Abstract
Objective
Although some studies have reported a higher incidence of HIV infection among non–US-born people than among US-born people, national data on this topic are scarce. We compared the epidemiology of HIV infection between US-born and non–US-born residents of the United States and examined the characteristics of non–US-born people with diagnosed HIV infection by region of birth (ROB).
Methods
We used a cross-sectional study design to produce national, population-based data describing HIV infection among US-born and non–US-born people. We analyzed National HIV Surveillance System data for people with HIV infection diagnosed during 2010-2017 and reported to the Centers for Disease Control and Prevention (CDC). We compared data on demographic characteristics, transmission risk category, and stage 3 infection (AIDS) classification within 3 months of HIV diagnosis, by nativity and ROB.
Results
During 2010-2017, 328 317 children and adult US residents were diagnosed with HIV infection and were reported to CDC: 214 973 (65.5%) were US-born, 50 301 (15.3%) were non–US-born, and 63 043 (19.2%) were missing data on country of birth. After adjusting for missing country of birth, 266 147 (81.1%) people were US-born and 62 170 (18.9%) were non–US-born. This group accounted for 15 928 of 65 645 (24.2%) HIV diagnoses among girls and women and 46 242 of 262 672 (17.6%) HIV diagnoses among boys and men. A larger percentage of non–US-born people than US-born people had stage 3 infection (AIDS) at HIV diagnosis (31.2% vs 23.9%). Among non–US-born people with HIV diagnoses, 19 876 (39.5%) resided in the South.
Conclusions
Characterizing non–US-born people with HIV infection is essential for developing effective HIV interventions, particularly in areas with large immigrant populations.
Keywords: HIV, immigrants, migrants, non–US-born, late diagnosis, disparities
HIV is a global epidemic characterized by stark disparities. In 2015, approximately 25.5 million people were living with HIV infection in sub-Saharan Africa, 5.1 million in Asia and the Pacific, and 1.1 million in the United States.1,2 Although the number of new HIV infections has declined in most regions of the world since 1998 because of increasing dissemination of antiretroviral therapy, it was estimated that by June 2019, only 24.5 million of the 37.9 million (65%) people living with HIV (PLWH) worldwide were receiving antiretroviral therapy.3
Global migration has almost doubled since 1990, increasing from 154 million international migrants in 1990 to 232 million international migrants in 2013.4 In 2013, half of all international migrants resided in 10 destination countries, primarily in North America, Europe, and the Middle East. The United States was home to the largest share of international migrants: 45.8 million, or roughly 20% of all international migrants in 2013.4 As global migration has increased, so has the proportion of the US population that is non–US-born: from 5.4% in 1970 to 13.5% in 2016.5,6
Although several local jurisdictions have reported a higher incidence of HIV infection among non–US-born people than among US-born people,7-10 some jurisdictions have reported lower HIV diagnosis rates among non–US-born people than among US-born people,11,12 and national data on this topic are scarce. A study of HIV infections among non–US-born people in the United States by Prosser et al13 found that 16.2% of HIV diagnoses during 2007-2010 were among non–US-born people. However, this analysis included only 46 US states and excluded several jurisdictions with large non–US-born populations, including Maryland and Massachusetts. The findings also predate the end of the so-called HIV immigration ban in 2010, after which PLWH who fulfilled appropriate immigration requirements were able to enter the United States regardless of their HIV status, and HIV testing was no longer required as part of US immigration-related medical examinations.14 Lifting the immigration ban might have had 2 important effects: (1) an increase in the number of non–US-born PLWH and (2) delayed diagnosis of HIV infection, resulting in higher proportions of people newly diagnosed with HIV infection who are concurrently diagnosed with stage 3 infection (AIDS) and/or higher proportions of non–US-born PLWH who are undiagnosed than was the case before the ban was lifted.
Our study sought to update the results of the Prosser et al13 article by including data from all 50 states and 6 US territories during 2010-2017. Given the disproportionate effect of HIV infection on some regions of the United States and the emphasis of the Ending the Epidemic initiative15 on jurisdictions disproportionately affected by HIV, we also expanded the analysis of Prosser et al to provide greater detail about the geographic distribution of non–US-born PLWH in the United States.
Methods
We analyzed National HIV Surveillance System data, including all 50 states, 6 US territories, and the District of Columbia, for all people with HIV infection diagnosed during 2010-2017 and reported to the Centers for Disease Control and Prevention (CDC) through June 30, 2019. As a part of HIV surveillance, data on demographic characteristics, HIV transmission category (ie, how HIV was transmitted), and clinical outcomes are collected by local health jurisdictions for each person newly reported with HIV. Data are transmitted to CDC without personal identifying information. Collecting information on country of birth (COB) is recommended but not required. We used US Census data as denominators for the population of people living in the United States who were born in other regions of the world, as well as the populations living in each region of the United States. Immigrants and refugees with HIV infection may have previously received a diagnosis outside the United States, but without documentation of a previous positive result, these people are considered to have their HIV diagnoses in the United States for the purposes of HIV surveillance.
We categorized non–US-born people into regions of birth (ROBs) based on definitions from the 2014 American Community Survey and Puerto Rico Community Survey Place of Birth, Migration, and Place of Work Code List.16 We grouped people born in Mexico or Central America into a Mexico/Central America category. The North America ROB includes people born in Canada, Bermuda, Greenland, and Saint Pierre and Miquelon. We categorized people born in US-dependent areas (American Samoa, Guam, Northern Mariana Islands, Puerto Rico, Republic of Palau, and the US Virgin Islands) as US-born.
For people missing data on COB or HIV transmission category, we applied multiple imputation to national HIV surveillance data to assign plausible HIV transmission categories and nativity (US-born, non–US-born).13,17 Multiple imputation is a statistical approach in which each missing value is replaced with a set of plausible values that represent the uncertainty about the true, but missing, value. We imputed missing values of HIV transmission category first. We then used imputed transmission category values to impute missing values of nativity. Variables used in both imputations included sex at birth, age, race/ethnicity, stage of disease at diagnosis, and region of residence. Multiple imputation does not attempt to estimate each missing value but instead draws a random sample from its distribution of plausible values. This process results in valid statistical inferences that properly reflect the uncertainty due to missing values. All analyses stratified by ROB exclude data on non–US-born people with a diagnosis of HIV during the study period and imputed nativity. To examine changes over time in the proportion of HIV diagnoses among non–US-born people, we completed a secondary analysis for which we restricted the data to the same states and territories included in the study by Prosser et al13 using data from 2007-2010. We calculated diagnosis rates by nativity, both overall and by sex at birth.
