Abstract
Introduction:
Alpha-mangostin (MAN) possesses a wide variety of pharmacological effects. In this study, we investigated its effect on cholinergic anti-inflammatory pathway (CAP), and tested if CAP regulation was involved in the therapeutic action on acute lung injury (ALI).
Methods:
Male Sprague Dawley rats were pre-treated with MAN (40 mg/kg) for 3 days and ALI was induced with an intraperitoneal injection of lipopolysaccharide (LPS). Certain rats received monolateral vagotomy or sham surgery. The effects on inflammatory reactions and relevant pathways in ALI rats or LPS pre-treated RAW 264.7 cells were investigated by histological, immunohistochemical, immunoblotting, RT-qPCR, and immunofluorescence assays, while levels of proinflammatory cytokines, acetylcholine (Ach) and the enzymatic activity of acetylcholinesterase (AchE) were determined by corresponding quantitative kits.
Results:
Oral administration of MAN reduced the severity of ALI, while vagotomy surgery antagonized this effect. MAN restored the decline in α7 nicotinic acetylcholine receptor (α7nAchR) in the lungs of ALI rats, and promoted the expression of α7nAchR and choline acetyltransferase (CHAT) in RAW 264.7 cells. Although AchE expression was barely affected by MAN at 5 μg/ml, its catalytic activity was reduced by almost 95%. Extracellular rather than intracellular Ach was notably raised shortly after MAN treatment. Furthermore, MAN at 5 μg/ml effectively inhibited LPS-induced increase in phosphorylation and nucleus translocation of p65 subunit, and secretion of TNF-α and IL-1β, which was then offset by methyllycaconitine citrate hydrate.
Conclusion:
MAN activated CAP by increasing peripheral Ach and up-regulating α7nAchR expression, which eventually led to NF-κB inhibition and remission of acute inflammations.
Keywords: α-mangostin, alpha7 nicotinic acetylcholine receptor, cholinergic anti-inflammatory pathway, immunity, inflammation
Introduction
α-Mangostin (MAN) is a bioactive xanthone isolated from the pericarp of mangosteen (Garcinia mangostana Linn.).1 It possesses various bioactivities and exhibits promising therapeutic potentials against infections, inflammations, tumors, malaria and allergies.1,2 Among these properties, its anti-inflammatory effect has been well investigated and documented.1,2 Our previous studies also demonstrated that MAN was effective in controlling both acute and chronic inflammations in vivo.3–5 However, the mechanism underlying its anti-inflammatory actions is still not fully understood.
The recently conceptualized cholinergic anti-inflammatory pathway (CAP) reflects the sophisticated interplay between the nerve system and inflammatory reactions.6 Sustained activation of CAP triggers rapid and systematic remission of inflammatory symptoms, which eventually reshapes the cytokines profile. As such, this signaling pathway has profound implication in anti-inflammatory therapeutics and numerous studies have solidly proven that CAP is a promising target for the treatment of inflammatory diseases, including sepsis, hemorrhagic shock, colitis, and arthritis.6,7 The molecular foundation of CAP is based on the interaction between acetylcholine (Ach) and alpha7 nicotinic acetylcholine receptor (α7nAchR) [7-8].6,7 α7nAchR is a member of the superfamily of cys-loop cationic ligand-gated channel. α7nAchR is highly expressed on immune cells, such as macrophages and lymphocytes.7,8 This is consistent to the vital role it plays in immune regulation.6–9 Accordingly, stimulation of α7nAChR has been confirmed to be beneficial for the alleviation of several inflammation and immune-related diseases.6
Based on the current understanding of CAP, electric stimulation of vagi could be a direct and effective tactic to achieve rapid alleviation of inflammations. In spite of numerous evidences regarding the successful tests on animal models, the application of this method is difficult in clinical practice, as it usually requires the inconvenient surgical implantation of nerve cuff electrode, whose long-term safety concerns has not been fully addressed.6 Upon comparison, pharmacological manipulation of either biosynthesis/release of Ach or α7nAchR expression becomes a more pragmatic option.
Previously, we observed that treatment of collagen-induced arthritis (CIA) with MAN rich mangosteen extract induced heavy sweating in rats shortly after the administration and increased the expression of α7nAchR.4 As well known, eccrine sweat gland mainly function in response to Ach stimulus.10 Inspired by these clues, we speculated that this extract could exert anti-inflammatory effects through the activation of CAP in vivo. However, the exact influence of the MAN on CAP is yet to be clarified. In this study, we attempted to identify the involvement of CAP regulation in the anti-inflammatory properties of MAN.
Materials and methods
Chemicals and reagents
MAN (purity > 98%) was purchased from SanHerb Bioscience Inc. (Chengdu, Sichuan, China). Lipopolysaccharide (LPS, from gram-negative bacteria E. coli 055:B5), nicotine (Nic) and methyl lycaconitine citrate hydrate (MLA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), RMPI-1640 medium, phosphate buffered saline (PBS), penicillin-streptomycin together with enhanced chemiluminescence (ECL) detection kit were supplied by Thermo Scientific (Rockford, IL, USA). Antibodies and BCA protein quantitative kit were purchased from Cell Signaling Technology (Beverly, MA, USA) and Keygen Biotech (Nanjing, Jiangsu, China), respectively. Ultra-pure water was prepared by using a Milli-Q purification system (Millipore, Bedford, MA, USA).
