
Regarding bone variables, adjusted age and tibial length results are presented in Figure 1. No differences were found among groups for total and cortical vBMD, total area, and Frac_X. The fit group presented higher values than the strong and unfit groups for the SSIPOL, cortical area, and total and cortical BMC (all P < 0.05) (Figure 1). Additionally, the fit group also presented higher values for cortical thickness than the unfit group (P < 0.05) (Figure 1). No differences were found between the strong and unfit groups for any of the measured bone variables.
Discussion
Bone Sexual Dimorphism
No bone sexual dimorphism, as reported later in life by previous studies,19,28 was found in our study. Very few studies have evaluated bone structure in the same age cohort. Moon et al,27 who measured at 38% of the nondominant tibia, found no differences between 6-year-old boys and girls. Binkley and Specker,4 in a study aiming to evaluate the validity of pQCT measures in 3- to 4-year-olds, reported no differences between boy and girl tibial values with the same device used in the present study (Stratec XCT 2000). These findings, in line with ours, suggest that BMC, vBMD, and bone structural sexual dimorphism could emerge later in life, although further research is needed in this population to confirm our results.
Associations Between Physical Fitness and Bone
The findings of the present study suggest that physical fitness might modify bone structure without stimulating vBMD, which is in line with previous studies. For example, Schoenau36 evaluated the association of handgrip and bone strength in 6- to 13-year-old children, finding age-dependent increases in bone strength indexes, cross-sectional areas, and cortical areas without increases in vBMD, and more importantly, positive associations between handgrip and structural parameters without associations between handgrip and vBMD. Similar results can be found in older populations, for example, when comparing the dominant radius with the nondominant radius of tennis players (mean age, 30 years). In this regard, Haapasalo et al21 found significant differences between both limbs for most of the structural variables without finding differences in vBMD. These findings, in line with ours, suggest that physical fitness and consequently physical activity or exercise stimulate bone structure and not vBMD. Increases in cortical thickness and in cross-sectional areas entail increases in areal BMD, as measured with DXA, which would explain why so many studies have found benefits of exercise interventions on areal BMD.16,29
The negative results of the balance test were expected, as Cadenas-Sanchez et al6 recently published a study evaluating the reliability of the tests included in the PREFIT battery and showed a low reliability for the single-leg balance test, suggesting that this test should be eliminated from the battery. Nonetheless, because data for the present study were already collected before the publication of the aforementioned study, it was decided to present all the collected physical fitness data and explore possible associations with bone variables. Similarly, results from the adapted shuttle run test, which is performed to test cardiorespiratory fitness, did not predict any of the measured bone variables. Cardiorespiratory fitness has shown controversial results when evaluating its association with bone variables in previous studies. Some researchers have found positive associations between cardiorespiratory fitness and bone variables measured with DXA in cross-sectional studies evaluating adolescents,12,43 while others in longitudinal studies found no association between cardiorespiratory fitness and the enhancement of bone mass.41 Data from long follow-up studies suggest that only neuromotor fitness (muscular strength and speed) is related to BMD in adulthood.2,25
Although there is no clear explanation for the controversial results found when examining the literature regarding cardiorespiratory fitness and bone, it is possible that if instead of analyzing each fitness component separately, a holistic analysis were performed, clearer results would emerge. The cluster analysis results support this idea, as those classified as fit who presented higher cardiorespiratory levels than both the strong and unfit, presented enhanced bone variables. These results are similar to those found by Duckham et al,9 who evaluated 7- to 9-year-old children and found that those classified as fit presented better structural bone variables than those classified as unfit. Summarizing, these results suggest that although when independently assessed muscular fitness and agility are determinants for bone mass, cardiorespiratory fitness is also important in preschool children. The fact that no differences were found between the strong and unfit groups for any of the measured bone variables suggests that for preschool children, global fitness (high levels of all fitness variables) is more important than just high muscular strength.
Similar results have been found in children9 and adolescents,18 where physical fitness has been shown to be critical to bone health. Additionally, previous studies have shown that differences in physical fitness during adolescence can lead to differences in BMD during adulthood.2 This might suggest that the differences in bone health found already at the preschool stage could be retained through childhood and adolescence or that those who are active at young ages are more likely to stay active during adolescence and consequently present improved bone mass during their entire life. Furthermore, long-term longitudinal studies are needed to confirm this hypothesis.
