Abstract
Inflammatory bowel diseases (IBDs), including ulcerative colitis and Crohn’s disease, are chronic inflammatory disorders of the gastrointestinal tract. With in-depth studies on the mechanisms of the initiation and development of IBD, increasing lines of evidence have focused on the intestinal microbiota in the pathogenesis of IBD. The imbalance between the host and intestinal microbiota induces dysregulated immune response in intestinal mucosa and plays a pivotal role in the initiation of disease and ongoing bowel destruction. This review focuses on recent advances in intestinal microbiota regulation of mucosal immune response as well as novel approaches based on intestinal microbiota alterations in the diagnosis and evaluation of therapeutic response in IBD.
Keywords: diagnosis, immune response, inflammatory bowel disease, microbiota, therapeutic response
Introduction
The gut microbiome is maintained in a state of mutual benefit and symbiosis with the host under physiological conditions and encodes a variety of functional genes, which enrich the human genome. Gut microbes, together with intestinal epithelial barrier and immune cells, form the complex intestinal microecosystem and perform two-sided functions, which do not merely contribute to systemic metabolism but also impact intestinal homeostasis. The intestinal microbes inhabit the surface of the lumen, which are mainly composed of plenty of bacteria, viruses, fungi, and parasites. The number of bacteria has been proved amount to about 1014, which is almost tenfold of human cells. The total mass of intestinal bacteria is about 0.2 kg, accounting for 60% of the dry mass of the stool, and there are more than 50 phylotypes and about 1100 species.1 Among them, Firmicutes, Bacteroidetes, and Actinobacteria form a major part of the gut microbiota, whereas the frequencies of Proteobacteria and Fusobacteria are correspondingly lower. The formation of a homeostatic microbial spectrum is closely associated with sex,2 maternal health status,3–5 the modes of childbirth (vaginal delivery or Cesarean delivery),6 the diet composition in early childhood,6,7 antibiotic use,7 and contact with pets.2,3 These factors at discrete stages display significant functions in gradually forming a balanced and harmonious state of the organism’s microbial community. Previous studies have demonstrated that the intestinal microbial diversity of infants under 1 year of age proliferates and tends to be stable by the age of 3.8,9 The complexity of the intestinal microbiota (number of taxa and functional genes) usually reaches the levels of adult before puberty. However, it is still different in taxonomy and function from adults. Generally, the number of Bacteroides is maintained at a relatively high level, whereas Bifidobacterium is lower.10 Moreover, various environmental factors such as smoking, air pollution, hygiene habits, psychological stress, diets, and drugs have been proven to be involved in modulating the composition of intestinal microorganisms.11
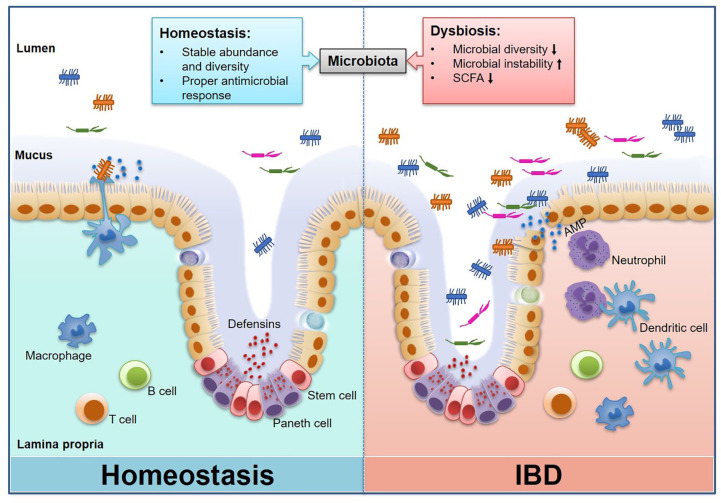
The intestinal microbiota interacts with the host physiological process to assist in carbohydrate and amino acid metabolism,12 energy harvest and storage from the diet,13 protection against epithelial cell damage,14 and induction of intestinal angiogenesis.15 Previous studies have demonstrated an increase of susceptibility to food allergy in germ-free (GF) mice,16 and antibiotic-treated wild-type mice, devoid of microbiota, also have an increased susceptibility to food allergy.17 In addition, the early exposure to microbial antigens and microorganism colonization modulates host intestinal immune system development,18 while microbial dysbiosis is generally considered to be associated with intestinal and systemic diseases, such as inflammatory bowel diseases (IBDs) (Figure 1), allergy, cancer, and metabolic abnormalities. In this review, we summarize the new concept on the relationship between intestinal microbiota and mucosal immunity in IBDs.
