Abstract
In a pooled population analysis, we investigated the pharmacokinetics of i.v. anidulafungin in four studies across a full range of adult and pediatric ages in patients with confirmed, suspected, or at high risk of invasive candidiasis (IC). Relationships between anidulafungin exposure and key efficacy end points (global response of success and all‐cause mortality) and safety end points (all‐cause hepatic or gastrointestinal adverse events) in all patients and separately in pediatric patients and the appropriate dosing regimen for IC treatment in pediatric patients were evaluated. Pediatric patients received a 3.0 mg/kg (maximum 200 mg) i.v. loading dose and 1.5 mg/kg (maximum 100 mg) daily thereafter. Adults received a 200 mg i.v. loading dose and 100 mg daily thereafter. Estimated systemic anidulafungin exposures were similar across age groups (neonates to adults) at the weight‐based doses studied in pediatric patients. No clear associations were identified between anidulafungin exposure and efficacy or safety end points.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Guidelines on treating pediatric invasive candidiasis (IC) are largely based on adult studies. A previous analysis of weight‐based anidulafungin treatment (1.5 mg/kg/day) in children at risk for fungal infections showed an anidulafungin concentration profile similar to that in adults receiving 100 mg/day.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This pooled population analysis investigated the pharmacokinetics of anidulafungin in pediatric and adult patients with suspected or confirmed IC, relationships between anidulafungin exposure and efficacy and safety end points, and the suitability of the 1.5 mg/kg maintenance dosing regimen for treating pediatric IC.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Estimated anidulafungin exposures in pediatric patients were within the range of those in adults at the approved dose and were not related to efficacy or significantly related to safety end points.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ An anidulafungin i.v. dosing regimen for IC (3.0 mg/kg loading dose followed by a daily maintenance dose of 1.5 mg/kg, maximum 100 mg) is proposed for children from 1 month of age to match adults.
Invasive candidiasis (IC) is a common fungal disease that affects upward of 250,000 people globally per year, with > 50,000 cases resulting in death. 1 The incidence of candidemia is age‐related, with the highest rates at the extremes of the lifespan. 1 In one study in the United States, candidemia was diagnosed in 43 of 100,000 pediatric hospital admissions and 30 of 100,000 adult admissions, with attributable mortality rates of 10% and 14.5%, respectively. 2 Although IC‐attributable mortality rates are generally lower in pediatric patients vs. adults, 2 , 3 rates of up to 29% and 40% have been reported in children (> 28 days and < 18 years of age) and neonates (≤ 28 days), respectively. 2 , 3 , 4 In addition to increased duration of hospital stays and healthcare costs in patients with IC vs. those without, 2 invasive fungal infections can also lead to long‐term neurodevelopmental impairment in preterm infants, 5 , 6 including cerebral palsy. 5
Guidelines from the Infectious Diseases Society of America and the European Society of Clinical Microbiology and Infectious Diseases recommend echinocandins for the first‐line treatment of IC in adults. 7 , 8 , 9 Because of a lack of well‐controlled pediatric studies, 9 recommendations for the treatment of IC in pediatric patients are largely based on the results of adult studies. 10 However, amphotericin B, echinocandins, and azoles can be used. 9 , 10 Of note, the pathophysiology of IC in neonates and premature infants differs from that in older children and adults, and hematogenous Candida meningoencephalitis (HCME) is common. 11 , 12
Anidulafungin is an i.v. echinocandin that is synthesized from a fermentation product of Aspergillus nidulans. It is approved for the treatment of IC in adults worldwide, with an adult dosing regimen of a 200 mg loading dose (LD) followed by a 100 mg maintenance dose (MD) once daily. 13 Anidulafungin is not metabolized by the liver, but rather undergoes slow chemical degradation in the blood at physiologic temperature and pH, and biliary excretion without renal involvement. 13 , 14 The pharmacokinetics (PKs) of anidulafungin have been previously evaluated in healthy adults, 15 adults with renal or hepatic impairment, 16 adults with serious fungal infections, 17 and in children (aged 2–17 years) with neutropenia, 18 neonates, and infants (aged < 30 and ≥ 30 postnatal days, respectively) 12 at high risk for invasive fungal infections. Anidulafungin has been shown to exhibit linear and predictable PKs in all of these populations, consistent with the clearance mechanism of the drug, with comparable exposures in adults with or without hepatic or renal insufficiency 16 and in children, 18 neonates, and infants 12 receiving weight‐adjusted doses. Animal studies have suggested a dose‐dependent penetration of anidulafungin into the central nervous system with rapid antifungal activity in infected cerebrums, indicating possible efficacy against HCME. 19
The PKs, safety, and efficacy of anidulafungin were evaluated in a recently completed phase IIIb, prospective, open‐label, noncomparative study in children aged 1 month to < 18 years of age with confirmed or at high risk of IC (study 1, NCT00761267). 20 , 21 Here, we describe the PKs of anidulafungin in a pooled population analysis in the first analysis of this type across a full range of ages, from neonates to older adults, in patients with confirmed, suspected, or at high risk of IC. Exposure‐response analyses in patients aged from 1 month to older adults with confirmed IC are also reported.
METHODS
Study design and patients
Pooled data from four studies were included in this analysis, including one pediatric (NCT00761267; study 1) clinical trial in patients with confirmed or at high risk of IC, and two adult (NCT00496197 and NCT00689338; studies 2 and 3, respectively) clinical trials in patients with confirmed IC, and one pediatric investigator‐initiated research (IIR) PK study in infants and neonates aged < 24 months with suspected IC, conducted by Duke University. Details of the study populations, study designs, and dosing regimens are presented in Supplementary Table S1. In these studies, all pediatric patients (< 18 years of age) received i.v. anidulafungin at a 3.0 mg/kg (maximum 200 mg) LD followed by 1.5 mg/kg (maximum 100 mg) MD q.d. for a minimum of 5 or 10 days in patients with suspected or microbiologically confirmed IC, respectively, in study 1, or 3–5 days in the pediatric IIR study, and all adults (≥ 18 years of age) received a 200 mg i.v. LD followed by 100 mg i.v. MD q.d. for a minimum of 5–10 days.
These studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines established by the International Council on Harmonization and approved by the Institutional Review Boards and Independent Ethics committees of the participating centers. Written informed consent, or assent where applicable, was obtained from all patients or their parents/guardians as appropriate.
The objectives of the pooled PK analysis of data from all four studies were to characterize the PKs of anidulafungin in pediatric and adult patients with confirmed, suspected, or at high risk of IC, and to predict individual exposure parameters based on the final PK parameter estimates. In addition, we sought to explore the relationship between anidulafungin exposure and key efficacy and safety end points in patients with confirmed IC from the three clinical studies, and to confirm the appropriate dosing regimen for treatment of IC in pediatric patients.
Study endpoints
Pharmacokinetics
Pooled data from all four studies were included in the population PK analysis. The sampling schedule for each study is detailed in Supplementary Table S2. Plasma samples were stored at −20°C or colder after collection and analyzed periodically at a centralized laboratory (PPD, Richmond, VA) using a validated high‐performance liquid chromatography‐tandem mass spectrometric method in compliance with Pfizer standard operating procedures. The bioanalytical assay had a dynamic range of 50–20,000 μg/L and a lower limit of quantification of 50 μg/L. For the three clinical studies, the between‐day assay accuracy (expressed as percent relative error) for quality control concentrations was ≤ 4.17%. Assay precision, expressed as the between‐day percent coefficient of variation of the mean estimated concentrations of quality control samples, was ≤ 8.68%.
The population PK analyses were conducted using a nonlinear mixed‐effects modeling approach (NONMEM version 7.3) and first‐order conditional estimation method with interaction. A previously described phase II/III PK two‐compartment model with first‐order elimination 17 , 22 was used as the base disposition model to fit the concentration data from all patients. Body weight was incorporated as a structural covariate on clearance (CL), central volume of distribution (V c), and peripheral volume of distribution (V p), based on the results of previous analyses, 12 , 18 , 22 and was expressed as power or linear functions in the base model, and the relationships were re‐evaluated with standard allometry and then as estimated power functions. The influence of additional covariates (age and sex) on anidulafungin PK was assessed during a subsequent covariate evaluation step. The final model was evaluated using the following criteria: Goodness‐of‐fit plots, plausibility of PK parameter estimates, precision of parameter estimates, objective function values, and visual predictive check.
Estimated systemic exposure parameters area under the curve (AUC) over a 24‐hour dosing interval at steady state (AUC0–24,ss) and trough (minimum) concentration at steady state (Cmin,ss) were calculated based on the MD administered to each patient using individual empirical Bayesian estimates from the final PK model and the equations listed in the Supplementary Information . Data were summarized by age groups across all four studies as well as for the pediatric clinical study (study 1) alone. Estimated systemic anidulafungin exposures (AUC0–24,ss) were also examined graphically by body weight in five quantile groups).
Exposure‐response relationship for efficacy and safety
Exposure‐response efficacy and safety analyses included data only from patients with PK samples in the three clinical studies (studies 1–3). Data from the pediatric IIR study, which was primarily designed as a PK study with short treatment duration, were excluded from these analyses.
Exposure‐response efficacy and safety graphical analyses were used to estimate the probability of response. Estimated systemic anidulafungin exposures (AUC0–24,ss) were examined graphically for five quantile groups (AUC0–24,ss quantile ≤ 20%, > 20–40%, > 40–60%, > 60–80%, and > 80%) across all three clinical studies and also separately for study 1. Once graphical analyses had demonstrated a possible exposure‐response relationship, exposure‐response logistic regression modeling was performed for efficacy and safety end points using the maximum likelihood method (LAPLACE estimation).
Exposure‐response efficacy
Patients within the modified intent‐to‐treat population, consisting of patients with confirmed Candida infection who received at least one dose of anidulafungin, and who had estimated systemic exposure parameters and efficacy data available, were included in the exposure‐response analysis for efficacy.
Efficacy end points included global response (success vs. failure) at the end of i.v. treatment and end of treatment (EOT), and all‐cause mortality (shown as survival vs. death) at EOT and end of study (EOS), including a follow‐up period. Global response of success was defined as resolution of signs and symptoms of IC and no need for additional systemic antifungal therapy, plus eradication or presumed eradication of Candida species present at baseline. Patients with an indeterminate response were excluded from the global response analysis.
Exposure‐response safety
All patients who received at least one dose of anidulafungin and had estimated PK exposure data available were included in the safety population for the exposure‐response safety analysis. This analysis focused only on the incidence of all‐cause hepatic and gastrointestinal (GI) adverse events (AEs) during the anidulafungin i.v. treatment period.
Hepatic AEs included hepatobiliary disorders (cholestasis, hepatitis acute, hepatomegaly, hyperbilirubinemia, and ocular icterus) and abnormal laboratory investigations (alanine aminotransferase or aspartate aminotransferase increased, γ‐glutamyltransferase increased, liver function tests abnormal/increased, prothrombin time prolonged, and transaminases increased) reported as AEs by the investigator. GI AEs included nausea, vomiting, diarrhea, abdominal pain, and abdominal distension. Data were treated as binary data (a patient had or did not have a relevant AE) for probability analyses with an assumption of independence of hepatic and GI AEs. Patients who had both GI and hepatic AEs were kept in both the hepatic and GI AE analysis groups; for analysis of hepatic AEs, patients reporting only GI AEs were treated as non‐AE patients and vice versa. Patients who did not experience hepatic or GI AEs and those who experienced AEs of interest within the post‐anidulafungin treatment period were included as non‐AE patients in the analysis.