LPS challenge in rats and treatments
Thirty-six male Sprague Dawley rats (7 weeks old) were supplied by Qinglongshan Laboratory Animal Company (Nanjing, Jiangsu, China). The animals were housed under standard conditions (with temperature and relative humidity at 24°C and 50%, respectively and a 12 h light and dark cycle). All the animal experimental procedures were approved by the Ethical Committee of Yijishan Hospital (Ethics approval number: YJS 2019-3-008), and strictly in accordance with the guideline for the care and use of laboratory animals (United States National Research Council, 2011). After 7 days of acclimatization, rats were randomly allocated to six groups (six rats per group).
The groupings are as follows:
Group 1: Healthy normal control
Group 2: Healthy rats receiving vagotomy (surgery)
Group 3; Healthy rats receiving sham operation (sham surgery)
Group 4: Acute lung injury (ALI) models (model control)
Group 5: MAN treated ALI rats (MAN treatment)
Group 6: ALI rats receiving MAN treatment together with vagotomy surgery (MAN + surgery).
Rats in groups 5 and 6 were given MAN at 40 mg/kg in the form of suspension by oral gavage for three consecutive days prior to the surgery and ALI induction. ALI (rats in groups 4–6) was induced by the administration of an intraperitoneal injection of LPS (5 mg/kg). Prior to the induction of ALI, monolateral vagi was deliberately cut to block the cholinergic nerve conduction in some rats (groups 2 and 6). At the same time, rats in group 3 received the same surgical procedures but without the vagotomy to rule out negative effects brought by operation injuries.
Sampling and histological/immunohistochemical examinations
Twelve hours after the induction of ALI, all the rats were anesthetized with chloral hydrate and blood samples were collected though the abdominal aorta. The blood samples were centrifuged at 3000 rpm for 5 min to obtain the serum. Levels of IL-1β, TNF-α, and Ach in the serum were measured by using commercially available ELISA or colorimetric assay kits (Multisciences Biotech, Hangzhou, China) according to the manufacturer’s protocols.
After the sacrifice, the lungs were quickly excised from the rats, washed in cold PBS and fixed in neutral buffered formalin. The specimens were subsequently cut into two parts, which were used for histological and immunohistochemical examinations respectively. Briefly, lungs embedded in paraffin were sectioned, mounted on glass slides and stained with hematoxylin/eosin (H&E) for the general histological examination. Main pathological characteristics related to ALI including alveolar wall incrassation, inflammatory infiltration, alveolar hemorrhage were observed and evaluated under a light microscope (Olympus BH-2, Tokyo, Japan). Some other dewaxed sections were subjected to rehydration, epitope retrieval and serum blocking procedures. Afterwards, the processed slides were incubated with anti- α7nAchR primary antibody, and followed by HRP-conjugated secondary antibody incubation. α7nAchR was visualized by 3,3-diaminobenzidine staining, and the counterstaining with hematoxylin was carried out finally.
Cell culture and cytokines secretion evaluation
Murine macrophage derived RAW264.7 cells were used for the in vitro experiments to validate the impacts of MAN on CAP. The cells were grown in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin under the normal culture conditions (37°C, humidified atmosphere with 5% CO2). The medium was replaced once a day and the passage was carried out every 2 days. The in vitro experiments were performed in triplicate.
To simulate inflammatory conditions, cells except for normal control were pre-treated with LPS (1 μg/ml) for 1 h. Afterwards, half of the supernatant in the wells was replaced with the test compounds containing fresh medium and further incubated for 24 h. The treatment arrangement was as follows: normal control, LPS stimulation, LPS stimulation plus MAN treatment (5 μg/ml), LPS stimulation plus Nic treatment (5 μM), LPS stimulation plus MAN+MLA co-treatment (5 μM for MLA). After the treatments, the supernatant was collected for the determination of IL-1β and TNF-α in the medium by using ELISA kits (Multisciences Biotech, Hangzhou, China).
Evaluation of Ach extracellular release and the enzymatic activity
Cells at the exponential growth stage were seeded in a 6-well plate at a density of 1 × 106 cells per well and allowed to attach overnight. Then, the cells were treated with MAN (5 μg/ml) at various time (0.5, 1, 2, 4, and 6 h) and untreated cells at each time interval were taken as normal control. After the treatment, the supernatant and cells were collected. The cells were subjected to three rounds of freeze-thaw cycle in PBS and the supernatant was obtained after centrifuging at 12000 rpm for 10 min at 4°C. Ach contents in the medium and cells were analyzed using a colorimetric assay kit supplied by Keygen Biotech (Nanjing, Jiangsu, China) according to the manufacturer’s instruction.
For the enzymatic activity assessment of acetylcholinesterase (AchE), attached cells were treated with MAN at various concentrations (0, 2.5, 5, and 10 μg/ml) for 4 h. The cells were then harvested by trypsinization and subjected to supersonic treatments in ice-water bath. The lysate obtained after centrifugation (8000 rpm for 10 min at 4°C) was subjected to quantitative analysis using a colorimetric assay kit (Solarbio, Beijing, China).