Figure 1.
Interaction between intestinal microbiota and host immune response in normal and IBD states. Gut microbes play an essential role in maintaining the intestinal mucosal immune homeostasis, including the maintenance of intact intestinal mucosal barrier and moderate immune response to antigens. Disturbances in the abundance and diversity of intestinal microorganisms drive impaired function of the host intestinal immune system, and thus become a potential cause of a series of intestinal and systemic diseases including IBDs.
IBD, inflammatory bowel disease.
Unique microbial signatures in IBD
A retrospective study has demonstrated that antibiotic use during pregnancy and childhood significantly increases the risk of very early onset of IBD,19 suggesting that the dysbiosis of intestinal microbiota is associated with the initiation of IBDs. An analysis of the intestinal microbiota within patients with IBDs also reveals a prominent alteration in their microbial spectrum, with reduced diversity [50% in Crohn’s disease (CD) and 30% in ulcerative colitis (UC)] and increased microbial instability.20,21
Alternations of microbiota in patients with CD
Actinobacteria and Proteobacteria have been found to significantly increase in fecal samples, especially the more adherent and invasive Enterobacteriaceae, which have been detected in new-onset of patients with CD.22 On the contrary, Bacteroides and Firmicutes have been observed to be markedly decreased, and particularly, the Lactobacillus capable of producing short-chain fatty acids (SCFAs) is notably reduced in these patients.20 Further analysis on fecal microbiota of patients with CD reveals a significant increase in Enterobacteriaceae. Consistently with the data from patients with CD, a subsequent murine colitis model also illustrates that tungstate could ameliorate the inflammatory responses via inhibiting the excessive growth of Enterobacteriaceae.23 Another study has demonstrated that Faecalibacterium prausnitzii is significantly reduced in the stools of patients with CD,24 which has been proven to be closely associated with disease relapse. Moreover, evidence has further demonstrated that F. prausnitzii transplanted into a murine model of chronic colitis could relieve inflammatory response in the intestinal mucosa. Importantly, the treatment with food-grade Lactococcus lactis, which delivers a plasmid encoding microbial anti-inflammatory molecules expressed by F. prausnitzii, elicits a significant remission of experimental colitis in mice, suggesting that anti-inflammatory substances secreted by F. prausnitzii play an essential role in regulating intestinal inflammatory response.24
Alternations of microbiota in patients with UC
Microbial products in the feces of patients with UC have been confirmed to promote intestinal mucosal inflammation, while dendritic cells from healthy volunteers stimulated by filter-sterilized fecal solution from feces of patients with UC and co-cultured with naïve T cells have been observed to profoundly increase the proportion of differentiated type 2 helper T (TH2) cells, which are closely related to the severity of the disease.21 Numbers of Bacteroides and Candida have been observed to significantly increase in feces of patients with UC.25,26 Another analysis has reported that less butyrate is synthesized by Clostridium in the luminal flora of patients with UC, suggesting a protective role of Clostridium in the initiation of UC.27 Moreover, a fecal metabolomics study further revealed an increased frequency of symbiotic Escherichia coli during intestinal inflammation, and its respiration and formate oxidation have been proved to participate in the occurrence of microbial imbalance and intestinal inflammatory response.27 In addition, the alterations of microbiota are also present in patients with defects in the IBD susceptibility genes. For instance, intestinal microbiota analysis of patients with defects in caspase recruitment domain family member 9 (CARD9), one of the admitted IBD susceptibility genes, has shown that the microbes capable of metabolizing tryptophan, such as Lactobacillus, are not adequate to process enough metabolites as aromatic hydrocarbon receptor (AhR) ligands. Analysis of fecal metabolites in patients with UC with CARD9 gene deficiency has also shown that the reduced density of tryptophan-derived indole derivatives elicits downregulated activation of the indole-related AhR signaling pathway and decreased interleukin (IL)-22 production,28 which contribute to the recovery from colitis.
