Abstract
Background.
There has been increased interest in the interplay of genetic and environmental factors in the development of problematic alcohol use, including socioeconomic conditions of the neighborhood. Using a co-twin design, we examined the extent to which contributions of genetic, shared environmental, and unique environmental influences on hazardous drinking differed according to levels of neighborhood socioeconomic deprivation.
Method.
Data came from 1,521 monozygotic (MZ) and 609 dizygotic (DZ) twin pairs surveyed in Washington State. A measure of neighborhood deprivation was created based on census-tract-level variables and the Alcohol Use Disorders Identification Test 3-item instrument was used to assess level of hazardous drinking. We tested a series of nested structural equation models to examine associations among hazardous drinking, neighborhood deprivation, and the variance components (genetic [A], shared [C] and unique environmental [E] influences) of these two constructs, testing for both main effects and moderation by neighborhood deprivation.
Results.
Neighborhood deprivation was significantly associated with increased hazardous drinking, after accounting for A and C variance common to both phenotypes. Adjusting for within-pair differences in income and education, neighborhood deprivation moderated the magnitude of variance components of hazardous drinking, with the variance attributable to shared environment and non-shared environment increasing in more deprived neighborhoods.
Conclusions.
Findings point to amplification of early childhood as well as unique adulthood environmental risk on hazardous drinking in areas of greater deprivation.
Keywords: alcohol, neighborhood, twin design, gene-environment interplay, heritability
Introduction
Alcohol misuse is common among adults in the United States with nearly 13% of adults having a past year alcohol use disorder diagnosis according to the National Epidemiology Study on Alcohol and Related Conditions (Grant et al., 2017). Understanding the etiology of alcohol misuse remains a public health priority. Risk factors for alcohol misuse exist across multiple levels of influence including at the neighborhood-level (Sudhinaraset, Wigglesworth, & Takeuchi, 2016). For example, a number of cross-sectional and longitudinal studies have found that neighborhood socioeconomic disadvantage (or deprivation) is associated with alcohol use and consequences (Brenner, Diez Roux, Barrientos-Gutierrez, & Borrell, 2015; Cerda, Diez-Roux, Tchetgen, Gordon-Larsen, & Kiefe, 2010; Rhew, Kosterman, & Lee, 2017). Also, using a co-twin design to account for genetic and environmental factors (Duncan et al., 2014; McGue, Osler, & Christensen, 2010), an earlier study found that twins living in neighborhoods with greater socioeconomic deprivation relative to their co-twin had higher levels of hazardous drinking (Rhew, Kosterman, Duncan, & Mair, 2018).
In addition to examining potential effects of environmental factors, co-twin designs have been commonly used to understand the relative contributions of genetic and environmental influences on alcohol misuse (Heath et al., 1997; Mbarek et al., 2015; Prescott & Kendler, 1999; Xian et al., 2008). With a sample of both monozygotic (MZ) and dizygotic (DZ) twins, one can decompose variance in alcohol misuse due to genetic (A), environmental factors shared by both twins (C; e.g., factors due to common upbringing during childhood), and unique environmental factors that make the twins within a pair different (E). Twin studies have consistently found substantial variance in alcohol misuse explained by genetic influences, with one meta-analysis suggesting that heritability accounted for 50% of the variance in alcohol use disorder (Verhulst, Neale, & Kendler, 2015).
Twin studies can also be extended to understand how A, C, and E variance components may differ depending on environmental characteristics, including neighborhood factors. For example, the influence of genetics on alcohol misuse may be dependent on environmental factors, which is suggestive of gene-x-environment interaction (Strachan, Duncan, Horn, & Turkheimer, 2016). Two main mechanisms through which environmental factors could modify genetic influences on alcohol misuse have been posited (Dick & Kendler, 2012; Young-Wolff, Enoch, & Prescott, 2011). The first suggests that environments exerting higher levels of social control will restrict availability of alcohol and lower the permissibility of excessive use, thereby reducing genetic expression (Shanahan & Hofer, 2005). The second theorized process indicates that more stressful environments will magnify expression of genetic susceptibility. For example, researchers suggest genetic variations in pathways regulating rewarding effects of alcohol and stress response may interact with environmental stressors to increase risk (Clarke, Nymberg, & Schumann, 2012). Consistent with the stress process, studies of measured genotypes have found stronger associations between candidate genes and alcohol-related behavior among those who experienced a greater number of stressful life events and higher overall levels of psychosocial stress (Bau, Almeida, & Hutz, 2000; Blomeyer et al., 2008; Covault et al., 2007; Madrid, MacMurray, Lee, Anderson, & Comings, 2001).
Neighborhoods with high levels of socioeconomic deprivation may also be area-level contexts characterized by less social control and increased psychosocial stressors as suggested by social disorganization theory (Sampson & Groves, 1989). Thus, it is possible that neighborhood deprivation may also increase genetic expression of alcohol misuse. This has not yet been examined to our knowledge. However, one recent twin study of young adults found that genetic influences on alcohol-related problems were stronger, but potentially shared and unique environmental influences weaker, among those living in census tracts with higher levels of alcohol outlet density (Slutske, Deutsch, & Piasecki, 2019). This finding further suggests that the neighborhood may be an important environmental context that moderates genetic vulnerabilities to a number of behaviors, including alcohol use and misuse.
Using a population based sample of adult twins living in Washington State, this study builds on earlier work by taking full advantage of the twin design in order to examine whether the contribution of genetic and environmental components of hazardous drinking in this sample varies according to level of neighborhood socioeconomic deprivation.
