Abstract
Purpose of review:
Sexual dysfunction occurs commonly in people with chronic kidney disease (CKD) and has been recognized as a research priority. We sought to evaluate the current state of the literature addressing sexual dysfunction in people with CKD and identify barriers and strategies to improve our management of this important symptom.
Sources of information:
OVID Medline and Google Scholar were searched for English, peer-reviewed studies using keywords and terms related to “Chronic Kidney Disease,” “sexuality,” and “sexual dysfunction OR function.”
Methods:
In this narrative review, we describe definitions of sexual dysfunction and contributors exacerbated by CKD, barriers to researching sexual dysfunction in people with CKD, and possible avenues for future research.
Key findings:
Sexual dysfunction is common in people with CKD and results from a combination of kidney disease itself, as well as its associated physical (ie, comorbidities) and nonphysical factors. Barriers to the study of sexual dysfunction in CKD include inconsistent disease definitions, stigma, variable efficacy and safety of established therapies, and evolving gender roles in sexual function. Potential avenues for future research to improve the sexual function in people with CKD may include evaluating the safety and efficacy of established therapies in people with CKD using a variety of observational and interventional study designs, engaging people with CKD and multidisciplinary team members in research, and using implementation science methods to translate what is known about sexual function into clinical practice. Concerted efforts are required to break down barriers and improve sexual function in people with CKD. Patients have identified this as an important research priority, and national networks need to direct efforts to reduce symptom burden.
Limitations:
This narrative review was limited by a paucity of high-quality studies examining sexual dysfunction specifically in people with kidney disease.
Keywords: chronic kidney disease, sexual function, sexual health, narrative review
Abrégé
Contexte motivant la revue:
Les dysfonctions sexuelles sont fréquentes chez les personnes atteintes d’insuffisance rénale chronique (IRC) et ont été reconnues comme une priorité de recherche. Cette revue avait pour objectif d’évaluer la documentation actuelle traitant du dysfonctionnement sexuel chez les personnes atteintes d’IRC et de répertorier les obstacles à la recherche dans ce domaine et les stratégies qui permettent d’améliorer la gestion de cet important symptôme.
Sources:
Les bases de données OVID Medline et Google Scholar ont été consultées à la recherche des études rédigées en anglais et évaluées par des pairs à l’aide de mots-clés et de termes liés à Chronic Kidney Disease (insuffisance rénale chronique), sexuality (sexualité) et sexual dysfunction OR function (dysfonction OU fonction sexuelle).
Méthodologie:
Cette revue narrative présente les définitions de la dysfonction sexuelle et les contributeurs exacerbés par l’IRC. Elle décrit également les obstacles limitant la recherche sur le dysfonctionnement sexuel chez les personnes atteintes d’IRC et cerne de possibles pistes pour les recherches futures.
Principaux résultats:
Le dysfonctionnement sexuel est fréquent chez les personnes atteintes d’IRC et résulte d’une combinaison de la néphropathie elle-même et de facteurs physiques (c.-à-d. les maladies concomitantes) et non physiques qui y sont associés. Les obstacles limitant l’étude de la dysfonction sexuelle en contexte d’IRC comprennent: 1) l’incohérence dans les différentes définitions de maladie; 2) la stigmatization; 3) l’efficacité et l’innocuité variables des thérapies existantes; et 4) le caractère évolutif des rôles des genres dans la fonction sexuelle. Les possibles axes de recherche pour les études futures pourraient inclure: 1) l’utilization d’une variété de modèles d’études observationnelles et interventionnelles pour évaluer l’innocuité et l’efficacité des thérapies existantes dans cette population de patients; 2) la participation de patients et de membres de l’équipe multidisciplinaire à la recherche, et 3) l’utilization de méthodes scientifiques de mise en œuvre afin de traduire les connaissances dans la pratique clinique. Des efforts concertés sont nécessaires pour éliminer les obstacles à la recherche et améliorer la fonction sexuelle des personnes atteintes d’IRC. Il s’agit d’une importante priorité de recherche pour les patients, et les réseaux nationaux se doivent d’orienter leurs efforts vers la réduction du fardeau des symptômes.
Limites:
Cette revue narrative était limitée par le manque d’études de haute qualité examinant spécifiquement la dysfonction sexuelle chez les personnes atteintes de néphropathies.
What was known before
Sexual dysfunction is a common symptom experienced by people with chronic kidney disease, although there is a paucity of research on the mechanistic and clinical aspects of sexual health in this population.
What this adds
There are a number of barriers to research on sexual function in persons living with chronic kidney disease, including varying disease definitions and treatment efficacy, stigma, and lack of patient voice in driving the research agenda. Future research in this area should apply a variety of observational, interventional, and qualitative study designs and engage with patients and the multidisciplinary team to translate knowledge into impactful, person-centered care.
