Abstract
Artificial intelligence (AI)-based applications have found widespread applications in many fields of science, technology, and medicine. The use of enhanced computing power of machines in clinical medicine and diagnostics has been under exploration since the 1960s. More recently, with the advent of advances in computing, algorithms enabling machine learning, especially deep learning networks that mimic the human brain in function, there has been renewed interest to use them in clinical medicine. In cardiovascular medicine, AI-based systems have found new applications in cardiovascular imaging, cardiovascular risk prediction, and newer drug targets. This article aims to describe different AI applications including machine learning and deep learning and their applications in cardiovascular medicine. AI-based applications have enhanced our understanding of different phenotypes of heart failure and congenital heart disease. These applications have led to newer treatment strategies for different types of cardiovascular diseases, newer approach to cardiovascular drug therapy and postmarketing survey of prescription drugs. However, there are several challenges in the clinical use of AI-based applications and interpretation of the results including data privacy, poorly selected/outdated data, selection bias, and unintentional continuance of historical biases/stereotypes in the data which can lead to erroneous conclusions. Still, AI is a transformative technology and has immense potential in health care.
Keywords: AI, machine learning, big data, precision medicine, cardiovascular disease
Introduction to Machine Learning, Deep Learning and Early Applications in Medicine
Early computer algorithms and information systems in medicine
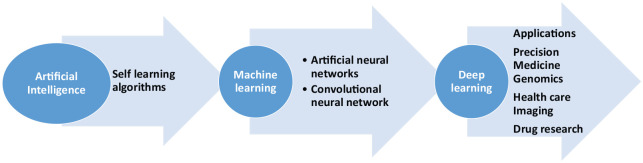
The role of computers, algorithms, and early AI information systems in medicine, especially in clinical decision making, has been under exploration since the 1960s. Especially with recent advances in AI, machine learning and deep learning computer programs are now able to simulate the neural activity of the neocortex in the brain where most of the reasoning, thinking, and cognitive functions happen1 (Figure 1). Today supercomputers such as IBM’s Watson can analyze terabytes of data and find patterns in it, with widespread applications in image, voice, and speech recognition used by global companies Facebook, Apple, and Amazon. These self-taught deep learning AI systems have already defeated the human world champion in complex Chinese game Go.1,2
Figure 1.
Relationship between artificial intelligence, machine learning, and deep learning.
One of the initial studies exploring the role of computer algorithms/mathematical programs in cardiovascular medicine were done by Warner et al3,4 in 1963 and later Gorry and Barnett5 in 1968. They studied the role of mathematical programs for the diagnosis of congenital heart disease. Warner et al3,4 described a mathematical model of clinical diagnosis of congenital heart disease based on Baye’s theorem of probability. Using their approach, congenital heart disease can be diagnosed with accuracy comparable to that by the physician and improved with refinements with symptom and physical signs—disease data matrix.3,4 However, there were several limitations to the use of diagnosis support systems.
Berner et al6 studied the diagnostic capabilities of 4 internal medicine diagnostic systems, namely Dxplain, Iliad, Meditel, and QMR. They suggested that these programs should be used by physicians who can judiciously use the information provided by these systems. They raised the concern that sometimes essential diagnoses may be obscured, leading to inappropriate and excessive investigations in inexperienced hands.6 Apart from clinical decision support systems, early AI systems helped in the interpretation of laboratory results. PUFF was probably the first computer program that was developed by researchers at Stanford University and Pacific Presbyterian Medical Center, San Francisco, for the interpretation of pulmonary function tests in patients with lung disease. It used computer algorithms to diagnose the presence and severity of lung disease and produce reports from the electronic patient data.7,8
There were several limitations to using computers in clinical decision making. First, most of these systems are based on the principle of “if this happens . . . then do this,” and after that apply mathematics to define the probability of different outcomes. However, in real world, clinical problems are complex, multifactorial, random yet interconnected. For example, fever and abdominal pain in a 40-yearold African American man may have similar or different etiology than in a 25-year-old Caucasian woman, and then to make things even more complicated, inclusion of socioeconomic factors, past medical/surgical history, travel history, drug intake, and personal habits can lead to endless possibilities. The same disease/s can manifest with different symptoms and signs in different individuals. On the other hand, diseases may manifest differently in the presence of a variety of extrinsic and intrinsic factors. Therefore, it is hard to make algorithms / clinical decision support systems. In addition, these clinical decision support systems lack clinical reasoning, intuition, and insight. Furthermore, there are concerns regarding the safety, reproducibility, usability, validity, and reliability of these systems.6,9
Machine learning, advanced AI algorithms and its applications in medicine
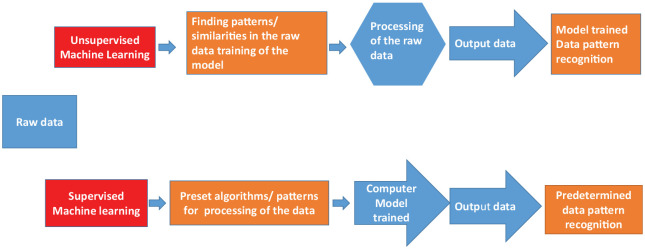
With the advancement in computing speeds, supercomputers, and newer AI learning techniques, AI is increasingly finding applications in health care. The term machine learning was first used by LA Samuel,10 an American pioneer in the field of computer gaming and artificial intelligence in 1959. It is an interdisciplinary field that uses techniques to give computer systems the ability to “learn” from a given data set, without being explicitly programmed in a particular manner (Table 1). Machine learning can be loosely classified into several categories (Figure 2). It can be supervised learning where algorithms are presented with inputs and outputs, the goal is to map an input to output; for example, identification of an image, handwriting recognition, electrocardiogram interpretation, and automated chest x-ray/computed tomographic (CT) scan findings interpretation.11-13 In supervised learning, an individual uses a database for a set of observations and their outcomes and then uses this to form a predictive model to classify outcomes from a given set of observations. For this purpose, there are several algorithms currently in use, which include statistical methods such as linear regression, logistic regression, survival analysis, and decision trees.11-13 Unsupervised learning is a type of machine learning where the goal is to learn about the inherent relationships and patterns in the data itself. The examples of unsupervised learning include clustering, principal component analysis, and self-organizing maps.11-13
Table 1.