To examine the geographic distribution of HIV diagnoses among non–US-born people in the United States, we calculated the proportion of non–US-born people from each ROB by region of residence in the United States (Northeast, Midwest, South, West, US-dependent areas). To identify the 5 metropolitan statistical areas (MSAs) with the largest number of HIV diagnoses from each ROB, we first calculated the proportion of HIV diagnoses accounted for by non–US-born PLWH by ROB for each MSA, and then we ranked MSAs by these proportions for each ROB. We used a threshold of ≥5% to highlight differences in percentage distributions when comparing populations. We used SAS version 9.4 (SAS Institute Inc) for all analyses.
This analysis used deidentified data collected for surveillance purposes and, thus, was not considered human subjects research.
Results
Nativity and Late Diagnosis
During 2010-2017, a total of 311 854 children and adult US residents received an HIV diagnosis, of whom 214 973 (65.5%) were US-born, 50 301 (15.3%) were non–US-born, and 63 043 (19.2%) were missing data on COB (Table 1). After imputation for missing COB, a total of 266 147 (81.1%) people were US-born and 62 170 (18.9%) were non–US-born. A greater percentage of non–US-born people than US-born people had HIV infection classified as stage 3 infection (AIDS) at diagnosis: 31.2% (19 399 of 62 170) versus 23.9% (63 691 of 266 147).
Table 1.
Characteristics of US-born and non–US-born people with HIV diagnosed during 2010-2017 in the United States and 6 dependent areasa ,b
| Characteristic | US-born | Non–US-born | ||
|---|---|---|---|---|
| No.c (%) | Adjusted no.c ,d (%) | No.c (%) | Adjusted no.c ,d (%) | |
| Sex | ||||
| Male | 174 768 (81.3) | 216 430 (81.3) | 37 147 (73.8) | 46 242 (74.4) |
| Female | 40 205 (18.7) | 49 717 (18.7) | 13 154 (26.2) | 15 928 (25.6) |
| Age at diagnosis, y | ||||
| <13 | 537 (0.2) | 637 (0.2) | 679 (1.3) | 781 (1.3) |
| 13-19 | 11 266 (5.2) | 13 634 (5.1) | 1052 (2.1) | 1399 (2.3) |
| 20-29 | 79 898 (37.2) | 97 676 (36.7) | 12 553 (25.0) | 15 928 (25.6) |
| 30-39 | 47 716 (22.2) | 59 674 (22.4) | 16 242 (32.3) | 19 430 (31.3) |
| 40-49 | 39 379 (18.3) | 49 190 (18.5) | 11 479 (22.8) | 14 056 (22.6) |
| 50-59 | 26 472 (12.3) | 33 147 (12.5) | 5744 (11.4) | 7349 (11.8) |
| ≥60 | 9705 (4.5) | 12 189 (4.6) | 2552 (5.1) | 3226 (5.2) |
| Race/ethnicity | ||||
| Non-Hispanic American Indian/Alaska Native | 1182 (0.5) | 1369 (0.5) | 19 (<0.1) | 27 (<0.1) |
| Non-Hispanic Asian | 1537 (0.7) | 1653 (0.6) | 3914 (7.8) | 5031 (8.1) |
| Non-Hispanic black/African American | 98 217 (45.7) | 124 053 (46.6) | 14 686 (29.2) | 17 230 (27.7) |
| Hispanic/Latinod | 39 350 (18.3) | 46 615 (17.5) | 26 970 (53.6) | 34 285 (55.1) |
| Non-Hispanic Native Hawaiian/other Pacific Islander | 273 (0.1) | 327 (0.1) | 84 (0.2) | 97 (0.2) |
| Non-Hispanic white | 64 559 (30.0) | 80 493 (30.2) | 3118 (6.2) | 3824 (6.2) |
| Non-Hispanic multiple races | 9855 (4.6) | 11 637 (4.4) | 1510 (3.0) | 1676 (2.7) |
| Transmission category e | ||||
| Boys and men | ||||
| Male-to-male sexual contact | 139 173 (79.6) | 173 888 (80.3) | 28 316 (76.2) | 35 669 (77.1) |
| Injection drug use | 8571 (4.9) | 10 419 (4.8) | 1103 (3.0) | 1419 (3.1) |
| Male-to-male sexual contact and injection drug use | 9494 (5.4) | 11 011 (5.1) | 880 (2.4) | 1096 (2.4) |
| Heterosexual contactf | 17 116 (9.8) | 20 626 (9.5) | 6456 (17.4) | 7579 (16.4) |
| Otherg | 414 (0.2) | 487 (0.2) | 392 (1.1) | 479 (1.0) |
| Girls and women | ||||
| Injection drug use | 6768 (16.8) | 8222 (16.5) | 583 (4.4) | 782 (4.9) |
| Heterosexual contactf | 32 995 (82.1) | 40 996 (82.5) | 12 086 (91.9) | 14 589 (91.6) |
| Otherg | 442 (1.1) | 499 (1.0) | 486 (3.7) | 558 (3.5) |
| Stage 3 infection (AIDS) classification within 3 months of HIV diagnosis | ||||
| Yes | 52 228 (24.3) | 63 691 (23.9) | 16 009 (31.8) | 19 399 (31.2) |
| No | 162 745 (75.7) | 202 457 (76.1) | 34 292 (68.2) | 42 770 (68.8) |
| Total | 214 973 (100.0) | 266 147 (100.0) | 50 301 (100.0) | 62 170 (100.0) |
aData source: Centers for Disease Control and Prevention.18
bData have been statistically adjusted to account for missing transmission category. As such, values may not sum to column subtotals and total.
cCases reported with missing country of birth and/or transmission risk were redistributed to nativity and transmission risk categories through multiple imputation.
dHispanic/Latino people can be of any race.
eNumbers adjusted for missing risk factors.
fHeterosexual contact with a person known to have, or to be at high risk for, HIV infection.
gIncludes hemophilia, blood transfusion, perinatal exposure, and risk factor not reported or not identified.
Total diagnosis rates per 100 000 population, adjusted for missing nativity data, were higher among non–US-born people overall than among US-born people overall (non–US-born people, 18.5; US-born people, 12.1) and among both male (non–US-born, 28.2; US-born, 19.9) and female (non–US-born, 9.2; US-born, 4.1) US residents.