Microbiota dysbiosis is associated with the disease progression of IBD
Previous studies have demonstrated that several microorganisms are involved in the pathogenic progression of IBD.29,30 A retrospective study has shown that the levels of Clostridium difficile, E. coli O157, Salmonella and Staphylococcus aureus toxins are significantly increased in the sera of patients with IBD with a longer course of disease, and that the concentrations of these toxins are also significantly higher in patients with active IBD than those in patients with IBD in remission or healthy volunteers,31 indicating that the toxins produced by the intestinal microbiota may be directly absorbed through the damaged intestinal mucosal barrier into the circulation and participate in the procession of IBD. Therefore, the serum levels of bacterial toxins can be used as indicators of IBD progression. In addition, we have also found that a large number of bacteria is capable of binding IgA or IgG in the fecal samples of patients with active IBD, and that the contents of soluble IgA and IgG in feces are significantly increased in patients with active IBD compared with healthy controls, which are also positively correlated with the disease activity of patients with IBD.32
Summary
Numerous studies have shown that there is a prominent variation in the diversity, abundance, and metabolite of intestinal microbes between patients with IBD and healthy individuals. The variation has been proven to contribute to the disease progression of IBD via impacting the immune response in gut mucosa. Taken together, these findings allow us with the possibility to further study the etiology, pathology, diagnosis and treatment of IBD using the intestinal flora as a breakthrough.
The Janus-faced properties of intestinal microbiota in gut immunity of IBD
Essential role of microbiota in initiating immune responses in gut mucosa
Microbiota has been proved to play an essential role in the formation of a well-developed immune system. Previous data have shown that there are diminished regulatory T (Treg) cells,33 fewer supervised CD4+CD8αα+ intraepithelial lymphocytes,34 impaired innate lymphoid cell function35 and increased susceptibility to bacterial infection in GF mice. Aside from the effects on immune response in mice, relevant data on human study have also shown that the intestinal microbiota displays various properties in promoting proper immune development.36,37 Microbial exposure at the juvenile stage has been found to contribute to the development of human TH cells, especially the establishment of intestinal TH2 immune response.33 Immune disorders caused by insufficient microbial exposure are considered to be one of the etiological agents of IBD. Epidemiological studies have also shown that underexposure of microbiota in juveniles is associated with immune-related diseases in adulthood, called the ‘hygiene hypothesis’,38 and that the underlying mechanisms may be ascribed to the inhibition of the excessive production of IgE from intestinal microbiota.39 An animal experiment has further demonstrated that colitis fails to occur when CD4+CD45RBhigh T cells from GF mice are reconstituted into Rag-1−/−mice, suggesting that the intestinal microbiota is involved in the activation of colitogenic CD4+ T cells and the induction of colitis.40 After oral administration of antibiotics for 7 days, abnormal activation of macrophages is also observed in the intestinal mucosa, which further affects the intestinal mucosal immune homeostasis, especially the immune response regulated by CD4+ T cells.41 Therefore, the intestinal bacterial antigens are involved in a variety of immune responses in the intestinal mucosa.
Role of microbiota-derived antigens in inducing T-cell differentiation in gut mucosa
Evidence has shown that certain microbiota-derived antigens play an immune-mediated destructive or protective role in inducing the differentiation of mucosal CD4+ T cells into TH1, TH17 or Treg cells. Transplantation of Bacteroides fragilis into GF mice induces the differentiation of TH1 cells.41,42 Polysaccharide (PSA) produced by B. fragilis is a prerequisite for the induction of TH1 cell differentiation, which also acts as a ligand for Toll-like receptor 2 on the surface of CD4+ T cells and participates in the activation of CD4+ T cells.41,42 In addition, segmented filamentous bacteria (SFB) have the potential to induce the differentiation into TH17 cells in the intestine.43 Once SFB infiltrates into the mucus layer and contacts with the intestinal epithelial cells (IECs), expression of TH17-related genes is observed to increase in IECs, which is in line with an enhanced proliferation and differentiation into TH17 cells.44 Other intestinal commensal microbes such as altered Schaedler flora could also promote the differentiation of mucosal CD4+ T cells into TH17 cells.45 However, some intestinal commensal microbes, such as Clostridium nucleus and Peptostreptococcus, could induce intestinal CD4+ T cells to differentiate into Treg cells.46 The underlying mechanisms whereby the microbiota-derived antigens induce the differentiation into Treg cells may be ascribed to the induction of IL-10 by intestinal CX3CR1+ phagocytic cells through the symbiotic antigens.47
Role of microbiota-derived antigens in maintaining intestinal homeostasis
The intestinal commensal microbiota is supposed to interact with intestinal immune cells and maintain the homeostasis as well as a stable internal environment. Previous studies have shown that PSA from the outer membrane vesicles (OMV) of B. fragilis activates dendritic cells and induces the release of IL-10 in Treg cells, which functions as an anti-inflammatory mediator in regulating intestinal mucosal immunity.48 Further, this process mainly depends on cellular autophagy-related proteins ATG16L1 and NOD2.49 Consistently, dendritic cells in the intestinal mucosa of patients with CD with ATG16L1 or NOD2 gene deficiency fail to recognize the OMV, which causes reduced Treg cell differentiation and decreased IL-10 secretion. In addition, intestinal commensal bacteria are also able to maintain a stable intestinal innate immune response by limiting excessive colonization and growth of pathogenic bacteria.