RT-qPCR, immunoblotting and immunofluorescence assays
In order to investigate the possible effects of MAN on CAP related signaling, we carried out RT-qPCR and western-blot analysis. Details about the experimental procedures were as reported previously.11 Primers were synthesized by Sangon Biological Engineering Technology and Service Company (Shanghai, China), and the oligonucleotide sequences were as follows: CHRNA7, F: 5’-GCGAGTTCCAGAGGAAGCTTTAC-3’, R: 5’-ACGGTGAGTGGTTGCGAGTC-3’; AchE, F: 5’-AATGACACAGAGCTGGTAGCCT-3’, R: 5’-CACGAAGGAGAACCGGAAGA-3’; CHAT (choline acetyltransferase), F: 5’-TGCCGCCTACTGAGAGCAAG-3’, R: 5’-GGGTCTGGCTGTTCTAGAGGCT-3’; GAPDH, F: 5’-GGCCTT‘CCGTGTTCCTACC-3’, R: 5’-TGCCTGCTTCACCACCTTC-3’.
By using GAPDH as a reference, the relative expression of target genes was normalized through the 2-DeltaDeltaCt calculation. To perform the immunoblotting analysis, cells were lysed in RIPA buffer to obtain whole protein samples, while the nucleus protein was extracted through fractional centrifugations using an extraction kit provided by Solarbio (Beijing, China). GAPDH and histone were adopted as the internal references in the two experiments, respectively. The separation of proteins was achieved through SDS-PAGE and signals were developed using the ECL approach after the immunoreactions.
The evaluation of NF-κB p65 subunit intracellular distribution was mainly achieved by immunofluorescence method. Briefly, cells in 6-wells plate were treated with LPS, MAN, or together with MLA for 6 h and then fixed with 0.5% Triton-X to allow antibodies/probes permeation. After extensive washing with cold PBS, the cells were blocked with normal goat serum, and incubated with primary antibodies overnight at 4°C. To observe p-p65, a further incubation with FITC tagged secondary antibodies was carried out, while a cy3 conjugated secondary antibody was used to visualize p65. Counterstaining with Hoechst was used to dye the nucleus and identify the location of p65 subunit with the aid of a BX53 fluorescence microscope (Olympus, Tokyo, Japan) or a LSM800 laser confocal microscope (Cal Zeiss, Göettingen, Germany).
Statistical analysis
The sample size was calculated based on the post-hoc power analyses by using the G*power 3.1 software (http://www.gpower.hhu.de/). The threshold of statistical power was set at 0.8. Statistical analyses were performed using SPSS statistical analysis software (SPSS, Chicago, IL, version 14.0). The differences among different groups were evaluated based on one-way analysis of variance coupled with Tukey post hoc test. Statistically significant was determined at *P < 0.05 and ** P < 0.01.
Results
CAP regulation was involved in therapeutic actions of MAN on ALI
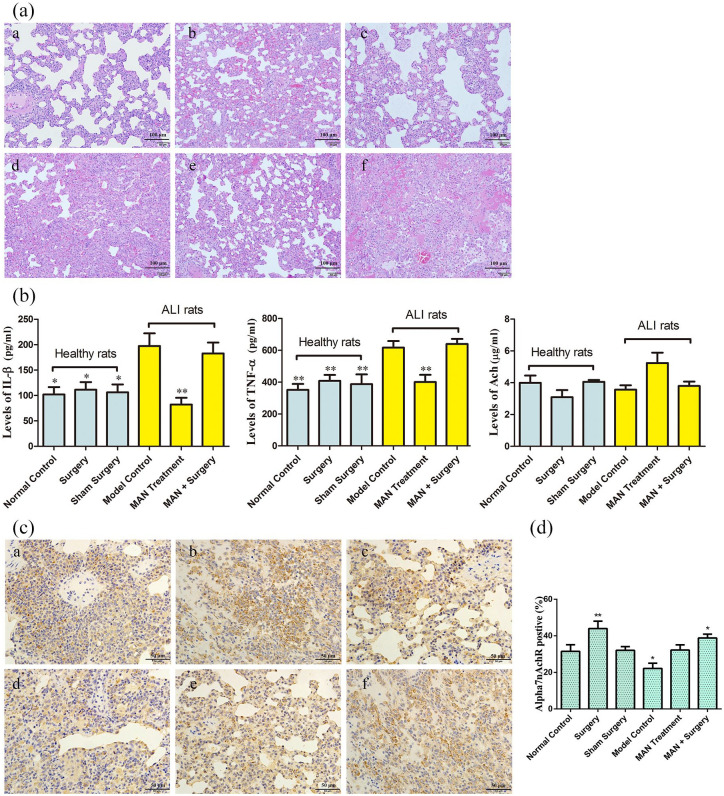
Treatment with MAN attenuated the pathological changes observed in the lungs of ALI rats. Alveolar wall thickening was greatly alleviated, while inflammatory cells infiltration and alveolar hemorrhage were also reduced to certain extent. All these therapeutic effects displayed by MAN treatments were totally antagonized by vagotomy, suggesting that the therapeutic actions of MAN on ALI could be mediated through vagi excitement and CAP activation. At the same time, we observed that both vagotomy and sham operation had little effects on the histological structure of the lungs (mainly internal hemorrhage). This observation basically ruled out the possibility that aggravated severity of ALI in rats receiving both MAN treatments and vagotomy was caused by surgical injuries or nerve disorders (Figure 1(a)).
Figure 1.