Demographic Characteristics and Transmission Risk
A lower percentage of non–US-born people with diagnosed HIV infection than US-born people with diagnosed HIV infection were male and of black race, and a higher percentage of non–US-born people with diagnosed HIV infection were of Hispanic/Latino ethnicity. Non–US-born people accounted for 12.2% (17 230 of 141 283) and 42.4% (34 285 of 80 900) of HIV diagnoses among people of black race and Hispanic/Latino ethnicity, respectively. Non–US-born girls and women accounted for 15 928 of 65 645 (24.3%) HIV diagnoses. A higher percentage of non–US-born boys and men than US-born boys and men acquired HIV infection via heterosexual contact (16.4% [7579 of 46 242] vs 9.5% [20 626 of 216 430]). Similarly, a higher percentage of non–US-born girls and women (91.6% [14 589 of 15 928] than US-born girls and women (82.4% [40 966 of 49 717] acquired HIV through heterosexual contact. A lower percentage of non–US-born girls and women than their US-born counterparts had HIV attributed to injection drug use (4.9% [782 of 15 928] vs. 16.5% [8222 of 49 717]).
Country of Birth
Among 50 301 non–US-born people with known COBs, the COBs with the most HIV diagnoses during the study period were Mexico (n = 11 368, 22.6%), Haiti (n = 3770, 7.5%), Cuba (n = 2926, 5.8%), Dominican Republic (n = 1765, 3.5%), El Salvador (n = 1731, 3.4%), Jamaica (n = 1470, 2.9%), Honduras (n = 1459, 2.9%), Ethiopia (n = 1416, 2.8%), Guatemala (n = 1407, 2.8%), and Colombia (n = 1319, 2.6%). The largest absolute numbers of non–US-born people with HIV diagnoses were born in the Central America/Mexico (16 660 of 50 301, 33.1%; Table 2) and Caribbean (10 778 of 50 301, 21.4%) regions. The ROB differed by sex. The largest numbers of non–US-born boys and men with diagnosed HIV infection were from Central America/Mexico (n = 14 503, 39.0%) and the Caribbean (n = 7200, 19.4%), whereas the largest numbers of non–US-born girls and women with diagnosed HIV infection were from Africa (n = 5355, 40.7%) and the Caribbean (n = 3578, 27.2%).
Table 2.
Characteristics of non–US-born people with HIV diagnosed in the United States and 6 dependent areas, by world region of birth, 2010-2017a
| Characteristics | Total | Africa | Asia | Europe | North America | Central America/Mexico | South America | Caribbean | Oceania | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||
| Male | 37 141 | 3685 (9.9) | 4495 (12.1) | 1851 (5.0) | 212 (0.6) | 14 503 (39.0) | 4682 (12.6) | 7200 (19.4) | 147 (0.4) | 366 (1.0) |
| Female | 13 152 | 5355 (40.7) | 874 (6.6) | 379 (2.9) | 23 (0.2) | 2157 (16.4) | 590 (4.5) | 3578 (27.2) | 22 (0.2) | 174 (1.3) |
| Age at diagnosis, y | ||||||||||
| <13 | 679 | 413 (60.8) | 75 (11.0) | 99 (14.6) | 1 (0.1) | 24 (3.5) | 11 (1.6) | 50 (7.4) | 0 | 6 (0.9) |
| 13-19 | 1052 | 245 (23.3) | 90 (8.6) | 47 (4.5) | 3 (0.3) | 341 (32.4) | 87 (8.3) | 219 (20.8) | 1 (0.1) | 19 (1.8) |
| 20-29 | 12 551 | 1513 (12.1) | 1607 (12.8) | 556 (4.4) | 42 (0.3) | 4857 (38.7) | 1544 (12.3) | 2263 (18.0) | 42 (0.3) | 127 (1.0) |
| 30-39 | 16 238 | 2965 (18.3) | 1741 (10.7) | 716 (4.4) | 59 (0.4) | 6035 (37.2) | 1755 (10.8) | 2740 (16.9) | 51 (0.3) | 176 (1.1) |
| 40-49 | 11 478 | 2097 (18.3) | 1196 (10.4) | 478 (4.2) | 71 (0.6) | 3687 (32.1) | 1201 (10.5) | 2587 (22.5) | 47 (0.4) | 114 (1.0) |
| 50-59 | 5743 | 1229 (21.4) | 459 (8.0) | 232 (4.0) | 36 (0.6) | 1274 (22.2) | 515 (9.0) | 1903 (33.1) | 24 (0.4) | 71 (1.2) |
| ≥60 | 2552 | 578 (22.6) | 201 (7.9) | 102 (4.0) | 23 (0.9) | 442 (17.3) | 159 (6.2) | 1016 (39.8) | 4 (0.2) | 27 (1.1) |
| Race/ethnicity | ||||||||||
| Non-Hispanic American Indian/Alaska Native | 19 | 4 (21.1) | 6 (31.6) | 2 (10.5) | 2 (10.5) | 0 | 4 (21.1) | 1 (5.3) | 0 | 0 |
| Non-Hispanic Asian | 3903 | 27 (0.7) | 3745 (96.0) | 13 (0.3) | 11 (0.3) | 1 (<0.1) | 32 (0.8) | 14 (0.4) | 17 (0.4) | 43 (1.1) |
| Non-Hispanic black/African American | 14 679 | 8308 (56.6) | 65 (0.4) | 101 (0.7) | 20 (0.1) | 55 (0.4) | 289 (2.0) | 5588 (38.1) | 10 (0.1) | 243 (1.7) |
| Hispanic/Latinob | 26 969 | 189 (0.7) | 252 (0.9) | 273 (1.0) | 12 (<0.1) | 16 522 (61.3) | 4626 (17.2) | 4915 (18.2) | 10 (<0.1) | 170 (0.6) |
| Non-Hispanic Native Hawaiian/other Pacific Islander | 84 | 2 (2.4) | 37 (44.0) | 1 (1.2) | 0 | 2 (2.4) | 1 (1.2) | 3 (3.6) | 36 (42.9) | 2 (2.4) |