Introduction
People with chronic kidney disease (CKD) are often affected by multiple chronic comorbidities, which cumulatively manifest in varied symptoms that impact quality of life.1 Sexual function can be negatively impacted by kidney disease itself and by common comorbidities such as diabetes and vascular disease.2 Defining sexual dysfunction for those with and without CKD has been challenging for both academic and clinical communities given the diverse drivers and difficulty separating sexual symptoms from physiological dysfunction.3 The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition; DSM-V) provides updated definitions of sexual dysfunction as a “heterogenous group of disorders that are typically characterized by a clinically significant disturbance in a person’s ability to respond sexually or to experience pleasure.”4 This includes disorders of physical function (genito-pelvic pain/penetration disorder in females, erectile disorder in males, and substance/medication-induced sexual dysfunction in either females or males) and disorders of interest, arousal, and orgasm (female sexual interest/arousal disorder, female orgasmic disorder, male hypoactive sexual desire disorder, premature ejaculation, and delayed ejaculation).5
The burden of sexual dysfunction among those with CKD is difficult to estimate due to shifting definitions and the sensitive nature of the topic,3 although it appears progressively more common as kidney function deteriorates and more frequently than in people with normal kidney function.2 Males with CKD have a high prevalence of erectile dysfunction, with 71% of people with estimated glomerular filtration rates (eGFR) below 60 mL/min/1.73 m2 affected.6 Male patients receiving maintenance hemodialysis have a similar reported prevalence, ranging from 68% to 92%.6,7 Difficulties with ejaculation or the inability to orgasm have been reported in 59% of sexually active male dialysis patients.8 Sexual dysfunction is also prevalent in women with CKD,9,10 with an estimated 56% of those on dialysis having difficulties achieving orgasm.8 Sexual desire has also been found to decrease in almost half of people receiving dialysis.11 Despite the prevalence of sexual symptoms or dysfunction in patients across the full range of kidney disease being higher than those without CKD, most of the kidney-specific literature has focused on dialysis populations.
Although an important element of overall symptom burden for persons living with CKD, sexual well-being is neither consistently integrated into CKD care nor addressed by kidney health research programs to the extent of other symptoms.12,13 A Canadian partnership of people living with CKD, their caregivers, clinicians, and policymakers identified CKD symptoms, including sexual dysfunction, as a top priority for research.14 However, the paucity of literature related to the mechanistic aspects of sexual health and interventions for treating sexual dysfunctions among the CKD population highlights a significant mismatch between the priorities of patients and those of the academic community. Therefore, the aim of this narrative review is to summarize the mechanisms of symptoms of sexual dysfunction, identify barriers, and solutions to improve research into sexual health in kidney disease and suggest specific research avenues to tackle this important problem.
Methods
We sought to evaluate the prevalence, contributors, and research gaps for sexual dysfunction in CKD in this narrative review of the literature. Following an initial meeting including a patient coresearcher, we searched OVID Medline and Google scholar databases from inception to February 3, 2020, with keywords “chronic kidney disease,” “sexuality,” and “sexual dysfunction OR function” for peer-reviewed English articles in adults. Internal and external peer review was performed as part of the Kidney Research Scientist Core Education and National Training (KRESCENT) program.
Contributors to Sexual Dysfunction in CKD
Common to Both Sexes
Many contributors to sexual dysfunction in people with CKD are common between male and female sexes and are also found in other patients with advancing age or chronic medical conditions (Figure 1). This includes psychosocial, psychological, and psychiatric contributors such as stress, grief, depression, and anxiety. Socioeconomic barriers to sexual activity can include institutionalized housing and lack of access to privacy. Physical ailments such as fatigue, anemia, or chronic pain can limit both sexual desire and performance. Comorbidities such as autonomic or peripheral neuropathy, diabetes, cardiovascular, or peripheral vascular disease can all have a negative impact on sexual function.2 Similarly, dysregulation of the hypothalamic-pituitary-gonadal axis that occurs as CKD progresses may contribute to sexual dysfunction. Although there is some evidence that improvement of the uremic milieu with intensive dialysis or kidney transplantation may reverse some of these abnormalities, whether this translates to improved sexual function is unclear.2,15-18 Medications including diuretics, β-blockers, histamine receptor antagonists, aldosterone antagonists, and glucocorticoids all negatively impact sexual function as recognized adverse effects.19 Contributors to sexual dysfunction unique to people receiving maintenance dialysis may include body image issues related to dialysis access devices (fistula, peritoneal, or hemodialysis catheters) or medicalization of the bedroom (eg, dialysis machines). In addition, polypharmacy and the potential for inappropriate drug dosing with impaired kidney function can complicate sexual function among people with CKD. While many of these contributors to sexual dysfunction are also implicated in people without CKD, they occur with greater frequency among people with CKD, particularly those with more advanced disease or higher comorbidity burden.1 Understanding the interaction between more prevalent comorbidities or socioeconomic factors associated with CKD with sexual health remains poorly delineated in the literature.
Figure 1.
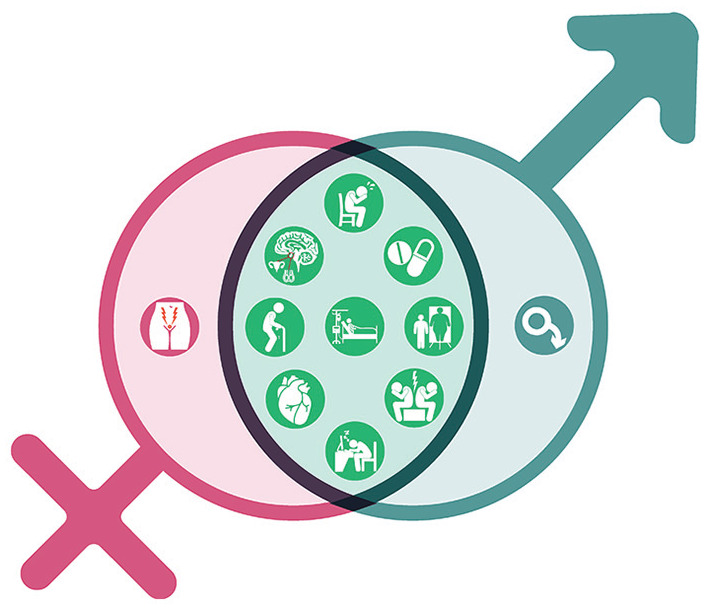
Contributors to sexual dysfunction in people with chronic kidney disease.