Methods
Participants
We used data from the Washington State Twin Registry (WSTR; formerly the University of Washington Twin Registry), a population-based sample of adult twins. The participants were identified from Washington State Department of Licensing records. Details about the construction of the Registry are described elsewhere (Afari et al., 2006; Strachan et al., 2013). The registry has been enrolling adult twins, 18 years and older, since 1999. The twin participants completed a survey with items on sociodemographics, general physical and mental health, and lifestyle behaviors. Standard questions about childhood similarity that determine zygosity with greater than 90% accuracy when compared with DNA-based methods were used to classify twins as identical (monozygotic; MZ) or fraternal (dizygotic; DZ) (Spitz et al., 1996; Torgersen, 1979). For this study, we used data from same sex pairs who completed surveys between 2008 and 2012. Surveys were completed by 4260 participants (2130 twin pairs) during this time frame.
Participants’ residential addresses were geocoded and linked to the census tracts in which they were located. In this study, 1189 census tracts were represented with a mean of 3.4 participants per tract (range: 1, 17).
Measures
Neighborhood socioeconomic deprivation
The Singh or Area Deprivation Index was used to characterize neighborhood socioeconomic disadvantage based on the census tract where one resided (Singh, 2003). In earlier work, the Singh Index was derived from a factor analysis of 2000 census data on17 different indicators (e.g., educational and occupational composition, income and employment distributions, unemployment rate, quality of housing and crowding). Because surveys were completed well after 2000 in this study, we used census tract data for the 17 indicators from the 2006–2010 5-year estimates from American Community Survey data. First, we standardized each of the 17 indicators such that each had a mean of 0 and standard deviation of 1. Factor loadings reported in the 2003 paper were applied to the 17 standardized indicators and then the sum of the loading-x-indicator projects were calculated to create participants’ index scores. Higher scores on the index indicate greater deprivation. In this sample, the Singh index scores ranged from −2.3 to 4.4, where 0 represents the average level of deprivation in this study sample. To aid in interpretation of the score, supplemental tables shows descriptive statistics for the 17 tract-level indicators in the full sample (Table S1) at different levels of the index score in this sample (Table S2).
Hazardous Drinking
The Alcohol Use Disorders Identification Test Consumption Scale (AUDIT-C) was used as a measure of hazardous drinking (Bush et al., 1998). The three items that comprise the AUDIT-C ask about frequency of any drinking, the number of drinks consumed on a typical drinking occasion, and the frequency of drinking six or more drinks on a single occasion with response category options scored from 0 to 4. The items are typically summed to yield a total scale score (range: 0 to 12) or used to screen for hazardous drinking and/or likely alcohol use disorder. The AUDIT-C has shown strong criterion validity when compared against DSM-IV and DSM-5 alcohol use disorder diagnoses in general population samples (Dawson, Grant, Stinson, & Zhou, 2005; Dawson, Smith, Saha, Rubinsky, & Grant, 2012). In this sample, internal consistency was good, particularly considering the few number of items (α = .75).
Covariates
Other covariates assessed in the survey include age (years divided by 10), sex (male=0, female=1), annual household income (8 categories ranging from <$20,000 to >$80,000), educational attainment (7 ordered categories), race (white=1, nonwhite=0), and an indicator for urbanicity (0 = rural [<1000 persons per square mile within one’s census tract]; 1 = urban [≥1000 persons per square mile]).
Analytic Plan
All analyses were conducted using Mplus version 8.2 with maximum likelihood estimation. We used likelihood ratio tests to compare nested models. For all models, we used data among those who had complete data (n = 4180; 98.1%)
Univariate biometric decomposition
We employed the classical twin model to decompose the variance of both hazardous drinking and the Singh index into additive genetic variance (A), shared environmental variance (C), and non-shared environmental variance (E). For each phenotypic construct, we specified that the A variance components are correlated at 1.0 between MZ twins within the same pair (who share 100% of their genes) and at 0.5 between DZ twins (who share on average 50% of their segregating alleles). For both MZ and DZ twins, the correlation of C variance components was fixed at 1.0 between twins within pairs under the equal environments assumption (Joseph, 2002; Mitchell et al., 2007). The correlation between E variance components was specified as zero. For this analysis, hazardous drinking was specified as a latent variable with the three AUDIT-C items as indicators. This was done to separate random measurement error in the measured indicators from non-shared environmental variance. Further, the latent variable specification reduces estimate bias related to skew that may arise from quantifying a phenotype with summed scores (Eaves & Verhulst, 2014; Molenaar & Dolan, 2014; Van Hulle & Rathouz, 2015).
Nested models of associations between neighborhood deprivation and hazardous drinking
We estimated four nested structural equation models (SEMs) to examine the role of neighborhood deprivation and its interplay with genetic and environmental influences on hazardous drinking. Model 1, the “phenotypic” model, used one parameter to capture the overall association between Singh index and hazardous drinking, adjusting for covariates, by constraining the specified effects of A, C, and E components of neighborhood deprivation on hazardous drinking to equality. Model 2, often referred to as a “quasi-causal” model, freed this constraint and examined the extent to which neighborhood deprivation and hazardous drinking covaried through genetic, shared environmental, and non-shared environmental pathways. As shown in Figure 1, the non-shared environmental overlap between these phenotypes is estimated while controlling for underlying genetic or environmental backgrounds that the Singh index and hazardous drinking share, and provides an approximation of the association of neighborhood deprivation with hazardous drinking independent of selection into neighborhood types due to genetic or shared environmental background. Selection processes are supported if Singh index and hazardous drinking are associated between twin pairs, but not within twin pairs. These processes may be the result of a shared genetic background (known as gene–environment correlation, or rGE, represented by the bA path in Figure 1) or a common underlying developmental environment (represented by the bC path). On the other hand, a stronger inference is supported by a non-zero within-pair association (represented by the bE path), reflecting that a non-shared environmental overlap between the phenotypes is present (Turkheimer & Harden, 2014).
Figure 1.