| Non-Hispanic white | 3121 | 127 (4.1) | 593 (19.0) | 1748 (56.0) | 171 (5.5) | 72 (2.3) | 220 (7.0) | 40 (1.3) | 79 (2.5) | 68 (2.2) |
| Non-Hispanic multiple races | 1510 | 383 (25.4) | 671 (44.4) | 92 (6.1) | 19 (1.3) | 8 (0.5) | 89 (5.9) | 217 (14.4) | 17 (1.1) | 14 (0.9) |
| Transmission category c | ||||||||||
| Boys and men | ||||||||||
| Male-to-male sexual contact | 28 315 | 1624 (5.7) | 3780 (13.3) | 1555 (5.5) | 189 (0.7) | 11 932 (42.1) | 4166 (14.7) | 4680 (16.5) | 127 (0.4) | 262 (0.9) |
| Injection drug use | 1103 | 149 (13.5) | 124 (11.2) | 43 (3.9) | 2 (0.2) | 490 (44.4) | 67 (6.1) | 211 (19.1) | 2 (0.2) | 15 (1.4) |
| Male-to-male sexual contact and injection drug use | 879 | 59 (6.7) | 121 (13.8) | 55 (6.3) | 7 (0.8) | 433 (49.3) | 82 (9.3) | 103 (11.7) | 9 (1.0) | 10 (1.1) |
| Heterosexual contactd | 6454 | 1618 (25.1) | 431 (6.7) | 154 (2.4) | 15 (0.2) | 1623 (25.1) | 360 (5.6) | 2166 (33.6) | 9 (0.1) | 78 (1.2) |
| Othere | 392 | 236 (60.2) | 39 (9.9) | 45 (11.5) | 1 (0.3) | 25 (6.4) | 6 (1.5) | 40 (10.2) | 0 | 0 |
| Girls and women | ||||||||||
| Injection drug use | 583 | 200 (34.3) | 38 (6.5) | 38 (6.5) | 3 (0.5) | 113 (19.4) | 31 (5.3) | 149 (25.6) | 2 (0.3) | 9 (1.5) |
| Heterosexual contactd | 12 084 | 4868 (40.3) | 782 (6.5) | 273 (2.3) | 18 (0.1) | 2024 (16.7) | 547 (4.5) | 3394 (28.1) | 20 (0.2) | 158 (1.3) |
| Othere | 484 | 287 (59.3) | 53 (11.0) | 67 (13.8) | 2 (0.4) | 20 (4.1) | 12 (2.5) | 35 (7.2) | 0 | 8 (1.7) |
| Stage 3 infection (AIDS) classification within 3 months of HIV diagnosis | ||||||||||
| Yes | 16 009 | 2933 (18.3) | 1598 (10.0) | 478 (3.0) | 53 (0.3) | 6464 (40.4) | 1172 (7.3) | 3113 (19.4) | 50 (0.3) | 148 (0.9) |
| No | 34 285 | 6107 (17.8) | 3771 (11.0) | 1752 (5.1) | 182 (0.5) | 10 196 (29.7) | 4100 (12.0) | 7665 (22.4) | 119 (0.3) | 392 (1.1) |
| Total | 50 293 | 9040 (18.0) | 5369 (10.7) | 2230 (4.4) | 235 (0.5) | 16 660 (33.1) | 5272 (10.5) | 10 778 (21.4) | 169 (0.3) | 540 (1.1) |
aData source: Centers for Disease Control and Prevention.18 All values presented are number (percentage).
bHispanic/Latino people can be of any race.
cNumbers have been adjusted for missing country of birth and missing risk factors.
dHeterosexual contact with a person known to have, or to be at high risk for, HIV infection.
eIncludes hemophilia, blood transfusion, perinatal exposure, and risk factor not reported or not identified.
Epidemiology of HIV by ROB
We observed substantial differences in the characteristics of non–US-born people with HIV infection by ROB. Africa and the Caribbean had the largest percentages of girls and women with diagnosed HIV infection (5355 of 9040 [59.2%] and 3578 of 10 778 [33.2%], respectively; Table 2). The lowest percentages of non–US-born boys and men who acquired HIV through male-to-male sexual contact were from Africa (1624 of 3685, 44.1%) and the Caribbean (4680 of 7200, 65.0%). More than 80% of boys and men from Asia (3780 of 4495, 84.1%), Europe (1555 of 1851, 84.0%), North America (189 of 212, 89.2%), Central America/Mexico (11 932 of 14 503, 82.3%), and Oceania (108 of 127; 85.0%) had HIV infection attributed to male-to-male sexual contact. The percentage of non–US-born people with stage 3 infection (AIDS) at diagnosis ranged from 21.4% (478 of 2230) in Europe to 38.8% (6464 of 16 660) in Central America/Mexico.
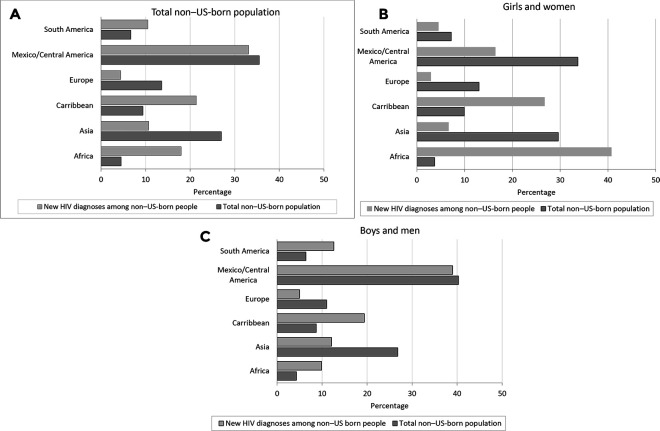
Although non–US-born people from Central America/Mexico accounted for the largest absolute number of HIV diagnoses, the proportion of HIV diagnoses among people from Central America/Mexico with known ROB was similar to the proportion of HIV diagnoses among people from Central America/Mexico in the entire non–US-born population (Figure, A). In contrast, people from the Caribbean and Africa were overrepresented among HIV diagnoses (21.4% and 18.0% of non–US-born PLWH, respectively). Girls and women from Africa and the Caribbean represented 40.7% (5355 of 13 154) and 27.2% (3578 of 13 154), respectively, of non–US-born girls and women with diagnosed HIV infection (Figure, B). African-born and Caribbean-born girls and women accounted for 16.7% (8933 of 53 359) of all HIV diagnoses (US-born and non–US-born) among girls and women with known ROB.
Figure.