Further analysis of hepatic and GI AEs was performed using the estimated individual systemic exposure parameters AUC0–24,ss and Cmin,ss in a proportional odds model (logistic regression). Linear and maximum effect (Emax) exposure effect relationships were investigated, with covariates of interest being age, sex, and analysis population (pediatric or adult).
RESULTS
Pharmacokinetics
A total of 163 patients (95 men (58.3%) and 68 women (41.7%)) from four studies were included in the population PK analysis (Table 1 and Supplementary Table S1). The median age of the overall population was 21 years and ranged from premature infants (1.8 days of age) to older adults (81 years of age). From this population, 14 patients from the pediatric IIR PK study, 66 pediatric patients from study 1, and 83 adults from studies 2 and 3 provided 797 anidulafungin plasma concentration data points, with 391 (49.1%) from pediatric patients. An intensive sampling schedule for PK analysis was undergone by six infants (1 month to < 2 years of age) in study 1, 22 adults in study 3, and all patients in the pediatric IIR study (Supplementary Table S2).
Table 1.
Patient demographics and characteristics
| Characteristic | Study 1 (n = 66) | Study 2 (n = 33) | Study 3 (n = 50) | Pediatric IIR (n = 14) | Total (N = 163) |
|---|---|---|---|---|---|
| Number of PK samples | 281 | 123 | 283 | 110 | 797 |
| Male, n (%) | 36 (54.55) | 20 (60.61) | 30 (60) | 9 (64.29) | 95 (58.28) |
| Race, n (%) | |||||
| White | 52 (78.79) | 26 (78.79) | 48 (96) | 10 (71.43) | 136 (83.44) |
| Black | 1 (1.52) | 4 (12.12) | 1 (2) | 4 (28.57) | 10 (6.13) |
| Asian | 6 (9.09) | 2 (6.06) | 0 (0) | 0 (0) | 8 (4.91) |
| Other | 7 (10.61) | 1 (3.03) | 0 (0) | 0 (0) | 8 (4.91) |
| Missing | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Age, years | |||||
| Mean (SD) | 5.9 (5.14) | 53.2 (18.83) | 58.2 (13.74) | 82.1 (124.10) a | 31.0 (28.38) |
| Median (range) | 4 (0.10–17) | 59 (20–81) | 58.5 (25–80) | 26.3 (1.8–451.1) a | 21 (1.8 a –81) |
| Body weight, kg | |||||
| Mean (SD) | 23.0 (19.09) | 77.8 (19.37) | 73.8 (27.31) | 3.2 (2.61) | 48.0 (35.51) |
| Median (range) | 16.6 (2.31–85.7) | 77.2 (37.8–122.1) | 70.0 (48.0–240.0) | 2.8 (0.75–9.47) | 54.0 (0.75–240.0) |
PK, pharmacokinetics; SD, standard deviation.
Age, days.
Anidulafungin PK was best characterized by a two‐compartment disposition model with first‐order elimination. Body weight was considered a structural covariate on CL, V c, and V p, with estimated power functions (percentage relative standard error (%RSE)) of 0.92 (2.98), 1.20 (4.95), and 0.77 (6.04), respectively. No other covariates (e.g., chronological age and sex) were identified as statistically significant. Race was not investigated because the majority of patients (83.4%) were white (Table 1 ) and no ethnic effect was identified in previous population PK studies. 17 , 22 The concentration data across the studies were adequately characterized by the final model (Figure 1 ). For a typical patient weighing 70 kg, the estimated (%RSE) CL, V c, V p, and intercompartmental CL were 1.16 (3.92) L/hour, 26.7 (6.5) L, 22.4 (12.9) L, and 2.37 (15.2) L/hour, respectively, with interindividual variability of 37.9%, 46.5%, 53.8%, and 52.2%, respectively. The residual variability was 21.2% (%RSE 6.77; Supplementary Table S3).
Figure 1.