Summary
These published data have indicated that the intestinal microbiota prominently affects both the initial development of the immune system and the immune responses in intestinal mucosa under physiological or pathogenic conditions. However, the exact roles of intestinal microbiota in regulating homeostasis in gut mucosa are still not fully understood and need further investigation in the future.
Relevance of intestinal microbial metabolites to regulating immune responses in gut mucosa
Role of microbiota-derived SCFAs in regulating intestinal mucosal immune responses
Immune regulation by microbial metabolites (e.g. SCFAs) on intestinal homeostasis has been paid more attention to better understand the pathogenesis of IBD.50,51 SCFAs mainly consist of acetic acid, propionic acid and butyrate, which are produced by intestinal bacteria through decomposing dietary fiber and play a critical role in intestinal immunity. SCFAs have been confirmed to regulate cell differentiation, proliferation, and exocrine functions via binding G protein-coupled receptors (GPR) 41, GPR43, and GPR109 on the surface of immune cells and IECs. They promote the production of acetyl-CoA and regulate metabolic receptors in B cells, leading to an increase of immunoglobulin production (e.g. IgA, IgG) in gut mucosa and a profound promotion of B cells to differentiate into plasma cells.52 In agreement with these findings, we have also found a decreased secretion of soluble IgA (sIgA) and poor capacity of binding bacteria in the intestinal lumen from Gpr43−/− mice, along with a decrease of intestinal IgA+ B cells. After feeding wild-type mice with acetic acid, sIgA and intestinal IgA+ B cells are observed to increase in feces. On the contrary, the levels of sIgA in feces and the frequencies of IgA+ B cells in intestinal mucosa are not found to recover in Gpr43−/− mice, indicating that acetic acid could induce sIgA secretion by intestinal B cells in a GPR43-dependent manner.53 Another study has also demonstrated that Clostridium, including clusters IV, XIVa, and XVIII, elicits a robust differentiation of mucosal CD4+ T cells into CD4+Foxp3+ Treg cells by producing SCFAs.54 Furthermore, SCFAs are also observed to facilitate intestinal mucosal CD4+ T cells to produce IL-10, which is reduced in Gpr43−/−CD4+ T cells, suggesting that SCFAs modulate CD4+ T-cell differentiation and promote IL-10 production through the GPR43-mediated pathway.55,56
Role of microbiota-derived indole derivatives in regulating intestinal mucosal immune responses
Accumulating lines of evidence have demonstrated that intestinal microbiota metabolizes tryptophan into indole derivatives, including indole, indole acetic acid, indole propionic acid, and indole acetaldehyde, which have been shown to be involved in intestinal homeostasis.57 Analysis of intestinal microbial metabolites in patients with IBD has revealed that reduced tryptophan metabolites (indole, indole propionic acid, and indole acetaldehyde) decrease the biological activity of AhR and cause intestinal inflammatory damage.58 Indole metabolites have been demonstrated to promote IECs to express IL-10R1 and reinforce the intestinal mucosal barrier, while tryptophan kinase-mutant E. coli fails to induce the expression of IL-10R1 in IECs.55 Animal studies also subsequently proved that the indole metabolite could activate the IL-10 signaling pathway and elicit attenuation of colitis.55 In addition, transplantation of fecal microbiota from Card9−/− or Il-22−/− mice into GF recipients promotes the initiation of colitis, while the administration with three Lactobacillus strains (L. murinus CNCM I-5020, L. reuteri CNCM I-5022, L. taiwanensis CNCM I-5019) competent in metabolizing tryptophan, or the remediation with AhR agonists [6-formylindolo (3,2-b) carbazole (Ficz)] elicits the recovery of IL-22 and amelioration of colitis.28 Interestingly, the underlying mechanisms of the colitis induced by fecal microbiota from Card9−/− or Il-22−/− mice are found to be associated with a reduced indole content in the intestinal stool. Other protective effects of Lactobacillus, especially L. murinus, are associated with the induction of Treg cells.59 Therefore, dysbiosis, especially the loss of intestinal microbiota capable of modulating intestinal inflammation, has been extensively proved to contribute to the compromised homeostasis in intestinal mucosa. Furthermore, the administration of specific microbiota, especially targeted therapy aiming at rectifying the microbial dysbiosis in patients with IBD, may provide a therapeutic approach in the management of disease.