Proportion of non–US-born people with newly diagnosed HIV infection during 2010-2017 and proportion of the 2016 non–US-born population, by region of birth, total, and sex at birth for regions of birth comprising the largest proportion of non–US-born HIV cases. Oceania and North America are not presented because of small numbers. Data source for non–US-born population: Pew Research Center.19 Data source for non–US-born population sizes by sex: Grieco et al.20
US Region of Residence
Among 50 301 non–US-born people with diagnosed HIV infection with known COB, the largest percentage (19 876, 39.5%) resided in the South (Table 3). However, region of residence varied by ROB; the largest proportions of people from Central America/Mexico and Asia resided in the West, and the largest proportion of people from Europe resided in the Northeast.
Table 3.
Diagnoses of HIV infection among non–US-born people, by world region of birth and region of residence at time of diagnosis, United States and 6 dependent areas, 2010-2017a
| Region of residence | Africa | Asia | Europe | North America |
Central America/Mexico | South America |
Caribbean | Oceania | Other | Total no. |
|---|---|---|---|---|---|---|---|---|---|---|
| Northeast | 2457 (19.6) | 1220 (9.7) | 743 (5.9) | 45 (0.4) | 1971 (15.7) | 2099 (16.7) | 3854 (30.7) | 30 (0.2) | 124 (1.0) | 12 548 |
| Midwest | 1646 (40.1) | 528 (12.9) | 190 (4.6) | 14 (0.3) | 1280 (31.2) | 159 (3.9) | 152 (3.7) | 10 (0.2) | 120 (2.9) | 4102 |
| South | 3397 (17.1) | 1201 (6.0) | 665 (3.3) | 86 (0.4) | 5787 (29.1) | 2329 (11.7) | 6232 (31.4) | 27 (0.1) | 152 (0.8) | 19 876 |
| West | 1540 (11.4) | 2400 (17.8) | 624 (4.6) | 90 (0.7) | 7613 (56.3) | 668 (4.9) | 337 (2.5) | 100 (0.7) | 142 (1.1) | 13 514 |
| US dependent areas | 0 | 20 (7.7) | 8 (3.1) | 0 | 9 (3.4) | 17 (6.5) | 203 (77.8) | 2 (0.8) | 2 (0.8) | 261 |
| Total | 9040 (18.0) | 5369 (10.7) | 2230 (4.4) | 235 (0.5) | 16 660 (33.1) | 5272 (10.5) | 10 778 (21.4) | 169 (0.3) | 540 (1.1) | 50 301 |
aData source: Centers for Disease Control and Prevention.18 All values presented are number (percentage), unless otherwise indicated.
In our analysis of MSAs, the New York–Jersey City–White Plains, New York–New Jersey MSA was 1 of the top 5 MSAs for all ROBs (Table 4). The proportion of all HIV diagnoses among people born in each ROB found in the 5 MSAs varied by ROB, from 36.2% (2948 of 8146) of African-born people to 72.8% (7339 of 10 081) of Caribbean-born people living in the 5 MSAs with the most HIV diagnoses for each respective ROB. Four of the 5 MSAs with the most HIV diagnoses among Caribbean-born people were in Florida.
Table 4.
Metropolitan statistical areas (MSAs) accounting for the largest numbers of HIV diagnoses among non–US-born people, by world region of birth, 2010-2016a ,b
| MSA | Africa | Asia | Europe | North America |
Central America/ Mexico |
South America |
Caribbean | Oceania | Other |
|---|---|---|---|---|---|---|---|---|---|
| MSA 1 | New York–Jersey City–White Plains, New York–New Jersey | New York–Jersey City–White Plains, New York–New Jersey | New York–Jersey City–White Plains, New York–New Jersey | Los Angeles–Long Beach-Glendale, California | Los Angeles–Long Beach-Glendale, California | New York–Jersey City–White Plains, New York–New Jersey | Miami–Miami Beach–Kendall, Florida | Los Angeles–Long Beach-Glendale, California | Chicago–Naperville–Arlington Heights, Illinois |
| MSA 2 | Washington–Arlington–Alexandria, District of Columbia–Virginia–Maryland–West Virginia | Los Angeles–Long Beach–Glendale, California | Los Angeles–Long Beach–Glendale, California | New York–Jersey City–White Plains, New York–New Jersey | Houston–The Woodlands–Sugar Land, Texas | Miami–Miami Beach–Kendall, Florida | New York–Jersey City–White Plains, New York–New Jersey | New York–Jersey City–White Plains, New York–New Jersey | Los Angeles–Long Beach–Glendale, California |
| MSA 3 | Houston–The Woodlands–Sugar Land, Texas | San Francisco–Redwood City–South San Francisco, California | Miami–Miami Beach–Kendall, Florida | Fort Lauderdale–Pompano Beach–Deerfield Beach, Florida | New York–Jersey City–White Plains, New York–New Jersey | Fort Lauderdale–Pompano Beach–Deerfield Beach, Florida | Fort Lauderdale–Pompano Beach–Deerfield Beach, Florida | San Francisco–Redwood City–South San Francisco, California | Newark, New Jersey–Pennsylvania |
| MSA 4 | Minneapolis–St. Paul–Bloomington, Minnesota–Wisconsin | Houston–The Woodlands–Sugar Land, Texas | Fort Lauderdale–Pompano Beach–Deerfield Beach, Florida | San Francisco–Redwood City–South San Francisco, California | San Diego–Carlsbad, California | Los Angeles–Long Beach–Glendale, California | West Palm Beach–Boca Raton–Delray Beach, Florida | Seattle–Bellevue–Everett, Washington | Philadelphia, Pennsylvania |
| MSA 5 | Silver Spring–Frederick–Rockville, Maryland | Seattle
|
San Francisco–Redwood City–South San Francisco, California | Miami–Miami Beach–Kendall, Florida | Dallas–Plano–Irving, Texas | Orlando–Kissimmee–Sanford, Florida | Orlando–Kissimmee–Sanford, Florida | Oakland–Hayward–Berkeley, California | New York–Jersey City–White Plains, New York–New Jersey |
| Total residing in these 5 MSAsc | 2948 | 2014 | 968 | 93 | 6409 | 3244 | 7339 | 82 | 211 |
| Total HIV diagnosesd | 8146 | 4868 | 1993 | 210 | 14 592 | 5005 | 10 081 | 151 | 476 |
| Percentage residing in these 5 MSAse | 36.2 | 41.4 | 48.6 | 44.3 | 43.9 | 64.8 | 72.8 | 54.3 | 44.3 |
aData source: Centers for Disease Control and Prevention.18
bMSA 1 is the MSA that accounts for the largest number of people with new HIV diagnoses during the study period from each region of birth. MSA 2 is the MSA that accounts for the second largest number of people newly diagnosed with HIV from each region of birth. MSA3, MSA4, and MSA5 account for the third, fourth, and fifth largest numbers of people with new HIV diagnoses from each region of birth, respectively.
cTotal number of people with newly diagnosed HIV infection from each region of birth (column total).
dTotal number of people with newly diagnosed HIV infection from each region of birth living in an MSA with population >500 000.
ePercentage of people with newly diagnosed HIV infection residing in an MSA with >500 000 population in these 5 MSAs.