Note. Sexual dysfunction includes physical limitations as well as psychosocial, emotional, and other organic contributors. Many factors are shared between people with CKD and all people with advancing age and chronic illness. Some factors are sex specific (eg, vaginal dyspareunia, erectile dysfunction), but many are common between males and females (eg, mental health, adverse drug effects and polypharmacy, physical limitations and frailty, medicalization of the bedroom, body image issues, fatigue, interpersonal stressors).
Unique to Females With CKD
Estrogen deficiency has been postulated as a major determinant of menstrual irregularities and early menopause in women with CKD.20 The hypoestrogenic state associated with menopause leads to decreased blood flow to the vulva and vagina, which reduces vaginal lubrication and results in pain with sex, termed dyspareunia.21,22 A study of females with grades 4 to 5 (G4-5) CKD showed that there were lower sexual function scores with pain and reduced lubrication compared with age-matched controls.23 Hormonal irregularities in the female CKD population have not been extensively studied, although in this study, transdermal estradiol treatment compared with control was associated with substantially improved sexual function.23
Unique to Males With CKD
Triggered by neurological signals, erection is primarily a vascular phenomenon requiring adequate arterial inflow and reduction of venous outflow. Not only is erectile dysfunction a marker of underlying vascular disease, the altered mineral metabolism in CKD can lead to ectopic vascular calcification exacerbating small vessel vascular disease.24 Generation of nitric oxide by nitric oxide synthase is an additional requirement for an erection, via the production of cyclic guanosine monophosphate (cGMP). The phosphodiesterase (PDE-5) inhibitors sildenafil, vardenafil, and tadalafil improve erectile response by increasing cGMP in the general population, and though evidence of safety in people with CKD is limited, there is some evidence that they are efficacious in improving erectile dysfunction for males with CKD.12 Finally, despite an incomplete understanding of pathophysiologic mechanisms, endocrine abnormalities are frequent in males with CKD, including hyperprolactinemia and reduced testosterone synthesis, which may contribute to sexual dysfunction.
Barriers to Research of Sexual Dysfunction in CKD
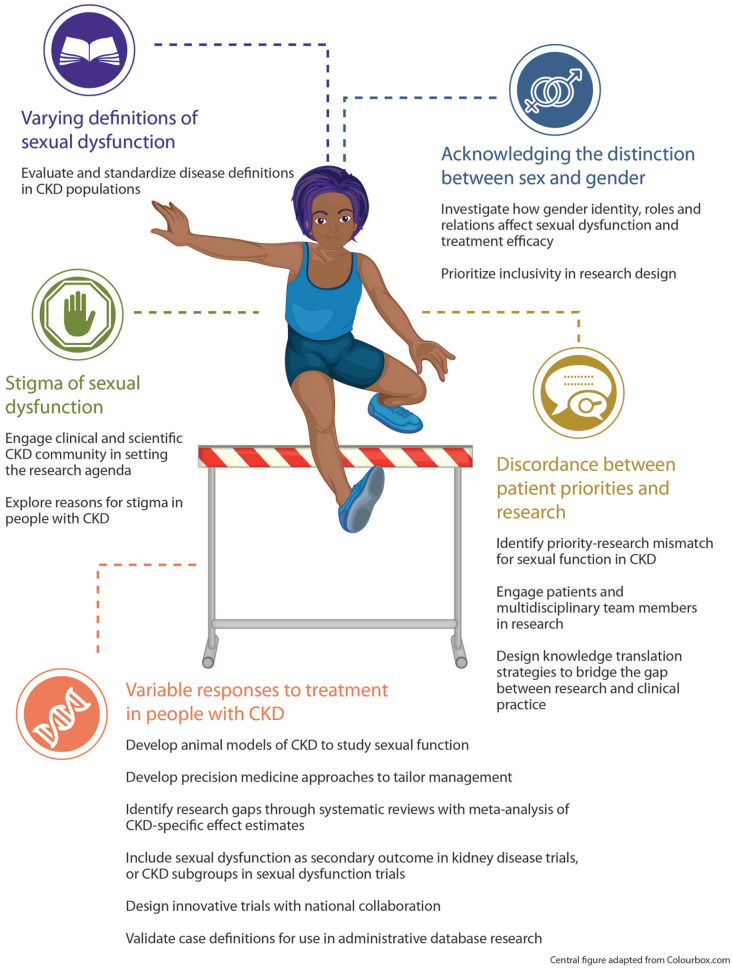
Although sexual dysfunctions are common among people with CKD and symptom causes and treatments have been identified as important avenues for CKD research, there are numerous barriers that the scientific community must overcome to study this area effectively (Figure 2).
Figure 2.
Barriers to improving sexual function in people with CKD and potential research avenues to address them.
Source. Central figure adapted from Colourbox.com.
Note. Several barriers to conducting sexual dysfunction research in people with CKD are presented here. We provide potential research avenues that could be used to target each barrier. CKD = chronic kidney disease.
Barrier 1: Defining Sexual Dysfunction in CKD
Standardizing the definitions of different sexual dysfunctions has been challenging, particularly as major guideline and classification bodies from differing clinical specialties organize sexual dysfunctions in different ways.25 These differences in descriptive taxonomies are largely the result of forced dichotomization of sexual dysfunctions as being psychiatric or medical in nature, even though etiological factors span both domains.25 The 2 most commonly used classification systems for sexual dysfunctions are the DSM-V and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) from the World Health Organization.25
The DSM-V diagnostic criteria for sexual dysfunctions separate male from female sexual dysfunctions and specify criteria for 8 dysfunctions that are present for at least 6 months and for more than 75% of sexual encounters.4 Although the DSM-V is primarily oriented for mental health conditions, conditions such as erectile dysfunction and genito-pelvic penetration disorder (which are explained primarily by medical/organic etiologies) are also defined. The ICD-10 classification system for sexual dysfunctions dichotomizes the disorders as organic or nonorganic.25 For each condition, an ICD-10 diagnostic alpha-numeric code and definition are assigned to standardize case definitions for use in health services research.26 However, sex-based specifications are not included in the ICD-10 definitions, nor are concessions made for specific underlying medical etiologies, such as CKD. With the notable differences in sexual dysfunction definitions between the 2 major classification bodies, another group (the International Consultation on Sexual Medicine) has generated a consensus statement on “Definitions of Sexual Dysfunctions.”25 These expert and literature-based definitions were largely composed of a combination of DSM-V and ICD-10 definitions, with several new definitions included as well. These definitions were stratified by sex to avoid the issue of the psychiatric/medical dichotomization.