Therapeutic effects of MAN on ALI rats and its regulatory effects on CAP in vitro. (a) Histological examination of rat lungs (H&E staining); (b) quantitative analyses of serological biomarkers in rats, assessed by the ELISA or colorimetric methods (n = 6); (c) α7nAchR expression in rat lungs, investigated by the immunohistochemical method; (d) quantification of experiment C (n = 3). For a and c: a-f represented healthy controls, healthy rats receiving vagotomy, healthy rats receiving sham surgery, ALI models, MAN treated ALI rats, and ALI rats receiving MAN treatment together with vagotomy surgery, respectively. Statistical significance: *P < 0.05 and **P < 0.01 compared with ALI models in b; *P < 0.05 and **P < 0.01 compared with normal healthy controls in d.
Cytokines network disturbance plays a major role in initiating, amplifying, and perpetuating ALI, and the resulting cytokines storm characterized by the accumulation of IL-β, TNF-α and other pro-inflammatory cytokines has been identified as the critical event accounting for the deterioration of ALI.12 MAN significantly reduced IL-β and TNF-α levels in the serum of ALI rats, which was then completely offset by vagotomy. Both vagotomy and sham operation exerted no effects on these cytokines. These clues together could serve as solid evidences supporting the involvement of CAP in the therapeutic actions of MAN on ALI. In addition, an increase in circulating Ach levels was observed in rats treated with MAN, although this change was not statistically significant (Figure 1(b)). Compared with the healthy control, α7nAchR expression in the lungs of ALI rats was obviously decreased, while MAN restored the expression of α7nAchR in the treated rats (Figure 1(c)). α7nAchR was significantly overexpressed in rats receiving vagotomy, which may be associated with a possible compensation feedback (Figure 1(d)). That is, the dissection of vagi caused an insufficient release of Ach, leading to an up-regulation of α7nAchR as a means of compensation.
MAN up-regulated α7nAchR expression in RAW 264.7 cells in vitro
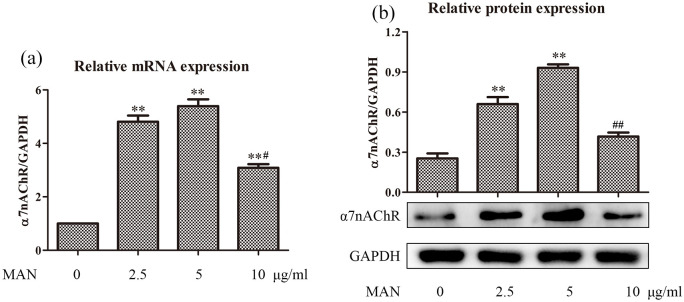
As indicated above, MAN simultaneously increased the circulating Ach and α7nAchR expression in the lungs of ALI rats, which would lead to sustained activation of CAP and consequently alleviate inflammations. To further consolidate this hypothesis, we carried out in vitro experiments. Firstly, we tested the effect of MAN on α7nAchR expression in RAW 264.7 cells. Our previous study found that MAN stimulus at concentrations below 10 μg/ml had no significant cytotoxicity on these cells within 24 h.5 Meanwhile, it exerted significant anti-inflammatory effects on LPS pre-treated RAW 264.7 cells at 3 μg/ml.3 As such, 2.5, 5, and 10 μg/ml were adopted as low, medium, and high treatment concentrations in the following experiments. As anticipated, the expression of gene CHRNA7 was significantly up-regulated upon treatment with MAN. The most effective regulation was observed at 5 μg/ml rather than 10 μg/ml (Figure 2(a)). Similar phenomenon was also observed concerning the expression of protein α7nAchR in vitro. Initially, MAN increased α7nAchR expression in a concentration dependent manner. However, the regulatory effect was greatly abrogated at 10 μg/ml (Figure 2(b)).
Figure 2.
MAN up-regulated α7nAchR/CHRNA7 expression in RAW 267.4 cells in vitro. (a) Levels of gene CHRNA7 expression, investigated by RT-qPCR (n = 3); (b) levels of protein α7nAchR expression, investigated by immunoblotting assay (n = 3). Statistical significance: **P < 0.01 compared with untreated cells; #P < 0.05 and ##P < 0.01 compared with cells treated by MAN at 5 μg/ml.