Discussion
We estimated that 62 170 non–US-born people received an HIV diagnosis during 2010-2017, accounting for 18.9% of HIV diagnoses in the United States during this time, after imputing nativity for the 19.2% of people who were missing data on COB. HIV diagnosis rates were higher among non–US-born people than among US-born people, both overall and by sex at birth. The differences in the epidemiology of HIV that we observed between non–US-born people and US-born people, and among non–US-born people from various ROBs, have important implications for HIV prevention and care. The variation in modes of HIV acquisition may require that HIV prevention messages be tailored differently for some groups of non–US-born people (ie, heterosexual people vs men who have sex with men). In addition, among racial/ethnic groups such as Asian/Pacific Islander people, prevention interventions should reflect that most people with diagnosed HIV infection in these groups are non–US-born. Non–US-born people may have less access to health care than US-born people and varying levels of English proficiency and health literacy. Therefore, HIV prevention strategies developed for US-born people may not be culturally appropriate for some non–US-born people.
Our results are similar to the results of Prosser et al,13 who used national HIV surveillance data for people diagnosed with HIV infection during 2007-2010. Although Prosser et al reported that 16.2% of HIV diagnoses were among non–US-born people,13 we found that 18.7% of HIV diagnoses during 2010-2017 were among non–US-born people when we restricted data to the same group jurisdictions used by Prosser et al. Given that our methods were similar to the methods of Prosser et al and we included the same jurisdictions, the slightly larger percentage we observed likely reflects an actual increase in the proportion of people with diagnosed HIV infection who are non–US-born.
Although we found that the distribution of non–US-born people diagnosed with HIV during the study period was similar to the overall distribution of non–US-born people in the United States, we found differences. The largest numbers of non–US-born people in the United States overall in 2016 were from the following 10 countries: Mexico (26.5%), China (6.2%), India (5.6%), the Philippines (4.4%), El Salvador (3.1%), Vietnam (3.1%), Cuba (2.9%), the Dominican Republic (2.5%), South Korea (2.4%), and Guatemala (2.1%).19 In contrast, the largest numbers of new HIV diagnoses reported from 2010-2017 among non–US-born people were from Mexico (22.6%), Haiti (7.5%), Cuba (5.8%), Dominican Republic (3.5%), El Salvador (3.4%), Jamaica (2.9%), Honduras (2.9%), Ethiopia (2.8%), Guatemala (2.8%), and Colombia (2.6%). We also found that the proportion of new HIV diagnoses among non–US-born people from some ROBs was disproportionate to the size of the non–US-born population from those ROBs. In particular, people from the Caribbean and Africa accounted for a greater share of new HIV diagnoses (21.4% and 18.0% of non–US-born PLWH, respectively) compared with the total non–US-born population (9.4% and 4.5% of the population in 2016, respectively).19 Girls and women from Africa and the Caribbean were especially overrepresented, comprising 40.7% and 27.2%, respectively, of non–US-born girls and women with diagnosed HIV infection, compared with 3.7% and 9.9%, respectively, of total non–US-born girls and women.20
We and others have found that non–US-born people are more likely than US-born people to have stage 3 infection (AIDS) within 1 year of HIV diagnosis.8,21-25 Concurrent stage 3 infection (AIDS) is often a marker for inadequate frequency of testing. A study using National Health Interview Survey data from 2013-2014 found that a slightly larger proportion of non–US-born people (40%) than US-born people (37%) reported ever testing for HIV.23 However, immigrants from areas with generalized HIV epidemics might be more likely than US-born residents to have been exposed to and tested for HIV infection, and Valverde et al26 noted that levels of ever testing for HIV infection were highest among people born in regions with high HIV prevalence. Although few studies have compared HIV testing among immigrants with HIV testing among US-born people, Ojikutu et al27 found less recent testing among black immigrants than among US-born black people in Massachusetts.
Despite evidence of delayed HIV diagnosis among non–US-born people, multiple studies have found that, generally, non–US-born people fare as well as or better than US-born people in outcomes of the HIV care continuum after initial diagnosis.8,11,28,29 This observation highlights the need to focus prevention efforts on increasing HIV testing and diagnosis. Although increased testing in the United States will not decrease the possibility of a delayed diagnosis among people with HIV infection acquired before they arrive in the United States, earlier HIV diagnoses among such people may reduce onward HIV transmission in the United States and will be important in averting the morbidity and mortality associated with HIV infection. Developing HIV testing interventions depends on characterizing the populations most at risk for HIV infection. For non–US-born people diagnosed with HIV infection, collecting data on the various characteristics of this population is a first step. Working with local jurisdictions to increase the collection and reporting of data on COB would be important progress toward better describing the population, both in size and distribution across ROBs. Basic sociodemographic information, such as how long people had lived in the United States before receiving an HIV diagnosis, health care use, literacy levels, and primary languages, would also be helpful in understanding how to reach non–US-born people who would benefit most from HIV testing.
