Abstract
Background:
Biomarkers are needed to estimate which patients benefit most from combination ipilimumab and nivolumab immunotherapy. Rigorous biomarker analyses from prior ipilimumab randomized studies without nivolumab are likely to inform which biomarker analyses should be prioritized when examining patients treated with the combination. For the first time, the current analyses investigate absolute lymphocyte count (ALC) in randomized, controlled trials of ipilimumab without nivolumab to assess whether ALC is prognostic or predictive of ipilimumab treatment benefit.
Methods:
Data included patients (n=1136) treated in the two randomized, controlled phase III studies MDX010–20 and CA184–024. ALC was measured at pre-treatment baseline and every 3 weeks for up to 12 weeks, prior to each dose of ipilimumab. Cox proportional hazards models were used to estimate and test associations between ALC measures and overall survival (OS).
Results:
In both randomized studies, baseline ALC and ALC halfway through induction (at week 6) were associated with OS not only in ipilimumab-treated patients, but also in patients treated with non-ipilimumab control treatments. ALC increased in patients receiving ipilimumab, but this degree of change was not predictive of ipilimumab treatment benefit.
Conclusions:
Using data from randomized, controlled studies, we were able to conclude for the first time that baseline ALC, ALC halfway through induction (week 6), and the degree of ALC change from baseline to week 6 are prognostic biomarkers in melanoma patients, and do not appear to be predictive of ipilimumab treatment benefit. This now more comprehensive understanding of ALC as a biomarker from ipilimumab trials will inform subsequent biomarker investigations in ongoing ipilimumab combination studies such as ipilimumab in combination with nivolumab.
Keywords: Absolute lymphocyte count, biomarker, ipilimumab, melanoma, nivolumab, overall survival
Introduction
In patients with metastatic melanoma, treatment with the combination of ipilimumab and nivolumab is associated with long-term survival [1,2], yet biomarkers are needed to help understand which patients benefit most from treatment. Rigorous biomarker analyses from prior ipilimumab randomized studies without nivolumab are likely to inform subsequent biomarker studies involving combination immunotherapy. We analyzed the absolute lymphocyte count (ALC) in two randomized, controlled phase III trials of patients with advanced melanoma receiving ipilimumab: MDX010–20 and CA184–024 [3,4]. The two randomized studies, with a combined sample size of more than 1100 patients, provided a unique opportunity for the first time to assess whether ALC was prognostic, predictive of treatment benefit, or both.
Of all putative ipilimumab biomarkers previously identified, we chose to investigate ALC in randomized controlled trials for three reasons. First, ALC is measured by a standard laboratory test available in peripheral blood and is easily evaluable in large numbers of patients which could facilitate subsequent evaluation in large, randomized ipilimumab combination studies. Second, our group and others have found reproducible associations between ALC and clinical outcomes following ipilimumab in smaller, non-controlled series [5,6]. Third, since ipilimumab targets cytotoxic T lymphocyte antigen-4 (CTLA-4) on T lymphocytes, there is mechanistic rationale to the hypothesis that the quantity of lymphocytes may be relevant.
Methods
Overall survival (OS) was defined as the time from randomization to death from any cause or to censoring event. We tested three ALC measures for their association with OS: pre-treatment baseline ALC (ALC1); rate of change of ALC from baseline to the time of the third ipilimumab dose/halfway through induction (Slope3); and ALC value at the time of the third ipilimumab dose/halfway through induction (ALC3). For OS analyses involving ALC3 or Slope3, only patients who provided ALC3 and Slope3 values were included. Thus, these were landmark analyses: any patient who died or was censored for OS before providing an ALC3 value was excluded. For MDX010–20, 592 (92.1%) of 643 treated patients had ALC1 values and 481 (74.8%) had ALC3 and Slope3 values. For CA184–024, 481 (96.6%) of 498 treated patients had ALC1 values and 422 (84.7%) had ALC3 and Slope3 values.
Cox proportional hazards (PH) models, which included linear effects of ALC measure and treatment group, and their interactions, were used to evaluate associations between ALC measures and OS. The models were stratified by baseline M-stage and either prior interleukin (IL)-2 therapy (MDX010–20) or Eastern Cooperative Oncology Group (ECOG) performance status (CA184–024). Thus, these all were multivariable models. The estimated associations between ALC measures and OS, which were constrained to be the same for each stratum, indicated incremental association after controlling for the stratification variables. Omnibus likelihood-ratio tests were used to test overall ALC measure and interaction effects. ALC1 and ALC3 were analyzed as both continuous and binary variables. A binary threshold of 1.0 × 109 cells/L was used to be consistent with prior literature [5,6].
The magnitudes of associations were summarized by hazard ratios (HRs). For analyses of ALC measures as continuous variables, reported HRs were scaled to compare the 75th and 25th percentiles of the measures, where these percentiles were from both studies combined. Because the effects of ALC measures were constrained to be linear in the Cox PH models, these HR estimates depended only on the difference between the 75th and 25th percentiles, not on their individual values. Thus, as long as the ALC measures had sufficient spread in a given treatment group, this scaling of HRs was sensible. For analyses of ALC measures as binary variables, the HR compared hazards for the two levels.