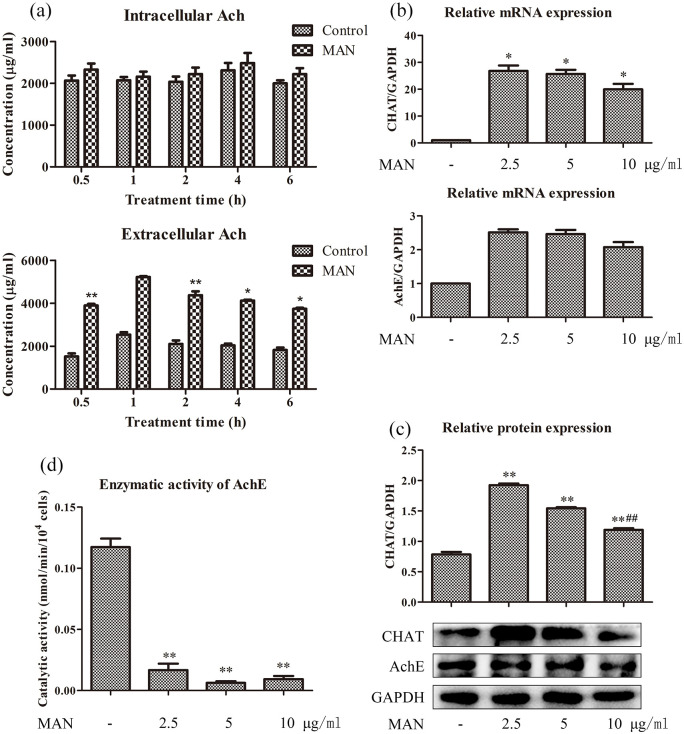
MAN increased intracellular Ach through multiple approaches
Aside the expression of α7nAchR, circulating Ach is another important factor determining CAP status.6,7 Therefore, we quantified Ach levels to assess the effects of MAN on the biosynthesis and release of Ach in RAW 264.7 cells. Upon comparison with untreated cells, we found that MAN had no influence on intracellular Ach, but it caused significant increase in extracellular Ach levels (Figure 3(a)). Two plausible theories were raised to explain this phenomenon: MAN promoted Ach biosynthesis and subsequent secretion; MAN slowed down the degradation velocity of Ach. Because CHAT and AchE serve as rate-limiting enzymes in the biosynthesis and degradation processes of Ach respectively, we investigated the two using PCR and immunoblotting methods. PCR analyses suggested that MAN significantly promoted mRNA expression of CHAT but not AchE, suggesting that the increase in production of Ach was theoretically plausible during MAN treatment (Figure 3(b)). Accordingly, MAN increased protein CHAT expression in RAW 264.7 cells, and had no influence on the expression of AchE. This effect was observed to be gradually weakened as the treatment concentration increased (Figure 3(c)). Although MAN had no effect on AchE expression, it could be an efficient antagonist of AchE, as it inhibited the catalytic activity of this enzyme with high efficacy in vitro (Figure 3(d)).
Figure 3.
Possible mechanism involved in the regulation of MAN on Ach levels. (a) Quantitative results of intracellular and extracellular Ach levels in RAW 264.7 cells under MAN treatments at 5 μ/ml for varied time, determined by the colorimetric method (n = 3); (b) mRNA expression of gene CHAT and AchE, investigated by RT-qPCR (n = 3); (c) protein expression of CHAT and AchE, investigated by immunoblotting assay (n = 3); (d) enzymatic activity of AchE in RAW 264.7 cells under MAN treatments in vitro (n = 3). Statistical significance: *P < 0.05 and **P < 0.01 compared with untreated cells; #P < 0.05 compared with cells treated by MAN at 2.5 μg/ml.
MAN reduced inflammatory actions through CAP activation in vitro
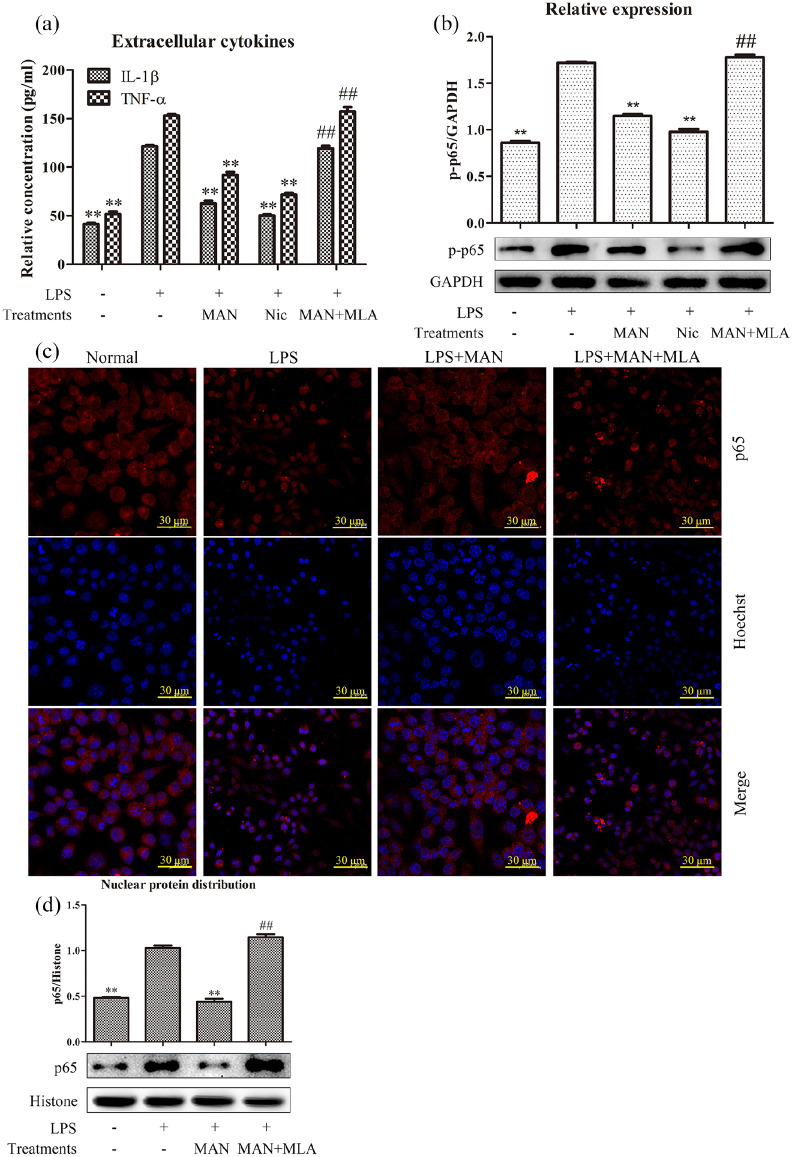
As a typical endotoxin, LPS greatly promoted the secretion of pro-inflammatory cytokines IL-1β and TNF-α in RAW 264.7 cells. This phenomenon was effectively reversed by MAN treatment. The well characterized α7nAchR agonist Nic exhibited similar anti-inflammatory effects compared with MAN, while the antagonist MLA completely restored the cytokines decline elicited by MAN (Figure 4(a)). It further confirmed that activation of α7nAchR is a pragmatic approach to treat inflammations, and manipulation of CAP was involved in the anti-inflammatory actions of MAN. As the production of many pro-inflammatory cytokines including IL-1β and TNF-α is controlled by NF-κB, we further investigated this pathway. Consistent to their effects on cytokines secretion, immunoblotting assay showed that both MAN and Nic efficiently suppressed the increased p-p65 expression induced by LPS, and the potent anti-inflammatory potentials of MAN was totally offset by MLA once again in this assay (Figure 4(b)). Results from immunofluorescence observation further consolidated this finding. As shown in Supplementary S1, significant phosphorylation of p65 occurred under LPS stimulus, which was then abrogated by MAN. Co-treatment with MLA attenuated the suppressive effects of MAN on p65 phosphorylation. Observation under the fluorescence microscope also