Artificial intelligence: terms and definitions.
| Artificial intelligence | Terms and definitions |
|---|---|
| Machine learning | Defined as an interdisciplinary field that uses statistical techniques to give computer systems the ability to “learn” from a given data set, without being explicitly programmed in a certain manner |
| Deep learning | A type of machine learning that uses algorithms in multilayered neural networks for processing large amount of raw data |
| Supervised learning | A type of machine learning that learns patterns from known data sets with known responses |
| Unsupervised learning | A type of machine learning that learns patterns from unlabeled data sets |
| Artificial neural networks (ANNs) | A framework for many different machine learning algorithms to work together and process complex data inputs |
| Convolutional neural networks (CNNs) | Consists of layers of hidden nodes for processing information and is a type of ANN which “learns” by different mechanisms and help in image processing and complex data processing |
Abbreviations: ANN, artificial neural network; CNN, convolutional neural network.
Figure 2.
Machine learning: unsupervised and supervised learning.
Deep learning is a new name for an approach to AI called neural networks. This area of AI was first proposed in 1944 by two University of Chicago researchers Walter Pitts and Warren McCullough, who later moved to Massachusetts Institute of Technology. They wrote a groundbreaking article entitled “A Logical Calculus of the Ideas Immanent in Nervous Activity” published in the Bulletin of Mathematical Biophysics and proposed the first mathematical model of artificial neural networks.14-16 They explained how the brain acts as an “information processor,” and how brain neurons can produce highly complex patterns with necessary information sensory inputs by interconnecting with each other and involving physics logic gates “and,” “or,” “not” analogy. In essence, they concluded that neurons act as logic gates taking in multiple inputs and processing a single output.14-17 This concept had a profound impact on our understanding of brain functioning, and later laid the foundation of artificial neural networks, machine learning and AI.
The artificial neural network (ANN) consists of interconnected “nodes” which are similar to biological neurons in that they get input, process it, and have an output. In any artificial neural network, there are three types of nodes – (1) input nodes, (2) hidden nodes, and (3) output nodes (Figure 3). The input nodes are like sensory neurons of the central nervous system they bring in a data set of information for processing. The hidden nodes process the information from the data set, and the output nodes represent the final interpretation of data. The convolutional neural network (CNN) consists of layers of hidden nodes for processing information and is a type of ANN which “learns” by different mechanisms such as “back propagation.”16-18 However, there are 2 features that are specific for CNN “parameter sharing” and “pooling,” which reduces the computational power required to process the data and improves image processing and sophisticated data processing.16-18 Basically, these machine learning (ML) methods help a network to learn from errors (back propagation) or learn from internodal relationships and reduce the complexity of the data/ images (parameter sharing and pooling).16-18 Whereas the machine learning algorithms almost always require structured data, deep learning networks requires a hierarchical representation of data in multilayered networks, where each layer is a representation that is a high-level abstraction of the representation from the previous layer of the neural networks (Figure 3). Therefore, deep learning algorithms are suited for reasoning and interpretation of images, analysis of complex images, and recognition of sound and voice samples.
Figure 3.
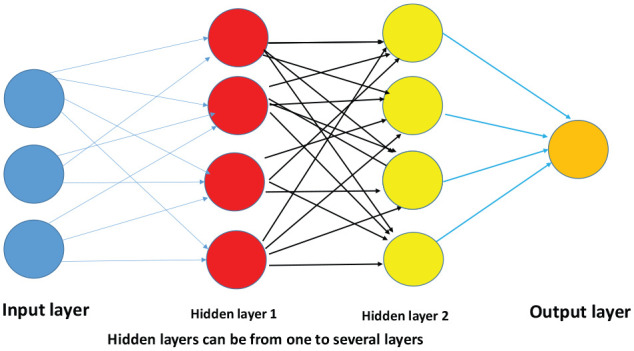
Deep learning network—multiple layers.
Hidden layers can be from one to several layers.
In cardiovascular medicine today ML/AI has found wide range of applications in cardiovascular drug therapy, pharmacogenomics, heart failure management, cardiovascular imaging, and diagnostics. AI can provide tools to apply precision medicine and big data in cardiovascular medicine therefore, augmenting the effectiveness of the cardiologist. AI/ML algorithms can analyze vastly heterogeneous clinical data without any assumptions accurately for prediction and classification. Therefore, cardiovascular medicine can benefit from the incorporation of AI. Here we have described the impact of AI in various fields of cardiovascular medicine.