Summary
Intestinal dysbiosis and alternations of microbial metabolites (such as SCFAs, indoles) have been proven to be involved in the pathogenesis of IBD. Studies on microbial metabolites may provide therapeutic approaches in the diagnosis and treatment of IBD.
Potentials of microbiota in evaluating clinical diagnosis, treatment and prognosis of IBD
Potential roles of microbiota in the diagnosis and assessment of disease activity of IBD
In recent years, accumulating data on intestinal microecology have been illustrated as a hot spot in the clinical translational study of IBD. Alterations of intestinal microbiota may provide new clues for evaluating clinical diagnosis, treatment, and prognosis of IBD. There are significant differences in the fecal microbial spectrum between patients with IBD and healthy donors, while fecal microbiota transplantation (FMT) in patients with IBD also needs to follow the selectivity of disease.60,61 In a cohort study, they collected feces from 2045 patients with IBD for 16S rRNA analysis, and conspicuous microbial dysbiosis was found in patients with CD, including decreased diversity and abundance. They finally screened out eight types of microbiota to differentiate CD from other diseases, showing that Faecalibacterium, an unknown genus of Peptostreptococcaceae, Anaerostipes, Methanobrevibacter and an unknown genus of Christensenellaceae were abundant in the normal control group and patients with UC, while Fusobacterium and Escherichia were enriched in the feces of patients with CD. Furthermore, Collinsella was detected only in the feces of patients with UC. In addition, the accuracy rate distinguishing CD and non-CD diseases reached 77% when the fecal microbiota was used as the marker, with the sensitivity of 60% and the specificity of 68%. In addition, the accuracy rate identifying CD and UC was about 64%, with the sensitivity of 60% and the specificity of 94.8%.62 Another cohort study collected the feces of 21 patients with remission CD, 17 siblings, and 19 healthy volunteers for microbial profile analysis. The results showed that the fecal microbiota of relatives of patients with CD had similar changes, and that the diversity was markedly decreased compared with healthy controls. Notably, F. prausnitzii was significantly decreased, along with a high risk of CD disease.63 Taken together, these results may partially explain the phenomenon of family aggregation in IBD. Moreover, the levels of microbial metabolites such as SCFAs and indole metabolites in the stool of patients with IBD are significantly decreased,64 which are found to be related to the changes in the intestinal microbial spectrum at the time of onset. Therefore, the alternations of microbial metabolites or microbial spectrum may be used as an indicator of clinical diagnosis and activity assessment of IBD.