Why do the different classification systems pose a barrier to sexual dysfunction research in CKD? Diagnostic or case definitions are a necessary part of epidemiologic research study designs.27 When definitions differ between studies, it is exceedingly difficult to compare results and contribute to the same understanding of the science of sexual dysfunction. This is true when studying sexual dysfunction alone or in relation to another medical condition, such as CKD. Use of specific case definitions derived from one classification database (ie, ICD-10) may not generalize well to clinical settings where different criteria are used.25
Barrier 2: Stigma of Sexual Dysfunction
Research of sexual function of people with kidney disease is likely affected by the stigma and sensitivity of the subject matter, though we could not find this explicitly mentioned in the literature. Rather, there was a plethora of evidence from the general medical literature suggesting that this was a barrier to the clinical management of these dysfunctions, which may translate to challenges with recruitment of participants for clinical research in this area.
In a qualitative study of older adults from a primary care practice in the United Kingdom, several barriers to seeking help for sexual health concerns were identified. Many older people had sexual problems that they wanted to address with their family physicians but were unable to because of shame, embarrassment, and fear related to these symptoms.28 These dysfunctions were often felt to be dismissed as part of normal aging, which was also a barrier to seeking care. Other research has found that the social stigma around female sexual function and the embarrassment that accompanies sexual dysfunction are major barriers to patient-clinician communication.29 Some CKD literature suggests that nephrologists do not routinely discuss sexual function with their patients, although cited reasons included lack of topic knowledge and insufficient time rather than their discomfort with this issue, per se.13 Similarly, a minority of nephrology nurses discuss sexual health with their dialysis patients due to barriers of ethnicity, religion, and older age of the patient,30 as well as inadequate training and lack of suitable time and setting to discuss this sensitive topic.31 Together, these patterns may translate into difficulties with enrollment of persons with CKD and sexual dysfunctions into research studies. Until the public and those in the scientific and clinical communities accept that these stigmas exist, patient recruitment and prioritization of these studies in CKD research will remain challenging.
Barrier 3: Response to Treatment for Sexual Dysfunction May Be Different in People With CKD
Altered pharmacokinetics and pharmacodynamics resulting from decreased kidney function could lead to increased side effects or reduced efficacy of therapies, which may pose challenges to the feasibility of clinical research in this area. Furthermore, as the etiology of sexual dysfunction in people with CKD is often multifactorial, applying therapies that target only a specific contributor may be an inadequate approach to addressing the broader issue of sexual dysfunction more holistically.2
Erectile dysfunction is the most studied sexual dysfunction in people with CKD, and we will use this as an example of how sexual dysfunctions are challenging to research. As described above, sildenafil, vardenafil, and tadalafil are all therapies that maintain relaxed smooth muscle in the corpus cavernosum.2 In interventional studies of erectile dysfunction therapies in patients with CKD, response to treatment varies, though generally there is evidence of efficacy.2,12 This is likely due to differences in defining the included population, variable outcome definitions to identify responders to therapy (ie, survey responses or functional biometric erection testing), and differences in the procedures used to identify or manage other causes of erectile dysfunction. People with CKD have a high prevalence of cardiovascular diseases, with up to two-thirds of patients with eGFR <30 mL/min/1.73 m2 having overt disease.32 Many of these patients have coronary artery disease or heart failure that is managed with nitrate-containing compounds. As the interaction of sildenafil and nitrate therapies may precipitate harmful side effects, such as severe hypotension, this combination is contraindicated.33 Patients with coronary artery disease are likely to have similar vascular insufficiency that may precipitate erectile dysfunction, and so this may present a barrier to inclusion of high-risk patients in erectile dysfunction CKD trials. As sexual dysfunctions in people with CKD are often multifactorial, the exclusion of patients from interventional studies on the basis of other contributors to dysfunction would severely limit the feasibility of such research and generalizability to the broader CKD population for whom this is an issue.
Barrier 4: Discordance Between Patient Priorities and Research
The perceived mismatch between what patients want to see researched and the research that is actually being done has prompted a number of large-scale initiatives to ensure the voices of patients are heard. For example, the James Lind Alliance supports partnerships between patients and other knowledge users to identify research priorities across a variety of health conditions. Although some patient-oriented research programs, such as the pan-Canadian network Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD), focus on addressing research priorities identified by patients, literature suggests that this discordance between patient priorities and active research persists. One study found that while a minority (18%) of early priority setting partnerships identified drug therapies as priorities, drug trials continue to comprise the majority of registered trials (37%-86%).34 Similarly, a scoping review of studies in people with kidney failure reported that less than one-quarter addressed topics consistent with the top 10 research priorities identified by people on or nearing dialysis.35,36
Sexual dysfunction appears among common CKD symptoms in the list of top research priorities for people with CKD that are not receiving maintenance dialysis.14 While a full systematic review to assess alignment of this priority with ongoing research is beyond the scope of this article, it is likely that the priorities-research mismatch extends to this topic as well. This may relate to a bias toward interventional studies with “hard” clinical endpoints (eg, mortality, kidney failure), whereas studies of sexual dysfunction tend to focus on outcomes of patient experience and quality of life. Furthermore, studies of sexual dysfunction may be overlooked by investigators due to a lack of appreciation of the impact of this issue on patient well-being or the lack of funding appeal to support such work. Even in the clinical domain, sexual dysfunction is not routinely assessed on CKD symptom questionnaires or patient-reported outcome measures, making this a challenging issue to measure reliably across clinical and research settings.