AI, clinical applications, and cardiovascular drug therapy
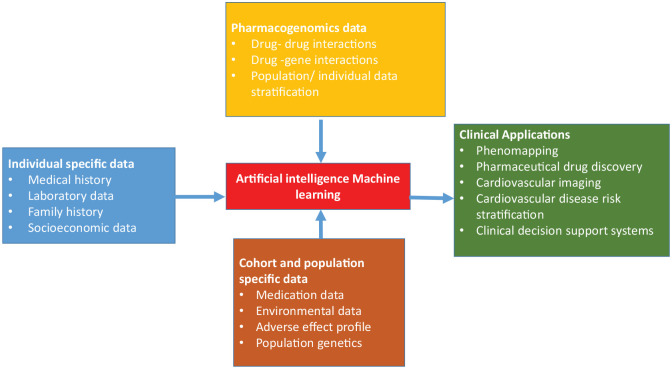
One of the earliest applications of the AI in cardiovascular medicine was in the area of cardiovascular drug therapy (Figure 4). Precision medicine has evolved with the use of AI applications in population genetics. AI applications, Big Data, and precision medicine have made a significant impact in newer drug development and is helping in finding effective treatments while minimizing the risk of developing side effects in a given individual.19-22 There are several cardiovascular drugs which are being explored as therapeutic targets including clopidogrel, warfarin, and statins particularly simvastatin.21-24
Figure 4.
Role of artificial intelligence in cardiovascular medicine and research.
Interestingly, pharmacogenomics and precision medicine has already made a big impact on the warfarin dosing in different patient populations as shown in the randomized clinical trials done by Pirmohamed et al23 and Syn et al.24 Pirmohamed et al23 showed that the patients on pharmacogenetic-based warfarin dosing had greater time in the therapeutic international normalized ratio (INR) range than the patients with standard dosing. Similar results were found by Syn et al24 in Asian patient population on warfarin therapy. Li et al25 have also utilized back propagation neural network model for predicting the warfarin maintenance dose after heart valve replacement. Furthermore, deep learning–based AI systems have potential groundbreaking applications in drug discovery, personalized drug therapy and precision medicine.19
Another emerging application of AI in cardiovascular therapeutics and disease management is in the management of heart failure. AI has led to a newer approach to cardiovascular risk stratification and phenotyping of heart failure, newer cardiovascular drug therapies for hypertension management and optimized medical drug therapy.26-31 Shah et al26 applied the principles of precision medicine in understanding the pathophysiology of heart failure and proposed a new classification of heart failure with preserved ejection fraction (HFpEF). This novel classification was based on “phenomapping” a technique in which all the relevant patient data including detailed clinical, laboratory tests, echocardiography, and imaging studies were analyzed by AI-based unsupervised deep learning algorithms.27
Shah et al26,27 classified HFpEF into three categories. The pheno-group 1 (natriuretic peptide deficiency syndrome phenotype) patients were the youngest with obesity but had the least cardiac abnormalities and low brain natriuretic peptide (BNP) levels and had the best outcomes. Pheno-group 2 (obesity-cardiometabolic phenotype) patients had the highest prevalence of diabetes and obesity with higher BNP levels, had the worse left ventricular relaxation (lowest e’ velocity) determined by echocardiography. The last pheno-group 3 (cardiorenal phenotype) patients had the highest prevalence of electrocardiographic, echocardiographic abnormalities and renal dysfunction, having the worst outcomes.27 The phenomapping of the HFpEF patients may lead to the development of novel targeted drug therapies and may also help in design future clinical trials to identify responders to different targeted drug therapies.26,27
Przewlocka-Kosmala et al31 used machine learning to study the association between cardiac left ventricular systolic reserve function and exercise intolerance in HFpEF patients. They found that decreased left ventricular systolic function was associated with poor reserve function. Overall, we can conclude that AI has already started to make a considerable impact on the way we treat several cardiovascular conditions and drug therapy. The studies by Li et al25 for warfarin dosing, applications in heart failure by Shah et al,26,27 and Przewlocka-Kosmala et al31 have shown that AI and precision medicine is here to stay. AI has, therefore, opened new avenues in cardiovascular therapeutics and drug therapeutics (Figure 4).
Applications of AI in cardiovascular imaging
AI and machine learning have the potential to revolutionize the field of cardiovascular medicine. AI has found applications in diagnosis of obstructive coronary artery disease, determination of left ventricular ejection fraction, prediction of abnormal fractional flow reserve in patients undergoing coronary computed tomography angiogram (CCTA), and readmission rates in heart failure patients (Tables 2 and 3). Recently, Zellweger et al32,33 studied the role of AI as a noninvasive tool for the diagnosis of coronary artery disease. They used an AI-based mimetic pattern–based algorithm (MPA) and found it to be better than the Framingham risk score in detecting patients with angiographically documented coronary artery disease (CAD). They found that the positive predictive value of the optimized MPA for the exclusion of CAD in the “training” and “test” population was 98% and 95%, respectively.32,33
Table 2.