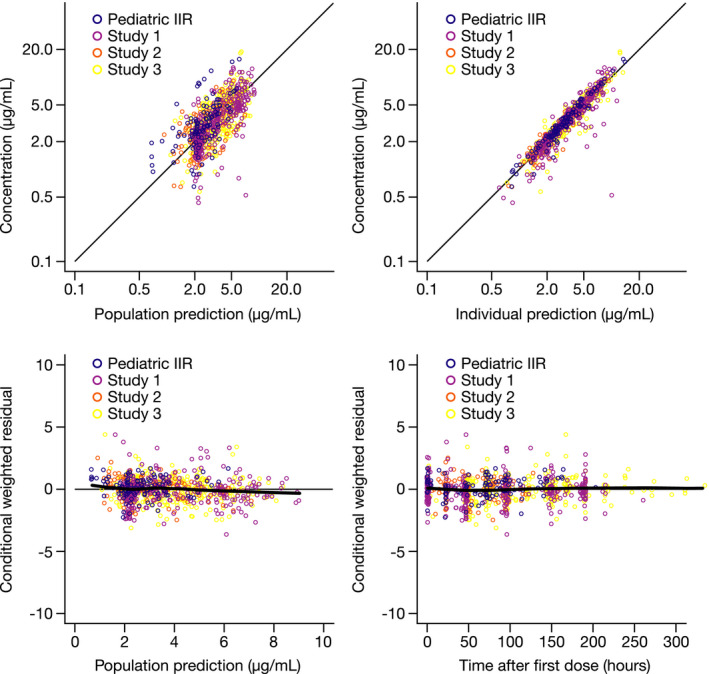
Goodness‐of‐fit plots for the final model by study. IIR, investigator‐initiated research. [Colour figure can be viewed at wileyonlinelibrary.com]
The final model generally predicted the data well across the studies, with the exception of underprediction in the pediatric IIR study, which consisted of a heterogeneous patient cohort with large observed variability. There was a good match between the median and 5th and 95th percentiles for observed and simulated data in all clinical studies, as indicated by a visual predictive check by study (Figure 2 ), and across the age groups, as indicated by a prediction‐corrected visual predictive check plot (Supplementary Figure S1). The estimated mean AUC0–24,ss for MDs (adult, 100 mg q.d.; pediatric, 1.5 mg/kg q.d.) ranged from 80.77 µg*hour/mL for neonates (aged < 30 days) to 95.24 µg*hour/mL for adults (≥ 65 years of age; Table 2 ). Estimated systemic exposures (AUC0–24,ss and Cmin,ss) and weight‐corrected mean PK parameters were comparable across the age groups, with the exception of volume of distribution at steady state, which was slightly higher in neonates, although generally consistent with the distribution range in the other groups (Table 2 ). Stratifying the data by body weight revealed lower exposures at extremes of body weight, with an overlap in the estimated AUC0–24,ss for MDs at body weights < 30 and ≥ 77 kg (Supplementary Figure S2).
Figure 2.
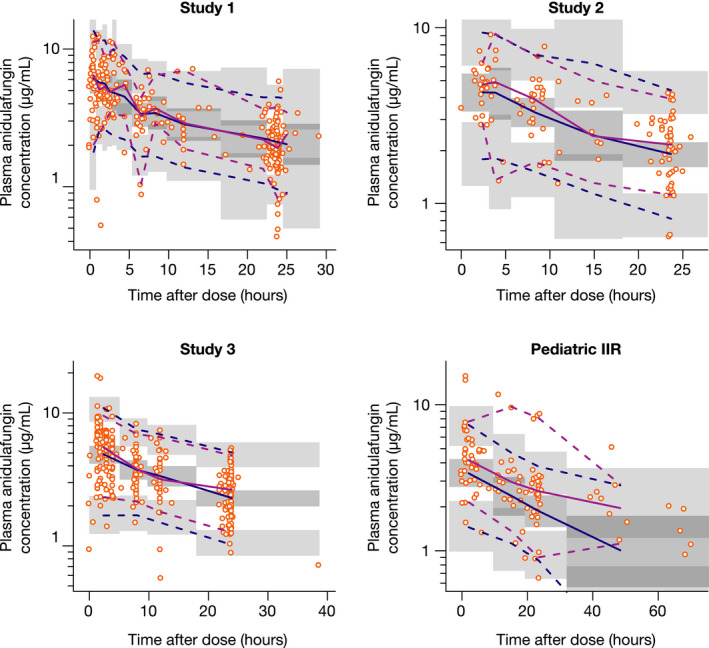
Visual predictive check for the final model, stratified by study, vs. time after dose. Dashed lines represent the 90% confidence intervals (95% upper limits and 5% lower limits) of observed and simulated data, respectively. Solid lines are medians (50%) of observed and simulated data, respectively. Dots represent observed data. Shadow areas are 95% confidence intervals for the 2.5th, 50th, and 97.5th percentile prediction intervals based on simulated data. IIR, investigator‐initiated research. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 2.
Summary of estimated systemic anidulafungin exposure and PK parameters in pediatric and adult patients by age groups (LD/MD: 3.0/1.5 mg/kg q.d. for pediatric patients and 200/100 mg q.d. for adults)
| Age group | AUC0–24,ss, μg*hour/mL | Cmin,ss, μg/mL | CL, L/hour/kg | Vss, L/kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (range) | 90% CI | Mean (SD) | Median (range) | 90% CI | Mean (SD) | Median (range) | 90% CI | Mean (SD) | Median (range) | 90% CI | |
| < 30 days; n = 8 | 80.77 (28.03) | 83.87 (29.27–124.97) | 41.68, 114.78 | 2.64 (0.99) | 2.59 (0.81–4.09) | 1.28, 3.88 | 0.023 (0.013) | 0.020 (0.012–0.053) | 0.014, 0.042 | 1.10 (0.41) | 1.02 (0.60–1.68) | 0.62, 1.66 |
| 1 month to < 2 years; n = 23 | 82.91 (43.74) | 67.52 (49.09–260.64) | 50.13, 111.98 | 2.46 (1.44) | 1.92 (1.17–8.12) | 1.27, 3.69 | 0.021 (0.006) | 0.021 (0.006–0.032) | 0.013, 0.030 | 0.77 (0.23) | 0.75 (0.25–1.32) | 0.48, 1.27 |
| 2 to < 5 years; n = 19 | 82.81 (31.90) | 80.01 (35.80–161.65) | 38.55, 138.86 | 2.51 (1.11) | 2.46 (1.01–5.36) | 1.02, 4.32 | 0.021 (0.009) | 0.021 (0.009–0.049) | 0.012, 0.035 | 0.90 (0.28) | 0.88 (0.59–1.72) | 0.63, 1.49 |
| 5 to < 18 years; n = 30 | 86.77 (31.12) | 80.49 (28.30–197.79) | 57.85, 124.45 | 2.52 (0.96) | 2.34 (0.62–5.90) | 1.59, 3.76 | 0.020 (0.008) | 0.019 (0.009‐0.053) | 0.012, 0.027 | 0.75 (0.16) | 0.78 (0.50–1.11) | 0.51, 0.95 |