Barrier 5: Acknowledging the Distinction Between Sex and Gender
Another potential barrier to research is the lack of clarity within the research community about the influence of sex and gender on sexual health for persons with CKD. Data suggest sex- and gender-based differences exist in rates of decline in kidney function, dialysis initiation, and transplantation between men and women,37 but research exploring the impact of sex and gender identity on other domains of CKD care is only starting to emerge. Whereas sex, categorized as male or female, refers to a person’s biological attributes (eg, karyotype, sex hormones, reproductive function), gender encompasses the sociocultural roles, behaviors, and identities of men, women, and gender-diverse people.38 Interventional and epidemiological studies in nephrology tend to emphasize what differences exist between biological sexes or self-identified genders, with little or no acknowledgment of how a person’s gender identity and related gender roles and relations might contribute to the observed effect. Furthermore, the typical categorization of pathophysiology and treatments for sexual dysfunction according to sex or gender tends to oversimplify the complex relationship between a person’s physiology and socially constructed gender identity. Although certain disorders such as erectile dysfunction are uniquely related to biological sex, others such as low sexual desire occur across men, women, gender-diverse, and nonbinary individuals. Even in disorders that occur in all people with CKD, the underlying causes and manifestations often differ.3 These subtleties highlight the challenges in tackling issues of sexual health in a research study unless careful attention is paid to the gendered nature of the problem and potential solutions.
Avenues for Future Research
People living with CKD care about their sexual health and have prioritized this as an important research focus. It is time for the academic community to overcome barriers to sexual health research for people with CKD and formalize these priorities into research. There are large gaps in what we know about sexual function and dysfunctions in people with CKD, and it is often within these gaps that we find opportunity for meaningful scientific growth. Here, we outline research avenues across the spectrum of scientific inquiry.
Animal Models for Sexual Dysfunction in CKD
The development of therapeutics in clinical medicine has depended on the establishment of animal models for studying pathophysiology, pharmacology, and toxicity. Development of sexual dysfunction models in kidney disease may provide valuable insight into future treatments. Erectile dysfunction has been studied in various animals including rats, mice, and rabbits.39 Rat models of cavernous nerve stimulation with subsequent intracavernous pressure measurements40 can be coupled with techniques of CKD modeling such as subtotal nephrectomy, unilateral ureteric obstruction, or folic acid.41 These CKD models used in larger rodents such as rats can be valuable for female sexual dysfunction as well. Rat models of pelvic or pudendal nerve stimulation can be used reliably to study genital arousal by examining clitoral blood flow, vaginal blood flow, and intracavernosal clitoral pressure.42 As described earlier, females with CKD note considerably decreased libido and orgasm. Nerve stimulation studies in rodent CKD models may help explore these pathways allowing for better application of therapies.
Precision Medicine for Sexual Dysfunction
The principles of precision medicine could theoretically be applied to sexual health in CKD. Precision medicine attempts to incorporate not only traditional disease markers such as demographics and comorbidities, but aims to include a detailed analysis of all available quantitative data including biomarkers, imaging, and electronic medical record data to refine the phenotype. Precision medicine recognizes that sexual dysfunction represents a continuous, evolving phenotype, not a dichotomized diagnosis, and the cause and severity will vary between people based on their specific contributing milieu. In other words, one treatment will not be appropriate for all.
Safety and Efficacy of Established Therapies in People With CKD
From our literature review, people with kidney disease do not appear to be included in research investigating the spectrum of sexual dysfunction, though there is a need to document these gaps more formally. As an example, Vecchio et al completed a systematic review in 2010 evaluating trials of sexual dysfunction therapy in CKD populations, though most summarized literature was focused on erectile dysfunction therapies.12 In addition to repeating a similar updated systematic review, researchers could develop an expanded search strategy to identify additional randomized controlled trials investigating erectile dysfunction therapies in the general population to identify potential kidney disease inclusion and exclusion from these trials. Furthermore, if these trials did provide effect estimates that were specific to subgroups of patients with kidney disease, these kidney disease-specific estimates could be combined via meta-analysis to compare the effectiveness of therapies in these groups.
It is no surprise to the nephrology community that conducting primary research studies such as randomized controlled trials in people with kidney disease can be difficult and is a barrier as described above. Fewer trials have historically been conducted in CKD populations than other chronic diseases,43 and when we look to studies that investigate sexual dysfunction, this is compounded further. The classic 2-arm randomized controlled trial with individual patient-level randomization has many practical challenges when applied to people with CKD. Recruitment of people with CKD with comorbidities that would exclude them from trials is difficult. Trials in people receiving maintenance dialysis are often completed with patients that are not representative of this population.44 Trials designed to target improvements in sexual dysfunctions may be hampered by reduced effect sizes in people with kidney disease, as these dysfunctions are often multifactorial and not addressed fully by a single intervention. This translates into higher required sample sizes for adequate statistical power to detect meaningful changes in sexual dysfunction outcomes. Thus, the cost and feasibility of traditional clinical trials of sexual dysfunction in people with CKD will be a challenge for future research. These barriers present opportunities to design innovative clinical trials in CKD populations. Within Canada, platforms exist to support collaborative, innovative clinical trial design and conduct through the Canadian Nephrology Trials Network (www.cntn.ca) and Innovative Clinical Trials in Hemodialysis Centers initiative.45 In particular, this latter group has developed pragmatic cluster-randomized dialysis trials that are embedded in routine care and use existing registry or administrative databases for cost efficiency. Applying this example to sexual dysfunction may allow researchers to investigate people with CKD in novel ways, overcome trial feasibility barriers, and generate high-quality evidence for managing these dysfunctions.