Different studies exploring the role of AI in cardiovascular medicine.
| Authors | Type of study | Type of AI/machine learning method used | PMID |
|---|---|---|---|
| Li et al25 | Predicting the warfarin maintenance dose after heart valve replacement with AI methods | Back propagation neural network model | 31586305 |
| Shah et al26,27 | Classification of HFpEF into different categories | Phenomapping and Big Data | 28585183 25398313 |
| Zellweger et al32,33 | Role of AI as a noninvasive tool for the diagnosis of coronary artery disease | AI-based mimetic pattern–based algorithm (MPA) | 30174760 |
| Khamis et al34 | Automatic apical view classification of echocardiograms | Multistage classification and supervised learning | 27816858 |
| Narula et al35 | Differential diagnosis of hypertrophic cardiomyopathy and physiological hypertrophy seen in the athletes | AI algorithms used random forest, support vector machines and artificial neural networks | 27884247 |
| Sanchez-Martinez et al36 | Left ventricular function in heart failure patients with preserved ejection fraction | Unsupervised machine learning methods | 29661795 |
| Sengupta et al37 | Differentiation of restrictive cardiomyopathy and constrictive pericarditis by machine learning | Associative memory classifier / Machine learning | 27266599 |
| Tabassian et al38 | Spatiotemporal effects of myocardial infarction and cardiac contractile function | Principal component analysis and automatic classification | 28321681 |
| Moghaddasi and Nourian39 | Assessment of Mitral regurgitation with echocardiography images | Support vector machines, template matching, linear discriminant analysis | 27082766 |
| Larroza et al40 | Differentiate between acute and chronic myocardial infarction using cardiac MRI images | Support vector machine, random forest, SVM with polynesial kernels | 28624024 |
| Dawes et al41 | Role of cardiac MRI in 3D measurement of right ventricular function and outcomes in pulmonary hypertension | Supervised learning and principal component analysis | 28092203 |
| Attia et al42 | Role of AI-based learning algorithms to diagnose asymptomatic left ventricular dysfunction. | Convolution neural networks based study | 30617318 |
| Kakadiaris et al43 | Machine learning (ML)-based risk calculator for cardiovascular risk prediction | Support Vector Machine | 30571498 |
Abbreviations: AI, artificial intelligence; ML, machine learning; MPA, mimetic pattern–based algorithm; MRI, magnetic resonance imaging; SVM, support vector machine.
Table 3.
Artificial intelligence in cardiovascular medicine: avenues and potential.
| Artificial intelligence in cardiovascular medicine: avenues and potential |
|---|
| • AI / Machine learning can look at the large set of complex data and help in predicting better cardiovascular risk score in angiographically documented CAD.33,44 |
| • AI-based systems have several applications in echocardiography,34–37 cardiac CT/ MRI, including AI-based diagnosis of cardiovascular conditions,40,41,46 teaching of the medical professionals,36 understanding myocardial contractile function and differentiating acute from chronic myocardial infarction.40 |
| • AI with Big data has opened up a field of precision medicine which can revolutionize cardiovascular risk stratification and population health.43,47,48 |
| • Another application of AI and big data is the application of genomics and phenotyping of heart failure.26-31 |
| • AI-based systems can help in improving health care outcomes and systems based practice.43,50 |
Abbreviations: AI, artificial intelligence; CAD, coronary artery disease; CT, computed tomography; MRI, magnetic resonance imaging.
van Rosendael et al44 studied patients included in the multicenter CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) registry who were suspected to have CAD and underwent 64-slice coronary computed coronary angiogram (CCTA). The investigators studied patient clinical characteristics and CCTA images. They collected the data from 35 CCTA variables including coronary vessels stenosis severity, plaque composition of 16 coronary segments, coronary dominance, etc. They found out that the risk score created by the AI algorithm that utilizes these data has greater prognostic accuracy than the current CCTA integrated risk scores.44
AI has found several applications in the field of echocardiography. These applications work on the principles of acquiring a data set of clinical variables, images, and use of different AI/machine learning methods such as supervised and unsupervised learning in finding relationships between them. Al’Aref et al45 studied the role of machine learning techniques used for developing data-driven predictive models in the field of cardiac imaging. Khamis et al34 studied the use of machine learning in the automatic apical view classification of echocardiograms. They used standard apical views automatic classification for cardiac function assessment with an accuracy of 95% in real-time implementation. Narula et al35 studied the role of machine learning in the differential diagnosis of hypertrophic cardiomyopathy and physiological hypertrophy seen in athletes. Sanchez-Martinez et al36 explored the role of AI in the diagnosis of HFpEF, particularly with trainee readers with limited experience. Sengupta et al37 studied the role of machine learning method associative memory classifier in differentiating restrictive cardiomyopathy and constrictive pericarditis. Tabassian et al38 have applied machine-based learning algorithms to study myocardial contractile function and study spatiotemporal effects of myocardial infarction. This helps in the formation of cardiac segmental deformation curves which help in diagnosing regions of infarcts from a healthy heart. Even valvular heart disease such as mitral regurgitation can be accurately diagnosed with AI learning systems.39
Similar to echocardiography, AI/ML algorithms have found useful applications in cardiac magnetic resonance image (MRI) and CT imaging. Larroza et al40 used machine learning techniques to differentiate acute from chronic myocardial infarction by using cardiac MRI image texture analysis. They used random forest and support vector models of unsupervised machine learning and found that acute myocardial infarction can be diagnosed with 81% sensitivity and 84% specificity with various models. Dawes et al41 found that an AI machine learning–based survival model that utilizes 3-dimensional cardiac motion in patients with newly diagnosed pulmonary hypertension independent of conventional risk factors such as the 6-minute walk test. They found that poor outcomes were associated with the failure of basal longitudinal shortening as well as transverse contraction of the septum and free wall.42 Cardiac image segmentation with the help of cardiac MRI and subsequent quantification of ventricular volume, wall thickness, ejection fraction, strain imaging and subsequent data analysis with the help of AI has opened new avenues in the diagnosis, management, and risk stratification of disorders such as pulmonary hypertension and cardiomyopathies.46