It is important to note that similar to previous studies developed in preschool children39 and older children and adolescents,15 we found a high number of pQCT blurred scans. When comparing our results with previous studies, it seems as though the percentage of blurry/moving scans decreases with an increase in participant age (studies with younger participants saw a higher percentage of loss of scans [51% of scans presented poor quality]39), while in studies including older participants, the percentage of moved scans seemed to decrease, as Moon et al27 and Cole et al8 found a 20.5% and 16% rate of moved scans, respectively, when measuring the diaphyseal tibia in 6- to 7-year-olds. It is important to acknowledge this loss of scans due to movement in our study and in previous ones so that further studies aiming to evaluate bone structure with pQCT in young children take this sample loss into account for sample size calculations.
Strengths and Limitations
Although this study presents several strengths, such as the measurement of tibial structure using pQCT and the assessment of physical fitness using a validated testing battery, it is not without limitations. First, this is a cross-sectional study, and therefore we cannot conclude that an increase in any of the physical fitness variables would improve bone structure or strength. Second, physical activity and nutrition, which could affect bone variables, were not registered. Finally, some physical fitness tests such as the balance test and the long jump test have shown poor reliability in previous studies developed in similar age samples.6
Conclusion
Regarding the influence of fitness on bone, relative upper and lower muscular strength and speed/agility predicted all the measured bone variables except for vBMD. Although balance and cardiorespiratory fitness did not directly influence any of the measured bone variables, high cardiorespiratory fitness was one of the main characteristics of the fit group that presented higher bone values than the strong and unfit groups. These results suggest that global fitness is a key determinant to bone structure and strength but not to vBMD in preschool children. Consequently, performing physical fitness tests could provide useful information related to bone health in preschoolers. Bone mass sexual dimorphism has still not emerged in 3- to 6-year-olds. Further similar studies with preschoolers are needed to corroborate the present results.
Acknowledgments
We thank the preschoolers, parents, and teachers who participated in this study.
Footnotes
The following authors declared potential conflicts of interest: J.M.-P. is supported by the Spanish Ministry of Education (FPU014/04302). C.C.-S. is supported by a grant from the Spanish Ministry of Economy and Competitiveness (BES-2014-068829). In addition, this study was further supported by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Unit of Excellence on Exercise and Health (UCEES). Additional funding was received from the SAMID III network, RETICS, funded by the PN I+D+I 2017-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion, the European Regional Development Fund (ERDF) (ref. RD16/0022), the EXERNET Research Network on Exercise and Health in Special Populations (DEP2005-00046/ACTI), the University of the Basque Country (GIU14/21), and the University of Zaragoza (JIUZ-2014-BIO-08).
References
- 1. Adams JE, Engelke K, Zemel BS, Ward KA; International Society of Clinical Densitometry. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:258-274. [DOI] [PubMed] [Google Scholar]
- 2. Barnekow-Bergkvist M, Hedberg G, Pettersson U, Lorentzon R. Relationships between physical activity and physical capacity in adolescent females and bone mass in adulthood. Scand J Med Sci Sports. 2006;16:447-455. [DOI] [PubMed] [Google Scholar]
- 3. Basterfield L, Reilly JK, Pearce MS, et al. Longitudinal associations between sports participation, body composition and physical activity from childhood to adolescence. J Sci Med Sport. 2015;18:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Binkley TL, Specker BL. pQCT measurement of bone parameters in young children: validation of technique. J Clin Densitom. 2000;3:9-14. [DOI] [PubMed] [Google Scholar]