Results
ALC1 and ALC3 were both significantly associated with OS, as either continuous or binary variables (Fig. 1). P values for the tests of overall association, after accounting for baseline M-stage and either prior IL-2 therapy (MDX010–20) or ECOG performance status (CA184–024), ranged from 0.037 to < 0.0001 for ALC1 and from 0.0049 to < 0.0001 for ALC3.
Fig. 1.
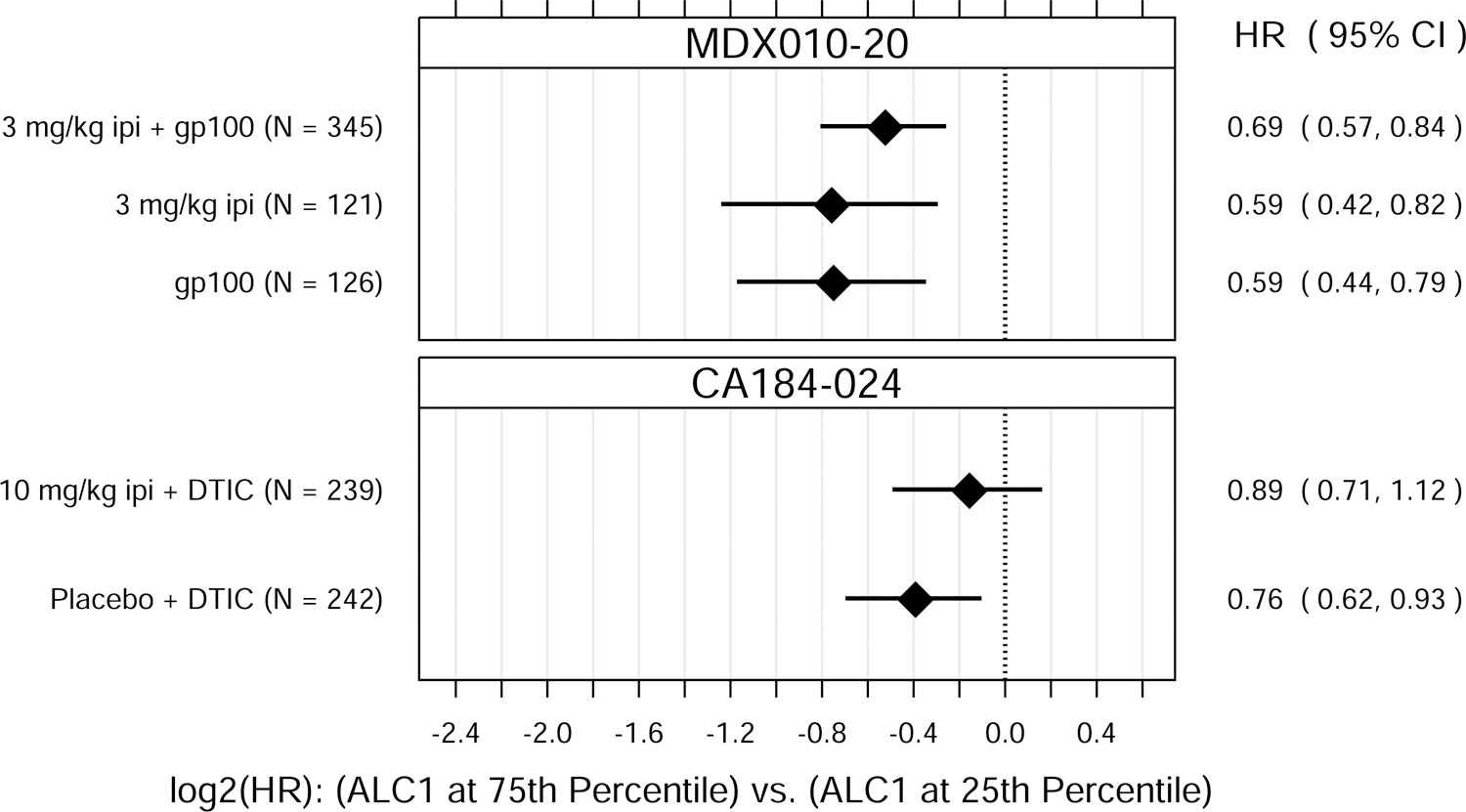
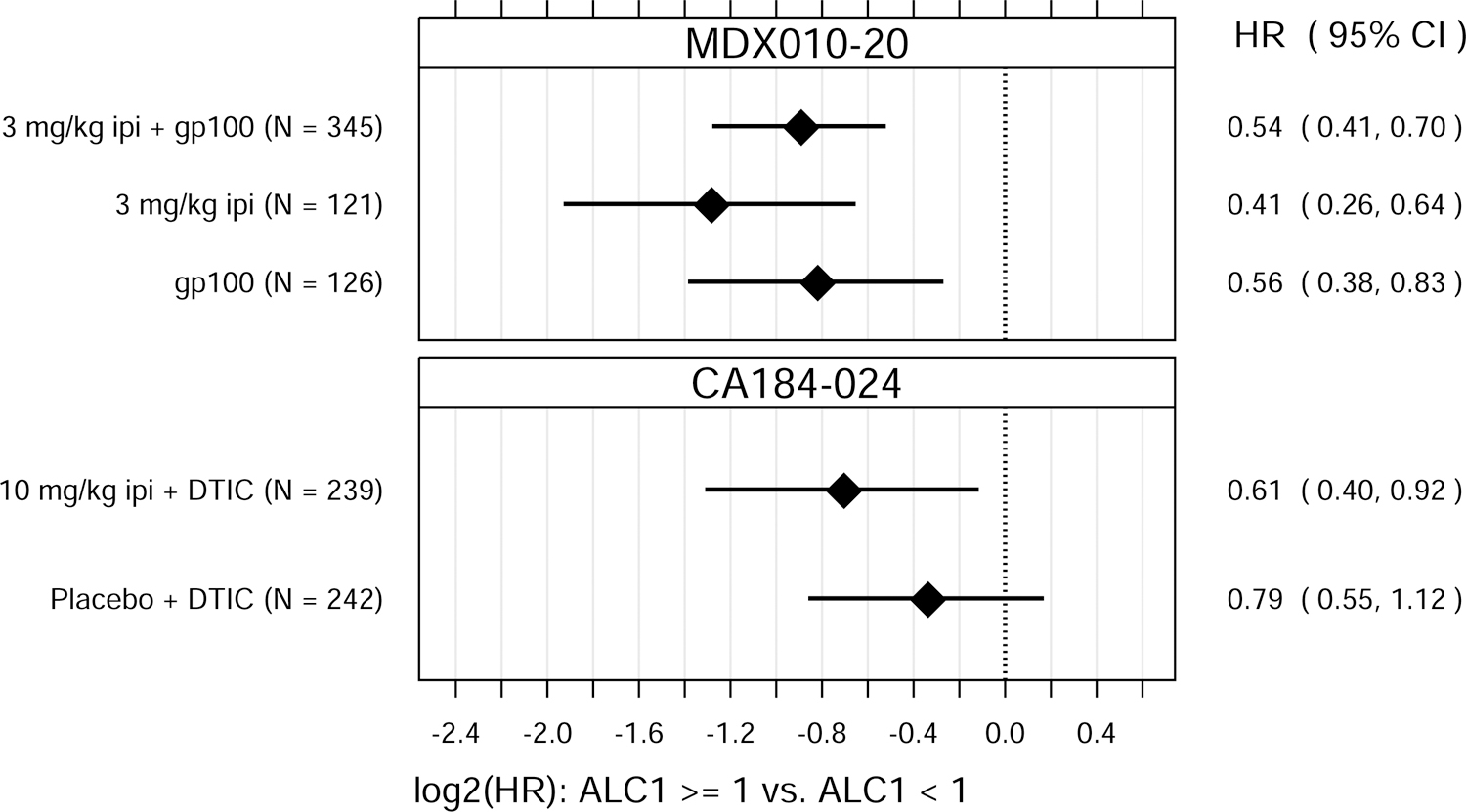
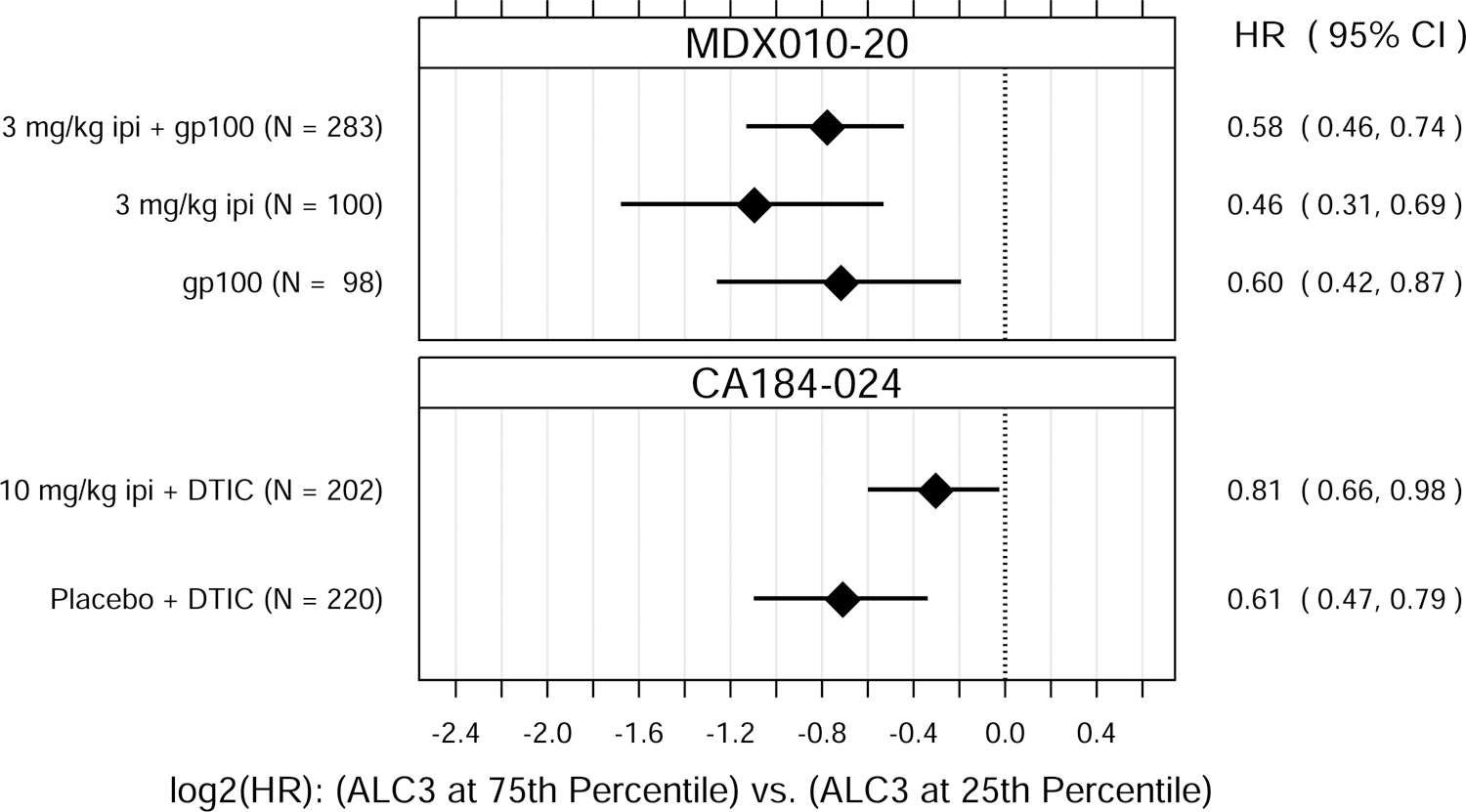
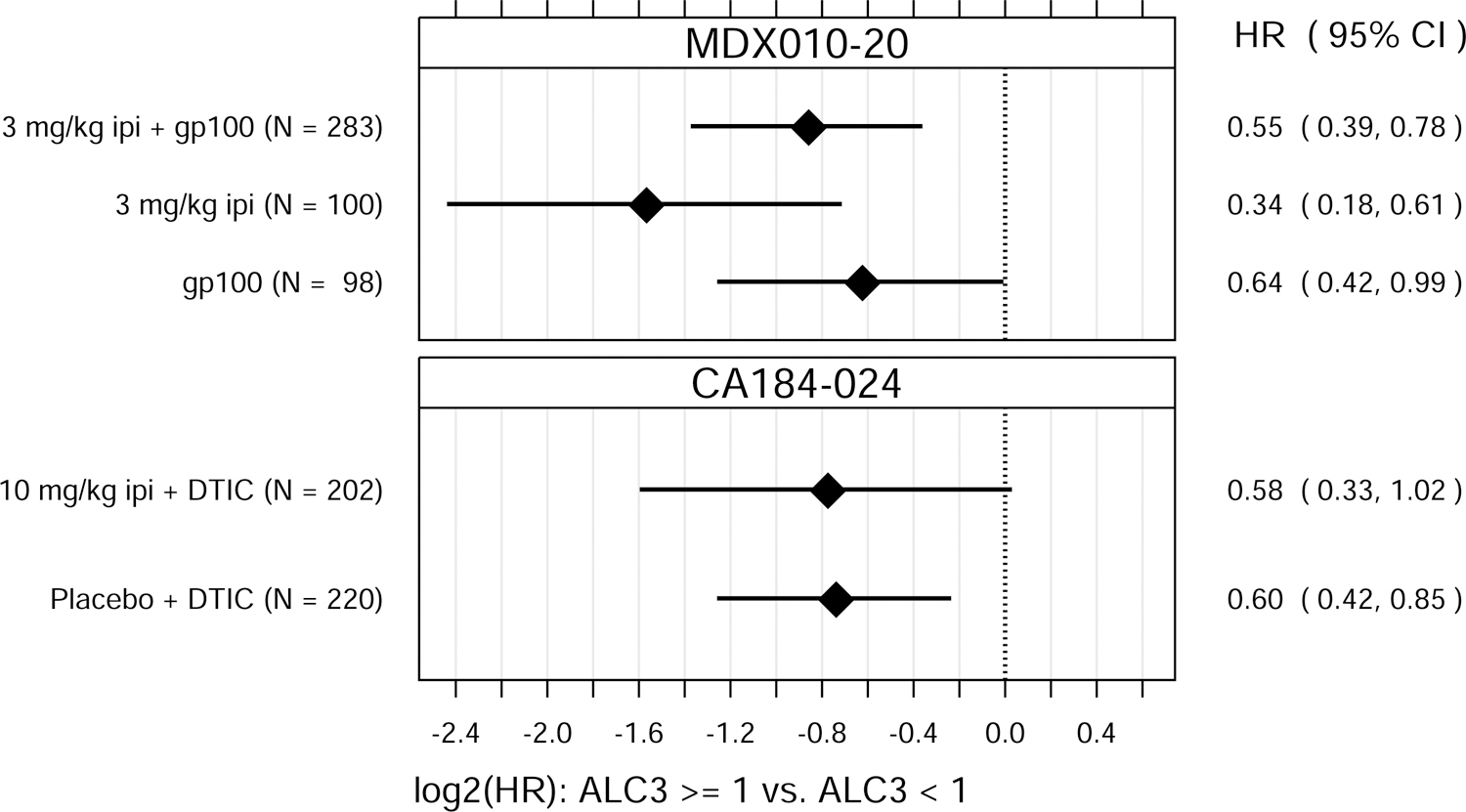
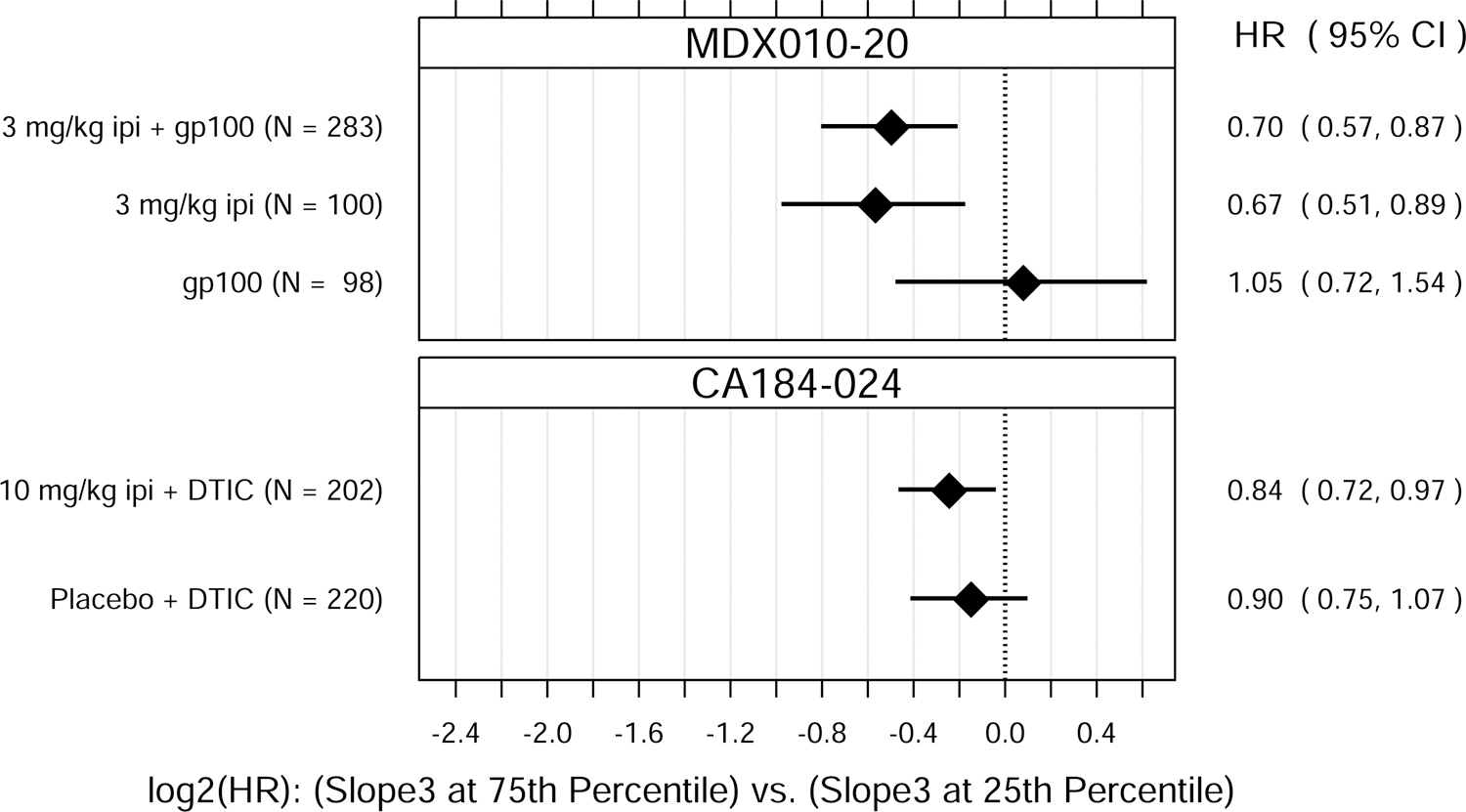