Another application of big data and AI is in the development of knowledge-based reconstruction of cardiac images for right ventricular volume measurements. Laser et al51 utilized knowledge-based reconstruction of the right ventricular volumes with echocardiography and cardiac MRI and compared them with the gold standard direct cardiac MRI and found that knowledge-based reconstruction has excellent accuracy and reproducibility for right ventricular 3D volumetry. Right ventricle has a complex crescent shape which many times could not be visualized with 2D imaging echocardiography techniques. 3D visualization and cardiac image reconstruction with the help of AI can help in the identification of patterns of diseases and it is also time-efficient.51 Similarly, calculation of left ventricular mass, papillary muscle identification, common carotid artery, and descending aorta measurements with fully automated AI programs have been performed with high accuracy and reproducibility.52,53
AI-based algorithms therefore have already made substantial impact in diagnosing coronary artery disease, risk stratification,32,33,44,45 cardiovascular imaging modalities such as echocardiography,39,45 and cardiac MRI.40,41,46,51-53 However, it has to be noted that most of these studies are descriptive studies and are done at the state of the art centers. Its widespread availability, reproducibility, and applicability in different patient populations remain to be seen. Still, AI has already made a considerable impact and can play an essential role in different cardiovascular imaging modalities and help in diagnosing several cardiovascular diseases. AI has the potential to maximize efficiency in overburdened health care systems by relieving physicians from time-consuming, repetitive activities and overall improving optimal patient care.
Miscellaneous applications of AI in cardiovascular medicine
AI-based learning systems are now increasingly employed in physical diagnosis and electrocardiography interpretation all around the world. Thompson et al54 tested an AI-based algorithm for diagnostic interpretation of the heart murmurs in pediatric population. The AI-based murmur detection algorithm had a sensitivity of 93% and a specificity of 81%. Attia et al42 studied the role of AI-based learning algorithms to diagnose asymptomatic left ventricular dysfunction. In this study, investigators attempted to diagnose asymptomatic left ventricular dysfunction by EKG alone using an AI-based CNN deep learning method. The authors first “trained” AI /CNN neural network to detect asymptomatic left ventricular dysfunction with the data from around 44 959 patients including 12-lead EKG and echocardiogram data such as the left ventricular ejection and then tested this CNN in an independent set of 52 870 patients. The network model showed sensitivity of 86.3% and specificity of 85.7%, and accuracy of 85.7%, respectively.42 Using the AI-based CNN method, Nirschl et al55 were able to identify heart failure in patients by the histological interpretation of the slides of endomyocardial biopsy with a sensitivity of 99% and specificity of 94%.
Precision medicine can be used for metabolic profiling of the atherosclerotic plaques and finding novel risk factors for predicting adverse outcomes. Jung et al56 did metabolic profiling of the atherosclerotic plaques to find out that certain lipid metabolites particularly quinic acid is markedly elevated in plaques. Using AI/precision medicine comprehensive metabolic profiling of human plaque samples can be done to open up new avenues to treat cardiovascular diseases. Diller et al47 explored the role of deep learning–based algorithms in diagnosing, estimating prognosis and guiding therapy in adult patients with congenital heart diseases. The deep learning–based algorithm was applied to >44 000 medical records from >10 000 patients over a period of 18 years. The analysis was used to characterize diagnosis, disease complexity and New York Heart Association (NYHA) class, of the adult congential heart disease and it showed an accuracy of 91.1%, 97.0%, and 90.6%, respectively, in the test sample.47 Balanescu et al48 did the AI based analysis of coronary angiography findings in the cancer patients. They found that cancer patients are less likely to get coronary angiography for diagnosis of CAD than noncancer patients. Cancer patients are less likely to have multivessel disease and involvement of left anterior descending and left circumflex disease than noncancer patients.48 Kakadiaris et al43 have proposed a machine learning (ML)-based risk calculator with the use of Support Vector Machine using the database of the Multiethnic Study of Atherosclerosis (MESA) study population. They utilized this method to study in a new cohort of FLEMENGHO study (the Flemish Study on Environment, Genes and Health Outcomes) population and compared the data to established American College of Cardiology (ACC)/American Heart Association (AHA) cardiovascular disease risk calculator. The ML-based risk calculator recommended lesser drug therapy still missed fewer cardiovascular events, therefore outperforming ACC/AHA risk calculator and showing the enormous premise of ML in cardiovascular risk prediction.43
AI and Data Privacy Concerns and Other Challenges
There are several limitations to the widespread use of AI/ machine in health care, especially cardiovascular medicine (Table 4). Improper dichotomization and improper calibration are known problems in the application of machine learning in health care.13 Even though the use of AI in medicine, especially in cardiovascular medicine, is in a nascent stage, yet there are concerns about using patient data and especially protected health information about patients. For example, identifiable patient health care data from Royal Free London NHS Foundation Trust was transferred to Google Deep Mind to develop an algorithm to study acute kidney injury without the patient’s consent.55,56 This case raised much concern. There are concerns about the transparency, data protection/breach and objectives of data used by the private organizations as most AI firms working in the field of healthcare are for-profit organizations. In Europe, the privacy laws are stricter with the introduction of 2016 General Data Protection Regulation (GDPR) by the European Union in 2016. GDPR is a European Union regulation on data protection and privacy for all individuals within the European Union and the European Economic Area.57 It relates to individual consent for use of personal data, what to do in a data breach, penalties when the required protocol is not followed, etc. With the increasing use of AI in health care especially with electronic medical records of the patients which along with the medical data also stores patient’s sociodemographic data, including social security numbers, and health insurance information. In the United States, we need better data protection laws. Unfortunately, presently there are no standard laws and uniform guidelines regarding individual data protection in the United States.