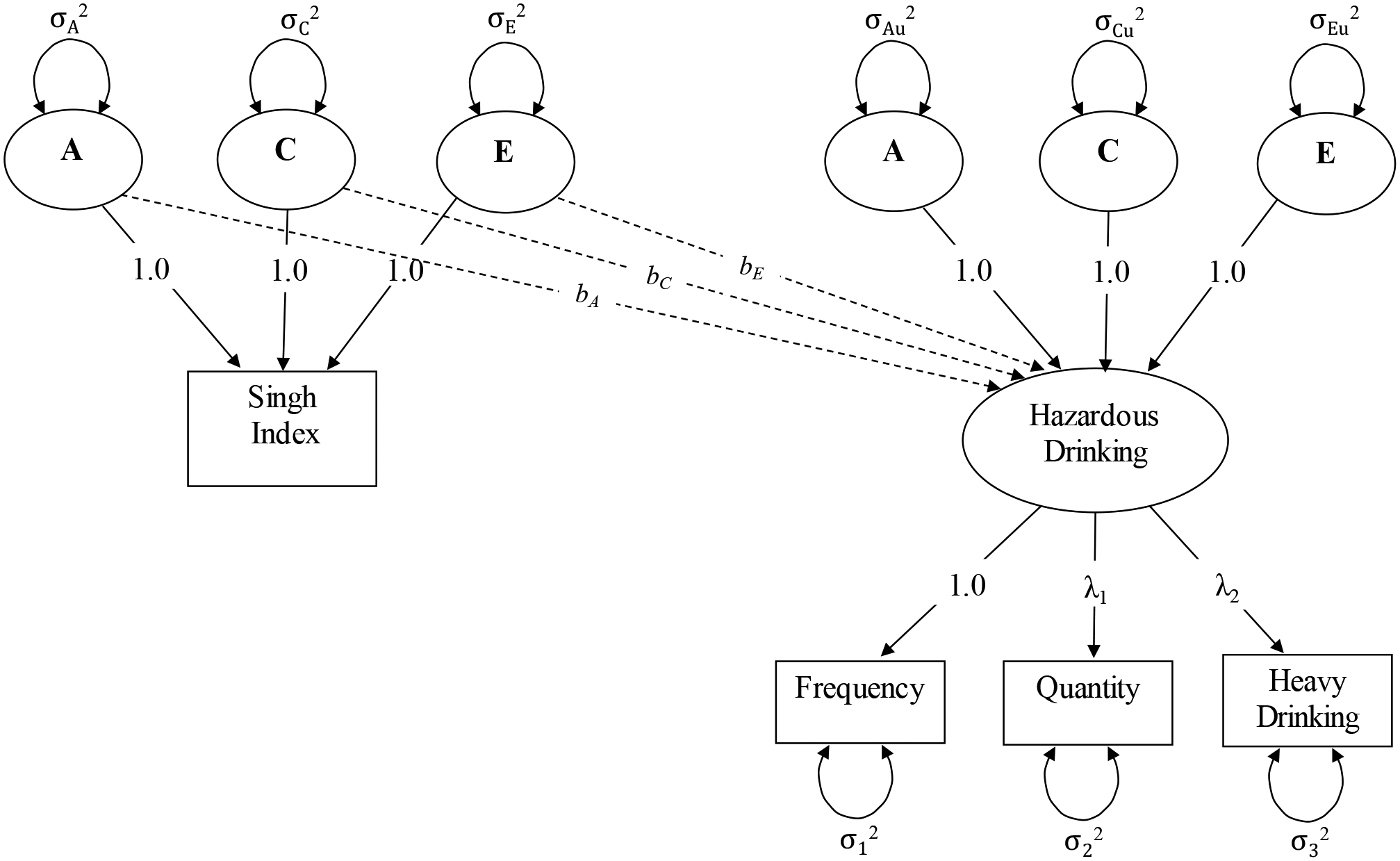
Path diagram for “quasi-causal” model of the effect of neighborhood deprivation on hazardous drinking
After accounting for the main effects of Singh index and sociodemographic covariates on hazardous drinking, the residual variation in hazardous drinking may be partitioned into A, C, and E components. In Model 3, the “residual variances” model, we estimated the extent to which these variances vary as a function of the Singh index (Purcell, 2002). The moderation of the residual variance components of hazardous drinking (represented by bAu1, bCu1, and bEu1 in Figure 2) reflects the extent to which neighborhood deprivation influences genetic and environmental influences on hazardous drinking behavior. It is possible, however, that these residual variances show heteroscedasticity with respect to neighborhood deprivation not because neighborhood socioeconomic circumstances impact on genetic and environmental risk for hazardous drinking, but instead because the main effect of neighborhood deprivation has a non-static influence on hazardous drinking. That is, this heteroscedasticity may be present due to the covariance between neighborhood deprivation and hazardous drinking depending on the level of neighborhood deprivation. To test this possibility, we ran a “saturated” model (Model 4) that allowed the regression of hazardous drinking on the ACE components of neighborhood deprivation to vary as a function of the neighborhood deprivation. That is, the effects of the three different components of neighborhood deprivation on hazardous drinking could depend on level of the Singh index as represented by the b1A, b1C, and b1E terms in Figure 2 (Johnson, 2007; van der Sluis, Posthuma, & Dolan, 2012). For each of the moderated paths, the Singh index is the moderating variable; the b0 terms are the values of the ACE variances (or main effects of the Singh index) where the Singh index = 0; and the b1 terms represent the rate of increase or decrease in a given variance component (or main effect) as a function of the Singh index.
Figure 2.