In many areas, data on non–US-born people are an important component of understanding the epidemiology of HIV. The South, the region most affected by HIV, was home to more than one-third of non–US-born people with diagnosed HIV infection. Many MSAs with the most non–US-born people with diagnosed HIV infection are in border states or states that have major ports of entry, such as Florida, Texas, California, and New York. These states also had some of the largest increases in the size of the non–US-born population during 2000-2010.5 However, as noted previously, it is unclear to what degree non–US-born people are arriving in the country with HIV infection or acquiring HIV after arrival. Studies conducted in Europe and in areas in the United States have found varying levels of postmigration HIV acquisition among immigrants,30-32 but studies that included immigrants from multiple ROBs found that rates of postmigration acquisition are typically lowest among African immigrants and higher among people born in Latin America and the Caribbean than among people from other ROBs.12,33-35 A study using US national molecular HIV surveillance data found that among people newly diagnosed with HIV infection during 2001-2011, only 28% of non–US-born people were linked with ≥1 other person in an HIV transmission cluster and that men who have sex with men and people who inject drugs were more likely than other non–US-born people to be part of an HIV transmission cluster.36
Limitations
Our study had several limitations. First, 19.2% of diagnoses were reported with missing data on COB. Although we used multiple imputation, a method that is used to estimate values for HIV transmission category, to estimate nativity for people missing data on COB, we may have overestimated or underestimated the proportion of non–US-born people if nativity-related factors were not accounted for in the imputation procedure. In addition, it was not possible to estimate COB, only nativity. As such, we excluded people who were imputed to be non–US-born but reported with missing data on COB from our analyses by ROB. Therefore, we may have overestimated or underestimated the proportions of people born in each ROB. Second, some non–US-born people experience more stigma than other non–US-born people related to same-sex sexual contact, and same-sex sexual behavior is criminalized in many countries.37-39 Therefore, HIV acquisition through male-to-male sexual contact may be underreported among non–US-born people. Finally, some diagnoses among non–US-born people may occur among people who received an HIV diagnosis before arrival in the United States, but lack of documentation of previous positive test results may lead to a later diagnosis date in the National HIV Surveillance System.8 Some late HIV diagnoses among non–US-born people might also reflect a lack of engagement in care in the United States or in other countries rather than true late diagnoses.
Conclusions
Our study found that compared with their US-born counterparts, non–US-born people with diagnosed HIV infection were more likely to be female, to have acquired HIV through heterosexual contact, or to have stage 3 infection (AIDS) at the time of diagnosis. Compared with people with diagnosed HIV infection from other ROBs, people with diagnosed HIV infection from Latin America were more likely to be male and have acquired HIV through male-to-male sexual contact, and people with diagnosed HIV infection from Africa were more likely to be female and to have acquired HIV via heterosexual contact. Although these data provide important information about HIV among non–US-born people, our understanding of HIV incidence in this population is limited. We do not know how many HIV infections among non–US-born people were acquired in the United States and, consequently, how many HIV infections might have been averted through local prevention efforts. Similarly, it is unclear to what degree onward transmission of HIV infection among, or from, non–US-born people could occur. Although evidence for delayed HIV diagnosis among non–US-born people suggests the possibility of onward transmission, data on sexual mixing patterns and sexual behavior in most immigrant groups are lacking. Despite these limitations, the growing non–US-born population and our observation that approximately one-third of non–US-born people had concurrent HIV infection/stage 3 infection (AIDS) diagnoses highlight the need to increase HIV testing and linkage to care among non–US-born people.
Footnotes
Authors’ Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Allergy and Infectious Disease (K01AI095060, R01AI127232).
ORCID iD
Roxanne P. Kerani https://orcid.org/0000-0002-4416-4280
References
- 1. Centers for Disease Control and Prevention Diagnoses of HIV infection in the United States and dependent areas, 2016. HIV Surveill Rep. 2017;28:1-125. [Google Scholar]
- 2. UNAIDS Global AIDS update 2016. Published 2017. Accessed May 4, 2020 https://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
- 3. UNAIDS Fact sheet—World AIDS Day, 2019. Published 2019. Accessed May 17, 2020 https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 4.United Nations Department of Economic and Social Affairs. Population Division World migration in figures. Paper presented at: United Nations High-Level Dialogue on International Migration and Development; October 3-4, 2013; New York.
- 5. Grieco EM., Trevelyan E., Larsen L. et al. The Size, Place of Birth, and Geographic Distribution of the Foreign-Born Population of the United States: 1960 to 2010. US Census Bureau; 2012. [Google Scholar]
- 6. Batalova J., Blizzard B., Bolter J. Frequently requested statistics on immigrants and immigration in the United States. 2020. Accessed May 30, 2020 http://www.migrationpolicy.org/article/frequently-requested-statistics-immigrants-and-immigration-united-states
- 7. Ashton C., Bernhardt SA., Lowe M., Mietchen M., Johnston J. Comparison of HIV/AIDS rates between U.S.-born blacks and African-born blacks in Utah, 2000-2009. Open AIDS J. 2012;6:156-162. 10.2174/1874613601206010156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kerani R., Bennett AB., Golden M., Castillo J., Buskin SE. Foreign-born individuals with HIV in King County, WA: a glimpse of the future of HIV? AIDS Behav. 2018;22(7):2181-2188. 10.1007/s10461-017-1914-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kerani RP., Kent JB., Sides T. et al. HIV among African-born persons in the United States: a hidden epidemic? J Acquir Immune Defic Syndr. 2008;49(1):102-106. 10.1097/QAI.0b013e3181831806 [DOI] [PubMed] [Google Scholar]
- 10. Sides TL. An epidemiologic update on the HIV/AIDS epidemic in Minnesota. Minn Med. 2003;86(6):33-37. [PubMed] [Google Scholar]
- 11. Wiewel EW., Torian LV., Nasrallah HN., Hanna DB., Shepard CW. HIV diagnosis and utilisation of HIV-related medical care among foreign-born persons in New York City, 2001-2009. Sex Transm Infect. 2013;89(5):380-382. 10.1136/sextrans-2012-050677 [DOI] [PubMed] [Google Scholar]