Table 4.
Artificial intelligence in cardiovascular medicine: challenges and pitfalls.
| Artificial intelligence in cardiovascular medicine: challenges and pitfalls |
|---|
| • Dichotomania and improper calibration are known problems of artificial intelligence (AI)-based machine learning methods13 |
| • AI-based systems needs to address data privacy concerns,49,50 and United States needs universal data protection laws like General Data Protection Regulation (GDPR) of the European Union57-61 |
| • AI-based systems needs data integrity to prevent poor data selection, selection bias, historical bias/stereotypes in data analysis58,59 |
| • AI-based systems needs to guard against the use of faulty algorithms such as assuming correlation to causation to prevent encoding of discrimination in the automated systems58,59 |
| • AI-based systems also have problems associated with lack of standardization, suitability to the problem, reproducibility and legal responsibilities which may limit widespread use60,61 |
The success of AI-based systems applications and data science depends upon the integrity of the data inputs. This is true for AI/ big data applications in health care too. In 2016, the White House issued 2 reports titled “Big Data: A Report on Algorithmic Systems, Opportunity, and Civil Rights” and “The Administration’s Report on the Future of Artificial Intelligence.” These reports highlighted the challenges and opportunities in the field of Big Data and AI.58,59 The first report “Big Data: A Report on Algorithmic Systems, Opportunity, and Civil Rights” explains the role of Big Data in several areas.58 It highlighted two systemic flaws in the Big Data which can lead to the encoding of discrimination in the automated decision algorithms. The first one is related to poor data entry. Poorly selected/outdated data, selection bias, and unintentional continuance of historical biases/stereotypes in the data can lead to erroneous assumptions and results. Second, the use of faulty algorithms for data analysis such as using algorithms that assume correlation to causation, personalization of the matching systems to the user profile, leading to decreased availability of options/services, hence encoding discrimination in the automated systems. Therefore, this report emphasized discretion in the use of Big Data as a widespread assumption that ‘numbers don’t lie’ and that data-related results and inferences are infallible may not always work.
With newer AI techniques used by different researchers there is also a question of reproducibility and standardization. Petersen et al60 highlighted these areas as potential challenges in widespread use of AI in health care. Lack of standardization, suitability to the problem, reproducibility and legal responsibilities are other challenges which are limiting widespread use. Forcier et al61 have also explored the concerns of data privacy and AI applications in the context of European, Canadian, and US health care data privacy laws. They concluded that legal reforms for digital data protection is required to protect individual personal information yet enabling responsible data sharing and cross-border data transfers that is beneficial for everyone. The key to do this is to have laws for obtaining valid unambiguous consent for the individual data use, put in place mandatory security breach notifications and higher penalties for the misuse of the data.
AI: Is It the Future? Or What Is the Future of AI?
AI is a rapidly growing field in every aspect of human endeavor ranging from science, sports, business, and medicine. It is important to not lose perspective of just gathering more information, but our endeavor should be to find areas where AI can provide innovative health care solutions in the field of cardiovascular medicine and drug therapy. The use of AI for data-centric applications may help in finding newer phenotypes of common diseases and lead to newer advances in cardiovascular drug therapies. It is important to note that AI helps in generating correlations and do not establish causal relationships. These are merely hypothesis generators for more rigorous clinical studies/trials.62,63 Therefore, clinical judgment, context, and rationale should be of paramount importance in the interpretation of studies using AI. There are also concerns about biased sampling in electronic health care (EHR) records related data studies as EHR oversample sicker individuals and individuals with health care access. It is notable that billing data from EHR identifies health care conditions that have higher compensation or easy billing. Therefore, one must be cautious when applying interpretations to the general population.62,63 Still, AI is a transformative technology that has immense potential in health care. AI with genomic medicine, phenomapping of cardiovascular diseases, and application of diagnostic tools such as echocardiograms and MRI/CT imaging has the potential to revolutionize early diagnosis and management of many cardiovascular diseases.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: PM and SS wrote the first draft of the manuscript, XX and JLM made critical revisions, suggestions and approval of the final version of the mansucript.
ORCID iD: Pankaj Mathur  https://orcid.org/0000-0001-8966-2868
https://orcid.org/0000-0001-8966-2868
References
- 1. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 2. Silver D, Schrittwieser J, Simonyan K, et al. Mastering the game of Go without human knowledge. Nature. 2017;550:354-359. [DOI] [PubMed] [Google Scholar]
- 3. Warner HR, Toronto AF, Veasy LG. Experience with Baye’s theorem for computer diagnosis of congenital heart disease. Ann N Y Acad Sci. 1964;115:558-567. [PubMed] [Google Scholar]
- 4. Toronto AF, Veasy LG, Warner HR. Evaluation of a computer program for diagnosis of congenital heart disease. Prog Cardiovasc Dis. 1963;5:362-377. [DOI] [PubMed] [Google Scholar]
- 5. Gorry GA, Barnett GO. Sequential diagnosis by computer. JAMA. 1968;205:849-854. [PubMed] [Google Scholar]
- 6. Berner ES, Webster GD, Shugerman AA, et al. Performance of four computer-based diagnostic systems. N Engl J Med. 1994;330:1792-1796. [DOI] [PubMed] [Google Scholar]
- 7. http://www.openclinical.org/aisp_puff.html Accessed 12 April 2020.