| 18 to < 46 years; n = 20 | 83.80 (25.50) | 75.77 (47.80–139.01) | 54.67, 127.48 | 2.44 (0.81) | 2.15 (1.35–4.21) | 1.54, 3.94 | 0.019 (0.008) | 0.017 (0.010–0.035) | 0.010, 0.035 | 0.79 (0.24) | 0.73 (0.42–1.32) | 0.49, 1.16 |
| 46 to < 65 years; n = 36 | 92.07 (39.80) | 86.27 (39.77–231.81) | 42.73, 153.44 | 2.71 (1.23) | 2.54 (1.16–6.91) | 1.29, 4.76 | 0.017 (0.006) | 0.016 (0.009–0.031) | 0.009, 0.027 | 0.72 (0.19) | 0.69 (0.34–1.19) | 0.46, 1.11 |
| ≥ 65 years; n = 27 | 95.24 (45.95) | 88.79 (30.80–246.90) | 43.61, 162.22 | 2.89 (1.68) | 2.60 (0.87–9.06) | 1.24–5.37 | 0.017 (0.006) | 0.015 (0.007–0.030) | 0.009, 0.027 | 0.76 (0.25) | 0.72 (0.46–1.49) | 0.47, 1.13 |
AUC0–24,ss, area under the curve over a 24‐hour dosing interval at steady state (maintenance dose); CI, confidence interval; CL, clearance; Cmin,ss, minimum concentration at steady state (maintenance dose); LD, loading dose; MD, maintenance dose; PK, pharmacokinetic; q.d., once daily; SD, standard deviation; V ss, volume of distribution at steady state.
Exposure‐response efficacy
Among 484 patients (64 pediatric and 420 adult) in the modified intent‐to‐treat populations from studies 1–3, a total of 134 patients (62 pediatric and 72 adult) had paired estimated PK parameter and efficacy data available and were included in the exposure‐response efficacy analyses. Of these, in studies 1, 2, and 3, respectively, the rate of global response of success at EOT was 72.6%, 83.9%, and 68.3%; furthermore, 88.7%, 96.8%, and 73.2% were alive at EOS.
Graphical quantile analysis of estimated systemic anidulafungin AUC0–24,ss data from all three clinical studies and from study 1 alone showed that the probability of treatment failure (Figure 3a ) or death (Figure 3b ) did not seem to be related to low systemic anidulafungin exposures. As there was no clear association observed between systemic anidulafungin exposure quantiles and all‐cause mortality or treatment failure, no further exposure‐response efficacy modeling analysis was performed.
Figure 3.
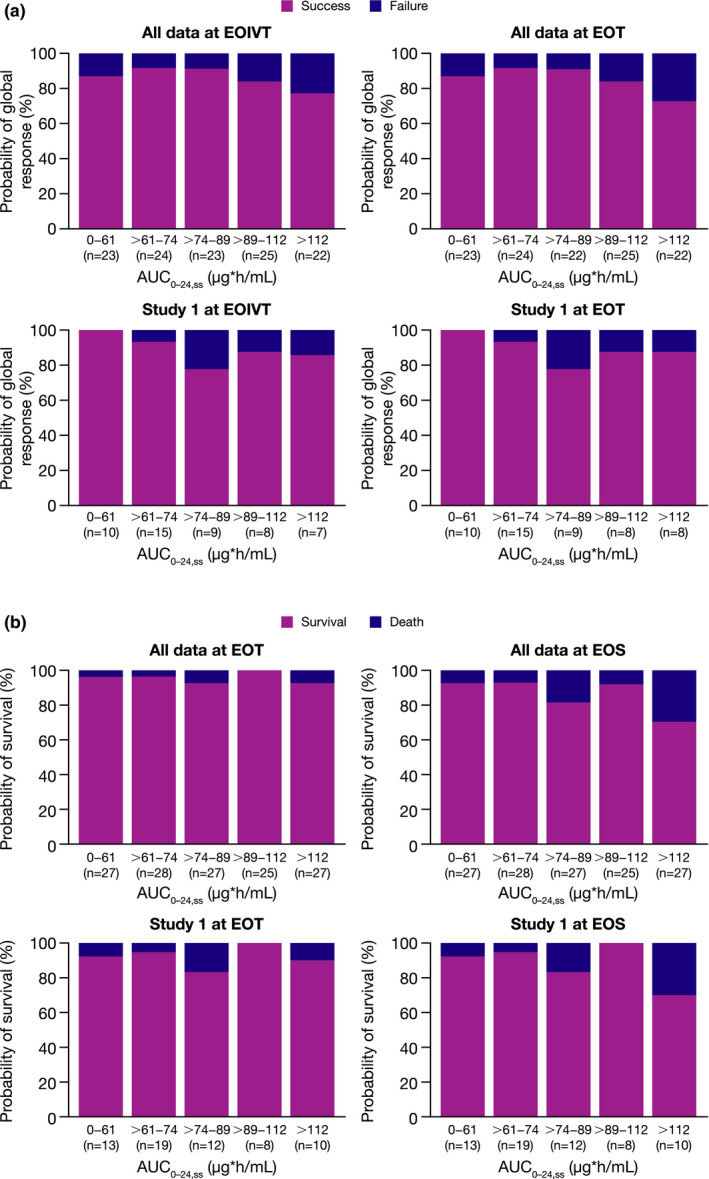
Probability of global response of success or all‐cause mortality for all three clinical studies and for study 1 alone. (a) Global response of success at EOIVT and EOT and (b) all‐cause mortality (shown as survival at EOT and EOS vs. anidulafungin AUC0–24,ss). Global response of success was defined as resolution of signs and symptoms of invasive candidiasis and no need for additional systemic antifungal therapy, plus eradication or presumed eradication of Candida species present at baseline. Patients with an indeterminate response were excluded. Data include patients with exposure data from the modified intent‐to‐treat populations, defined as all patients with confirmed Candida infection who received at least one dose of study drug. AUC0–24,ss, area under the curve over a 24‐hour dosing interval at steady state; EOIVT, end of intravenous therapy; EOS, end of study, including follow‐up period; EOT, end of treatment. [Colour figure can be viewed at wileyonlinelibrary.com]
Exposure‐response safety
Among 566 patients (68 pediatric and 498 adult) in the overall safety populations from studies 1–3, a total of 149 patients (66 pediatric and 83 adult) had paired estimated PK parameter and safety data available and were included in the exposure‐response safety analyses. Of these, in studies 1, 2, and 3, respectively, 48.5%, 78.8%, and 62.0% of patients had neither hepatic nor GI AEs; 18.2%, 9.1%, and 14.0% had all‐cause hepatic AEs; and 40.9%, 18.2%, and 30.0% had all‐cause GI AEs.