- 12. Wiewel EW., Torian LV., Hanna DB., Bocour A., Shepard CW. Foreign-born persons diagnosed with HIV: where are they from and where were they infected? AIDS Behav. 2015;19(5):890-898. 10.1007/s10461-014-0954-1 [DOI] [PubMed] [Google Scholar]
- 13. Prosser AT., Tang T., Hall HI. HIV in persons born outside the United States, 2007-2010. JAMA. 2012;308(6):601-607. 10.1001/jama.2012.9046 [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention Final rule removing HIV infection from U.S. immigration screening. 2010. Accessed May 18, 2020 https://www.cdc.gov/immigrantrefugeehealth/laws-regs/hiv-ban-removal/final-rule.html
- 15. Fauci AS., Redfield RR., Sigounas G., Weahkee MD., Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844-845. 10.1001/jama.2019.1343 [DOI] [PubMed] [Google Scholar]
- 16. US Census Bureau American Community Survey and Puerto Rico Community Survey: 2014 code list. 2014. Accessed September 21, 2018 https://www2.census.gov/programs-surveys/acs/tech_docs/code_lists/2014_ACS_Code_Lists.pdf
- 17. Harrison KM., Kajese T., Hall HI., Song R. Risk factor redistribution of the national HIV/AIDS surveillance data: an alternative approach. Public Health Rep. 2008;123(5):618-627. 10.1177/003335490812300512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention HIV case surveillance. Published 2019. Accessed May 28, 2020 https://www.cdc.gov/hiv/statistics/surveillance/systems/index.html#HIV%20Case%20Surveillance
- 19. Radford J., Budiman A. 2016, foreign-born population in the United States statistical portrait. Published September 14, 2018. Accessed May 28, 2020 https://www.pewresearch.org/hispanic/2018/09/14/2016-statistical-information-on-foreign-born-in-united-states
- 20. Grieco EM., Acosta YD., de la Cruz GP. et al. The Foreign-Born Population of the United States: 2010. US Census Bureau; 2012. [Google Scholar]
- 21. Espinoza L., Hall HI., Hu X. Diagnoses of HIV infection among Hispanics/Latinos in 40 states and Puerto Rico, 2006-2009. J Acquir Immune Defic Syndr. 2012;60(2):205-213. 10.1097/QAI.0b013e31824d9a29 [DOI] [PubMed] [Google Scholar]
- 22. Johnson AS., Hu X., Dean HD. Epidemiologic differences between native-born and foreign-born black people diagnosed with HIV infection in 33 U.S. states, 2001-2007. Public Health Rep. 2010;125(Suppl 4):61-69. 10.1177/00333549101250S410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheehan DM., Trepka MJ., Fennie KP., Maddox LM. Rate of new HIV diagnoses among Latinos living in Florida: disparities by country/region of birth. AIDS Care. 2015;27(4):507-511. 10.1080/09540121.2014.978731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demeke HB., Johnson AS., Wu B., Nwangwu-Ike N., King H., Dean HD. Differences between U.S.-born and non-U.S.-born black adults reported with diagnosed HIV infection: United States, 2008-2014. J Immigr Minor Health. 2019;21(1):30-38. 10.1007/s10903-018-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demeke HB., Johnson AS., Zhu H., Gant Z., Duffus WA., Dean HD. HIV infection-related care outcomes among U.S.-born and non–U.S.-born blacks with diagnosed HIV in 40 U.S. areas: the National HIV Surveillance System, 2016. Int J Environ Res Public Health. 2018;15(11):pii:E2404. 10.3390/ijerph15112404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valverde E., DiNenno E., Oraka E., Bautista G., Chavez P. HIV testing among foreign-born men and women in the United States: results from a nationally representative cross-sectional survey. J Immigr Minor Health. 2018;20(5):1118-1127. 10.1007/s10903-017-0655-8 [DOI] [PubMed] [Google Scholar]
- 27. Ojikutu B., Nnaji C., Sithole J. et al. All black people are not alike: differences in HIV testing patterns, knowledge, and experience of stigma between U.S.-born and non–U.S.-born blacks in Massachusetts. AIDS Patient Care STDS. 2013;27(1):45-54. 10.1089/apc.2012.0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levison JH., Regan S., Khan I., Freedberg KA. Foreign-born status as a predictor of engagement in HIV care in a large US metropolitan health system. AIDS Care. 2017;29(2):244-251. 10.1080/09540121.2016.1210077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Page LC., Goldbaum G., Kent JB., Buskin SE. Access to regular HIV care and disease progression among black African immigrants. J Natl Med Assoc. 2009;101(12):1230-1240. 10.1016/S0027-9684(15)31134-2 [DOI] [PubMed] [Google Scholar]
- 30. Brännström J., Sönnerborg A., Svedhem V., Neogi U., Marrone G. A high rate of HIV-1 acquisition post immigration among migrants in Sweden determined by a CD4 T-cell decline trajectory model. HIV Med. 2017;18(9):677-684. 10.1111/hiv.12509 [DOI] [PubMed] [Google Scholar]
- 31. Burns FM, Arthur G, Johnson AM, Nazroo J, Fenton KA, Group SC, SONHIA collaboration group United Kingdom acquisition of HIV infection in African residents in London: more than previously thought. AIDS. 2009;23(2):262-266. 10.1097/QAD.0b013e32831c546b [DOI] [PubMed] [Google Scholar]
- 32. Desgrées-du-Loô A., Pannetier J., Ravalihasy A. et al. Sub-Saharan African migrants living with HIV acquired after migration, France, ANRS PARCOURS study, 2012 to 2013 [published correction appears in Euro Surveill. 2015;20(47). doi:10.2807/1560-7917.ES.2015.20.47.30069]. Euro Surveill. 2015;20(46). doi:10.2807/1560-7917.ES.2015.20.46.30065 [DOI] [PubMed] [Google Scholar]
- 33. Alvarez-Del Arco D., Fakoya I., Thomadakis C. et al. High levels of postmigration HIV acquisition within nine European countries. AIDS. 2017;31(14):1979-1988. 10.1097/QAD.0000000000001571 [DOI] [PubMed] [Google Scholar]
- 34. Rice BD., Elford J., Yin Z., Delpech VC. A new method to assign country of HIV infection among heterosexuals born abroad and diagnosed with HIV. AIDS. 2012;26(15):1961-1966. 10.1097/QAD.0b013e3283578b80 [DOI] [PubMed] [Google Scholar]
- 35. Dougan S., Elford J., Rice B. et al. Epidemiology of HIV among black and minority ethnic men who have sex with men in England and Wales. Sex Transm Infect. 2005;81(4):345-350. 10.1136/sti.2004.012328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valverde EE., Oster AM., Xu S., Wertheim JO., Hernandez AL. HIV transmission dynamics among foreign-born persons in the United States. J Acquir Immune Defic Syndr. 2017;76(5):445-452. 10.1097/QAI.0000000000001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilbert PA., Barrington C., Rhodes SD., Eng E. Saliendo adelante: stressors and coping strategies among immigrant Latino men who have sex with men in a nontraditional settlement state. Am J Mens Health. 2016;10(6):515-525. 10.1177/1557988316647704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beyrer C. Pushback: the current wave of anti-homosexuality laws and impacts on health. PLoS Med. 2014;11(6):e1001658. 10.1371/journal.pmed.1001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poteat T., Diouf D., Drame FM. et al. HIV risk among MSM in Senegal: a qualitative rapid assessment of the impact of enforcing laws that criminalize same sex practices. PLoS One. 2011;6(12):e28760. 10.1371/journal.pone.0028760 [DOI] [PMC free article] [PubMed] [Google Scholar]