revealed the increased distribution of p65 in nucleus (Supplementary S1). Subsequently, we further characterize changes of p65 intracellular distribution using a laser confocal microscope. It was found that p65 subunit was extensively distributed in cytoplasm but not nucleus in normal cells. LPS caused visible aggregation of p65 in the nucleus, which is essential for the transcriptional regulatory activity of NF-κB, while MAN posed a negative effect on this process. The effect elicited by MAN on p65 nucleus translocation was completely antagonized by MLA (Figure 4(c)). The immunoblotting assay obtained similar results, and further validated the findings mentioned above (Figure 4(d)).
Figure 4.
CAP activation contributed to the inhibition of MAN on NF-κB signaling in vitro. (a) Levels of pro-inflammatory cytokines secretion in LPS pre-treated RAW 264.7 cells receiving either LPS or in combination with test chemicals, determined by the ELISA method (n = 3); (b) levels of p-p65 expression in RAW 264.7 cells receiving treatments with either LPS or in combination with test chemicals, assessed by immunoblotting assay (n = 3); (c) effects of MAN and α7nAchR agonist/antagonist on p65 intracellular distribution in LPS pre-treated RAW 264.7 cells, investigated by immunofluorescence assay; D, nuclear distribution of p65 subunit in RAW 264.7 cells under different treatments, investigated by immunoblotting assay (n = 3). The concentration of MAN, Nic and MLA were 5 μg/ml, 5 μM, and 5 μM, respectively. Statistical significance: **P < 0.01 compared with LPS treated cells, ##P < 0.01 compared with LPS+MAN treated cells.
Discussion
Sufficient evidence has suggested that the anti-inflammatory properties of MAN in vivo is closely related to its effects on cytokines profiles and macrophages seem to be its main target.13,14 This notion was supported by our previous study, as MAN exhibited promising therapeutic effects on ALI, which is believed to be mainly mediated by macrophages.3 Because macrophages do not only sense and cope with infections directly, but also initiate adaptive immune reactions by functioning as antigen presenting cells. Thus, the influence of MAN on macrophage inevitably affects lymphocytes, and thereby has profound impact on immunity. In a recent report, we confirmed that MAN suppressed the differentiation of Th17 cells and restored the immune homeostasis in CIA rats through its regulatory effect on macrophages.15 This study also shed additional insight on elucidating the anti-inflammatory mechanism of MAN from an novel perspective. Many researches focused on traditional inflammatory pathways, and NF-κB was usually identified as an important therapeutic target of MAN because of its undisputed role in cytokines production and inflammatory reactions.16,17 Any changes leading to NF-κB inhibition in macrophages will benefit the improvement of immune milieu theoretically. However, down-regulation of NF-κB in immune cells is not always time efficient and sustainable, due to the redundancy and complexity of upstream signal cascades. As such, the mystery behind the fast and effective anti-inflammatory actions of MAN is still largely unknown. It has been demonstrated that CAP activation significantly contributed to the anti-arthritic effect of MAN in CIA rats.15 CAP responds rapidly to external stimulus. Under this context, we speculated that CAP activation might be involved in the therapeutic actions of MAN on NF-κB-controlled acute inflammation. Results obtained from this study basically supported this hypothesis.