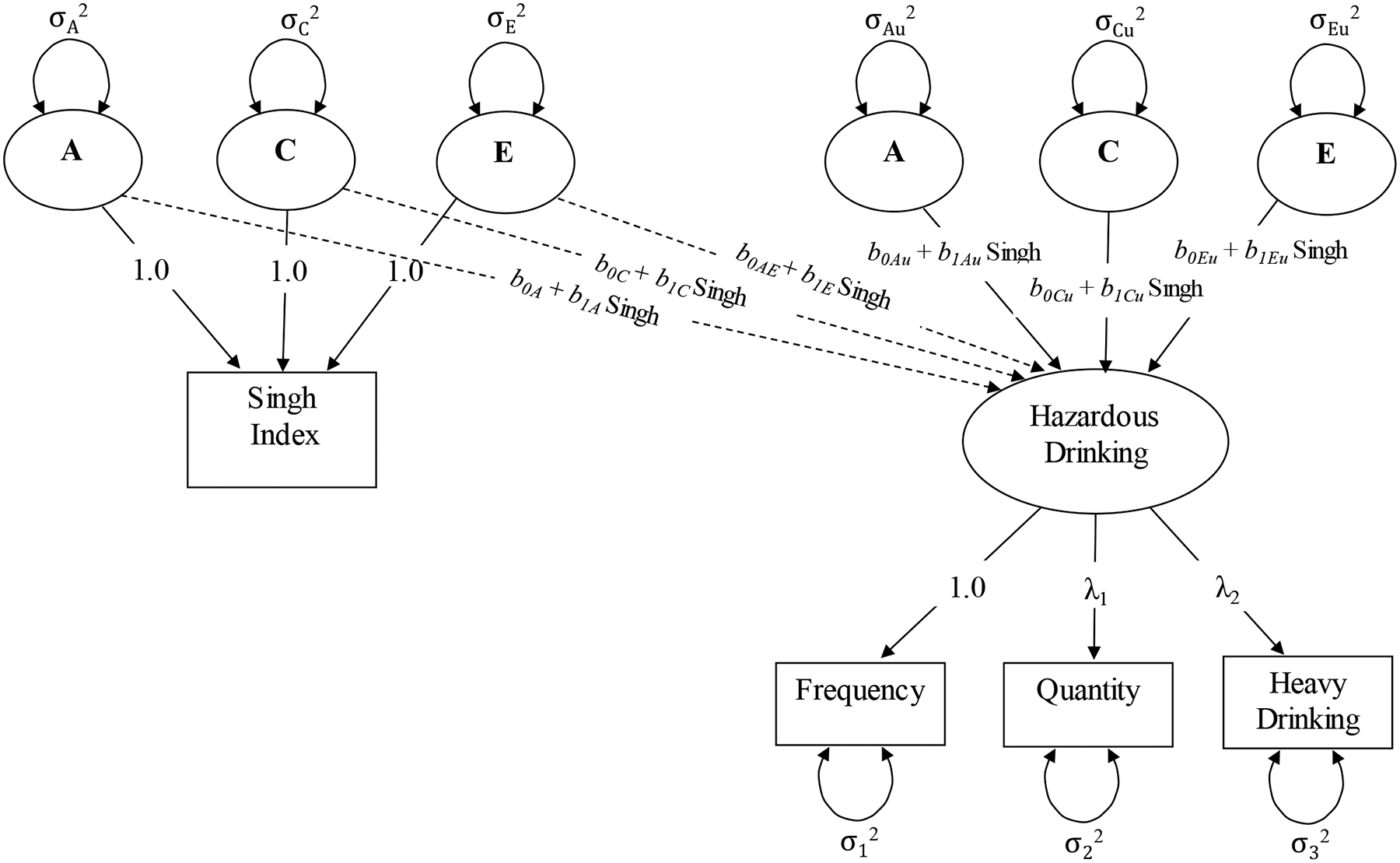
Path diagram for saturated model of the moderating role of neighborhood deprivation on variance components of hazardous drinking
Results
In the final analysis sample of same sex twin pairs, 65.6% were female. The average age was 36.6 years (SD=17.4). The median household income was between US $50,000 and $60,000, 95.3% had graduated from high school, and 31.9% had at least a bachelor’s degree. The sample was predominantly white (90.1%). The socio-demographic characteristics of the sample were similar to the population of Washington State at the time period of data collection.
Table 1 provides descriptive statistics, twin correlations by zygosity, and standardized A, C, and E variances for hazardous drinking as well as neighborhood deprivation. Heritability accounted for over one third (38%) of variance in hazardous drinking, while close to a quarter (25%) was due to shared environment. There was also substantial variance in neighborhood deprivation due to genetic (30%) and shared environmental (31%) influences. The presence of both between- and within-family variability in each construct leaves open the possibility that hazardous drinking and the Singh index may be correlated via genetic or shared environmental confounders in addition to (or to the exclusion of) causal pathways.
Table 1.
Descriptive statistics, twin correlations, and standardized variance components for alcohol problems and area-level socioeconomic deprivation.
| Hazardous Drinkinga | Singh Index | |
|---|---|---|
| Mean (SD) | 2.50 (2.38) | 0.00 (0.91) |
| rmz | .63 | .61 |
| rdz | .44 | .46 |
| a2 | .38 | .30 |
| c2 | .25 | .31 |
| e2 | .37 | .39 |
Standard errors in parentheses.
ACE estimates: additive genetic (a), variance attributable to the additive effect of individual genes; shared environmental (c), variance attributable to environmental influences shared by twins raised in the same family; and non-shared environmental (e), variance attributable to environmental influences unique to the individual.
Descriptive statistics for hazardous drinking here are based on a summed score of the three AUDIT-C items, but the measurement model used in the biometric decomposition and primary analyses specifies a continuous, normal distribution of latent scores on the alcohol problems continuum.