Hazard ratio (HR) estimates from Cox PH models, for each treatment group in each trial. HR estimates compare OS hazards for (A) ALC1 at the 75th and 25th percentiles; (B) ALC1 dichotomized as ≥ 1 vs. < 1 × 109 cells/L; (C) ALC3 at the 75th and 25th percentiles; (D) ALC3 dichotomized as ≥ 1 vs. < 1 × 109 cells/L; and (E) Slope3 at the 75th and 25th percentiles. Diamonds show point estimates. Horizontal bars show 95% confidence intervals (CI), based on Wald statistics. DTIC = dacarbazine; gp100 = glycoprotein 100 peptide vaccine; ipi = ipilimumab; N = number of patients included in the analysis per treatment group. All patients with a baseline (no more than 28 days prior to confirmed treatment initiation) and at least one post-baseline ALC evaluation were included in the ALC1 analyses. Of those patients, all with ≥ 1 ALC evaluation at least 7 days after dose 2 and prior to dose 3 were included in the ALC3 and Slope3 analyses. Percentiles are from the ALC1, ALC3, or Slope3 distributions over both trials combined. For ALC1, 25th percentile = 1.01 × 109 cells/L; 75th percentile = 1.80 × 109 cells/L; interquartile range = 0.79 cells/L. For ALC3, 25th percentile = 1.15 × 109 cells/L; 75th percentile = 2.08 × 109 cells/L; interquartile range = 0.93 cells/L. For Slope3, 25th percentile = −0.021 × 109 cells/L/week; 75th percentile = 0.070 × 109 cells/L/week; interquartile range = 0.091 cells/L/week. For each patient, Slope3 was estimated by simple linear regression, using all ALC assessments in the period from ALC1 to ALC3, and actual assessment dates. Examination of scaled Schoenfeld residuals suggested that the PH assumption was reasonable for all Cox PH models.