Therapeutic approach of FMT in the treatment of IBD
Recently, numerous emerging therapeutic approaches have been applied in the treatment of IBD, including small-molecule inhibitors, monoclonal antibodies against pathogenic proinflammatory cytokines and chemokines, and FMT. With the in-depth understanding of intestinal microbiota, FMT has been implemented as a treatment strategy for several diseases, especially for the treatment of multiply recurrent Clostridioides difficile infection.65 In a randomized controlled study of FMT for active UC, the response rate of the FMT-treated group (24%) at week 7 was significantly higher than that in the placebo group (5%), and the intestinal bacterial diversity of FMT-treated patients was significantly higher than controls.60 A double-blind, controlled study of patients with active UC who received treatment with fecal enema from healthy volunteers demonstrated that the rate of steroid-free clinical remission and endoscopic remission in the FMT group was higher than that in the placebo group at week 8 [risk ratio = 3.6, 95% confidence interval (CI) 1.1–11.9, p = 0.021], and that the intestinal microbial diversity of patients with UC receiving FMT was also higher compared with that in the placebo group.66 A randomized clinical trial of 8-week FMT in patients with active UC using anaerobically treated feces demonstrated that steroid-free remission was achieved in 32% patients (12 of 38) receiving mixed unrelated donor feces compared with 9% patients (3 of 35) receiving autologous feces (difference = 23%, 95% CI 4–42%, odds ratio = 5.0, 95% CI 1.2–20.1, p = 0.03).67 The anaerobic treatment of feces effectively avoided the loss of beneficial obligate anaerobic microbiota, such as F. prausnitzii, which is associated with better clinical remissions in patients with UC.68 However, several limitations still prevent the clinical application of FMT for UC, including inconsistent clinical trial protocol, small sample size, and unstable efficacy. The efficacy of FMT is currently determined in the active phase of treatment in patients with UC, but the long-term tolerance or safety is still less predictable. However, the evidences are still not available on the efficacy of FMT in patients with CD.69,70 A meta-analysis of FMT in patients with CD has demonstrated a clinical remission rate of 52% (95% CI 31–71%, p = 0.063, I2 = 52%) among 71 patients with CD receiving FMT.71 Moreover, the only clinical trial referring to endoscopic remission in patients with CD showed no significant improvement at week 8 among the patients receiving FMT.72 The prospect of FMT in the treatment of patients with CD is still modest giving to the current scanty research data.73 In order to apply the introduction of FMT in clinical treatment for IBD, many questions still need to be solved, including the timing of FMT in patients, individual selectivity, dose of induce remission and long-term maintenance.
The application prospect of intestinal microbiota in IBD
Alterations of intestinal microbiota may be used to predict the therapeutic response in the treatment of IBD. A prospective study of anti-tumor necrosis factor-α in the treatment of UC has demonstrated that the intestinal microbiota before treatment shows less dysbiosis and higher abundance of F. prausnitzii in the responders than that in the nonresponders. Importantly, F. prausnitzii also increases in stool during induction therapy.74 In another study of vedolizumab (i.e. anti-α4β7 monoclonal antibody) in the treatment of IBD, the α diversity of fecal microbiota in patients with CD who responded at week 14 was significantly increased, and the patients with an increased abundance of Roseburia and Burkholderiales species before treatment had higher efficacy.75 Moreover, the intestinal microbiota could also predict the risk of postoperative complications and recurrence in surgically treated patients with IBD. Rumenococcus gnavus, Bacteroides vulgatus, and Clostridium perfringens were observed to increase in feces of patients with UC with pouchitis after ileal pouch-anal anastomosis, while Lacnospiraceae genera and Roseburia were markedly reduced.76 After ileocolonic resection in patients with CD, microbiota changes in the small intestine and anastomotic region directly affected the recurrence of postoperative inflammatory reactions, while F. prausnitzii and Ruminococcus gnavus had been found to have protective roles in this process.77 Another study on intestinal microbiota alterations of patients with CD after ileal resection has proven that the ileectomy elicited a reduction in the α diversity of ileal mucosa-associated microbiota. Proteobacteria (especially Alphaproteobacteria), Coribacteriaceae, and Enterococcus were found to be significantly increased, while Firmicutes (especially Lachnospiraceae) and Ruminococcaceae (especially Eubacterium, Ruminococcus, Butyricoccus, Dorea, and Blautia) were reduced. These changes were observed to be related to endoscopic recurrence and could be a predicted indicator of postoperative recurrence.78
Summary
With the in-depth study of intestinal microbiota, the microbial differences present in patients with IBD are expected to provide a novel approach for the diagnosis, treatment and prognosis of IBD. Although there are still some limitations that could not be ignored in the treatment of IBD through adjusting the composition of intestinal microbiota such as FMT. As our understanding of intestinal flora is getting more thorough, the proper treatment of IBD in virtue of intestinal microbiota will be just around the corner.
Conclusion
Intestinal microbiota plays a key role in regulating immune homeostasis in the enteric internal environment and interacts with mucosal various immune cells responsive to the initiation of IBD. Probing the roles of intestinal microbiota in the initiation and development of mucosal inflammation will provide a new theoretical basis to better understand the pathogenesis of IBD, and importantly, open a novel avenue for future clinical diagnosis, treatment, and prognosis.