Graphical quantile analysis of systemic anidulafungin exposure parameter AUC0–24,ss as a potential predictor for hepatic and GI AEs revealed a slight trend toward an increased incidence of all‐cause hepatic AEs with higher estimated systemic exposure in the pooled data from all three clinical studies, but not in study 1 alone (Figure 4 ). There was no apparent relationship between estimated systemic exposure and all‐cause GI AEs.
Figure 4.
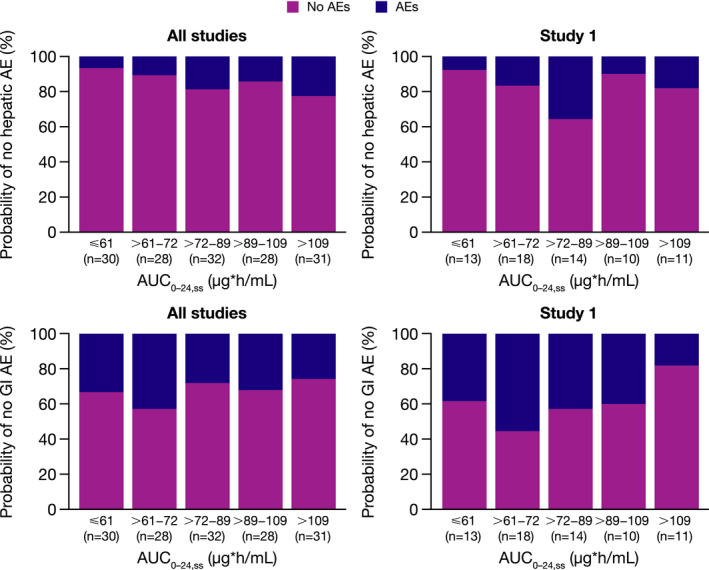
Probability of all‐cause hepatic or GI AEs during i.v. anidulafungin treatment vs. anidulafungin AUC0–24,ss for all three clinical studies and for study 1. Data include patients with exposure data from the safety populations, defined as all patients with confirmed Candida infection who received at least one dose of study drug. AE, adverse event; AUC0–24,ss, area under the curve over a 24‐hour dosing interval at steady state; GI, gastrointestinal. [Colour figure can be viewed at wileyonlinelibrary.com]
Neither AUC0–24,ss nor Cmin,ss was identified as a statistically significant predictor for the incidence of all‐cause hepatic or GI AEs using logistic regression. Furthermore, none of the exposure parameters was identified as a statistically significant predictor for hepatic AE incidence using either the linear effect relationship or Emax model, and no other covariates of interest (i.e., age, sex, or analysis population (pediatric or adult)) were identified as statistically significant on the linear drug‐effect model.
DISCUSSION
Results of this pooled population PK analysis showed that estimated systemic anidulafungin exposures were similar across age groups, from premature infants to older adults, receiving the same weight‐based (patients < 18 years of age) or fixed (patients ≥ 18 years of age) dosing regimens. The estimated mean AUC0–24,ss ranged from 80.77 μg*hour/mL at a pediatric MD of 1.5 mg/kg q.d. to 95.24 μg*hour/mL at an adult MD of 100 mg q.d., with comparable exposure ranges in pediatric and adult patients. Across the age groups, the CL and volume of distribution at steady state (V ss) ranged from 0.017–0.023 L/hour/kg and 0.72–1.1 L/kg, respectively. When given the same weight‐normalized dose, the estimated systemic exposures are generally within the range of those reported previously for overlapping populations. In pediatric patients aged 2–11 and 12–17 years old at high risk for invasive fungal infections, the mean AUCss was reported as 96.1 and 102.9 mg*hour/mL, the mean CL was reported as 0.016 and 0.016 L/hour/kg, and the mean V ss was reported as 0.42 and 0.45 L/kg, respectively. 18 In neonatal and infant patients at high risk for invasive fungal infections, the median AUCss was 74.9 and 97.7 μg*hour/mL, the mean CL was 0.020 and 0.015 L/kg/hour, and the mean V ss was 1.7 and 0.9 L/kg, respectively, 12 and in adult patients with serious fungal infections, the mean AUCss was reported as 110.3 μg*hour/L and the mean CL was reported as 1.0 L/hour. 22
Anidulafungin undergoes slow chemical degradation in the blood at physiological pH and temperature. 13 , 14 With a lack of ontogenic influence in the chemical degradation, and because of negligible renal CL (< 1%) and lack of hepatic metabolism, 14 the anidulafungin pediatric dose can be predicted from the adult dose by adjusting for size using body weight on a linear scale. As expected given the nonrenal clearance mechanism, 14 after weight adjustment on CL, V c, and V p, neither age nor sex was identified as significant covariates on either CL or V c in the final PK model. The CL and V c exponents were 0.92 and 1.2, supporting a near‐linear relationship to body weight in this population over a very wide weight range (0.75–240.0 kg). However, stratifying anidulafungin AUC0–24,ss by body weight revealed a trend toward lower exposures at extremes of body weight. Compared with patients weighing 30–77 kg, the lower exposure in the small group of patients with body weight ≥ 77 kg is consistent with adults receiving fixed LD/MD of 200/100 mg, irrespective of body weight. The results of previous studies have suggested increasing anidulafungin LDs and MDs in morbidly obese adult patients to augment exposure. 23 , 24 Our results also showed a trend toward a lower median exposure in patients weighing < 30 kg; however, a target AUC for efficacy was not determined in a previous study and lower AUC (< 40 mg*hour/L) did not impact on treatment success. 22