Neuro-immune feedback CAP evades the redundancy of classic immunologic processes and rapidly inhibits pro-inflammatory cytokines secretion under some critical conditions such as sepsis.18 Hence, manipulation of CAP has become a fascinating anti-inflammatory option especially for acute inflammations considering its great clinical significance. Treatments with Nic effectively reduced various inflammations in vivo.19,20 However, such regimen also raises concerns about addiction and other side effects associated with unselective bonding with other receptors subtypes in the nervous system. Consequently, more emphases have been attached to the synthesis and identification of selective α7nAchR agonist in recent years. As expected, these compounds exhibited encouraging anti-inflammatory potentials in animal models.21,22 Unfortunately, even the most investigated reagents cannot be used in clinical practices in the near future unless the safety concerns are fully addressed. Comparatively, exploration of bioactive compounds with good safety profile such as MAN seems to be a more feasible approach. Although molecular docking studies suggested that MAN did not directly bind to α7nAchR (data not provided), it substantially activated CAP through several other mechanisms. Firstly, MAN could act as an antagonist of AchE, and the accumulated Ach would elicit sustained activation of CAP locally. Secondly, it promoted the production and release of Ach, which is also essential for the extracellular increase of Ach. Furthermore, it promoted the expression of α7nAchR at both mRNA and protein levels. These evidences indicated that MAN as well as certain compounds sharing similar structural resemblance could be good candidates as novel anti-inflammatory agents by targeting CAP.
Mounting reports solidly confirm xanthone derivatives as effective AchE antagonists.23,24 We further demonstrated that MAN promoted CHAT expression in vitro. These results suggested that MAN crippled the degradation and increased the biosynthesis of Ach simultaneously. Because intracellular Ach was barely affected by MAN, it is highly plausible that Ach accumulation observed in this study was mainly caused by the inhibition on AchE, while the direct consequences from CHAT increase are still needed to be investigated. It was also observed that MAN treatment caused an increase in both circulating Ach and α7nAchR expression at the same time. These two factors reinforce the effects of MAN on CAP without doubt. Inspired by the overexpression of α7nAchR in rats receiving vagotomy surgery, we assume that there exists a compensation mechanism in CAP (Figure 1(c)). That is, reduced circulating Ach will increase α7nAchR expression through an unspecified feedback. Accordingly, increased Ach under MAN treatments would pose a negative effect on α7nAchR. As such, it is reasonable to deduce that MAN directly promoted the expression of α7nAchR, while the underlying mechanism is totally unknown. Nonetheless, MAN was effective in activating CAP, which will have promising clinical applications in the treatments of many diseases, including inflammations, metabolic disorders, and cholinergic-dependent cognitive deficits.25–27
The current study revealed a novel anti-inflammatory mechanism of MAN involving CAP regulation. As the selective receptor of Ach accounting for the anti-inflammatory properties, the change of α7nAchR is essential for the clinical outcomes of ALI rats upon MAN treatments. Unfortunately, this study did not provide enough useful clues to clarify the mechanism underling the regulation of MAN on α7nAchR expression. Besides, RAW 264.7 cells were adopted in experiments in vitro in this study because of the convenience. However, their properties and immune functions are not exactly the same with macrophages derived from disease models in vivo, and thereby cannot perfectly mimic the inflammatory conditions in ALI rats. These issues should be addressed in the researches in future.
Conclusion
In conclusion, this study further confirmed the therapeutic effects of MAN on systematic acute inflammations, and identified CAP as an important therapeutic target in this process. Different from α7nAchR agonist, MAN did not interact with this receptor directly, but achieved sustained activation of CAP by increasing peripheral Ach and α7nAchR expression. These evidences partially elucidated the anti-inflammatory mechanism of MAN and shed light on the possible clinical application of MAN on many other cholinergic system related diseases.
Supplemental Material
Supplemental material, S1 for Activation of cholinergic anti-inflammatory pathway involved in therapeutic actions of α-mangostin on lipopolysaccharide-induced acute lung injury in rats by Zhe Yang, Qin Yin, Opeyemi Joshua Olatunji, Yan Li, Shu Pan, Dan-dan Wang and Jian Zuo in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81973823, 81603388, and 81173596), Key Project of Natural Science Foundation of Anhui Province for College Scholar (KJ2018A0249), Major Project of Natural Science Foundation of the Department of Education of Anhui province (KJ2019ZD32), and Funding of “Peak” Training Program for Scientific Research of Yijishan Hospital, Wannan Medical College (GF2019J01).
Ethics approval: Ethical approval for this study was obtained from the Ethical Committee of Yijishan Hospital (Ethics approval number: YJS 2019-3-008).
Animal welfare: The present study followed the guideline for the care and use of laboratory animals (United States National Research Council, 2011).
ORCID iD: Jian Zuo  https://orcid.org/0000-0003-4940-9408
https://orcid.org/0000-0003-4940-9408
Supplemental material: Supplemental material for this article is available online.