Table 2 shows parameter estimates and standard errors from the four SEMs. The phenotypic regression of hazardous drinking on the Singh index (Model 1) showed a statistically significant, but small, positive association. On average, each 1-SD unit increase in Singh index was associated with a 0.04 unit increase in hazardous drinking. The initial quasi-causal model (data not shown in Table 2) showed better fit than the phenotypic model (LR test p = .013). The pathway from the E component of neighborhood deprivation to hazardous drinking was statistically significant, which indicates that the effect of neighborhood deprivation remained after accounting for the genetic and environmental influences common to both the Singh index and hazardous drinking (bE = 0.07; p < 0.001). Although neither the genetic nor the shared environmental pathways were statistically significant (b0A = 0.05, p = .603; b0C = −0.08, p = .439), they were non-zero, opposite in direction, and had large standard errors. These observations reflect a high degree of correlation between A and C components of neighborhood deprivation and a lack of power to differentiate between these sources of covariation. To improve model power and stability of parameter estimates, A and C pathways from neighborhood deprivation to hazardous drinking were constrained to be equal (i.e., the total between-family effect was estimated rather than individual between-family components A and C). No statistically significant change in model fit was observed (LR test p = 0.498, df = 1). As shown in Table 2, Model 2 parameter estimates appeared more stable (bA = bC = −0.01, p = .633; b0E = 0.07, p < .001), and the same conclusions were reached as in the non-constrained model. Therefore, the remaining models fit to the data followed this protocol for estimating between- and within-family main effects of Singh Index on hazardous drinking.
Table 2.
Parameter estimates and their standard errors and fit indices for adjusteda SEM models of hazardous drinking latent variable.
| Parameter | Model 1: Phenotypic Model | Model 2: Quasi-Causal Modelb | Model 3: Moderation of Residual Variance (“residual variance” model)b | Model 4: Moderation of Main Effects (“saturated” model”)b† |
|---|---|---|---|---|
| Main Effect of Singh Index on Alcohol Problems | ||||
| A Regression | ||||
| b0A | .04 (.01) | ….01 (.02) | .001 (.03) | ….003 (.03) |
| b1A | ― | ― | ― | .002 (.03) |
| C Regression | ||||
| b0C | .04 (.01) | ….01 (.02) | .001 (.03) | ….003 (.03) |
| b1C | ― | ― | ― | .002 (.03) |
| E Regression | ||||
| b0E | .04 (.01) | .07 (.02) | .05 (.02) | .04 (.02) |
| b1E | ― | ― | ― | .05 (.02) |
| Effect of Singh Index on Residual ACE Components of Alcohol Problems | ||||
| A Component | ||||
| b0Au | .68 (.11) | .67 (.11) | .73 (.10) | .73 (.11) |
| b1Au | ― | ― | ….08 (.04) | ….08 (.04) |
| C Component | ||||
| b0Cu | .47 (.16) | .48 (.16) | .39 (.17) | .39 (.18) |
| b1Cu | ― | ― | .18 (.03) | .17 (.03) |
| E Component | ||||
| b0Eu | .57 (.03) | .57 (.03) | .57 (.03) | .57 (.03) |
| b1Eu | ― | ― | .03 (.01) | .03 (.01) |
| Model Fit | ||||
| −2LL | 89332 | 89324 | 89261 | 892545 |
| Δ−2LL (Δdf) | ― | 8 (+1) | 63 (+3) | 7 (+2) |
| p-value | ― | .005 | <.001 | .036 |
Adjusted for age, sex, annual household income, educational attainment, and race
bA and bC parameters were constrained to be equal in models
Denotes best-fitting model.
Note: Statistically significant (p < .05) parameter estimates bolded.
The next two models examined moderation of A, C, and E components of hazardous drinking by neighborhood deprivation. The residual variances model (Model 3) showed significantly improved model fit compared to Model 2 (p < 0.001), suggesting that residual variance in hazardous drinking varies by level of neighborhood deprivation. Contrary to expectations, residual genetic variance on hazardous drinking decreased by 0.08 standard deviation units per unit increase in the Singh index, but this did not reach statistical significance. Residual variance in hazardous drinking due to shared environment (b1Cu= 0.17, p < 0.001) and non-shared environment (b1Eu=0.03, p = 0.032) appeared to vary significantly according to level of neighborhood deprivation such that the shared and non-shared environmental risk increased with higher levels of neighborhood deprivation.
Finally, we ran a final model that further allowed the paths from variance components of neighborhood deprivation to hazardous drinking to vary by level of neighborhood deprivation (Model 4). This model showed further improvement in model fit relative to Model 3 (p = 0.036), which suggested that influence of the main effects of the A, C, and E components of neighborhood deprivation on hazardous drinking depend on level of neighborhood deprivation. Specifically, we observed that the unique environmental influence of neighborhood was stronger in more deprived neighborhoods, whereas the between-family effects were essentially zero across all levels of Singh Index. Further, we observed that, in spite of these non-static main effects that contribute to heteroscedasticity, similar to Model 3 findings the shared and unique environmental risk for hazardous drinking increased with increasing neighborhood deprivation. Based on results from the fully saturated model (Model 4), Figure 3 depicts how variance components of hazardous drinking vary across levels of neighborhood deprivation.
Figure 3.