Footnotes
Author contributions: ZL was responsible for conception, literature review and revising the manuscript. CH and HL drafted the manuscript and interpreted the results. All authors agreed to the final version.
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was support by the grants from the National Natural Science Foundation of China (81630017, 91740117, 91942312).
ORCID iD: Zhanju Liu  https://orcid.org/0000-0002-0326-543X
https://orcid.org/0000-0002-0326-543X
Contributor Information
Huimin Chen, Department of Pediatrics, Jiaozuo Women and Children Hospital, Jiaozuo, China; Department of Gastroenterology, The Shanghai Tenth People’s Hospital of Tongji University, Shanghai, China.
Hongfen Li, Department of Pediatrics, Jiaozuo Women and Children Hospital, Jiaozuo, China.
Zhanju Liu, Department of Gastroenterology, The Shanghai Tenth People’s Hospital of Tongji University, No. 301 Yanchang Road, Shanghai, 200072, China; Department of Gastroenterology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, 450014, China.
References
- 1. Tamburini S, Shen N, Wu HC, et al. The microbiome in early life: implications for health outcomes. Nat Med 2016; 22: 713–722. [DOI] [PubMed] [Google Scholar]
- 2. Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016; 22: 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durack J, Kimes NE, Lin DL, et al. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun 2018; 9: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu DM, Ma J, Prince AL, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017; 23: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 2018; 9: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Backhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015; 17: 852. [DOI] [PubMed] [Google Scholar]
- 7. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016; 8: 343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vatanen T, Kostic AD, d’Hennezel E, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016; 165: 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hollister EB, Riehle K, Luna RA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018; 562: 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petersen LM, Bautista EJ, Nguyen H, et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017; 5: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J Innate Immun 2018; 10: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018; 50: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reinhardt C, Bergentall M, Greiner TU, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012; 483: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozakova H, Schwarzer M, Tuckova L, et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell Mol Immunol 2016; 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shu SA, Yuen AWT, Woo E, et al. Microbiota and food allergy. Clin Rev Allergy Immunol 2019; 57: 83–97. [DOI] [PubMed] [Google Scholar]
- 18. Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016; 351: 1296–1302. [DOI] [PubMed] [Google Scholar]
- 19. Ortqvist AK, Lundholm C, Halfvarson J, et al. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut 2019; 68: 218–225. [DOI] [PubMed] [Google Scholar]
- 20. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017; 152: 327–339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mar JS, LaMere BJ, Lin DL, et al. Disease severity and immune activity relate to distinct interkingdom gut microbiome states in ethnically distinct ulcerative colitis patients. MBio 2016; 7: e01072–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haberman Y, Schirmer M, Dexheimer PJ, et al. Age-of-diagnosis dependent ileal immune intensification and reduced alpha-defensin in older versus younger pediatric Crohn disease patients despite already established dysbiosis. Mucosal Immunol 2019; 12: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu W, Winter MG, Byndloss MX, et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018; 553: 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016; 65: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryan FJ, Ahern AM, Fitzgerald RS, et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat Commun 2020; 11: 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah J, Dutta U, Rudramurthy S, et al. Colonic mucosa-associated candida assessed by biopsy culture is associated with disease severity in ulcerative colitis: a prospective study. J Dig Dis 2019; 20: 642–648. [DOI] [PubMed] [Google Scholar]
- 27. Hughes ER, Winter MG, Duerkop BA, et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 2017; 21: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016; 22: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rapozo DC, Bernardazzi C, de Souza HS. Diet and microbiota in inflammatory bowel disease: the gut in disharmony. World J Gastroenterol 2017; 23: 2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Britton GJ, Contijoch EJ, Mogno I, et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt regulatory T cells and exacerbate colitis in mice. Immunity 2019; 50: 212–224.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu H, Sun X, Sun M, et al. Serum bacterial toxins are related to the progression of inflammatory bowel disease. Scand J Gastroenterol 2014; 49: 826–833. [DOI] [PubMed] [Google Scholar]
- 32. Lin R, Chen H, Shu W, et al. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. J Transl Med 2018; 16: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohnmacht C, Park JH, Cording S, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 2015; 349: 989–993. [DOI] [PubMed] [Google Scholar]