Inclusion of sexual function outcomes as secondary outcomes in trials of therapies that may have an effect on sexual function or contributors to dysfunction (eg, trials of cardiovascular interventions, treatment of depression or anxiety) is also important. Outside of trials designed to investigate sexual dysfunctions specifically in people with CKD, there are other opportunities for researchers to generate rigorous evidence in this population. Advocacy for inclusion of people with CKD in larger sexual dysfunction trials and inclusion of CKD as a prespecified subgroup are avenues worth pursuing.
Outside of trials and primary data collection, there are opportunities to use existing administrative databases to investigate sexual dysfunction in people with CKD. One of the common challenges to using administrative databases for health research is that validated case definitions for conditions or diseases are often not available. Case definitions for defining comorbidities within administrative data are usually based on combinations of ICD codes found over a prespecified time period.46 Case definitions should be validated before use against a gold standard such as chart review to ensure that the algorithm performs adequately in administrative sources.46,47 An ICD code being associated with each sexual dysfunction is unlikely to perform well in identifying cases of sexual dysfunction in people with CKD. These algorithms would likely underestimate sexual dysfunction prevalence as they rely on capture of health care utilization or physician claims in the data sources, and we know that these conditions tend to be underdiagnosed and underreported by patients.2 Furthermore, as these diagnoses are more often made as outpatients, we may rely on physician claims for these diagnostic codes which are not as high quality as other sources.46,47
Another possible avenue that could be examined would be to use Canadian administrative data holdings to study harms of therapies prescribed for sexual dysfunctions in people with CKD. The Canadian Network for Observational Drug Effect Studies (CNODES) is a nationwide collaborative where rare adverse drug effects can be investigated on a large scale.48 Researchers could design a research study where the adverse effects of commonly prescribed sexual dysfunction therapies (eg, sildenafil) could be examined in existing administrative databases nationwide to understand what the expected risk of these therapies may be for people with CKD.
Engaging People With CKD and Multidisciplinary Collaboration in Sexual Dysfunction Research
Although evidence is sparse for treatment of sexual dysfunction for people with CKD, existing literature tends to focus on pharmaceutical interventions for the most common physical symptoms (eg, erectile dysfunction therapy). With this research focus, psychosocial contributors to sexual dysfunction are less frequently investigated.2 From our evaluation of the sexual dysfunction in CKD literature, the patient and partner voice has not been included in research, which would be critical for understanding the psychosocial contributors to this issue. Engaging patients and their partners in research is an important consideration for sexual dysfunction research, particularly as it involves such interpersonal and sensitive content. People with CKD are increasingly being incorporated into research programs; however, it is important to ensure engagement is not tokenistic and to identify in partnership appropriate and meaningful opportunities for inclusion at key points across the research spectrum (eg, question generation and priority setting, research design, data collection, summary of main messages with patient lens, manuscript preparation).49 As an example, engaging patients to lead sexual dysfunction focus groups with patient-prioritized discussion scripts may encourage open conversation and provide rich and grounded research findings. Canada is a leader in patient-oriented research, with several national and provincial organizations providing support for patients engaging as coresearchers in kidney health research (eg, Kidney Foundation of Canada, Strategy for Patient-Oriented Research, Can-SOLVE CKD). Research that is designed with a patient-oriented and prioritized lens aligns well with the mandates of these organizations and may provide avenues to secure financial support for sexual dysfunction research.
Patient engagement may be extremely beneficial in sexual dysfunction research; however, collaboration with other professional disciplines is also highly important. This is analogous to the clinical realm, where physicians may not be the most important professionals in identifying and managing sexual dysfunctions. Instead, the social workers, psychologists, occupational and physical therapists, and nurses who are often most connected to these CKD patient sexual dysfunctions are better positioned to direct much of the nonpharmacological management approaches. Incorporation of these multidisciplinary professionals as collaborators in sexual dysfunction research may also provide unique perspectives on how best to answer sexual dysfunction research questions in ways that address their practical needs as care providers.
Translating Knowledge to Improved Sexual Health in People With CKD
Bridging the gap between mechanistic and clinical research is the first step toward developing precision health approaches and implementing person-centered tools for people with CKD living with sexual dysfunction. The overall lack of literature in this area identified by this review highlights important knowledge gaps, but also opportunities to explore novel care approaches. Even when high-quality data exist, knowledge users, including health care providers and patients, must be aware and willing to apply these data in practice. For example, a survey of nephrologists found that they addressed issues of sexual dysfunction in less than half of their new patients with CKD.13 Thus, in addition to the barriers to research into sexual dysfunction described above, the individual and organizational barriers to integrating sexual health into patient care require further exploration.
Increasing awareness of this issue among the health care team and its importance to patients is critical, as is the availability of accessible tools and resources for healthcare providers and patients. First, asking about sexual function on clinic intake questionnaires or collecting CKD-specific patient-reported outcome measures that include sexual health is a practical way to assess patients’ sexual health concerns in routine care.50 Second, providing reliable information and tools can enhance patients’ kidney health knowledge and support self-management as they relate to sexual health. The availability of online and print materials related to sexual health through some national kidney health organizations provides a starting point, but interactive, tailored resources that integrate sexual health into a patient’s overall CKD care strategy would be an important next step. Given the patient priority of symptom management in CKD, which includes sexual dysfunction, engaging patients and knowledge users in the development of such resources is necessary to ensure their needs and knowledge gaps are addressed. Research into how best to implement these resources in the clinical setting would be critical for promoting their routine use in practice.