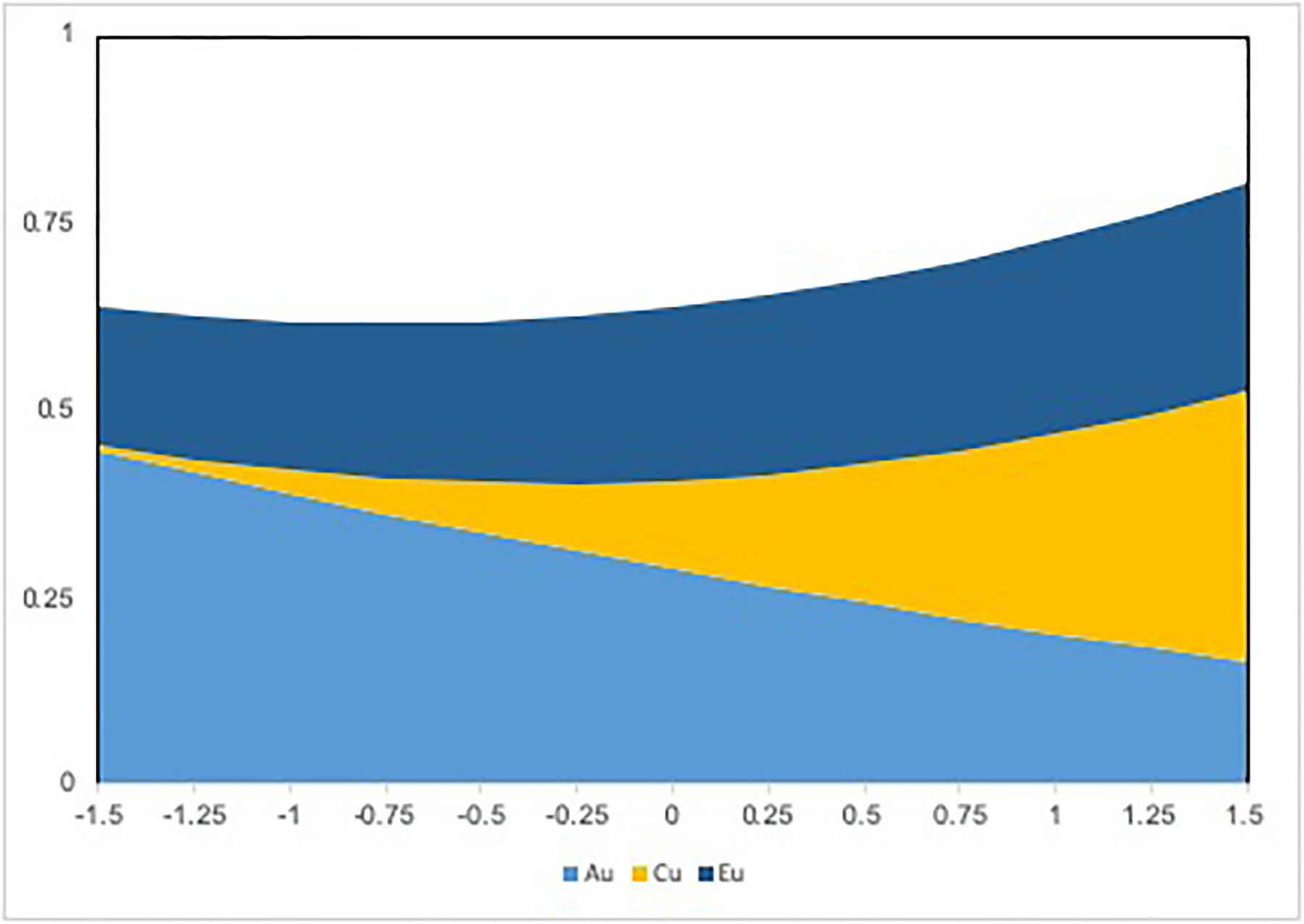
Variance components of hazardous drinking by Singh neighborhood deprivation score
Discussion
There is emerging evidence to suggest that neighborhood deprivation may play a role in the development of hazardous drinking and other forms of disordered alcohol use. However, less is known about for whom neighborhood deprivation may be most salient. This study extended prior work by taking full advantage of a co-twin design to examine the interplay between neighborhood deprivation and genetic and environmental influences on hazardous drinking. In this study, the influence of shared and unique environmental influences appeared to be stronger in neighborhoods with higher levels of deprivation, while the influence of genetic influences appeared to be somewhat weaker. Results from this study may provide more clarity about how neighborhood deprivation interacts with risk factors across the developmental course to shape alcohol behaviors. Life course research has shown that early childhood environmental factors (e.g., childhood socioeconomic status, family members’ substance use) may place individuals at risk for alcohol and other substance use problems in adulthood (Evans-Polce, Doherty & Ensminger, 2014; Jones et al, 2016; Poulton et al, 2002). There may also be numerous social and environmental factors; including various forms of psychosocial stressors such as discrimination, traumatic events, and general life stressors; experienced in adulthood that may make individuals vulnerable to hazardous and disordered drinking (Keyes et al., 2012). Studies have found that those from stigmatized minority groups may be more vulnerable to effects of neighborhood deprivation on alcohol use and problems (Karriker-Jaffe et al., 2012). It has been theorized that this may be due to a lack of sufficient material and psychosocial resources in disadvantaged areas available to buffer against stigmatized individuals’ own elevated levels of psychosocial stress. Similarly, those with elevated risk due to childhood and/or unique adulthood factors may also be experiencing high levels of psychosocial adversity that, without sufficient resources in their neighborhood environment available, may make them more prone to use alcohol as a coping strategy for the experience of stress.
With regards to moderation of genetic influences, the results were somewhat surprising. Other twin studies have found heritability of alcohol use and misuse was stronger in environmental contexts that could be characterized by less social control (Dick et al., 2009) or greater physical availability of alcohol (Slutske, Deutsch, & Piasecki, 2019). It might have been expected, then, that genetic influences on hazarous drinking would also be stronger in areas with greater deprivation. However, we did not find statistically moderation of genetic influences, which may suggest that neighborhood deprivation may not be a salient contextual factor that activates genetic risk.
Strengths and limitations
This study had multiple strengths. Using a twin design, we were able to control for genetic and shared environmental factors that may have predisposed individuals to select into more deprived neighborhoods and also engage in high-risk drinking, thus reducing the potential for confounding. Further, this sample of twins was relatively large (>2000 twin pairs) and from a population-based twin registry in Washington State. Finally, our neighborhood deprivation measure, derived from publicly available Census data, was a validated measure that has been used in numerous studies and can be reproduced in future research studies allowing for comparison of results across studies.
Study findings should also be considered in light of multiple limitations. Although we were able to account for genetic and environmental factors as potential confounders, data were cross-sectional and it was therefore impossible to definitively determine the temporal ordering of the deprivation-hazardous drinking association and thus causation could not be established. It is possible that individuals with alcohol problems show limited socioeconomic mobility and were restricted to reside in neighborhoods with greater deprivation. Further, we captured neighborhoods during one point in time during adulthood. Exposure to disadvantaged neighborhoods assessed earlier during or cumulatively over the life course may be relevant. This study was conducted in Washington State and results may not be generalizable to other regions of the country. Of particular note, although representative of Washington, there was limited racial/ethnic diversity of this study sample and there was some indication that this sample showed greater socioeconomic advantage relative to the broader U.S. population. The measure of hazardous drinking was only three items. A more comprehensive measure with greater variability may have been more sensitive to effects of neighborhood deprivation and its moderation of ACE components. Finally, individuals may traverse multiple environments as part of their routine activities (e.g., work, school, recreation). Thus, individuals may be exposed to other important environmental characteristics outside of their neighborhood of residence that could contribute to alcohol hazardous drinking.