- 8. Aikins JS, Kunz JC, Shortliffe EH, Fallat RJ. PUFF: an expert system for interpretation of pulmonary function data. Comput Biomed Res. 1983;16:199-208. [DOI] [PubMed] [Google Scholar]
- 9. Szolovits P, Patil RS, Schwartz WB. Artificial intelligence in medical diagnosis. Ann Intern Med. 1988;108:80-87. [DOI] [PubMed] [Google Scholar]
- 10. Samuel LA. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3:210-229. [Google Scholar]
- 11. Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69:2657-2664. [DOI] [PubMed] [Google Scholar]
- 12. Deo RC. Machine learning in medicine. Circulation. 2015;132:1920-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668-2679. [DOI] [PubMed] [Google Scholar]
- 14. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5:115-133. [PubMed] [Google Scholar]
- 15. http://news.mit.edu/2017/explained-neural-networks-deep-learning-0414. Accessed 12 April 2020.
- 16. http://nautil.us/issue/21/information/the-man-who-tried-to-redeem-the-world-with-logic. Accessed 12 April 2020.
- 17. http://www.mind.ilstu.edu/curriculum/mcp_neurons/mcp_neuron_5.php?modGUI=212&compGUI=1749&itemGUI=3022. Accessed 12 April 2020.
- 18. Dilsizian ME, Siegel EL. Machine meets biology: a primer on artificial intelligence in cardiology and cardiac imaging. Curr Cardiol Rep. 2018;20:139. doi: 10.1007/s11886-018-1074-8. [DOI] [PubMed] [Google Scholar]
- 19. Kalinin AA, Higgins GA, Reamaroon N, et al. Deep learning in pharmacogenomics: from gene regulation to patient stratification. Pharmacogenomics. 2018;19:629-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cavallari LH, Weitzel K. Pharmacogenomics in cardiology—genetics and drug response: 10 years of progress. Future Cardiol. 2015;11:281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521-1537. [DOI] [PubMed] [Google Scholar]
- 22. Kitzmiller JP, Mikulik EB, Dauki AM, Murkherjee C, Luzum JA. Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med. 2016;9:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294-2303. [DOI] [PubMed] [Google Scholar]
- 24. Syn NL, Wong AL, Lee SC, et al. Genotype-guided versus traditional clinical dosing of warfarin in patients of Asian ancestry: a randomized controlled trial. BMC Med. 2018;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q, Wang J, Tao H, et al. The prediction model of warfarin individual maintenance dose for patients undergoing heart valve replacement, based on the back propagation neural network. Clin Drug Investig. 2020;40:41-53. [DOI] [PubMed] [Google Scholar]
- 26. Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah SJ. Precision medicine for heart failure with preserved ejection fraction: an overview. J Cardiovasc Transl Res. 2017;10:233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156-1164. [DOI] [PubMed] [Google Scholar]
- 29. Lee KT, Hour AL, Shia BC, Chu PH. The application and future of big database studies in cardiology: a single-center experience. Acta Cardiol Sin. 2017;33:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krittanawong C, Bomback AS, Baber U, Bangalore S, Messerli FH, Tang WW. Future direction for using artificial intelligence to predict and manage hypertension. Curr Hypertens Rep. 2018;20:75. [DOI] [PubMed] [Google Scholar]
- 31. Przewlocka-Kosmala M, Marwick TH, Dabrowski A, Kosmala W. Contribution of cardiovascular reserve to prognostic categories of heart failure with preserved ejection fraction: a classification based on machine learning. J Am Soc Echocardiogr. 2019;32:604-615. [DOI] [PubMed] [Google Scholar]
- 32. Zellweger MJ, Tsirkin A, Vasilchenko V, et al. A new non-invasive diagnostic tool in coronary artery disease: artificial intelligence as an essential element of predictive, preventive, and personalized medicine. EPMA J. 2018;9:235-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zellweger MJ, Brinkert M, Bucher U, Tsirkin A, Ruff P, Pfisterer ME. A new memetic pattern based algorithm to diagnose/exclude coronary artery disease. Int J Cardiol. 2014;174:184-186. [DOI] [PubMed] [Google Scholar]
- 34. Khamis H, Zurakhov G, Azar V, Raz A, Friedman Z, Adam D. Automatic apical view classification of echocardiograms using a discriminative learning dictionary. Med Image Anal. 2017;36:15-21. [DOI] [PubMed] [Google Scholar]
- 35. Narula S, Shameer K, Salem Omar AM, Dudley JT, Sengupta PP. Machine-learning algorithms to automate morphological and functional assessments in 2D-echocardiography. J Am Coll Cardiol. 2016;68:2287-2295. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 36. Sanchez-Martinez S, Duchateau N, Erdei T, et al. Machine learning analysis of left ventricular function to characterize heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2018;11:e007138. [DOI] [PubMed] [Google Scholar]
- 37. Sengupta PP, Huang YM, Bansal M, et al. Cognitive machine-learning algorithm for cardiac imaging: a pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging. 2016;9:e004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tabassian M, Alessandrini M, Herbots L, et al. Machine learning of the spatio-temporal characteristics of echocardiographic deformation curves for infarct classification. Int J Cardiovasc Imaging. 2017;33:1159-1167. [DOI] [PubMed] [Google Scholar]