References
- 1. Pedrazachaverri J, Cárdenasrodríguez N, Orozcoibarra M, et al. (2008) Medicinal properties of mangosteen ( Garcinia mangostana). Food and Chemical Toxicology 46: 3227–3239. [DOI] [PubMed] [Google Scholar]
- 2. Chen G, Li Y, Wang W, et al. (2018) Bioactivity and pharmacological Properties of α-mangostin from the mangosteen fruit: A review. Expert Opinion on Therapeutic Patents 28: 13543776. [DOI] [PubMed] [Google Scholar]
- 3. Tao M, Jiang J, Wang L, et al. (2018) α-Mangostin alleviated lipopolysaccharide induced acute lung injury in rats by suppressing NAMPT/NAD controlled inflammatory reactions. Evidence-based Complementary and Alternative Medicine 2018: 5470187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuo J, Yin Q, Wang L, et al. (2018) Mangosteen ethanol extract alleviated the severity of collagen-induced arthritis in rats and produced synergistic effects with methotrexate. Pharmaceutical Biology 56: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang K, Yin Q, Mao Q, et al. (2019) Metabolomics analysis reveals therapeutic effects of α-mangostin on collagen-induced arthritis in rats by down-regulating nicotinamide phosphoribosyltransferase. Inflammation 42: 741–753. [DOI] [PubMed] [Google Scholar]
- 6. Martelli D, Mckinley MJ, Mcallen RM. (2014) The cholinergic anti-inflammatory pathway: A critical review. Autonomic Neuroscience-Basic & Clinical 182: 65–69. [DOI] [PubMed] [Google Scholar]
- 7. Wang DW, Zhou RB, Yao YM. (2009) Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chinese Journal of Traumatology 12: 355–364. [PubMed] [Google Scholar]
- 8. Beckmann J, Lips KS. (2013) The non-neuronal cholinergic system in health and disease. Pharmacology 92: 286–302. [DOI] [PubMed] [Google Scholar]
- 9. Cheng Q, Yakel JL. (2015) The effect of alpha7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochemical Pharmacology 97: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimura K, Low DA, Keller DM, et al. (2007) Cutaneous blood flow and sweat rate responses to exogenous administration of acetylcholine and methacholine. Journal of Applied Physiology 102: 1856–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zuo J, Dou DY, Wang HF, et al. (2017) Reactive oxygen species mediated NF-κB/p38 feedback loop implicated in proliferation inhibition of HFLS-RA cells induced by 1,7-dihydroxy-3,4-dimethoxyxanthone. Biomedicine & Pharmacotherapy 94: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RB, Pugin J, Lee JS, et al. (2004) Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews 14: 523–535. [DOI] [PubMed] [Google Scholar]
- 13. Kasemwattanaroj P, Moongkarndi P, Pattanapanyasat K. (2013) Immunomodulatory activities of alpha-mangostin on peripheral blood mononuclear cells. Natural Product Communications 8: 1257–1260. [PubMed] [Google Scholar]
- 14. Ge Y, Xu X, Liang Q, et al. (2019) α-Mangostin suppresses NLRP3 inflammasome activation via promoting autophagy in LPS-stimulated murine macrophages and protects against CLP-induced sepsis in mice. Inflammation Research 68: 471–479. [DOI] [PubMed] [Google Scholar]
- 15. Yin Q, Wu YJ, Pan S, et al. (2020) Activation of cholinergic anti-inflammatory pathway in peripheral immune cells involved in therapeutic actions of α-mangostin on collagen induced arthritis in rats. Drug Design, Development and Therapy 14: 1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You BH, Chae HS, Song J, et al. (2017) α-Mangostin ameliorates dextran sulfate sodium-induced colitis through inhibition of NF-κB and MAPK pathways. International Immunopharmacology 49: 212–221. [DOI] [PubMed] [Google Scholar]
- 17. Mohan S, Syam S, Abdelwahab SI, et al. (2018) An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food & Function 9: 3860–3871. [DOI] [PubMed] [Google Scholar]
- 18. Gallowitsch-Puerta M, Pavlov VA. (2007) Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sciences 80: 2325–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang J, Shi SQ, Shi L, et al. (2014) Nicotine, an α7 nAChR agonist, reduces lipopolysaccharide-induced inflammatory responses and protects fetuses in pregnant rats. American Journal of Obstetrics and Gynecology 211: 538. [DOI] [PubMed] [Google Scholar]
- 20. Chen XM, Li FQ, Yan S, et al. (2016) Nicotine alleviates the liver inflammation of non-alcoholic steatohepatitis induced by high-fat and high-fructose in mice. Journal of Peking University 48: 777–782. [PubMed] [Google Scholar]
- 21. Papke RL, Zheng G, Horenstein NA, et al. (2005) The characterization of a novel rigid nicotine analog with α7-selective nAChR agonist activity and modulation of agonist properties by boron inclusion. Bioorganic & Medicinal Chemistry Letters 15: 3874–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiong J, Yuan YJ, Xue FS, et al. (2012) Postconditioning with α7nAChR agonist attenuates systemic inflammatory response to myocardial ischemia-reperfusion injury in rats. Inflammation 35: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 23. Nag G, Das S, Das S, et al. (2015) Antioxidant, anti-acetylcholinesterase and anti-glycosidase properties of three species of Swertia, their xanthones and amarogentin: A comparative study. Pharmacognosy Journal 2: 117–123. [Google Scholar]
- 24. Qin J, Lan W, Liu Z, et al. (2013) Synthesis and biological evaluation of 1,3-dihydroxyxanthone mannich base derivatives as anticholinesterase agents. Chemistry Central Journal 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pavlov VA, Tracey KJ. (2012) The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nature Reviews Endocrinology 8: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan H, Gu R, Wei D. (2015) The α7 nAChR Selective Agonists as Drug Candidates for Alzheimer’s Disease. Advances in Experimental Medicine and Biology 827: 353–365. [DOI] [PubMed] [Google Scholar]
- 27. Russo P, Taly A. (2012) α7-Nicotinic acetycholine receptors: An old actor for new different roles. Current Drug Targets 13: 574–578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, S1 for Activation of cholinergic anti-inflammatory pathway involved in therapeutic actions of α-mangostin on lipopolysaccharide-induced acute lung injury in rats by Zhe Yang, Qin Yin, Opeyemi Joshua Olatunji, Yan Li, Shu Pan, Dan-dan Wang and Jian Zuo in International Journal of Immunopathology and Pharmacology