After establishing that ALC1 and ALC3 were associated with OS, we next sought to assess whether ALC1 and/or ALC3 were predictive of ipilimumab treatment benefit within these clinical trials. ALC1 and ALC3 were also associated with OS in patients in these two trials who did not receive ipilimumab. There were no significant differences in these associations between treatment groups (ipilimumab-containing vs. non-ipilimumab-containing arms), suggesting that ALC1 and ALC3 were prognostic of OS but not predictive of ipilimumab’s treatment benefit in improving OS over the control treatments tested.
An increase in ALC is a known pharmacodynamic effect of ipilimumab [5], which we also observed in these randomized, controlled studies (Fig. 2). Accordingly, we evaluated the potential association between OS and Slope3, defined as the rate of change in ALC between the dates of ALC1 and ALC3 assessments. Similar to ALC1 and ALC3, Slope3 was significantly associated with OS (Fig. 1), with P values of 0.00032 (MDX010–20) and 0.030 (CA184–024). We used an interaction test to assess whether Slope3 was predictive of ipilimumab treatment benefit. There were no significant interactions between treatment group and Slope3 in these randomized studies (P = 0.14 and P = 0.57, respectively), suggesting Slope3, similar to ALC1 and ALC3, does not appear to be predictive of ipilimumab treatment benefit.