- 39. Moghaddasi H, Nourian S. Automatic assessment of mitral regurgitation severity based on extensive textural features on 2D echocardiography videos. Comput Biol Med. 2016;73:47-55. [DOI] [PubMed] [Google Scholar]
- 40. Larroza A, Materka A, López-Lereu MP, Monmeneu JV, Bodí V, Moratal D. Differentiation between acute and chronic myocardial infarction by means of texture analysis of late gadolinium enhancement and cine cardiac magnetic resonance imaging. Eur J Radiol. 2017;92:78-83. [DOI] [PubMed] [Google Scholar]
- 41. Dawes TJW, de Marvao A, Shi W, et al. Machine learning of three-dimensional right ventricular motion enables outcome prediction in pulmonary hypertension: a cardiac MR imaging study. Radiology. 2017;283:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70-74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 43. Kakadiaris IA, Vrigkas M, Yen AA, Kuznetsova T, Budoff M, Naghavi M. Machine learning outperforms ACC / AHA CVD risk calculator in MESA. J Am Heart Assoc. 2018;7:e009476. doi: 10.1161/JAHA.118.009476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Rosendael AR, Maliakal G, Kolli KK, et al. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr. 2018;12:204-209. [DOI] [PubMed] [Google Scholar]
- 45. Al’Aref SJ, Anchouche K, Singh G, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. 2019;40:1975-1986. [DOI] [PubMed] [Google Scholar]
- 46. Peng P, Lekadir K, Gooya A, Shao L, Petersen SE, Frangi AF. A review of heart chamber segmentation for structural and functional analysis using cardiac magnetic resonance imaging. MAGMA. 2016;29:155-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diller GP, Kempny A, Babu-Narayan SV, et al. Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: data from a single tertiary centre including 10 019 patients. Eur Heart J. 2019;40:1069-1077. doi: 10.1093/eurheartj/ehy915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Balanescu DV, Monlezun DJ, Donisan T, et al. A cancer paradox: machine-learning backed propensity-score analysis of coronary angiography findings in cardio-oncology. J Invasive Cardiol. 2019;31:21-26. [DOI] [PubMed] [Google Scholar]
- 49. Powles J, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health Technol. 2017;7:351-367. doi: 10.1007/s12553-017-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Constantinides P, Fitzmaurice DA. Artificial intelligence in cardiology: applications, benefits and challenges. Br J Cardiol. 2018;25:86-87. doi: 10.5837/bjc.2018.024. [DOI] [Google Scholar]
- 51. Laser KT, Horst JP, Barth P, et al. Knowledge-based reconstruction of right ventricular volumes using real-time three-dimensional echocardiographic as well as cardiac magnetic resonance images: comparison with a cardiac magnetic resonance standard. J Am Soc Echocardiogr. 2014;27:1087-1097. [DOI] [PubMed] [Google Scholar]
- 52. Kirschbaum S, Aben JP, Baks T, et al. Accurate automatic papillary muscle identification for quantitative left ventricle mass measurements in cardiac magnetic resonance imaging. Acad Radiol. 2008;15:1227-1233. [DOI] [PubMed] [Google Scholar]
- 53. Gao S, van’t Klooster R, Brandts A, et al. Quantification of common carotid artery and descending aorta vessel wall thickness from MR vessel wall imaging using a fully automated processing pipeline. J Magn Reson Imaging. 2017;45:215-228. [DOI] [PubMed] [Google Scholar]
- 54. Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol. 2019;40:623-629. doi: 10.1007/s00246-018-2036-z. [DOI] [PubMed] [Google Scholar]
- 55. Nirschl JJ, Janowczyk A, Peyster EG, et al. A deep-learning classifier identifies patients with clinical heart failure using whole-slide images of H&E tissue. PLoS ONE. 2018;13:e0192726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jung S, Song SW, Lee S, et al. Metabolic phenotyping of human atherosclerotic plaques: metabolic alterations and their biological relevance in plaque-containing aorta. Atherosclerosis. 2018;269:21-28. [DOI] [PubMed] [Google Scholar]
- 57. https://gdpr.eu/. Accessed 12 April 2020.
- 58. https://obamawhitehouse.archives.gov/blog/2016/10/12/administrations-report-future-artificial-intelligence. Accessed 12 April 2020.
- 59. https://obamawhitehouse.archives.gov/blog/2016/05/04/big-risks-big-opportunities-intersection-big-data-and-civil-rights. Accessed 12 April 2020.
- 60. Petersen SE, Abdulkareem M, Leiner T. Artificial intelligence will transform cardiac imaging-opportunities and challenges. Front Cardiovasc Med. 2019;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Forcier MB, Gallois H, Mullan S, Joly Y. Integrating artificial intelligence into health care through data access: can the GDPR act as a beacon for policymakers? J Law Biosci. 2019;6:317-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maddox TM, Rumsfeld JS, Payne PRO. Questions for artificial intelligence in health care. JAMA. 2019;321:31-32. [DOI] [PubMed] [Google Scholar]
- 63. Israni ST, Verghese A. Humanizing artificial intelligence. JAMA. 2019;321:29-30. [DOI] [PubMed] [Google Scholar]