- 34. Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science 2017; 357: 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 2013; 498: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spencer SP, Fragiadakis GK, Sonnenburg JL. Pursuing human-relevant gut microbiota-immune interactions. Immunity 2019; 51: 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gensollen T, Iyer SS, Kasper DL, et al. How colonization by microbiota in early life shapes the immune system. Science 2016; 352: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 2018; 18: 105–120. [DOI] [PubMed] [Google Scholar]
- 39. Cahenzli J, Koller Y, Wyss M, et al. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013; 14: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev 2001; 182: 190–200. [DOI] [PubMed] [Google Scholar]
- 41. Scott NA, Andrusaite A, Andersen P, et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med 2018; 10: eaao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122: 107–118. [DOI] [PubMed] [Google Scholar]
- 43. Tan TG, Sefik E, Geva-Zatorsky N, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA 2016; 113: E8141–E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Atarashi K, Tanoue T, Ando M, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015; 163: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu W, Chen F, Liu Z, et al. Microbiota-specific Th17 cells: Yin and Yang in regulation of inflammatory bowel disease. Inflamm Bowel Dis 2016; 22: 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sefik E, Geva-Zatorsky N, Oh S, et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 2015; 349: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim M, Galan C, Hill AA, et al. Critical role for the microbiota in CX3CR1(+) intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity 2018; 49: 151–163.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramakrishna C, Kujawski M, Chu H, et al. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat Commun 2019; 10: 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu H, Khosravi A, Kusumawardhani IP, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016; 352: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 51. Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017; 14: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim M, Qie Y, Park J, et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016; 20: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu W, Sun M, Chen F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 2017; 10: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell 2017; 168: 928–943.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun M, Hao T, Li X, et al. Direct observation of selective autophagy induction in cells and tissues by self-assembled chiral nanodevice. Nat Commun 2018; 9: 4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen L, Sun M, Wu W, et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis 2019; 25: 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018; 23: 716–724. [DOI] [PubMed] [Google Scholar]
- 58. Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The aryl hydrocarbon receptor (AhR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol. Epub ahead of print 11 April 2020. DOI: 10.1007/s12016-020-08789-3. [DOI] [PubMed] [Google Scholar]
- 59. Tang C, Kamiya T, Liu Y, et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 2015; 18: 183–197. [DOI] [PubMed] [Google Scholar]
- 60. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149: 102–109.e6. [DOI] [PubMed] [Google Scholar]
- 61. Sokol H, Landman C, Seksik P, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome 2020; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn’s disease. Gut 2017; 66: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hedin C, van der Gast CJ, Rogers GB, et al. Siblings of patients with Crohn’s disease exhibit a biologically relevant dysbiosis in mucosal microbial metacommunities. Gut 2016; 65: 944–953. [DOI] [PubMed] [Google Scholar]
- 64. Lee D, Albenberg L, Compher C, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015; 148: 1087–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saha S, Khanna S. Management of Clostridioides difficile colitis: insights for the gastroenterologist. Therap Adv Gastroenterol 2019; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017; 389: 1218–1228. [DOI] [PubMed] [Google Scholar]
- 67. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019; 321: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cammarota G, Ianiro G. FMT for ulcerative colitis: closer to the turning point. Nat Rev Gastroenterol Hepatol 2019; 16: 266–268. [DOI] [PubMed] [Google Scholar]
- 69. Levy AN, Allegretti JR. Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Therap Adv Gastroenterol 2019; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen T, Zhou Q, Zhang D, et al. Effect of faecal microbiota transplantation for treatment of Clostridium difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of cohort studies. J Crohns Colitis 2018; 12: 710–717. [DOI] [PubMed] [Google Scholar]
- 71. Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2017; 11: 1180–1199. [DOI] [PubMed] [Google Scholar]
- 72. Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis 2016; 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ooijevaar RE, Terveer EM, Verspaget HW, et al. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med 2019; 70: 335–351. [DOI] [PubMed] [Google Scholar]
- 74. Magnusson MK, Strid H, Sapnara M, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis 2016; 10: 943–952. [DOI] [PubMed] [Google Scholar]
- 75. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 2017; 21: 603–610.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Machiels K, Sabino J, Vandermosten L, et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut 2017; 66: 79–88. [DOI] [PubMed] [Google Scholar]
- 77. Mondot S, Lepage P, Seksik P, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut 2016; 65: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sokol H, Brot L, Stefanescu C, et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut 2019; 69: 462–472. [DOI] [PubMed] [Google Scholar]