Fig. 2.
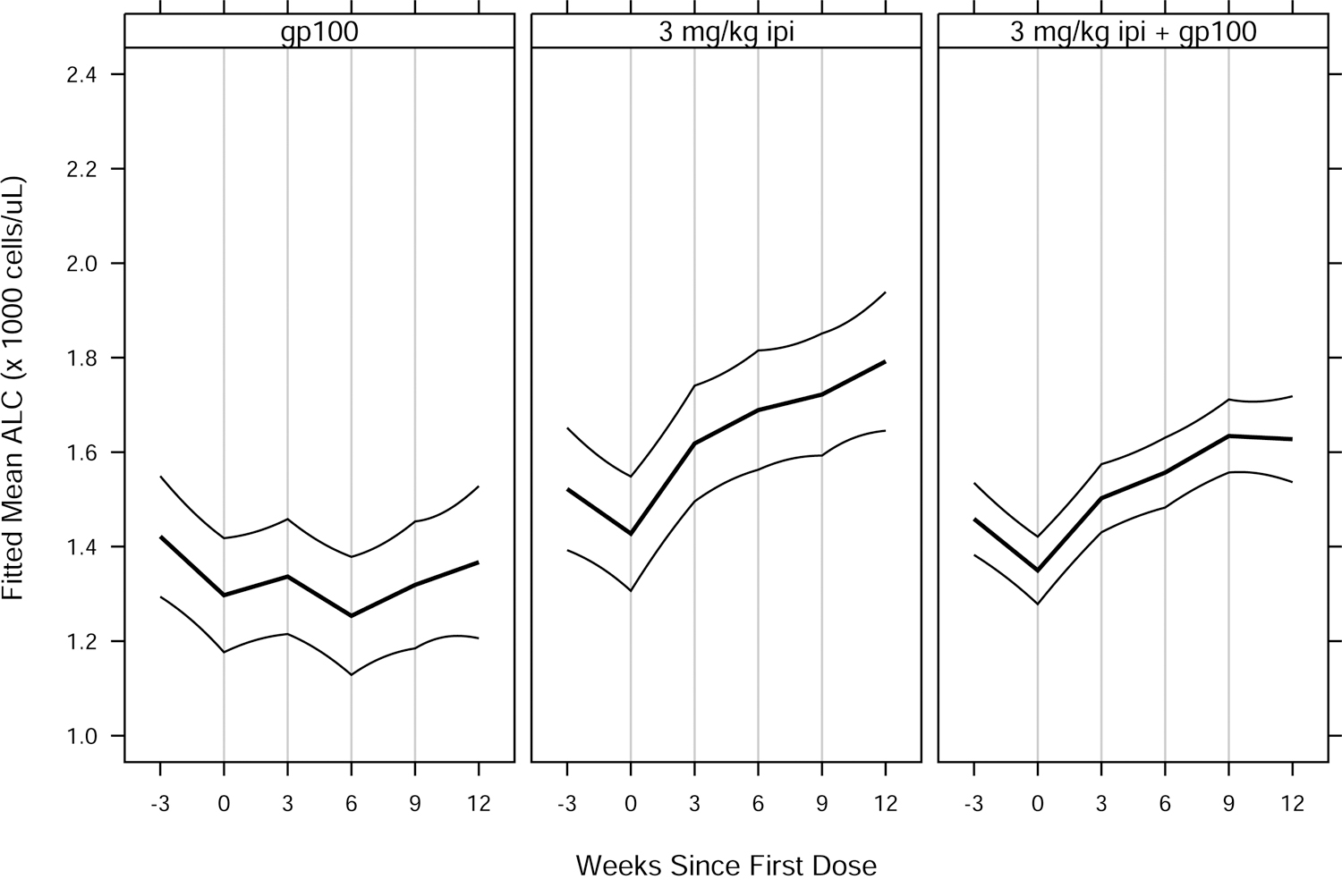
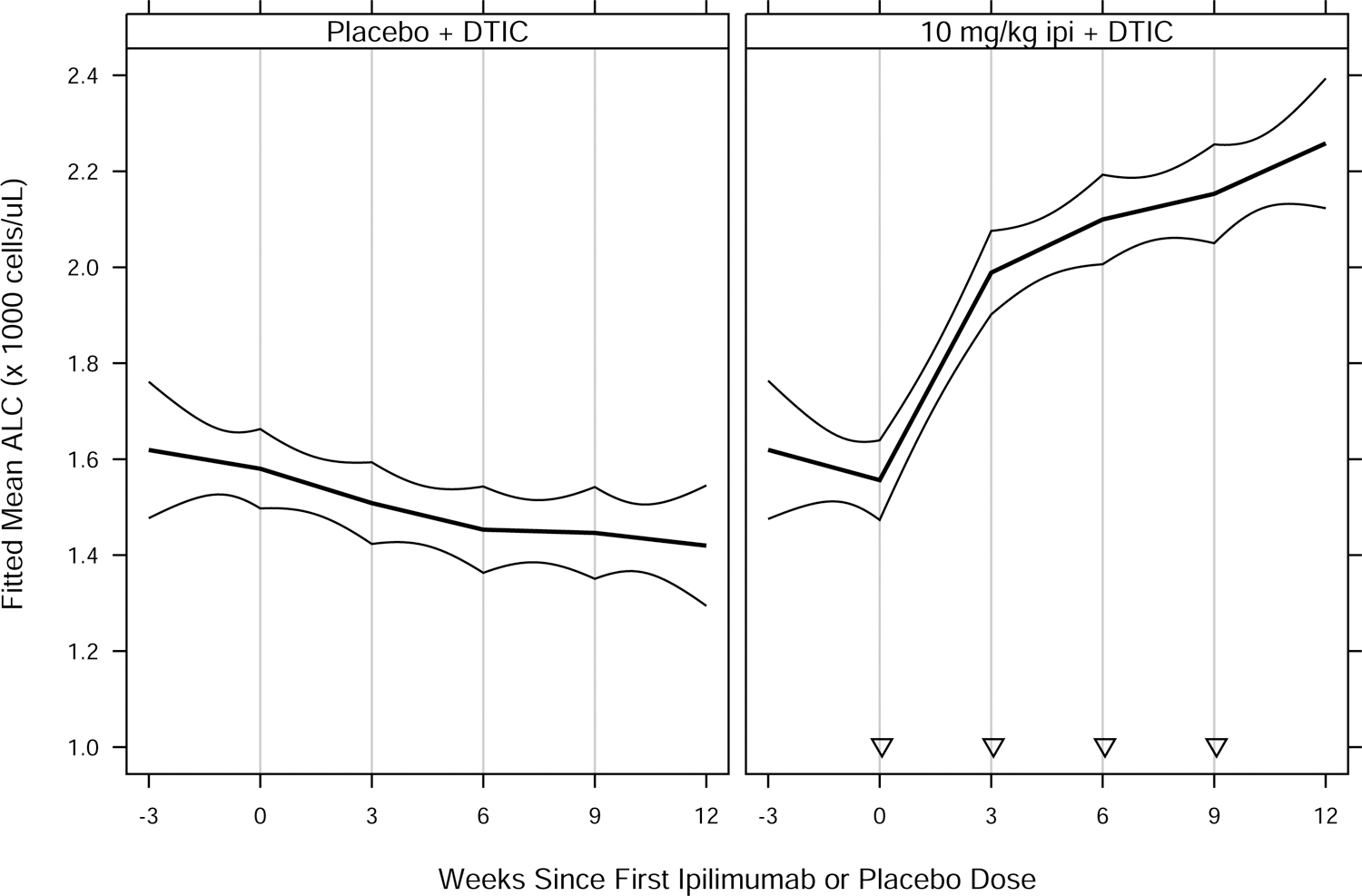
Fitted mean ALC vs. weeks since first ipilimumab or placebo dose, by treatment group for (A) MDX010–20; and (B) CA184–024. Thick curves show fitted means and thin curves show pointwise two-sided 95% confidence intervals for the means. Vertical lines show nominal dosing days. Inverted triangles in (B) indicate ipilimumab doses, in contrast to placebo doses. Patients included in the analyses — 639 of 643 (MDX010–20) and 497 of 498 (CA184–024) — had ≥ 1 ALC assessment in the period from 28 days prior to a confirmed date of treatment initiation to 28 days after dose 4 of study drug. For each study, estimated means and confidence intervals are from an extended linear model, fit by restricted maximum likelihood. The model had fixed effects of treatment group and days since first dose (DSFD). The effect of DSFD was modeled by linear regression splines with, for each patient, a knot at the actual day of each dose, and interaction between treatment group and the splines. Within-patient correlations were modeled by a spatial exponential structure with Euclidean distance, and within-patient variances were inversely proportional to the number of ALC measurements on a given day. DTIC = dacarbazine; gp100 = glycoprotein 100 peptide vaccine; ipi = ipilimumab.