- 5. Cadenas-Sanchez C, Intemann T, Labayen I, et al. ; PREFIT Project Group. Physical fitness reference standards for preschool children: the PREFIT project. J Sci Med Sport. 2019;22:430-437. [DOI] [PubMed] [Google Scholar]
- 6. Cadenas-Sanchez C, Martinez-Tellez B, Sanchez-Delgado G, et al. Assessing physical fitness in preschool children: feasibility, reliability and practical recommendations for the PREFIT battery. J Sci Med Sport. 2016;19:910-915. [DOI] [PubMed] [Google Scholar]
- 7. Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2007;23:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole ZA, Harvey NC, Kim M, et al. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone. 2012;50:562-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duckham RL, Rantalainen T, Ducher G, et al. Effects of habitual physical activity and fitness on tibial cortical bone mass, structure and mass distribution in pre-pubertal boys and girls: the Look study. Calcif Tissue Int. 2016;99:56-65. [DOI] [PubMed] [Google Scholar]
- 10. Eshghi A, Haughton D, Legrand P, Skaletsky M, Woolford S. Identifying groups: a comparison of methodology. J Data Sci. 2011;9:271-291. [Google Scholar]
- 11. Esteban-Cornejo I, Tejero-Gonzalez CM, Sallis JF, Veiga OL. Physical activity and cognition in adolescents: a systematic review. J Sci Med Sport. 2015;18:534-539. [DOI] [PubMed] [Google Scholar]
- 12. Ginty F, Rennie KL, Mills L, Stear S, Jones S, Prentice A. Positive, site-specific associations between bone mineral status, fitness, and time spent at high-impact activities in 16- to 18-year-old boys. Bone. 2005;36:101-110. [DOI] [PubMed] [Google Scholar]
- 13. Gómez-Bruton A, Gonzalez-Agüero A, Casajús JA, Rodríguez GV. Swimming training repercussion on metabolic and structural bone development; benefits of the incorporation of whole body vibration or pilometric training; the RENACIMIENTO project. Nutr Hosp. 2014;30:399-409. [DOI] [PubMed] [Google Scholar]
- 14. Gómez-Bruton A, González-Agüero A, Gómez-Cabello A, et al. Bone structure of adolescent swimmers; a peripheral quantitative computed tomography (pQCT) study. J Sci Med Sport. 2016;19:707-712. [DOI] [PubMed] [Google Scholar]
- 15. Gómez-Bruton A, González-Agüero A, Matute-Llorente A, et al. Effects of whole body vibration on tibia strength and structure of competitive adolescent swimmers: a randomized controlled trial. PM R. 2018;10:889-897. [DOI] [PubMed] [Google Scholar]
- 16. Gómez-Bruton A, Matute-Llorente A, González-Agüero A, Casajús JA, Vicente-Rodríguez G. Plyometric exercise and bone health in children and adolescents: a systematic review. World J Pediatr. 2017;13:112-121. [DOI] [PubMed] [Google Scholar]
- 17. Gordon CM, Bachrach LK, Carpenter TO, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43-58. [DOI] [PubMed] [Google Scholar]
- 18. Gracia-Marco L, Vicente-Rodríguez G, Casajús JA, Molnar D, Castillo MJ, Moreno LA. Effect of fitness and physical activity on bone mass in adolescents: the HELENA Study. Eur J Appl Physiol. 2011;111:2671-2680. [DOI] [PubMed] [Google Scholar]
- 19. Gracia-Marco L, Vicente-Rodriguez G, Valtuena J, et al. Bone mass and bone metabolism markers during adolescence: the HELENA Study. Horm Res Paediatr. 2010;74:339-350. [DOI] [PubMed] [Google Scholar]
- 20. Gu X, Chang M, Solmon MA. Physical activity, physical fitness, and health-related quality of life in school-aged children. J Teach Phys Educ. 2016;35:117-126. [Google Scholar]
- 21. Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351-357. [DOI] [PubMed] [Google Scholar]