Exposure‐response efficacy analyses showed no relationship between systemic anidulafungin exposure measures and global response or all‐cause mortality at end of i.v. treatment, EOT, or EOS. Patients in study 1, and in all data combined, had a similar probability of efficacy at low estimated systemic exposures, and pediatric patients did not have a greater incidence of hepatic or GI AEs at higher exposures. The lack of an exposure‐response relationship is not surprising because the studies were not designed as dose‐ranging. Doses were chosen to be efficacious in the severely ill populations included, with pediatric dosing designed to provide similar exposures to those achieved in the adult studies with a fixed LD/MD of 200/100 mg (approved dose). The lack of any exposure‐response for both efficacy and safety across the pooled population supports the proposed weight‐based pediatric i.v. dosing regimen (3.0 mg/kg LD followed by 1.5 mg/kg MD q.d.) that is exposure‐matched to adults. A previous population PK/pharmacodynamic analysis of data pooled from four studies in adults with esophageal candidiasis or IC showed a positive association between anidulafungin exposure and efficacy in all participants overall (87 patients and 211 concentrations), but not with AUC/minimum inhibitory concentration (71 patients), in a dose‐ranging IC study that used MDs of 50, 75, and 100 mg/day. 22 However, no trend in AUC was observed in clinically evaluable patients using graphical analysis. 22
Laboratory abnormalities in liver function tests have been noted in healthy volunteers and patients receiving anidulafungin treatment, and the causal relationship has not been established. 13 In the current investigation, exposure‐response safety analyses revealed a trend toward an increased incidence of all‐cause hepatic AEs with higher estimated systemic anidulafungin exposure in the combined data. However, no statistically significant associations between exposure and hepatic or GI AEs were identified in this pooled pediatric and adult population analysis using either linear or Emax relationships, consistent with previous exposure‐response safety findings in adults treated for IC. 22 Taking the graphical and modeling analysis results together, the incidence of all‐cause hepatic or GI AEs was not associated with systemic anidulafungin exposures and was not related to age or sex in this dataset. Because there was no statistically significant age effect, adult and pediatric populations seem similar with regard to the incidence of these AEs.
The main strength of this PK analysis is the inclusion of anidulafungin data from patients across the lifespan from neonates to older adults, with a wide range of body weights, and the same indication of suspected, proven, or at high risk of IC. Limitations are that the exposure‐response efficacy and safety analyses in adults were conducted using PK data from only a subset of patients, and that no efficacy data were collected from the pediatric IIR study, which was primarily designed as a PK study. 12 Furthermore, in the pediatric IIR study, patients were treated for 3–5 days for suspected serious infections, compared with a minimum of 5–10 days of anidulafungin treatment for confirmed or at high risk of Candida infection in the other studies; therefore, safety data from the pediatric IIR study, although collected and reported in the original publication, 12 were not included in the current safety analysis. Another potential limitation in evaluating exposure‐response was that the same weight‐based dose in children and fixed dose in adults was administered due to the study designs. This lack of dose‐ranging information limited the full characterization of exposure‐response.
In summary, PK data from pediatric and adult patients with confirmed, suspected, or at high risk of IC were characterized by a two‐compartment PK model with first‐order elimination. Body weight was a structural covariate on CL, V c, and V p. PK parameters were not affected by age or sex in this analysis. Estimated systemic anidulafungin exposure parameters were not related to efficacy or significantly related to the incidence of anidulafungin treatment‐emergent, all‐cause hepatic, or GI AEs in this pooled population. Based on these results, an i.v. dosing regimen of a 3.0 mg/kg LD followed by 1.5 mg/kg MD q.d., capped at the adult approved doses of 200 mg LD/100 mg MD q.d., is proposed for children from 1 month of age to match adults. The estimated systemic anidulafungin exposures achieved with this regimen in pediatric patients aged 1 month to < 18 years old with IC (study 1) 21 were comparable to exposures demonstrated to be efficacious in adults with IC at the recommended adult dosing regimen (200 mg LD/100 mg MD q.d.). The optimal anidulafungin dosing for neonatal treatment is so far unknown and animal studies suggest that higher doses are required to achieve central nervous system coverage for the treatment of HCME in premature infants and neonates. 19 As the data presented in the current study are based on comparative simulations of exposure with no exposure‐response relationships determined in the context of these studies, and in light of the limited experience with anidulafungin in pediatric patients, dosing in children should be approached with caution.
Funding
This study was funded by Pfizer Inc.