Conclusions
This study of adult twins found that influences of shared environmental) and unique environmental factors on hazardous drinking among adults may vary according to residential neighborhood deprivation. Consistent with socioecological models for health (Bronfenbrenner, 1979), these findings highlight the complex interplay of factors across multiple levels of influence, from genetics to individual and family environments to neighborhoods, in shaping alcohol use behaviors. Continued research that investigates how multiple factors work together across the ecological levels and across the life course, rather than focusing on isolated effects of singe factors, may help yield a clearer understanding of the underlying mechanisms that give rise to hazardous drinking in the population (Keyes & Galea, 2016).
Supplementary Material
Acknowledgements
This study was supported with funding from the University of Washington Royalty Research Fund (A101837) and grants from the NIH (R01 AG042176 awarded to Glen Duncan and R01DA033956 awarded to Rick Kosterman). The content of this manuscript does not necessarily represent views of the funding agencies. The authors thank Ally Avery for her assistance with data management and other support for this study.
Footnotes
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Afari N, Noonan C, Goldberg J, Edwards K, Gadepalli K, Osterman B, … Buchwald D (2006). University of Washington Twin Registry: Construction and characteristics of a community-based twin registry. Twin Research and Human Genetics, 9(6), 1023–1029. doi:DOI 10.1375/twin.9.6.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bau CHD, Almeida S, & Hutz MH (2000). The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: Association and interaction with stress and harm avoidance on severity prediction. American Journal of Medical Genetics, 96(3), 302–306. doi:Doi [DOI] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, & Laucht M (2008). Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biological Psychiatry, 63(2), 146–151. doi:DOI 10.1016/j.biopsych.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Brenner AB, Borrell LN, Barrientos-Gutierrez T, & Diez Roux AV (2015). Longitudinal associations of neighborhood socioeconomic characteristics and alcohol availability on drinking: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Soc Sci Med, 145, 17–25. doi: 10.1016/j.socscimed.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AB, Diez Roux AV, Barrientos-Gutierrez T, & Borrell LN (2015). Associations of Alcohol Availability and Neighborhood Socioeconomic Characteristics With Drinking: Cross-Sectional Results From the Multi-Ethnic Study of Atherosclerosis (MESA). Subst Use Misuse, 50(12), 1606–1617. doi: 10.3109/10826084.2015.1027927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U (1979). The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press. [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, & Project ACQI (1998). The AUDIT alcohol consumption questions (AUDIT-C) - An effective brief screening test for problem drinking. Archives of Internal Medicine, 158(16), 1789–1795. doi:DOI 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- Cerda M, Diez-Roux AV, Tchetgen ET, Gordon-Larsen P, & Kiefe C (2010). The relationship between neighborhood poverty and alcohol use: estimation by marginal structural models. Epidemiology, 21(4), 482–489. doi: 10.1097/EDE.0b013e3181e13539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Nymberg C, & Schumann G (2012). Genetic and Environmental Determinants of Stress Responding. Alcohol Research-Current Reviews, 34(4), 484–494. [PMC free article] [PubMed] [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AHN, & Kranzler HR (2007). Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biological Psychiatry, 61(5), 609–616. doi:DOI 10.1016/j.biopsych.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, & Zhou Y (2005). Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism-Clinical and Experimental Research, 29(5), 844–854. doi:Doi 10.1097/01.Alc.0000164374.32229.A2 [DOI] [PubMed] [Google Scholar]
- Dawson DA, Smith SM, Saha TD, Rubinsky AD, & Grant BF (2012). Comparative performance of the AUDIT-C in screening for DSM-IV and DSM-5 alcohol use disorders. Drug and Alcohol Dependence, 126(3), 384–388. doi:DOI 10.1016/j.drugalcdep.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bernard M, Aliev F, Viken R, Pulkkinen L, Kaprio J, & Rose RJ (2009). The Role of Socioregional Factors in Moderating Genetic Influences on Early Adolescent Behavior Problems and Alcohol Use. Alcoholism-Clinical and Experimental Research, 33(10), 1739–1748. doi:DOI 10.1111/j.1530-0277.2009.01011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, & Kendler KS (2012). The Impact of Gene-Environment Interaction on Alcohol Use Disorders. Alcohol Research-Current Reviews, 34(3), 318–324. [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Mills B, Strachan E, Hurvitz P, Huang R, Moudon A, & Turkheimer E (2014). Stepping towards causation in studies of neighborhood and environmental effects: How twin research can overcome problems of selection and reverse causation. Health & Place, 27, 106–111. doi: 10.1016/j.healthplace.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L, & Verhulst B (2014). Problems and pit-falls in testing for G x E and epistasis in candidate gene studies of human behavior. Behav Genet, 44(6), 578–590. doi: 10.1007/s10519-014-9674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Polce RJ, Doherty EE, & Ensminger ME (2014). Taking a life course approach to studying substance use treatment among a community cohort of African American substance users. Drug and alcohol dependence, 142, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou PS, Saha TD, Pickering RP, Kerridge BT, Ruan JW, … Hasin DS (2017). Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, … Martin NG (1997). Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine, 27(6), 1381–1396. doi:Doi 10.1017/S0033291797005643 [DOI] [PubMed] [Google Scholar]
- Johnson W (2007). Genetic and environmental influences on behavior: capturing all the interplay. Psychol Rev, 114(2), 423–440. doi: 10.1037/0033-295X.114.2.423 [DOI] [PubMed] [Google Scholar]
- Joseph J (2002). Twin studies in psychiatry and psychology: science or pseudoscience? Psychiatr Q, 73(1), 71–82. [DOI] [PubMed] [Google Scholar]