Conclusions
Sexual dysfunction is common in people with CKD and has been identified by people living with CKD as an important research gap. In this narrative review, we describe the mechanisms of sexual dysfunction in people with CKD and the barriers to conducting research in this population. Utilization of trans-disciplinary research methods may help the scientific and clinical communities understand sexual dysfunction in people with CKD and identify ways to increase awareness and manage these dysfunctions more effectively. Once the evidence gaps outlined in this review are addressed, we must direct our focus to translating this new knowledge into comprehensive, person-centered CKD care.
Acknowledgments
The authors thank the internal KRESCENT reviewers for their valuable peer review. In addition, Ms. Sarah Gil provided invaluable graphic design expertise for figure development.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: All authors consent to publication.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.B.L. has received compensation as a speaker and advisory board member for Otsuka Pharmaceutical. No other disclosures to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: T.G.H. and M.S. are supported by a Kidney Research Scientist Core Education and National Training (KRESCENT) Program postdoctoral fellowship (cosponsored by the Kidney Foundation of Canada, the Canadian Society of Nephrology, and Canadian Institutes of Health Research). T.G.H. is also supported by the Clinician Investigator Program at the University of Calgary. M.S. is supported by the Canadian Society of Transplantation research training award. M.B.L. and M.J.E. are supported by KRESCENT New Investigator Awards.
ORCID iD: Meghan J. Elliott  https://orcid.org/0000-0002-5434-2917
https://orcid.org/0000-0002-5434-2917
References
- 1. Fraser SDS, Taal MW. Multimorbidity in people with chronic kidney disease: implications for outcomes and treatment. Curr Opin Nephrol Hypertens. 2016;25(6):465-472. [DOI] [PubMed] [Google Scholar]
- 2. Finkelstein FO, Shirani S, Wuerth D, Finkelstein SH. Therapy insight: sexual dysfunction in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(4):200-207. [DOI] [PubMed] [Google Scholar]
- 3. Sungur MZ, Gündüz A. A comparison of DSM-IV-TR and DSM-5 definitions for sexual dysfunctions: critiques and challenges. J Sex Med. 2014;11(2):364-373. [DOI] [PubMed] [Google Scholar]
- 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 5. Ishak WW, Tobia G. DSM-5 Changes in diagnostic criteria of sexual dysfunctions. Reprod Syst Sex Disord. 2013;2:1-3. [Google Scholar]
- 6. Costa MR, Ponciano VC, Costa TR, de Oliveira AM, Gomes CP, de Oliveira EC. Prevalence and factors associated with erectile dysfunction in patients with chronic kidney disease on conservative treatment. Int J Impot Res. 2017;29(6):219-224. [DOI] [PubMed] [Google Scholar]
- 7. Gorsane I, Amri N, Younsi F, Helal I, Kheder A. Erectile dysfunction in hemodialysis patients. Saudi J Kidney Dis Transpl. 2016;27:23-28. [DOI] [PubMed] [Google Scholar]
- 8. Steele TE, Wuerth D, Finkelstein S, et al. Sexual experience of the chronic peritoneal dialysis patient. J Am Soc Nephrol. 1996;7(8):1165-1168. [DOI] [PubMed] [Google Scholar]
- 9. Basok EK, Atsu N, Rifaioglu MM, Kantarci G, Yildirim A, Tokuc R. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int Urol Nephrol. 2009;41(3):473-481. [DOI] [PubMed] [Google Scholar]
- 10. Bellinghieri G, Santoro D, Mallamace A, Savica V. Sexual dysfunction in chronic renal failure. J Nephrol. 2008;21(suppl 13):S113-S117. [PubMed] [Google Scholar]
- 11. Fryckstedt J, Hylander B. Sexual function in patients with end-stage renal disease. Scand J Urol Nephrol. 2008;42:466-471. [DOI] [PubMed] [Google Scholar]
- 12. Vecchio M, Navaneethan SD, Johnson DW, et al. Treatment options for sexual dysfunction in patients with chronic kidney disease: a systematic review of randomized controlled trials. Clin J Am Soc Nephrol. 2010;5(6):985-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Ek GF, Krouwel EM, Nicolai MP, et al. Discussing sexual dysfunction with chronic kidney disease patients: practice patterns in the office of the nephrologist. J Sex Med. 2015;12(12):2350-2363. [DOI] [PubMed] [Google Scholar]
- 14. Hemmelgarn BR, Pannu N, Ahmed SB, et al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2017;32:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bass A, Ahmed SB, Klarenbach S, Culleton B, Hemmelgarn BR, Manns B. The impact of nocturnal hemodialysis on sexual function. BMC Nephrol. 2012;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Bahnasawy MS, El-Assmy A, El-Sawy E, et al. Critical evaluation of the factors influencing erectile function after renal transplantation. Int J Impot Res. 2004;16(6):521-526. [DOI] [PubMed] [Google Scholar]
- 17. Anantharaman P, Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:119-125. [DOI] [PubMed] [Google Scholar]
- 18. Tavallaii SA, Mirzamani M, Heshmatzade Behzadi A, et al. Sexual function: a comparison between male renal transplant recipients and hemodialysis patients. J Sex Med. 2009;6(1):142-148. [DOI] [PubMed] [Google Scholar]
- 19. Imprialos KP, Stavropoulos K, Doumas M, Tziomalos K, Karagiannis A, Athyros VG. Sexual dysfunction, cardiovascular risk and effects of pharmacotherapy. Curr Vasc Pharmacol. 2018;16:130-142. [DOI] [PubMed] [Google Scholar]