- 22. Herrmann D, Buck C, Sioen I, et al. Impact of physical activity, sedentary behaviour and muscle strength on bone stiffness in 2–10-year-old children—cross-sectional results from the IDEFICS study. Int J Behav Nutr Phys Act. 2015;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janz KF, Letuchy EM, Burns TL, Francis SL, Levy SM. Muscle power predicts adolescent bone strength: Iowa Bone Development Study. Med Sci Sports Exerc. 2015;47:2201-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalkwarf HJ, Laor T, Bean JA. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA). Osteoporos Int. 2011;22:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kemper HC, Twisk JW, van Mechelen W, Post GB, Roos JC, Lips P. A fifteen-year longitudinal study in young adults on the relation of physical activity and fitness with the development of the bone mass: the Amsterdam Growth and Health Longitudinal Study. Bone. 2000;27:847-853. [DOI] [PubMed] [Google Scholar]
- 26. Lozano-Berges G, Matute-Llorente Á, Gómez-Bruton A, González-Agüero A, Vicente-Rodríguez G, Casajús JA. Bone geometry in young male and female football players: a peripheral quantitative computed tomography (pQCT) study. Arch Osteoporos. 2018;13:57. [DOI] [PubMed] [Google Scholar]
- 27. Moon RJ, Cole ZA, Crozier SR, et al. Longitudinal changes in lean mass predict pQCT measures of tibial geometry and mineralisation at 6-7 years. Bone. 2015;75:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen TV, Maynard LM, Towne B, et al. Sex differences in bone mass acquisition during growth: the Fels Longitudinal Study. J Clin Densitom. 2001;4:147-157. [DOI] [PubMed] [Google Scholar]
- 29. Nogueira R, Weeks B, Beck B. Exercise to improve pediatric bone and fat: a systematic review and meta-analysis. Med Sci Sports Exerc. 2014;46:610-621. [DOI] [PubMed] [Google Scholar]
- 30. Ortega FB, Cadenas-Sánchez C, Sánchez-Delgado G, et al. Systematic review and proposal of a field-based physical fitness-test battery in preschool children: the PREFIT battery. Sports Med. 2015;45:533-555. [DOI] [PubMed] [Google Scholar]
- 31. Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes. 2008;32:1-11. [DOI] [PubMed] [Google Scholar]
- 32. Ortega FB, Ruiz JR, Hurtig-Wennlöf A, et al. Cardiovascular fitness modifies the associations between physical activity and abdominal adiposity in children and adolescents: the European Youth Heart Study. Br J Sports Med. 2010;44:256-262. [DOI] [PubMed] [Google Scholar]
- 33. Prokasky A, Rudasill K, Molfese VJ, Putnam S, Gartstein M, Rothbart M. Identifying child temperament types using cluster analysis in three samples. J Res Pers. 2017;67:190-201. [Google Scholar]
- 34. Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294-305. [DOI] [PubMed] [Google Scholar]
- 35. Sanson A, Letcher P, Smart D, Prior M, Toumbourou JW, Oberklaid F. Associations between early childhood temperament clusters and later psychosocial adjustment. 2009;55:26-54. [Google Scholar]
- 36. Schoenau E. The development of the skeletal system in children and the influence of muscular strength. Horm Res Paediatr. 1998;49:27-31. [DOI] [PubMed] [Google Scholar]
- 37. Sievänen H. A physical model for dual-energy X-ray absorptiometry–derived bone mineral density. Invest Radiol. 2000;35:325-330. [DOI] [PubMed] [Google Scholar]
- 38. Smith JJ, Eather N, Morgan PJ, Plotnikoff RC, Faigenbaum AD, Lubans DR. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. 2014;44:1209-1223. [DOI] [PubMed] [Google Scholar]
- 39. Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Min Res. 2003;18:885-892. [DOI] [PubMed] [Google Scholar]
- 40. Tan VP, Macdonald HM, Kim S, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Min Res. 2014;29:2161-2181. [DOI] [PubMed] [Google Scholar]
- 41. Vicente-Rodriguez G, Ara I, Perez-Gomez J, Serrano-Sanchez JA, Dorado C, Calbet JA. High femoral bone mineral density accretion in prepubertal soccer players. Med Sci Sports Exerc. 2004;36:1789-1795. [DOI] [PubMed] [Google Scholar]
- 42. Vicente-Rodriguez G, Urzanqui A, Mesana MI, et al. Physical fitness effect on bone mass is mediated by the independent association between lean mass and bone mass through adolescence: a cross-sectional study. J Bone Min Metab. 2008;26:288-294. [DOI] [PubMed] [Google Scholar]
- 43. Vlachopoulos D, Ubago-Guisado E, Barker AR, et al. Determinants of bone outcomes in adolescent athletes at baseline: the PRO-BONE study. Med Sci Sports Exerc. 2017;49:1389-1396. [DOI] [PubMed] [Google Scholar]