- Karriker-Jaffe KJ (2011). Areas of disadvantage: a systematic review of effects of area-level socioeconomic status on substance use outcomes. Drug Alcohol Rev, 30(1), 84–95. doi: 10.1111/j.1465-3362.2010.00191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karriker-Jaffe KJ, Zemore SE, Mulia N, Jones-Webb R, Bond J, & Greenfield TK (2012). Neighborhood disadvantage and adult alcohol outcomes: differential risk by race and gender. J Stud Alcohol Drugs, 73(6), 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, & Hasin DS (2012). Stress and alcohol: epidemiologic evidence. Alcohol research: current reviews. [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, & Galea S (2016). Population health science. Oxford University Press. [Google Scholar]
- Jones TM, Hill KG, Epstein M, Lee JO, Hawkins JD, & Catalano RF (2016). Understanding the interplay of individual and social–developmental factors in the progression of substance use and mental health from childhood to adulthood. Development and psychopathology, 28(3), 721–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid GA, MacMurray J, Lee JW, Anderson BA, & Comings DE (2001). Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol, 23(2), 117–122. doi:Doi 10.1016/S0741-8329(00)00138-5 [DOI] [PubMed] [Google Scholar]
- Mbarek H, Milaneschi Y, Fedko IO, Hottenga J-J, de Moor MHM, Jansen R, … Vink JM (2015). The genetics of alcohol dependence: Twin and SNP-based heritability, and genome-wide association study based on AUDIT scores. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics, 168(8), 739–748. doi: 10.1002/ajmg.b.32379 [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, & Christensen K (2010). Causal Inference and Observational Research: The Utility of Twins. Perspectives on Psychological Science, 5(5), 546–556. doi:Doi 10.1177/1745691610383511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KS, Mazzeo SE, Bulik CM, Aggen SH, Kendler KS, & Neale MC (2007). An investigation of a measure of twins’ equal environments. Twin Research and Human Genetics, 10(6), 840–847. doi: 10.1375/twin.10.6.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar D, & Dolan CV (2014). Testing systematic genotype by environment interactions using item level data. Behav Genet, 44(3), 212–231. doi: 10.1007/s10519-014-9647-9 [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, & Moffitt TE (2002). Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. The lancet, 360(9346), 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, & Kendler KS (1999). Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry, 156(1), 34–40. [DOI] [PubMed] [Google Scholar]
- Purcell S (2002). Variance components models for gene-environment interaction in twin analysis. Twin Research, 5(6), 554–571. doi:Doi 10.1375/136905202762342026 [DOI] [PubMed] [Google Scholar]
- Rhew IC, Kosterman R, Duncan GE, & Mair C (2018). Examination of Cross-Sectional Associations of Neighborhood Deprivation and Alcohol Outlet Density With Hazardous Drinking Using a Twin Design. J Stud Alcohol Drugs, 79(1), 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhew IC, Kosterman R, & Lee JO (2017). Neighborhood Typologies Associated with Alcohol Use among Adults in Their 30s: a Finite Mixture Modeling Approach. J Urban Health. doi: 10.1007/s11524-017-0161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson RJ, & Groves WB (1989). Community Structure and Crime - Testing Social-Disorganization Theory. American Journal of Sociology, 94(4), 774–802. doi:Doi 10.1086/229068 [DOI] [Google Scholar]
- Shanahan MJ, & Hofer SM (2005). Social context in gene-environment interactions: Retrospect and prospect. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 60, 65–76. [DOI] [PubMed] [Google Scholar]
- Singh GK (2003). Area deprivation and widening inequalities in US mortality, 1969–1998. American Journal of Public Health, 93(7), 1137–1143. doi:Doi 10.2105/Ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Deutsch AR, & Piasecki TM (2019). Neighborhood density of alcohol outlets moderates genetic and environmental influences on alcohol problems. Addiction. doi: 10.1111/add.14534 [DOI] [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, & Carlier M (1996). Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behav Genet, 26(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Strachan E, Duncan G, Horn E, & Turkheimer E (2016). Neighborhood deprivation and depression in adult twins: genetics and genexenvironment interaction. Psychol Med, 1–12. doi: 10.1017/S0033291716002622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, … Buchwald D (2013). University of Washington Twin Registry: Poised for the Next Generation of Twin Research. Twin Research and Human Genetics, 16(1), 455–462. doi:Doi 10.1017/Thg.2012.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhinaraset M, Wigglesworth C, & Takeuchi DT (2016). Social and Cultural Contexts of Alcohol Use: Influences in a Social-Ecological Framework. Alcohol research : current reviews, 38(1), 35–45. [PMC free article] [PubMed] [Google Scholar]
- Torgersen S (1979). The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma), 28(3), 225–236. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, & Harden KP (2014). Behavior genetic research methods: Testing quasi-causal hypotheses using multivariate twin data In Reis HT & Judd CM (Eds.), Handbook of Research Methods in Social and Personality Psychology (2nd ed.). New York, NY: Cambridge University Press. [Google Scholar]
- van der Sluis S, Posthuma D, & Dolan CV (2012). A note on false positives and power in G x E modelling of twin data. Behav Genet, 42(1), 170–186. doi: 10.1007/s10519-011-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, & Rathouz PJ (2015). Operating Characteristics of Statistical Methods for Detecting Gene-by-Measured Environment Interaction in the Presence of Gene-Environment Correlation under Violations of Distributional Assumptions. Twin Research and Human Genetics, 18(1), 19–27. doi: 10.1017/thg.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. doi: 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, & Bucholz KK (2008). Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction, 103(8), 1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, & Prescott CA (2011). The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review, 31(5), 800–816. doi:DOI 10.1016/j.cpr.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