- 20. Holley JL. The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis. 2004;11(4):337-341. [PubMed] [Google Scholar]
- 21. Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9(1):32-40. [DOI] [PubMed] [Google Scholar]
- 22. Miro F, Parker SW, Aspinall LJ, et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89(10):4910-4915. [DOI] [PubMed] [Google Scholar]
- 23. Prescott L, Eidemak I, Harrison AP, Molsted S. Sexual dysfunction is more than twice as frequent in Danish female predialysis patients compared to age- and gender-matched healthy controls. Int Urol Nephrol. 2014;46(5):979-984. [DOI] [PubMed] [Google Scholar]
- 24. Diaconu CC, Manea M, Marcu DR, Socea B, Spinu AD, Bratu OG. The erectile dysfunction as a marker of cardiovascular disease: a review. Acta Cardiol. 2020;75:286-292. [DOI] [PubMed] [Google Scholar]
- 25. McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13(2):135-143. [DOI] [PubMed] [Google Scholar]
- 26. Jetté N, Quan H, Hemmelgarn B, et al. The development, evolution, and modifications of ICD-10: challenges to the international comparability of morbidity data. Med Care. 2010;48(12):1105-1110. [DOI] [PubMed] [Google Scholar]
- 27. Dohoo IR, Wayne Martin S, Stryhn H. Methods in epidemiologic research. Charlottetown, P.E.I.: VER, Incorporated; 2012. [Google Scholar]
- 28. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20(6):690-695. [DOI] [PubMed] [Google Scholar]
- 29. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28(4):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Ek GF, Gawi A, Nicolai MPJ, et al. Sexual care for patients receiving dialysis: a cross-sectional study identifying the role of nurses working in the dialysis department. J Adv Nurs. 2018;74(1):128-136. [DOI] [PubMed] [Google Scholar]
- 31. Yodchai K, Hutchinson AM, Oumtanee A. Nephrology nurses’ perceptions of discussing sexual health issues with patients who have end-stage kidney disease. J Ren Care. 2018;44(4):229-237. [DOI] [PubMed] [Google Scholar]
- 32. Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Ren Care. 2010;36(suppl 1):4-8. [DOI] [PubMed] [Google Scholar]
- 33. Ishikura F, Beppu S, Hamada T, Khandheria BK, Seward JB, Nehra A. Effects of sildenafil citrate (Viagra) combined with nitrate on the heart. Circulation. 2000;102:2516-2521. [DOI] [PubMed] [Google Scholar]
- 34. Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients,’ clinicians’ and the research communities’ priorities for treatment research: there is an important mismatch. Res Involv Engagem. 2015;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manns B, Hemmelgarn B, Lillie E, Dip SCPG, Cyr A, Gladish M, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jun M, Manns B, Laupacis A, et al. Assessing the extent to which current clinical research is consistent with patient priorities: a scoping review using a case study in patients on or nearing dialysis. Can J Kidney Health Dis. 2015;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151-164. [DOI] [PubMed] [Google Scholar]
- 38. Tannenbaum C, Greaves L, Graham ID. Why sex and gender matter in implementation research. BMC Med Res Methodol. 2016;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gajbhiye SV, Jadhav KS, Marathe PA, Pawar DB. Animal models of erectile dysfunction. Indian J Urol. 2015;31:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sironi G, Colombo D, Poggesi E, et al. Effects of intracavernous administration of selective antagonists of α1-adrenoceptor subtypes on erection in anesthetized rats and dogs. J Pharmacol Exp Ther. 2000;292:974-981. [PubMed] [Google Scholar]
- 41. Bao Y-W, Yuan Y, Chen J-H, Lin W-Q. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39:72-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai RS, Alexander MS, Marson L. Activation of somatosensory afferents elicit changes in vaginal blood flow and the urethrogenital reflex via autonomic efferents. J Urol. 2008;180(3):1167-1172. [DOI] [PubMed] [Google Scholar]
- 43. Strippoli GFM, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15(2):411-419. [DOI] [PubMed] [Google Scholar]
- 44. Smyth B, Haber A, Trongtrakul K, et al. Representativeness of randomized clinical trial cohorts in end-stage kidney disease: a meta-analysis. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee EJ, Patel A, Acedillo RR, et al. Cultivating innovative pragmatic cluster-randomized registry trials embedded in hemodialysis care: workshop proceedings from 2018. Can J Kidney Health Dis. 2019;6. doi: 10.1177/2054358119894394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bello A, Hemmelgarn B, Manns B, Tonelli M, Alberta Kidney Disease Network. Use of administrative databases for health-care planning in CKD. Nephrol Dial Transplant. 2012;27(suppl 3):iii12-iii18. [DOI] [PubMed] [Google Scholar]
- 48. Suissa S, Henry D, Caetano P, et al. CNODES: the Canadian Network for Observational Drug Effect Studies. Open Med. 2012;6:e134-40. [PMC free article] [PubMed] [Google Scholar]
- 49. Molnar AO, Barua M, Konvalinka A, Schick-Makaroff K. Patient engagement in kidney research: opportunities and challenges ahead. Can J Kidney Health Dis. 2017;4:doi: 10.1177/2054358117740583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang E, Bansal A, Novak M, Mucsi I. Patient-reported outcomes in patients with chronic kidney disease and kidney transplant-part 1. Front Med (Lausanne). 2017;4:254. [DOI] [PMC free article] [PubMed] [Google Scholar]