Conflict of interest
Rujia Xie is an employee of Pfizer Asia Manufacturing Pte Ltd. Lynn McFadyen is an employee and shareholder of Pfizer R&D, UK, Ltd. Susan Raber, Margaret Tawadrous, and Heidi Leister‐Tebbe are employees and shareholders of Pfizer Inc. Robert Swanson is contracted by, and is a shareholder of, Pfizer Inc. Michael Cohen‐Wolkowiez has received grant support from Pfizer Inc. Daniel K. Benjamin Jr. has received grant support from Pfizer Inc. Ping Liu is a former employee of Pfizer Inc. and current employee of Clinical Pharmacology, Linking Truth Technology Co. Ltd.
Author contributions
R.X. wrote the manuscript. R.S., M.T., P.L., M.C.‐W., and D.K.B. designed the research. L.M., S.R., M.T., H.L.‐T., P.L., M.C.‐W., and D.K.B. performed the research. R.X., L.M., S.R., R.S., M.T., H.L.‐T., M.C.‐W., and D.K.B. analyzed the data.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
Supplementary Material
Acknowledgments
The authors thank all patients who participated in this study and their families and the study investigators and staff at each participating site. Medical writing support, under the direction of the authors, was provided by Claire Cairney, PhD, of CMC Connect, McCann Health Medical Communications, funded by Pfizer, in accordance with Good Publication Practice (GPP3) guidelines.
References
- 1. Kullberg, B.J. & Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 373, 1445–1456 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Zaoutis, T.E. , Argon, J. , Chu, J. , Berlin, J.A. , Walsh, T.J. & Feudtner, C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41, 1232–1239 (2005). [DOI] [PubMed] [Google Scholar]
- 3. Pappas, P.G. et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37, 634–643 (2003). [DOI] [PubMed] [Google Scholar]
- 4. Santolaya, M.E. et al. Active surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome. Pediatr. Infect. Dis. J. 33, e40–e44 (2014). [DOI] [PubMed] [Google Scholar]
- 5. Adams‐Chapman, I. et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J. Pediatr. 163, 961–967 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin, D.K. Jr et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117, 84–92 (2006). [DOI] [PubMed] [Google Scholar]
- 7. Cornely, O.A. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non‐neutropenic adult patients. Clin. Microbiol. Infect. 18 (suppl. 7), 19–37 (2012). [DOI] [PubMed] [Google Scholar]
- 8. Ullmann, A.J. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 18 (suppl. 7), 53–67 (2012). [DOI] [PubMed] [Google Scholar]
- 9. Pappas, P.G. et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1–e50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hope, W.W. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 18 (suppl. 7), 38–52 (2012). [DOI] [PubMed] [Google Scholar]
- 11. Benjamin, D.K. Jr , Poole, C. , Steinbach, W.J. , Rowen, J.L. & Walsh, T.J. Neonatal candidemia and end‐organ damage: a critical appraisal of the literature using meta‐analytic techniques. Pediatrics 112, 634–640 (2003). [DOI] [PubMed] [Google Scholar]
- 12. Cohen‐Wolkowiez, M. et al. Safety and pharmacokinetics of multiple‐dose anidulafungin in infants and neonates. Clin. Pharmacol. Ther. 89, 702–707 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfizer Inc. ERAXIS® (anidulafungin) . Highlights of prescribing information. <http://labeling.pfizer.com/showlabeling.aspx?id=566> (2018). Accessed October 2, 2019.
- 14. Damle, B.D. , Dowell, J.A. , Walsky, R.L. , Weber, G.L. , Stogniew, M. & Inskeep, P.B. In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin. Antimicrob. Agents Chemother. 53, 1149–1156 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dowell, J.A. , Stogniew, M. , Krause, D. , Henkel, T. & Damle, B. Lack of pharmacokinetic interaction between anidulafungin and tacrolimus. J. Clin. Pharmacol. 47, 305–314 (2007). [DOI] [PubMed] [Google Scholar]
- 16. Dowell, J.A. , Stogniew, M. , Krause, D. & Damle, B. Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J. Clin. Pharmacol. 47, 461–470 (2007). [DOI] [PubMed] [Google Scholar]
- 17. Dowell, J.A. , Knebel, W. , Ludden, T. , Stogniew, M. , Krause, D. & Henkel, T. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44, 590–598 (2004). [DOI] [PubMed] [Google Scholar]
- 18. Benjamin, D.K. Jr. et al. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob. Agents Chemother. 50, 632–638 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warn, P.A. et al. Anidulafungin for neonatal hematogenous Candida meningoencephalitis: identification of candidate regimens for humans using a translational pharmacological approach. Antimicrob. Agents Chemother. 56, 708–714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roilides, E. et al. A prospective, open‐label study to assess the safety, tolerability and efficacy of anidulafungin in the treatment of invasive candidiasis in children 2 to <18 years of age. Pediatr. Infect. Dis. J. 38, 275–279 (2019). [DOI] [PubMed] [Google Scholar]
- 21. Roilides, E. et al. Safety, efficacy and pharmacokinetics of anidulafungin in patients 1 month to <2 years of age with invasive candidiasis, including candidemia. Pediatr. Infect. Dis. J. 39, 305–309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu, P. Population pharmacokinetic‐pharmacodynamic analysis of anidulafungin in adult patients with fungal infections. Antimicrob. Agents Chemother. 57, 466–474 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wasmann, R.E. et al. Pharmacokinetics of anidulafungin in obese and normal‐weight adults. Antimicrob. Agents Chemother. 62, e00063–e00018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lempers, V.J. et al. Does weight impact anidulafungin pharmacokinetics? Clin. Pharmacokinet. 55, 1289–1294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Supplementary Material