Conclusions
Our results demonstrated that ALC values were associated with OS in the two randomized, phase III studies as a prognostic factor [3,4]. Since the ALC associations with OS were not specific to ipilimumab, we are now able — for the first time — to conclude that ALC is not a predictive factor for ipilimumab treatment benefit over the control agents tested in these trials. It is possible these studies were underpowered to definitively exclude ALC as predictive of treatment benefit. Nonetheless, in the largest dataset yet available from randomized trials, we did not find convincing evidence for ALC as a predictive marker.
The evidence of ALC as a prognostic biomarker underscores the immune system’s importance in the general outcome of patients with advanced melanoma. Yet the search for predictive biomarkers for ipilimumab and ipilimumab combination immunotherapy remains challenging. It is possible that a collection of biomarkers, including readily available routine peripheral blood tests such as the neutrophil to lymphocyte ratio and tissue-based assays, will ultimately be shown to be predictive for ipilimumab or ipilimumab combination immunotherapy [7], but this will require evaluation in randomized trials as we have performed for ALC in this manuscript.
As initial studies suggest possible association with OS or other outcomes, we advocate for rigorous testing of putative biomarkers’ predictive ability in randomized, controlled immunotherapy trials. These studies would be of even greater interest in more recent anti-PD1 randomized trials such as the Checkmate 067 study. The ultimate identification of predictive biomarkers will become increasingly important as ipilimumab combination regimens expand influence in the treatment of patients with many different malignancies beyond melanoma [8,9].
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, Ludwig Collaborative and Swim Across America Laboratory, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Parker Institute for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Weill Cornell Medical College, New York, NY 10065, USA. We thank David M. Berman, MD, PhD, for contributions to the analyses and interpretation of the data. Professional editorial assistance was provided by Ward A. Pedersen, PhD, at StemScientific, an Ashfield Company, and was funded by Bristol-Myers Squibb.
Conflicts of Interest and Source of Funding: Dr. Postow has received consulting fees from 2015-present from Bristol-Myers Squibb, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, and Aduro; honoraria from Bristol-Myers Squibb and Merck; and institutional support from RGenix, Infinity, Bristol-Myers Squibb, Merck, Array BioPharma, and Novartis. Dr. Panageas discloses stock ownership in the following companies: Catalyst Biotech, Johnson & Johnson, Pfizer, and Viking Therapeutics. Dr. Wolchok has been a consultant for Adaptive Biotech, Advaxis, Amgen, Apricity, Array BioPharma, Ascentage Pharma, Astellas, Bayer, Beigene, Bristol-Myers Squibb, Celgene, Chugai, Elucida, Eli Lilly, F Star, Genentech, Imvaq, Janssen, Kleo Pharma, MedImmune, Merck, Neon Therapeutics, Ono, Polaris Pharma, Polynoma, Psioxus, Puretech, Recepta, Trieza, Sellas Life Sciences, Serametrix, Surface Oncology, and Syndax; has received research support from Bristol-Myers Squibb, MedImmune, Merck Pharmaceuticals, and Genentech; and has equity in Potenza Therapeutics, Tizona Pharmaceuticals, Adaptive Biotechnologies, Elucida, Imvaq, Beigene, Trieza. Dr. Wolchok also discloses the following patents: Xenogeneic DNA Vaccines, ALPHAVIRUS REPLICON PARTICLES EXPRESSING TRP2, Myeloid-derived suppressor cell (MDSC) assay, NEWCASTLE DISEASE VIRUSES FOR CANCER THERAPY, Genomic Signature to Identify Responders to Ipilimumab in Melanoma, Engineered Vaccinia Viruses for Cancer Immunotherapy, Anti-CD40 agonist mAb fused to Monophosphoryl Lipid A (MPL) for cancer therapy, and CAR+ T cells targeting differentiation antigens as means to treat cancer. The study was supported by Bristol-Myers Squibb.
Footnotes
Data Sharing:
Bristol-Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 5.Ku GY, Yuan J, Page DB, Schroeder SEA, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010; 116:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013; 24:1697–1703. [DOI] [PubMed] [Google Scholar]
- 7.Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral blood clinical laboratory variables associated withoutcomes following combination nivolumab and ipilimumab immunotherapy. Cancer Med 2018; 7:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]


